Abstract
Symptomatic neurocysticercosis, a major cause of epilepsy worldwide, results from inflammation around Taenia solium larvae, but the mechanisms are unknown. Eotaxin, not previously reported in cases of human infection, and interleukin-5 (IL-5) but not IL-8 concentrations were elevated in patient serum, and IL-5 levels were also elevated in cerebrospinal fluid (CSF). Eosinophil-selective mediators may be involved in the pathogenesis of cysticercosis. IL-6 concentrations were also elevated in patient CSF, possibly indicative of an acute-phase response.
Cysticercosis causes 20 to 30% of cases of adult-onset epilepsy in Peru (10) and is an emerging infection in the United States (22, 23, 29). Ingestion of Taenia solium (pork tapeworm) eggs causes larval encystment in human tissues, most importantly in the brain. Morbidity usually develops years later as larvae degenerate or are killed by antiparasite therapy (8) when there is inflammation with leukocyte influx. High-dose corticosteroid is the recognized treatment but has limited efficacy, and intracerebral inflammation can prove to be fatal (30). Immunopathogenesis is poorly understood. Downregulation of the Th1 response in favor of Th2 responses was observed in one study of murine infection with Taenia crassiceps (28), while a mixed Th1-Th2 response has been reported more recently (19). In humans, raised soluble interleukin-2 (IL-2) concentrations have been found in cerebrospinal fluid (CSF), suggesting Th1 activity (20).
Histopathological examination of degenerating cysticerci reveals numerous eosinophils, and eosinophilic masses may develop (1). In addition, eosinophils are frequently found in CSF samples from patients (25). Therefore, we have focused on detection of the eosinophil-selective mediators eotaxin and IL-5 (13) and the neutrophil and T-cell attractant IL-8 (15, 31). Eotaxin, first identified in guinea pig lung (11), binds to human CCR3 receptors (14) and is critical in eosinophil recruitment in cellular and animal models (11, 18). Actions of IL-5 include stimulation of eosinophil precursor differentiation in bone marrow, priming of eosinophils, and prolonging of eosinophil survival (17). IL-5 has been detected in the Mazzotti reaction that can follow treatment of onchocerciasis (16). Since this reaction has some similarities to the treatment-associated eosinophilic inflammatory responses around degenerating parasites which may be life threatening in neurocysticercosis, it is possible that eosinophil-selective cytokines are involved in immune responses in cysticercosis. In this study, we also measured concentrations of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) and the acute-phase reactant IL-6 in neurocysticercosis patients.
Study samples were archived material from patients at the Instituto de Ciencias Neurologicas, the main neurological hospital in Lima, Peru. Fourteen neurocysticercosis patients (providing 9 serum and 14 CSF samples) and 23 control subjects (20 serum and 9 CSF samples), with mean ages (± standard deviation) of 30 (± 19) and 46 (± 15) years, respectively, were studied. Seven patients and nine control subjects were male. Neurocysticercosis was diagnosed with standard clinical, radiological, and serological criteria (6). Thirteen neurocysticercosis patients (but no controls) had epilepsy, and one had progressive dementia due to frontal lobe cysticerci. All patients and no controls had T. solium-specific antibodies in serum and/or CSF detected by electroimmunotransfer blotting (26). Computerized tomography brain scans of 11 patients (of whom 2 had obstructive hydrocephalus) revealed multiple viable cysticerci, calcified cysticerci, and contrast-enhancing granulomas. Two patients had single lesions. Fourteen control subjects, from the same geographical area of Peru as the patients, had mechanical, noninflammatory spinal disease and nine had headache, the investigations of which excluded inflammatory or infective etiology.
Serum from antecubital venipuncture was stored in pyrogen-free tubes at −20°C in Peru, transferred on dry ice to the United Kingdom, and kept at −80°C thereafter. Cytokine bioactivity is stable in samples processed in this manner (9). Reliable eosinophil counts were not consistently available at the time of study. CSF was collected by sterile lumbar puncture following administration of local anesthetic. Informed consent from all patients and ethics committee approval from Johns Hopkins University, Baltimore, Md., and AB Prisma, Lima, Peru, were obtained.
Cytokine measurements were performed in duplicate with appropriate controls in laboratories blinded to clinical details. Specific enzyme-linked immunosorbent assays (ELISAs) were used to measure eotaxin and IL-8 (5), and a commercial ELISA was used to measure IL-5 (Biosource, Camarillo, Calif.). Eotaxin was measured in a sandwich ELISA using monoclonal capture and polyclonal detector antibodies (10a). The ELISA was shown to be specific and did not cross-react with any other chemokines available. TNF-α levels were measured with the highly sensitive WEHI 164 cell line, subclone 13 (gift of A. Waage) (7), and IL-6 levels were measured with the specific B9 cell proliferation assay (27). The lower limits of sensitivities for the above assays were 33.6, 50, 11.4, 36, and 7 pg/ml, respectively. Nonparametric data were analyzed with the Mann-Whitney U test.
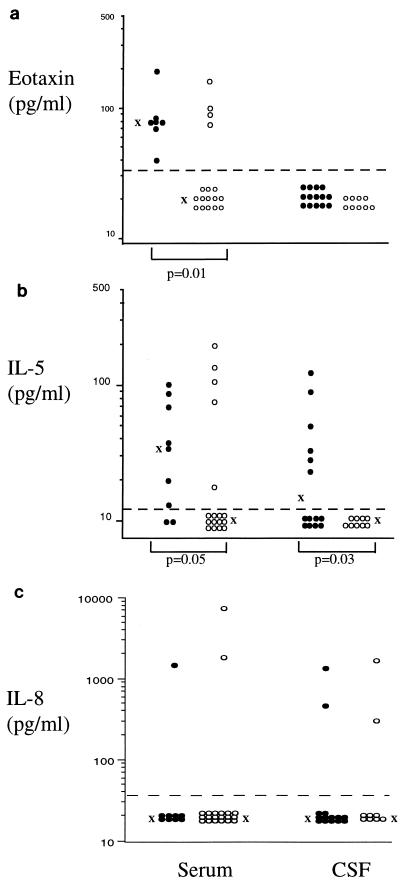
The eosinophil-selective mediator eotaxin was detected in serum of all patients and 23.5% of controls, and concentrations were significantly higher in patients (P = 0.01) (Fig. 1a). Eotaxin was not detected in CSF. IL-5 was detected in serum of 78% of patients compared to 29% of controls. Mean IL-5 concentrations were higher in patients (P = 0.05) (Fig. 1b). IL-5 was detected in 43% of patient CSF and in no controls (P = 0.03). In contrast, IL-8 was only occasionally detected in serum or CSF (Fig. 1c) and was not associated with known pathology.
FIG. 1.
Concentrations of eotaxin (a), IL-5 (b), and IL-8 (c) in serum and CSF of neurocysticercosis patients (•) and control subjects (○). Geometric means of immunoreactive cytokine concentrations are indicated by “X.” Only statistically significant differences between groups are shown.
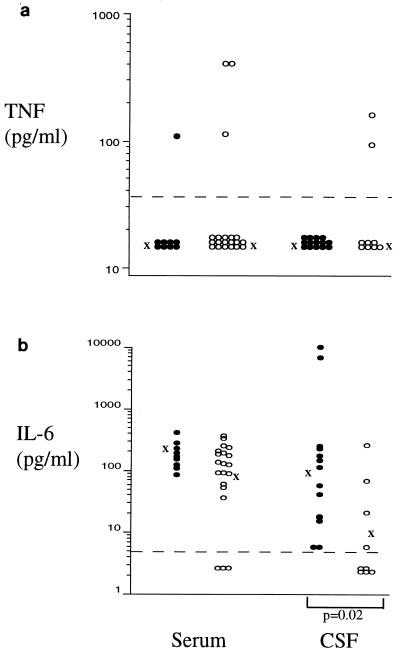
The proinflammatory mediator TNF-α was detected infrequently (Fig. 2a) and was not associated with any known pathology. IL-6 was detected in serum of most patients and control subjects, and concentrations were similar in both groups (Fig. 2b). IL-6 was present in CSF of all patients but in only 44% of controls and at significantly elevated concentrations (P = 0.02).
FIG. 2.
Concentrations of the proinflammatory cytokines TNF-α (a) and IL-6 (b) in serum and CSF of neurocysticercosis patients (•) and control subjects (○). Geometric means of bioactive cytokine concentrations are indicated by “X.” Only statistically significant differences between groups are shown.
The detection of eotaxin and IL-5 but not IL-8 in this study is consistent with a central role for eosinophils in immunity against some helminths. In one series of neurocysticercosis patients, eosinophils were found in over 57% of 326 inflammatory CSF samples (25). Peripheral blood eosinophilia is less common, occurring in 3 to 40% of neurocysticercosis patients, but may develop following surgical removal of cysticerci from the brain, presumably because of parasite antigen release (1). To our knowledge, this is the first report of circulating eotaxin in an infectious disease of humans. However, the physiological significance of eotaxin in cysticercosis cannot be determined from this study. It may have a role in host defense or contribute to inflammatory damage in cysticercosis. It is even possible that both eotaxin and IL-5 secretion represent no more than a bystander phenomenon in this infection. Certainly, in a murine model of schistosomiasis, abrogation of eosinophil recruitment did not result in overwhelming infection (24). However, in other parasitic diseases, such as Angiostrongylus cantonensis CNS infection in mice, eosinophils do appear to have a role in the killing of intracranial worms (21). Full resolution of these issues requires prospective sequential studies.
The presence of eotaxin in serum but not CSF was unexpected. CSF does not contain an inhibitor of the eotaxin in ELISA, since recombinant eotaxin added to CSF was detectable. However, all patients with neurocysticercosis had numerous asymptomatic cysticerci in muscle and subcutaneous tissues, and the assay may only have been sensitive enough to detect eotaxin in the more densely parasitized periphery. Alternatively, eotaxin may be released only during initiation of cellular recruitment and rapidly cleared from CSF. It is also possible that other parasitic infections may contribute to the elevated eotaxin levels, since their presence could not be fully excluded.
The elevated IL-5 concentrations found were consistent with the many reports of IL-5 in parasitic and allergic disorders (17). The predominant action of IL-5 may be to increase the circulating pool of eosinophils while eotaxin acts as a local chemoattractant (4), although recent data suggest eotaxin also has direct regulatory effects on bone marrow. IL-5 was found in the periphery of some control subjects, which may have reflected the presence of intestinal parasites; this could not be excluded in the present study. It is also possible that a second parasite may have contributed to the elevated IL-5 levels detected in patient serum. However, this would not explain the raised CSF IL-5 concentrations which were often found in these patients and the fact that elevated CSF IL-5 levels were usually associated with raised serum IL-5 and eotaxin concentrations. The low levels of IL-8 detected indicate that this chemokine is not an important T-cell attractant in cysticercosis.
Elevated IL-6 concentrations were consistent with the acute-phase protein response seen in many systemic helminth infections. The source of IL-6 is most likely to be intracerebral macrophages and glial cells (2), which may be activated directly by the parasite. TNF-α may stimulate IL-6 secretion, but TNF-α concentrations were seldom elevated in patients. It is possible that transient TNF-α secretion was missed or that bioactive TNF-α may be present only in tissues. It is likely that TNF-α is involved in inflammatory responses in cysticercosis, since these are granulomatous in nature and TNF-α has a central role in granuloma formation in parasitic diseases (3, 12). The patients with hydrocephalus had the two highest IL-6 concentrations and the only TNF-α detected, suggesting that raised intracranial pressure may contribute to proinflammatory responses. We are investigating this observation further in prospective studies.
In summary, this study provides the first data on eotaxin in human infection, and the data are consistent with the hypothesis that eosinophil-selective mediators have a role in either immune responses or inflammatory injury in cysticercosis. However, definitive proof is awaited. In addition, neurocysticercosis is characterized by raised CSF IL-6 levels, probably driving an acute-phase protein response. Longitudinal data on concentrations of eosinophil-selective mediators and their relation to clinical symptoms in cysticercosis patients are now required, since antagonists to eotaxin or IL-5 (currently being considered as potential therapies in asthma and inflammatory bowel disease) may be of value in future management of treatment-associated inflammatory responses.
Acknowledgments
This research was supported in part by a Wellcome Trust biomedical research collaboration grant (H.H.G., R.H.G., and J.S.F.), by Wellcome Trust program grant 038775/Z/93/z/1.27/MP/NOS/jf (T.J.W. and P.J.J.), by National Institutes of Health grants GM 44918 (D.G.R.) and 1-U01 A135894-01 (R.H.G. and H.H.G.), by FDA grant FD-R-001107-01 (R.H.G. and H.H.G.), and by the National Asthma Campaign of the United Kingdom (T.J.W. and P.J.J.).
REFERENCES
- 1.Ali-Khan Z, Siboo R, Meerovitch E, Faubert G, Faucher M G. Cysticercosis racemosus in an eosinophilic phlegmon in the brain. Trans R Soc Trop Med Hyg. 1981;75:774–779. doi: 10.1016/0035-9203(81)90408-9. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 3.Amiri P, Locksley R M, Parslow T G, Sadick M, Rector E, Ritter D, McKerrow J H. Tumour necrosis factor α restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 4.Collins P D, Marleau S, Griffiths-Johnson D A, Jose P J, Williams T J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeForge L E, Remick D G. Sandwich ELISA for detection of picogram quantities of interleukin-8. Immunol Investig. 1991;20:89–97. doi: 10.3109/08820139109054928. [DOI] [PubMed] [Google Scholar]
- 6.Del Brutto O H, Wadia N H, Dumas M, Cruz M, Tsang V C W, Schantz P M. Proposal of diagnostic criteria for human cyticercosis and neurocysticercosis. J Neurol Sci. 1996;142:1–6. doi: 10.1016/0022-510x(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 7.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 8.Evans C, Gonzalez A E, Gilman R H, Verastegui M, Garcia H H, Chavera A . the Cysticercosis Working Group in Peru. Cysticercosis: immunology and immunotherapy. In: Rose F C S, editor. Recent advances in tropical neurology. London, England: Elsevier; 1996. pp. 155–174. [Google Scholar]
- 9.Friedland J S, Suputtamongkol Y, Remick D G, Chaowagul W, Strieter R M, Kunkel S L, White N J, Griffin G E. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun. 1992;60:2402–2408. doi: 10.1128/iai.60.6.2402-2408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia H H, Gilman R, Martinez M, Tsang V C W, Pilcher J B, Herrara G, Diaz F, Alvardo M, Miranda E the Cysticercosis Working Group in Peru. Cysticercosis as a major cause of epilepsy in Peru. Lancet. 1993;341:197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Jose, P. J., et al. Unpublished data.
- 11.Jose P J, Griffiths-Johnson D A, Collins P D, Walsh D T, Moqbel R, Totty N F, Truong O, Hsuan J J, Williams T J. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airway inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph A L, Boros D L. Tumor necrosis factor plays a role in Schistosoma mansoni egg-induced granulomatous formation. J Immunol. 1993;151:5461–5471. [PubMed] [Google Scholar]
- 13.Kita H, Gleich G J. Chemokines active on eosinophils: potential roles in allergic inflammation. J Exp Med. 1996;183:2421–2426. doi: 10.1084/jem.183.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany H L, Murphy P M, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725–7730. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 15.Larsen C G, Anderson A O, Appella E, Oppenheim J J, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 16.Limaya A P, Abrams J S, Silver J E, Awadzi K, Francis H F, Ottesen E A, Nutman T B. Interleukin-5 and posttreatment eosinophilia in patients with onchocerciasis. J Clin Investig. 1991;88:1418–1421. doi: 10.1172/JCI115449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahanty S, Nutman T. Interleukin-5 and its receptor. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. New York, N.Y: Marcel Dekker; 1997. pp. 67–79. [Google Scholar]
- 18.Ponath P D, Qin S, Ringler D J, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo J-A, Newman W, Gutierrez-Ramos J-C, Mackay C R. Cloning of the human eosinophil chemoattractant eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Investig. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson P, Atmar R L, Lewis D E, White A C. Granuloma cytokines in murine cysticercosis. Infect Immun. 1997;65:2925–2931. doi: 10.1128/iai.65.7.2925-2931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolfs A, Muhlschlegel F, Jansen-Rocsseck R, Martins A R, Bedaque E A, Tamburus W M, Pedretti L, Schulte G, Feldmeier H, Kremsner P. Clinical and immunologic follow-up study of patients with neurocysticercosis after treatment with praziquantel. Neurology. 1995;45:532–538. doi: 10.1212/wnl.45.3.532. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 22.Schantz P M, Moore A C, Munoz J L, et al. Neurocysticercosis in an orthodox Jewish community. N Engl J Med. 1992;327:692–695. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- 23.Shandera W X, White A C, Chen J C, Diaz P, Armstrong R. Neurocyticercosis in Houston, Texas: a report of 112 cases. Medicine (Baltimore) 1994;73:37–52. doi: 10.1097/00005792-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Sher A, Coffman R L, Hieny S, Scott P, Cheever A W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotello J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. Arch Intern Med. 1985;145:442–445. [PubMed] [Google Scholar]
- 26.Tsang V C W, Brand J A, Boyer A E. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cyticercosis. J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 27.Ulich T R, Guo K, Remick D G, del Castillo J, Yin S. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 28.Villa O F, Kuhn R E. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1995;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 29.White A C. Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–115. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 30.Woo E, Yu Y L, Huang C Y. Cerebral infarct precipitated by praziquantel in neurocysticercosis: a cautionary note. Trop Geogr Med. 1987;40:143–146. [PubMed] [Google Scholar]
- 31.Yoshimura T, Matsushima K, Tanaka S, Robinson E A, Appella E, Oppenheim J J, Leonard E J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]