Abstract
Two synthetic peptides, corresponding to the N-terminal sequence of the 45-kDa subunit of the protective 9B antigen of Schistosoma mansoni and differing in only one amino acid residue, were synthesized. These peptides were recognized by the protective monoclonal antibody 152-66-9B, as well as by sera of mice and humans infected with schistosomiasis. The peptides were coupled to a protein carrier and used for immunization. One of the peptides, 9B-peptide1, induced in mice significant protection against challenge infection, manifested in a 40 to 50% reduction in worm burden.
Schistosomiasis is a serious parasitic disease that infects over 200 million people worldwide and causes an estimated 500,000 deaths per year. Despite the fact that its global distribution has changed significantly in the past 50 years, particularly in regions where control strategies have been successfully employed, the disease remains endemic in over 70 developing countries (22). Chemotherapy, although effective, does not prevent reinfection, and in addition, partial drug resistance to the most commonly used chemotherapeutic agent against schistosomiasis, praziquantel (7, 11), has been reported. Immunological intervention in the form of a vaccine would contribute to the success of present efforts if added to existing control strategies (22). At present, there are no practical vaccines against any human helminth infection (1, 2, 5). In the case of schistosomiasis, even a partially protective vaccine would be beneficial, since the parasite does not reproduce in the mammalian host and morbidity is correlated with worm burden (23, 24). It has been demonstrated that injection into experimental animals of live cercariae attenuated by sublethal doses of radiation results in high levels of resistance against subsequent reinfection (21). However, though effective, such an irradiated vaccine is not a practical approach, and the search continues for defined antigen vaccines that would protect from initial infection and/or egg-granuloma-associated pathology (5). Development of either recombinant or synthetic peptide vaccines would be a suitable approach for vaccine production based on protective components of the parasite.
Peptide vaccines against many microbial agents have been widely investigated in the past two decades (4) and have been shown to induce partial protection in vivo, e.g., in the cases of measles and influenza (3, 13). In the realm of parasite vaccines, efforts have been made to prepare synthetic peptide vaccines against malaria (6, 17). Against schistosomiasis, efforts are being made to direct specific B-cell and T-helper-cell responses by identifying different epitopes in protective antigens such as Sm23 and triose-phosphate isomerase (19, 20). A cytotoxic T-cell C-terminal lipopeptide has been derived from the vaccine candidate Sm28GST (16) and employed as well in the form of multiple antigen peptide (8).
In our laboratory, we have purified an antigen from Schistosoma mansoni extract by affinity chromatography on a highly protective monoclonal antibody (MAb), 152-66-9B. This antigen, denoted 9B-Ag, is 450 kDa in its native form but migrates as a 200-kDa band in the presence of sodium dodecyl sulfate and, under reducing conditions, exhibits two main subunits, of 45 and 30 kDa (27). We have previously demonstrated that vaccination of mice with 9B-Ag resulted in high levels of protection, ca. 45%, against challenge infection (14, 15, 27). It was of interest, therefore, to explore whether peptide segments of this antigen are capable of inducing a protective effect.
Herewith we report that a 14-residue peptide, corresponding to the N-terminal region of the 9B-Ag 45-kDa subunit and denoted 9B-peptide1, was capable of inducing a significant level of protection of mice (30 to 50%) against challenge infection. A similar peptide, denoted 9B-peptide2 and differing in only the residue in position 7, showed no protective activity. These results are discussed in view of the potential of such a simple, relatively short peptide to serve as a vaccine candidate against schistosomiasis.
Parasite.
A Puerto Rican strain of S. mansoni was maintained in outbred CD1 mice and Biomphalaria glabrata snails. S. mansoni cercariae and schistosomula were prepared as described previously (15). Adult worms were obtained by liver perfusion from chronically infected mice at 6 to 7 weeks postinfection, as previously described (23).
Antisera.
The following mouse sera were obtained from CD1 and C57BL/6J mice: normal mouse serum and serum from acutely infected mice taken 9 weeks after exposure to 300 cercariae. In addition, mouse ascitic fluid was obtained for preparation of the monoclonal antibody (152-66-9B) (27). Individual human serum samples (a gift from Zvi Bentwich, The Kaplan Hospital, Rehovot, Israel) were obtained from Ethiopian immigrants to Israel who had been exposed previously to schistosomiasis. All patients suffered from relatively mild chronic infections, and the samples were obtained before any treatment was applied.
RIA.
Solid-phase radioimmunoassay (RIA) was performed essentially as described by Pierce and Klinman (18), with a slight modification: the antigens were stuck to the plate in the presence of 2% glutaraldehyde–phosphate-buffered saline solution at a concentration of 5 μg of 9B-peptide1 or 9B-peptide2.
ELISA.
For enzyme-linked immunosorbent assay (ELISA), whole-parasite sonicate (10 μg/well), peptide-protein conjugates (5 μg/well) in sodium carbonate buffer (pH 9.6), or free 9B-peptide1 or 9B-peptide2 in 2% glutaraldehyde–phosphate-buffered saline solution served as antigen adsorbed to the plates.
Immunization and protection studies.
Groups of 8 to 10 mice (C57BL/6J) were immunized intradermally and in the foot pads with bovine serum albumin (BSA)-peptide conjugate (50 μg/100 μl), as follows. The first injection was in complete Freund adjuvant (CFA), into the foot pads and in the base of the tail, with a booster injection with the same conjugate in incomplete Freund adjuvant (IFA) by intraperitoneal or subcutaneous injection. Four weeks after the last booster, infection with 70 to 90 cercaria was performed by the ring method (23). Control groups included one of the following: carrier alone (BSA or ovalbumin [OVA]) or adjuvant alone, injected by the same regimen as described above.
Complement-dependent cytotoxicity assay.
The susceptibility of schistosomula to antibodies and complement was determined as previously described (27).
Statistical analysis.
Statistical analysis was performed with the Stat View II program (Abacus Concepts Inc., Berkeley, Calif.).
Peptide sequences and preparation.
N-terminal sequencing analysis was performed on samples, obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, of the 45-kDa subunit of 9B-Ag (∼20 μg) after blotting to polyvinylpyrrolidone paper. Sequences were determined by the Edman degradation method in a 475A Protein Microsequencer (Applied Biosystems) with a 120PTH analyzer and a 900A control data analysis module. The 14-residue peptides were synthesized by M. Fridkin of the Department of Organic Chemistry at The Weizmann Institute by the solid-phase method with a multipeptide synthesizer (Abimed AMS 422) and were purified by high-performance liquid chromatography. Repeated sequencing of this 45-kDa subunit showed two sequences with a difference in one amino acid, at position 7. Accordingly, two peptides were synthesized: 9B-peptide1 (GFTTNEERYNVFAE) and 9B-peptide2 (GFTTNEPRYNVFAE). The peptides were coupled to BSA or OVA by using the water-soluble carbodiimide reagent EDCI for protein conjugation (26). A 1:38 molar ratio of carrier to peptide was used. The lack of lysine residues in either peptide favors binding to the carrier and not cross-linking to itself.
Recognition of the 9B peptides by the 155-66-9B MAb.
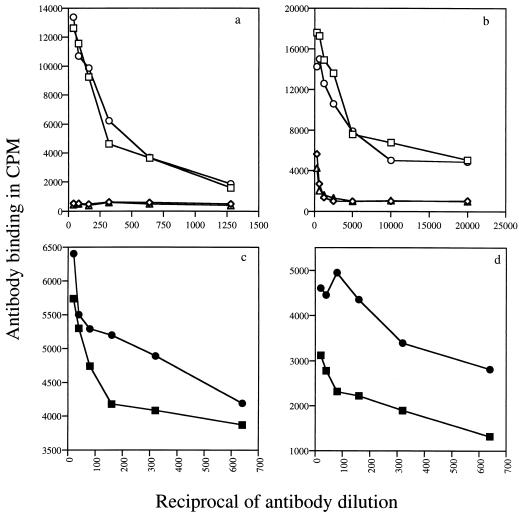
The two synthetic peptides of the N-terminal region of the 45-kDa subunit of 9B-Ag, namely 9B-peptide1 and 9B-peptide2, were bound in their free forms (5 μg/well) or as BSA conjugates to RIA plates and incubated with different concentrations of MAb 152-66-9B immunoglobulin (Ig) over a range of dilutions: 1/10 to 1/1,280 for free peptide and 1/50 to 1/20,000 for the conjugates. Quantitation with 125I-goat anti-mouse Ig showed that the peptides are recognized by the MAb to similar levels (Fig. 1a) and that the binding obtained with the conjugated 9B peptides was higher than that of the free peptides (Fig. 1b).
FIG. 1.
9B peptide recognition by MAb 152-66-9B (a and b) and by sera from acutely infected mice (c and d). Recognition was measure by monitoring antibody binding to free 9B-peptide1 (circles) or 9B-peptide2 (squares) (a and c) or to 9B peptides coupled to OVA (b and d). Binding of nonrelated MAb anti-2,4-dinitrophenol to 9B-peptide1 (▵) or to 9B-peptide2 (◊) is also shown. Results in panels c and d are presented after subtraction of background with control serum.
Reactivity of 9B peptides with infected sera from mouse or human origin.
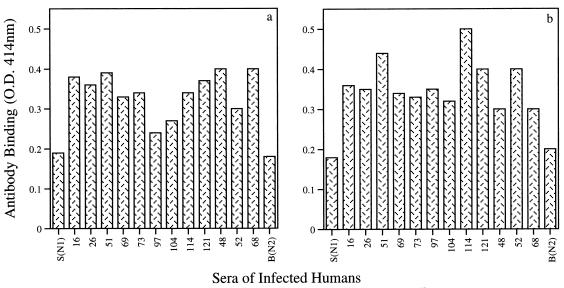
Figure 1c and d demonstrate the recognition of both peptides by infected mouse sera: free 9B-peptide1 is better recognized by the infected mouse sera than 9B-peptide2 (Fig. 1c). The results are in counts per minute of the goat anti-mouse Ig antibody bound to the infected serum after subtraction of counts per minute obtained with the noninfected serum. Similar results were obtained when peptides conjugated to OVA were used as the antigen. Again, 9B-peptide1–OVA is better recognized than 9B-peptide2–OVA by infected mouse sera (Fig. 1d). Free 9B-peptide1 and 9B-peptide2 are also recognized by individual sera from human patients infected with S. mansoni (n = 12), as detected by ELISA. All of the human sera at a 1:80 dilution showed significant binding to the two peptides (Fig. 2). Nonrelated peptides (a commercial mixture of human immunodeficiency virus peptides used for serum evaluation) did not show any binding even when tested with nondiluted serum samples (data not shown).
FIG. 2.
9B peptide recognition by sera from infected humans. Recognition was quantitated by monitoring antibody binding to free 9B-peptide1 (a) or free 9B-peptide2 (b) by ELISA. Bars represent sera from infected individuals at a 1/80 dilution. S(N1) and B(N2) are controls from noninfected individuals. O.D., optical density.
Protection conferred by 9B peptides.
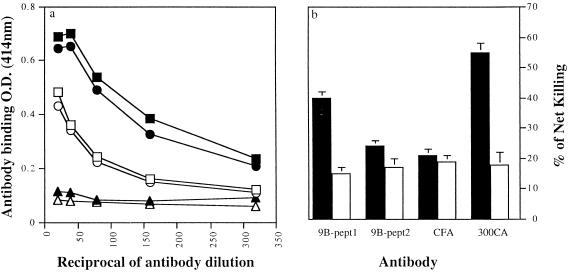
The most important issue was whether the synthetic peptides could induce protection against challenge infection with the parasite. To analyze the protective properties of the 9B peptides, we immunized mice with 50 μg of the BSA-conjugated peptides twice at a 1-month interval and challenged them 1 month later by the ring assay with 70 to 90 cercariae. As shown in Table 1, worm burden was reduced by an average of 45% ± 3% after immunization with 9B-peptide1 conjugate (P, 0.0001 to 0.005) and by only 5% ± 3% after immunization with 9B-peptide2 conjugate, as compared with control mice that either received only carrier or adjuvant alone or were not immunized at all. Sera from vaccinated mice showed significant antibody titers against the respective peptides, as monitored by ELISA with OVA-conjugated peptides as matrix-bound antigen. Anti-9B-peptide1 antisera gave higher titers than anti-9B-peptide2 antisera. No binding to the OVA carrier alone was detected (Fig. 3a).
TABLE 1.
Vaccination of mice with conjugates of the two 9B peptides
| Expt. | No. of mice/group | Treatmenta | Worm burden (mean ± SD) | % Protection |
|---|---|---|---|---|
| 1 | 8 | 9B-Pept1–BSA | 21 ± 2 | 44 |
| 1 | 9 | 9B-Pept2–BSA | 35 ± 3 | 5 |
| 1 | 9 | BSA | 37 ± 3 | |
| 1 | 9 | None | 30 ± 4 | |
| 2 | 9 | 9B-Pept1–BSA | 15 ± 3 | 53 |
| 2 | 11 | BSA | 32 ± 3 | |
| 2 | 9 | CFA | 28 ± 4 | |
| 3 | 16 | 9B-Pept1–BSA | 19 ± 2 | 37 |
| 3 | 9 | 9B-Pept2–BSA | 27 ± 3 | 10 |
| 3 | 8 | BSA | 30 ± 4 | |
| 3 | 8 | CFA | 30 ± 5 | |
| 4 | 5 | 9B-Pept1–BSA | 16 ± 3 | 46 |
| 4 | 6 | 9B-Pept2–BSA | 35 ± 4 | |
| 4 | 7 | BSA | 29 ± 3 |
Mice were immunized with 50 μg of conjugate peptide in CFA as described in the text. One month after the last injection, mice were infected precutaneously with 70 to 90 cercariae. Six or 7 weeks after infection, their livers were perfused and the numbers of adult worms were determined. The number of adult worms in 9B-peptide1 (9B-Pept1)–BSA-treated mice relative to BSA-treated mice in all experiments was significant at 95%, as calculated by Fisher’s exact test.
FIG. 3.
Antibody titers and complement-dependent cytotoxicities of sera from mice vaccinated with BSA conjugates of 9B-peptide1 or 9B-peptide2. (a) Antibody binding, quantitated by ELISA, of anti-9B-peptide1–BSA antibody with OVA conjugates of 9B-peptide1 (■) and 9B-peptide2 (•), as well as unconjugated OVA (▴), and 9B-peptide2–BSA antibody with OVA conjugates of 9B-peptide1 (□) and 9B-peptide2 (○), as well as unconjugated OVA (▵). (b) Complement-mediated cytotoxic effects on schistosomula. Sera from vaccinated mice were added to 200 schistosomula and incubated for 30 min. The susceptibility to complement lysis was determined in the presence of guinea pig complement. Results are expressed as percent net killing of schistosomula, namely, the difference between mortality in the presence of active (filled bars) and of heat-inactivated (open bars) complement for anti-9B-peptide1–BSA and anti-9B-peptide2–BSA. CFA, negative-control sera from mice immunized with CFA alone; 300CA, positive-control sera from mice infected with 300 cercaria.
Complement-dependent cytotoxicity of anti-9B-peptide1.
Antisera produced by vaccinated mice against 9B-peptide1 or 9B-peptide2, were investigated for their capacities to mediate complement-dependent killing of schistosomula. Sera from mice immunized with CFA alone or from normal, untreated mice were used as negative controls, and sera from infected mice were used as positive controls. Experiments were performed with fresh guinea pig serum as a source of complement and with heat-inactivated serum as an internal control. The extent of killing was taken as the difference between the percentages of dead schistosomula in the presence of active and of inactivated complement. The results (Fig. 3b) show that anti-9B-peptide1 antibodies induced significant levels of cytotoxicity (40% of net killing). This value is close to the level of cytotoxicity caused by the positive controls (an average of 55% of killing). The toxic effect of anti-9B-peptide1 mouse antibody was manifested also by tegumental blebbing and granulation of the parasite in the presence of fresh guinea pig complement. In contrast, in the presence of anti-9B-peptide2 antiserum, only 20 to 24% of killing was observed, similarly to the nonspecific killing by control (CFA-injected) serum. Similar low-level killing was observed in the internal controls in the presence of heat-inactivated serum. These results corroborate the protective effect of the 9B-peptide1 conjugate described above.
Synthetic peptides as vaccine candidates have several advantages. They are easy and inexpensive to produce, and therefore, high quantities of a homogeneous product may be obtained while avoiding complicated purification procedures (2, 4). Another advantage is that T-cell and B-cell epitopes may be selected. In recent years, the approach of using peptide epitopes for vaccines against S. mansoni has been developed in several laboratories (12, 16, 19, 20). We have therefore tested the protective effects of peptide epitopes derived from the protective 9B antigen investigated in our laboratory. Our approach to deriving such peptides was by sequencing the N-terminal region of the 45-kDa subunit of the 9B antigen obtained upon affinity purification with the 152-66-9B MAb.
In two separate experiments, N-terminal sequencing revealed two peptides with a single difference in position 7. These two peptides, which have been investigated in this study, seem to be exclusive to the parasite, since the 14-residue sequence did not correlate with any described sequence in protein data banks. B-cell responses are important in the course of schistosomiasis, and the use of antibodies to identify potential vaccine candidates is widely applied. In this realm, serum from mice vaccinated with irradiated cercariae, which can confer partial resistance when transferred to naive recipients, was used to identify other potential vaccine candidates (25). Similarly, the surface antigen glyceraldehyde-3-phosphate dehydrogenase, which is preferentially recognized by serum of resistant patients, is also being investigated as a vaccine candidate (10). The 152-66-9B MAb, which is a protective antibody (27), recognizes the two N-terminal peptides (Fig. 1). Recognition by this MAb indicates that the 9B N-terminal peptide comprises a B-cell epitope, leading us to believe that these peptides may themselves protect against the disease.
Serum from acutely infected CD1 mice, as well as sera from infected humans, recognized the two N-terminal 9B peptides, preferentially 9B-peptide1 (Fig. 1 and 2). The presence of antibodies toward 9B peptides in natural infections thus indicates that in the course of the disease antibodies of this specificity are generated. Vaccination with these peptides might enhance this response.
To investigate the protective role of the 9B-derived peptides, the two peptides coupled to BSA were used to immunize mice. Upon challenge infection, it was observed that 9B-peptide1, which is also better recognized by the infected mice sera, led to 40 to 50% protection, whereas 9B-peptide2 led to only 5 to 10% protection (Table 1). The vaccinated protected mice showed high antibody titers in their sera against 9B-peptide1 and 9B-peptide2 with significant cross-reaction between these two peptides (Fig. 3a), demonstrating some structural similarity of the two B-cell epitopes. However, in their cytotoxic effects the two peptides differed: only 9B-peptide1 induced antibodies capable of specific killing of schistosomula in the presence of complement. Although specific toward a single surface component, the extent of killing by anti-9B-peptide1 serum is high and is comparable with killing by infected mouse serum, which is reactive with multiple parasite antigens (Fig. 3b). Antibodies and complement cause damage by interacting with surface membrane components (9). The damage to the tegument that we observed corroborates these findings.
The protection data are encouraging especially because of the simplicity of peptide synthesis and the relatively small size of the peptide. They also reinforce the notion that even a single epitope, if relevant, could serve as a suitable target for effective immune damage to the parasite. Immunopotentiation of this response by employing appropriate carriers and/or adjuvants could augment the protective effect and should be further investigated. However, even the presently observed level of decrease in worm burden can lead to reduced morbidity and may thus lead to a successful vaccine.
Acknowledgments
This work was supported in part by grant I-0354-080.02-94 from the German-Israel Fund (GIF) and by grant 12603 from The Center for Molecular Biology of Tropical Diseases.
We are indebted to Zvi Bentwich and Sonia Zlotnikov of The Kaplan Hospital, Rehovot, for their collaboration in testing the interaction of the peptides with human serum samples. This paper was written while Ruth Arnon was a Scholar-in-Residence at the Fogarty International Center for Advanced Study in the Health Sciences, National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Arnon R. Immuno-parasitological parameters in schistosomiasis—a perspective view of a vaccine-oriented immunochemist. Vaccine. 1991;9:379–394. doi: 10.1016/0264-410x(91)90123-n. [DOI] [PubMed] [Google Scholar]
- 2.Arnon R. Synthetic peptides as the basis for vaccine design. Mol Immunol. 1991;28:209–215. doi: 10.1016/0161-5890(91)90063-p. [DOI] [PubMed] [Google Scholar]
- 3.Arnon R, Levi R. Synthetic recombinant vaccines induce anti-influenza long-term immunity and cross-strain protection. In: Cohen S, Shafferman A, editors. Novel strategies in design and production of vaccines. New York, N.Y: Plenum Press; 1996. pp. 23–29. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yedidia T, Arnon R. Design of peptide and polypeptide vaccines. Curr Opin Biotechnol. 1997;8:442–448. doi: 10.1016/s0958-1669(97)80066-3. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist N R. Controlling schistosomiasis by vaccination: a realistic option? Parasitol Today. 1995;11:191–194. [Google Scholar]
- 6.de Oliveira G, Clavijo P, Nussenzweig R S, Nardin E H. Immunogenicity of an alum-adsorbed synthetic multiple-antigen peptide based on B- and T-cell epitopes of the Plasmodium falciparum CS protein: possible vaccine application. Vaccine. 1994;12:1012–1017. doi: 10.1016/0264-410x(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Fallon P G, Sturrock R F, Niang A C, Doenhoff M J. Diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:61–62. [PubMed] [Google Scholar]
- 8.Ferru I, Georges B, Bossus M, Estaquier J, Delacre M, Harn D A, Tartar A, Capron A, Grassmasse H, Auriault C. Analysis of the immune response elicited by a multiple antigen peptide (MAP) composed of two distinct protective antigens derived from the parasite Schistosoma mansoni. Parasite Immunol. 1997;19:1–11. doi: 10.1046/j.1365-3024.1997.d01-138.x. [DOI] [PubMed] [Google Scholar]
- 9.Fishelson Z. Complement and parasitic trematodes. Parasitol Today. 1989;5:19–25. doi: 10.1016/0169-4758(89)90218-4. [DOI] [PubMed] [Google Scholar]
- 10.Goudot C V, Caillol D, Jabali M D, Dessein A J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989;170:2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail M, Metwally A, Farghaly A, Bruce J, Tao L F, Bennett J L. Characterization of isolates of Schistosoma mansoni from Egyptian villager that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic D, Aslund L, Oswald I P, Caspar P, Champion C, Pearce E, Coligan J E, Strand M, Sher A, James S L. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996;157:806–814. [PubMed] [Google Scholar]
- 13.Levi R, Pirak-Aboud E, Leclerc C, Lowell G H, Arnon R. Intranasal immunization of mice against influenza with synthetic peptides anchored to proteosomes. Vaccine. 1995;13:1353–1358. doi: 10.1016/0264-410x(94)00083-y. [DOI] [PubMed] [Google Scholar]
- 14.Mendlovic F, Arnon R, Tarrab-Hazdai R, Puri J. Genetic control of immune response to a purified Schistosoma mansoni antigen. II. Establishment and characterization of specific I-A and I-E restricted T-cell clones. Parasite Immunol. 1989;11:683–694. doi: 10.1111/j.1365-3024.1989.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 15.Mendlovic F, Tarrab-Hazdai R, Puri J, Arnon R. Genetic control of immune response to a purified Schistosoma mansoni antigen. I. Effect of MHC class II antigens on the cellular, humoral and protective responses. Parasite Immunol. 1989;11:667–682. doi: 10.1111/j.1365-3024.1989.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 16.Pancre V, Gras-Masse H, Delanoye A, Herno J, Capron A, Auriault C. Induction of cytotoxic T-cell activity by the protective antigen of Schistosoma mansoni Sm28GST or its derived C-terminal lipopeptide. Scand J Immunol. 1996;44:485–492. doi: 10.1046/j.1365-3083.1996.d01-340.x. [DOI] [PubMed] [Google Scholar]
- 17.Patarroyo M E, Romero P, Torres M L, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabazas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 18.Pierce S K, Klinman N R. Allogenic carrier-specific enhancement of hapten-specific secondary B-cell responses. J Exp Med. 1967;114:1254–1261. doi: 10.1084/jem.144.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds S R, Dahl C E, Harn D A. T and B epitope determination and analysis of multiple antigenic peptides for Schistosoma mansoni. Experimental vaccine triose-phosphate isomerase. J Immunol. 1994;152:193–200. [PubMed] [Google Scholar]
- 20.Reynolds S R, Schoemaker C B, Harn D A. T and B cell epitope mapping of Sm23, an integral membrane protein of Schistosoma mansoni. J Immunol. 1992;149:3995–4001. [PubMed] [Google Scholar]
- 21.Richter D, Harn D A, Matuschka F R. The irradiated cercariae vaccine model: looking on the bright side of radiation. Parasitol Today. 1995;8:288–293. doi: 10.1016/0169-4758(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 22.Savioli I, Renganathan E, Montresor A, Davis A, Behbehani K. Control of schistosomiasis—a global picture. Parasitol Today. 1997;13:444–448. doi: 10.1016/s0169-4758(97)01141-1. [DOI] [PubMed] [Google Scholar]
- 23.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of S. mansoni and the recovery of adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 24.Smithers S R, Terry R J. Immunity in schistosomiasis. Am J Acad Sci. 1969;160:826–840. doi: 10.1111/j.1749-6632.1969.tb15904.x. [DOI] [PubMed] [Google Scholar]
- 25.Soisson L M, Masterson C P, Tom T D, McNally M T, Lowell G H, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–3620. [PubMed] [Google Scholar]
- 26.Staros J V, Wright R W, Swingle D M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 27.Tarrab-Hazdai R, Levi-Schaffer F, Brenner V, Horowitz S, Eshhar Z, Arnon R. Protective monoclonal antibodies against Schistosoma mansoni antigen isolation, and suitability for active immunization. J Immunol. 1985;135:2772–2779. [PubMed] [Google Scholar]