Abstract
Pancreatitis, encompassing acute and chronic forms, and pancreatic cancer pose significant challenges to the exocrine tissue of the pancreas. Recurrence rates and complications following acute pancreatitis episodes can lead to long-term risks, including diabetes mellitus. Chronic pancreatitis can develop in approximately 15% of cases, regardless of the initial episode’s severity. Alcohol-induced pancreatitis, idiopathic causes, cigarette smoking, and hereditary pancreatitis contribute to the progression to chronic pancreatitis. Chronic pancreatitis is associated with an increased risk of pancreatic cancer, with older age at onset and smoking identified as risk factors. This scoping review aims to synthesise recent publications (2017–2022) on the diagnostic differentiation between pancreatitis and pancreatic cancer while identifying knowledge gaps in the field. The review focuses on biomarkers and imaging techniques in individuals with pancreatitis and pancreatic cancer. Promising biomarkers such as faecal elastase-1 and specific chemokines offer non-invasive ways to assess pancreatic insufficiency and detect early biomarkers for chronic pancreatitis. Imaging techniques, including computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), and positron emission tomography (PET), aid in differentiating between chronic pancreatitis and pancreatic cancer. However, accurately distinguishing between the two conditions remains a challenge, particularly when a mass is present in the head of the pancreas. Several knowledge gaps persist despite advancements in understanding the association between pancreatitis and pancreatic cancer, including the correlation between histopathological grading systems, non-invasive imaging techniques, and biomarkers in chronic pancreatitis to determine the risk of progression to pancreatic cancer, as well as differentiating between the two conditions. Further research is necessary to enhance our understanding of these aspects, which can ultimately improve the diagnosis and management of pancreatitis and pancreatic cancer.
Keywords: pancreatitis, chronic pancreatitis, pancreatic adenocarcinoma, pancreatic carcinoma, pancreatic cancer
1. Introduction
Acute and chronic pancreatitis (CP), together with pancreatic cancer (PC), account for a significant proportion of diseases affecting the exocrine tissue of the pancreatic gland [1]. A nationwide study of acute pancreatitis (AP) in Japan showed a recurrence rate of 20% and a complication rate with diabetes mellitus of up to 54% following index AP [2]. The same study demonstrated a transition to CP in up to 15% of cases, with no difference in transition rates based on the severity stratification of AP [2]. A different study showed that recurrent acute pancreatitis (RAP) progresses to CP, with progressive acute pancreatitis (PAP) accounting for 24% of AP [3]. A retrospective cohort study in Beijing demonstrated that independent risk factors for progression to CP included more than four episodes of RAP, idiopathic pancreatitis, and pseudocysts [4].
This progression is worse with alcohol-induced pancreatitis (48%), idiopathic causes (47%), cigarette smoking, and hereditary pancreatitis than other aetiologies [3]. In the Japanese study, the transition rate was found to be significantly higher in the alcohol-induced AP cohort compared to all other causes [2]. Alcohol and cigarette use increase the susceptibility of AP to recurring episodes by reducing the threshold for triggering trypsinogen activation in acinar cells, hindering the secretion of pancreatic duct cells, or affecting the immune system, resulting in chronic inflammation. This is thought to be due to the failure of protective mechanisms in the normal healing process, such as DNA repair, apoptosis, and the immune-mediated elimination of dysplastic cells [5]. The overall mortality in the AP cohort is worse in the PAP than in the nonprogressive acute pancreatitis cohorts [3].
Several risk factors for PC have been identified, including cigarette smoking, alcohol use, diabetes mellitus, and CP (see Figure 1 for the risk factors and relative risk) [6,7]. The latter is responsible for up to a 16-fold increased risk of PC [8]. In one study, Korpela et al. found the incidence of PC in CP cohorts to be 6.6%, with older age at onset and smoking as the risk factors. They also found features of CP in 38.8% of histopathological specimens following surgery for PC [9]. The classic histopathological features used to diagnose CP include fibrosis, acinar atrophy, and ductal abnormalities. However, there are no specific distinguishing features for different aetiologies of CP based on histopathology, which underscores the significance of correlation with clinical and radiological findings. Furthermore, there is no consensus on the histopathological grading system for the severity of CP. Some pathologists classify CP into mild, moderate, and severe CP using the fibrosis scoring system proposed by Klöppel and Mallet in 1991 [10].
Figure 1.
The average lag period between CP and PC is reported to be about 20 years [1]. However, there is a linear relationship between the proportion of CP patients developing PC over time, with 1.8% of patients diagnosed with PC after 10 years of diagnosis with pancreatitis and 4% after 20 years [11].
It is still unclear how much of this observed risk factor of CP is confounded by cigarette smoking and alcohol use, both of which are independent risk factors for PC [8]. Even though 70% of cases of CP are attributed to alcohol abuse, a large majority of alcohol users (95%) never suffer from CP [5].
The relationship between CP and PC is further illustrated by the observation that a significant proportion of PC (~5%) is misdiagnosed as CP, leading to potentially poorer outcomes occasioned by delay in diagnosis [8]. There are usually diagnostic difficulties between PC and CP in the case of a mass in the head of the pancreas due to the similar features of a hard mass, vascular invasion, or adjacent organ invasion seen in both diseases. The incidence of malignancy in CP patients with an apparent inflammatory mass in the head of the pancreas may be as high as 33.7% [12]. Epidemiology studies in the United States have demonstrated an overlap between patients presenting with recurrent acute pancreatitis and the development of CP and PC [13].
This association between CP and PC follows other patterns of association between chronic inflammation and subsequent malignancies [14]. Ninety percent of PCs have K-ras oncogene mutations, which are also found in CP sufferers, suggesting a likely common pathophysiology between the two diseases [8].
This review aims to qualitatively synthesise recent literature on the preoperative diagnostic differentiation between pancreatitis and PC and identify potential knowledge gaps.
2. Methodology
2.1. Search Strategy
We searched four online databases (Cochrane, PubMed, Web of Science, and Google Scholar) plus grey literature. The combination of medical subject headings (MeSH) used in the search was “pancreatitis”, or “chronic pancreatitis” and “pancreatic cancer” or “pancreatic carcinoma” or “pancreatic adenocarcinoma” appearing in the title of the publication. We used the Boolean operators (‘AND’ and ‘OR’) to combine keywords effectively and applied filters to limit the search results to the years 2017 to 2022. The search criteria were modified post hoc to focus on preoperative diagnostic differentiation between pancreatitis and pancreatic cancer and were applied to all citations for assessment of relevance. The citation lists obtained were added to a reference manager, and an analysis of text words appearing in the title and abstracts was initially conducted.
PDF copies of all the references were uploaded, and duplicates were removed. NvivoR 12 version 1.7.1 (Lumivero) was used to code the literature under the following headings and subheadings.
2.2. Study Type
Case reports.
Randomised controlled studies.
Cohort studies.
Systematic reviews and meta-analyses.
2.3. Diagnostic Differentiation
Biomarkers.
Imaging.
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
All publications from 2017 to October 2022, including case reports, cohort studies, randomised controlled studies, and systematic reviews and meta-analyses, with a combination of MeSH appearing in the title of the publication, were included.
2.4.2. Exclusion Criteria
Animal studies, non-English language publications, e-posters, conference reports, letters, supplements, textbook chapters, or theses were excluded.
The literature search findings are reported using a flow diagram following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews (PRISMA-ScR) [15]. The protocol of the scoping review has been registered on Open Science Framework registries https://doi.org/10.17605/OSF.IO/UVHZX accessed on 12 February 2023.
3. Results
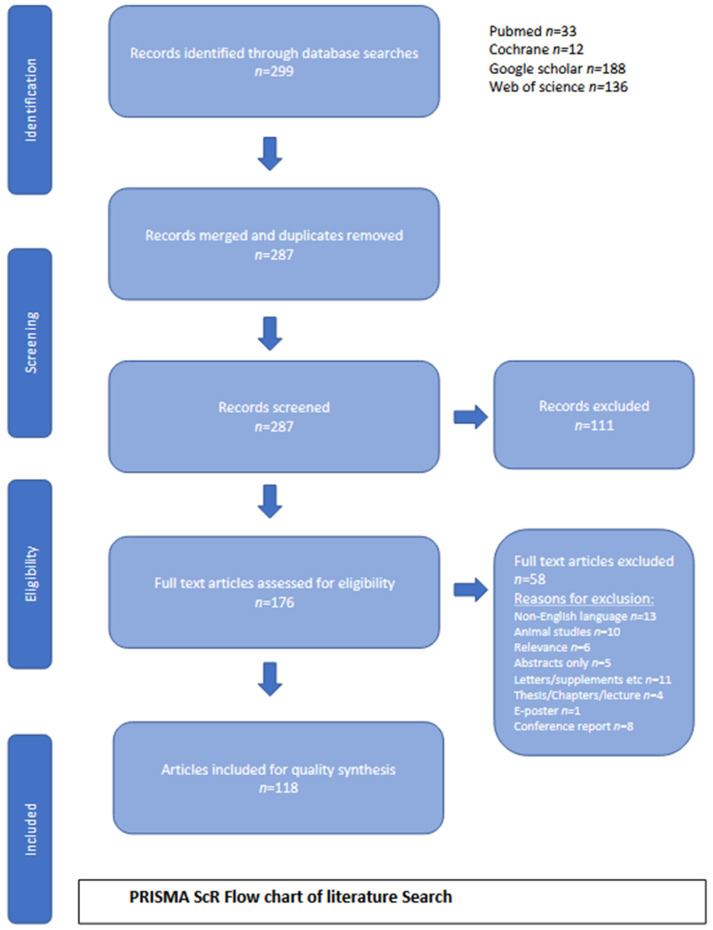
Figure 2 represents a comprehensive flow chart outlining the outcomes of our literature search. A total of 299 articles were initially identified from the Pubmed, Cochrane, Google Scholar, and Web of Science databases. After merging records and removing duplicates, the abstracts of the remaining articles were screened to determine their relevance. Among these, 176 full-text articles were assessed for eligibility, and 58 were excluded based on the predefined exclusion criteria. Ultimately, 118 articles were included in the study for qualitative synthesis. Among the included articles, there were 75 cohort studies, 31 reviews/meta-analyses, and 12 case reports. Notably, no randomised controlled trials were identified during the search process.
Figure 2.
Prisma-ScR flow chart.
3.1. Data Analysis and Presentation
3.1.1. Diagnostic Differentiation
There were 92 out of 118 articles that covered the subject of diagnosis with overlap across the two subheadings coded for diagnosis, namely, “Biomarkers” (in 60 articles out of 92) and “Imaging” (46 out of 92).
3.1.2. Biomarkers
Data charting tool included:
Authors.
Year of publication.
Country of origin.
Biomarkers identified for diagnostic differentiation.
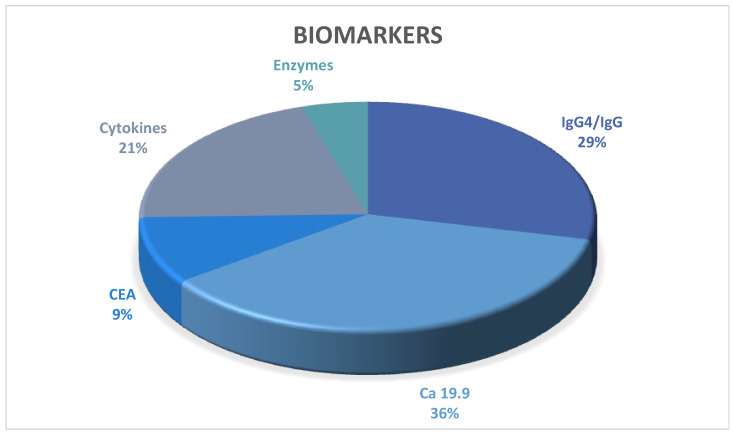
The absolute number of publications that presented biomarkers were as follows:
Ca 19.9 (23), IgG4+/IgG (18), Cytokines and chemokines (13), CEA (6), and Enzymes, including faecal elastase-1 and amylase (3). Figure 3 summarises biomarkers published for diagnostic differentiation in the period under review.
Figure 3.
Biomarkers.
See Table 1 for the biomarker data extracted from each publication.
Table 1.
Studies included for review of biomarkers.
| First Author | Year | Country | Biomarkers |
|---|---|---|---|
|
2019 | China | IgG4+/IgG+ plasma cell ratio > 40% |
|
2018 | China | overexpression of galectin-1 promotes PSC activity |
|
2018 | Germany | PDAC stroma > mucin content than CP |
|
2019 | Czech Republic | Plasmatic IgG4 levels >135 mg/dL in PC |
|
2020 | China | Pancreatic stellate cell (PSC)-stimulating factors |
|
2019 | Portugal | CA19-9 and IgG4 |
|
2019 | Romania | CA 199 and glycosylation alterations |
|
2017 | Finland | P-suPAR was significantly higher in PC |
|
2018 | China | IgG4 high specificity and low sensitivity (AIPvsPC) |
|
2020 | Taiwan | use of mass spectrometry for protein biomarkers |
|
2022 | China | CA19-9 and KRAS mutations in blood |
|
2021 | France | nuclear protein 1 (NUPR1 |
|
2020 | USA | unique immune signature panels |
|
2018 | Germany | matrix metalloproteinase inhibitor TIMP1 |
|
2019 | Germany | Interleukin-18 |
|
2017 | Czech Republic | IgG4 levels |
|
2021 | Denmark | Low and high amylase is associated with PC and CP |
|
2018 | USA | transferrin, ER-60 protein, proapolipoprotein, tropomyosin 1, alpha 1 actin precursor, ACTB protein, and gamma 2 propeptide, aldehyde dehydrogenase 1A1, pancreatic lipase, and annexin A1 |
|
2019 | China | ratio of IgG4/IgG and CA 19-9 |
|
2017 | China | Ca19-9 |
|
2020 | USA | Cytokines IL-4, IL-5, IL-6, IL-13, IL-15, IL-17, IL-18, IFN-γ, TNF-α, and chemokines |
|
2020 | Japan | serum IgG4 and CA19-9 |
|
2018 | Denmark | anti-plasminogen binding peptide, anticarbonic anhydrase II, IgG4 |
|
2020 | Poland | MMP-2, MMP-9, CA19-9, and CEA |
|
2021 | Poland | αSMA expression higher in tumours > than 3 cm |
|
2020 | Taiwan | CA19-9, CEA, CRP and IgG4 |
|
2017 | Finland | Proteomics |
|
2022 | China | sodium–glucose cotransporter 2 inhibitors vs. dipeptidyl peptidase-4 inhibitors |
|
2019 | China | serum CEA and CA19-9 |
|
2021 | China | Macrophage Migration Inhibitory Factor |
|
2021 | China | serum exosomal microRNAs |
|
2021 | Kazakhstan | IgG4, IL-4, IL-5, IL-13, IL-10, and TGF-β |
|
2019 | UK | IgG4 immunohistochemistry |
|
2020 | Germany | Novel Autoantibody Signatures |
|
2019 | India | cytokines |
|
2022 | Germany | scRNAseq and bioinformatics analyses |
|
2017 | Sweden | Glycocholic acid, N-palmitoyl glutamic acid and hexanoylcarnitine |
|
2020 | Poland | pentraxin 3 (PTX3) |
|
2018 | Denmark | cytokines and chemokines |
|
2020 | China | CA19-9 |
|
2021 | Finland | polyamines—acetylputrescine, diacetylspermidine, N8-acetylspermidine and diacetylputrescine |
|
2022 | USA | CA19-9, IgG4 |
|
2018 | China | CA19-9 |
|
2021 | Czech Republic | cytotoxin-associated gene A |
|
2021 | Czech Republic | IgG4 |
|
2020 | Finland | CA19-9, CEA |
|
2020 | Italy | CA19-9 |
|
2020 | China | T Lymphocytes |
|
2017 | USA | CA19-9 |
|
2018 | India | CA19-9 |
|
2018 | Taiwan | IgG and IgG4 levels |
|
2021 | India | Cytokines and chemokines |
|
2018 | Canada | CA19-9 and IgG4/IgG |
|
2021 | Japan | CA19-9 and IgG4/IgG |
|
2022 | South Korea | CA19-9 |
|
2017 | China | CA19-9 |
|
2019 | Lithuania | Faecal elastase-1 |
|
2021 | Netherlands | CA19-9 |
|
2021 | Japan | CEA, CA19-9, IgG4 |
|
2019 | Japan | CEA, CA19-9, IgG4 |
3.1.3. Imaging
Data charting tool included:
Authors.
Year of publication.
Country of origin.
Cross-sectional imaging identified for diagnostic differentiation.
The absolute number of publications that presented on cross-sectional imaging were as follows: CT/enhanced CT radiomics (31), EUS/CEUS/EUS FNA/EUS FNB (20), MRI/MRCP/MR elastography or tomoelastography (16), FDG PET (8), ERCP (7), TAUS (3), and Infrared spectroscopy (1). Figure 4 summarises imaging types published for diagnostic differentiation in recent publications.
Figure 4.
Imaging.
See Table 2 for the imaging data extracted from each publication.
Table 2.
Studies included for review of imaging.
| First Author | Year of Publication | Country | Modalities |
|---|---|---|---|
|
2017 | China | 18F-FDG PET/CT |
|
2021 | Netherlands | CT, EUS, MRI |
|
2021 | China | CT and MRI |
|
2020 | Finland | CT, MRCP, US, FDG PET/CT, EUS |
|
2021 | China | CT |
|
2021 | Turkey | CT, ERCP |
|
2022 | USA | CT, ERCP, EUS |
|
2018 | Canada | US, MRI, CT, ERCP |
|
2020 | Taiwan | CT, MRCP |
|
2019 | China | CT |
|
2021 | Japan | 18F- FDG PET/CT |
|
2021 | Japan | CT |
|
2018 | South Korea | CEUS |
|
2020 | Thailand | CT and MRI |
|
2020 | Lithuania | CT- and MRI |
|
2022 | Italy | EUS |
|
2019 | UK | CT |
|
2019 | Japan | CT, MRI, FDG-PET |
|
2018 | Germany | MDCT, B-mode EUS, ESE, CELMI-EUS, EUS-FNA |
|
2021 | China | MR elastography and tomoelastography |
|
2018 | Canada | EUS |
|
2019 | China | ERCP |
|
2018 | China | CT |
|
2020 | Italy | EUS-FNB and EUS-FNA |
|
2018 | Netherlands | EUS |
|
2021 | Spain | CT |
|
2020 | USA | CT |
|
2022 | South Korea | CT and MRI |
|
2022 | Germany | infrared spectroscopy |
|
2018 | Taiwan | CT, MRI, FDG-PET, EUS |
|
2017 | France | CT |
|
2018 | India | CT, MRI, FDG-PET, EUS, CE-EUS |
|
2019 | Czech Republic | US, EUS |
|
2017 | USA | CT, MRCP, EUS, ERCP |
|
2018 | India | FDG-PET, EUS |
|
2019 | USA | CT and MRI, and MRCP |
|
2022 | China | Enhanced CT Radiomics |
|
2020 | Japan | EUS-FNA, CE-EUS, CT |
|
2019 | USA | EUS-FNA, MRI, CT |
|
2017 | Czech Republic | CT, EUS, ERCP |
|
2020 | China | US, CEUS |
|
2021 | China | EUS |
|
2020 | Turkey | CT |
|
2019 | Portugal | CT, ERCP |
|
2019 | China | CT, MRI, FDG-PET |
|
2018 | Denmark | EUS |
4. Discussion
We searched recent publications to establish if there are new studies on biomarkers and imaging that may help with the preoperative diagnostic differentiation between CP and PC. Although there are many studies on Ca 19-9 as a biomarker of CP and PC, there is still uncertainty with respect to sensitivity and specificity regarding its use. Ca 19-9 is commonly used for the diagnosis of PDAC, but its routine use is not recommended in patients with CP due to its low specificity. Faecal elastase-1 and specific chemokines offer non-invasive ways to assess pancreatic insufficiency and detect early biomarkers for CP. There are a few novel biomarkers that hold promise but more research is still needed.
Direct markers of pancreatic exocrine function are invasive because they involve obtaining pancreatic juices via endoscopy or Dreiling tube following stimulation by secretin or cholecystokinin. Bicarbonate, lipase, or trypsin is then measured, and these measurements are highly sensitive for late CP but have a lower sensitivity range of 70–75% for early chronic pancreatitis [25]. Indirect markers, on the other hand, are non-invasive tests of pancreatic insufficiency. One example is faecal elastase-1, with a cutoff of 100 micrograms having a sensitivity of 46.5% and a specificity of 88% for the diagnosis of CP [25]. Early biomarkers for CP include chemokines like transforming growth factor-Beta 1 (TGF- β1), platelet-derived growth factor BB, and chemerin, which are elevated in CP [25]. Other reported markers are des-Leu albumin, which is present in 68% of CP, and YKL-40, a mammalian chitinase-like protein, which is elevated in CP [25].
The serologic marker for autoimmune pancreatitis (AIP) is immunoglobulin G4 (IgG4). Its elevation supports the diagnosis of AIP, but normal levels do not exclude it. It can also be elevated in 10% of patients with pancreatic cancer [24,57].
There are no highly sensitive nor specific tumour markers for PC [25]. Although serum carbohydrate antigen 19-9 (Ca 19-9) is commonly used for the diagnosis of pancreatic ductal adenocarcinoma (PDAC), its routine use is not recommended in the cohort of CP patients due to its low specificity because inflammation is found in both conditions [72]. The reported sensitivity and specificity of Ca 19-9 are 78% and 83%, respectively, while those of carcinoembryonic antigen (CEA) are 44% and 85%, respectively [25,36].
Pancreatic stellate cells are the main cells involved in the fibrosis observed in PDAC and CP by activating the α-smooth muscle actin (αSMA), which is strongly expressed in PDAC and moderately in CP when contrasted to the weak expression observed in healthy individuals. This immunoexpression of the αSMA protein is higher in larger tumours and higher grades of differentiation of tumours [40].
There are other novel biomarkers for PC with promising early results like tissue inhibitor of metalloproteinase 1 (TIMP-1), matrix metalloproteinase 9 (MMP-9), urokinase-type plasminogen activator receptor (uPAR), osteopontin, heat shock protein 70 (HSP 70), and macrophage inhibitor cytokine (MIC-1) [25,36].
Imaging modalities such as CT, MRI, EUS, and PET/CT are used to distinguish between CP and PC. EUS-guided FNB was found to have higher diagnostic accuracy than EUS-guided FNA for differentiating pseudo-tumour-like pancreatitis from PC. CEH-EUS improves specificity when differentiating focal AIP from PC, and FDG PET has a higher sensitivity for AIP than for PC. It is important to differentiate between PC and CP for early detection and appropriate management.
Korpela et al. recommend that patients with a biliary stricture and other risk factors for PC, including higher age at onset of CP, should undergo assessment with computed tomography (CT) and endoscopic ultrasound (EUS) [9]. Several imaging features may help distinguish CP from PC on CT and magnetic resonance imaging (MRI). A hypovascular mass associated with a smooth dilatation of an upstream pancreatic duct and parenchymal atrophy is more suggestive of PC as opposed to an irregular pancreatic duct, and it is a penetrating duct sign that is more in keeping with focal chronic pancreatitis [80]. A double duct sign is nonspecific for PC, as it does occur in benign pathologies like choledocholithiasis and CP. However, the common bile duct stricture in the head of the pancreas tends to be longer and tapered in CP versus the short, abrupt cutoff stenosis observed in PC [80]. Isolated pancreatic duct dilatation has a 35% higher probability of being PC in the absence of CP [80]. The presence of a mass on CT or MRI in chronic calcifying pancreatitis associated with a dilated common bile duct (CBD) is suggestive of a malignancy [89].
Diffusion-weighted imaging and apparent diffusion coefficient values alone cannot distinguish PC from CP [80]; however, combined detection sensitivity and specificity of CT and Diffusion-Weighted Imaging–Magnetic Resonance Imaging (DWI-MRI) with Magnetic Resonance Cholangiopancreatography (MRCP) for mass-forming pancreatitis and PC is higher than either modality on their own [76].
Compared to conventional ultrasound, contrast-enhanced ultrasound time–intensity curves (TICs) with SonoVue® [bracco imaging] contrast is better at distinguishing pseudo-tumour-like pancreatitis from PC [94].
Contrast-enhanced high mechanical index EUS has a higher sensitivity and specificity (96% and 91%, respectively) to discriminate CP from PDAC than B-mode EUS (92% and 63%, respectively), endoscopic sonoelastography (96% and 38%, respectively), and multidetector contrast-enhanced CT (89% and 70%, respectively) [82]. EUS-guided fine-needle biopsy (FNB) has higher diagnostic accuracy and sensitivity for differentiating pseudo-tumour-like pancreatitis from PC than EUS-guided fine-needle aspiration (FNA) [61]. A recent retrospective study suggested that EUS should still form part of the diagnostic algorithm for evaluating acute, idiopathic, or CPof an unclear cause even when CT/MRI does not show a mass lesion because there is still a 5.3% risk of PC [93].
The combination of endoscopic retrograde cholangiopancreatography (ERCP) with tumour markers (Ca 19-9 and CEA) improves the diagnostic accuracy and sensitivity of PC from pseudo-tumour-like pancreatitis, thereby lowering the rate of a missed diagnosis of PC [44].
Even though imaging signs overlap between AIP and PC, CT values for AIP are significantly higher than those for PC [77]. Where there is a pancreatic mass, typical features of PC should be excluded on CT or MRI, those typical features being hypoattenuating lesion, pancreatic parenchymal compression by mass, abrupt cutoff of the dilated pancreatic duct with distal atrophy of the gland, vessel involvement, double-duct signs, and lymphadenopathy [41]. The CT values in an AIP cohort were statistically higher than in a PC cohort [77]. PDAC has a significantly higher stiffness and fluidity than AIP and healthy patients on MRI tomoelastography [83]. Although AIP resembles PC on cross-sectional imaging, EUS may differentiate between the two pathologies owing to peripancreatic hypoechoic margins (PHMs), which are present in 40% of focal AIP1 patients but not seen in PC [81]. The pancreatic duct wall was thickened in 67% of a focal AIP1 cohort compared to 6.7% of a PC cohort [81]. Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) improved specificity when differentiating focal AIP from PC [79].
Studies on AIP and PC show that cross-sectional imaging using early [18] F-FDG PET/CT scans (PET60min) has a predominant focal metabolic avidity with a higher average uptake (SUVmax of 7.30 ± 3.21) in PC when compared to a predominantly diffuse pattern of avidity in AIP with an average SUVmax of 5.24 ± 1.81. Just over 50% of cases in PC tend to have pancreatic duct dilatation, which is only observed in a quarter of cases in AIP [75]. FDG PET has a higher sensitivity for AIP than for PC [68].
5. Conclusions
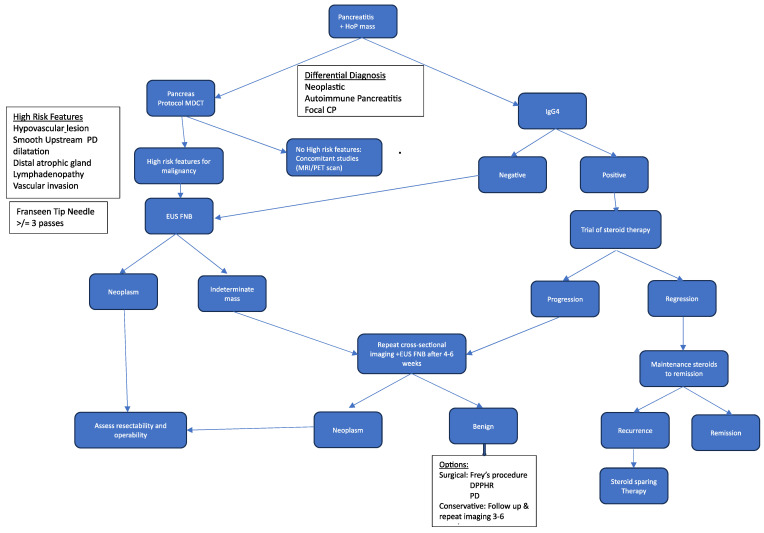
A review of research on pancreatitis and PC in the last five years demonstrates extensive exploratory work. However, much is unknown and poorly understood regarding their association with regard to disease progression from pancreatitis to PC, and there is still a significant challenge in differentiating between the two entities. A misdiagnosis of pancreatitis as PC is accompanied by high morbidity resulting from inappropriate management, and a missed diagnosis of PC is accompanied by mortality due to the delay in management. Some of the areas needing further exploration are the correlation between the histopathological grading systems with non-invasive imaging and biomarkers of CP and whether the risk of progression to PC in CP cohorts is associated with a specific grading or severity scoring. Because of the low sensitivity and specificity in the current imaging modalities and biomarkers in use, there is a need for further research on radiomics, metabolomics, PC cytokines, and liquid biopsy to improve accuracy for both imaging modalities and biomarkers for diagnostic differentiation between pancreatitis and pancreatic cancer (see Figure 5 for a proposed diagnostic algorithm for pancreatitis with a head-of-pancreas mass).
Figure 5.
Proposed diagnostic algorithm for pancreatitis with head of pancreas mass.
Abbreviations
| AP | Acute pancreatitis |
| AIP | Autoimmune pancreatitis |
| CA19-9 | Serum carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CEH-EUS | Contrast-enhanced harmonic endoscopic ultrasound |
| CP | Chronic pancreatitis |
| CT | Computed tomography |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| DPPHR | Duodenal-preserving pancreatic head resection |
| DWI-MRI | Diffusion-Weighted Imaging–Magnetic Resonance Imaging |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EUS | Endoscopic ultrasound |
| FAP | Familial adenomatous polyps |
| 18F-FDG | 2-deoxy-2-[fluorine-18]fluoro-D-glucose |
| FNA | Fine-needle aspiration |
| FNB | Fine-needle biopsy |
| HBROCS | Hereditary breast and ovarian cancer syndrome |
| HoP Head of pancreas | |
| HP | Hereditary pancreatitis |
| HSP | Heat shock protein 70 |
| IgG4 | Immunoglobulin G4 |
| MDCT | Multi-detector computed tomography |
| MIC-1 | Macrophage inhibitor cytokine |
| MMP-9 | Matrix metalloproteinase 9 |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| MRI | Magnetic resonance imaging |
| PAP | Progressive acute pancreatitis |
| PC | Pancreatic cancer |
| PD | Pancreatico-duodenectomy |
| PDAC | Pancreatic ductal adenocarcinoma |
| PET | Positron emission tomography |
| PHM | Peripancreatic hypoechoic margins |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping review |
| RAP | Recurrent acute pancreatitis |
| αSMA | α-smooth muscle actin |
| SUVmax | Maximum standardised uptake value |
| TAUS | Transabdominal ultrasound |
| TGF-1 | Transforming growth factor-Beta 1 |
| TIC | Time–intensity curves |
| TIMP 1 | Tissue inhibitor of metalloproteinase 1 |
| uPAR | Urokinase-type plasminogen activator receptor |
Author Contributions
Conceptualisation, F.M. and C.A.; methodology, F.M.; validation, F.M., L.F. and C.A.; formal analysis, F.M.; investigation, F.M.; resources, F.M.; writing—original draft preparation, F.M.; writing—review and editing, F.M., L.F. and C.A.; supervision, L.F. and C.A.; project administration, F.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not relevant to this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review of published research. No new data were generated. The full list of articles reviewed is under References. Alternatively, this information may be requested from the corresponding author in writing.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Raimondi S., Lowenfels A.B., Morselli-Labate A.M., Maisonneuve P., Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010;24:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin. Gastroenterol. Hepatol. 2009;7:S15–S17. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Nøjgaard C., Becker U., Matzen P., Andersen J.R., Holst C., Bendtsen F. Progression from acute to chronic pancreatitis: Prognostic factors, mortality, and natural course. Pancreas. 2011;40:1195–1200. doi: 10.1097/MPA.0b013e318221f569. [DOI] [PubMed] [Google Scholar]
- 4.Tao H., Xu J., Li N., Chang H., Duan L. Early identification of high-risk patients with acute recurrent pancreatitis progression to chronic pancreatitis. Arch. Med. Sci. 2022;18:535–539. doi: 10.5114/aoms/146262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb D.C. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am. J. Physiol. Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 6.Yamada R., Tsuboi J., Murashima Y., Tanaka T., Nose K., Nakagawa H. Advances in the Early Diagnosis of Pancreatic Ductal Adenocarcinoma and Premalignant Pancreatic Lesions. Biomedicines. 2023;11:1687. doi: 10.3390/biomedicines11061687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhobayb T., Peravali R., Ashkar M. The Relationship between Acute and Chronic Pancreatitis with Pancreatic Adenocarcinoma: Review. Diseases. 2021;9:93. doi: 10.3390/diseases9040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkegård J., Mortensen F.V., Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 9.Korpela T., Udd M., Mustonen H., Ristimäki A., Haglund C., Seppänen H., Kylänpää L. Association between chronic pancreatitis and pancreatic cancer: A 10-year retrospective study of endoscopically treated and surgical patients. Int. J. Cancer. 2020;147:1450–1460. doi: 10.1002/ijc.32971. [DOI] [PubMed] [Google Scholar]
- 10.Esposito I., Hruban R.H., Verbeke C., Terris B., Zamboni G., Scarpa A., Morohoshi T., Suda K., Luchini C., Klimstra D.S., et al. Guidelines on the histopathology of chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and the European Pancreatic Club. Pancreatology. 2020;20:586–593. doi: 10.1016/j.pan.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Lowenfels A.B., Maisonneuve P., Cavallini G., Ammann R.W., Lankisch P.G., Andersen J.R., DiMagno E.P., Andren-Sandberg A., Domellof L. Pancreatitis and the risk of pancreatic cancer. N. Engl. J. Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 12.Perumal S., Palaniappan R., Pillai S.A., Velayutham V., Sathyanesan J. Predictors of malignancy in chronic calcific pancreatitis with head mass. World J. Gastrointest. Surg. 2013;5:97–103. doi: 10.4240/wjgs.v5.i4.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav D., Lowenfels A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisonneuve P., Lowenfels A.B. Chronic pancreatitis and pancreatic cancer. Dig. Dis. 2002;20:32–37. doi: 10.1159/000063165. [DOI] [PubMed] [Google Scholar]
- 15.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 16.Li G., Liu T., Zheng J., Kang W., Xu J., Gao Z., Ma J. Untypical autoimmune pancreatitis and pancreatic cancer: Differential diagnosis experiences extracted from misdiagnose of two cases. Orphanet J. Rare Dis. 2019;14:245. doi: 10.1186/s13023-019-1217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang D., Wu Q., Zhang J., Zhang H., Yuan Z., Xu J., Chong Y., Huang Y., Xiong Q., Wang S., et al. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway. Oncol. Rep. 2018;39:1347–1355. doi: 10.3892/or.2018.6202. [DOI] [PubMed] [Google Scholar]
- 18.Haeberle L., Steiger K., Schlitter A.M., Safi S.A., Knoefel W.T., Erkan M., Esposito I. Stromal heterogeneity in pancreatic cancer and chronic pancreatitis. Pancreatology. 2018;18:536–549. doi: 10.1016/j.pan.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Dite P., Novotny I., Dvorackova J., Kianicka B., Blaho M., Svoboda P., Uvirova M., Rohan T., Maskova H., Kunovsky L. Pancreatic solid focal lesions: Differential diagnosis between autoimmune pancreatitis and pancreatic cancer. Dig. Dis. 2019;37:416–421. doi: 10.1159/000499762. [DOI] [PubMed] [Google Scholar]
- 20.Jin G., Hong W., Guo Y., Bai Y., Chen B. Molecular mechanism of pancreatic stellate cells activation in chronic pancreatitis and pancreatic cancer. J. Cancer. 2020;11:1505–1515. doi: 10.7150/jca.38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinho R., Alves A., Pignatelli N., Nunes V. Unclassified autoimmune pancreatitis mimicking pancreatic cancer. J. Surg. Case Rep. 2019;2019:rjy340. doi: 10.1093/jscr/rjy340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negoi I., Beuran M., Hostiuc S., Sartelli M., El-Hussuna A., De-Madaria E. Glycosylation alterations in acute pancreatitis and pancreatic cancer: CA19-9 expression is involved in pathogenesis and maybe targeted by therapy. Ann. Transl. Med. 2019;7((Suppl. S8)):S306. doi: 10.21037/atm.2019.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronen A., Aittoniemi J., Huttunen R., Nikkola A., Rinta-Kiikka I., Nikkola J., Limnell O., Nordback I., Sand J., Laukkarinen J. Plasma suPAR may help to distinguish between chronic pancreatitis and pancreatic cancer. Scand. J. Gastroenterol. 2021;56:81–85. doi: 10.1080/00365521.2020.1849383. [DOI] [PubMed] [Google Scholar]
- 24.Dai C., Cao Q., Jiang M., Sun M.-J. Serum immunoglobulin G4 in discriminating autoimmune pancreatitis from pancreatic cancer: A diagnostic meta-analysis. Pancreas. 2018;47:280–284. doi: 10.1097/MPA.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 25.Chou C., Chang C., Chen C. Analytically validated protein biomarkers of chronic pancreatitis and pancreatic cancer for potential clinical diagnosis with mass spectrometry. Rapid Commun. Mass Spectrom. 2019;34:e8580. doi: 10.1002/rcm.8580. [DOI] [PubMed] [Google Scholar]
- 26.Li W., Wang J., Li Y., Yue Q., Cui M., Liu J. KRAS Mutations in Peripheral Blood (with or without CA19-9) for Differential Diagnosis of Pancreatic Cancer and Chronic Pancreatitis: A Systematic Review and Meta-analysis. Indian J. Surg. 2022;84:615–622. doi: 10.1007/s12262-022-03475-4. [DOI] [Google Scholar]
- 27.Huang C., Iovanna J., Santofimia-Castaño P. Targeting fibrosis: The bridge that connects pancreatitis and pancreatic cancer. Int. J. Mol. Sci. 2021;22:4970. doi: 10.3390/ijms22094970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park W.G., Li L., Appana S., Wei W., Stello K., Andersen D.K., Hughes S.J., Whitcomb D.C., Brand R.E., Yadav D., et al. Unique circulating immune signatures for recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer: A pilot study of these conditions with and without diabetes. Pancreatology. 2019;20:51–59. doi: 10.1016/j.pan.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prokopchuk O., Grünwald B., Nitsche U., Jäger C., Prokopchuk O.L., Schubert E.C., Friess H., Martignoni M.E., Krüger A. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer. 2018;18:128. doi: 10.1186/s12885-018-4055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Yu X., Werner J., Bazhin A.V., D’haese J.G. The role of interleukin-18 in pancreatitis and pancreatic cancer. Cytokine Growth Factor Rev. 2019;50:1–12. doi: 10.1016/j.cytogfr.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Macinga P., Pulkertova A., Bajer L., Maluskova J., Oliverius M., Smejkal M., Heczkova M., Spicak J., Hucl T. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J. Gastroenterol. 2017;23:2185–2193. doi: 10.3748/wjg.v23.i12.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen S.E.J., Langsted A., Varbo A., Madsen C.M., Tybjærg-Hansen A., Nordestgaard B.G. Low and high pancreatic amylase is associated with pancreatic cancer and chronic pancreatitis. Eur. J. Epidemiol. 2021;36:975–984. doi: 10.1007/s10654-021-00801-0. [DOI] [PubMed] [Google Scholar]
- 33.Sanh N., Fadul H., Hussein N., Lyn-Cook B.D., Hammons G., Ramos-Cardona X.E., Mohamed K., Mohammed S. Proteomics profiling of pancreatic cancer and pancreatitis for biomarkers discovery. J. Cell Sci. Ther. 2018;9:4. doi: 10.4172/2157-7013.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C., Lou J., Bao M., Yang S., Lou J. Autoimmune pancreatitis masquerading as pancreatic cancer: Case report of a Chinese man. Int. J. Clin. Exp. Pathol. 2019;12:4354. [PMC free article] [PubMed] [Google Scholar]
- 35.Yan T., Ke Y., Chen Y., Xu C., Yu C., Li Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS ONE. 2017;12:e0174735. doi: 10.1371/journal.pone.0174735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kandikattu H.K., Venkateshaiah S.U., Mishra A. Chronic pancreatitis and the development of pancreatic cancer. Endocr. Metab. Immune Disord.-Drug Targets. 2020;20:1182–1210. doi: 10.2174/1871530320666200423095700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa T., Kawashima H., Ohno E., Iida T., Suzuki H., Uetsuki K., Yamada K., Yashika J., Yoshikawa M., Gibo N., et al. Risks and characteristics of pancreatic cancer and pancreatic relapse in autoimmune pancreatitis patients. J. Gastroenterol. Hepatol. 2020;35:2281–2288. doi: 10.1111/jgh.15163. [DOI] [PubMed] [Google Scholar]
- 38.Detlefsen S., de Vos J.D., Tanassi J.T., Heegaard N.H.H., Fristrup C., de Muckadell O.B.S. Value of anti-plasminogen binding peptide, anti-carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine. 2018;97:e11641. doi: 10.1097/MD.0000000000011641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dranka-Bojarowska D., Lewinski A., Lekstan A., Gajda M., Ciosek J., Mrowiec S. The assessment of serum and diagnostic peritoneal lavage concentration of matrix metalloproteinase-2, matrix metalloproteinase-9, carbohydrate antigen 19-9, and carcinoembryonic antigen in patients with pancreatic cancer and chronic pancreatitis. J. Physiol. Pharmacol. 2020;71:689–704. doi: 10.26402/jpp.2020.5.09. [DOI] [PubMed] [Google Scholar]
- 40.Winter K., Dzieniecka M., Strzelczyk J., Wągrowska-Danilewicz M., Danilewicz M., Małecka-Wojciesko E. Alpha smooth muscle actin (αSMA) immunohistochemistry use in the differentiation of pancreatic cancer from chronic pancreatitis. J. Clin. Med. 2021;10:5804. doi: 10.3390/jcm10245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh T.-S., Lin T.-A., Chen T.-C., Tseng J.-H. Autoimmune pancreatitis type 2: Mimicking pancreatic cancer. Formos. J. Surg. 2020;53:113–116. doi: 10.4103/fjs.fjs_104_19. [DOI] [Google Scholar]
- 42.Saraswat M., Joenväärä S., Seppänen H., Mustonen H., Haglund C., Renkonen R. Comparative proteomic profiling of the serum differentiates pancreatic cancer from chronic pancreatitis. Cancer Med. 2017;6:1738–1751. doi: 10.1002/cam4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou O.H.I., Zhou J., Mui J.V., Satti D.I., Chung C.T., Lee T.T.L., Lee S., Dee E.C., Ng K., Cheung B.M.Y., et al. Lower risks of new-onset acute pancreatitis and pancreatic cancer in sodium glucose cotransporter 2 (SGLT2) inhibitors compared to dipeptidyl peptidase-4 (DPP4) inhibitors: A propensity score-matched study with competing risk analysis. Diabetes Epidemiol. Manag. 2023;9:100115. doi: 10.1016/j.deman.2022.100115. [DOI] [Google Scholar]
- 44.Luo B., Peng F., Hong M., Su S., Fang C., Yang X., Xia G., Li B. ERCP combined with tumor markers in differential diagnosis of pancreatic cancer and pseudotumor-like pancreatitis. J. Buon. 2019;24:1568–1573. [PubMed] [Google Scholar]
- 45.Wen Y., Cai W., Yang J., Fu X., Putha L., Xia Q., Windsor J.A., Phillips A.R., Tyndall J.D.A., Du D., et al. Targeting macrophage migration inhibitory factor in acute pancreatitis and pancreatic cancer. Front. Pharmacol. 2021;12:638950. doi: 10.3389/fphar.2021.638950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng L.-P., Han C.-Q., Nie C., Xu T., Zhang K., Li X.-J., Xie X.-R., Lin R., Ding Z. Identification of potential serum exosomal microRNAs involved in acinar-ductal metaplasia that is a precursor of pancreatic cancer associated with chronic pancreatitis. Medicine. 2021;100:e25753. doi: 10.1097/MD.0000000000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poddighe D. Autoimmune pancreatitis and pancreatic cancer: Epidemiological aspects and immunological considerations. World J. Gastroenterol. 2021;27:3825–3836. doi: 10.3748/wjg.v27.i25.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickerson L.D., Farooq A., Bano F., Kleeff J., Baron R., Raraty M., Ghaneh P., Sutton R., Whelan P., Campbell F., et al. Differentiation of autoimmune pancreatitis from pancreatic cancer remains challenging. Mol. Med. 2019;43:1604–1611. doi: 10.1007/s00268-019-04928-w. [DOI] [PubMed] [Google Scholar]
- 49.Ghassem-Zadeh S., Hufnagel K., Bauer A., Frossard J.-L., Yoshida M., Kutsumi H., Acha-Orbea H., Neulinger-Muñoz M., Vey J., Eckert C., et al. Novel autoantibody signatures in sera of patients with pancreatic cancer, chronic pancreatitis and autoimmune pancreatitis: A protein microarray profiling approach. Int. J. Mol. Sci. 2020;21:2403. doi: 10.3390/ijms21072403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mungamuri S.K., Pasupulati A.K., Mavuduru V.A. Exploring Pancreatic Metabolism and Malignancy. Springer; Berlin/Heidelberg, Germany: 2019. Immunotherapy for Diabetogenic Pancreatitis and Pancreatic Cancer: An Update; pp. 215–236. [DOI] [Google Scholar]
- 51.Sunami Y., Chen Y., Trojanowicz B., Sommerer M., Hämmerle M., Eils R., Kleeff J. Single Cell Analysis of Cultivated Fibroblasts from Chronic Pancreatitis and Pancreatic Cancer Patients. Cells. 2022;11:2583. doi: 10.3390/cells11162583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindahl A., Heuchel R., Forshed J., Lehtiö J., Löhr M., Nordström A. Discrimination of pancreatic cancer and pancreatitis by LC-MS metabolomics. Metabolomics. 2017;13:61. doi: 10.1007/s11306-017-1199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gluszek S., Matykiewicz J., Grabowska U., Chrapek M., Nawacki L., Wawrzycka I., Gluszek-Osuch M., Kozieł D. Clinical usefulness of pentraxin 3 (PTX3) as a biomarker of acute pancreatitis and pancreatic cancer. Med. Stud. 2020;36:6–13. doi: 10.5114/ms.2020.94082. [DOI] [Google Scholar]
- 54.Bang U.C., Watanabe T., Bendtsen F. The relationship between the use of statins and mortality, severity, and pancreatic cancer in Danish patients with chronic pancreatitis. Eur. J. Gastroenterol. Hepatol. 2018;30:346–351. doi: 10.1097/MEG.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X., Lang R., Zhang Z., Zhao W., Ji Z., Tan H., Zhou X. Exploring and validating the clinical risk factors for pancreatic cancer in chronic pancreatitis patients using electronic medical records datasets: Three cohorts comprising 2,960 patients. Transl. Cancer Res. 2020;9:629–638. doi: 10.21037/tcr.2019.11.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissinen S.I., Venäläinen M., Kumpulainen P., Roine A., Häkkinen M.R., Vepsäläinen J., Oksala N., Rantanen T. Discrimination between pancreatic cancer, pancreatitis and healthy controls using urinary polyamine panel. Cancer Control. 2021;29:1–8. doi: 10.1177/10732748211039762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal K.K., Jassal R., Browne A., Hossain M., Akhtar R. Autoimmune Pancreatitis Masquerading as Pancreatic Cancer: A Case Report and Literature Review. Cureus. 2022;14:e21900. doi: 10.7759/cureus.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H., Han W., Jin M., Lai Y., Wang X., Wang J., Yao Y., Wu D., Qian J., Yang H. Establishment and verification of a scoring model for the differential diagnosis of pancreatic cancer and chronic pancreatitis. Pancreas. 2018;47:459–465. doi: 10.1097/MPA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 59.Kunovsky L., Dite P., Jabandziev P., Dolina J., Vaculova J., Blaho M., Bojkova M., Dvorackova J., Uvirova M., Kala Z., et al. Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 2021;13:835–844. doi: 10.4251/wjgo.v13.i8.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macinga P., Bajer L., Del Chiaro M., Chari S.T., Dite P., Frulloni L., Ikeura T., Kamisawa T., Kubota K., Naitoh I., et al. Pancreatic cancer in patients with autoimmune pancreatitis: A scoping review. Pancreatology. 2021;21:928–937. doi: 10.1016/j.pan.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Grassia R., Imperatore N., Capone P., Cereatti F., Forti E., Antonini F., Tanzi G.P., Martinotti M., Buffoli F., Mutignani M., et al. EUS-guided tissue acquisition in chronic pancreatitis: Differential diagnosis between pancreatic cancer and pseudotumoral masses using EUS-FNA or core biopsy. Endosc. Ultrasound. 2020;9:122–129. doi: 10.4103/eus.eus_75_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q., Tao X., Xia S., Guo F., Pan C., Xiang H., Shang D. T Lymphocytes: A promising immunotherapeutic target for pancreatitis and pancreatic cancer? Front. Oncol. 2020;10:382. doi: 10.3389/fonc.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walling A., Freelove R. Pancreatitis and pancreatic cancer. Prim. Care Clin. Off. Pract. 2017;44:609–620. doi: 10.1016/j.pop.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Rana S.S., Gorsi U., Gupta P., Sharma R., Basher R., Dhalaria L., Gupta R. Pancreatic cancer masked by acute pancreatitis as well as an unusual iatrogenic complication. J. Dig. Endosc. 2018;09:088–091. doi: 10.4103/jde.JDE_95_17. [DOI] [Google Scholar]
- 65.Hsu W.-L., Chang S.-M., Wu P.-Y., Chang C.-C. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J. Int. Med. Res. 2018;46:1657–1665. doi: 10.1177/0300060517742303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalayarasan R., Narayanan S., Sahoo J., Mohan P. Impact of surgery for chronic pancreatitis on the risk of pancreatic cancer: Untying the Gordian knot. World J. Gastroenterol. 2021;27:4371–4382. doi: 10.3748/wjg.v27.i27.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang H.Y., Kohtakangas E.L., Mitrovic B., Asai K., Shum J.B. Autoimmune pancreatitis masquerading as pancreatic cancer: When in doubt, cut it out. J. Gastrointest. Cancer. 2017;49:365–372. doi: 10.1007/s12029-017-9924-y. [DOI] [PubMed] [Google Scholar]
- 68.Ohtani M., Ofuji K., Akazawa Y., Saito Y., Nosaka T., Ozaki Y., Takahashi K., Naito T., Matsuda H., Hiramatsu K., et al. Clinical Usefulness of [18F]-Fluoro-2-Deoxy-d-Glucose–Positron Emission Tomography/Computed Tomography for Distinguishing Between Autoimmune Pancreatitis and Pancreatic Cancer. Pancreas. 2021;50:1014–1019. doi: 10.1097/MPA.0000000000001873. [DOI] [PubMed] [Google Scholar]
- 69.Kim H.S., Gweon T.-G., Park S.H., Kim T.H., Kim C.W., Chang J.H. Incidence and risk of pancreatic cancer in patients with chronic pancreatitis: Defining the optimal subgroup for surveillance. Sci. Rep. 2023;13:106. doi: 10.1038/s41598-022-26411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S., Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine. 2017;96:e5908. doi: 10.1097/MD.0000000000005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bieliuniene E., Frøkjær J.B., Pockevicius A., Kemesiene J., Lukosevicius S., Basevicius A., Atstupenaite V., Barauskas G., Ignatavicius P., Gulbinas A., et al. CT- and MRI-based assessment of body composition and pancreatic fibrosis reveals high incidence of clinically significant metabolic changes that affect the quality of life and treatment outcomes of patients with chronic pancreatitis and pancreatic cancer. Medicina. 2019;55:649. doi: 10.3390/medicina55100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Umans D.S., A Hoogenboom S., Sissingh N.J., Lekkerkerker S.J., Verdonk R.C., E van Hooft J. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J. Gastroenterol. 2021;27:3148–3157. doi: 10.3748/wjg.v27.i23.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsubayashi H., Satoh T., Ishikawa K., Ishiwatari H., Endo M., Urikura A., Kishida Y., Imai K., Hotta K., Yabuuchi Y., et al. Comparison of five-phase computed tomography images of type 1 autoimmune pancreatitis and pancreatic cancer: Emphasis on cases with atypical images. Pancreatology. 2021;21:666–675. doi: 10.1016/j.pan.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Miyoshi H., Kano M., Kobayashi S., Ito T., Masuda M., Mitsuyama T., Nakayama S., Ikeura T., Shimatani M., Uchida K., et al. Diffuse pancreatic cancer mimicking autoimmune pancreatitis. Intern. Med. 2019;58:2523–2527. doi: 10.2169/internalmedicine.2689-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J., Jia G., Zuo C., Jia N., Wang H. 18F- FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer. 2017;17:695. doi: 10.1186/s12885-017-3665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang S., Li Y. A comparative analysis of CT and MRI in differentiating pancreatic cancer from mass pancreatitis. Am. J. Transl. Res. 2021;13:6431–6438. [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y., Li F., An N., Peng Z. Atypical enhanced computed tomography signs of pancreatic cancer and its differential diagnosis from autoimmune pancreatitis. Gland. Surg. 2021;10:347–354. doi: 10.21037/gs-20-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ergin E., Oruc N., Özütemiz Ö. Autoimmune Pancreatitis after a Seven-Year History of Suspicious Pancreatic Cancer. Case Rep. Gastroenterol. 2021;15:195–201. doi: 10.1159/000511286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho M.K., Moon S.-H., Song T.J., Kim R.E., Oh D.W., Park D.H., Lee S.S., Seo D.W., Lee S.K., Kim M.-H. Contrast-enhanced endoscopic ultrasound for differentially diagnosing autoimmune pancreatitis and pancreatic cancer. Gut Liver. 2018;12:591–596. doi: 10.5009/gnl17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srisajjakul S., Prapaisilp P., Bangchokdee S. CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol. Medica. 2020;125:356–364. doi: 10.1007/s11547-019-01132-7. [DOI] [PubMed] [Google Scholar]
- 81.Tacelli M., Zaccari P., Petrone M., Della Torre E., Lanzillotta M., Falconi M., Doglioni C., Capurso G., Arcidiacono P. Differential EUS findings in focal type 1 autoimmune pancreatitis and pancreatic cancer: A proof-of-concept study. Endosc. Ultrasound. 2022;11:216–222. doi: 10.4103/EUS-D-21-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harmsen F.-J., Domagk D., Dietrich C., Hocke M. Discriminating chronic pancreatitis from pancreatic cancer: Contrast-enhanced EUS and multidetector computed tomography in direct comparison. Endosc. Ultrasound. 2018;7:395–403. doi: 10.4103/eus.eus_24_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu L., Guo J., Jin Z., Xue H., Dai M., Zhang W., Sun Z., Xu J., Garcia S.R.M., Asbach P., et al. Distinguishing pancreatic cancer and autoimmune pancreatitis with in vivo tomoelastography. Eur. Radiol. 2020;31:3366–3374. doi: 10.1007/s00330-020-07420-5. [DOI] [PubMed] [Google Scholar]
- 84.Wyse J.M., Sahai A.V. Endoscopic ultrasound-guided management of pain in chronic pancreatitis and pancreatic cancer: An update. Current Treatment Options in Gastroenterology. Curr. Treat. Options Gastroenterol. 2018;16:417–427. doi: 10.1007/s11938-018-0193-z. [DOI] [PubMed] [Google Scholar]
- 85.Konings I.C., Cahen D.L., Harinck F., Fockens P., van Hooft J.E., Poley J.-W., Bruno M.J. Evolution of features of chronic pancreatitis during endoscopic ultrasound-based surveillance of individuals at high risk for pancreatic cancer. Endosc. Int. Open. 2018;6:E541–E548. doi: 10.1055/a-0574-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enjuto D.T., Herrera N., Ceinos C.J., Bonilla A.R., Llorente-Lázaro R., Guerreiro J.G., Castro-Carbajo P. Hereditary pancreatitis related to SPINK-1 mutation. Is there an increased risk of developing pancreatic cancer? J. Gastrointest. Cancer. 2021;54:268–269. doi: 10.1007/s12029-021-00729-4. [DOI] [PubMed] [Google Scholar]
- 87.Jeon C.Y., Chen Q., Yu W., Dong E.Y., Chung J., Pandol S.J., Yadav D., Conwell D.L., Wu B.U. Identification of individuals at increased risk for pancreatic cancer in a community-based cohort of patients with suspected chronic pancreatitis. Clinical and Translational Gastroenterology. Clin. Transl. Gastroenterol. 2020;11:e00147. doi: 10.14309/ctg.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teske C., Kahlert C., Welsch T., Liedel K., Weitz J., Uckermann O., Steiner G. Label-free differentiation of human pancreatic cancer, pancreatitis, and normal pancreatic tissue by molecular spectroscopy. J. Biomed. Opt. 2022;27:075001. doi: 10.1117/1.JBO.27.7.075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed A., Ayav A., Belle A., Orry X., Chevaux J.-B., Laurent V. Pancreatic cancer in patients with chronic calcifying pancreatitis: Computed tomography findings—A retrospective analysis of 48 patients. Eur. J. Radiol. 2016;86:206–212. doi: 10.1016/j.ejrad.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 90.Rashid S., Singh N., Gupta S., Rashid S., Nalika N., Sachdev V., Bal C.S., Datta Gupta S., Chauhan S.S., Saraya A. Progression of chronic pancreatitis to pancreatic cancer: Is there a role of gene mutations as a screening tool? Pancreas. 2018;47:227–232. doi: 10.1097/MPA.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 91.Tirkes T., Shah Z.K., Takahashi N., Grajo J.R., Chang S.T., Venkatesh S.K., Conwell D.L., Fogel E.L., Park W., Topazian M., et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: The consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology. 2019;290:207–215. doi: 10.1148/radiol.2018181353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma X., Wang Y.-R., Zhuo L.-Y., Yin X.-P., Ren J.-L., Li C.-Y., Xing L.-H., Zheng T.-T. Retrospective Analysis of the Value of Enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis. Int. J. Gen. Med. 2022;15:233–241. doi: 10.2147/IJGM.S337455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartell N., Bittner K., Vetter M.S., Kothari T., Kaul V., Kothari S. Role of endoscopic ultrasound in detecting pancreatic cancer missed on cross-sectional imaging in patients presenting with pancreatitis: A retrospective review. Dig. Dis. Sci. 2019;64:3623–3629. doi: 10.1007/s10620-019-05807-z. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Xu W. The advantage of using CEUS time-intensity curves vs. conventional ultrasound in the differential diagnosis of mass pancreatitis and pancreatic cancer. Int. J. Clin. Exp. Med. 2020;13:8578–8584. [Google Scholar]
- 95.Yang A., Guo T., Xu T., Zhang S., Lai Y., Wu X., Wu D., Feng Y., Jiang Q., Wang Q., et al. The role of EUS in diagnosing focal autoimmune pancreatitis and differentiating it from pancreatic cancer. Endosc. Ultrasound. 2021;10:280–287. doi: 10.4103/EUS-D-20-00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konur S., Ozkahraman A., Surmeli N., Gunduz I., Iliklerden U.H., Dertli R., Kayar Y. The severity of acute pancreatitis according to modified balthazar classification in patients with pancreatic cancer. Tumori J. 2020;106:356–361. doi: 10.1177/0300891620948961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review of published research. No new data were generated. The full list of articles reviewed is under References. Alternatively, this information may be requested from the corresponding author in writing.