Abstract
The intestinal flora has been the focus of numerous investigations recently, with inquiries not just into the gastrointestinal aspects but also the pathomechanism of other diseases such as nervous system disorders and mitochondrial diseases. Mitochondrial disorders are the most common type of inheritable metabolic illness caused by mutations of mitochondrial and nuclear DNA. Despite the intensive research, its diagnosis is usually difficult, and unfortunately, treating it challenges physicians. Metabolites of the kynurenine pathway are linked to many disorders, such as depression, schizophrenia, migraine, and also diseases associated with impaired mitochondrial function. The kynurenine pathway includes many substances, for instance kynurenic acid and quinolinic acid. In this review, we would like to show a possible link between the metabolites of the kynurenine pathway and mitochondrial stress in the context of intestinal flora. Furthermore, we summarize the possible markers of and future therapeutic options for the kynurenine pathway in excitotoxicity and mitochondrial oxidative stress.
Keywords: kynurenine pathway, intestinal flora, excitotoxicity, oxidative stress
1. Introduction
The gut microbiota, also referred to as the intestinal flora, has become a focal point in healthcare. One of the most noteworthy advancements in recent gut microbiota research has been the revelation that the assembly of microorganisms in our digestive system has the capacity to influence various aspects of brain development and function.
Examining the neurobiological mechanisms responsible for the degree of influence exerted by microbial organisms on host physiology, brain function, and behavior has become a top research priority. Researchers are currently exploring various pathways and potential mechanisms that may govern the interactions between intestinal flora and the brain.
One central focus in this area of investigation is how the microbiota regulates the availability of circulating tryptophan (Trp). Trp is an essential amino acid that is responsible for the synthesis of serotonin (5-HT) and, moreover, is an initial compound of the kynurenine pathway (KP). The KP has a crucial role in many disorders, such as immunological [1], neurological [2], and mitochondrial diseases [3]. Mitochondrial disorders are chronic genetic illnesses that occur when mitochondria in cells fail to produce sufficient energy due to damage to their own DNA.
There is limited direct evidence establishing a clear and causal connection between the gut microbiota and mitochondrial disorders. However, research in both areas has been progressing, and there is growing interest in understanding potential links between them. This review focuses on the possible role of the gut microflora in the production of KP metabolites, and the role of the two systems in the pathophysiology of mitochondrial disorders.
2. Connections between the Intestinal Flora and the KP
2.1. Intestinal Flora
Intestinal flora, also known as the gut microbiota or gut microbiome, refers to the diverse community of microorganisms that inhabit the human gastrointestinal tract, primarily the colon. These microorganisms consist of bacteria, viruses, fungi, and other microorganisms, with bacteria being the most abundant and well-studied component. The human gut is home to trillions of microorganisms, with an estimated 1000 to 1150 different species of bacteria.
The initial colonization of microbes primarily occurs during the process of childbirth. Newborns delivered through the vaginal route are exposed to bacteria from their mother’s feces and vaginal region, while infants born via cesarean section initially encounter bacteria from the hospital environment and their mother’s skin [4]. It is important to note that despite the long-lasting belief that the prenatal environment is completely sterile, recent research has shown that before breastfeeding, the placenta, the amniotic fluid, and meconium can carry a certain amount of bacteria [5]. Studies have detected, in the infant gut, the presence of bacteria such as Enterobacteriaceae, Bifidobacterium and Bacteroides [6].
The infant gut microbiota remains highly changeable and dynamic until around the age of two when the consumption of solid foods begins [4]. Afterward, around the third year of life, as the diet becomes more diverse, the microbiota stabilizes and starts resembling the microbial compositions found in adults [5]. In adulthood, healthy people’s gut microbiota is primarily populated by four main phyla: Firmicutes, Actinobacteria, Bacteroidetes, and Verrucomicrobia [7]. The composition of the gut microbiota in healthy young adults and middle-aged individuals is characterized by a high diversity of bacterial species [8]. However, as individuals age, their gut microbiota undergoes changes, with a higher proportion of Bacteroides spp. and distinct patterns of Clostridium groups identified in the elderly compared to younger adults [9]. Bacteria in these species can also metabolize Trp into substances of the KP [10].
Therefore, during infancy and old age, gut microbiota composition is highly dynamic and experiences significant shifts, whereas in healthy young adults and middle-aged individuals, it tends to be more stable and diverse. Even during adulthood, the composition of the gut microbiota can undergo significant changes over the course of a year [11]. This has led to debates regarding the best way to characterize and monitor an individual’s gut microbiota composition. The concept of “enterotypes” (three core clusters of bacterial genera: Bacteroides, Prevotella, and Ruminococcus) is not universally accepted due to variations between individuals and challenges in categorizing an individual’s gut microbial composition within one specific enterotype [11]. An alternative approach suggests that the gut microbiota composition reflects a core set of functional profiles, where certain bacterial species play a more critical role in influencing health and disease [12].
This diversity plays a crucial role in maintaining a healthy gut. So gut microorganisms are an intricate microbial community recognized as a crucial influencer of neurodevelopment [13], and their involvement in the development of excitotoxicity is highly probable [14]. The potential contribution of gut microbiota to brain development can be described as follows [15]. On the one hand, Trp serves as a precursor for various biologically significant metabolites with crucial physiological roles, and it holds significant implications for the development of the central nervous system (CNS). Serotonin (5-hydroxytryptamine, 5-HT), originating from Trp via the action of Trp hydroxylase, regulates essential neurodevelopmental approaches [15]. These processes are under the influence of short-chain fatty acids (SCFAs) produced by the gut microbiome, such as acetate, propionate, and butyrate [16,17]. These substances can influence the brain by modifying the levels of neurotransmitter precursors [18] and via the vagal nerve [19]. On the other hand, neurotrophins such as brain-derived neurotrophic factor (BDNF), which are closely associated with neurodevelopment and neuroprotection, play crucial roles in promoting neuronal survival, synaptic plasticity, and cognitive function [15]. Recently, it has been also shown that the KP is changed in BDNF knock-in mice [20], so there is a connection between the substances of the KP and the BDNF pathway. Additionally, it is proposed that gut microbiota may influence the expression of N-methyl-D-aspartate (NMDA) receptors and contribute to brain development [21].
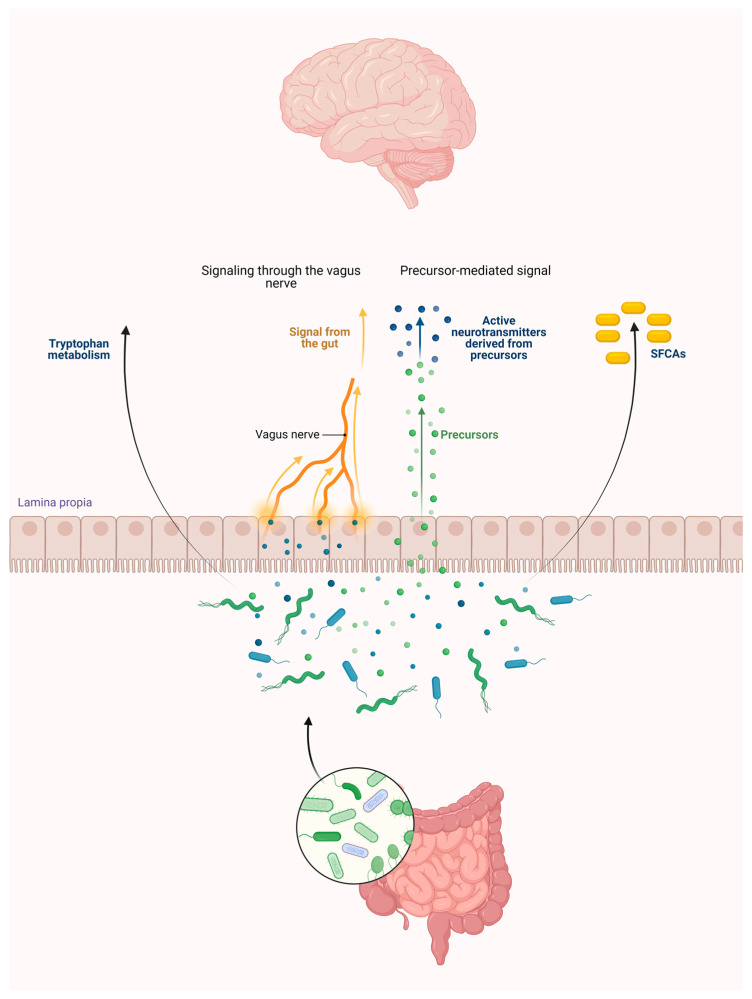
To sum up, bacteria in the gut microbiome can metabolize Trp, leading to the production of various metabolites within the gut (Figure 1).
Figure 1.
The gut microbiota influences the brain through tryptophan, short-chain fatty acids via the vagus nerve, and the precursor of neurotransmitters. Abbreviation: SCFAs—short-chain fatty acids.
2.2. The KP and Its Receptors
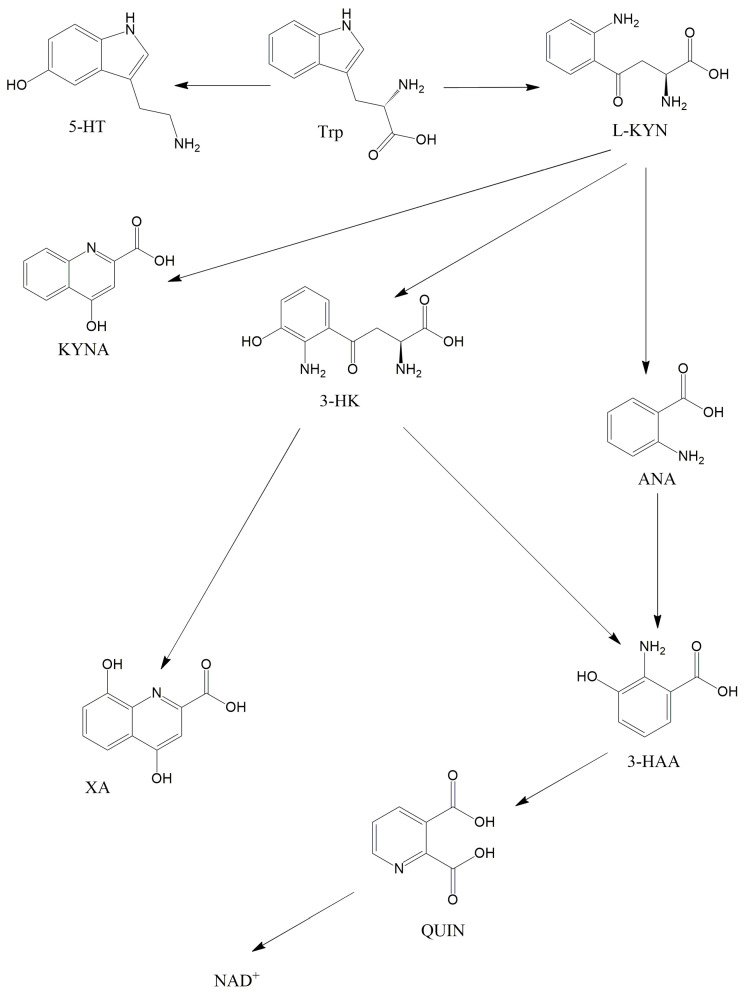
Trp is an essential amino acid that is crucial in the brain as it is the precursor of 5-HT. Several bacterial strains have the capability to enhance the production of Trp in the body, potentially playing a role in maintaining regular gut motility [22]. Nonetheless, the majority (more than 90%) of Trp in mammalian cells metabolizes in the KP and not towards the 5-HT (Figure 2). Kynurenic acid (KYNA) is one of the best-known substances of the KP, which was first described by Justus von Liebig in 1853 [23]. Liebig was a German scientist, who discovered KYNA in dog urine. It is important to know that KYNA is an endogenous glutamate receptor antagonist and has neuroprotective effects. This substance is created by kynurenine aminotransferases (KATs) from L-kynurenine (L-KYN), which is constructed by the formamidase enzyme from N-formylkynurenine. N-formylkynurenine, and is formed from L-Trp by two iron-dependent enzymes: indolamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2) and tryptophan 2,3-dioxygenase (TDO). It is noteworthy that, besides KYNA, L-KYN can transform into anthranilic acid (ANA) in reaction to kynureninase (3-HAO) and to 3-hydroxykynurenine (3-HK) when reacting to kynurenine 3-monooxygenase (KMO). KMO, in eukaryotic cells, is a mitochondrial protein located in the outer membrane of mitochondria [24]. ANA can further transform into 3-hydroxyanthranilic acid (3-HAA) when exposed to 3-hydroxyanthranilic acid hydroxylase. Furthermore, 3-HK can also be converted to 3-HAA by the kynureninase enzyme. In addition to this, 3-HK can also transform into xanthurenic acid. 3-HAA can further be converted to quinolinic acid (QUIN) when exposed to 3-hydroxyanthranillic acid 3,4-dioxygenase. In the last step of the KP, QUIN is transformed into nicotinamide adenine dinucleotide (NAD+) in reaction to quinolinic acid phosphoribosyl transferase. NAD+ has a pivotal role in mitochondrial energy management and redox reactions [25]. Different from KYNA, QUIN is an endogenous glutamate receptor agonist, which when produced by microglia [26], can provoke lipid peroxidation [27] and has a relevant role in the neurodegenerative process [28]. QUIN can exert its effects, probably by stimulating NMDA receptors, yielding the overproduction of free radicals and inhibiting the respiratory chain. Research shows that the enzymes and substances of the KP have pivotal roles in the pathomechanisms of migraine [29], Parkinson’s disease [30], schizophrenia [31] and mitochondrial dysfunction [32,33,34].
Figure 2.
The kynurenine pathway. This figure illustrates the main metabolites of the KP. Abbreviations: 3-HAA—3-hydroxyanthranilic acid, 3-HK—3-hydroxykynurenine, 5-HT—serotonin, ANA—anthranilic acid, KYNA—kynurenic acid, L-KYN—L-kynurenine, NAD+—nicotinamide adenine dinucleotide, QUIN—quinolinic acid, Trp—tryptophan, XA—xanthurenic acid.
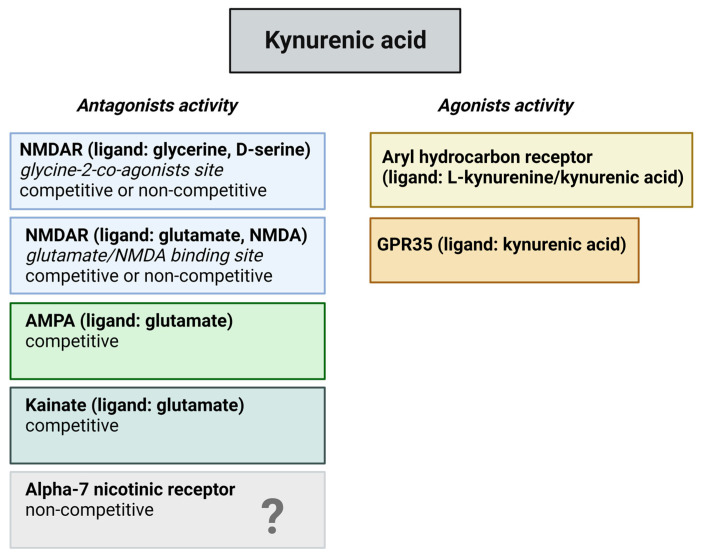
Kynurenines exert their effects on different receptors, such as glutamate receptors in a dose dependent manner, aryl hydrocarbon (AhR) receptors, G protein-coupled receptor 35 (GPR35), and α-7 nicotinic (α7 nACh) receptors (Figure 3). Glutamate receptors are a class of neurotransmitter receptors found in the CNS of animals, hence humans. Glutamate is the most abundant excitatory neurotransmitter in the brain, and its receptors play a fundamental role in various aspects of neuronal communication and synaptic plasticity. There are several types of glutamate receptors, but they are generally categorized into two major classes. The ionotropic glutamate receptors function as ligand-gated ion channels. This implies that upon binding with glutamate, they facilitate the movement of ions, including the influx of sodium (Na+) and calcium (Ca2+) as well as the efflux of potassium (K+) through the cell membrane. Ionotropic receptors mediate fast synaptic transmission and include three subtypes: NMDA receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors. The other types of glutamate receptors are metabotropic glutamate receptors, which are G protein-coupled receptors that are indirectly linked to ion channels through intracellular signaling pathways. They modulate synaptic transmission and play a role in the regulation of neuronal excitability. KYNA is an antagonist at the strychnine-insensitive glycine-binding site of NMDA receptors at low doses [35] and can also inhibit the glutamate-binding site of the NMDA receptors at higher doses [36]. In addition to this, KYNA has an antagonistic impact on kainate- and AMPA receptors [35]. The effect of KYNA on AMPA receptors is also concentration dependent, which means that KYNA can stimulate the receptors at nanomolar and micromolar concentrations but in different circumstances, namely between micromolar and millimolar concentrations, can inhibit the AMPA receptors [37,38].
Figure 3.
Potential binding sites for kynurenic acid. The figure provides an overview of potential receptors for kynurenic acid and their corresponding actions. Recent inquiries have raised uncertainties regarding its impact on the alpha-7 nicotinic receptor. Abbreviations: NMDAR—N-methyl-D-aspartate receptors, AMPA—α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, GPR35—G protein-coupled receptor 35.
AhR receptors are a family of proteins found in many species, including humans. AhR is a ligand-activated transcription factor that can bind to various aromatic hydrocarbons, including environmental pollutants like dioxins and polycyclic aromatic hydrocarbons (PAHs). When AhR binds to its ligands, it undergoes a conformational change and translocates to the cell nucleus, where it regulates the expression of various genes. The AhR receptor activation promotes equilibrium in host-microbe interactions, with indole derived from microbial Trp serving as a crucial ligand for this transcription factor [39]. Additionally, the AhR receptor is involved in the regulation of IDO and TDO expression [40,41]. Elevated AhR receptor activation has been noted in individuals with post-acute sequelae of SARS-CoV-2 infection, correlating with heightened IDO2 activity [42]. Additionally, a decline in mitochondrial function is evident in these patients. Experimental findings indicate that AhR receptor antagonists can diminish IDO2 activation [42]. Consequently, it can be inferred that modifications in the KP might impact mitochondrial function through AhR receptors.
GPR35 is a member of the G protein-coupled receptor family. The exact function of GPR35 is not fully understood, and it appears to have diverse roles in different tissues. Research has implicated GPR35 in immune responses, inflammatory processes, and gastrointestinal functions.
The α7 nACh receptor is a type of nicotinic acetylcholine receptor that plays a crucial role in neurotransmission within the CNS. Nicotinic receptors are ionotropic receptors that respond to the neurotransmitter acetylcholine, and they are named after nicotine, which also binds to and activates these receptors. Doubts regarding the effects of KYNA on α7 nACh receptors have been raised by Stone [43].
2.3. Role of the Intestinal Flora in the KP Metabolism
The relationship between diet, the KP, and the gut microbiome is a complex and emerging area of research in the fields of nutrition, metabolism, and microbiology. Various diets can significantly influence the composition and function of the gut microbiome [44]. For example, diets rich in fiber from fruits, vegetables, and whole grains can promote the growth of beneficial bacteria in the gut [45]. On the other hand, high-fat and high-sugar diets can alter the microbiome composition and may contribute to the development of metabolic disorders [46].
Research has shown that Trp, and thus the KP, has a unique role in the dual communication between microorganisms of the gut and the various organs of the host [47]. Bacteria in the gut can influence the activity of IDO1 in the gut, and some of these bacteria have enzymes very similar to those of the KP and thus contribute to the production of the metabolites of the KP [47]. It is widely recognized that the bidirectional communication between the microbiota and the immune system plays a crucial role in shaping the host’s intestinal immune response [48]. Consequently, mice that lack gut microbiota display a deficiency in their innate immune system. When compared to conventional mice, germ-free mice exhibit decreased degradation of Trp via the KP, leading to higher levels of available Trp and lower levels of KYNA in their bloodstream [49,50]. Additionally, the levels of circulating Trp and KYNA return to normal after microbial colonization in mice immediately after weaning [50].
In a recent study, Sun and his colleagues found that long-term high-fat diets disrupted the metabolism of Trp in the bloodstream in mice [51]. This disruption was characterized by a reduction in Trp levels and an increase in the activity of the KP. Notably, this aberrant Trp metabolism strongly correlated with the proliferation of the Proteobacteria phylum in the colon, so deviations in Trp metabolism can induce changes in the KP metabolism [51] as well. In this interesting research, the authors could abrogate the long-term high-fat diet shift with antibiotic treatment, but changes induced by the long-term high-fat diet could be transferred to mice with a standard diet through fecal transplantation, thus revealing the role of gut microbiota in this process. In another experimental setting, it was shown that the administration of Bacillus infantis to rats elevates Trp and KYNA levels but decreases the level of 5-Hydroxyindoleacetic acid [52], which is the main metabolite of 5-HT [53]. These data obviously prove that the gut microbiome influences the KP.
Alterations in the KP have been documented in several diseases linked to a disrupted microbiome. Patients diagnosed with irritable bowel syndrome (IBS) have reported heightened KP metabolism and altered microbiome [54,55,56], providing evidence of a connection between these two systems. The roles of altered microbiome and oxidative stress have also been described in patients with Crohn’s disease [57,58]. In a recent study, it has been shown that Trp has a protective impact on Crohn’s disease and IBS [59], by strengthening the role of the KP against mitochondrial stress and supporting a normal microbiome.
However, it is important to note that the interplay between diet, kynurenine, and the gut microbiome is still an active area of research and that the mechanisms involved are not fully understood. It is also important to consider individual variations in microbiome composition and responses to diet.
3. Mitochondrial Disorders, and Oxidative Stress: Possible Role of the KP
3.1. Mitochondria
Mitochondria are the provider and warehouse of energy in eukaryotic organ-isms; they have a cylinder shape, and almost every cell (except red blood cells) contains thousands of them. The origin of mitochondria is described by the endosymbiotic theory, which states that in the distant past a proteobacterium was swallowed up via endocytosis, affording the host with the skill to produce energy in the form of adenosine triphosphate (ATP). This double-membrane cell-forming cell has no nucleus, but it has its own genetic material called (mtDNA). The role of mitochondria is essential in many cellular processes, e.g., calcium homeostasis [60], apoptosis [61], free radical formation, and, of course, the production of ATP via oxidative phosphorylation. In this process, electrons are transported to molecular oxygen via the mitochondrial respiratory chain, namely complex I to V, which develops a proton gradient through the mitochondrial membrane. Thereafter, the complex V (or ATP synthase) can generate ATP. Malfunction of the oxidative phosphorylation process can increase production of highly reactive free radicals (redox homeostasis), yielding oxidative stress, lipid peroxidation, DNA damage, and cell death.
3.2. Mitochondrial Disorders and Oxidative Stress
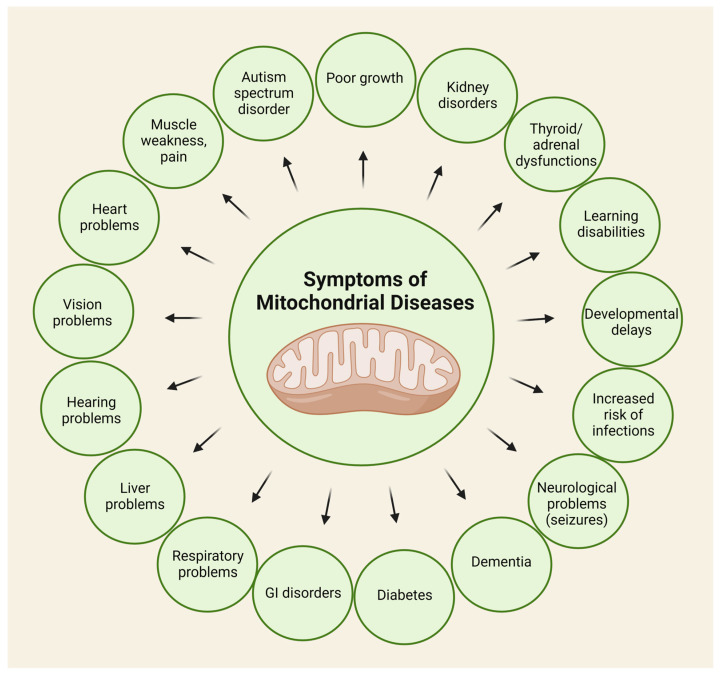
Mitochondrial disorders are chronic, uncurable conditions, the diagnosis of which is very difficult. These disorders can affect various organs and systems in the body, and their severity can vary widely. They have many, non-specific symptoms, from muscle weakness to seizures (Figure 4).
Figure 4.
Potential symptoms of mitochondrial disorders. This figure shows that almost all organs can be affected.
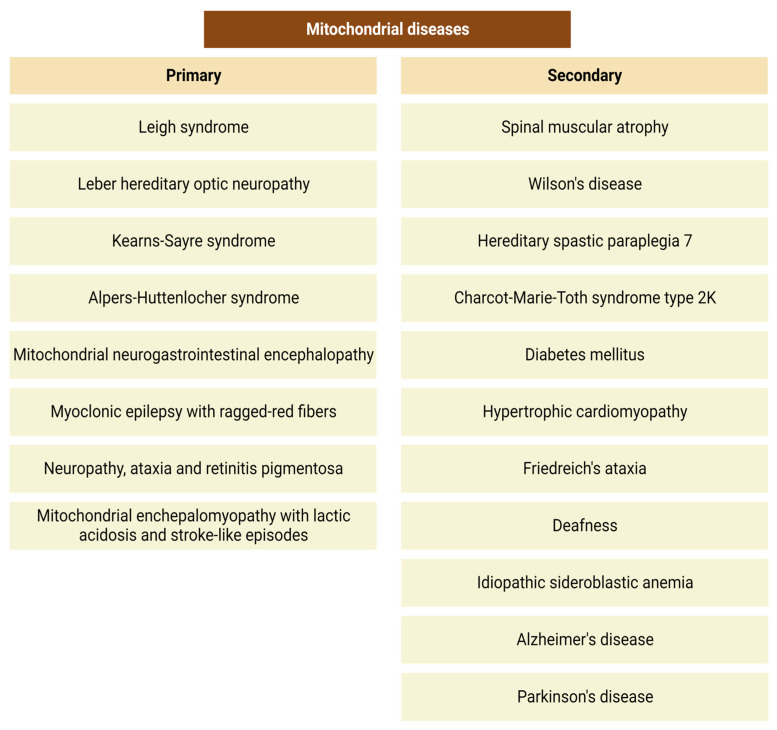
The causes underlying mitochondrial disorders include gene mutations as well as environmental factors. These mutations can affect both nuclear and mitochondrial DNA, with more than 350 genes currently identified as bases for mitochondrial diseases [62]. We can divide mitochondrial disorders into primary and secondary mitochondrial diseases. Primary mitochondrial conditions (Figure 5) are genetically determined and identified by mutations in the nuclear or mtDNA.
Figure 5.
Most common primary and secondary mitochondrial disorders.
In addition to this, secondary mitochondrial disorders (Figure 5) are triggered by mutations or several environmental factors, such as alcohol [63], smoking [64], carbon monoxide [65], and antiretroviral [66] or aminoglycoside [67] therapy.
What increases the difficulty of diagnosis is that we have no specific laboratory or diagnostic tests to prove the diagnosis of mitochondrial disorders. The age of onset, affected organs, and severity of symptoms can vary significantly among individuals. Symptoms can involve the nervous system, muscles, heart, liver, kidneys, and other organs [68]. The first patient with mitochondrial disorder was described by Luft in 1959 [69]. He examined a 30-year-old woman with exudation and raised fluid intake, full-body weakness, and inability to gain weight despite a huge caloric intake. The symptoms of the patient began at the age of 7. Laboratory tests showed normal thyroid function and that the basal metabolic rate was extremely high. Muscle biopsy showed high ATPase activity, elevated level of cytochrome oxidase, and low levels of coenzyme Q in the mitochondria, which strongly pointed towards intensified mitochondrial synthesis.
The diagnosability of these disorders has improved recently, with the most trustworthy method being genetic testing. The first step is usually the examination of mtDNA, and then analyses of nuclear DNA for genes affected in mitochondrial diseases. It is possible to completely examine the nuclear DNA with whole genome sequencing. In addition to this, clinicians can also use additional tests, such as a muscle biopsy, biochemical tests on blood, urine, cerebrospinal fluid, and MRI [70]. Despite the intensive research, we have no completely reliable testing methods [71] that can be used in all patients suspected of mitochondrial disorder.
At present, an effective and targeted treatment for the majority of patients with mitochondrial disease is still unavailable. Various approaches, primarily involving nutritional supplements like creatine, co-enzyme Q10, carnitine, and vitamin combinations, have been extensively utilized, relying on individual case reports [72]. Treatment also focuses on managing symptoms and supportive care, including physical therapy and occupational therapy [73].
In conclusion, mitochondrial disorders represent a complex group of genetic diseases with diverse clinical presentations. Ongoing research is essential for advancing our understanding and developing potential therapeutic interventions for these challenging conditions.
Oxidative stress is a cellular condition that occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to eliminate them or neutralize their harmful effects. ROS, including free radicals like superoxide and hydrogen peroxide, are natural products of metabolic processes, including those in the mitochondria. Under normal conditions, the body has defense mechanisms to contain ROS and prevent cellular damage. However, when this balance is disrupted, oxidative stress can occur, leading to damage to lipids, proteins, and DNA within cells. Oxidative stress, if not managed effectively by the cell’s antioxidant defenses, can lead to cellular damage, including damage to mitochondrial components such as mtDNA. This can further exacerbate mitochondrial dysfunction and create a vicious cycle of increased ROS production and cellular damage; thus, mitochondria patients can suffer continuous ROS harm.
Mitochondrial stress and intestinal flora exhibit a complex relationship that influences overall health and homeostasis in the body. In response to elevated glucose stress, the irregular structure and/or function of mitochondria can trigger the creation of a significant quantity of ROS [74] by driving mitochondria towards heightened oxygen consumption and increased redox potential [75]. Additionally, hyperglycemia alters oxygen transport, favoring the respiratory chain complex II [76]. The process of converting ADP to ATP reduces membrane potential, but hyperglycemia promotes ADP regeneration and consumption rates. This leads to a decline in ATP formation, a persistent rise in membrane potential, and an amplification of ROS production [77]. These ROS can activate ryanodine receptor 2 and suppress the activity of sarcoplasmic reticulum calcium transport ATPase [78], resulting in elevated apoptosis levels and reduced mitophagy levels [79], which contribute to mitochondrial damage [80]. Mitochondrial dysfunction can compromise the integrity of the gut barrier [81]. The gut barrier prevents the entry of harmful substances into the bloodstream and plays a crucial role in immune regulation. Mitochondrial stress-induced damage may lead to increased intestinal permeability, allowing the translocation of bacterial components and toxins [82].
To sum up, there is no direct link between mitochondrial stress and intestinal flora; however, some studies suggest that the gut microbiota can influence host mitochondrial function indirectly through several mechanisms as previously summarized. On the one hand, gut bacteria can produce SCFAs through the fermentation of dietary fiber [83]. SCFAs have been shown to positively impact mitochondrial function and overall cellular health [84]. On the other hand, dysbiosis, an imbalance in the gut microbiota, can lead to inflammation [85]. Chronic inflammation may affect mitochondrial function and contribute to mitochondrial stress. In addition, the gut microbiota produces various metabolites that can enter the bloodstream and potentially affect distant organs, including mitochondria [81]. Finally, the gut microbiota plays a role in regulating the immune system. Immune responses, particularly inflammation, can impact mitochondrial function [86].
3.3. The Role of the Kynurenine Pathway in the Pathophysiology of Mitochondrial Diseases
The last step of the KP is the conversion of QUIN to NAD+, which plays a regulatory role in various cellular functions, including mitochondrial function, DNA repair, and the response to hypoxia by adjusting the cell’s energy state [87,88,89]. Additionally, intermediate activity within the KP can also affect NAD+ conversion and mitochondrial action by influencing levels of ROS [25]. The prevailing understanding suggests that the primary role of the KP is the generation of NAD+, a molecule closely associated with the control of critical oxidative stress processes. In addition, elevated KMO expression results in the formation of intermediary substances that trigger glutamate receptors, culminating in oxidative stress [90]. Moreover, within the peripheral nervous system, 3-HK is transported across the blood-brain barrier (BBB), consequently enhancing its presence and availability in the brain [91]. Both in vivo and in vitro investigations have revealed that the neuronal cell death induced by 3-HK is alleviated by antioxidants like glutathione, catalase, and deferoxamine [92]. These findings illustrate that the detrimental impacts of 3-HK are manifested by imposing oxidative stress.
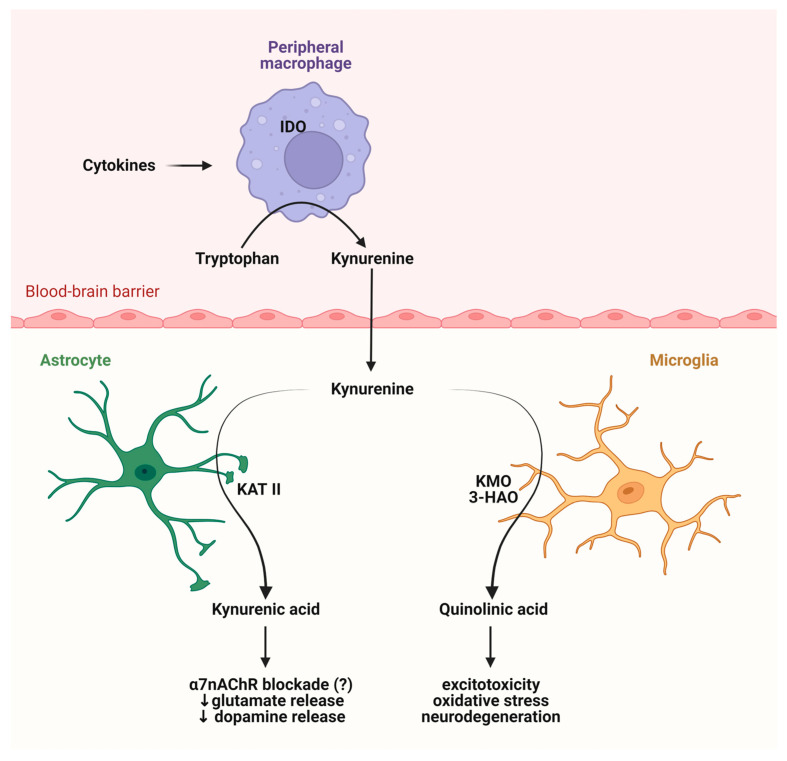
KYNA and QUIN constitute a vital pair responsible for upholding equilibrium in oxidative stress levels [93]. QUIN’s neurotoxicity arises from heightened glutamate signaling, lipid peroxidation, and its role as an activator of NMDA receptors [94], so increased levels of QUIN lead to NMDA receptor activation. Findings from animal research have validated that QUIN triggers inflammation and oxidative stress in rats, potentially resulting in neurodegeneration within the striatum [95]. In contrast, KYNA counteracts the neurotoxic impact of QUIN and serves as a physical shield for neurons [96]. Consequently, maintaining the balance of metabolites within the KP is imperative for preserving normal mitochondrial function (Figure 6).
Figure 6.
The role of the KP metabolites in the CNS. The KP plays a major role in the immune system, and immune cells can influence the production of the KP metabolites. Abbreviations: IDO—indolamine 2,3-dioxygenase, KAT II—kynurenine aminotransferase II, KMO—kynurenine 3-monooxygenase, 3-HAO—kynureninase, α7 nAChR—alpha7 nicotinic acetylcholine receptor.
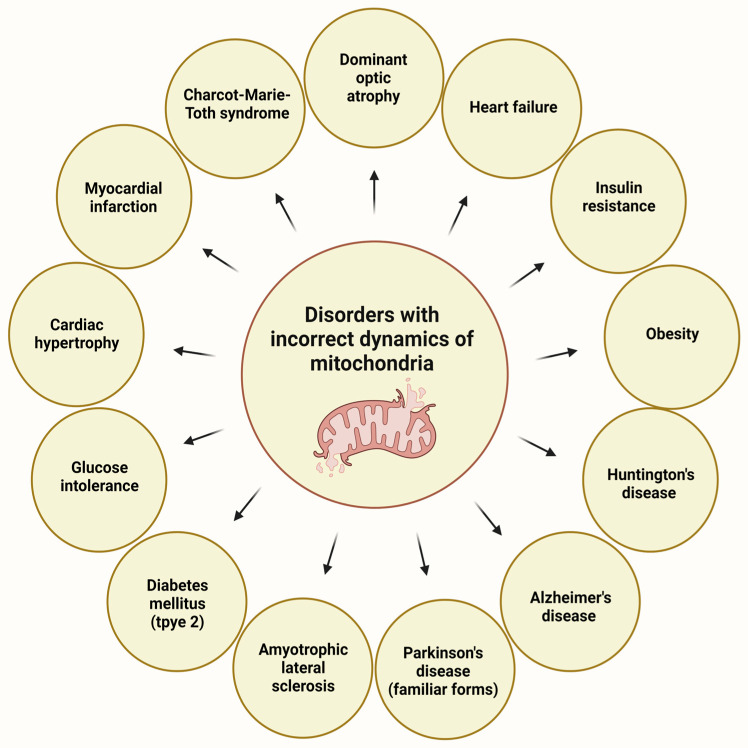
Some of the KP metabolites can influence mitochondrial dynamics, particularly the fusion and fission cycles. These processes can regulate the function and morphology of the mitochondria because the damaged mitochondria can fuse with normal neighboring mitochondria, resulting in its reconstruction. The process of fission means the transport of seriously damaged mitochondria to lysosomes for breakdown, a process also called mitophagy. Mitophagy represents a specialized variant of autophagy designed to discern and eliminate impaired or unneeded mitochondria that features essential components and key proteins shared with the broader autophagic process. The unique mechanism responsible for enveloping mitochondria within phagophores is established through separate mechanisms [97]. Forms of mitophagy include PINK1-PRKN axis-dependent mitophagy, receptor-mediated mitophagy, FUNDC1-mediated mitophagy, BNIP3- and BNIP3L-mediated mitophagy, BCL2L13-mediated mitophagy, FKBP8-mediated mitophagy, and PHB2-mediated mitophagy, as discussed in Zhang’s review article [97]. These processes representing the dynamics of mitochondria have a crucial role in cellular homeostasis [98]. Several metabolic, cardiovascular problems, and neurodegenerative diseases can be traced back to the malfunctioning of these dynamics (Figure 7).
Figure 7.
Disorders with impaired mitochondrial dynamics [3].
Mitochondrial fission is modulated by the fission 1 protein and dynamin related protein (Drp1). It is important to note that the overexpression of KMO can regulate the phosphorylation of Drp1, elevating mitochondrial fission [34]; so KMO can influence the dynamics and homeostasis of mitochondria. In addition to this, knockdown of the KMO gene causes a distortion of shape in mitochondria [99] and increases the number of mitochondria with reduced respiratory activity. Taken together, KMO regulates mitochondrial dynamics, probably via interaction with Drp1 [34].
On the other hand, metabolites of the KP can influence the level of NAD+, which has a role in mitochondrial function, dynamics, and the production of free radicals [25]. Supplemental Trp can elevate lifespan in mice [100] and in Caenorhabditis elegans [101], probably by increasing the level of NAD+ [102].
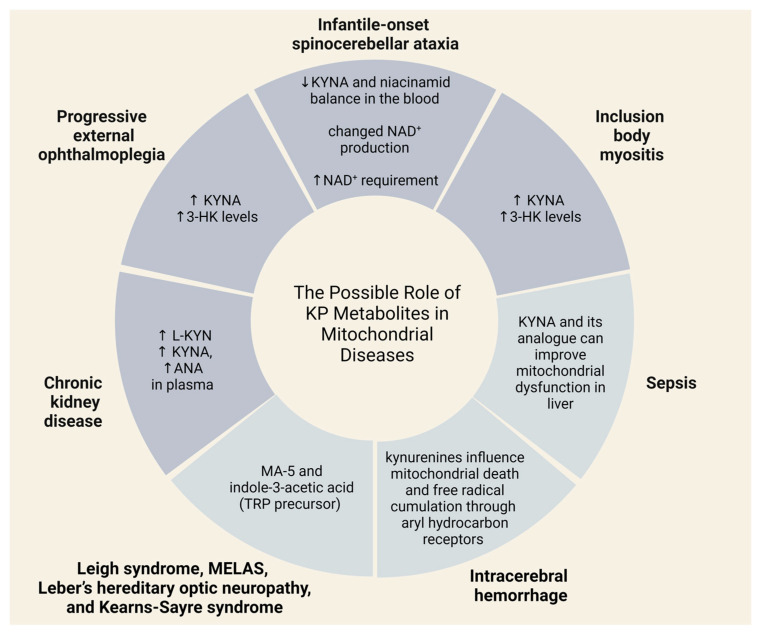
Leigh syndrome, the most common mitochondrial disease in childhood, is a neurodegenerative disorder caused by over 75 known genetic mutations [103]. In this paper, the authors provide a comprehensive overview of the genetic, biochemical, clinical, metabolic and neuroradiological heterogeneity of Leigh syndrome. A recent study revealed that individuals with French Canadian variants of Leigh syndrome exhibited decreased levels of L-KYN and 3-HAA in their blood [104]. Furthermore, in these patients, there was an elevation in indoxyl sulfate levels [104], indicating a potential shift in Trp metabolism toward the indole pathway. It is worth mentioning that Trp can undergo metabolism not only to kynurenine or 5-HT but also to indoxyl sulfate. Trp can be transformed into indole by tryptophanase, and indole can further metabolize to indoxyl through cytochrome P450 2E1. Subsequently, sulfotransferase enzymes can convert indole to indoxyl sulfate.
Leber hereditary optic neuropathy is characterized by the degeneration of retinal ganglion cells, leading to vision loss. Patients with this condition typically carry mutations in genes that encode complex I within the electron transport chain. Studies of Leber hereditary optic neuropathy patients have identified reduced levels of Trp and glutamate [105], indicating potential involvement of the KP in the disease’s pathomechanism. Trp is the precursor of the KP, as mentioned above, and KYNA is a glutamate receptor antagonist. Myoclonic epilepsy with ragged red fibers is a primary mitochondrial disorder. This is an extremely rare illness, and can affect different parts of the body, especially the nervous system and the muscles. One of the main symptoms of the disorder is myoclonus epilepsy, and we know that 5-HT and the KP pathways change in patients with Unverricht–Lundborg disease, which is a type of progressive myoclonus epilepsy, and in cystatin B (CSTB)-deficient mice [106].
A recent study has shown that the metabolites of the KP can be used as biomarkers of several mitochondrial diseases. Buzkova and her colleagues found a decrease of KYNA and niacinamid balance in the blood of infantile-onset spinocerebellar ataxia patients, which suggests altered NAD+ production and elevated NAD+ demand [107]. This is a severe, progressive neurodegenerative condition that appears usually at the age of 1. The symptoms of the disease are ataxia, muscle hypotonia, ophthalmoplegia, deafness, and epileptic seizures. In addition to this, researchers also found an increase in KYNA and 3-HK levels in patients with progressive external ophthalmoplegia and inclusion body myositis [107]. Progressive external ophthalmoplegia attacks and causes weakness in eye muscles. This condition usually has several accompanying symptoms, such as deafness, neuropathy, ataxia, parkinsonism, and depression.
The other illness that correlates with mitochondrial dysfunction is chronic kidney disease (CKD), which is a general name for functional or structural malfunction of kidneys. Research has shown that CKD is also connected to the KP. For instance, increased L-KYN, KYNA, ANA levels were found in the plasma of patients with CKD [108].
Sporadic inclusion body myositis is a complex disease that has inflammatory, degenerative, and mitochondrial aspects [109]. The main manifestation of the disorder is debility of limbs.
Some neurodegenerative disorders are also linked to mitochondrial abnormalities, oxidative stress, and alterations of the KP metabolism [110,111]. Reduced levels of KYNA have been found in the plasma of patients with Alzheimer’s disease [112], and the same changes have been detected in patients with Parkinson’s and Huntington’s disorders [113]. This observation holds significant importance as reduced KYNA levels may trigger insufficient anti-inflammatory reaction, leading to heightened tissue damage and excessive cell proliferation in inflammatory conditions [114,115], which can be seen in neurodegenerative disorders. In addition to this, elevated levels of QUIN in the cerebrospinal fluid have been observed in patients with AIDS-associated dementia [116,117]. This finding confirms the existence of heightened mitochondrial damage linked to alterations in KYNA-QUIN levels.
In conjunction with this information, it is noteworthy that cannabinoid receptor agonists can hinder QUIN-induced mitochondrial dysfunction, lipid peroxidation, and the formation of ROS [118]. It is also important to highlight that KYNA, in an indirect and region-specific manner within the brain, enhances the presence of functional cannabinoid receptor 1 without altering the overall binding and activity of the receptor [119].
Data from animal models have pointed out that KYNA and its analogues can improve mitochondrial dysfunction in the liver, produced by sepsis, probably via the reduction of mitochondrial dysfunction of the CNS [120]. The synthetic compound mitochonic acid-5 (MA-5) is a candidate drug for the treatment of sporadic inclusion body myositis. This substance can ameliorate cell survival, elevate the level of ATP, and repair the dynamics of mitochondria in samples from patients with this disease [121]. MA-5 is also effective in the survival of fibroblasts sampled from patients with Leigh syndrome, MELAS (myopathy encephalopathy lactic acidosis and stroke-like episodes), Leber’s hereditary optic neuropathy, and Kearns-Sayre syndrome [122]. It is worthy of note that MA-5 is a derivative of indole-3-acetic acid [121], which is a plant hormone. The precursor of indole-3-acetic acid is Trp, which is also a precursor of the KP, so MA-5 and Trp have a common formation pathway. Another recent animal study has shown that kynurenines influence mitochondria death and free radical accumulation in mice after intracerebral hemorrhage through aryl hydrocarbon receptors [123] (Figure 8).
Figure 8.
Possible roles of KP metabolites in mitochondrial diseases. Abbreviations: KYNA—kynurenic acid, NAD+—nicotinamide adenine dinucleotide, 3-HK—3-hydroxykynurenine, MA-5—mitochonic acid-5, TRP—tryptophan, L-KYN—l-kynurenine, ANA—anthranilic acid.
Regardless, it is important to recognize that KYNA has restricted passage through the BBB. Potential therapeutic approaches could involve aiding penetration of the BBB and impeding or delaying the release of KYNA from the CNS. Elevating KYNA levels can be achieved by administering L-KYN. However, heightened KYNA levels come with increased elimination. Various methods can inhibit this process, and one potential agent with promise is probenecid. Animal studies have demonstrated its success in maintaining elevated KYNA levels [124,125,126]. Different chemical modifications can ease the passage of KYNA through the BBB. The resulting compounds, known as analogues, exhibit a heightened affinity for crossing the BBB. Examples of such analogues include 7-chloro-KYNA and 4-chloro-L-KYN or AV-101. Although 7-chloro-KYNA does not exhibit significantly improved BBB crossing compared to KYNA, 4-chloro-L-KYN serves as a BBB-crossing precursor for 7-chloro-KYNA. Additionally, 4-chloro-L-KYN has the ability to inhibit the synthesis of QUIN [127], suggesting its potential as a viable target for alleviating the adverse effects QUIN imposes on mitochondria. It is crucial to emphasize that 4-chloro-L-KYN can be generated through bacterial synthesis, specifically by the marine bacterium Saccharomonospora sp. CNQ-490 [128]. This strain has the capability to convert Trp into 4-chloro-L-KYN within three steps [128]. This development could potentially pave the way for utilizing microbiome-based therapy in addressing various neurodegenerative diseases.
4. Conclusions
In summary, the gut microbiota not only affect the nervous system but also play a significant role in the functioning of the body. The connection between the gastrointestinal system and the kynurenine pathway may yield previously unseen results in the treatment of various diseases; influencing the gut flora could provide a new therapeutic target for managing different conditions.
Acknowledgments
All figures were created using BioRender.com (accessed on 10 December 2023).
Author Contributions
Conceptualization, G.N.-G. and E.S.; writing—original draft preparation, G.N.-G. and E.S.; writing—review and editing, L.V.; visualization, E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Eleonóra Spekker currently works as a researcher at Pharmacoidea, and contributed to the drafting of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
The research was supported by the Incubation Competence Centre of the Centre of Excellence for Interdisciplinary Research, Development and Innovation of the University of Szeged. G.N-G. is a member of the Preventive Health Sciences Research Group.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pires A.S., Sundaram G., Heng B., Krishnamurthy S., Brew B.J., Guillemin G.J. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol. Ther. 2022;236:108055. doi: 10.1016/j.pharmthera.2021.108055. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y.S., Ogbechi J., Clanchy F.I., Williams R.O., Stone T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020;11:388. doi: 10.3389/fimmu.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sas K., Szabó E., Vécsei L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules. 2018;23:191. doi: 10.3390/molecules23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borre Y.E., O’Keeffe G.W., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez J.M., Murphy K., Stanton C., Ross R.P., Kober O.I., Juge N., Avershina E., Rudi K., Narbad A., Jenmalm M.C., et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adlerberth I., Wold A.E. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 7.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112 Pt B:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Knights D., Ward T.L., McKinlay C.E., Miller H., Gonzalez A., McDonald D., Knight R. Rethinking “enterotypes”. Cell Host Microbe. 2014;16:433–437. doi: 10.1016/j.chom.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 13.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S., Awasthi A., Singh S. Altered gut microbiota and intestinal permeability in Parkinson’s disease: Pathological highlight to management. Neurosci. Lett. 2019;712:134516. doi: 10.1016/j.neulet.2019.134516. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Yuan X., Kang Y., Song X. Tryptophan-kynurenine pathway as a novel link between gut microbiota and schizophrenia: A review. Trop. J. Pharm. Res. 2019;18:897–905. doi: 10.4314/tjpr.v18i4.30. [DOI] [Google Scholar]
- 16.Reigstad C.S., Salmonson C.E., Rainey J.F., Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand N., Gorantla V.R., Chidambaram S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells. 2022;12:54. doi: 10.3390/cells12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 19.de Lau L.M., Bornebroek M., Witteman J.C., Hofman A., Koudstaal P.J., Breteler M.M. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- 20.Ieraci A., Beggiato S., Ferraro L., Barbieri S.S., Popoli M. Kynurenine pathway is altered in BDNF Val66Met knock-in mice: Effect of physical exercise. Brain Behav. Immun. 2020;89:440–450. doi: 10.1016/j.bbi.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Maqsood R., Stone T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016;41:2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 22.Legan T.B., Lavoie B., Norberg E., Ley I.C., Tack S., Tompkins T.A., Wargo M.J., Mawe G.M. Tryptophan-synthesizing bacteria enhance colonic motility. Neurogastroenterol. Motil. 2023;35:e14629. doi: 10.1111/nmo.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebig J. Uber Kynurensäure. Ann. Chem. 1853;86:125–126. doi: 10.1002/jlac.18530860115. [DOI] [Google Scholar]
- 24.Hirai K., Kuroyanagi H., Tatebayashi Y., Hayashi Y., Hirabayashi-Takahashi K., Saito K., Haga S., Uemura T., Izumi S. Dual role of the carboxyl-terminal region of pig liver l-kynurenine 3-monooxygenase: Mitochondrial-targeting signal and enzymatic activity. J. Biochem. 2010;148:639–650. doi: 10.1093/jb/mvq099. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Portuguez R., Sutphin G.L. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020;132:110841. doi: 10.1016/j.exger.2020.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillemin G.J., Smythe G.A., Veas L.A., Takikawa O., Brew B.J. A beta 1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14:2311–2315. doi: 10.1097/00001756-200312190-00005. [DOI] [PubMed] [Google Scholar]
- 27.Behan W.M.H., McDonald M., Darlington L.G., Stone T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: Protection by melatonin and deprenyl. Br. J. Pharmacol. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lugo-Huitrón R., Ugalde Muñiz P., Pineda B., Pedraza-Chaverrí J., Ríos C., Pérez-de la Cruz V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell Longev. 2013;2013:104024. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy-Grócz G., Zádor F., Dvorácskó S., Bohár Z., Benyhe S., Tömböly C., Párdutz Á., Vécsei L. Interactions between the kynurenine and the endocannabinoid system with special emphasis on migraine. Int. J. Mol. Sci. 2017;18:1617. doi: 10.3390/ijms18081617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim C.K., Fernández-Gomez F.J., Braidy N., Estrada C., Costa C., Costa S., Bessede A., Fernandez-Villalba E., Zinger A., Herrero M.T., et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017;155:76–95. doi: 10.1016/j.pneurobio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Zádor F., Nagy-Grócz G., Kekesi G., Dvorácskó S., Szűcs E., Tömböly C., Horvath G., Benyhe S., Vécsei L. Kynurenines and the Endocannabinoid System in Schizophrenia: Common Points and Potential Interactions. Molecules. 2019;24:3709. doi: 10.3390/molecules24203709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González Esquivel D., Ramírez-Ortega D., Pineda B., Castro N., Ríos C., Pérez de la Cruz V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology. 2017;112 Pt B:331–345. doi: 10.1016/j.neuropharm.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Baran H., Staniek K., Bertignol-Spörr M., Attam M., Kronsteiner C., Kepplinger B. Effects of Various Kynurenine Metabolites on Respiratory Parameters of Rat Brain, Liver and Heart Mitochondria. Int. J. Tryptophan Res. 2016;9:17–29. doi: 10.4137/IJTR.S37973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddison D.C., Alfonso-Núñez M., Swaih A.M., Breda C., Campesan S., Allcock N., Straatman-Iwanowska A., Kyriacou C.P., Giorgini F. A novel role for kynurenine 3-monooxygenase in mitochondrial dynamics. PLoS Genet. 2020;16:e1009129. doi: 10.1371/journal.pgen.1009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birch P.J., Grossman C.J., Hayes A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988;154:85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 36.Kessler M., Terramani T., Lynch G., Baudry M. A Glycine Site Associated with N-Methyl-d-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 37.Prescott C., Weeks A.M., Staley K.J., Partin K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006;402:108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 38.Rózsa E., Robotka H., Vécsei L., Toldi J. The Janus-face kynurenic acid. J. Neural Transm. 2008;115:1087–1091. doi: 10.1007/s00702-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 39.Hubbard T.D., Murray I.A., Bisson W.H., Lahoti T.S., Gowda K., Amin S.G., Patterson A.D., Perdew G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015;5:12689. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M., Macchiarulo A., Vacca C., et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaronen M., Quintana F.J. Immunological Relevance of the Coevolution of IDO1 and AHR. Front. Immunol. 2014;5:521. doi: 10.3389/fimmu.2014.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L., Appelman B., Mooij-Kalverda K., Houtkooper R.H., van Weeghel M., Vaz F.M., Dijkhuis A., Dekker T., Smids B.S., Duitman J.W., et al. COVID-19 Biobank study Group. Prolonged indoleamine 2,3-dioxygenase-2 activity and associated cellular stress in post-acute sequelae of SARS-CoV-2 infection. EBioMedicine. 2023;94:104729. doi: 10.1016/j.ebiom.2023.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020;152:627–649. doi: 10.1111/jnc.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spekker E., Nagy-Grócz G. All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine. Neurol. Int. 2023;15:1174–1190. doi: 10.3390/neurolint15030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cronin P., Joyce S.A., O’Toole P.W., O’Connor E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients. 2021;13:1655. doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy E.A., Velazquez K.T., Herbert K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:515–520. doi: 10.1097/MCO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehhaghi M., Kazemi Shariat Panahi H., Guillemin G.J. Microorganisms’ Footprint in Neurodegenerative Diseases. Front. Cell Neurosci. 2018;12:466. doi: 10.3389/fncel.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gensollen T., Iyer S.S., Kasper D.L., Blumberg R.S. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 50.Desbonnet L., Clarke G., Traplin A., O’Sullivan O., Crispie F., Moloney R.D., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Sun P., Wang M., Liu Y.X., Li L., Chai X., Zheng W., Chen S., Zhu X., Zhao S. High-fat diet-disturbed gut microbiota-colonocyte interactions contribute to dysregulating peripheral tryptophan-kynurenine metabolism. Microbiome. 2023;11:154. doi: 10.1186/s40168-023-01606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014;20:14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke G., McKernan D.P., Gaszner G., Quigley E.M., Cryan J.F., Dinan T.G. A Distinct Profile of Tryptophan Metabolism along the Kynurenine Pathway Downstream of Toll-Like Receptor Activation in Irritable Bowel Syndrome. Front. Pharmacol. 2012;3:90. doi: 10.3389/fphar.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzgerald P., Cassidy E.M., Clarke G., Scully P., Barry S., Quigley E.M.M., Shanahan F., Cryan J., Dinan T.G. Tryptophan catabolism in females with irritable bowel syndrome: Relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol. Motil. 2008;20:1291–1297. doi: 10.1111/j.1365-2982.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 56.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 57.Bernstein C.N. Treatment of IBD: Where we are and where we are going. Am. J. Gastroenterol. 2015;110:114–126. doi: 10.1038/ajg.2014.357. [DOI] [PubMed] [Google Scholar]
- 58.Xu S., Li X., Zhang S., Qi C., Zhang Z., Ma R., Xiang L., Chen L., Zhu Y., Tang C., et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: A multi-omics Mendelian randomization study. BMC Med. 2023;21:179. doi: 10.1186/s12916-023-02878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu F., Du Y., Li C., Zhang H., Lai W., Li S., Ye Z., Fu W., Li S., Li X.G., et al. Association between metabolites in tryptophan-kynurenine pathway and inflammatory bowel disease: A two-sample Mendelian randomization. Sci. Rep. 2024;14:201. doi: 10.1038/s41598-023-50990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopper R.K., Carroll S., Aponte A.M., Johnson D.T., French S., Shen R.F., Witzmann F.A., Harris R.A., Balaban R.S. Mitochondrial matrix phosphoproteome: Effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCormick E.M., Zolkipli-Cunningham Z., Falk M.J. Mitochondrial disease genetics update: Recent insights into the molecular diagnosis and expanding phenotype of primary mitochondrial disease. Curr. Opin. Pediatr. 2018;30:714–724. doi: 10.1097/MOP.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerrison J.B., Miller N.R., Hsu F., Beaty T.H., Maumenee I.H., Smith K.H., Savino P.J., Stone E.M., Newman N.J. A case-control study of tobacco and alcohol consumption in Leber hereditary optic neuropathy. Am. J. Ophthalmol. 2000;130:803–812. doi: 10.1016/S0002-9394(00)00603-6. [DOI] [PubMed] [Google Scholar]
- 64.Giordano L., Deceglie S., d’Adamo P., Valentino M.L., La Morgia C., Fracasso F., Roberti M., Cappellari M., Petrosillo G., Ciaravolo S., et al. Cigarette toxicity triggers Leber’s hereditary optic neuropathy by affecting mtDNA copy number, oxidative phosphorylation and ROS detoxification pathways. Cell Death Dis. 2015;6:e2021. doi: 10.1038/cddis.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang J.M., Park H.W. Carbon monoxide poisoning as an epigenetic factor for Leber’s hereditary optic neuropathy. Korean J. Ophthalmol. 1996;10:122–123. doi: 10.3341/kjo.1996.10.2.122. [DOI] [PubMed] [Google Scholar]
- 66.Shaikh S., Ta C., Basham A.A., Mansour S. Leber hereditary optic neuropathy associated with antiretroviral therapy for human immunodeficiency virus infection. Am. J. Ophthalmol. 2001;131:143–145. doi: 10.1016/S0002-9394(00)00716-9. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen T., Jeyakumar A. Genetic susceptibility to aminoglycoside ototoxicity. Int. J. Pediatr. Otorhinolaryngol. 2019;120:15–19. doi: 10.1016/j.ijporl.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Klopstock T., Priglinger C., Yilmaz A., Kornblum C., Distelmaier F., Prokisch H. Mitochondrial Disorders. Dtsch. Arztebl. Int. 2021;118:741–748. doi: 10.3238/arztebl.m2021.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luft R. The development of mitochondrial medicine. Proc. Natl. Acad. Sci. USA. 1994;91:8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koenig M.K. Presentation and diagnosis of mitochondrial disorders in children. Pediatr. Neurol. 2008;38:305–313. doi: 10.1016/j.pediatrneurol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parikh S., Karaa A., Goldstein A., Bertini E.S., Chinnery P.F., Christodoulou J., Cohen B.H., Davis R.L., Falk M.J., Fratter C., et al. Diagnosis of ‘possible’ mitochondrial disease: An existential crisis. J. Med. Genet. 2019;56:123–130. doi: 10.1136/jmedgenet-2018-105800. [DOI] [PubMed] [Google Scholar]
- 72.Ng Y.S., Turnbull D.M. Mitochondrial disease: Genetics and management. J. Neurol. 2016;263:179–191. doi: 10.1007/s00415-015-7884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirano M., Emmanuele V., Quinzii C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018;62:467–481. doi: 10.1042/EBC20170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018;1859:940–950. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Anupam K., Kaushal J., Prabhakar N., Bhatnagar A. Effect of redox status of peripheral blood on immune signature of circulating regulatory and cytotoxic T cells in streptozotocin induced rodent model of type I diabetes. Immunobiology. 2018;223:586–597. doi: 10.1016/j.imbio.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 77.Bayrami G., Alihemmati A., Karimi P., Javadi A., Keyhanmanesh R., Mohammadi M., Zadi-Heydarabad M., Badalzadeh R. Combination of Vildagliptin and Ischemic Postconditioning in Diabetic Hearts as a Working Strategy to Reduce Myocardial Reperfusion Injury by Restoring Mitochondrial Function and Autophagic Activity. Adv. Pharm. Bull. 2018;8:319–329. doi: 10.15171/apb.2018.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J., Chen P., Cao Q., Wang W., Chang X. Traditional Chinese Medicine Ginseng Dingzhi Decoction Ameliorates Myocardial Fibrosis and High Glucose-Induced Cardiomyocyte Injury by Regulating Intestinal Flora and Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2022;2022:9205908. doi: 10.1155/2022/9205908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novak E.A., Mollen K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015;3:62. doi: 10.3389/fcell.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kłos P., Dabravolski S.A. The Role of Mitochondria Dysfunction in Inflammatory Bowel Diseases and Colorectal Cancer. Int. J. Mol. Sci. 2021;22:11673. doi: 10.3390/ijms222111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q., Sperandio M., Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoo J.Y., Groer M., Dutra S.V.O., Sarkar A., McSkimming D.I. Gut Microbiota and Immune System Interactions. Microorganisms. 2020;8:1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajman L., Chwalek K., Sinclair D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belenky P., Bogan K.L., Brenner C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y., Sauve A.A. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parrott J.M., O’Connor J.C. Kynurenine 3-Monooxygenase: An Influential Mediator of Neuropathology. Front. Psychiatry. 2015;6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skorobogatov K., De Picker L., Verkerk R., Coppens V., Leboyer M., Müller N., Morrens M. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front. Immunol. 2021;12:716980. doi: 10.3389/fimmu.2021.716980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colín-González A.L., Maldonado P.D., Santamaría A. 3-Hydroxykynurenine: An intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology. 2013;34:189–204. doi: 10.1016/j.neuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 93.García-Lara L., Pérez-Severiano F., González-Esquivel D., Elizondo G., Segovia J. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J. Neurosci. Res. 2015;93:1423–1433. doi: 10.1002/jnr.23595. [DOI] [PubMed] [Google Scholar]
- 94.Bryleva E.Y., Brundin L. Kynurenine pathway metabolites and suicidality. Neuropharmacology. 2017;112 Pt B:324–330. doi: 10.1016/j.neuropharm.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mishra J., Kumar A. Improvement of mitochondrial function by paliperidone attenuates quinolinic acid-induced behavioural and neurochemical alterations in rats: Implications in Huntington’s disease. Neurotox. Res. 2014;26:363–381. doi: 10.1007/s12640-014-9469-9. [DOI] [PubMed] [Google Scholar]
- 96.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 97.Zhang T., Liu Q., Gao W., Sehgal S.A., Wu H. The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy. 2022;18:1216–1239. doi: 10.1080/15548627.2021.1975914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marzetti E., Csiszar A., Dutta D., Balagopal G., Calvani R., Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H459–H476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ivatt R.M., Sanchez-Martinez A., Godena V.K., Brown S., Ziviani E., Whitworth A.J. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc. Natl. Acad. Sci. USA. 2014;111:8494–8499. doi: 10.1073/pnas.1321207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., D’Amico D., Ropelle E.R., Lutolf M.P., Aebersold R., et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 101.Fang E.F., Kassahun H., Croteau D.L., Scheibye-Knudsen M., Marosi K., Lu H., Shamanna R.A., Kalyanasundaram S., Bollineni R.C., Wilson M.A., et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katsyuba E., Mottis A., Zietak M., De Franco F., van der Velpen V., Gariani K., Ryu D., Cialabrini L., Matilainen O., Liscio P., et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–359. doi: 10.1038/s41586-018-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lake N.J., Compton A.G., Rahman S., Thorburn D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016;79:190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 104.Thompson Legault J., Strittmatter L., Tardif J., Sharma R., Tremblay-Vaillancourt V., Aubut C., Boucher G., Clish C.B., Cyr D., Daneault C., et al. A Metabolic Signature of Mitochondrial Dysfunction Revealed through a Monogenic Form of Leigh Syndrome. Cell Rep. 2015;13:981–989. doi: 10.1016/j.celrep.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rozen T.D. Can the effects of the mitochondrial DNA mutations found in Leber’s hereditary optic neuropathy be protective against the development of cluster headache in smokers? Cephalalgia Rep. 2020;3 doi: 10.1177/2515816320939571. [DOI] [Google Scholar]
- 106.Arbatova J., D’Amato E., Vaarmann A., Zharkovsky A., Reeben M. Reduced serotonin and 3-hydroxyanthranilic acid levels in serum of cystatin B-deficient mice, a model system for progressive myoclonus epilepsy. Epilepsia. 2005;46((Suppl. S5)):49–51. doi: 10.1111/j.1528-1167.2005.01008.x. [DOI] [PubMed] [Google Scholar]
- 107.Buzkova J., Nikkanen J., Ahola S., Hakonen A.H., Sevastianova K., Hovinen T., Yki-Järvinen H., Pietiläinen K.H., Lönnqvist T., Velagapudi V., et al. Metabolomes of mitochondrial diseases and inclusion body myositis patients: Treatment targets and biomarkers. EMBO Mol. Med. 2018;10:e9091. doi: 10.15252/emmm.201809091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pawlak K., Tankiewicz J., Mysliwiec M., Pawlak D. Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul. Fibrinolysis. 2009;20:590–594. doi: 10.1097/MBC.0b013e32832da16d. [DOI] [PubMed] [Google Scholar]
- 109.De Paepe B. Sporadic Inclusion Body Myositis: An Acquired Mitochondrial Disease with Extras. Biomolecules. 2019;9:15. doi: 10.3390/biom9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 111.Trushina E., McMurray C.T. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 112.Solvang S.H., Nordrehaug J.E., Aarsland D., Lange J., Ueland P.M., McCann A., Midttun Ø., Tell G.S., Giil L.M. Kynurenines, Neuropsychiatric Symptoms, and Cognitive Prognosis in Patients with Mild Dementia. Int. J. Tryptophan Res. 2019;12:1178646919877883. doi: 10.1177/1178646919877883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boros F.A., Vécsei L. Immunomodulatory Effects of Genetic Alterations Affecting the Kynurenine Pathway. Front. Immunol. 2019;10:2570. doi: 10.3389/fimmu.2019.02570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanaka M., Bohár Z., Vécsei L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules. 2020;25:564. doi: 10.3390/molecules25030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mor A., Tankiewicz-Kwedlo A., Krupa A., Pawlak D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells. 2021;10:1603. doi: 10.3390/cells10071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heyes M.P., Saito K., Lackner A., Wiley C.A., Achim C.L., Markey S.P. Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J. 1998;12:881–896. doi: 10.1096/fasebj.12.10.881. [DOI] [PubMed] [Google Scholar]
- 117.Chao C.C., Hu S., Gekker G., Lokensgard J.R., Heyes M.P., Peterson P.K. U50,488 protection against HIV-1-related neurotoxicity: Involvement of quinolinic acid suppression. Neuropharmacology. 2000;39:150–160. doi: 10.1016/S0028-3908(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 118.Rangel-López E., Colín-González A.L., Paz-Loyola A.L., Pinzón E., Torres I., Serratos I.N., Castellanos P., Wajner M., Souza D.O., Santamaría A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience. 2015;285:97–106. doi: 10.1016/j.neuroscience.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 119.Zádor F., Nagy-Grócz G., Dvorácskó S., Bohár Z., Cseh E.K., Zádori D., Párdutz Á., Szűcs E., Tömböly C., Borsodi A., et al. Long-term systemic administration of kynurenic acid brain region specifically elevates the abundance of functional CB1 receptors in rats. Neurochem. Int. 2020;138:104752. doi: 10.1016/j.neuint.2020.104752. [DOI] [PubMed] [Google Scholar]
- 120.Juhász L., Rutai A., Fejes R., Tallósy S.P., Poles M.Z., Szabó A., Szatmári I., Fülöp F., Vécsei L., Boros M., et al. Divergent Effects of the N-Methyl-D-Aspartate Receptor Antagonist Kynurenic Acid and the Synthetic Analog SZR-72 on Microcirculatory and Mitochondrial Dysfunction in Experimental Sepsis. Front. Med. 2020;7:566582. doi: 10.3389/fmed.2020.566582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oikawa Y., Izumi R., Koide M., Hagiwara Y., Kanzaki M., Suzuki N., Kikuchi K., Matsuhashi T., Akiyama Y., Ichijo M., et al. Mitochondrial dysfunction underlying sporadic inclusion body myositis is ameliorated by the mitochondrial homing drug MA-5. PLoS ONE. 2020;15:e0231064. doi: 10.1371/journal.pone.0231064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki T., Yamaguchi H., Kikusato M., Matsuhashi T., Matsuo A., Sato T., Oba Y., Watanabe S., Minaki D., Saigusa D., et al. Mitochonic acid 5 (MA-5), a derivative of the plant hormone indole-3-acetic acid, improves survival of fibroblasts from patients with mitochondrial diseases. Tohoku J. Exp. Med. 2015;236:225–232. doi: 10.1620/tjem.236.225. [DOI] [PubMed] [Google Scholar]
- 123.Ren R., Fang Y., Sherchan P., Lu Q., Lenahan C., Zhang J.H., Zhang J., Tang J. Kynurenine/Aryl Hydrocarbon Receptor Modulates Mitochondria-Mediated Oxidative Stress and Neuronal Apoptosis in Experimental Intracerebral Hemorrhage. Antioxid. Redox Signal. 2022;37:1111–1129. doi: 10.1089/ars.2021.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nozaki K., Beal M.F. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J. Cereb. Blood Flow Metab. 1992;12:400–407. doi: 10.1038/jcbfm.1992.57. [DOI] [PubMed] [Google Scholar]
- 125.Gigler G., Szénási G., Simó A., Lévay G., Hársing L.G., Sas K., Vécsei L., Toldi J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007;564:116–122. doi: 10.1016/j.ejphar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 126.Fejes-Szabó A., Bohár Z., Nagy-Grócz G., Vámos E., Tar L., Pődör B., Tajti J., Toldi J., Vécsei L., Párdutz Á. Effect of probenecid on the pain-related behaviour and morphological markers in orofacial formalin test of the rat. CNS Neurol. Disord. Drug Targets. 2015;14:350–359. doi: 10.2174/1871527314666150225141229. [DOI] [PubMed] [Google Scholar]
- 127.Parli C.J., Krieter P., Schmidt B. Metabolism of 6-chlorotryptophan to 4-chloro-3-hydroxyanthranilic acid: A potent inhibitor of 3-hydroxyanthranilic acid oxidase. Arch. Biochem. Biophys. 1980;203:161–166. doi: 10.1016/0003-9861(80)90164-2. [DOI] [PubMed] [Google Scholar]
- 128.Luhavaya H., Sigrist R., Chekan J.R., McKinnie S.M.K., Moore B.S. Biosynthesis of l-4-Chlorokynurenine, an Antidepressant Prodrug and a Non-Proteinogenic Amino Acid Found in Lipopeptide Antibiotics. Angew. Chem. Int. Ed. Engl. 2019;58:8394–8399. doi: 10.1002/anie.201901571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.