Abstract
Background and Objectives: In SSc, ILD is a major cause of morbidity and mortality. We aimed to investigate the performance of DLCO (diffusing capacity of lung carbon monoxide) and FVC (forced vital capacity) delta change (Δ) and baseline values in predicting the development of SSc-ILD. Methods: Longitudinal data of DLCO, FVC, and ILD on the HRCT of SSc patients from the EUSTAR database were evaluated at baseline (t0) and after 12 (±4) (t1) and 24 (±4) (t2) months. Results: 474/17805 patients were eligible for the study (403 females); 46 (9.7%) developed ILD at t2. Positivity for anti-topoisomerase antibodies (117 patients) showed an association with ILD development at t2 (p = 0.0031). Neither the mean t0 to t1 change (Δ) of DLCO nor the mean t0 to t1 FVCΔ predicted the appearance of ILD at t2. Investigating the possible role of baseline DLCO and FVC values in predicting ILD appearance after 24 (±4) months, we observed a moderate predictive capability of t0 DLCO < 80%, stronger than that of FVC < 80%. Conclusions: We suggest that an impaired baseline DLCO may be predictive of the appearance of ILD after 2 years of follow-up. This result advances the hypothesis that a reduction in gas exchange may be considered an early sign of lung involvement. However, further rigorous studies are warranted to understand the predictive role of DLCO evaluation in the course of SSc.
Keywords: computed tomography (CT), interstitial lung disease, scleroderma, pulmonary function test
1. Introduction
Systemic sclerosis (SSc) is characterized by small-vessel vasculopathy, immune dysregulation, and fibroblast dysfunction leading to the fibrosis of skin and internal organs [1]. Interstitial lung disease (ILD), together with pulmonary arterial hypertension (PAH), represents the main cause of SSc-related deaths [2,3,4], and it occurs more frequently in the diffuse cutaneous subset (dcSSc) [5]. Despite the recent advances in SSc-ILD treatment, lung involvement represents a fearsome manifestation of the disease, and the optimal timing to start an immunosuppressive and antifibrotic treatment still remains debated [6]. Recent studies also highlighted a certain involvement of a small airway in SSc patients, which may be associated with ILD [7]. A study on a Norwegian SSc cohort reported an ILD prevalence on HRCT (high-resolution computed tomography) of 50% [8]. The clinical course and the timing of onset of ILD are unpredictable among SSc patients, and ILD may occur in the early stages, particularly in dcSSc patients [2,9,10,11]. Symptoms due to lung involvement, such as dyspnoea and cough, can be delayed and are not specific to ILD. Therefore, an early diagnosis, a regular follow-up [12], and the identification of predictors of evolution are crucial and eagerly awaited to improve SSc-ILD management. In this context, besides HRCT which represents the mainstay for ILD diagnosis, other non-ionizing methods of lung evaluation such as lung ultrasound are under study [13].
The evidence-based expert European consensus confirmed HRCT as the primary tool for the screening and diagnosis of SSc-ILD, with pulmonary function tests (PFTs) and clinical symptoms providing supporting evidence [14]. The baseline forced vital capacity (FVC) value has been proposed as a reliable predictor of SSc pulmonary function deterioration [15,16,17]. In addition, the recent functional criteria for progressive pulmonary fibrosis reported an absolute decline in FVC of ≥5% or in diffusion of the lung for carbon oxide (DLCO) ≥10% within 1 year of follow-up as physiological evidence of disease progression, when not explained by other causes [18]. In SSc, a change in DLCO or in DLCO per unit alveolar volume (DLCO/AV) change ≥15% has been proposed as a marker of poor ILD prognosis in patients with signs of ILD at enrolment in clinical trials [19]. In addition, the combination of lung fibrotic involvement greater than 30% on HRCT or between 10 and 30% fibrotic extent with a predicted FVC <70% are associated with premature mortality [20]. Indeed, in patients with extensive disease, a decline in FVC ≥10% or a decline in FVC of 5–9% together with a decline in DLCO > 15% predicted mortality over a 15-year follow-up period [21]. Despite the confirmed important and complementary role of HRCT and PFTs in SSc-ILD diagnosis and management, there has been controversy regarding the usefulness of the DLCO and FVC as predictors of ILD onset [22,23,24]. In fact, although PFTs are recommended for the early detection of ILD progression, they seem inadequate as the only screening tool for the diagnosis of ILD [25,26].
The aim of our study was to investigate the role of DLCO and FVC in predicting the development of ILD by HRCT after a follow-up of 2 years, using the prospectively collected, longitudinal European Scleroderma Trial and Research (EUSTAR) database. The primary endpoint of this study was to evaluate the efficiency of the DLCO (DLCOΔ) and FVC (FVCΔ) delta change over 12 months from baseline to predict the subsequent ILD onset at 24 months. As secondary endpoints, the study evaluated and compared the clinical characteristics of patients with and without ILD at the end of observation, including the predictive capability of baseline DLCO and FVC values.
2. Patients and Methods
We evaluated SSc patients from the EUSTAR database who satisfied the 1980 American Rheumatology Association (ARA) and/or the 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for SSc [27,28]. Each EUSTAR centre received approval by the local ethics committee, and written informed consent was locally acquired for registered patients. Patients with available longitudinal data of DLCO, FVC, and HRCT at baseline (t0) and after 12 (±4) (t1) and 24 (±4) (t2) months were eligible for the study, but only patients with a negative HRCT at t0 and t1 were included.
HRCT was defined as negative when no signs of ILD were evident according to the local radiology evaluation, as entered in the database. Exclusion criteria included the following: an absence of PFTs and HRCT at t0, t1, and t2, juvenile disease onset, age < 18 years, the presence of ILD on HRCT at t0 or t1, a lack of follow-up visit, and a diagnosis of PH anytime during the study period (as entered in the database or a value of mPAP ≥ 25 mmHg).
The DLCOΔ (change from t0 to t1) and FVCΔ (change from t0 to t1) for the prediction of ILD appearance at t2 were calculated. We considered DLCO > 80% and FVC > 80% as normal values [29].
Statistical Analysis
Mean ± SDs were reported for continuous variables, while absolute and relative frequencies for each category were reported for categorical or dichotomous variables.
To evaluate differences between continuous variables, Student’s t-test, t-test with Satterthwaite adaptation, or Mann–Whitney Test were used, according to the results of Shapiro–Wilk’s test for normality distribution and Bartlett’s test for homoskedasticity.
To evaluate association between categorical variables, the chi-square test or Fisher exact test were used according to the frequencies in table cells. To assess the association between binary outcomes and risk factors, a logistic regression model was used. For each risk factor OR, 95% confidence intervals (CI95%) and p-values were reported. In addition, the predictive capability of each risk factor was represented by area under the ROC curve (AUROC) and its CI95% (considering the predictive capability to be scarce for an AUROC of 0.5–0.6; moderate for 0.6–0.7; good for 0.7–0.8; high for 0.8–0.9; and excellent for 0.9–1).
3. Results
3.1. Study Population
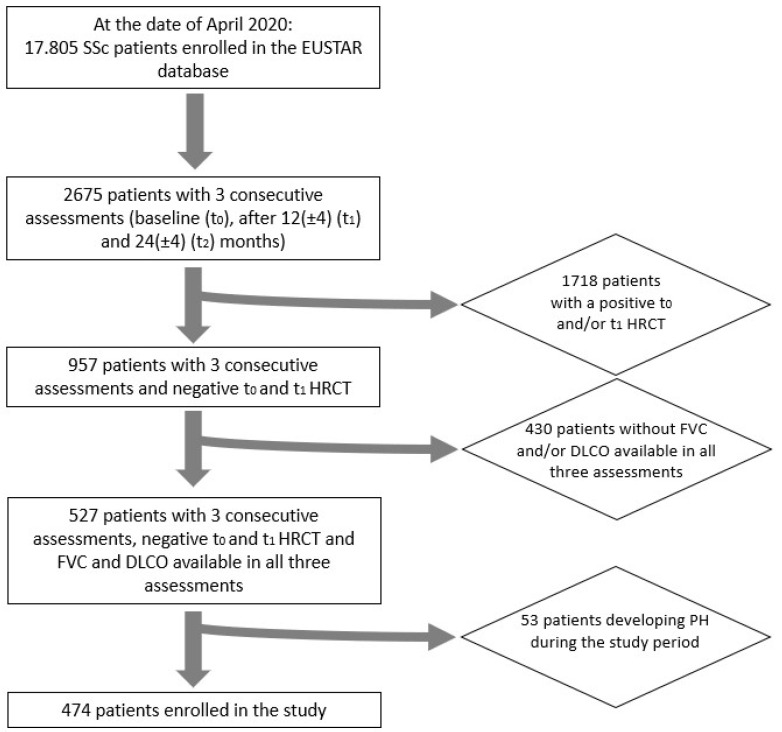
On 30 April 2020, among 17.805 SSc patients in the EUSTAR database, 527 patients had complete longitudinal data on PFTs and HRCT at t0, t1, and t2. Out of 527 patients, 53 were excluded for developing PH during the observation period, leading to the analysis of 474 patients from 39 EUSTAR centres (Figure 1). As expected, we noted a high female predominance (403 females, 85%), 220 (48.0%) patients were anticentromere (ACA)-positive, and 117 (25.4%) anti-topoisomerase I (Topo-I)-positive. Among the skin disease subsets, the limited cutaneous one (lcScc) was the most frequent (58.3%) followed by the dcSSc (26%), and 11.9% of patients presented only sclerodactyly. Regarding the capillaroscopic evaluation, the active scleroderma pattern was the most frequent (44.6%). The demographic and clinical characteristics are summarized in Table 1.
Figure 1.
Flowchart of patient enrolment.
Table 1.
Demographical and clinical characteristics of the enrolled population at t0.
| Features | Number of Patients (% of Patients with Available Data) |
|---|---|
| Sex (F) | 403 (85.0%) |
| Cigarette smoking ever | 33 (20.8%) |
| N.A. | 315 |
| Current cigarette smoker | 14 (26.4%) |
| N.A. | 421 |
| Disease duration (from Raynaud’s phenomenon onset) in months (median; Q1–Q3) | 77.4 (28.0–165.8) |
| Dysphagia | 286 (60.5%) |
| N.A. | 1 |
| Renal crisis | 6 (1.3%) |
| N.A. | 2 |
| Dyspnoea NYHA stage | |
|
353 (79.0%) |
|
89 (19.9%) |
|
5 (1.1%) |
|
0 |
| N.A. | 27 |
| Palpitations | 63 (13.6%) |
| N.A. | 9 |
| Conduction block | 50 (11.6%) |
| N.A. | 42 |
| Skin involvement | |
|
122 (26.0%) |
|
274 (58.3%) |
|
56 (11.9%) |
|
18 (3.8%) |
| N.A. | 4 |
| mRSS (mean ± SD) | 7.6 ± 7.1 |
| Digital pitting scars | |
|
36 (26.3%) |
|
26 (19.0%) |
|
75 (54.7%) |
| N.A. | 337 |
| Digital ulcers | |
|
16 (11.6%) |
|
44 (31.9%) |
|
78 (56.5%) |
| N.A. | 336 |
| Telangiectasia | 58 (37.9%) |
| N.A. | 321 |
| SSc-specific Antibody positivity | |
|
220 (48.0%) (N.A. 18) |
|
117 (25.4%) (N.A. 16) |
|
12 (4.7%) (N.A. 224) |
| CRP Elevation | 50 (10.9%) |
| N.A. | 16 |
| Presence of proteinuria | 15 (3.3%) |
| N.A. | 9 |
| Capillaroscopic scleroderma pattern | |
|
52 (28.3%) |
|
82 (44.6%) |
|
50 (27.2%) |
| N.A. | 290 |
Legend: F: female; N.A.: not available; dcSSc: diffuse cutaneous systemic sclerosis; lcSSc: limited cutaneous systemic sclerosis; mRSS: modified Rodnan skin score; ACA: anticentromere antibodies, Topo-I: anti-topoisomerase I antibodies; RNA-Pol III: anti-RNA polymerase III antibodies.
Among the enrolled population, 46 (9.7%) developed HRCT signs of ILD at t2.
The comparison of patients with and without ILD at t2 showed some different features at baseline (Table 2). Positivity for Topo-I antibodies was associated with ILD development (16.7% vs. 7.8%, p = 0.0031), contrarily to the positivity for ACA antibodies, which was negatively associated (4.4% vs. 14.4%, p = 0.0001). The disease duration and the extent of skin involvement were different in the two groups, without reaching statistically significant values (Table 2). In our population, the progression of skin involvement assessed by the modified Rodnan skin score (mRSS) from t0 to t1 did not correlate with ILD appearance [(OR (IC): 0.713 (0.35–1.46), p = 0.3521)].
Table 2.
Clinical and instrumental characteristics at t0 in patients with negative and positive t2 HRCT.
| Features | Patients with Negative t2 HRCT n (%) |
Patients with Positive t2 HRCT n (%) |
p-Value |
|---|---|---|---|
| Gender | |||
|
365 (91.0%) | 36 (9.0%) | 0.2101 |
|
63 (86.3%) | 10 (13.7%) | |
| Disease duration (from Raynaud onset) in months (median; Q1–Q3) (n) | 77.8 (28.4–161.7) (206) | 71.2 (17.4–194.1) (18) | 0.7537 |
| Skin involvement | |||
|
106 (86.9%) | 16 (13.1%) | |
|
250 (91.2%) | 24 (8.8%) | |
|
52 (92.9%) | 4 (7.1%) | 0.2832 |
|
18 | 0 | |
| mRSS (mean ± SD) (median; Q1-Q3) (n) | 6 (3–10) (393) | 7 (3–13) | 0.1563 |
| SSc-specific Antibody positivity | |||
|
211 (95.9%) | 9 (4.1%) | 0.0001 |
|
203 (85.3%) | 35 (14.7%) | |
|
97 (82.9%) | 20 (17.1%) | 0.0031 |
|
317 (92.4%) | 26 (7.6%) | |
|
9 (75.0%) | 3 (25.0%) | 0.1003 |
|
222 (91.0%) | 22 (9%) | |
| PFTs (values reported as %predicted ±SD) | |||
|
79.0 (±16.6) | 69.9 (±17.4) | 0.0006 |
|
102.2 (±17.3) | 94.6 (±16.2) | 0.0052 |
|
78.4 (±16.8) | 68.9 (±18.6) | 0.0005 |
|
101.9 (±17.9) | 94.7 (±16.5) | 0.0092 |
|
78.0 (±17.0) | 65.1 (±19.1) | <0.0001 |
|
101.6 (±17.6) | 94.5 (±20.0) | 0.126 |
Legend: dcSSc: diffuse cutaneous systemic sclerosis; lcSSc: limited cutaneous systemic sclerosis; mRSS: modified Rodnan skin score; ACA: anticentromere antibodies, Topo-I: anti-topoisomerase I antibodies; RNA-Pol III: anti-RNA polymerase III antibodies.
3.2. Pulmonary Function Trends
The mean value of DLCO and FVC as a percent (%) predicted at t0, t1, and t2 in patients with positive and negative HRCT for lung involvement at t2 are shown in Table 2. Interestingly, it must be pointed out that patients with a positive HRCT at t2 also had significantly lower values of DLCO and FVC at t0 and t1 in comparison to patients with a negative HRCT at t2 (Table 2).
3.3. Primary End Point
We found no statistically significant difference in the mean DLCOΔ and FVCΔ from t0 to t1 in the two populations (negative vs. positive t2 HRCT). The mean DLCOΔ in patients with negative t2 HRCT and positive t2 HRCT was −0.5 (±12.6) and −1.0 (±15.1), respectively. The mean FVCΔ in patients with negative t2 HRTC and positive t2 HRCT was −0.2 (±10.6) and 0.1 (±11.5), respectively. None of them predicted the appearance of ILD at t2 [DLCOΔ: OR 0.997 (95%CI 0.97–1.02), p = 0.8024; FVCΔ: OR 1.002 (95%CI 0.97–1.03), p = 0.8664].
3.4. Secondary End Point
The enrolled population was divided into four groups according to DLCO trends from t0 to t1 (normal cut-off of DLCO ≥ 80%):
161 patients maintained a normal DLCO;
47 moved from a normal to a reduced DLCO;
38 patients presented a reduced DLCO at t0 and recovered beyond normality at t1;
228 patients had a reduced DLCO both at t0 and at t1.
A higher percentage of patients with ILD at t2 was found in groups III (5, 13.3%) and IV (31, 13.3%) [OR 2.56 (95%CI 0.81–8.13), p = 0.1112 and OR: 2.66 (95%CI 1.23–5.75), p = 0.0130, respectively]. Therefore, it should be noted that among patients with a positive t2 HRCT, the majority had a reduced DLCO at baseline (Table 3).
Table 3.
Percentage of positive-HRCT patients at t2 in the populations identified according to the trend in DLCO and FVC from t0 to t1.
| Trend in DLCO from t0 to t1 | Pts with Negative t2 HRCT | Pts with Positive t2 HRCT | Odds Ratio (Confidence Limits) | p-Value |
|---|---|---|---|---|
| DLCO ≥ 80% at t0 and t1 | 152 (94.4%) | 9 (5.6%) | Ref | |
| DLCO ≥ 80% at t0 and <80% at t1 | 46 (9.7%) | 1 (2.2%) | 0.37 (0.05–2.98) | 0.3479 |
| DLCO < 80% at t0 and ≥80% at t1 | 33 (86.6%) | 5 (13.3%) | 2.56 (0.81–8.13) | 0.1112 |
| DLCO < 80% at t0 and at t1 | 197 (86.6%) | 31 (13.3%) | 2.66 (1.23–5.75) | 0.0130 |
| Trend in FVC from t0 to t1 | Pts with Negative t2 HRCT | Pts with Positive t2 HRCT | Odds Ratio (Confidence Limits) | p-Value |
| FVC ≥ 80% at t0 and t1 | 380 (91.0%) | 35 (8.9%) | Ref | |
| FVC ≥ 80% at t0 and <80% at t1 | 12 (75.5%) | 4 (25.5%) | 3.62 (1.11–11.82) | 0.0331 |
| FVC < 80% at t0 and ≥80% at t1 | 10 (91.0%) | 1 (8.9%) | 1.09 (0.14–8.73) | 0.9384 |
| FVC < 80% at t0 and at t1 | 26 (81.0%) | 6 (18.9%) | 2.51 (0.97–6.50) | 0.0588 |
The same analysis was performed for FVC% predicted (the normal cut-off of FVC% predicted is ≥80%), identifying four similar subgroups:
415 patients maintained a normal FVC;
16 patients moved from a normal to a reduced FVC;
11 patients presented a reduced FVC at t0 and recovered beyond a normality of ≥80% at t1;
32 patients had a reduced FVC both at t0 and at t1.
Considering FVC trend subgroups, the higher percentages of patients with a positive HRCT at t2 were in groups II (4, 25.5%) and IV (6, 18.9%) [OR 3.62 (95%CI 1.11–11.82), p = 0.0331 and OR 2.51 (95%CI 0.97–6.50), p = 0.0588, respectively]. Among patients with ILD at t2, the majority had a normal FVC both at t0 and at t1 (Table 3).
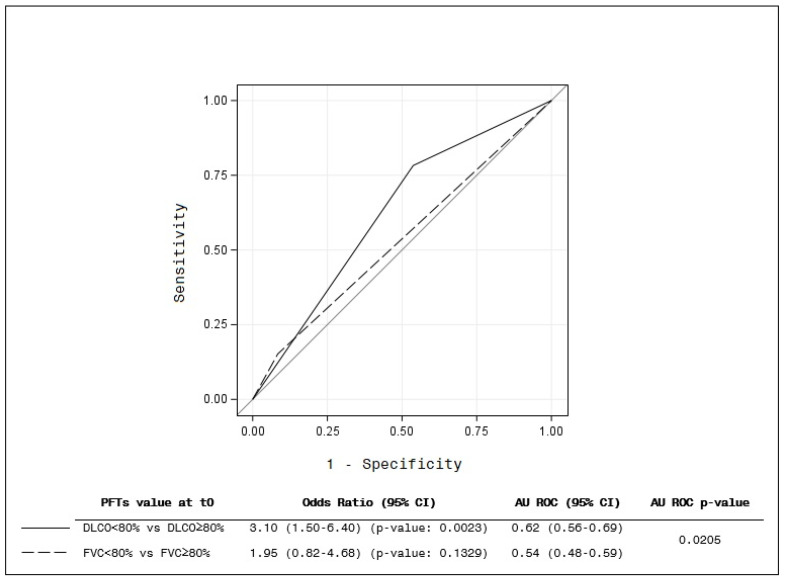
The most interesting result is that, at baseline (t0), the distinction between DLCO < 80% and DLCO ≥ 80% showed the best prediction for ILD development at t2 HRCT (AUROC IC95%) (p = 0.0205) (Figure 2). The figure shows that an impaired baseline DLCO value (<80%) had more predictive capability of a positive HRCT after 2 years of follow-up than an abnormal baseline FVC (<80%).
Figure 2.
Prediction of t0 value of DLCO and FVC. The figure shows that an impaired baseline DLCO value (<80%) had more predictive capability of a positive HRCT after 2 years of follow-up than an abnormal baseline FVC (<80%) (p = 0.0205).
4. Discussion
In SSc, the prevalence of ILD ranges from 47 to 84% and it is associated with significant mortality and morbidity [3,30,31,32]. It is now well known that SSc-lung involvement may be progressive or remain stable for longer periods [33]. The heterogeneity of ILD evolution is still a challenge, and it is therefore of primary importance to identify the best tool and the correct timing to detect and predict lung involvement.
Many efforts have been made to identify the predictors of ILD onset and in this context; the main result of our study suggests a certain role of baseline DLCO in its prediction. In fact, it is pivotal to identify ILD predictors, and this might help to follow-up SSc patients, identifying those at higher risk of developing ILD [34,35]. Some investigations focused on the possibility to predict the progression of lung involvement in SSc-ILD patients. Among these, the SPAR (SPo2 ARthritis) model predicted ILD progression, helping to detect patients at risk for progressive fibrosis with the combination of lower peripheral capillary oxygen saturation (after a 6 min walking test) and arthritis [36]. Ahmed et al. reported an association between baseline FVC and DLCO values and survival in SSc-ILD patients, identifying a threshold of 70% FVC% predicted and 77% DLCO% predicted [37]. In SSc-ILD patients, Volkmann et al. found that the decline in FVC and DLCO over 2 years was a better predictor of mortality compared to baseline FVC and DLCO values [38]. Morisset J et al. suggested the SADL (smoking history, age, and DLCO% predicted) model as a mortality risk predictor for SSc-ILD patients [30]. Clearly, these data highlight the importance of PFTs values in the prediction of ILD prognosis.
The present study was focused on predicting the appearance of SSc-ILD on HRCT by using PFT parameters. The primary endpoint evaluating DLCOΔ and FVCΔ between t0 and t1 as a predictor of ILD onset at t2 HRCT was not met, and this might be related to the mean disease duration of the study population (about 6 years at t0), too long to use the Δ change to predict ILD. However, the secondary endpoint analysis suggested that even when signs of ILD are not yet present on HRCT, patients with a low baseline DLCO may have an increased risk of developing ILD after 2 years of follow-up.
Our population presented mild lung involvement showing an average non-significant functional decline in DLCO and FVC at one year of follow-up, and this probably influenced the success of the primary endpoint analysis. To evaluate the predicting role of DLCOΔ and FVCΔ, a larger population with a follow-up longer than two years would probably be required. On the other hand, the straightforward use of the baseline mean values of DLCO and FVC showed that the DLCO was better than the FVC for predicting a new ILD after 2 years. Dichotomizing these values (with abnormal defined as <80% for both DLCO and FVC), “abnormal” DLCO patients had a higher risk than “abnormal” FVC patients of developing HRCT-ILD after 2 years of follow-up. Into clinical practice, this datum may indicate the need to follow-up with HRCT SSc patients with a baseline impaired DLCO for at least 2 years. However, considering the “within-patient” variability in PFTs, perhaps it would be better to rely on more than a single measure to predict the development of ILD. Unfortunately, the design of our study ruled out all patients with ILD at HRCT at t1, preventing the possibility to evaluate the risk of developing ILD in a shorter follow-up.
At t2, our data showed normal mean FVC values in patients with ILD; however, despite the normal mean value, patients with ILD had a lower FVC than those without this complication. Considering DLCO, our data showed that in patients with ILD, the mean DLCO value over the study period was significantly lower than in patients with negative HRCT at t2. It is remarkable that patients without signs of ILD at t2 showed a DLCO near the normal cut-off (80%) at all the three assessments. This evidence may suggest an early impairment of the alveolar epithelium, probably confirming inflammatory involvement of the alveolar membrane as the primum movens in SSc-ILD pathogenesis [39]. Our data are in line with Tashkin et al., who found that DLCO provides the best overall estimate of HRCT-measured lung disease in SSc patients [34].
Our study confirmed some previous evidence, indicating a higher prevalence of Topo-I antibodies and the dcSSc subset among patients who developed ILD during the follow-up. In line with our results, previous data reported the association between dcSSc, African American ethnicity, shorter disease duration, and an older age at disease onset and the risk of developing SSc-ILD [40,41].
An association between progressive skin fibrosis within one year and a decline in lung function has been reported via analysing dcSSc patients from the EUSTAR cohort [42]. In our study, the change in mRSS seemed not to predict ILD onset. However, our population was probably too heterogeneous and with mild skin involvement, mostly presenting with only sclerodactyly or a limited subset.
Our study presents some limitations, mostly related to the data collected in the EUSTAR database. Data regarding the extent of ILD and qualitative data about pulmonary involvement in patients with ILD at t2 were mostly missing (no available data about reticulation changes, traction, and/or honeycombing changes; information about ground-glass opacities was only recorded in about 37%). PFTs were performed in 39 different centres, between 2003 and 2019, making the percent predicted less uniform and more variable. In addition, absolute values were not recorded, the haemoglobin value was not available for all patients, and the sample size was reduced when patients were divided into groups according to the trends in DLCO and FVC over time. In fact, some patients might have a negative baseline HRCT with a lower PFTs value, probably due to an incorrect execution of PFTs, as some of these patients presented a normal value of FVC at t1. In addition, the absence of a centralized reading of the HRCT scans or the presence of different HRCT acquisition protocols, as well as the absence of data on smoking (or other environmental factors) for all patients, must be listed. It is important to note that, in our study, no other correlations between ILD and biological data, other than autoantibodies, were determined as, unfortunately, some biological features were missing for enrolled patients. Beyond these weaknesses, the strengths of our study are represented by the number of enrolled patients (474 patients) and the long follow-up, as the study focused on PFTs compared to HRCT for at least 48 months in all patients, suggesting a predictive value of baseline DLCO.
Predictors of ILD onset are eagerly awaited to improve SSc-ILD management. Our study confirms the known risk factors in the identification of an SSc population at higher risk of developing ILD, including Topo-I positivity, the dcSSc subset, and a shorter disease duration from the appearance of Raynaud’s phenomenon. Our data suggest that the baseline DLCO value is linked to an increased risk of ILD development after 2 years of follow-up, while the DLCOΔ and FVCΔ do not predict ILD development.
5. Conclusions
We provide the evidence of the reduction of gas exchange as an early sign of lung involvement before any HRCT signs are evident. At earlier time points, our study could not differentiate the predictive value of FVC from DLCO. In the future, further comparisons of FVC and DLCO as predictors will require prospective studies and a careful consideration of potential confounders, such as pulmonary hypertension, cardiac disease, and/or concomitant ILD treatment. The confirmation of a predictive value of decreased DLCO or FVC in SSc patients without confounders may drive the clinician to perform or repeat HRCT to detect ILD as early as possible and to treat it in the patient’s window of opportunity.
Abbreviations
| SSc | Systemic sclerosis |
| ILD | Interstitial lung disease |
| HRCT | High-resolution computed tomography |
| PFTs | Pulmonary function tests |
| DLCO | Diffusion of the lung for carbon oxide |
| FVC | Forced vital capacity |
| EUSTAR | European Scleroderma Trial and Research |
| dcSSc | Diffuse cutaneous subset of SSc |
| lcSSc | Limited cutaneous subset of SSc |
| ACA | Anticentromere antibodies |
| Topo-I | Anti-topoisomerase I |
| RNA-Pol III | Anti-RNA polymerase III antibodies |
Appendix A
List of EUSTAR collaborators:
-
-
Basel (Switzerland) Ulrich Walker, Dr. Bettina Bannert (002);
-
-
Bari (Italy) Florenzo Iannone, Fabio Cacciapaglia fabio.cacciapaglia79@gmail.com (004);
-
-
Genova (Italy) Maurizio Cutolo, Sabrina Paolino sabrina.paolino@unige.it (011);
-
-
Pavia (Italy), Carlomaurizio Montecucco, Roberto Caporali caporali@smatteo.pv.it (019);
-
-
Madrid (Spain), Patricia Carreira, Beatriz E. Joven (023);
-
-
Padova (Italy), Andrea Doria, Elisabetta Zanatta (031);
-
-
Paris (France), Dominique Farge Bancel, Adrian Hij adrian.hij@aphp.fr (035);
-
-
Torino (Italy), Raffaele Pellerito, Torino, Italy (049);
-
-
Tübingen (Germany), Jörg Henes, Ann-Christian Pecher Ann-Christin.Pecher@Med.Uni-Tuebingen.de (056);
-
-
Niska Banja (Serbia and Montenegro), Bojana Stamenkovic, Aleksandra Stankovic ackana@junis.ni.ac.rs (073);
-
-
Moscow (Russia), Lidia P.Ananieva, Ludmila Garzanova lyuda-garzanova@yandex.ru (078);
-
-
Bad Nauheim (Germany), Ulf Müller-Ladner (081);
-
-
Lille (France), David Launay (093);
-
-
Bucharest (Romania), Ruxandra Maria Ionescu, Daniela Opris danaopris0103@yahoo.com (096);
-
-
Bucharest (Romania), Ana Maria Gheorghiu, Mihai Bojinca (100);
-
-
Erlangen (Germany), Jörg Distler (106);
-
-
Milan (Italy); Francesca Ingegnoli (110);
-
-
Gent (Belgium), Vanessa Smith (113);
-
-
Foggia (Italy), Francesco Paolo Cantatore, Addolorata Corrado a.corrado@mail.unifg.it (115);
-
-
Copenhagen (Denmark), Susanne Ullman (116);
-
-
Brussels (Belgium), Marie Vanthuyne, Houssiau frédéric frederic.houssiau@uclouvain.be (122);
-
-
Beijing (China), Mengtao Li (154);
-
-
Alexandria (Egypt), Walid Ahmed Abdel Atty Mohamed (155);
-
-
Roma (Italy), Edoardo Rosato, Antonietta Gigante (158);
-
-
Madrid (Spain), Paloma García de la Peña Lefebvre, Dr. Jorge Juan Gonzalez Martin (169);
-
-
Buenos Aires (Argentina), Eduardo Kerzberg, Fabiana Montoya sfabianamontoya@gmail.com (178);
-
-
Barcelona (Spain), Ivan Castellví, Milena Millan (180);
-
-
New Brunswick, USA, Vivien M. Hsu (188);
-
-
Lubeck (Germany), Gabriela Riemekasten, Sabine Sommerlatte (199).
Author Contributions
G.L. coordinated the study and participated in the design of the study and the drafting of the manuscript; C.B. participated in the design of the study and the drafting of the manuscript; L.T. participated in the drafting of the manuscript and performed the statistical analysis; A.M.-P., M.O., S.T., M.H., F.D.G., R.I., O.D., V.R., Y.A., A.M.G., E.S., J.D.V.-B., E.H., M.T., N.D., F.S., L.M., A.-M.H.-V., A.G. and S.G. participated in the drafting of the manuscript; M.M.-C. conceived the study and participated in the design of the study and the drafting of the manuscript; D.F. participated in the design of the study and the drafting of the manuscript; S.B.-R. conceived the study, coordinated the study, and participated in the design of the study and the drafting of the manuscript; EUSTAR Collaborators: participated to the EUSTAR database. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Each EUSTAR centre received approval from the local ethics committee, and written informed consent was locally acquired for registered patients (ethics committee approval number of the lead centre: 2008/0017054; approval date: 9 May 2008).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The access to the anonymized raw data that support the findings of this study are available on reasonable request, and subject to review by EUSTAR committee. Data requests may be sent to the corresponding author.
Conflicts of Interest
Gemma Lepri: none declared. Cosimo Bruni: C.B. is a Guest Editor for Diagnostics (Special Issue “Advances in Identification and Management of Systemic Sclerosis”); C.B. reports personal fees from Boehringer Ingelheim, grants from the European Scleroderma Trial and Research (EUSTAR) group, grants from the New Horizon Fellowship, grants from the Foundation for Research in Rheumatology (FOREUM), grants from Gruppo Italiano Lotta alla Sclerodermia (GILS), and grants from the Scleroderma Clinical Trial Consortium (SCTC) and the Scleroderma Research Foundation (SRF) outside the submitted work. Lorenzo Tofani: none declared. Alberto Moggi Pignone: none declared. Martina Orlandi: none declared. Tomasetti Sara: speaker’s fees from Roche and Boehringer Ingelheim. Michael Hughes: none declared. Francesco Del Galdo: none declared. Rosaria Irace: none declared. Oliver Distler O.D. (last three years) has/had consultancy relationship and/or has received research funding in the area of potential treatments for systemic sclerosis and its complications from (last three years): Abbvie, Acceleron Pharma, Amgen, AnaMar, Arxx Therapeutics, Baecon Discovery, Blade Therapeutics, Bayer, Boehringer Ingelheim, ChemomAb, Corbus Pharmaceuticals, CSL Behring, Galapagos NV, Glenmark Pharmaceuticals, GSK, Horizon (Curzion) Pharmaceuticals, Inventiva, iQvia, Italfarmaco, iQone, Kymera Therapeutics, Lilly, Medac, Medscape, Mitsubishi Tanabe Pharma, MSD, Novartis, Pfizer, Roche, Sanofi, Serodapharm, Topadur, Target Bioscience and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Valeria Riccieri: none declared. Yannick Allanore received personal fees from Boehringer, Sanofi, Menarini, and Medsenic and grants from Alpine with regard to the management of systemic sclerosis. Ana Maria Gheorghiu: none declared. Elise Siegert: none declared. Jeska de Vries-Bouwstra: none declared. Eric Hachulla: none declared. Mohammed Tikly: none declared. Nemanja Damjanov: none declared. Francois Spertini: none declared. Luc Mouthon: none declared. Anna-Maria Hoffmann-Vold received consulting fees from Actelion, ARXX, Bayer, Boehringer Ingelheim, Lilly, Medscape, Merck Sharp & Dohme, and Roche, and grants from Boehringer Ingelheim. Armando Gabrielli: none declared. Serena Guiducci: none declared. Marco Matucci-Cerinic has received consulting fees or an honorarium from Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, and Roche. Daniel Furst: none declared. Silvia Bellando Randone: none declared.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Varga J., Trojanowska M., Kuwana M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017;2:137–152. doi: 10.5301/jsrd.5000249. [DOI] [Google Scholar]
- 2.Volkmann E.R., Fischer A. Update on morbidity and mortality in systemic sclerosis–related interstitial lung disease. J. Scleroderma Relat. Disord. 2020;6:11–20. doi: 10.1177/2397198320915042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen V.D., Medsger T.A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perelas A., Silver R.M., Arrossi A.V., Highland K.B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 2020;8:304–320. doi: 10.1016/S2213-2600(19)30480-1. [DOI] [PubMed] [Google Scholar]
- 5.Walker U.A., Tyndall A., Czirjak L., Denton C., Farge-Bancel D., Kowal-Bielecka O., Muller-Ladner U., Bocelli-Tyndall C., Matucci-Cerinic M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR scleroderma trials and research group database. Ann. Rheum. Dis. 2007;66:754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanatta E., Moccaldi B., Szucs G., Spagnolo P. Should we use nintedanib as early therapy in patients with SSc-ILD? Autoimmun. Rev. 2023 doi: 10.1016/j.autrev.2023.103463. in press . [DOI] [PubMed] [Google Scholar]
- 7.Panagopoulos P.K., Goules A.V., Georgakopoulou V.E., Kallianos A., Chatzinikita E., Pezoulas V.C., Malagari K., Fotiadis D.I., Vlachoyiannopoulos P., Vassilakopoulos T., et al. Small airways dysfunction in patients with systemic sclerosis and interstitial lung disease. Front. Med. 2022;9:1016898. doi: 10.3389/fmed.2022.1016898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann-Vold A.M., Fretheim H., Halse A.K., Seip M., Bitter H., Wallenius M., Garen T., Salberg A., Brunborg C., Midtvedt Ø., et al. Tracking Impact of Interstitial Lung Disease in Systemic Sclerosis in a Complete Nationwide Cohort. Am. J. Respir. Crit. Care Med. 2019;200:1258–1266. doi: 10.1164/rccm.201903-0486OC. [DOI] [PubMed] [Google Scholar]
- 9.Cottin V., Brown K.K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD) Respir. Res. 2019;20:13. doi: 10.1186/s12931-019-0980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man A., Davidyock T., Ferguson L.T., Ieong M., Zhang Y., Simms R.W. Changes in forced vital capacity over time in systemic sclerosis: Application of group-based trajectory modelling. Rheumatology. 2015;54:1464–1471. doi: 10.1093/rheumatology/kev016. [DOI] [PubMed] [Google Scholar]
- 11.Assassi S., Sharif R., Lasky R.E., McNearney T.A., Estrada-Y-Martin R.M., Draeger H., Nair D.N., Fritzler M.J., Reveille J.D., Arnett F.C., et al. Predictors of interstitial lung dis-ease in early systemic sclerosis: A prospective longitudinal study of the GENISOS cohort. Arthr Res. Ther. 2010;12:R166. doi: 10.1186/ar3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Distler O., Volkmann E.R., Hoffmann-Vold A.M., Maher T.M. Current and future perspectives on management of systemic scle-rosis-associated interstitial lung disease. Expert. Rev. Clin. Immunol. 2010;15:1009–1017. doi: 10.1080/1744666X.2020.1668269. [DOI] [PubMed] [Google Scholar]
- 13.Ruaro B., Baratella E., Confalonieri P., Confalonieri M., Vassallo F.G., Wade B., Geri P., Pozzan R., Caforio G., Marrocchio C., et al. High-Resolution Computed Tomography and Lung Ultrasound in Patients with Systemic Sclerosis: Which One to Choose? Diagnostics. 2021;11:2293. doi: 10.3390/diagnostics11122293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann-Vold A.M., Maher T.M., Philpot E.E., Ashrafdazadeh A., Barake R., Barsotti S., Bruni C., Carducci P., Carreira P.E., Castellvi I., et al. The identification and man-agement of interstitial lung disease in systemic sclerosis: Evidence-based European consensus statements. Lancet Rheumatol. 2020;2:e71–e83. doi: 10.1016/S2665-9913(19)30144-4. [DOI] [PubMed] [Google Scholar]
- 15.Plastiras S.C., Karadimitrakis S.P., Ziakas P.D., Vlachoyiannopoulos P.G., Moutsopoulos H.M., Tzelepis G.E. Scleroderma lung: Initial forced vital capacity as predictor of pulmonary function decline. Arthritis Rheumatol. 2006;55:598–602. doi: 10.1002/art.22099. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman A.M., Aaløkken T.M., Brit L.M., Garen T., Midtvedt O., Brunborg C., Tore G.J., Molberg Ø. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol. 2015;67:2205–2212. doi: 10.1002/art.39166. [DOI] [PubMed] [Google Scholar]
- 17.Caron M., Hoa S., Hudson M., Schwartzman K., Steele R. Pulmonary function tests as outcomes for systemic sclerosis interstitial lung disease. Eur. Respir. Rev. 2018;27:170102. doi: 10.1183/16000617.0102-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghu G., Remy-Jardin M., Richeldi L., Thomson C.C., Inoue Y., Johkoh T., Kreuter M., Lynch D.A., Maher T.M., Martinez F.J., et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaenmuang P., Navasakulpong A. Short-Term Lung Function Changes and Predictors of Progressive Systemic Sclerosis–Related Interstitial Lung Disease. Tuberc. Respir. Dis. 2020;83:312–320. doi: 10.4046/trd.2020.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh N.S., Desai S.R., Veeraraghavan S., Hansell D.M., Copley S.J., Maher T.B., Corte T.J., Sander C.R., Ratoff J., Devaraj J., et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am. J. Respir. Crit. Care Med. 2008;177:1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 21.Goh N.S., Hoyles R.K., Denton C.P., Hansell D.M., Renzoni E.A., Maher T.M., Nicholson A.G., Wells A.U. Short-Term Pulmonary Function Trends Are Predictive of Mortality in Interstitial Lung Disease Associated With Systemic Sclerosis. Arthritis Rheumatol. 2017;69:1670–1678. doi: 10.1002/art.40130. [DOI] [PubMed] [Google Scholar]
- 22.Naidu G.S.R.S.N.K., Sharma S.K., Adarsh M.B., Dhir V., Sinha A., Dhooria S., Jain S. Effect of mycophenolate mofetil (MMF) on sys-temic sclerosis-related interstitial lung disease with mildly impaired lung function: A double-blind, placebo-controlled, ran-domized trial. Rheumatol. Int. 2020;40:207–216. doi: 10.1007/s00296-019-04481-8. [DOI] [PubMed] [Google Scholar]
- 23.Showalter K., Hoffmann A., Rouleau G., Aaby D., Lee J., Richardson C., Dematte J., Agrawal R., Chang R.W., Hinchcliff M. Performance of Forced Vital Capacity and Lung Diffusion Cutpoints for Associated Radiographic Interstitial Lung Disease in Systemic Sclerosis. J. Rheumatol. 2018;45:1572–1576. doi: 10.3899/jrheum.171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna D., Seibold J.R., Wells A., Distler O., Allanore Y., Denton C., Furst D.E. Systemic sclerosis-associated interstitial lung disease: Lessons from clinical trials, outcome measures, and future study design. Curr. Rheumatol. Rev. 2010;6:138–144. doi: 10.2174/157339710791330768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein E.J., Jaafar S., Assassi S., Domsic R.T., Frech T.M., Gordon J.K., Broderick R.J., Hant F.N., Hinchcliff M.E., Shah A.A., et al. PFTs alone are an inadequate screening tool for the diagnosis of ILD in patients with early dcSSc. Arthritis Rheumatol. 2020;72:1892–1896. doi: 10.1002/art.41415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna S.A., Nance J.W., Suliman S.A. Detection and Monitoring of Interstitial Lung Disease in Patients with Systemic Sclerosis. Curr. Rheumatol. Rep. 2022;24:166–173. doi: 10.1007/s11926-022-01067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., Matucci-Cerinic M., Naden R.P., Medsger T.A., Jr., Carreira P.E., et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 28.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Com-mittee Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 29.Owens G.R., Fino G.J., Herbert D.L., Steen V.D., Medsger Jr T.A., Pennock B.E., Cottrell J.J., Rodnan G.P., Rogers R.M. Pulmonary function in progressive systemic sclerosis. Comparison of CREST syndrome variant with diffuse scleroderma. Chest. 1983;84:546–550. doi: 10.1378/chest.84.5.546. [DOI] [PubMed] [Google Scholar]
- 30.Morisset J., Vittinghoff E., Elicker B.M., Hu X., Le S., Ryu J.H., Jones K.D., Haemel A., Golden J.A., Boin F., et al. Mortality Risk Prediction in Scleroderma-Related Interstitial Lung Disease: The SADL Model. Chest. 2017;152:999–1007. doi: 10.1016/j.chest.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzog E.L., Mathur A., Tager A.M., Feghali-Bostwick C., Schenider F., Varga J. Review: Interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: How similar and distinct? Arthritis Rheumatol. 2014;66:1967–1978. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knarborg M., Hyldgaard C., Bendstrup E., Davidsen J.R., Løkke A., Shaker S.B., Hilberg O. Incidence, prevalence and regional distribution of systemic sclerosis and related interstitial lung Disease: A nationwide retrospective cohort study. Chronic Respir. Dis. 2022;19:14799731221125559. doi: 10.1177/14799731221125559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George P.M., Wells A.U. Disease staging and sub setting of interstitial lung disease associated with systemic sclerosis: Impact on therapy. Expert. Rev. Clin. Immunol. 2018;14:127–135. doi: 10.1080/1744666X.2018.1427064. [DOI] [PubMed] [Google Scholar]
- 34.Tashkin D.P., Volkmann E.R., Tseng C.-H., Kim H.J., Goldin J., Clements P., Furst D., Khanna D., Kleerup E., Roth M.D., et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann. Rheum. Dis. 2016;75:374–381. doi: 10.1136/annrheumdis-2014-206076. [DOI] [PubMed] [Google Scholar]
- 35.Nagy T., Toth N.M., Palmer E., Polivka L., Csoma B., Nagy A., Eszes N., Vincze K., Bárczi E., Bohács A., et al. Clinical Predictors of Lung-Function Decline in Systemic-Sclerosis-Associated Interstitial Lung Disease Patients with Normal Spirometry. Biomedicines. 2022;10:2129. doi: 10.3390/biomedicines10092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu W., Jordan S., Becker M.O., Dobrota R., Maurer B., Fretheim H., Ye S., Siegert E., Allanore Y., Hoffmann-Vold A.-M., et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: The SPAR model. Ann. Rheum. Dis. 2018;77:1326–1332. doi: 10.1136/annrheumdis-2018-213201. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed S.S., Johnson S.R., Meaney C., Chau C. Lung function and survival in systemic sclerosis interstitial lung disease. J. Rheumatol. 2014;41:2326–2328. doi: 10.3899/jrheum.140156. [DOI] [PubMed] [Google Scholar]
- 38.Volkmann E.R., Tashkin D.P., Sim M., Li N., Goldmuntz E., Keyes-Elstein L., Pinckney A., Furst D.E., Clements P.J., Khanna D., et al. Short-teOh dear IBoy they’re not gonna be happyrm progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann. Rheum. Dis. 2019;78:122–130. doi: 10.1136/annrheumdis-2018-213708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nihtyanova S.I., Denton C.P. Pathogenesis of systemic sclerosis associated interstitial lung disease. J. Scleroderma. Relat. Disord. 2020;5:6–16. doi: 10.1177/2397198320903867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steen V., Domsic R.T., Lucas M., Fertig N., Medsger T.A. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–2994. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nihtyanova S.I., Schreiber B.E., Ong V.H., Rosenberg D., Moinzadeh P., Coghlan J.G., Wells A.U., Denton C.P. Prediction of Pulmonary Complications and Long-Term Survival in Systemic Sclerosis. Arthritis Rheumatol. 2014;66:1625–1635. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 42.Wu W., Jordan S., de Oliveira Graf N., Pena J.d.O., Curram J., Allanore Y., Matucci-Cerinic M., Pope J.E., Denton C.P., Khanna D., et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann. Rheum. Dis. 2019;78:648–656. doi: 10.1136/annrheumdis-2018-213455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The access to the anonymized raw data that support the findings of this study are available on reasonable request, and subject to review by EUSTAR committee. Data requests may be sent to the corresponding author.