Abstract
Trimetazidine (TMZ), used for treating stable angina pectoris, has garnered attention in the realm of sports due to its potential performance-enhancing properties, and the World Anti-Doping Agency (WADA) has classified TMZ on the S4 list of prohibited substances since 2014. The purpose of this narrative mini-review is to emphasize the biochemical aspects underlying the abusive use of TMZ among athletes as a metabolic modulator of cardiac energy metabolism. The myocardium’s ability to adapt its energy substrate utilization between glucose and fatty acids is crucial for maintaining cardiac function under various conditions, such as rest, moderate exercise, and intense effort. TMZ acts as a partial inhibitor of fatty acid oxidation by inhibiting 3-ketoacyl-CoA thiolase (KAT), shifting energy production from long-chain fatty acids to glucose, reducing oxygen consumption, improving cardiac function, and enhancing exercise capacity. Furthermore, TMZ modulates pyruvate dehydrogenase (PDH) activity, promoting glucose oxidation while lowering lactate production, and ultimately stabilizing myocardial function. TMZs role in reducing oxidative stress is notable, as it activates antioxidant enzymes like glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD). In conclusion, TMZs biochemical mechanisms make it an attractive but controversial option for athletes seeking a competitive edge.
Keywords: trimetazidine, doping, metabolic modulator, oxidative stress
1. Introductions
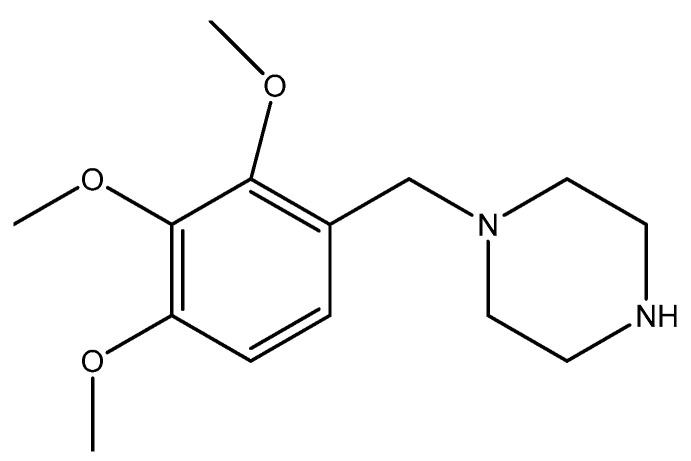
Trimetazidine (TMZ) [1-(2.3.4-trimethoxybenzyl)-piperazine] is a piperazine derivative with a structure shown in Figure 1. It was introduced on the market in 1963 in France for the treatment of (stable) angina pectoris [1].
Figure 1.
Trimetazidine structure.
TMZ is part of the class of anti-anginal drugs with protective effects against ischemia-induced heart injury, acting as a modulator of cardiac energy metabolism between obtaining ATP from the beta-oxidation of fatty acids and glycolysis, maintaining the intracellular levels of phosphocreatine (PCr) and ATP, reducing the level of lactate and cellular acidosis, preventing calcium overload, and reducing the concentration of free radicals [2,3]. As cellular oxygen consumption in the case of glucose oxidation is lower than in the case of long-chain fatty acids, TMZ increases the survival of cardiomyocytes in ischemic conditions [3,4,5]. Other studies show a reduction in oxidative stress parameters resulting mainly from lipid peroxidation [6]. To our knowledge, there is no literature available that addresses the potential risks associated with the use of TMZ in sports, whether by professional athletes or those engaging in recreational activities. Even though TMZ has protective effects on cardiomyocytes, the principle of fair play in sports prohibits the use of substances that provide athletes with unjustifiable and direct advantages.
All these mechanisms make TMZ a compound with potential uses for improving performance in athletes. TMZ is included in the list of prohibited substances during and outside the competition of World Anti-Doping Agency (WADA) S4 hormone and metabolic modulators since 2014, together with aromatase inhibitors, anti-estrogens, selective estrogen receptor modulators (SERM), agents preventing activin receptor IIB activation, and metabolic modulators [7].
International media reports cases of athletes doped with TMZ (Chinese swimmer Sun Yang in 2014, Russian bobsledder Nadezhda Sergeeva in 2018 at the Pyeongchang Olympics, or Russian skater Kamila Valieva in 2022 at the Beijing Winter Olympics). The literature focus was more on detection methods from biological samples but was incomplete regarding the pharmacological reasons behind this abusive use. To compensate for this, the aim of this mini-review is to highlight the most important biochemical mechanisms that could bring unfair benefits to athletes doped with TMZ during or out of competition [8,9].
2. Methodology
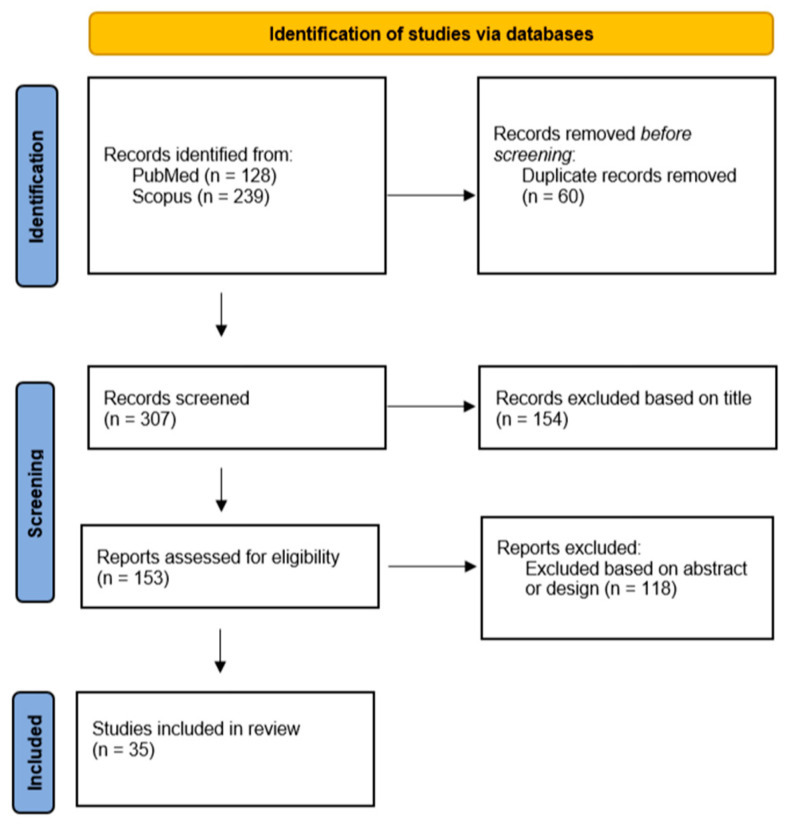
For this narrative review, a systematic search on Scopus and PubMed was performed using combinations of the following keywords: ‘‘trimetazidine’’, ‘‘doping’’, ‘‘oxidative stress’’, and ‘‘metabolic modulator’’. EndNote 9 software was used to collect the articles, and after removing duplicates, screening the title, abstract, and study design, articles were retained for the narrative review (Figure 2). Non-English articles as well as gray literature were excluded. Additionally, articles unrelated to the biochemical aspects of trimetazidine or its use in doping were excluded based on their title, abstract, and study design. Review articles as well as original articles of research, both preclinical and clinical studies, written in English, were included, with an emphasis on the latest published articles guided by the experience of the authors. This approach aimed to ensure an up-to-date synthesis of the literature, with a specific focus on the biochemical mechanisms that explain the use of trimetazidine as a metabolic modulator.
Figure 2.
Flow diagram of the literature review process; n = number of articles.
3. Energy Metabolism of the Myocardium
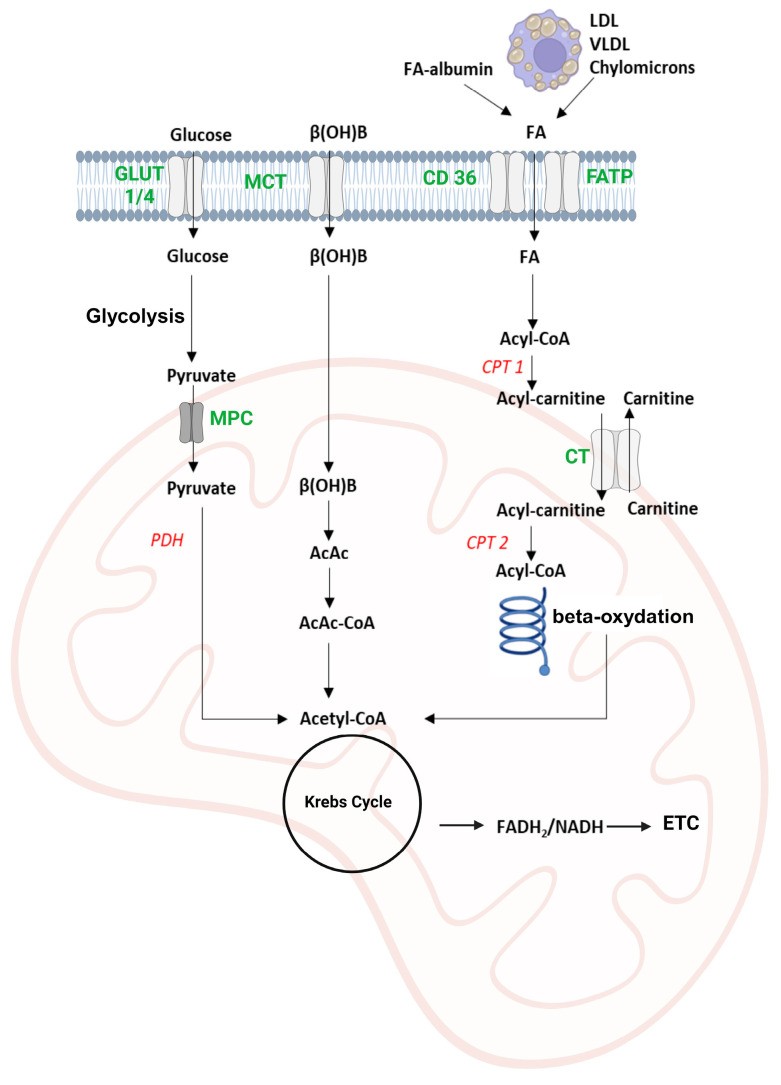
Maintaining the contractile function of the myocardium consumes significant amounts of ATP, mainly obtained from the combined use of beta-oxidation of fatty acids, metabolism of glucose, ketone bodies, and amino acids, as presented in Figure 3 [10].
Figure 3.
Energy metabolism of cardiomyocytes. The figure describes the uptake of the most important energy substrates in the myocardium: carbohydrates (glucose), fatty acids, and ketone bodies (beta-hydroxybutyrate). Glucose is processed by glycolysis in the cytoplasm, followed by the entry of pyruvate into the mitochondria, where it is oxidatively decarboxylated with the formation of acetyl-CoA. The fatty acids are activated into acyl-CoA and transferred to the mitochondria linked to carnitine. In mitochondria, the process of beta-oxidation will generate more molecules of acetyl-CoA, and the ketone bodies represented by beta-hydroxybutyrate and acetoacetate are metabolized by ketolysis to acetyl-CoA. The acetyl-CoA formed is metabolized in the Krebs cycle with the formation of reduced coenzymes (NADH and FADH2) that will be oxidized in the electron transport chain with energy formation, a part of which is stored in the form of ATP. (β(OH)B–β-hydroxybutyrate, FA–fatty acid, MCT–monocarboxylic acid transporter, CD 36–cluster of differentiation 36, FATP–fatty acid transport protein, GLUT–glucose transporter, MPC–mitochondrial pyruvate carrier, CPT 1 and 2–carnitine palmitoyl transferase 1 and 2, CT–carnitine translocase, PDH–pyruvate dehydrogenase, AcAc–acetoacetate, ETC–electron transfer chain). The text in red letters signifies enzymes and in green letters signifies transporters.
3.1. Beta-Oxidation of Fatty Acids
The fatty acids used for energy purposes by the myocardium originate from adipose tissue following the process of lipolysis of triglycerides by hormone-sensitive lipase or from lipoprotein fractions such as chylomicrons or very low-density lipoproteins (VLDL) catalyzed by lipoprotein lipase [11]. They are transported in the blood in free form or bound to plasma albumin for uptake by cardiomyocytes via transporters such as cluster of differentiation 36 (CD 36) and fatty acid transport proteins (FATP) [12]. In the cytoplasm, fatty acids are activated by fatty acyl-CoA synthetase through an ATP-dependent reaction with coenzyme A, leading to the formation of acyl-CoA, which can be bound to glycerol to form deposit triglycerides, used for the synthesis of membrane phospholipids, or transported in the mitochondria to be oxidized for energy purposes [13,14]. Since the mitochondrial double membrane layer is impermeable to acyl-CoA, transport is carried out with the help of the carnitine transporter and 3 enzymes: carnitine palmitoyl transferase 1 (CPT-1) which catalyzes the esterification of fatty acids with carnitine, a translocase that transfers acyl-carnitine in the mitochondria, and CPT-2 located in the inner membrane of the mitochondria that releases the acyl residue from carnitine and binds it to coenzyme A [15]. The beta-oxidation of fatty acids includes four reactions: an oxidation reaction catalyzed by acyl-CoA dehydrogenase with the formation of NADH and an unsaturated compound, enoyl-CoA; a hydration with the formation of 3-hydroxyacyl-CoA; an oxidation catalyzed by 3-hydroxyacyl-CoA dehydrogenase, FAD-dependent, in which a 3-ketoacyl-CoA is formed; and a thiolytic cleavage by 3-ketoacyl-CoA thiolase (KAT) with the formation of acetyl-CoA and an acyl-CoA residue with (n-2) carbon atoms [16]. Acetyl-CoA will be oxidized in the Krebs Cycle with the formation of CO2 and reduced coenzymes (NADH and FADH2) that will supply electrons to the electron transport chain (ETC) with energy formation, stored in the form of ATP through the oxidative phosphorylation process [17].
3.2. Carbohydrate Metabolism
Glucose can enter the cardiac myocytes with the help of insulin-dependent GLUT-4 and insulin-independent GLUT-1 transporters and is phosphorylated with the formation of glucose-6-phosphate [18]. Glycolysis takes place in the cytoplasm with the formation of a minimal amount of energy: 2 ATP/mol of glucose and 2 molecules of pyruvate. Pyruvate can be transformed into lactate, or in the presence of oxygen, it is converted to acetyl-CoA in the mitochondrial matrix by oxidative decarboxylation via the pyruvate dehydrogenase (PDH) pathway [19]. PDH is controlled covalently by PDH kinase, by the energy status of the cell, and by the NADH/NAD+ and acetyl-CoA/CoA-SH ratios >1, which have an activating effect on PDH kinase and an inhibitory effect on PDH. The myocardium contains glycogen deposits, with a still-debated purpose, but it is assumed that it has an energy-buffer role, involved in the fine regulation of the energy flow of glucose in the heart, as well as an important role in determining myocardial metabolic health [20,21].
3.3. Ketone Bodies Metabolism
Acetoacetate and beta-hydroxybutyrate are uptaken in cardiomyocytes by passive diffusion or via monocarboxylic acid transporters. Beta-hydroxybutyrate is oxidized to acetoacetate, which will be esterified with coenzyme A in a reaction catalyzed by succinyl CoA 3-ketoacid-CoA transferase (SCOT). The formed acetoacetyl-CoA is cleaved, resulting in the formation of two molecules of acetyl-CoA, which will enter the Krebs cycle [22].
4. Biochemical Changes during Exercise
The myocardium can permanently switch between the use of glucose or fatty acids for energy purposes; this competition has been the subject of many studies since 1963 when the “Randle Cycle” was first mentioned [23,24]. Cardiac cells present many mitochondria in which the synthesis of ATP from different substrates takes place, so, during an acute, intense effort, carbohydrates will be the main source of energy, while in resting conditions, fatty acids will be used [21].
During fasting, lipolysis is initiated with the release of fatty acids, which can, ultimately, generate ketone bodies in the liver. Fatty acids and ketone bodies have a glucose conservation effect, which is extremely important, especially for maintaining brain function during periods of starvation. The inhibitory effect on carbohydrate metabolism is directed especially to myocardial, hepatic, and beta-pancreatic cell PDH, which thus leads to the accumulation of lactate and pyruvate, both important precursors for gluconeogenesis [25].
In cases of physical effort or after a meal rich in lipids, the increased concentration of free fatty acids and ketone bodies in the blood has an inhibitory effect on glycolysis, with glucose being redirected to form glycogen deposits, which explains the rapid replenishment of glycogen deposits post-physical effort [26]. This metabolic switch is due to the inhibitory effect on PDH through the ratios NADH/NAD+ and Acetyl-CoA/CoA-SH >1, but also through the direct, allosteric inhibitory action of citrate formed in excess in the mitochondria on the key enzymes of glycolysis, 6-phosphofructo-1 kinase (PFK-1) and PFK-2. PFK-2 catalyzes the formation of fructose 2,6-bisphosphate (F-2,6-P2), which is an activator of PFK-1. Therefore, the accumulation of citrate in the cytoplasm has a double inhibitory effect on glycolysis: allosteric and by decreasing the concentration of F-2,6-P2 [19,27].
In conditions of metabolic stress such as acute, high-intensity cardiac effort, the inhibitory effect of beta-oxidation of fatty acids on glycolysis is removed and AMP-activated protein kinase (AMPK) is activated, with an essential role in maintaining caloric homeostasis [28]. AMPK is activated during metabolic stress induced either by glucose or oxygen deprivation or by increased cellular energy demands during intense muscular effort, both of which are characterized by an AMP/ATP ratio >1. It determines the activation of ATP-generating catabolic processes (beta-oxidation of fatty acids or glycolysis) and the inactivation of energy-consuming anabolic processes. AMPK can determine in an insulin-independent way the uptake of glucose into the ischemic heart cell, but it can also directly modulate by phosphorylating key enzymes in carbohydrate, lipid, or protein metabolism, such as the inactivation of acetyl-CoA carboxylase (ACC), the enzyme that catalyzes the key step in the de novo synthesis of fatty acids, the transformation of acetyl-CoA into malonyl-CoA, or the activation of PFK-2 and implicitly of glycolysis, but also of the Akt substrate 160, a protein that determines the incorporation of GLUT-4 transporters for glucose in the cell membrane [29,30].
Metabolic stress can also be induced by stimulating the myocardium with epinephrine. This will cause increased glycolysis via the second messenger, cyclic adenosine monophosphate (cAMP), and intramitochondrial [Ca2+]. cAMP determines the activation of cyclic AMP-dependent protein kinase (PKA) and thus, through phosphorylation, decreases the activity of ACC, favors glucose uptake, activates glycogen phosphorylase, and PDH. In these conditions, glucose is preferentially used as an energy substrate to support the cardiac effort induced by epinephrine [31].
5. TMZ Mechanism of Action
TMZ is an antianginal drug that belongs to the group of partial inhibitors of fatty acid oxidation (PFox inhibitors). Even if the mechanism of action is not fully elucidated, it has demonstrated cardioprotective effects similar to nifedipine or propranolol in stable pectoral angina by modulating cardiac energy metabolism [32]. TMZ is a 3-ketoacyl CoA thiolase (KAT) inhibitor, the enzyme involved in the process of mitochondrial beta-oxidation of fatty acids, thus reducing the use of long-chain fatty acids as an energy substrate in the myocardium, leading to a better use of carbohydrates through the process of glycolysis and thereby decreasing proton and Na+ accumulation [33]. Even if the beta-oxidation of fatty acids produces a greater amount of energy than glycolysis, it still increases the oxygen consumption of the cell [34]. Paradoxically, in conditions of hypoxia, when both beta-oxidation and aerobic use of glucose are modified, in the cells the use of fatty acids for energy purposes is preferred, which will exacerbate oxygen consumption, and the end product of glycolysis is lactate, responsible in severe cases for metabolic acidosis [35].
Another important mechanism of action of TMZ is the activation of PDH, the enzyme that couples intracytoplasmic glycolysis with pyruvate transformation in the mitochondria into acetyl-CoA through oxidative decarboxylation. Compared to the beta-oxidation process, energy obtained in this way decreases the oxygen consumption of the cell, decreases the production of [H+], increases the risk of acidosis, and increases the intracellular accumulation of Ca2+ with a stabilizing effect on the membrane [36,37].
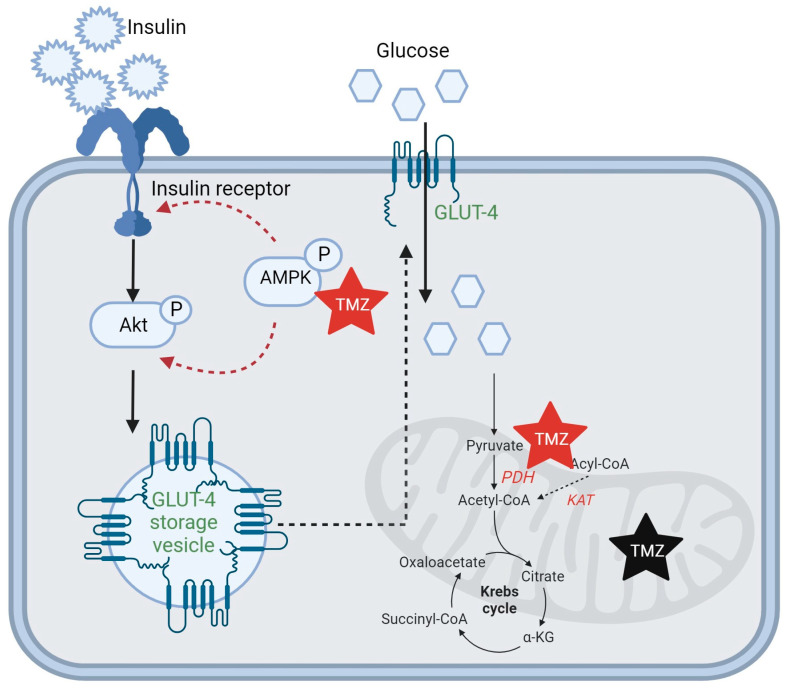
TMZ activates AMPK, the key enzyme in carbohydrate metabolism, and reduces insulin resistance in the skeletal muscle by externalizing GLUT-4 receptors in the cell membrane and favoring their expression through a positive loop with peroxisome proliferator-activated receptor β (PPARβ) [38]. Phosphorylated AMPK activates Akt through phosphorylating IRS1 at Ser794; see Figure 4 [39].
Figure 4.
TMZ mechanism of action. TMZ is an inhibitor of KAT, decreasing fatty acid β-oxidation, and an activator of PDH, an enzyme that catalyzes the transformation of pyruvate to acetyl-CoA, making it a metabolic modulator from using fatty acids as a source of energy to glucose. TMZ activates AMPK and Akt through phosphorylation. GLUT-4 insulin-activated receptors for glucose are externalized from storage vesicles to the cell membrane. (TMZ–trimetazidine, KAT–3 ketoacyl-CoA thiolase, Akt–protein kinase B, GLUT-4-insulin-regulated glucose transporter, AMPK–AMP-activated protein kinase, PDH–pyruvate dehydrogenase). Red stars represent an activator effect and black star represents an inhibitor effect of TMZ on specific enzymes. A red dotted arrow signifies a process involving several steps activated by TMZ and a black dotted arrow signifies a process involving several steps inhibited by TMZ.
The improvement of cardiac function, especially of the systolic phase, by switching the use of energy substrates in favor of glucose was highlighted by a study on mice with cardiomyocyte-specific deficiency of PDH. In these conditions, the oxidative decarboxylation of pyruvate with the formation of acetyl-CoA is reduced, and the fatty acids through beta-oxidation will provide the energy necessary for cardiac function. The study shows that in cases of insufficient use of glucose for energy purposes, the amount of ATP available is sufficient for the diastolic function but not for the systolic one [40]. The importance of glucose in cardiac energy metabolism is very well documented in the case of type 2 diabetes mellitus, where the combined use of alternative energy substrates such as fatty acids, ketone bodies, and branched-chain amino acids is responsible for the occurrence of cardiomyocyte dysfunction and the development of diabetic cardiomyopathy [41]. However, this mechanism alone cannot explain the energy use of other substrates than glucose in diabetic patients. Studies show that almost all enzymes activated by insulin secretion are acetylated. High blood sugar levels produce insulin secretion and hyperacetylation of some enzymes, especially those involved in the beta-oxidation of fatty acids, leading to a decrease in their stability and activity [42].
6. TMZ and Oxidative Stress
Oxidative stress is an important characteristic of cardiovascular diseases, especially in the ischemic myocardium, but at the same time also in moderate to intense muscular effort. The most important sources of reactive oxygen species (ROS) are electron losses that can occur at the level of the ETC and nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase/NOX) [43]. TMZ as a modulator of cardiac energy metabolism, can influence different markers of oxidative stress. In a study conducted on mice subjected to cardiac hypoxia following acute myocardial infarction, TMZ demonstrated protective effects against cardiac rupture (CR), a severe complication. This ischemic condition triggered the generation of ROS, activation of inflammatory cytokines, matrix metalloproteinases (MMPs), and collagen deposition, culminating in structural changes within the damaged myocardium. Furthermore, the disruption of extracellular matrix (ECM) proteins emerged as a crucial mechanism in the pathogenesis of CR, with MMPs playing a recognized role in regulating ECM protein turnover. The activation of MMPs has been identified as a key factor in the progression of post-myocardial infarction (CR). In the same experimental study, the group that received TMZ pretreatment exhibited a reduction in the expression of MMPs, specifically MMP-2 and MMP-9. Additionally, TMZ pretreatment demonstrated a significant decrease in both cardiac hydrogen peroxide (H2O2) generation and malondialdehyde (MDA) levels, indicating a notable antioxidative effect of TMZ [44].
In another study on rats, TMZ (30 and 60 mg/kg) administered before exhaustive exercise (prolonged swimming training) conferred cardioprotection by modulating key markers associated with oxidative stress-related injury. TMZ elevated the levels of glutathione (GSH) and enhanced the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), while reducing the level of MDA. TMZ inhibited oxidative stress and cardiomyocyte apoptosis by activating the Nrf2/HO1 ((nuclear factor (erythroid-derived 2)-like 2)/heme oxygenase 1) signaling pathway and deactivating the NF-κB (nuclear factor NF-kappa-B p65 subunit) signaling pathway. Top of Form Apoptosis was also inhibited by reducing the Bax/Bcl-2 (B-cell lymphoma 2/Bcl-2-associated X protein) ratio. Bcl-2 and Bax, both significant members of the Bcl-2 family, play crucial roles in the regulation of apoptosis. Bax functions as a proapoptotic protein, whereas Bcl-2 acts as an anti-apoptotic protein. Furthermore, the levels of cleaved caspase-3, cleaved poly (ADP-ribose) polymerase (PARP), and cytochrome c, critical indicators of apoptosis, were found to be decreased in the pretreated group. The levels of cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP), which serve as biomarkers for myocardial damage, were also decreased by pretreatment with TMZ, highlighting TMZs positive effects on myocardial protection [45].
Other studies show that TMZ reduces oxidative stress by directly activating enzymes such as GSH-Px and SOD, with a concomitant reduction of MDA levels [46]. TMZ reduces the level of Bax and increases the expression of caspase-3 and Bcl-2 in a concentration-dependent manner in an animal model of myocardial infarction, decreasing the risk of myocardial cell apoptosis [47].
A study on the influence of chronic treatment with 60 mg/day TMZ, divided into three doses, in patients with end-stage renal disease shows that the serum levels of MDA were significantly lower after 6 months of treatment, and the mortality rate due to cardiovascular complications was significantly reduced compared to the control group [48]. The administration of 20 mg of TMZ 3 times a day to patients with chronic cor pulmonale shows that after 3 months of treatment, the activities of plasma SOD, erythrocyte catalase (CAT), and GSH-Px (erythrocyte and plasma) were increased, and the amount of MDA and brain natriuretic peptide (BNP) was lower in the group treated with TMZ [49].
Additionally, there are reports indicating that mitochondrial permeability transition plays a crucial role in cardiomyocyte apoptosis. This phenomenon is a consequence of the opening of the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane, and it is stated that inhibiting the opening of mPTP could serve as an additional mechanism for providing cardioprotection. TMZ can also be considered a mitochondrial permeability transition pore (mPTP) inhibitor, thus having a cardioprotective effect during ischemia [50].
7. TMZ and Doping
TMZ was added to the WADA list of prohibited substances in January 2014 as a metabolic modulator due to a decrease in coronary vascular resistance, increased coronary blood flow, and cytoprotective/anti-ischemic effects in other organs [1].
The abuse of metabolic modulators such as TMZ in sports started with the similarities regarding the use of energy substrates with ischemic heart disease and heart failure, as the predominant use of anaerobic glycolysis as well as its decoupling from mitochondrial oxidative metabolism has an essential role in cardiac inefficiency (oxygen consumed per work performed) and functional impairment. The biochemical approaches proposed in this case would favor the oxidative metabolism of glucose and/or inhibit the beta-oxidation process [51].
During rest, the beta-oxidation of fatty acids is the main energy source (up to 80%), and approximately 20% of the maximum oxidative capacity of the myocardium cell is used. During low to moderate intensity effort, an increase in the uptake of glucose and lactate in the myocardium was observed, while in the case of high-intensity effort, the use of glucose decreases, the aerobic limit is reached, anaerobic metabolism is triggered, and ventricular performance declines [52].
By inhibiting the key enzyme of beta-oxidation, KAT, TMZ determines a metabolic switch between the predominant use of fatty acids and the use of glucose for energy purposes, which lowers demand for oxygen and improves left ventricular ejection fraction, cardiac diastolic function, quality of life, and exercise capacity [1]. For example, in the case of running medium distances (800–5000 m), the intensity of the effort is very high, ∼95% to 130% of VO2 max, and for performance athletes who present primarily Type IIa/IIx fiber morphology, glycogen is almost exclusively used for energy purposes both aerobically and anaerobically [53]. The amount of lactate produced following the anaerobic use of glucose is relatively high, up to 20 mmol/L, and the muscle pH value is low, up to 6.6. An important buffer in this case is muscle carnosine [54]. In these conditions, the activation of PDH by TMZ can have a favorable impact not only on the aerobic metabolism of the cell but also on the maintenance of muscle pH. In the case of ultra-marathon runners (distances higher than 42.2 km), food intake and the use of carbohydrates for energy purposes are essential for the body’s adaptation to training [55,56]. Glycogen, in addition to its role as an energy deposit, is also a regulator of metabolic signaling through direct or indirect mediation of the AMPK and p38 mitogen-activated protein kinase (MAPK) pathways. AMPK and p38 MAPK control the expression and activity of the peroxisome proliferator activated receptor γ coactivators (PGCs), which directly increase the mitochondrial mass in the muscles and implicitly the exercise capacity [57]. Intense sprinting requires rapid energy turnover, mainly originating from small sex-differences in anaerobic metabolism. The relative aerobic contribution becomes more important as the distance increases, in men from 20.4 ± 7.9% in the 100 m race to 41.3 ± 10.9% in the 400 m race and in women from 25.0 ± 7.4% in the 100 m race up to 44.5 ± 7.6% in the 400 m race [58].
Regarding the performance-enhancing effect of TMZ on the myocardium, a study conducted on patients with heart failure with preserved ejection fraction who were administered 20 mg of TMZ three times a day shows that by reducing beta-oxidation and favoring glucose oxidation, more moles of ATP/mol of oxygen consumed will be obtained, which improves mitochondrial function, reduces oxidative stress, and increases the value of the myocardial PCr/ATP ratio [59]. Studies conducted with 31P-magnetic resonance spectroscopy (31P-MRS) show that TMZ increases the value of the PCr/ATP ratio, a measure of myocardial energetics; the low value of this ratio is due to the imbalance between the intake and the oxygen requirement of the cell [60].
In the case of performance athletes, diet, dietary supplements, and prescribed medications are recommended by physicians specializing in sports medicine. However, for those engaging in recreational sports activities, concern arises regarding the debatable origin of TMZ as a prescription substance (black market or online sources), with risks of adulteration or overdose. Additionally, there is the potential danger of combining TMZ with other prohibited substances, which influences cardiac metabolism and poses risks to the life and health of the athlete.
8. Synergism and Antagonism of TMZ with Natural Compounds in Sports
Many athletes use natural extracts to enhance their athletic performance. The choice of these extracts is based on the supposed anabolic properties of the various natural compounds [61]. Aside from the naturally occurring substances that WADA prohibits (cocaine, cathinone, ephedrine, strychnine, and cannabinoids), there are still various classes of active principles that can have a variety of outcomes, eventually enhancing athletic performance. Dietary supplements that contain natural compounds are very popular among athletes, and there are some hypotheses why these compounds are combined with synthetic drugs. Some natural substances can have synergistic effects with trimetazidine. For example, saponins are a class of active principles that have amphiphilic properties. These compounds usually promote the absorption of other active substances [45]. Frequently used by athletes are the sterol saponins found in various plant products, such as Tribulus terrestris, Discorea sp., etc. [62,63].
Beetroot juice is also very popular among athletes due to its nitrate content. Following ingestion, anaerobic bacteria in the oral cavity use nitrate reductase to convert NO3 to nitrite (NO2), which is then converted to nitric oxide (NO) in the stomach. Numerous physiological processes that affect the use of oxygen during striated muscle contraction are triggered by NO. Hypoxic conditions facilitate the physiological mechanisms to reduce NO2, therefore NO (a vasodilator) is created in muscle regions that use or require more oxygen [64]. The nitrate-nitrite pathway has proven to have an important role in exercise efficiency [65]. In connection with TMZ, a synergistic effect may be expected between supplements containing nitrates and TMZ. Both categories are used in the management of angina pectoris. However, based on our current knowledge, there is a lack of studies that validate this synergistic effect.
Related to the TMZ mechanism of action that activates AMPK, facilitating glucose uptake, several natural compounds have been proven to have similar effects. Naringenin, a bioflavonoid with multiple effects and frequently encountered in dietary supplements used by athletes, activates AMPK and stimulates glucose uptake in skeletal muscle cells [66]. Also, diosgenin, a sterolic saponin, increases AMPK and acetyl-CoA carboxylase phosphorylation [67]. All these compounds could act in synergy when co-administered with TMZ.
An alternative hypothesis is that some natural compounds can antagonize the effects of TMZ. This is the case for all the compounds that stimulate fatty acid oxidation. In vitro and in vivo animal studies have demonstrated that resveratrol improves mitochondrial fatty acid beta-oxidation and acts in the opposite way compared to TMZ [68,69]. The same effect was noticed for some flavonoids [70].
However, more studies are needed to determine if these hypotheses are confirmed.
9. TMZ and Restrictive Diets in Sports
In the case of performance athletes, the diet occupies an extremely important place because it must ensure the energy and nutrients necessary during training, adaptation, and recovery between training sessions to have an optimal weight and to maintain their long-term health [71]. The impact of restrictive diets on sports performance is the subject of numerous studies, from the anti-inflammatory effect of the Mediterranean diet to the insulin-sensitizing effect of the high-fat diet [72,73]. A high-fat diet is often adopted by performance athletes not only for weight loss but also for the insulin-sensitizing effect. Studies show that the ketogenic diet (<50 g/day carbohydrate, >75% fat) could be beneficial both in increasing short-duration, vigorous-intensity endurance tests and in weight loss, which also enhances athletic performance [74]. A comparative study on the effect of TMZ and physical exercise in an animal model of insulin resistance shows common mechanisms by which serum glucose is reduced, glucose metabolism in the cell increases, and skeletal muscle insulin signaling-related protein ratios of p-IRS1/IRS1 and p-AKT/AKT increase. The mechanism of action is based on the nuclear factor erythroid 2 related factor 2 (Nrf2) signaling pathway, a main antioxidant signaling regulator that could prevent the development of metabolic syndrome and related cardiovascular diseases, as well as the inhibition by TMZ of the KAT enzyme [72]. Exercise can increase oxidative stress in an exercise-type and intensity-dependent manner, and the body’s ability to respond depends on each individual and the level of training [75]. TMZ, through upregulation of Nrf2/heme oxygenase-1 (HO-1) and downregulation of nuclear factor kappa B signaling, decreases the level of oxidative stress and apoptosis induced by exhaustive exercise [45]. Another study carried out in mice fed a high-fat diet shows that the expression of carbohydrate responsive element binding protein (ChREBP) and the de novo biosynthesis of fatty acids were reduced by decreasing the activity of enzymes like fatty acid synthase and acetyl CoA carboxylase in the high-fat diet with co-administration of the TMZ group compared with the diet group by increasing AMPK activity [76,77]. Studies show that TMZ alone or with exercise can improve mitochondrial quality in an obesity-induced skeletal muscle dysfunction characterized by mitochondrial dysfunction. A high-fat diet for 8 weeks induced obesity that was reduced by physical exercise, while TMZ did not affect body weight. Both physical exercise and TMZ prevented the accumulation of visceral fat when administering a high-fat diet [78].
10. Training, Competition, and “Fuel” Type? The Athlete’s Dilemma: Glucose or Fatty Acids?
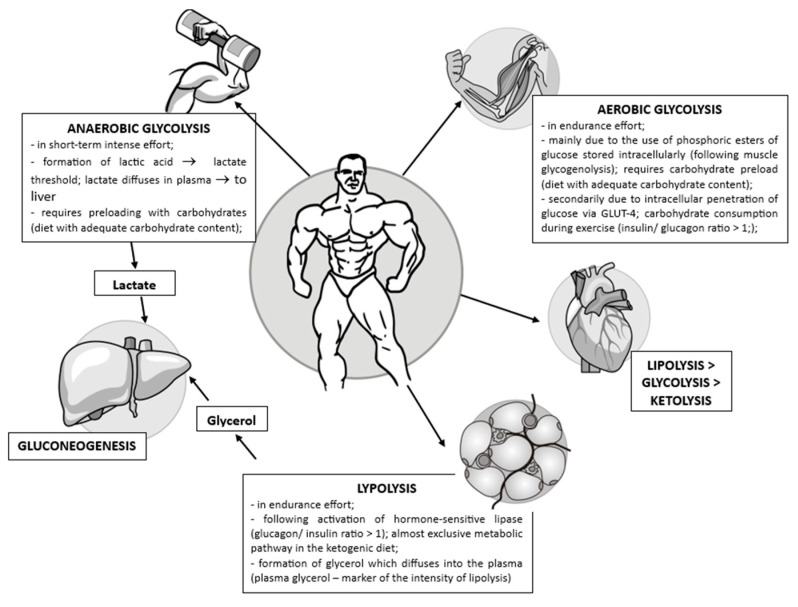
The utilization of the phosphagen (PCr-ATP) system and anaerobic glycolysis plays a crucial role in distinguishing performance levels in specific sports such as weightlifting, bodybuilding, and sprinting (e.g., running 100 m). Therefore, the significance of muscle glycogen stores is highlighted, emphasizing the essential nature of a carbohydrate-rich diet and the importance of avoiding exhausting effort within 48 h before a competition to optimize athletic performance. During effort, the PCr-ATP system provides energy only for a few seconds, and although anaerobic glycolysis can be considered a “waste” of energy and lead to the formation of lactic acid as an end product, it has two major advantages: fast response and ATP formation in the absence of oxygen [79]. However, even during short-term intense efforts (of a few minutes), the release of energy is not necessarily sequential (PCr-ATP system, anaerobic glycolysis, aerobic glycolysis) [80].
In endurance efforts, things are more complicated. The balance between lipolysis and aerobic glycolysis can be adjusted according to different criteria, such as the quality of the “fuel” used, energy efficiency, oxygen consumption, the risk of oxidative stress, the type of diet (especially regarding carbohydrate intake and the ketogenic diet), and can be quantified using the respiratory quotient (RQ) as a parameter.
The respiratory quotient (RQ) represents the ratio between the volume of CO2 released/Vol O2 consumed during respiration, a dimensionless number used to establish the basal metabolic rate. The RQ value varies from 1 in the case of aerobic use of carbohydrates, to 0.7 in the case of lipids, to 0.8 in the case of amino acids, and is 0 for the anaerobic use of glucose (see Figure 5) [81].
Figure 5.
The use of different energy sources during endurance efforts.
TMZ can induce metabolic flexibility to modulate the switch between energy substrates in ATP synthesis, as it increases intracellular glucose availability and decreases fatty acid oxidation; this is easily proven by measuring the RQ [82].
Preclinical studies on the rat heart performed at high altitude hypoxia with the combination of L-carnitine (a specific transporter of fatty acids in the mitochondria) and TMZ showed a clear improvement in cardiac metabolism by facilitating the beta-oxidation of fatty acids and promoting aerobic glycolysis [83]. On the other hand, clinical trials show that a balanced use of fatty acid beta-oxidation and glycolysis is essential for normal cardiac function, although the preferential use of glucose as an energy substrate has a favorable effect on myocardial ischemia [84].
On another note, the primary role of mitochondrial dysfunctions in age-related skeletal muscle loss (sarcopenia) is an important subject of consideration. Optimizing energy production via metabolic modulation could be an intriguing strategy to counteract mitochondrial dysfunctions and enhance stem cell function, thereby improving myogenesis during the aging process. Regarding the connection between myogenesis and oxidative metabolism, a study conducted on an experimental model of cachexia shows that reprogramming metabolism through 12 consecutive days of TMZ treatment induces the expression of myogenic genes in the skeletal muscles of mice, enhancing the levels of mitochondrial proteins and promoting oxidative metabolism in aging muscles [85]. Preclinical studies in mice show that it acts as an "exercise mimetic" and prevents sarcopenia and muscle dysfunction following cancer cachexia [86]. Therefore, TMZ enhances myoblast differentiation and fosters myogenesis, leading to the formation of new myofibers. This serves as an additional reason for its use as a performance-enhancing substance.
Additionally, Bucci et al. [87] postulate that human obesity is characterized by increased use of fatty acids by skeletal muscles concomitant with subcutaneous fat storage disorder, a phenomenon that leads to increased exposure of lean mass to oxidative stress. This maladaptation is effectively counteracted by TMZ.
We still do not have a final answer to the question of whether lipids or glucose are the most efficient energy substrate for sustained physical effort, but it is certain that TMZ as a metabolic modulator between lipo- and glycolysis improves sports performance, which is why TMZ is included on the prohibited list of WADA [7].
11. Conclusions
The mechanism of action of TMZ is complex, involving a metabolic modulation of lipid metabolism by inhibiting KAT while simultaneously activating biochemical pathways that favor the use of glucose for energy purposes. Key enzymes such as PDH or AMPK activated by TMZ connect glycolysis with mitochondrial metabolism, reducing the oxygen consumption of the cell. Even if this metabolic modulation does not bring substantial advantages in the case of occasional athletes, in the case of performance athletes, during competition, sometimes the difference is made in thousandths of a second. That is why TMZ is included by WADA on the list of substances prohibited for performance athletes during and outside the competition.
Author Contributions
Conceptualization, A.P. and A.T.-V.; methodology, C.-E.V., C.F. and B.-E.Ő.; writing—original draft preparation, M.-G.B., J.K.B. and R.Ș.; writing—review and editing, A.T.-V.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the University of Medicine, Pharmacy, Science, and Technology "George Emil Palade” of Târgu Mureș Research Grant number 164/23/10.01.2023.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sigmund G., Koch A., Orlovius A.-K., Guddat S., Thomas A., Schänzer W., Thevis M. Doping Control Analysis of Trimetazidine and Characterization of Major Metabolites Using Mass Spectrometric Approaches: Analysis of Trimetazidine in Doping Controls. Drug Test. Anal. 2014;6:1197–1205. doi: 10.1002/dta.1680. [DOI] [PubMed] [Google Scholar]
- 2.Dai Z.-L., Song Y.-F., Tian Y., Li Y., Lin M., Lin J., Wang Q., Wang P., Gao W.-L. Trimetazidine Offers Myocardial Protection in Elderly Coronary Artery Disease Patients Undergoing Non-Cardiac Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Cardiovasc. Disord. 2021;21:473. doi: 10.1186/s12872-021-02287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam A., Lopaschuk G. Anti-Anginal Effects of Partial Fatty Acid Oxidation Inhibitors. Curr. Opin. Pharmacol. 2007;7:179–185. doi: 10.1016/j.coph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z., Chen J.-M., Huang H., Kuznicki M., Zheng S., Sun W., Quan N., Wang L., Yang H., Guo H.-M., et al. The Protective Effect of Trimetazidine on Myocardial Ischemia/Reperfusion Injury through Activating AMPK and ERK Signaling Pathway. Metabolism. 2016;65:122–130. doi: 10.1016/j.metabol.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClellan K.J., Plosker G.L. Trimetazidine: A Review of Its Use in Stable Angina Pectoris and Other Coronary Conditions. Drugs. 1999;58:143–157. doi: 10.2165/00003495-199958010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Iskesen I., Saribulbul O., Cerrahoglu M., Var A., Nazli Y., Sirin H. Trimetazidine Reduces Oxidative Stress in Cardiac Surgery. Circ. J. 2006;70:1169–1173. doi: 10.1253/circj.70.1169. [DOI] [PubMed] [Google Scholar]
- 7.World Anti Doping Agency The Prohibited List. [(accessed on 15 June 2023)]. Available online: https://www.wada-ama.org/en/prohibited-list.
- 8.Jeoung M.K., Kim K.S., Kim C.S., Kim N.H., Chung Y., Hong J.T., Moon D. An HPLC Determination of Trimetazidine in Human Plasma Using Liquid-Liquid Extraction for Sample Clean-Up. J. Liq. Chromatogr. Relat. Technol. 2005;28:1299–1309. doi: 10.1081/JLC-200054785. [DOI] [Google Scholar]
- 9.Okano M., Miyamoto A., Ota M., Kageyama S., Sato M. Doping Control Analysis of Trimetazidine in Dried Blood Spot. Drug Test. Anal. 2022 doi: 10.1002/dta.3414. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima A., Lopaschuk G.D. Cardiac Fatty Acid Oxidation in Heart Failure Associated with Obesity and Diabetes. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids. 2016;1861:1525–1534. doi: 10.1016/j.bbalip.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Alves-Bezerra M., Cohen D.E. Triglyceride Metabolism in the Liver. In: Terjung R., editor. Comprehensive Physiology. Wiley; Hoboken, NJ, USA: 2017. pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepino M.Y., Kuda O., Samovski D., Abumrad N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adeva-Andany M.M., Carneiro-Freire N., Seco-Filgueira M., Fernández-Fernández C., Mouriño-Bayolo D. Mitochondrial β-Oxidation of Saturated Fatty Acids in Humans. Mitochondrion. 2019;46:73–90. doi: 10.1016/j.mito.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Fritzen A.M., Lundsgaard A.-M., Kiens B. Tuning Fatty Acid Oxidation in Skeletal Muscle with Dietary Fat and Exercise. Nat. Rev. Endocrinol. 2020;16:683–696. doi: 10.1038/s41574-020-0405-1. [DOI] [PubMed] [Google Scholar]
- 15.Longo N., Frigeni M., Pasquali M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloway G.P., Bezaire V., Heigenhauser G.J.F., Tandon N.N., Glatz J.F.C., Luiken J.J.F.P., Bonen A., Spriet L.L. Mitochondrial Long Chain Fatty Acid Oxidation, Fatty Acid Translocase/CD36 Content and Carnitine Palmitoyltransferase I Activity in Human Skeletal Muscle during Aerobic Exercise: Human Skeletal Muscle Mitochondrial Fatty Acid Oxidation. J. Physiol. 2006;571:201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luptak I., Sverdlov A.L., Panagia M., Qin F., Pimentel D.R., Croteau D., Siwik D.A., Ingwall J.S., Bachschmid M.M., Balschi J.A., et al. Decreased ATP Production and Myocardial Contractile Reserve in Metabolic Heart Disease. J. Mol. Cell. Cardiol. 2018;116:106–114. doi: 10.1016/j.yjmcc.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto T., Sano M. Deranged Myocardial Fatty Acid Metabolism in Heart Failure. Int. J. Mol. Sci. 2022;23:996. doi: 10.3390/ijms23020996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honka H., Solis-Herrera C., Triplitt C., Norton L., Butler J., DeFronzo R.A. Therapeutic Manipulation of Myocardial Metabolism. J. Am. Coll. Cardiol. 2021;77:2022–2039. doi: 10.1016/j.jacc.2021.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandramouli C., Varma U., Stevens E.M., Xiao R.-P., Stapleton D.I., Mellor K.M., Delbridge L.M. Myocardial Glycogen Dynamics: New Perspectives on Disease Mechanisms. Clin. Exp. Pharmacol. Physiol. 2015;42:415–425. doi: 10.1111/1440-1681.12370. [DOI] [PubMed] [Google Scholar]
- 21.Pasqua T., Rocca C., Giglio A., Angelone T. Cardiometabolism as an Interlocking Puzzle between the Healthy and Diseased Heart: New Frontiers in Therapeutic Applications. J. Clin. Med. 2021;10:721. doi: 10.3390/jcm10040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwell A.A., Gopal K., Ussher J.R. Myocardial Energy Metabolism in Non-Ischemic Cardiomyopathy. Front. Physiol. 2020;11:570421. doi: 10.3389/fphys.2020.570421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hue L., Taegtmeyer H. The Randle Cycle Revisited: A New Head for an Old Hat. Am. J. Physiol.-Endocrinol. Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randle P.J., Garland P.B., Hales C.N., Newsholme E.A. The Glucose Fatty-Acid Cycle Its Role In Insulin Sensitivity And The Metabolic Disturbances Of Diabetes Mellitus. Lancet. 1963;281:785–789. doi: 10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 25.Umbarawan Y., Syamsunarno M.R.A.A., Koitabashi N., Yamaguchi A., Hanaoka H., Hishiki T., Nagahata-Naito Y., Obinata H., Sano M., Sunaga H., et al. Glucose Is Preferentially Utilized for Biomass Synthesis in Pressure-Overloaded Hearts: Evidence from Fatty Acid-Binding Protein-4 and -5 Knockout Mice. Cardiovasc. Res. 2018;114:1132–1144. doi: 10.1093/cvr/cvy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong S., Zhou W., Fang B., Lu W., Loro E., Damle M., Ding G., Jager J., Zhang S., Zhang Y., et al. Dissociation of Muscle Insulin Sensitivity from Exercise Endurance in Mice by HDAC3 Depletion. Nat. Med. 2017;23:223–234. doi: 10.1038/nm.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimova N., Long A., Scafidi S., Kristian T. Interplay between NAD+ and acetyl-CoA Metabolism in Ischemia-Induced Mitochondrial Pathophysiology. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019;1865:2060–2067. doi: 10.1016/j.bbadis.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin G.W., Taylor C.S., Taegtmeyer H. Regulation of Energy Metabolism of the Heart during Acute Increase in Heart Work. J. Biol. Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 29.Balakrishnan P., Liebe T., Amro E., Dhaka L., Heinzel T., Heller R. The Science of Hormesis in Health and Longevity. Elsevier; Amsterdam, The Netherlands: 2019. Metabolic Stress—Signaling and Metabolic Adaptation; pp. 139–148. [Google Scholar]
- 30.Wang H., Arias E.B., Treebak J.T., Cartee G.D. Exercise Effects on Γ3-AMPK Activity, Akt Substrate of 160 kDa Phosphorylation, and Glucose Uptake in Muscle of Normal and Insulin-Resistant Female Rats. J. Appl. Physiol. 2022;132:140–153. doi: 10.1152/japplphysiol.00533.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartee G.D. Mechanisms for Greater Insulin-Stimulated Glucose Uptake in Normal and Insulin-Resistant Skeletal Muscle after Acute Exercise. Am. J. Physiol.-Endocrinol. Metab. 2015;309:E949–E959. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M., Wei W., Stone C., Zhang L., Tian G., Ding J.N. Beneficial Effects of Trimetazidine on Expression of Serotonin and Serotonin Transporter in Rats with Myocardial Infarction and Depression. Neuropsychiatr. Dis. Treat. 2018;14:787–797. doi: 10.2147/NDT.S157441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chrusciel P., Rysz J., Banach M. Defining the Role of Trimetazidine in the Treatment of Cardiovascular Disorders: Some Insights on Its Role in Heart Failure and Peripheral Artery Disease. Drugs. 2014;74:971–980. doi: 10.1007/s40265-014-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guarini G., Huqi A., Morrone D., Capozza P.F., Marzilli M. Trimetazidine and Other Metabolic Modifiers. Eur. Cardiol. Rev. 2018;13:1. doi: 10.15420/ecr.2018.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fantini E., Demaison L., Sentex E., Grynberg A., Athias P. Some Biochemical Aspects of the Protective Effect of Trimetazidine on Rat Cardiomyocytes During Hypoxia and Reoxygenation. J. Mol. Cell. Cardiol. 1994;26:949–958. doi: 10.1006/jmcc.1994.1116. [DOI] [PubMed] [Google Scholar]
- 36.Eyassu F., Angione C. Modelling Pyruvate Dehydrogenase under Hypoxia and Its Role in Cancer Metabolism. R. Soc. Open Sci. 2017;4:170360. doi: 10.1098/rsos.170360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantor P.F., Lucien A., Kozak R., Lopaschuk G.D. The Antianginal Drug Trimetazidine Shifts Cardiac Energy Metabolism From Fatty Acid Oxidation to Glucose Oxidation by Inhibiting Mitochondrial Long-Chain 3-Ketoacyl Coenzyme A Thiolase. Circ. Res. 2000;86:580–588. doi: 10.1161/01.RES.86.5.580. [DOI] [PubMed] [Google Scholar]
- 38.Shu H., Hang W., Peng Y., Nie J., Wu L., Zhang W., Wang D.W., Zhou N. Trimetazidine Attenuates Heart Failure by Improving Myocardial Metabolism via AMPK. Front. Pharmacol. 2021;12:707399. doi: 10.3389/fphar.2021.707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzatsos A., Tsichlis P.N. Energy Depletion Inhibits Phosphatidylinositol 3-Kinase/Akt Signaling and Induces Apoptosis via AMP-Activated Protein Kinase-Dependent Phosphorylation of IRS-1 at Ser-794. J. Biol. Chem. 2007;282:18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- 40.Gopal K., Almutairi M., Al Batran R., Eaton F., Gandhi M., Ussher J.R. Cardiac-Specific Deletion of Pyruvate Dehydrogenase Impairs Glucose Oxidation Rates and Induces Diastolic Dysfunction. Front. Cardiovasc. Med. 2018;5:17. doi: 10.3389/fcvm.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renguet E., Bultot L., Beauloye C., Horman S., Bertrand L. The Regulation of Insulin-Stimulated Cardiac Glucose Transport via Protein Acetylation. Front. Cardiovasc. Med. 2018;5:70. doi: 10.3389/fcvm.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Zhou F., Bai M., Liu Y., Zhang L., Zhu Q., Bi Y., Ning G., Zhou L., Wang X. The Pivotal Role of Protein Acetylation in Linking Glucose and Fatty Acid Metabolism to β-Cell Function. Cell Death Dis. 2019;10:66. doi: 10.1038/s41419-019-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jîtcă G., Ősz B.E., Tero-Vescan A., Miklos A.P., Rusz C.-M., Bătrînu M.-G., Vari C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants. 2022;11:572. doi: 10.3390/antiox11030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong W., Ma Y., Li A., Shi H., Nie S. Trimetazidine Suppresses Oxidative Stress, Inhibits MMP-2 and MMP -9 Expression, and Prevents Cardiac Rupture in Mice with Myocardial Infarction. Cardiovasc. Ther. 2018;36:e12460. doi: 10.1111/1755-5922.12460. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H., Liu M., Zhang Y., Li X. Trimetazidine Attenuates Exhaustive Exercise-Induced Myocardial Injury in Rats via Regulation of the Nrf2/NF-κB Signaling Pathway. Front. Pharmacol. 2019;10:175. doi: 10.3389/fphar.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassanzadeh G., Hosseini A., Pasbakhsh P., Akbari M., Ghaffarpour M., Takzare N., Zahmatkesh M. Trimetazidine Prevents Oxidative Changes Induced in a Rat Model of Sporadic Type of Alzheimer’s Disease. Acta Med. Iran. 2015;53:17–24. [PubMed] [Google Scholar]
- 47.Luo X., Zhong Z., Chong A., Zhang W., Wu X. Function and Mechanism of Trimetazidine in Myocardial Infarction-Induced Myocardial Energy Metabolism Disorder Through the SIRT1–AMPK Pathway. Front. Physiol. 2021;12:645041. doi: 10.3389/fphys.2021.645041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aygen B., Celiker H., Dogukan A., Ilhan N. The Effects of Trimetazidine on Lipid Peroxidation in Patients Withend-Stage Renal Disease. Methods Find. Exp. Clin. Pharmacol. 2008;30:757. doi: 10.1358/mf.2008.30.10.1316831. [DOI] [PubMed] [Google Scholar]
- 49.Bayram E., Atalay C., Kocatürk H., Yücel O. Effects of Trimetazidine on Lipid Peroxidation, Antioxidant Enzyme Activities and Plasma Brain Natriuretic Peptide Levels in Patients with Chronic Cor Pulmonale. J. Int. Med. Res. 2005;33:612–619. doi: 10.1177/147323000503300602. [DOI] [PubMed] [Google Scholar]
- 50.Argaud L., Gomez L., Gateau-Roesch O., Couture-Lepetit E., Loufouat J., Robert D., Ovize M. Trimetazidine Inhibits Mitochondrial Permeability Transition Pore Opening and Prevents Lethal Ischemia–Reperfusion Injury. J. Mol. Cell. Cardiol. 2005;39:893–899. doi: 10.1016/j.yjmcc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Lopaschuk G.D. Metabolic Modulators in Heart Disease: Past, Present, and Future. Can. J. Cardiol. 2017;33:838–849. doi: 10.1016/j.cjca.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Rosano G.M.C., Vitale C., Spoletini I. Metabolic Approach to Heart Failure: The Role of Metabolic Modulators. Egypt. Heart J. 2015;67:177–181. doi: 10.1016/j.ehj.2015.03.004. [DOI] [Google Scholar]
- 53.Stellingwerff T., Bovim I.M., Whitfield J. Contemporary Nutrition Interventions to Optimize Performance in Middle-Distance Runners. Int. J. Sport Nutr. Exerc. Metab. 2019;29:106–116. doi: 10.1123/ijsnem.2018-0241. [DOI] [PubMed] [Google Scholar]
- 54.Perim P., Marticorena F.M., Ribeiro F., Barreto G., Gobbi N., Kerksick C., Dolan E., Saunders B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019;6:135. doi: 10.3389/fnut.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi R., Zhang J., Fang B., Tian X., Feng Y., Cheng Z., Fu Z., Zhang J., Wu J. Runners’ Metabolomic Changes Following Marathon. Nutr. Metab. 2020;17:19. doi: 10.1186/s12986-020-00436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiller N.B., Roberts J.D., Beasley L., Chapman S., Pinto J.M., Smith L., Wiffin M., Russell M., Sparks S.A., Duckworth L., et al. International Society of Sports Nutrition Position Stand: Nutritional Considerations for Single-Stage Ultra-Marathon Training and Racing. J. Int. Soc. Sports Nutr. 2019;16:50. doi: 10.1186/s12970-019-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baar K., McGee S. Optimizing Training Adaptations by Manipulating Glycogen. Eur. J. Sport Sci. 2008;8:97–106. doi: 10.1080/17461390801919094. [DOI] [Google Scholar]
- 58.Slater G.J., Sygo J., Jorgensen M. SPRINTING. Dietary Approaches to Optimize Training Adaptation and Performance. Int. J. Sport Nutr. Exerc. Metab. 2019;29:85–94. doi: 10.1123/ijsnem.2018-0273. [DOI] [PubMed] [Google Scholar]
- 59.Van De Bovenkamp A.A., Bakermans A.J., Allaart C.P., Nederveen A.J., Kok W.E.M., Van Rossum A.C., Handoko M.L. TrimetaziDine as a Performance-enhancING Drug in Heart Failure with Preserved Ejection Fraction (DoPING-HFpEF): Rationale and Design of a Placebo-Controlled Cross-over Intervention Study. Neth. Heart J. 2020;28:312–319. doi: 10.1007/s12471-020-01407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fragasso G., Perseghin G., De Cobelli F., Esposito A., Palloshi A., Lattuada G., Scifo P., Calori G., Del Maschio A., Margonato A. Effects of Metabolic Modulation by Trimetazidine on Left Ventricular Function and Phosphocreatine/Adenosine Triphosphate Ratio in Patients with Heart Failure. Eur. Heart J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 61.Deldicque L., Francaux M. Potential Harmful Effects of Dietary Supplements in Sports Medicine. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:439–445. doi: 10.1097/MCO.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 62.Ma Y., Guo Z., Wang X. Tribulus Terrestris Extracts Alleviate Muscle Damage and Promote Anaerobic Performance of Trained Male Boxers and Its Mechanisms: Roles of Androgen, IGF-1, and IGF Binding Protein-3. J. Sport Health Sci. 2017;6:474–481. doi: 10.1016/j.jshs.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernández-Lázaro D., Fernandez-Lazaro C., Seco-Calvo J., Garrosa E., Adams D., Mielgo-Ayuso J. Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review. Int. J. Environ. Res. Public. Health. 2022;19:9533. doi: 10.3390/ijerph19159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirmiran P., Houshialsadat Z., Gaeini Z., Bahadoran Z., Azizi F. Functional Properties of Beetroot (Beta vulgaris) in Management of Cardio-Metabolic Diseases. Nutr. Metab. 2020;17:3. doi: 10.1186/s12986-019-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones A.M. Dietary Nitrate Supplementation and Exercise Performance. Sports Med. 2014;44:35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zygmunt K., Faubert B., MacNeil J., Tsiani E. Naringenin, a Citrus Flavonoid, Increases Muscle Cell Glucose Uptake via AMPK. Biochem. Biophys. Res. Commun. 2010;398:178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 67.Cheng S., Liang S., Liu Q., Deng Z., Zhang Y., Du J., Zhang Y., Li S., Cheng B., Ling C. Diosgenin Prevents High-Fat Diet-Induced Rat Non-Alcoholic Fatty Liver Disease through the AMPK and LXR Signaling Pathways. Int. J. Mol. Med. 2017;41:1089–1095. doi: 10.3892/ijmm.2017.3291. [DOI] [PubMed] [Google Scholar]
- 68.Aires V., Delmas D., Le Bachelier C., Latruffe N., Schlemmer D., Benoist J.-F., Djouadi F., Bastin J. Stilbenes and Resveratrol Metabolites Improve Mitochondrial Fatty Acid Oxidation Defects in Human Fibroblasts. Orphanet J. Rare Dis. 2014;9:79. doi: 10.1186/1750-1172-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gimeno-Mallench L., Mas-Bargues C., Inglés M., Olaso G., Borras C., Gambini J., Vina J. Resveratrol Shifts Energy Metabolism to Increase Lipid Oxidation in Healthy Old Mice. Biomed. Pharmacother. 2019;118:109130. doi: 10.1016/j.biopha.2019.109130. [DOI] [PubMed] [Google Scholar]
- 70.Jung U.J., Lee M.-K., Park Y.B., Kang M.A., Choi M.-S. Effect of Citrus Flavonoids on Lipid Metabolism and Glucose-Regulating Enzyme mRNA Levels in Type-2 Diabetic Mice. Int. J. Biochem. Cell Biol. 2006;38:1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine. J. Am. Diet. Assoc. 2000;100:1543–1556. doi: 10.1016/S0002-8223(00)00428-4. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W., Dun Y., You B., Qiu L., Ripley-Gonzalez J.W., Cheng J., Fu S., Li C., Liu S. Trimetazidine and Exercise Offer Analogous Improvements to the Skeletal Muscle Insulin Resistance of Mice through Nrf2 Signaling. BMJ Open Diabetes Res. Care. 2022;10:e002699. doi: 10.1136/bmjdrc-2021-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaufman M., Nguyen C., Shetty M., Oppezzo M., Barrack M., Fredericson M. Popular Dietary Trends’ Impact on Athletic Performance: A Critical Analysis Review. Nutrients. 2023;15:3511. doi: 10.3390/nu15163511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McSwiney F.T., Doyle L., Plews D.J., Zinn C. Impact of Ketogenic Diet on Athletes: Current Insights. Open Access J. Sports Med. 2019;10:171–183. doi: 10.2147/OAJSM.S180409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirupathi A., Wang M., Lin J.K., Fekete G., István B., Baker J.S., Gu Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. BioMed Res. Int. 2021;2021:1947928. doi: 10.1155/2021/1947928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Li C., Li X., Wu C., Zhou H., Lu S., Liu X. Trimetazidine Improves Hepatic Lipogenesis and Steatosis in Non-alcoholic Fatty Liver Disease via AMPK-ChREBP Pathway. Mol. Med. Rep. 2020;22:2174–2182. doi: 10.3892/mmr.2020.11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ussher J.R., Keung W., Fillmore N., Koves T.R., Mori J., Zhang L., Lopaschuk D.G., Ilkayeva O.R., Wagg C.S., Jaswal J.S., et al. Treatment with the 3-Ketoacyl-CoA Thiolase Inhibitor Trimetazidine Does Not Exacerbate Whole-Body Insulin Resistance in Obese Mice. J. Pharmacol. Exp. Ther. 2014;349:487–496. doi: 10.1124/jpet.114.214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W., You B., Qi D., Qiu L., Ripley-Gonzalez J.W., Zheng F., Fu S., Li C., Dun Y., Liu S. Trimetazidine and Exercise Provide Comparable Improvements to High Fat Diet-Induced Muscle Dysfunction through Enhancement of Mitochondrial Quality Control. Sci. Rep. 2021;11:19116. doi: 10.1038/s41598-021-98771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spurway N.C. Aerobic Exercise, Anaerobic Exercise and the Lactate Threshold. Br. Med. Bull. 1992;48:569–591. doi: 10.1093/oxfordjournals.bmb.a072564. [DOI] [PubMed] [Google Scholar]
- 80.Gastin P.B. Energy System Interaction and Relative Contribution during Maximal Exercise. Sports Med. 2001;31:725–741. doi: 10.2165/00007256-200131100-00003. [DOI] [PubMed] [Google Scholar]
- 81.Patel H., Kerndt C.C., Bhardwaj A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Physiology, Respiratory Quotient. [PubMed] [Google Scholar]
- 82.Galgani J.E., Bergouignan A., Rieusset J., Moro C., Nazare J.-A. Editorial: Metabolic Flexibility. Front. Nutr. 2022;9:946300. doi: 10.3389/fnut.2022.946300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie H., Xu G., Aa J., Gu S., Gao Y. Modulation of Perturbed Cardiac Metabolism in Rats Under High-Altitude Hypoxia by Combination Treatment With L-Carnitine and Trimetazidine. Front. Physiol. 2021;12:671161. doi: 10.3389/fphys.2021.671161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaswal J.S., Keung W., Wang W., Ussher J.R., Lopaschuk G.D. Targeting Fatty Acid and Carbohydrate Oxidation--a Novel Therapeutic Intervention in the Ischemic and Failing Heart. Biochim. Biophys. Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Gatta L., Vitiello L., Gorini S., Chiandotto S., Costelli P., Giammarioli A.M., Malorni W., Rosano G., Ferraro E. Modulating the Metabolism by Trimetazidine Enhances Myoblast Differentiation and Promotes Myogenesis in Cachectic Tumor-Bearing C26 Mice. Oncotarget. 2017;8:113938–113956. doi: 10.18632/oncotarget.23044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molinari F., Pin F., Gorini S., Chiandotto S., Pontecorvo L., Penna F., Rizzuto E., Pisu S., Musarò A., Costelli P., et al. The Mitochondrial Metabolic Reprogramming Agent Trimetazidine as an ‘Exercise Mimetic’ in Cachectic C26-bearing Mice. J. Cachexia Sarcopenia Muscle. 2017;8:954–973. doi: 10.1002/jcsm.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bucci M., Borra R., Någren K., Maggio R., Tuunanen H., Oikonen V., Del Ry S., Viljanen T., Taittonen M., Rigazio S., et al. Human Obesity Is Characterized by Defective Fat Storage and Enhanced Muscle Fatty Acid Oxidation, and Trimetazidine Gradually Counteracts These Abnormalities. Am. J. Physiol.-Endocrinol. Metab. 2011;301:E105–E112. doi: 10.1152/ajpendo.00680.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.