Abstract
The Yersinia pestis pH 6 antigen was expressed by, and purified from, Escherichia coli containing cloned psa genes. By an enzyme-linked immunosorbence-based assay, purified pH 6 antigen bound to gangliotetraosylceramide (GM1A), gangliotriaosylceramide (GM2A), and lactosylceramide (LC) (designations follow the nomenclature of L. Svennerholm [J. Neurochem. 10:613–623, 1963]). Binding to GM1A, GM2A, and LC was saturable, with 50% maximal binding occurring at 498 ± 4, 390, and 196 ± 3 nM, respectively. Thin-layer chromatography (TLC) overlay binding confirmed that purified pH 6 antigen bound to GM1A, GM2A, and LC and also revealed binding to hydroxylated galactosylceramide. Intact E. coli cells which expressed the pH 6 antigen had a specificity similar to that of purified pH 6 in the TLC overlay assay except that nonhydroxylated galactosylceramide was also bound. The binding patterns observed indicate that the presence of β1-linked galactosyl residues in glycosphingolipids is the minimum determinant required for binding of the pH 6 antigen.
Members of the yersiniae express a range of adhesins termed YadA, Ail, invasin, and pH 6 antigen which are associated with virulence (14, 16). Other virulence attributes include a range of outer membrane proteins termed Yops. Some synergism may exist between the adhesins and Yops of the yersiniae since it has been found that adherence mediated by YadA is a prerequisite for the entry of at least two of the Yops (YopE and YopH) into eukaryotic cells (16). Yersinia pestis is a gram-negative facultative intracellular parasite and is the causative agent of plague. The pH 6 antigen is the only known putative adhesin expressed by Yersinia pestis. Expression of the pH 6 antigen, on the surface of the bacterium, is induced when the bacteria are grown between pH 5 and 6.7 and between 35 and 41°C (13). These conditions are encountered in the phagolysome, and synthesis at this site in macrophages has been demonstrated (13). The pH 6 antigen expressed on bacteria released from these macrophages might facilitate the colonization of other cell types and might also contribute to the delivery of Yops into these cells, by promoting bacterium-host cell contact (16).
The pH 6 antigen was first identified as a virulence determinant when derivatives of Y. pestis lacking the pH 6 antigen showed reduced virulence in mice (1). Structurally, the pH 6 antigen was found to consist of PsaA, a subunit protein, which assembles to form homopolymeric fibrillar structures on the bacterial surface (6, 7). A cosmid containing a 42-kbp DNA fragment which encodes PsaA and accessory loci necessary for pH 6 antigen biosynthesis (pDG1) has previously been cloned and expressed in Escherichia coli (6, 7). E. coli organisms expressing the pH 6 antigen have been shown to produce fibrillar structures which appear identical to those produced by Y. pestis expressing pH 6 antigen, and the fibrillar structures from both bacteria reacted with antiserum against the pH 6 antigen (6). Y. pestis cells, E. coli cells expressing the pH 6 antigen, and crude potassium isothiocyanate extracts of bacterial cells expressing the pH 6 antigen were all found to agglutinate erythrocytes from a range of species (2). The pH 6 antigen has been reported to bind to several human immunoglobulin G subclasses by acting as a bacterial Fc receptor (18).
This study examined the binding specificities of the pH 6 antigen of Y. pestis for a range of purified glycosphingolipids. The approach used was based on the binding pattern observed with both purified and cell-bound pH 6 antigen. It is suggested that the binding specificity observed in vitro reflects the receptor specificity in vivo.
E. coli HB101 containing pDG1 has previously been shown to express the pH 6 antigen (7). The pH 6 antigen structural gene (psaA) and a putative chaperone (psaB) are found within the cloned DNA fragment (6) and are expressed from the Y. pestis gene promoter (7). E. coli HB101 cells with or without pDG1 were grown with shaking (185 rpm) at 37°C in Luria-Bertani broth either supplemented or not supplemented with 100 μg of ampicillin per ml. Only E. coli HB101 cells containing pDG1 were able to cause the agglutination of sheep erythrocytes (2.5% vol/vol) in phosphate-buffered saline (PBS; 8 g of NaCl per liter, 0.2 g of KCl per liter, 1.15 g of Na2HPO4 per liter, 0.2 g of KH2PO4 per liter [pH 7.1]) after 2 h of incubation at 4°C in a microtiter tray assay. Aliquots of culture were taken at various time points, and their optical densities at 600 nm (OD600) and their levels of expression of the pH 6 antigen were determined (11). Maximum expression (equivalent to 272 μg/ml of culture) occurred during early stationary phase.
Crude pH 6 antigen extracts were prepared from cells of E. coli HB101 containing pDG1 with crude KSCN as potassium isothiocyanate the chaotropic agent (2). The extract was dialyzed against distilled water and mixed overnight at 4°C with ammonium sulfate (50% final concentration), and the precipitated material was pelleted by centrifugation at 5,000 × g for 10 min at 4°C before being exhaustively dialyzed against PBS. The minimal concentration of pH 6 antigen at this stage of preparation causing hemagglutination was 6.25 μg/ml.
The pH 6 antigen was further purified by isoelectric precipitation. The preparation described above was adjusted to pH 5.8 by the dropwise addition of 5 M HCl and incubated overnight with gentle stirring at 4°C. The resultant precipitate was pelleted by low-speed centrifugation and washed three times in PBS (pH 5.8) before finally being resuspended in PBS (pH 7.1). The minimal concentration of the final preparation of pH 6 antigen that caused hemagglutination was 4.7 ± 0.9 μg/ml (mean ± the standard error of the mean).
pH 6 antigen, which was not boiled prior to analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (10 or 15% acrylamide gels) and stained with Coomassie blue, appeared as a single band which migrated with a molecular mass of >100 kDa and did not enter the running gel. When samples were boiled prior to analysis, a predominant band with a molecular mass of 15 kDa was observed and was recognized by antiserum raised against pH 6 antigen (Fig. 1). The higher-molecular-mass bands did not react with pH 6 antiserum. N-terminal sequence analysis was carried out on Coomassie blue-stained (30 s) blots of SDS-polyacrylamide gels loaded with 6 μg of purified pH 6 antigen per lane. With a model 476A sequencer (Applied Biosystems Inc., Warrington, United Kingdom), the 15-kDa band had an N-terminal sequence of STVINSKDVS. This sequence was in agreement with the previously reported N-terminal amino acid sequence of PsaA, the structural subunit of the pH 6 antigen (6).
FIG. 1.
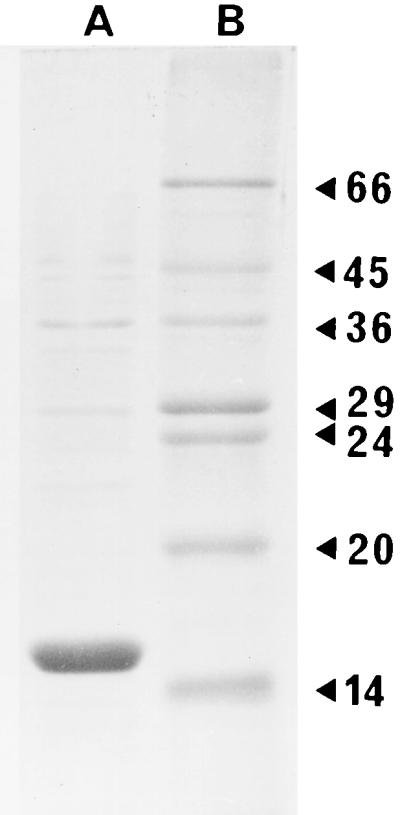
SDS-PAGE of purified pH 6 antigen (5 μg). Arrowheads on the right indicate molecular masses of marker proteins in kilodaltons. Track A contains purified pH 6 antigen, and track B contains molecular mass markers.
The pH 6 antigen is known to be able to agglutinate erythrocytes from a range of species (2). As few as 4.1 × 107 cells of E. coli HB101 containing pDG1 were able to cause agglutination of sheep erythrocytes (2.5%, vol/vol, in PBS), indicating that the pH 6 antigen is surface exposed. No hemagglutination was visible with 1010 cells of E. coli HB101. d-Mannose-specific adhesins are expressed by many gram-negative bacteria, including E. coli and Salmonella typhimurium (3, 15). Hemagglutination by E. coli containing pDG1 was unaffected by the presence of 1%, wt/vol, d-mannose. Hemagglutination was stable over a pH range of 4 to 10, indicating that the adhesin would be functional in a range of intracellular and extracellular environments. Hemagglutination of sheep erythrocytes by the purified pH 6 antigen was resistant to heating up to 80°C, above which temperature they rapidly pelleted. However, on cooling and remixing, stable erythrocyte mats were formed once again. Pretreatment of sheep erythrocytes with a range of proteases (10 mg of Aspergillus oryzae protease per ml, 10 mg of bovine pancreatic protease per ml, 5 mg of trypsin per ml, 1 mg of Streptomyces caespitosus per ml, and 1 mg of Streptomyces griseus protease per ml) for 1 h at 37°C (4) had no effect on the ability of the pH 6 antigen to agglutinate them, indicating that protein was unlikely to be an important component of the pH 6 receptor structure. Pretreatment of erythrocytes (5%, vol/vol, in PBS plus 1 mM glycine) with sodium metaperiodate (10 mM) for 20 min at 22°C, which disrupts saccharide structures containing neighboring hydroxyl residues, had no effect on hemagglutination by the pH 6 antigen (4, 12).
The ability of purified pH 6 antigen to bind to 15 glycosphingolipids (Table 1) was determined in two independent experiments, each involving quadriplicate assays based on a solid-phase enzyme-linked immunosorbent assay (ELISA) according to the method of Payne et al. (10). Briefly, glycosphingolipids (0.1 mg/ml) were suspended in methanol and the wells of a 96-well microtiter plate (Dynatech Immulon 2) were coated with 50-μl aliquots. The wells were blocked with 2% bovine serum albumin (BSA) in PBS for 2 h at room temperature and then washed twice with the same buffer. Purified pH 6 antigen was adjusted to 10 μg/ml in PBS–1% (wt/vol) skimmed-milk powder (PBS-blotto), and 100-μl aliquots were added to microtiter plate wells. After incubation at 37°C for 1 h and washing in 0.02% Tween 20–PBS, bound pH 6 antigen was detected with rabbit anti-pH 6 serum in combination with a goat anti-rabbit horseradish peroxidase conjugate and with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) as the chromogenic substrate (8). The OD414 of the microtiter tray wells were measured, and an increase of 0.1 over the OD414 of the control wells was considered to indicate binding. Of the glycosphingolipids tested (Table 1), only lactosylceramide (LC), gangliotriaosylceramide (GM2A), and gangliotetraosylceramide (GM1A) bound the pH 6 antigen (designations used are according to the terminology of Svennerholm [17]). The binding of the pH 6 antigen to GM1A, GM2A, and LC was saturable, and 50% binding occurred at 498 ± 4 nM (GM1A), 390 nM (GM2A), and 196 ± 3 nM (LC). Glycolipids containing sialic acid residues (gangliosides) either bound poorly or were unable to bind purified pH 6 antigen.
TABLE 1.
Ability of various glycosphingolipids to recognize the pH 6 antigen
| Structure of glycosphingolipida | Name or designation of glycosphingolipidb | Result of binding after separation by TLC with:
|
Result of binding in an assay based on ELISAc | |
|---|---|---|---|---|
| pH 6 antigen | E. coli/pDG1 | |||
| Galβ1-1Cer (hydroxylated fatty acids) | Galactosylceramide | + | + | N/A |
| Galβ1-1Cer (nonhydroxylated fatty acids) | Galacosylceramide | − | + | N/A |
| Galβ1-1Cer | Glucosylceramide | − | − | N/A |
| Galβ1-4Glcβ1-1Cer | LC | + | + | + |
| GalNAcβ1-4Galβ1-4Glcβ1-1Cer | GM2A | + | + | + |
| Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1Cer | GM1A | + | + | + |
| Galα-4Galβ1-4Glcβ1-1Cer | Globotriaosylceramide | − | − | − |
| GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer | Globotetraosylceramide (globoside) | − | − | − |
| GalNAcβ1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer | Globopentaosylceramide (Forssman glycolipid) | − | − | − |
| Galβ1-3GalNAcβ1-4Gal3→2NeuAc)β1-4Glcβ1-1Cer | GM1 | − | − | − |
| GalNAcβ1-4Gal(3←2NeuAc)β1-4Glcβ1-1Cer | GM2 | − | − | − |
| NeuAcα1-3Galβ1-4Glcβ1-1Cer | GM3 | − | − | − |
| NeuAcα2-3Galβ1-3GalNAcβ1-4Gal(3←2αNeuAc)β1-4Glcβ1-1Cer | GD1a | − | − | − |
| Galβ1-3GalNAcβ1-4Gal(3←2αNeuAc8←2αNeuAc)β1-4Glcβ1-1-Cer | GD1b | − | − | − |
| GalNAcβ1-4Gal(3→2NeuAc8←2αNeuAc)β12-4Glcβ1-1Cer | GD2 | − | − | − |
| NeuAcα2-8NeuAcα2-3Galβ1-4Glcβ1-1Cer | CD3 | − | − | − |
| NeuAcα2-8NeuAcα2-3Galβ13GalNAcβ1-4Gal(3←2NeuAc8←2αNeuAc) β1-4Glcβ1-1Cer | GQ1b | − | − | − |
| NeuAcα2-3Galβ1-3GalNAcβ1-4Gal(3β1-4Glcβ1-1Cer | GT1b | − | − | − |
Underlining indicates minimum binding determinant.
Abbreviations for gangliosides follow the nomenclature system of Svennerholm (17).
Purified glycosphingolipids and enzymes were obtained from Sigma Chemical Co. (Poole, United Kingdom).
An OD414 of >0.1 above those of controls was considered a positive result. N/A, not applicable.
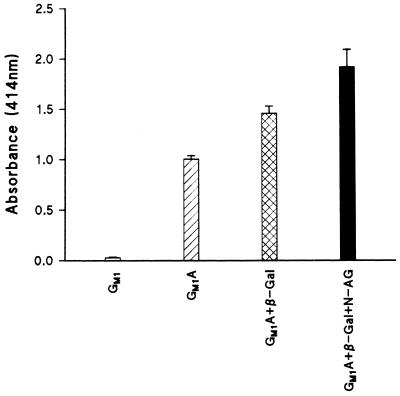
Differences between LC, GM1A, and GM2A are confined to the nonreducing ends of their oligosaccharide chains. The binding of the pH 6 antigen to LC is reduced by the addition of a GalNAc residue (e.g., in GM2A), and the binding is further reduced when a β-galactose (Galβ) residue is added to GM2A (e.g., in GM1A). To confirm the specificity of purified pH 6 antigen, GM1A was sequentially degraded from its nonreducing end. Wells containing immobilized glycosphingolipid were sequentially treated (1 h, 37°C) with 0.5 U of β-galactosidase [in 10 mM Tris–1.7 M (NH4)2SO4–10 mM MgCl2 (pH 7.3)], and the wells were washed three times with PBS and then treated (1 h, 37°C) with 0.1 U of N-acetylgalactosaminidase (in 5 mM sodium citrate–100 mM KH2PO4 [pH 3.7]) to remove galactose and then N-acetylgalactosamine residues (10). The wells were washed three times with PBS and then blocked for 1 h at room temperature with 200 μl of 2% BSA in PBS per well. The plate was washed twice with the BSA-PBS buffer, and 100 μl of purified pH 6 antigen was added (10 μg/ml in PBS-blotto). After incubation, bound pH 6 antigen was detected as described above. Removal of the Galβ residue from GM1A increased binding by 45%. The further removal of GalNAc resulted in a 91% increase in binding over the binding of GM1A. These results supported the direct binding studies and indicated that the affinity of the pH 6 antigen for glycosphingolipids is in the order LC > GM2A > GM1A (Fig. 2).
FIG. 2.
GM1A was sequentially treated with bovine β-galactosidase (β-Gal) and then N-acetylgalactosaminidase (N-AG). The ability of purified pH 6 antigen to bind to the products of enzymatic treatment, i.e., GM2A and LC, was determined by ELISA, and results were compared with the binding of the pH 6 antigen to GM1 and GM1A. Results are means ± standard errors of duplicates.
Eighteen glycosphingolipids were tested for the ability to bind purified pH 6 antigen by thin-layer chromatography (TLC) analysis (Table 1). Glycosphingolipids were separated with silica G60 (Merck Ltd., Leicester, United Kingdom) with chloroform-methanol-distilled water (60:35:8, vol/vol/vol) as the solvent. Glycosphingolipids on control plates were visualized by spraying the plates with p-anisaldehyde-sulfuric acid-ethanol (5:5:90, vol/vol/vol) and heating them at 110°C for 10 min. Test TLC plates were washed twice by immersion with 3% polyvinylpyrrolidone in acetate-buffered saline (ABS; 0.1 M sodium acetate, 0.2% CaCl2 [pH 7.2]) before being incubated either with or without purified pH 6 antigen (0.5 μg/ml in ABS). Unbound material was removed by washing the plates in ABS. Bound material was visualized with rabbit anti-pH 6 antiserum as described above except that a mixture of 3,3′-diaminobenzidine (3.3 mM) and urea hydrogen peroxide (7.4 mM) in 0.06 M Tris buffer was used as the chromogenic substrate. GM1A, GM2A, and LC all bound to the purified pH 6 antigen. In addition, galactosylceramide with hydroxylated fatty acids but not galactosylceramide with nonhydroxylated fatty acids or glucosylceramide bound the pH 6 antigen.
The 18 glycosphingolipids were also tested for the ability to bind E. coli expressing the pH 6 antigen (Table 1). Glycosphingolipids were prepared, separated, and visualized as described above. Binding was determined after the incubation for 2 h at 37°C of ABS suspensions (2 × 109 cells/ml) of E. coli HB101 either with or without the pDG1 plasmid. Binding was visualized as described above and observed only with E. coli containing the pDG1 plasmid. Specificity was similar to that of purified pH 6 antigen except that galactocerebrosides containing both hydroxylated and nonhydroxylated fatty acids were recognized. The degree of hydroxylation of the ceramide portion of glycosphingolipids has been demonstrated previously to affect binding characteristics (9), and the results found in this study are in agreement with this concept. It seems that the absence of a hydroxylated fatty acid chain alters the presentation of the attached galactose residue in such a way as to prevent binding of the purified pH 6 antigen but not of the pH 6 antigen which is expressed on the cell surface. It is possible that other E. coli cell surface components contribute to this altered conformation of the pH 6 antigen and therefore to the altered binding properties.
The role of the pH 6 antigen in the pathogenesis of infections caused by Y. pestis is not known (7). For many years it has been assumed that the pH 6 antigen allows the bacteria to bind to host cells. The data we present here indicate that the only common saccharide moiety of glycosphingolipids found to bind the pH 6 antigen is β1-linked galactose (Galβ1), which is hypothesized to represent the minimum receptor structure of the pH 6 antigen. However, as has been noted with other adhesin systems, the presence of the minimum receptor structure in glycosphingolipids does not necessarily correlate with binding (5). Thus, despite containing Galβ1 in their oligosaccharide sequence, globoside, the Forssman glycolipid, GM1, GM2, GM3, GD1a, GD1b, GD2, GD3, GQ1b, and GT1b do not bind or bind poorly to the pH 6 antigen. The inability of the pH 6 antigen to bind to gangliosides may have been attributable in part to charge repulsion between the sialosyl groups on the gangliosides and the negative charge on the pH 6 antigen (pI 5.8) at physiological pH. However, this is unlikely to apply to either globoside or the Forssman glycolipid since neither contains sialic acid residues. Our findings also indicate that the hydroxylation status of fatty acid chains present in the ceramide portion is critical for the binding of the pH 6 antigen. The glycosphingolipids recognized by the pH 6 antigen are common and are likely to be found on a range of host cell types. Accordingly, the receptor specificity of the pH 6 antigen is unlikely to play a direct role in cell tropism associated with the pathogenesis of Y. pestis. It seems more likely that the site and timing of the expression of pH 6, rather than its specificity, are important in determining which cells are bound by Y. pestis. In this respect, it is interesting that expression of the pH 6 antigen is induced only in acidic environments such as those found within abscesses (buboes or lesions in the liver and spleen) of animals infected with Y. pestis (13). It is known that Y. pestis cells are released from these environments, and a role for the pH 6 antigen might be in maximizing the colonization of naive macrophages or other nearby host cells.
Acknowledgments
We thank L. Lindler (Walter Reed Army Institute of Research, Washington, D.C.) for the provision of pDG1.
REFERENCES
- 1.Ben-Efraim S, Aronson M, Bichowsky-Slomnicki L. New antigenic component of Pasteurella pestis formed under specified conditions of pH and temperature. J Bacteriol. 1961;81:704–714. doi: 10.1128/jb.81.5.704-714.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bichowsky-Slomnicki L, Ben-Efraim S. Biological activities in extracts of Pasteurella pestis and their relation to the “pH 6 antigen.”. J Bacteriol. 1963;86:101–111. doi: 10.1128/jb.86.1.101-111.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craven S E, Williams D D. Inhibition of Salmonella typhimurium attachment to chicken cecal mucus by intestinal isolates of Enterobacteriaceae and Lactobacilli. Avian Dis. 1997;41:548–558. [PubMed] [Google Scholar]
- 4.Goldhar J. Bacterial lectinlike adhesins: determination and specificity. In: Clark V L, Bavoil P M, editors. Bacterial pathogenesis. London, United Kingdom: Academic Press; 1994. pp. 211–231. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson K A. Current experience from the interaction of bacteria with glycosphingolipids. In: Switalski L, Hook M, Beachey E, editors. Molecular mechanisms of microbial adhesion. New York, N.Y: Springer-Verlag; 1989. pp. 77–96. [Google Scholar]
- 6.Lindler L, Tall B D. Yersinia pestis pH 6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol Microbiol. 1993;8:311–324. doi: 10.1111/j.1365-2958.1993.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindler L E, Klempner M S, Straley S C. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makita A, Taniguchi N. Glycosphingolipids. In: Weigandt H, editor. Glycolipids. Oxford, United Kingdom: Elsevier; 1985. pp. 1–100. [Google Scholar]
- 9.Payne D. A study of K88-mediated haemagglutination by enterotoxigenic Escherichia coli (ETEC) Microbiologica. 1994;17:99–110. [PubMed] [Google Scholar]
- 10.Payne D, O’Reilly M, Williamson D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to β1-linked galactosyl residues in glycosphingolipids. Infect Immun. 1993;61:3673–3677. doi: 10.1128/iai.61.9.3673-3677.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne D W, Moore N, Lambert P. Use of an enzyme-linked immunosorbent assay (ELISA) to measure the expression of the K88 fimbrial antigen by enterotoxigenic Escherichia coli (ETEC) J Immunol Methods. 1993;159:283–289. doi: 10.1016/0022-1759(93)90169-8. [DOI] [PubMed] [Google Scholar]
- 12.Pazur J H. Neutral polysaccharides. In: Chaplin M F, Kennedy J F, editors. Carbohydrate analysis: a practical approach. Oxford, United Kingdom: IRL Press; 1986. pp. 55–96. [Google Scholar]
- 13.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierson D E. Mechanisms of Yersinia entry into mammalian cells. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: ASM Press; 1994. pp. 235–247. [Google Scholar]
- 15.Schaeffer A J, Chmiel J S, Duncan J L, Falkowski W S. Mannose-sensitive adherence of Escherichia coli to epithelial cells from women with recurrent urinary tract infections. J Urol. 1984;131:906–910. doi: 10.1016/s0022-5347(17)50706-5. [DOI] [PubMed] [Google Scholar]
- 16.Straley S C. Adhesins in Yersinia pestis. Trends Microbiol. 1993;1:285–286. doi: 10.1016/0966-842x(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 17.Svennerholm L. Chromatographic separation of human brain gangliosides. J Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 18.Zav’yalov V P, Abramov V M, Cherepanov P G, Spirina G V, Chernovskaya T V, Vasiliev A M, Zav’yalov G A. pH6 antigen (PsaA protein) of Yersinia pestis, a novel bacterial Fc-receptor. FEMS Immunol Med Microbiol. 1996;14:53–57. doi: 10.1111/j.1574-695X.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]