Abstract
Low levels of triiodothyronine (T3) in the brain lead to increased dopamine receptor sensitivity, potentially resulting in schizophrenia. Iodothyronine deiodinase 2 (DIO2) is the only enzyme which converts tetraiodothyronine (T4) to T3 in the brain. DIO2 polymorphism of rs225014 results in the expression of non-functioning DIO2. Therefore, this study aimed to investigate the association of rs255014 with schizophrenia and its impact on thyroid hormone levels. This study included 150 schizophrenia cases and 150 controls. DNA was extracted from blood and subjected to PCR and amplicon sequencing. Serum thyroid profiles were determined using chemiluminescent magnetic microparticle immunoassay. Statistical analyses involved independent sample t-tests, Chi-square, and Pearson’s correlation tests. The results revealed a higher frequency of the reference genotype (TT) in controls compared to cases (p < 0.05). However, rs225014 did not influence serum thyroid levels or the severity of schizophrenia (p > 0.05). Interestingly, control subjects exhibited significantly higher T3 levels (p < 0.001) than cases. Regardless of the genotype (TT or CC), the control group had higher mean T3 levels than the corresponding case group (p < 0.05). In conclusion, rs225014 is associated with schizophrenia and has no effect on serum thyroid hormone levels.
Keywords: tetraiodothyronine (T4), triiodothyronine (T3), PANSS, genotyping, iodothyronine deiodinase 2, rs225014
1. Introduction
The brain needs an adequate supply of thyroid hormones to facilitate its metabolic and environmental adaptations [1]. An inherent homeostasis mechanism regulates local thyroid hormone levels [2]. Tetraiodothyronine (T4) is a physiologically inactive form of a thyroid hormone which enters the brain through the blood–brain barrier (BBB) and cerebrospinal fluid (CSF) barrier. Inactive T4 in the brain is locally converted to triiodothyronine (T3) through the action of the iodothyronine deiodinase 2 (DIO2) enzyme. Within the brain, thyroid hormones regulate the sensitivity of dopamine receptors and the activity of tyrosine hydroxylase, the rate-limiting enzyme of the catecholaminergic pathway, and myelination and inflammatory processes [3,4]. Apart from this, a hypothyroid state in the brain reduces cognitive abilities [5] and increases dopamine (DP) levels [6], an important etio-pathological factor of schizophrenia [7]. Decreased thyroid hormone levels have also been shown to increase dopamine receptor sensitivity and the severity of schizophrenia [8]. Additionally, dopamine agonists have been shown to decrease thyroid hormone levels [9].
Further, serum thyroid hormone levels are not representative of thyroid hormone levels in the brain [10]. Rodent studies have reported that serum T3 accounts for only 20% of T3 in the cerebral cortex; the rest is made available by the local conversion of T4 to T3 by the DIO2 enzyme, which regulates thyroid hormones under normal physiological and metabolic conditions. Studies have reported three different forms of DIO enzymes (DIO1, DIO2, and DIO3) with different physiological roles in the brain. DIO1 is not expressed in the brain. DIO3 has inhibitory effects: it converts T4 to rT3 and T3 into T2. Hence, in neural cells, DIO2 is the only enzyme available for the deiodination of T4 to T3 [10]. Therefore, normal DIO2 expression is imperative to maintain normal T3 levels in the brain [9]. DIO2 is the primary and only available enzyme in the brain for T4-to-T3 conversion; its deregulation strongly impacts the brain’s levels of T3 but does not influence serum thyroid hormone levels because of the availability of DIO1 as well. It has been reported that serum thyroid hormone levels are not the true representative of brain thyroid hormone levels [3].
A single-nucleotide polymorphism (rs225014) in the DIO2 gene has been found to be associated with impaired psychological well-being [11]. It is present at exon3 of the DIO2 gene and results in the substitution of Thr92Ala. This substitution results in the ubiquitination of the enzyme DIO2, which is then no longer available to perform its normal physiological function, i.e., local conversion of T4 to T3. It has also been reported that DIO2 rs225014 plays an important role in determining the brain’s ability to respond to serum T4 [11,12]. The importance of the DIO2 gene in the regulation of brain thyroid hormone levels became more evident after studies reported that circulating thyroid hormone levels are not affected by rs225014 [11]. However, the DIO2 gene does disrupt normal thyroid levels in the brain [9,13]. Hence, it can be assumed that the detection of DIO2 polymorphism may be helpful in predicting the status of T3 levels in the brain. Consequently, previous studies have reported the association of DIO2 rs225014 with bipolar disorder [14] and impaired psychological well-being [11]. However, to date, no study has examined the association of this polymorphism with schizophrenia and its effect on thyroid hormone levels in schizophrenia patients.
To the best of our knowledge, only one conference abstract is available showing the association of rs225014 (Trh92Ala) polymorphism with schizophrenia in a Turkish population [15]. No study has been conducted to date on DIO2 gene polymorphism and its association with thyroid hormone levels in schizophrenia patients. Further, the abstract does not indicate the association of rs225014 variants with schizophrenia severity, nor does it analyze the impact of polymorphism on thyroid hormone levels. Therefore, the present study aimed to analyze the relationship of DIO2 gene polymorphism with schizophrenia and its impact on the severity of the disease and thyroid hormone levels.
2. Results
2.1. Basic Demographics of Study Participants
Significant differences of age (p < 0.001) were found among the cases and controls (33.86 ± 9.63 and 29.5 ± 9.52). Mean age at disease manifestation was 25.67 ± 8.44 years, showing the onset of schizophrenia at a younger age. Regarding gender distribution among the case group, a significant difference was observed between male and female schizophrenia patients (p < 0.05). In the cohort of 150 schizophrenia patients, 72% were males and 28% were females (Table 1), indicating a difference in the prevalence of schizophrenia between the sexes.
Table 1.
Age and gender distribution among schizophrenia cases and controls.
| Characteristics | Cases | Controls | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 33.86 ± 9.63 | 29.57 ± 9.52 | <0.001 |
| BMI | 22.96 ± 5.75 | 23.20 ± 5.08 | 0.711 |
| Age at Onset (year) | 25.67 ± 8.44 | - | - |
| Gender | N (%) | N (%) | 0.028 |
| Male | 108 (72.0%) | 90 (60.0%) | |
| Female | 42 (28.0%) | 60 (40.0%) |
p < 0.05 considered as statistically significant.
Various ethnic backgrounds were observed, and no significant differences were found between cases and controls (p = 0.344). Family history of a psychiatric disorder was found to be strongly associated with the onset of schizophrenia (p < 0.01). Further, a lower education status was found to be associated with schizophrenia (p < 0.001). Moreover, a higher education status was rarely found in the case group compared to the control group i.e., 26% vs. 74% respectively. A higher ratio of broken marriages was found in schizophrenia patients in this study, showing an association between divorce rate and schizophrenia (p < 0.001) (Table 2).
Table 2.
Basic demographic features of study participants.
| Characteristics | Cases N (%) | Controls N (%) | p-Value |
|---|---|---|---|
| Family history of psychiatric disorder | <0.001 | ||
| Yes | 47 (31.3%) | 15 (10.0%) | |
| No | 103 (68.7%) | 135 (90.0%) | |
| Education | <0.001 | ||
| Nil | 44 (29.3%) | 15 (10.0%) | |
| School | 66 (44.0%) | 24 (16.0%) | |
| College and above | 40 (26.7%) | 111 (74.0%) | |
| Ethnicity | 0.344 | ||
| Sindhi | 47 (31.30%) | 37 (24.7%) | |
| Punjabi | 14 (9.3%) | 21 (14.0%) | |
| Urdu Speaking | 66 (44.0%) | 73 (48.7%) | |
| Others | 23 (15.3%) | 19 (12.7%) | |
| Marital status | <0.001 | ||
| Married | 39 (26.0%) | 52 (34.7%) | |
| unmarried | 88 (58.7%) | 97 (64.7%) | |
| Divorced | 23 (15.3%) | 1 (0.7%) | |
p < 0.05 considered as statistically significant.
2.2. Genotyping
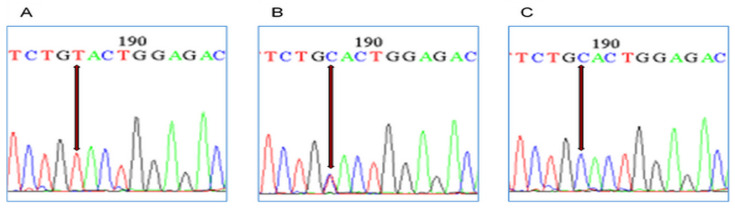
The electropherogram data generated after sequencing revealed all three variants of DIO2, rs225014, i.e., homozygous reference variant (TT), heterozygous (TC), and homozygous mutated genotype (CC). All three variants are shown in (Figure 1).
Figure 1.
Electropherogram of representative regions of DIO2 rs225014. (A) Arrows showing homozygous wild type (TT). (B) Arrow showing heterozygous (TC. (C) Arrow showing homozygous mutated (CC). The coloured peaks represent nucleotide bases of DNA. Blue, red, black and green represent Cytosine (C), Thymine (T), Guanine (G), and Adenine (A), respectively.
Genotype Frequencies of DIO2 (rs225014) Gene Polymorphism in Schizophrenia Patients and Controls
The genotype distribution of rs225014 for the control group was in Hardy-Weinberg equilibrium (HWE) (p-value > 0.05). In contrast, the genotype distribution for the case group deviated from HWE (p-value < 0.05). Interestingly, a significant difference was found between DIO2 (rs225014) gene polymorphisms and schizophrenia patients and controls (p = 0.019), with a higher frequency of the reference variant (TT) and heterozygous (TC) in controls and in cases, respectively (Table 3).
Table 3.
Genotype frequency of DIO2, rs225014 among schizophrenia cases and healthy controls.
| SNP ID | Genotype Frequency (%) | p-Value | p-Value (HWE) | ||
|---|---|---|---|---|---|
| rs225014 | TT | TC | CC | 0.019 | |
| Cases | 27 (18%) | 91 (60.7%) | 32 (21.3%) | 0.008 | |
| Controls | 44 (29.3%) | 68 (45.3%) | 38 (25.3%) | 0.260 | |
p < 0.05 considered as statistically significant. SNP: Single Nucleotide Polymorphism; Confidence Interval = 95%
2.3. Association of PANSS Score with Genotype Frequency
To observe the effect of genetic polymorphism on the severity of psychotic symptoms in schizophrenia patients, the Positive and Negative Syndrome Scale (PANSS) was administered. Mean sub-scale scores for positive, negative, cognitive, and PANSS total scores were calculated and expressed along with standard deviations. A statistical analysis revealed no significant association of PANSS score with any of the genotype variants of DIO2, rs225014 (p > 0.05) (Table 4).
Table 4.
Association of PANSS score with genotype variants of DIO2 rs225014.
| PANSS Score | Positive | Negative | Cognitive | Total | ||||
|---|---|---|---|---|---|---|---|---|
| rs225014 | Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value |
| 0.524 | 0.787 | 0.370 | 0.911 | |||||
| TT | 63.5 ± 17.9 | 62.8 ± 16.7 | 73.9 ± 14.3 | 66.5 ± 15.7 | ||||
| TC | 68.9 ± 17.2 | 61.0 ± 15.5 | 67.8 ± 16.4 | 62.7 ± 17.0 | ||||
| CC | 66.8 ± 11.7 | 64.5 ± 19.1 | 71.0 ± 12.5 | 70.7 ± 14.5 | ||||
p < 0.05 considered as statistically significant.
2.4. Thyroid Hormone Profile of Cases and Controls
Serum thyroid profiling (total T3, T4, and TSH levels) was done for both cases and controls. We found statistically significant differences in T3 levels between cases and controls (p < 0.001), with higher T3 levels in the control group. However, there was no significant difference regarding T4 and TSH levels among cases and controls (p = 0.817 and 0.691), respectively. A subgroup analysis with respect to gender between cases and controls revealed higher levels of T3 in males belonging to the control group (1.70 ± 0.24 vs. 1.50 ± 0.30) (p < 0.001). Moreover, significantly increased T3 levels were also found in females belonging to controls compared to those belonging to cases (1.53 ± 0.30 vs. 1.35 ± 0.37), respectively (p = 0.04) (Table 5).
Table 5.
Mean levels of total thyroid hormones among males and females belonging to schizophrenia cases and controls.
| Thyroid Hormone | Cases (Mean ± SD) | Controls (Mean ± SD) | p-Value |
|---|---|---|---|
| T3 | 1.46 ± 0.33 | 1.63 ± 0.28 | <0.001 |
| T4 | 7.52 + 1.69 | 7.46 ± 1.62 | 0.817 |
| TSH | 2.17 + 6.0 | 1.81 ± 6.02 | 0.691 |
| Male | |||
| T3 | 1.50 ± 0.30 | 1.70 ± 0.24 | <0.001 |
| T4 | 7.66 ± 1.62 | 7.29 ± 1.53 | 0.226 |
| TSH | 1.23 ± 0.66 | 2.10 ± 7.76 | 0.405 |
| Female | |||
| T3 | 1.35 ± 0.37 | 1.53 ± 0.30 | 0.040 |
| T4 | 7.22 ± 1.83 | 7.72 ± 1.74 | 0.284 |
| TSH | 4.27 ± 10.6 | 1.36 ± 0.85 | 0.097 |
p < 0.05 considered as statistically significant.
Mean Levels of Thyroid Hormones with Respect to Gender
Significant differences (p = 0.004) were found with respect to T3 levels in both males and females, with higher T3 levels in males (1.6 ± 0.29) than in females (1.4 ± 0.34). Meanwhile, similar levels of T4 and TSH were observed in both genders. (Table 6).
Table 6.
Mean levels of total thyroid hormones with respect to gender.
| Gender | T3 | T4 | TSH | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | |
| 0.004 | 0.858 | 0.370 | ||||
| Male | 1.6 ± 0.29 | 7.4 ± 1.58 | 1.6 ± 5.52 | |||
| Female | 1.4 ± 0.34 | 7.5 ± 1.78 | 2.5 ± 6.78 | |||
p < 0.05 considered as statistically significant.
2.5. Effect of DIO2, rs225014 on Thyroid Hormone Profile
A subgroup analysis was conducted to detect the effect of the individual variant of DIO2, rs225014, on serum thyroid hormone levels. Mean levels of T3, T4, and TSH were compared between subgroups based on the genotype. A statistically significant difference was found in mean T3 levels between cases and controls for individuals carrying the DIO2, rs225014 TT, and CC genotypes (p = 0.035 and 0.001), respectively, with higher mean T3 levels in controls with the TT and CC genotypes of the SNP. No significant difference in T3 levels was found in individuals with the TC genotype, rs225014, between cases and controls (p = 0.121). Regarding T4 levels, no significant difference was found between individuals carrying the TT, TC, and CC genotypes (p = 0.05, 0.464, and 0.417) between cases and controls. Further, p = 0.53 showed no significant difference among any of the genotype variants for DIO2, rs225014, or mean TSH levels both for cases and controls. Thyroid hormone levels were also compared among cases carrying different genotypes, and similar thyroid levels (p > 0.05) were found among individuals with different genotypes belonging to the case group (Table 7).
Table 7.
Difference in total thyroid hormone profile among genetic variants of DIO2 (rs225014) between cases and controls.
| Genotype | T3 | T4 | TSH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases Mean ± SD |
Controls Mean ± SD |
p-Value | Cases Mean ± SD |
Controls Mean ± SD |
p-Value | Cases Mean ± SD |
Controls Mean ± SD |
p-Value | |
| TT | 1.43 ± 0.33 | 1.61 ± 0.29 | 0.035 | 7.82 ± 1.61 | 6.91 ± 1.70 | 0.05 | 1.78 ± 2.13 | 1.35 ± 0.68 | 0.304 |
| TC | 1.51 ± 0.35 | 1.64 ± 0.31 | 0.121 | 7.33 ± 1.79 | 7.62 ± 1.40 | 0.464 | 1.69 ± 1.16 | 2.79 ± 9.75 | 0.531 |
| CC | 1.41 ± 0.30 | 1.65 ± 0.22 | 0.001 | 7.46 ± 1.65 | 7.84 ± 1.69 | 0.417 | 3.21 ± 10.83 | 1.05 ± 0.63 | 0.290 |
| p-value | 0.433 | - | - | 0.600 | - | - | 0.636 | ||
p < 0.05 is considered as statistically significant.
2.6. Correlation between PANSS Score and T3, T4, and TSH
To estimate the correlation between thyroid profile and severity of schizophrenia, we analyzed Pearson’s correlation between PANSS scores and the thyroid hormone profile (T3, T4, and TSH) of schizophrenia patients. No correlation was found among any of the PANSS subscale scores and serum levels of T3, T4, and TSH (Table 8).
Table 8.
Pearson’s Correlation between PNASS score and thyroid hormone profile of schizophrenia cases.
| Pearson Correlation | T3 | T4 | TSH |
|---|---|---|---|
| PANSS Positive | 0.166 | 0.116 | −0.060 |
| PANSS Negative | 0.040 | 0.179 | 0.032 |
| General Psychopathology | −0.033 | 0.136 | −0.013 |
| PANSS Total | 0.071 | 0.181 | −0.015 |
3. Discussion
This is the first comprehensive study to report an association of DIO2 rs225014 polymorphism with schizophrenia and its effect on serum thyroid hormone levels in schizophrenia patients. Our findings indicate a potential link between schizophrenia and variants of DIO2 rs225014. We found a higher frequency of the reference genotype (TT) in the control group, inferring its protective effect against schizophrenia. Further, we also determined that DIO2 rs225014 does not affect serum T3 levels.
Our analysis of demographic data revealed a higher prevalence of schizophrenia in males than in females among the recruited schizophrenia patients. This finding is consistent with other studies reporting a higher incidence of schizophrenia in males [16,17]. The presence of estrogen in females may be the underlying cause of this protective mechanism, ref. [18] as estrogen modulates the release and binding of dopamine to its receptors [19,20]. Moreover, estrogen therapy has also shown improvement in PANSS score in women suffering from schizophrenia [21]. As far as ethnicity is concerned, Urdu and Sindhi speaking individuals were recruited in larger numbers, probably because our single centered study was carried at IBS, DUHS, Karachi, Pakistan. Being a cosmopolitan city, Karachi’s inhabitants represent all ethnic groups in Pakistan, with the Urdu speaking population being the predominant group, followed by Punjabi, Sindhi, and other ethnic groups [22]. Individuals belonging to the Pathan, Balochi, Memon/Gujarati, Saraiki, and Hindko ethnicities were also recruited in the study, albeit in comparatively smaller numbers, and were therefore grouped into one group.
In relation to the effect of family history on psychiatric disorders, we found more of the participating patients with a positive family history of psychiatric illness compared to controls. This is also confirmed by previous twin studies and a number of other studies reporting a strong genetic association for schizophrenia [23,24].
Most patients in our schizophrenia case group were illiterate or had only received basic education, which may be due to their poor mental health and financial circumstances. This finding is consistent with the findings of Escott et al., who reported a lower level in academic achievement among individuals with schizophrenia [25]. Additionally, the control group in our study comprised peers and hospital staff, which might have contributed to the significant differences in educational level between the two groups.
Compromised cognitive abilities and the inability to meet the requirements of married life lead to increased marital disharmony, causing a higher divorce ratio in persons with mental illnesses such as schizophrenia [26,27,28]. The current study also found that schizophrenia patients had a greater divorce rate than the control group.
We also analyzed the effect of gender differences on thyroid hormone profile and found lower levels of T3 in females (p = 0.004). This observation is supported by studies reporting that a hypothyroid state is more common in females compared to males [29,30,31]. This observation contradicts the findings of other researchers who concluded that levels of T3 and TSH are not influenced by gender [32,33]. Further studies to reveal the impact of gender on thyroid hormone levels are therefore recommended.
Colak et al. also reported a higher frequency of the TT genotype in controls compared to the CC genotype in Turkish schizophrenia patients [15], indicating its protective effect against schizophrenia. However, their study was limited to an abstract, and it did not analyze the effect of DIO2 rs225014 on the severity of the disease or on serum thyroid hormone levels. SNP rs225014 has been studied in other psychiatric disorders before, like bipolar disorder. For example, Bing He et al. reported a higher frequency of the T allele in the control group compared to the bipolar case group [14].
Further, it has been shown that a non-synonymous polymorphism of the DIO2 gene (rs225014, T/C) replaces Thr92Ala at exon 3, resulting in the production of a physiologically inactive form of the DIO2 enzyme, subsequently leading to a hypothyroid state in the brain [11]. At this point, it should be kept in mind that DIO2 is the only enzyme available in the brain for the local conversion of T4 to T3 [10]. Further, this hypothyroid state in the brain affects the severity of psychological well-being [34,35]. Moreover, a strong relationship between DIO2 rs225014 polymorphism (Thr92Ala) and bipolar mood disorder, mental retardation, hypertension, and the risk of osteoarthritis has also been reported by researchers [34]. Further, another study reported that hypothyroid patients with the CC genotype at rs225014 presented with better psychological wellbeing when given T3 therapy compared to T4 therapy. This further supports the rationale that patients with the CC genotype (92Ala) of rs225014 might have a hypothyroid environment in the brain, as T4 might not be converted to T3 because of the nonfunctional DIO2 enzyme. Many studies have shown altered levels of thyroid hormone in schizophrenia patients [36,37,38,39]. However, so far, no study has analyzed the association between DIO2 gene polymorphism rs225014 and its effect on peripheral TH levels in schizophrenia patients.
Thyroid hormones (TH), both T3 and T4, are important for neural development, as well as for the day-to-day activities of the nervous system. DIO enzymes are required to convert T4 to T3. Low levels of thyroid hormone have been found to be associated with increased dopamine levels in schizophrenia patients; this is recognized as an important pathophysiological cause of schizophrenia [8]. Many treatment-resistant schizophrenia patients have shown improvement when given combined therapy of anti-psychotics and thyroid hormone [4,40]. Therefore, levels of thyroid hormones, especially in the brain, may help to determine the severity of the illness.
In the present study, we found similar levels of serum T4 and TSH between schizophrenia cases and controls. However, significantly low levels of serum T3 were found in cases compared to controls. Low levels of T3 in schizophrenia patients were also reported by Polat et al. [31], whereas the opposite result was reported by Singh et al. [41].
To find the effect of variants of DIO2, rs225014, on serum thyroid hormone levels, we conducted a subgroup analysis on serum thyroid hormone profiles (total T3, T4, and TSH) among cases and controls carrying different genotypes (TT, TC, and CC) of DIO2, rs225014. We found significantly low levels of mean T3 in both subgroups of cases carrying either the TT or the CC genotype compared to the respective control subgroups. These findings suggest that lower T3 levels in cases compared to controls are independent of this functional DIO2 polymorphism, as subgroups carrying either the TT or the CC genotype had similar T3 levels, which may be because of the availability of other T4-converting enzymes in the periphery, like DIO1 [34,42]. Moreover, similar levels of T3, T4, and TSH were also reported in schizophrenia patients carrying the TT, TC, or CC genotypes (Table 7). Further, no significant difference was found in T3 levels between cases and controls with the TC genotype; this may have been because of heterozygosity, further confirming that serum T3 levels are independent of DIO2 genotypes. Therefore, it is suggested that peripheral thyroid hormone levels, particularly T3, do not reflect the hypothyroid state of the brain, where only the DIO2 enzyme is available. Thus, detecting functional DIO2 polymorphisms in schizophrenia patients is important. If patients with DIO2 polymorphisms are given thyroid therapy, they should be given T3 rather than T4. Santos et al. also reported that the deregulation of DIO2 activity has no effect on circulating thyroid hormone levels but may affect the spatiotemporal distribution and regulation of thyroid hormone [3]. In our study, we found no significant difference regarding T4 and TSH levels in any of the subgroups of cases or controls.
To evaluate the relationship between the DIO2 rs225014 polymorphism and the severity of schizophrenia, we administered PANSS to patients. A statistical analysis showed no significant association between variants of DIO2 rs225014 (Thr92Ala) and the PANSS score. Replication studies with large sample sizes will be required to validate these findings. To the best of our knowledge, no previous study has discussed the association of this polymorphism with the PANSS score.
We also investigated the correlation of serum thyroid hormone levels (T3, T4, and TSH) with the severity of schizophrenia and did not find any correlation between the levels and PANSS subscale scores. This shows that serum thyroid hormone levels are not related to the severity of schizophrenia and supports our hypothesis that serum thyroid hormone levels do not reflect true schizophrenia pathology and symptoms. As such, the association of brain thyroid hormone with schizophrenia cannot be ruled out. However, it has been reported that the deregulation of DIO2 activity can influence the spatiotemporal distribution and regulation of TH, but it has no impact on circulating thyroid hormone levels [3]. These findings are in line with those of Telo et al., who did not find any correlation between PANSS subscale scores and levels of T3 and T4, although they reported a mild correlation between PANSS (negative symptoms score) and levels of TSH [43]. A few researchers have also reported a correlation between free T3 and improved cognitive function [44,45], suggesting that free T3 may improve cognitive function [44]. Again, these studies represented peripheral thyroid hormone levels, which are not a true representation of brain thyroid levels [9,11,13]. Our study concludes that DIO2 polymorphism rs225014 is associated with schizophrenia but does not influence peripheral thyroid hormone levels.
Additional research focused on brain thyroid hormone levels is necessary to confirm the influence of DIO2 polymorphism. This is crucial, because DIO2 is the sole enzyme in the brain responsible for converting T4 to T3, and approximately 80% of the intracellular T3 in the brain is generated locally through the deiodination of circulating T4 [11].
4. Materials and Methods
4.1. Ethical Approval and Consent to Participate
Our sample size was calculated using openepi.com with 80% power of test and a case-control study design. The calculated sample size was 135 individuals in each group, which was rounded up to 150 individuals per group [46]. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board, Dow University of Health Sciences Karachi, Pakistan. Informed written consent was obtained from patients or guardians or relatives of patients for participation in this study and the publication of clinical details.
4.2. Recruitment of Cases and Controls
A total of 300 participants, 150 cases and 150 controls, were recruited for this study from January 2018 to December 2021. Schizophrenia cases were recruited from the in-patient and outpatient departments of the Dr. Abdul Qadeer Khan Institute of Behavioural Sciences at the Dow University of Health Sciences. Diagnoses of patients with schizophrenia were carried out by a practicing psychiatrist following the guidelines of the Diagnostic and Statistical Manual of Mental Disorders-V (DSM-V) [47]. Patients with any other diagnosed endocrine or neurological disorders or pre-existing thyroid dysfunction were not included in the study. Controls without any diagnosed neurological, endocrinal, or psychological disorders were recruited from peers and hospital staff.
4.3. Demographic Details
Basic demographic details of study participants, like age, gender, ethnicity, education level, marital status, family history of any psychiatric disorder, medical history regarding any other comorbidity, duration of illness, and current medication, were recorded using a predesigned questionnaire.
4.4. Evaluation of Clinical and Psychopathological Symptoms
To assess the severity of illness, positive and negative syndrome scale scores (PANSS) were administered to patients by a psychiatrist, while the Beck Depression Inventory Scale II (BDI II) form was filled out by participating controls to rule out depression in the control group as one of the exclusion criteria.
4.5. Blood Collection and Thyroid Profile
Altogether, 6 mL of blood was drawn from each participant. Of this, 3 mL was collected in EDTA tubes and later used for DNA extraction. Another 3 mL was collected in gel tubes to obtain serum after centrifugation. The serum was separated and stored at −80 °C until used for thyroid profile assays.
Serum samples were sent to DOW Diagnostic Research and Reference Laboratories (DDRRL) where thyroid profile tests (total T3, T4, and TSH) were carried on an Abbott, ARCHITECT i2000SR via chemiluminescent magnetic microparticle immunoassay (Abbott Park, IL, USA).
4.6. DNA Extraction
DNA was extracted from whole blood using a DNA purification kit from Promega (Madison, WI, USA). as per the manufacturer’s protocol. The purity and quantity of DNA were checked using a nanodrop spectrophotometer (Thermofisher Scientific, Waltham, MA, USA). All DNA samples were then stored at −20 °C until further use for PCR.
4.7. Polymerase Chain Reaction (PCR) and Genetic Analysis
To amplify the genomic region encompassing DIO2 (rs225014), the following primers were designed using the Primer 3 software: Forward (5′CCTCATCAATGTAGACCAGCAGGAA3′) and reverse (5′ AATGTGAATTCAAGTGGCAATGTGTTT3′). A reaction mixture with a final volume of 40 µL was prepared for each DNA sample or single PCR reaction. A negative control (containing all reagents except DNA) was also run to confirm that there was no contamination in the reaction mixture. Once the reaction mixture or master mix had been prepared, 38 µL of the mixture was transferred to each PCR tube (200 µL) which already contained 2 µL of DNA sample. The PCR tubes were then subjected to a short spin and transferred to a thermal cycler (Applied Biosystem SimpliAmp Thermal Cycler, Thermofisher Scientific, Waltham, MA, USA) for amplification. PCR was run according to the following conditions: initial denaturation at 95 °C for 5 min, followed by 35 cycles consisting of denaturation at 94 °C for 30 s, annealing at 54.5 °C for 45 s, extension at 72 °C for 45 s, and then final extension at 72 °C for 10 min.
4.8. Sanger Sequencing
After confirming specific amplification through gel electrophoresis, PCR products encompassing DIO2 (rs225014) were sent to Macrogen Inc., Seoul, Republic of Korea, for purification and subsequent Sanger sequencing using ABI PRISM 3.0 Big Dye terminator chemistry.
4.9. Multiple Sequence Alignment of Nucleotides
The nucleotide sequences obtained for all samples were analyzed using MEGA 7.0 software. Sequences were matched with the reference sequence of DIO2 (GenBank accession no: NC_000014.9) from the NCBI nucleotide data bank through the CLUSTAL W tool of the software [48], and allelic variants were identified.
4.10. Statistical Analysis
The data were analyzed using SPSS software version 21. Mean and standard deviation were used for continuous variables between cases and controls, while frequency and percentage were used for all categorical variables. To compare the means of the two groups, we performed an independent sample t-test. The Chi square test was used to compare the frequency (%) of two categorical variables. Pearson’s correlation was used to check the relationship between two continuous variables. A p-value < 0.005 was considered the level of significance.
5. Conclusions
The present study concludes that DIO2, rs225014, is associated with schizophrenia but does not affect serum thyroid hormone levels or the PANSS score. Further studies should be conducted to confirm the effect of DIO2 polymorphism on CSF and brain thyroid hormone levels.
Author Contributions
Conceptualization, F.H.; methodology, Q.A. and W.W.; data curation, Q.A.; writing—original draft preparation, Q.A.; writing—review and editing, F.H.; visualization, F.H.; supervision, F.H.; project administration, F.H.; funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board, Dow University of Health Sciences Karachi (IRB-928/DUHS/Approval/2017/157).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Higher Education Commission Pakistan under National Research Grant Program for Universities (NRPU), grant number 7238/Sindh/NRPU/R and D/HEC/2017 and the APC was supported by Dow University of Health Sciences.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gothié J.-D., Demeneix B., Remaud S. Comparative approaches to understanding thyroid hormone regulation of neurogenesis. Mol. Cell. Endocrinol. 2017;459:104–115. doi: 10.1016/j.mce.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Stohn J.P., Martinez M.E., Hernandez A. Decreased anxiety-and depression-like behaviors and hyperactivity in a type 3 deiodinase-deficient mouse showing brain thyrotoxicosis and peripheral hypothyroidism. Psychoneuroendocrinology. 2016;74:46–56. doi: 10.1016/j.psyneuen.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos N.C., Costa P., Ruano D., Macedo A., Soares M.J., Valente J., Pereira A.T., Azevedo M.H., Palha J.A. Revisiting thyroid hormones in schizophrenia. J. Thyroid Res. 2012;2012:569147. doi: 10.1155/2012/569147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleem S., Arooj M., Basit A., Parveen G., Rasool R., Ahmad S., Waquar S., Qazi M., Ali S.S., Malik A. Inter-Relationship of Thyroid Disorders and Schizophrenia: An Extended Review. Pak. J. Mol. Med. 2015;1:49–62. [Google Scholar]
- 5.Luo M., Zhou X., Zou T., Keyim K., Dong L. Type II deiodinase polymorphisms and serum thyroid hormone levels in patients with mild cognitive impairment. Genet. Mol. Res. 2015;14:5407–5416. doi: 10.4238/2015.May.22.10. [DOI] [PubMed] [Google Scholar]
- 6.Wysokiński A., Kłoszewska I. Level of thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipolar disorder. Neurochem. Res. 2014;39:1245–1253. doi: 10.1007/s11064-014-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howes O., McCutcheon R., Stone J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sykora C., Amor M., Schlenker E. Age and hypothyroidism affect dopamine modulation of breathing and D2 receptor levels. Respir. Physiol. Neurobiol. 2013;185:257–264. doi: 10.1016/j.resp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Pereira J.C., Jr., Pradella-Hallinan M., Pessoa H.D. Imbalance between thyroid hormones and the dopaminergic system might be central to the pathophysiology of restless legs syndrome: A hypothesis. Clinics. 2010;65:547–554. doi: 10.1590/S1807-59322010000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J. Endocrinol. 2017;232:R83–R97. doi: 10.1530/JOE-16-0424. [DOI] [PubMed] [Google Scholar]
- 11.Panicker V., Saravanan P., Vaidya B., Evans J., Hattersley A.T., Frayling T.M., Dayan C.M. Common Variation in the DIO2 Gene Predicts Baseline Psychological Well-Being and Response to Combination Thyroxine Plus Triiodothyronine Therapy in Hypothyroid Patients. J. Clin. Endocrinol. Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 12.Hage M.P., Azar S.T. The link between thyroid function and depression. J. Thyroid Res. 2012;2012:590648. doi: 10.1155/2012/590648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gałecka E., Talarowska M., Orzechowska A., Górski P., Bieńkiewicz M., Szemraj J. Association of the DIO2 gene single nucleotide polymorphisms with recurrent depressive disorder. Acta Biochim. Pol. 2015;62:297–302. doi: 10.18388/abp.2015_1002. [DOI] [PubMed] [Google Scholar]
- 14.He B., Li J., Wang G., Ju W., Lu Y., Shi Y., He L., Zhong N. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:986–990. doi: 10.1016/j.pnpbp.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Colak A., Akan G., Oncu F., Yanbay H., Acar S., Yesilbursa D., Turkcan S., Atalar F. 1508–Association study of the dio2 gene as a susceptibility candidate for schizophrenia in the turkish population; A case-control study. Eur. Psychiatry. 2013;28:1. doi: 10.1016/S0924-9338(13)76526-X. [DOI] [Google Scholar]
- 16.Seeman M.V. Schizophrenia in Women as Compared to Men. Women Psychos. Multidiscip. Perspect. 2019;187 [Google Scholar]
- 17.Ahmad I., Khalily M.T., Hallahan B., Shah I. Factors associated with psychotic relapse in patients with schizophrenia in a Pakistani cohort. Int. J. Ment. Health Nurs. 2017;26:384–390. doi: 10.1111/inm.12260. [DOI] [PubMed] [Google Scholar]
- 18.Seeman M.V. Women who suffer from schizophrenia: Critical issues. World J. Psychiatry. 2018;8:125. doi: 10.5498/wjp.v8.i5.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoest K.E., Cummings J.A., Becker J.B. Oestradiol influences on dopamine release from the nucleus accumbens shell: Sex differences and the role of selective oestradiol receptor subtypes. Br. J. Pharmacol. 2018;176:4136–4148. doi: 10.1111/bph.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoest K.E., Quigley J.A., Becker J.B. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav. 2018;104:119–129. doi: 10.1016/j.yhbeh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lascurain M.B., Camuñas-Palacín A., Thomas N., Breadon C., Gavrilidis E., Hudaib A., Gurvich C., Kulkarni J. Improvement in depression with oestrogen treatment in women with schizophrenia. Arch. Women’s Ment. Health. 2019;23:149–154. doi: 10.1007/s00737-019-00959-3. [DOI] [PubMed] [Google Scholar]
- 22.Khan I., Burke F., Nawaz-ul-Huda S. Spatiotemporal Concentration of Homicides in Karachi. Pak. J. Criminol. 2019;11:68–83. [Google Scholar]
- 23.Howes O.D., Murray R.M. Schizophrenia: An integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilker R., Helenius D., Fagerlund B., Skytthe A., Christensen K., Werge T.M., Nordentoft M., Glenthøj B. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide Danish twin register. Biol. Psychiatry. 2018;83:492–498. doi: 10.1016/j.biopsych.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Escott-Price V., Bracher-Smith M., Menzies G., Walters J., Kirov G., Owen M.J., O’Donovan M.C. Genetic liability to schizophrenia is negatively associated with educational attainment in UK Biobank. Mol. Psychiatry. 2019;25:703–705. doi: 10.1038/s41380-018-0328-6. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P., Sharma N., Ghai S., Grover S. Perception about marriage among caregivers of patients with schizophrenia and bipolar disorder. Indian J. Psychol. Med. 2019;41:440. doi: 10.4103/IJPSYM.IJPSYM_18_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastrup L.H., Simonsen E., Ibsen R., Kjellberg J., Jennum P. Societal Costs of Schizophrenia in Denmark: A Nationwide Matched Controlled Study of Patients and Spouses Before and After Initial Diagnosis. Schizophr. Bull. 2019;46:68–77. doi: 10.1093/schbul/sbz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behere P.B. Effect of marriage on pre-existing psychoses. Indian J. Soc. Psychiatry. 2019;35:10. doi: 10.4103/ijsp.ijsp_9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng Z., Liu M., Zhang Q., Liu L., Song K., Tan J., Jia Q., Zhang G., Wang R., He Y. Gender and age impacts on the association between thyroid function and metabolic syndrome in Chinese. Medicine. 2015;94:e2193. doi: 10.1097/MD.0000000000002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer M., Glenn T., Pilhatsch M., Pfennig A., Whybrow P.C. Gender differences in thyroid system function: Relevance to bipolar disorder and its treatment. Bipolar Disord. 2014;16:58–71. doi: 10.1111/bdi.12150. [DOI] [PubMed] [Google Scholar]
- 31.Polat I.E., Kuzay D., Konar N.M. Analysing Biochemical Parameters and Developing Risk Prediction Models in Patients with Schizophrenia and Bipolar Disorder. Electron. J. Med. Educ. Technol. 2021;14:em2112. [Google Scholar]
- 32.Muslim S., Khalil Z. Effect of Age, Sex, Salt, Water and Climate on T3, T4 and TSH in Healthy Individuals. J. Ayub. Med. Coll. 2000;21 [Google Scholar]
- 33.Abdel R. Developments in Radioimmunoassay and Related Procedures. IAEA; Vienna, Austria: 1992. Effect of age and sex on thyroid function tests. Establishment of norms for the Egyptian population. [Google Scholar]
- 34.Verloop H., Dekkers O.M., Peeters R.P., Schoones J.W., Smit J.W. Genetics in endocrinology: Genetic variation in deiodinases: A systematic review of potential clinical effects in humans. Eur. J. Endocrinol. 2014;171:R123–R135. doi: 10.1530/EJE-14-0302. [DOI] [PubMed] [Google Scholar]
- 35.Schneider M.J., Fiering S.N., Pallud S.E., Parlow A.F., St. Germain D.L., Galton V.A. Targeted disruption of the type 2 selenodeiodinase gene (DIO 2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 36.Shrivastava A., Jadhav V., Karia S., Shah N., De Sousa A. Serum thyroid stimulating hormone levels and suicidal tendency in patients with first-episode schizophrenia: An exploratory study. Thyroid Res. Pract. 2016;13:63. [Google Scholar]
- 37.Baumgartner A., Pietzcker A., Gaebel W. The hypothalamic–pituitary–thyroid axis in patients with schizophrenia. Schizophr. Res. 2000;44:233–243. doi: 10.1016/S0920-9964(99)00187-5. [DOI] [PubMed] [Google Scholar]
- 38.Othman S.S., Kadir K.A., Hassan J., Hong G.K., Singh B.B., Raman N. High prevalence of thyroid function test abnormalities in chronic schizophrenia. Aust. N. Z. J. Psychiatry. 1994;28:620–624. doi: 10.3109/00048679409080785. [DOI] [PubMed] [Google Scholar]
- 39.Kalinowska S., Trześniowska-Drukała B., Safranow K., Pełka-Wysiecka J., Kłoda K., Misiak B., Samochowiec J. Association between thyroid function and metabolic syndrome in male and female schizophrenia patients. Psychiatry Res. 2019;274:167–175. doi: 10.1016/j.psychres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Steibliene V., Bunevicius A., Savickas A., Prange Jr A.J., Nemeroff C.B., Bunevicius R. Triiodothyronine accelerates and enhances the antipsychotic effect of risperidone in acute schizophrenia. J. Psychiatr. Res. 2016;73:9–16. doi: 10.1016/j.jpsychires.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Singh S.G., Debbarma S., Singh N.H., Singh T.B., Lenin R., Devi K.S. A comparative study of Thyroid Hormone levels among the Normal Healthy Persons, Depression and Schizophrenia. EJP. 2010;13:10–18. doi: 10.5005/EJP-13-1--2-25. [DOI] [Google Scholar]
- 42.Van Der Deure W.M., Hansen P.S., Peeters R.P., Uitterlinden A.G., Fenger M., Kyvik K.O., Hegedüs L., Visser T.J. The effect of genetic variation in the type 1 deiodinase gene on the interindividual variation in serum thyroid hormone levels: An investigation in healthy Danish twins. Clin. Endocrinol. 2009;70:954–960. doi: 10.1111/j.1365-2265.2008.03420.x. [DOI] [PubMed] [Google Scholar]
- 43.Telo S., Bilgic S., Karabulut N. Thyroid hormone levels in chronic schizophrenic patients: Association with psychopathology. West Indian Med. J. 2016;65:312–315. doi: 10.7727/wimj.2015.186. [DOI] [PubMed] [Google Scholar]
- 44.Yazici K., Yazici A.E., Taneli B. Different neuroendocrine profiles of remitted and nonremitted schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2002;26:579–584. doi: 10.1016/S0278-5846(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 45.Ichioka S., Terao T., Hoaki N., Matsushita T., Hoaki T. Triiodothyronine may be possibly associated with better cognitive function and less extrapyramidal symptoms in chronic schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;39:170–174. doi: 10.1016/j.pnpbp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Asadi M., Sadeghi A., Rezvanfar M.R., Talaie A., Rafiei F. The genetics study of gestational diabetes in Iranian women and DIO2 gene. Mol. Med. J. 2016;2:43–47. [Google Scholar]
- 47.Tandon R. The nosology of schizophrenia: Toward DSM-5 and ICD-11. Psychiatr. Clin. 2012;35:557–569. doi: 10.1016/j.psc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.