Abstract
Construction of a stable Shigella sonnei vaccine has been complicated by the instability of the virulence phenotype caused by the spontaneous loss of the invasion plasmid. To select a suitable candidate for vaccine construction, 16 S. sonnei strains were screened for stability of the virulence phenotype. A stable strain, S. sonnei Mosely, was selected for further work. pΔvirG2, a deletion derivative of the virG gene in the sacB suicide vector pCVD442, was used to generate an S. sonnei virG deletion strain, WRSS1, which was invasive in HeLa cells but negative in the Sereny test. WRSS1 was found to be both immunogenic and protective in the guinea pig keratoconjunctivitis model.
During the recent Desert Storm operation, shigellosis was responsible for 26.2% of the diarrheal disease in U.S. military personnel, with Shigella sonnei being responsible for about 80% of the shigellosis cases. Of the troops affected by shigellosis, 60% were unable to perform their assigned duties (18). Civilian and military travelers to countries of shigella endemicity are also at risk for infection (6, 18). The development of a vaccine directed against S. sonnei is thus an important goal to ensure protection of troops deployed in areas where shigellosis is endemic. S. sonnei is also a major cause of illness in developed countries, particularly in institutional settings, such as day care centers and prisons, and in military field settings (5). The increasing occurrence of resistance and multiple-drug resistance among clinical Shigella isolates to commonly used antimicrobial agents such as ampicillin, tetracycline, trimethroprim-sulfamethoxazole, and nalidixic acid is particularly prevalent in developing countries and provides an additional impetus for the development of vaccines directed against Shigella species (3, 7, 21, 29, 34). Further emphasizing the need for vaccines against bacterial infection, the development of a successful Haemophilus influenzae vaccine has significantly decreased concern about antibiotic resistance in areas where the vaccine is being widely used (26).
Previous studies have shown that killed whole-cell vaccines administered parenterally or orally did not confer protective immunity in humans or in monkeys (12, 17, 37). Since natural infections confer serotype-specific protection in approximately 75% of cases of reinfection (10, 13), it was thought that live attenuated vaccines for oral administration would effectively produce protective immunity against shigellosis. However, noninvasive live vaccines that have been tested required multiple high doses and frequent boosting immunizations for efficacy and provided varying rates of protection, making them too impractical for continued use (24, 25). Therefore, current attempts to construct vaccines to be administered orally have centered on designing live vaccines which are attenuated by specific deletions that retain the invasiveness of the bacteria but affect intra- and intercellular dissemination. Genes affecting biochemical pathways, such as aroA, aroD, or thyA (1, 22, 39), or specific virulence genes, such as virG (icsA), whose expression is required for intra- and intercellular spread of shigellae (4, 20), have been targets for deletions in live attenuated Shigella vaccines. Shigella strains with virG deletions do not produce a positive Sereny reaction in guinea pigs or plaques in tissue culture monolayers, since both assays are indicators of invasion followed by intracellular multiplication and intra- and intercellular spread (28, 33). Escherichia coli-Shigella flexneri 2a hybrid vaccine strains with a deletion in the virG locus were attenuated and protective in the guinea pig keratoconjunctivitis model (2). CVD1203, a double mutant for aroA and virG, elicited a moderate immune response at a dose that produced only mild constitutional symptoms (14, 27). An S. flexneri 2a vaccine, SC602, a double mutant for virG and iuc (which eliminates the production of aerobactin and impairs growth in tissues) is immunogenic in humans at nonreactogenic doses and shows promising efficacy against challenge with wild-type S. flexneri 2a (8, 9, 14, 31, 32). Thus, the construction of an S. sonnei strain with a virG deletion seemed a logical choice for a suitable vaccine candidate.
Construction of a stable S. sonnei vaccine strain has been complicated by the instability of the virulence phenotype caused by spontaneous loss of the large invasion plasmid. It has been noted in previous studies that S. sonnei strains lose the form I invasion plasmid at a frequency ranging from 1 to 2% to about 50% (30), which is greater than that seen with other Shigella serotypes. Since the genes encoding the O antigen are located on the invasion plasmid in S. sonnei, in contrast to the chromosomal locations of the O antigen in other Shigella serotypes, loss of this plasmid in S. sonnei results in form II colonies, which are avirulent and rough. To select a suitable candidate for vaccine construction, 16 S. sonnei strains from the Walter Reed Army Institute of Research collection were tested for stability of virulence expression. A single form I colony from each strain was grown overnight in Luria-Bertani broth at 37°C, and appropriate dilutions were plated on tryptic soy agar plates the following day. After overnight incubation at 37°C, colonies were counted and scored for the percentage of form I and form II colonies present. At least two trials were done for most strains. Stable strains were defined as strains with greater than 85% form I colonies after overnight growth. With this criterion, 7 of the 16 strains were designated stable (Table 1). The percentage of form I colonies for individual strains ranged from 87 to 98% for the stable strains and from 52 to 80% for the unstable strains. Stable strains retain >85% stability even after several passages.
TABLE 1.
Stability of virulent S. sonnei strains
| Strain | Form I (%)a | Stabilityb |
|---|---|---|
| Mosely | 87.2 ± 4.0 | S |
| Rudy | 89.6 ± 9.2 | S |
| VTi66 | 93.1 ± 2.8 | S |
| 53G | 61.7 ± 17.5 | U |
| 13-40 (82) | 78.0 | U |
| 212 | 74.8 | U |
| 5002-13 | 79.8 | U |
| MBI | 61.9 ± 13.7 | U |
| Patty | 71.2 | U |
| W107 | 73.1 ± 10.6 | U |
| LB | 97.9 ± 1.4 | S |
| 1041 | 87.5 | S |
| 482-79 | 87.6 ± 3.7 | S |
| EJ | 98.6 ± 0.7 | S |
| B120 | 55.5 ± 13.8 | U |
| 3175 | 51.7 ± 9.7 | U |
The method used to calculate form I percentage is described in the text. Standard deviations of the mean are given when more than one experiment was done per strain.
Stable strains (S), strains with >85% form I colonies after overnight incubation; unstable strains (U), strains with <85% form I colonies after overnight incubation.
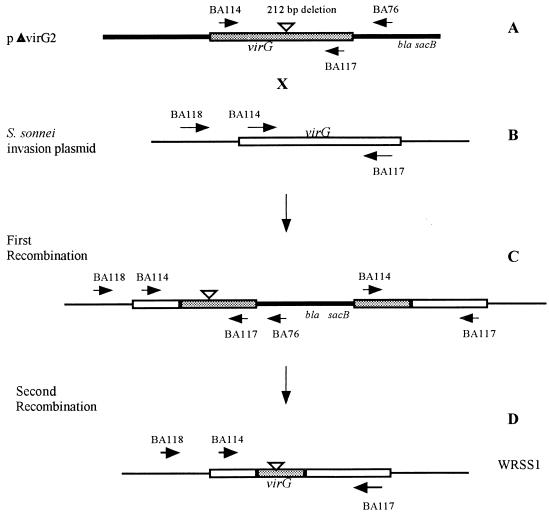
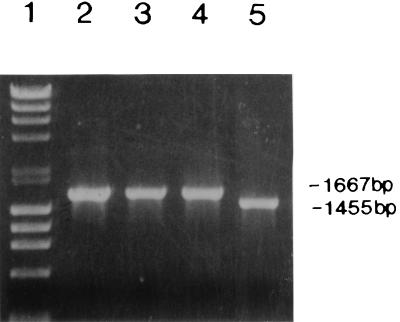
All stable strains were tested for antibiotic sensitivity, since strains sensitive to kanamycin or ampicillin could not be used with the suicide vector system and strains resistant to antibiotic(s) are not suitable for vaccine strains. S. sonnei Mosely, which was fully virulent in the Sereny test (Table 2) and was resistant only to tetracycline, was selected as the recipient strain in a filter mating experiment with donor strain SM10λpir(pΔvirG2). This strain was chosen over other stable strains because of its tetracycline resistance, which can be removed with fusaric acid (23). pΔvirG2 contains a 212-bp deletion within the virG structural gene cloned into the suicide vector pCVD442 as previously described (Fig. 1A and references 2 and 9). The deleted virG gene in pΔvirG2 contains stop codons in all three reading frames to prevent any expression beyond the deletion point. A schematic representation of the steps used in the construction of WRSS1, a virG deletion mutant of the S. sonnei Mosely strain, is shown in Fig. 1. A total of two crossover events, one on either side of the deletion, would result in the replacement of the wild-type virG allele on the invasion plasmid with the deleted version of pΔvirG2. Plasmid pΔvirG2 (Fig. 1A) was introduced from the donor strain SM10λpir(pΔvirG2) into the recipient strain S. sonnei Mosely (Fig. 1B) by an overnight filter mating and selection for ampicillin and tetracycline resistance as previously described (2). Individual colonies were selected and screened by PCR analysis. The first recombination event was monitored by the use of primers BA118 and BA76. These primers produce a 1.8-kb PCR product from the tetracycline-resistant, ampicillin-resistant strains, indicating that the insertion of the pΔvirG2 plasmid occurred in the virG gene (Fig. 1C). Neither the donor strain [SM10λpir(pΔvirG2)] nor the recipient strain (S. sonnei Mosely) contains both of these primer sequences, and thus these strains do not produce a PCR product (Fig. 1A and B). Primers BA114 and BA117 were also used to confirm the integration, since these primers yield two PCR products when integration has occurred, a 1,667-bp product corresponding to the wild-type gene and a 1,455-bp band corresponding to the 212-bp deleted gene (Fig. 1C and 2). A tetracycline-resistant, ampicillin-resistant strain with an insertion of pΔvirG2 into the virG gene was then put through sucrose selection as previously described (2). The presence of the sacB gene in pCVD442 inhibits growth on 5% sucrose. Therefore, growth on sucrose is used as a positive selection for the loss of vector sequences. Sucrose-resistant, tetracycline-resistant, ampicillin-sensitive colonies were tested for loss of suicide vector sequences, and the presence of the deleted virG gene was tested by PCR analysis as previously described (2). Figure 2 shows the result of PCR analysis of the second recombination event (Fig. 1D) with primers BA114 and BA117 and confirms that the S. sonnei recombinant strain contained a deleted virG gene. No product was obtained with primers BA118 and BA76, indicating the loss of vector sequences. The S. sonnei strain containing the deleted virG gene was plated on fusaric acid plates (21), and tetracycline-sensitive colonies were selected. PCR analysis as described above confirmed that the tetracycline-sensitive strain contained the deleted virG gene (Fig. 2). This strain was designated WRSS1.
TABLE 2.
Sereny test of vaccine strain WRSS1 and of WRSS1 complemented with wild-type virG gene
| Strain | Genotype | No. of eyes inoculated | No. of eyes with indicated ratinga:
|
|||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| S. sonnei Mosely | Wild type | 4 | 0 | 0 | 0 | 4 |
| WRSS1 | ΔvirG | 8 | 8 | 0 | 0 | 0 |
| WRSS1(pHS3188)b | virG+ | 4 | 0 | 0 | 1 | 3 |
| WRSS1(pHS3192)b | virG+ | 4 | 0 | 0 | 4 | 0 |
The rating system is described in the text.
pHS3188 contains a 7.6-kb EcoRI fragment, with the entire wild-type virG gene ligated into pBR322 (4). pHS3192 contains a 6.1-kb EcoRI-SalI fragment, with the entire virG gene from pHS3188 ligated into pBR322.
FIG. 1.
Construction of strain WRSS1. (A) Plasmid pΔvirG contains a virG gene with a 212-bp deletion cloned into pCVD442. The deleted version is shaded to distinguish it from the wild-type gene. Primer BA76 is located in the oriR6K portion of pCVD442. (B) The virG gene located on the invasion plasmid of S. sonnei Mosely with the positions of the primers used to monitor recombinants. BA118 is located in the 5′ noncoding region of virG. (C) The product of the first recombination event. Primers BA118 and BA76 were used to monitor insertion of the pΔvirG plasmid into virG. (D) The product of the second recombination event generating WRSS1. Sequences homologous to primers BA114 and BA117 are present in panels A, B, C, and D. Sequences homologous to primer BA118 are present only in panels B, C, and D. Sequences homologous to primers BA76 are present only in panels A and C.
FIG. 2.
PCR analysis of the virG gene in wild-type Shigella and vaccine strain WRSS1. PCR analysis of the size of virG was carried out with primers BA114 and BA117. Products were run on an ethidium-bromide-stained 0.8% agarose gel. Lane 1, molecular size markers; lane 2, S. flexneri 2a strain 2457T; lane 3, S. flexneri 5a strain M90T-W; lane 4, S. sonnei Mosely; lane 5, WRSS1. The sizes of the wild-type virG gene and the deleted gene found in WRSS1 are shown at the right.
Individual colonies of WRSS1 were first tested on colony immunoblots with monoclonal antibodies to IpaB and IpaC virulence proteins as previously described (38). All form I colonies of WRSS1 were found to express both proteins and agglutinated with S. sonnei antiserum. WRSS1 was invasive, as indicated by the HeLa cell invasion assay (11). WRSS1 consistently had >95% form I colonies after overnight growth, indicating that it had retained the stable phenotype of the parent strain. Unlike the parent strain, WRSS1 did not react with VirG-specific antiserum on Western blots (2, 38) and did not form plaques on epithelial cells in tissue culture (28), indicating the absence of intra- and intercellular spread, which is dependent on the expression of VirG.
The guinea pig keratoconjunctivitis test (Sereny reaction [33]) was used to test for attenuation of WRSS1 compared to that of the virulent parent strain. Four guinea pigs were inoculated with 4 × 108 CFU of the attenuated WRSS1 strain, and two guinea pigs were inoculated with 4 × 108 of the parent wild-type S. sonnei Mosely strain, and development of disease was monitored for 5 days. The following rating scheme for development of disease was used: 0, no disease or mild irritation; 1, mild conjunctivitis; 2, keratoconjunctivitis with no purulence; 3, fully developed keratoconjunctivitis with purulence (15, 16). Guinea pigs infected ocularly with 4 × 108 CFU of the wild-type strain developed fully developed keratoconjunctivitis in both eyes within 2 to 3 days. In contrast, animals receiving WRSS1 showed no signs of irritation or disease over the observation period (a rating of 0 compared to a rating of 3 with the wild-type strain) (Table 2). In efficacy tests with larger groups of animals, to be discussed below, inoculation with WRSS1 resulted in the complete absence of irritation or disease (Table 3). WRSS1 complemented with either pHS3188 or pHS3192, which contain the entire wild-type virG gene (4), restored the Sereny reaction, indicating that the virG deletion in WRSS1 is responsible for the attenuation of its virulence (Table 2).
TABLE 3.
Protective efficacy of WRSS1 measured with the guinea pig keratoconjunctivitis model
| Immunizing straina | No. of eyes with indicated ratingb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Postimmunizationc
|
Postchallenged
|
|||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Expt 1 | ||||||||
| WRSS1 | 16 | 0 | 0 | 0 | 11 | 5 | 0 | 0 |
| None (nonimmunized) | NAe | NA | NA | NA | 0 | 0 | 0 | 16 |
| Expt 2 | ||||||||
| WRSS1 | 16 | 0 | 0 | 0 | 10 | 4 | 2 | 0 |
| None (nonimmunized) | NA | NA | NA | NA | 0 | 0 | 1 | 15 |
In each experiment, 16 eyes were inoculated with WRSS1 and 16 were left uninoculated.
Ratings are described in the text.
Ratings after the first and second immunizations were identical.
Animals were challenged 4 weeks after the second immunization with virulent strain S. sonnei 53G.
NA, not applicable.
The protective efficacy and immunogenicity of WRSS1 were measured with the guinea pig keratoconjunctivitis model (15, 16). Ocular immunization with 3 × 108 to 4 × 108 CFU of WRSS1/eye on days 0 and 14 was followed by challenge with virulent S. sonnei. The immunizing inoculum was obtained from overnight growth plates (experiment 1) as previously described (2, 15, 16) or from rehydration of lyophilized product manufactured at the Walter Reed Army Institute of Research pilot vaccine production facility with Good Manufacturing Procedures (GMP) (experiment 2). Four weeks after the last immunization, both the immunized animals and the unimmunized control animals were challenged with 4 × 108 CFU of virulent S. sonnei 53G/eye. Animals were rated over a 5-day period as to time of development and severity of disease (Table 3) with the rating scheme described above. Percentage of protection was defined as follows: full, percentage of eyes with rating of 0; partial, percentage of eyes with rating of 1; combined, percentage of eyes with rating of 0 or 1 (sum of full and partial percentage). Results of both experiments are shown in Table 3. In animals immunized with WRSS1 grown from overnight plate cultures, 13 of 16 eyes showed no signs of disease (83% complete protection), while 3 eyes showed mild conjunctivitis (17% partial protection). When reconstituted lyophilized cultures were used, 10 of 16 eyes did not develop disease (63% complete protection), while 4 eyes developed mild disease (25% partial protection). In both cases, protection against challenge was significant by the Fisher exact test (P < 0.001), and there was no significant difference in the levels of protection conferred by the two formulations.
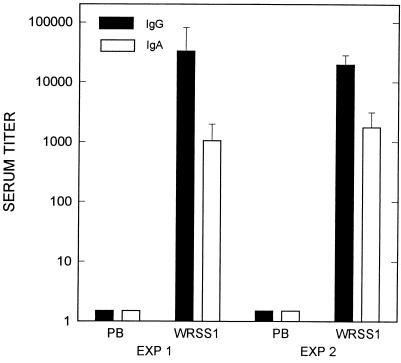
Immunogenicity of the vaccine was measured by determining levels of serum immunoglobulin G (IgG) and IgA antibodies specific for the S. sonnei O antigen. Two weeks after the last immunization, animals were bled and the serum antibody response against Plesiomonas shigelloides lipopolysaccharide (LPS), whose O antigen is immunologically identical to that of S. sonnei (35), was determined. Polyvinyl chloride microtiter plates were coated with 50 μl of P. shigelloides LPS at a concentration of 10 μg/ml in carbonate buffer (pH 9.6), and serum titers against P. shigelloides O antigen were measured by enzyme-linked immunosorbent assay as described previously (2, 16). Optical density was read at 405 and 570 nm. Endpoint titers were defined as the last dilution having an optical density at least 0.1 above that of the background well. Titers of prebleed sera used to obtain background titer values were <50 for both IgG and IgA. The geometric mean titers of experiments 1 and 2, shown in Fig. 3, demonstrate that both formulations of WRSS1 are immunogenic and produce comparable serum IgG and IgA titers against the O antigen.
FIG. 3.
Serum IgG and IgA titers against the S. sonnei O antigen 2 weeks after the boosting immunization of guinea pigs with WRSS1. The geometric mean titers from experiments 1 and 2 are shown. Background titers were determined from preimmunization bleeds (PB) and were <50 for both IgG and IgA. Standard errors of the mean are shown. Exp, experiment.
In volunteer studies, EcSf2a-2, an aroD deletion E. coli-S. flexneri 2a hybrid vaccine strain, was too reactogenic at doses required for a good immune response (19, 36). A virG deletion derivative of this strain has been tested in guinea pigs and found to be immunogenic and protective, although higher doses were required (2). Since the guinea pig keratoconjunctivitis test measures both development of disease and inflammation, a major feature of shigellosis in humans, the degree of inflammation in the eye evoked by different Shigella strains may be an indication of possible reactogenicity in humans. Other virG deletion strains of S. flexneri 2a have produced some reactogenicity in guinea pig eyes; this was reflected in the human safety trials. CVD1203, containing deletions in aroA and virG, produced mild conjunctivitis in 4 of 16 animals and moderate conjunctivitis in 1 of 16 animals within 24 h, although symptoms disappeared by 48 h (27). This strain was reactogenic in humans at doses of 108 and 109 but was tolerated at 106, although some symptoms of malaise and headache occurred in 4 of 10 volunteers. The 106 dose produced moderate LPS-specific IgA antibody-secreting cells (13). Ocular immunization of guinea pigs with SC602, containing deletions in virG and iuc, resulted in mild irritation that cleared up within 24 h (14). In recent human trials of the S. flexneri 2a vaccine SC602, doses of 106 CFU or greater resulted in some reactogenicity (8, 14). However, a single dose of 104 CFU was well tolerated, immunogenic, and protective (8, 14). In contrast to SC602 and CVD1203, the same dose of WRSS1 produced no reaction at all in guinea pigs, indicating that WRSS1 may be safer in humans at doses that were reactogenic for either CVD1203 or SC602.
Tests have shown that WRSS1 is as stable as the parent strain, and this stability was maintained in the lot of WRSS1 produced under GMP conditions. The GMP product was also safe, immunogenic, and protective in guinea pigs. Phase 1 safety trials followed by phase II efficacy trials in North American volunteers are being planned for the GMP product. Data from these trials, along with the results from the SC602 phase I and phase II trials, will indicate whether virG deletion mutants of the most prevalent Shigella serotypes may be used to formulate multivalent Shigella vaccines.
Acknowledgments
We thank E. V. Oaks for testing WRSS1 in the plaque assay and for running the Western blot assay with VirG-specific antisera. We also thank T. L. Hale for his support in this project.
REFERENCES
- 1.Ahmed Z U, Sarker M R, Sack D A. Protection of rabbits and monkeys from lethal shigellosis by oral immunization with a thymine-requiring and temperature-sensitive mutant of Shigella flexneri Y. Vaccine. 1990;8:153–158. doi: 10.1016/0264-410x(90)90139-d. [DOI] [PubMed] [Google Scholar]
- 2.Alexander W A, Hartman A B, Oaks E V, Venkatesan M M. Construction and characterization of virG (icsA)-deleted Escherichia coli K12-Shigella flexneri hybrid vaccine strains. Vaccine. 1996;14:1053–1061. doi: 10.1016/0264-410x(96)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi S, May-Zahav M, Sulkes J, Zilberberg R, Samra Z. Increasing antimicrobial resistance of Shigella isolates in Israel during the period 1984–1992. Antimicrob Agents Chemother. 1995;39:819–823. doi: 10.1128/aac.39.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardini M L, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri, that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black R E, Craun G F, Blake P A. Epidemiology of common-source outbreaks of shigellosis in the United States, 1961–1975. Am J Epidemiol. 1978;108:47–52. [PubMed] [Google Scholar]
- 6.Black R E. Epidemiology of travelers’ diarrhoea and relative importance of various pathogens. Rev Infect Dis. 1990;12:S73–S79. doi: 10.1093/clinids/12.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y-W, Houang E T S, Lyon D J, Ling J M, Ng T-K, Cheng A F B. Antimicrobial resistance in Shigella flexneri and Shigella sonnei in Hong Kong, 1986 to 1995. Antimicrob Agents Chemother. 1998;42:440–443. doi: 10.1128/aac.42.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coster T S, Sansonetti P J, Cohen D, Hale T L, Van De Verg L L, Hartman A B, Oaks E V, Venkatesan M M, Hoge C W. Abstracts of 1997 Cold Spring Harbor Laboratory Meeting on Microbial Pathogenesis and Host Response. N.Y: Cold Spring Harbor; 1997. Clinical trials of Shigella flexneri 2a candidate vaccine SC602p. 27. [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont H L, Hornick R B, Snyder M J, Libonati J P, Formal S B, Gangarosa E J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125:12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 12.Formal S B, Maenza R M, Austin S, LaBrec E H. Failure of parental vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol Med. 1967;25:347–353. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- 13.Hale T L. Shigella vaccines. In: Ala’Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 179–204. [Google Scholar]
- 14.Hale T L, Coster T S, Trofa A, Van De Verg L L, Oaks E V, Hartman A B, Venkatesan M M, Sansonetti P J. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Human safety and immunogenicity studies of SC602, an icsA-iuc deletion mutant of Shigella flexneri 2a, abstr. E-34; p. 272. [Google Scholar]
- 15.Hartman A B, Powell C P, Schultz C L, Oaks E V, Eckels K H. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59:4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman A B, Van De Verg L L, Collins H H, Jr, Tang D B, Bendiuk N O, Taylor D N, Powell C P. Local immune response and protection in the guinea pig keratoconjunctivitis model following immunizations with Shigella vaccines. Infect Immun. 1994;62:412–420. doi: 10.1128/iai.62.2.412-420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins A R, Floyd T M, Kader M A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am J Trop Med Hyg. 1955;4:281–288. [PubMed] [Google Scholar]
- 18.Hyams K C, Bourgeois A L, Merrell B R, Rozmajzi R, Escamilla J, Thornton S A, Wasserman G M, Burke A, Echeverria P, Green K Y, Kapikian A Z, Woody J N. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 19.Kotloff K L, Herrington D A, Hale T L, Newland J W, Van De Verg L, Cogan J P, Snoy P J, Sadoff J C, Formal S B, Levine M M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigens. Infect Immun. 1992;60:2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lett M-C, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989;171:353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima A A M, Lima M, Pinho M C N, Barros E A, Jr, Teixeira M J, Martins M C V, Guerrant R L. High frequency of strains multiply resistant to ampicillin, trimethoprim-sulfamethoxazole, streptomycin, chloramphenicol, and tetracycline isolated from patients with shigellosis in northeastern Brazil during the period 1988 to 1993. Antimicrob Agents Chemother. 1995;39:256–259. doi: 10.1128/aac.39.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindberg A A, Karnell A, Pal T, Sweiha H, Hultenby K, Stocker B A D. Construction of an auxotrophic Shigella flexneri strain for use as a live vaccine. Microb Pathog. 1990;8:433–440. doi: 10.1016/0882-4010(90)90030-t. [DOI] [PubMed] [Google Scholar]
- 23.Maloy S R, Nunn W D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981;145:1110–1112. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meitert T, Pencu E, Ciudin L, Tonciu M. Vaccine strain Sh. flexneri T32-Istrati. Studies in animals and volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen. Arch Roum Pathol Exp Microbiol. 1984;43:251–278. [PubMed] [Google Scholar]
- 25.Mel D M, Arsic B L, Nikolic B D, Radovanovic M L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull W H O. 1968;39:375–380. [PMC free article] [PubMed] [Google Scholar]
- 26.Murray B A. Can antibiotic resistance be controlled? N Engl J Med. 1994;330:1229–1230. doi: 10.1056/NEJM199404283301710. [DOI] [PubMed] [Google Scholar]
- 27.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sack, R. B., M. Rahman, M. Yunus, and E. H. Khan. 1997. Antimicrobial resistance in organisms causing disease. Clin. Infect. Dis. 24(Suppl. 1):S102–S105. [DOI] [PubMed]
- 30.Sansonetti P J, Kopecko D J, Formal S B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981;34:75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sansonetti P J, Aroundel J. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine. 1989;7:443–450. doi: 10.1016/0264-410x(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti P J, Arondel J, Fontaine A, d’Hauteville H, Bernardini M L. ompB (osmo-regulation) and icsA (cell-to-cell spread) mutants of S. flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–421. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 33.Sereny, B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol. Acad. Sci. Hung. 4:367–376. [PubMed]
- 34.Shears P. Shigella infections. Ann Trop Med Parasitol. 1996;90:105–114. doi: 10.1080/00034983.1996.11813034. [DOI] [PubMed] [Google Scholar]
- 35.Shimada T, Sakazaki R. On the serology of Plesiomonas shigelloides. Jpn J Med Sci Biol. 1978;31:135–142. doi: 10.7883/yoken1952.31.135. [DOI] [PubMed] [Google Scholar]
- 36.Taylor D N, Philip D F, Zapor M, Trofa A, Van De Verg L, Hartman A, Bendiuk N, Newland J W, Formal S B, Sadoff J C, Hale T L. Outpatient studies of the safety and immunogenicity of an auxotrophic Escherichia coli K-12-Shigella flexneri 2a hybrid vaccine candidate, EcSf2a-2. Vaccine. 1994;12:565–568. doi: 10.1016/0264-410x(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 37.Thomson D, Thomson R, Morrison J T. Oral vaccines and immunization by other unusual routes. Baltimore, Md: The Williams & Wilkins Co.; 1948. Bacillary dysentery (oral vaccines) pp. 84–92. [Google Scholar]
- 38.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. Infect Immun. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma N K, Lindberg A A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991;9:6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]