Abstract
Bacterial capsular polysaccharides (CP) are carbohydrate polymers comprised of repeating saccharide units. Several of these CP have side chains attached to their backbone structures. The side chains may include O-acetyl, phosphate, sialic acid, and other moieties. Those moieties represent the immunodominant epitopes and the most functional ones. The clinically significant Staphylococcus aureus type 5 CP (CP 5) and type 8 CP (CP 8) are comprised of a trisaccharide repeat unit with one O-acetyl group attached to each repeat unit. The immunogenicity of these CP and the functionality of antibodies to the backbone and the O-acetyl moieties were investigated. Immunization with the native CP conjugates (CP with 75% O-acetylation) elicited a high proportion of antibodies directed against the O-acetyl moiety. Nonetheless, all of the vaccinees produced antibodies to the backbone moieties as well. Conjugate vaccines made of de-O-acetylated CP elicited backbone antibodies only. Antibodies to both backbone and O-acetyl groups were found to be opsonic against S. aureus strains which varied in their O-acetyl content. Absorption studies with O-acetylated and de-O-acetylated CP showed that (i) native CP conjugates generated antibodies to both backbone and O-acetyl groups and (ii) O-acetylated isolates were opsonized by both populations of antibodies while the non-O-acetylated strains were predominantly opsonized by the backbone antibodies. These results suggest that S. aureus CP conjugate vaccines elicit multiple populations of antibodies with diverse specificities. Moreover, the antibodies of different specificities (backbone or O-acetyl) are all functional and efficient against the variations in bacterial CP that may occur among clinically significant S. aureus pathogenic isolates.
Staphylococcus aureus is a major cause of nosocomial infections (24, 30). Clinical isolates of S. aureus, like other invasive gram-positive pathogens, have been shown to possess capsular polysaccharides (CP) (16, 17). Of the more than 11 CP types, types 5 and 8 comprise the majority of clinical isolates (2, 11, 31). These two CP types were isolated, and their chemical structures were elucidated (10, 12, 22). The repeat units of both types were found to be comprised of three monosaccharides: 2-acetamido-2-deoxy-d-mannuronic acid, 2-acetamido-2-deoxy-l-fucose, and 2-acetamido-2-deoxy-d-fucose (12, 16, 22). Both polysaccharides are O acetylated at the uronic acid moiety (22). They differ in the glycosidic linkages and the site of O acetylation. Nonetheless, these two CP are totally distinct, with no detectable immunological cross-reactivity (33).
To make these CP immunogenic, conjugate vaccines were prepared (28, 29). S. aureus CP 5 and CP 8 were covalently coupled to a nontoxic recombinant Pseudomonas aeruginosa exoprotein A (rEPA). Conjugates were evaluated in animals and humans for their safety and immunogenicity (6, 8). Polyclonal antibodies generated by these conjugate vaccines in humans as well as in animals were found to mediate type-specific opsonophagocytic killing of the appropriate S. aureus types (6, 18). Antibodies to these CP, either administered by passive immunization or elicited by vaccination, were shown to protect mice against lethal challenge by S. aureus. Moreover, these antibodies were shown to protect mice against bacteremia and organ infection in a sublethal-challenge mouse model (9). Vaccine-induced antibodies were also effective in protecting rats against bacteremia and organ infections in a model of endocarditis in rats (21).
Since the S. aureus CP conjugate vaccine currently in use in clinical studies is comprised of highly O-acetylated CP, it is important to explore the usefulness of the different CP antibody populations elicited by this vaccine. In this study we investigated the immunological determinants of types 5 and 8 S. aureus CP and the interaction of CP-specific antibodies with other immunological determinants on the CP. The role of O-acetyl groups in eliciting protective immunity was also investigated.
MATERIALS AND METHODS
Bacterial strains.
Strain Lowenstein (type 5) and strain Wright (type 8) were used for the preparation of the CP and the conjugate vaccines as previously described (7). The following isolates were used in the in vitro opsonophagocytosis assay: type 5 strain Reynolds, a prototype strain from the collection of W. W. Karakawa, isolated from a blood culture of a patient at Kaiser Permanente Hospital, North Hollywood, California; strain JL232, a mutant derived from S. aureus strain Reynolds and received from J. C. Lee, Channing Labs, which lost its ability to O acetylate its CP and produced CP lacking the O-acetyl groups; and type 4 strain 7007, a bacteremic strain received from the W. W. Karakawa collection. In the original serotyping scheme, this isolate produced CP that gave a line of partial identity with CP 5 (17). We had purified CP from this isolate and compared it to CP 5 in sugar analysis, nuclear magnetic resonance (NMR), and chemical assays. Our unpublished data showed identical NMR shifts, identical sugar composition, and identical serological reactions. The only difference that we were able to find was the degree of acetylation (20 to 25%) of this CP compared to that of prototype 5 CP (60 to 75%). Therefore, we assumed that this strain was a variant of type 5.
Vaccines and antisera.
Human and rabbit sera were generated by immunizing animals or humans with type 5 or type 8 CP conjugated to rEPA (CP 5-rEPA and CP 8-rEPA) as previously described (6, 7). Monospecific sera for backbone type 5 CP were generated in rabbits immunized with conjugate vaccines made of de-O-acetylated type 5 CP conjugated to rEPA (CP 5-OH-rEPA) as previously described (7). Immunoglobulin G (IgG) for opsonophagocytosis was purified by using protein G gel (Pharmacia Biotech AB, Uppsala, Sweden). IgG preparations were absorbed by adding equal volumes of the appropriate CP solution at increasing concentrations and incubating for 1 h at 37°C and then overnight at 2 to 8°C. The precipitate from the absorbed IgG was removed by centrifugation at 1,500 × g for 10 min.
De-O-acetylation of CP.
The O-acetyl groups were hydrolyzed by treating type 5 or type 8 CP with 0.1 N NaOH for 4 h at 37°C. The reaction mixture was neutralized by the addition of 1 N HCl. The removal of the O-acetyl groups to generate de-O-acetylated CP (CP 5-OH and CP 8-OH) was confirmed by direct measurement of O-acetyl groups in the colorimetric assay (see below) and in some instances by running NMR spectra for the treated CP.
O-acetyl determination.
The O-acetyl content of the polysaccharides was determined by the Hestrin colorimetric method (14) with acetylcholine used as a standard. O acetylation was expressed as percent repeat units with an O-acetyl group attached.
Binding of carboxyl groups to ONPH.
The reactivity of the carboxylic groups of O-acetylated and de-O-acetylated S. aureus CP with 2-nitro-phenylhydrazine (ONPH) was measured as described by Murata et al. (23). Briefly, increasing concentrations of the tested CP were prepared and mixed with ONPH (10 mM in 0.15 M HCl). After the pH was adjusted to 4.8, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide was added to the reaction mixture to achieve a 10 mM final concentration. The absorbance at 530 nm was measured in a spectrophotometer. A glucuronic acid solution was used as a positive control.
Determination of antibodies to the backbone in sera from humans immunized with the S. aureus conjugate vaccine.
Sera obtained 6 weeks postimmunization from healthy human subjects immunized (6) with CP 5-rEPA or CP 8-rEPA S. aureus conjugate vaccines were absorbed with increasing amounts of CP 5 or CP 5-OH. These sera were subsequently tested in an anti-CP 5 enzyme-linked immunosorbent assay (ELISA) as follows. Each serum was diluted to a concentration that would result in an optical density at 450 nm of ∼2.0 in ELISA. The diluted serum was absorbed with increasing amounts of either CP 5 or CP 5-OH and tested in the CP 5 IgG ELISA. The absorbed samples were diluted down the plate and, by parallel line analysis, quantified for percent absorption by comparison to an unabsorbed reference (100% of IgG antibody detected). The maximum percent absorption with CP 5 was determined. This represents absorption of both O-acetyl-specific and backbone-specific antibodies. The serum was also absorbed with the same concentration of CP 5-OH. The percent absorption by the same amount of CP 5-OH is theorized to represent that component of the CP 5 antibodies that are backbone specific. For example, if CP 5 absorbs 100% of the CP 5 antibodies at a certain concentration and CP 5-OH absorbs 30% of the CP 5 antibodies at the same concentration, we may theorize that 30% of the CP 5 antibodies are backbone specific and the remaining 70% are O-acetyl specific.
Opsonophagocytosis assay.
The opsonophagocytosis assays for the different strains of S. aureus were performed by using a modified version of the assay previously described (18, 26). Briefly, in a 200-μl reaction mixture, 105 CFU of the appropriate S. aureus strain were mixed with 5 × 105 HL60 cells in RPMI 1640 medium containing 10% fetal calf serum and complement from a human source. After the purified IgG preparation was added, a sample was drawn for plating on tryptic soy agar (TSA) plates and the reaction mixture was incubated at 37°C. Another sample was drawn at 60 min and plated on TSA plates for bacterial counting. The survival of the bacteria was expressed as CFU at 60 min compared to CFU at time zero. Each experiment included two controls: the one for complement included complement, phagocytes, and bacteria, and the other included bacteria, complement, phagocytes, and normal rabbit IgG.
RESULTS
Availability of the carboxyl groups for reaction with ONPH.
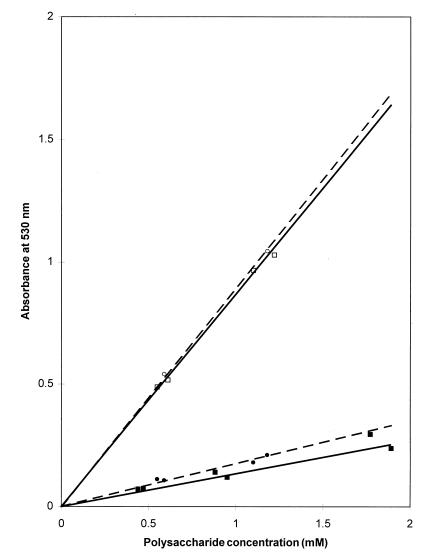
The results obtained from the ONPH reaction with acetylated and de-O-acetylated type 5 and type 8 CP are shown in Fig. 1. The number of carboxylic groups available for derivatization on O-acetylated type 5 or type 8 CP was found to be linearly proportional to the concentration of the CP in the reaction mixture. The degrees of O acetylation of the CP used in these experiments, measured by the colorimetric method, were similar (70 to 75%). Removal of the O-acetyl groups by treating the CP with NaOH resulted in an increased availability of reactive carboxylic groups. The increases in ONPH binding were 6.5- and 5.1-fold for CP 5 and CP 8, respectively. The binding of ONPH to the de-O-acetylated CP was also linearly proportional to the CP concentration. CP 5 and CP 8 behaved similarly in the de-O-acetylation process, and the amounts of reactive carboxyls per given CP concentration were almost identical for type 5 and type 8 CP.
FIG. 1.
Reactivity of native O-acetylated (solid symbols) and de-O-acetylated (open symbols) S. aureus CP 5 (squares) and CP 8 (circles) with ONPH as a function of CP concentration. CPs were activated with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide in the presence of ONPH, and the absorption at 530 nm was measured.
Backbone and O-acetyl antibodies elicited by S. aureus type 5 conjugate vaccine in adult human volunteers.
Sera from adult human volunteers immunized 6 weeks earlier with the CP5-rEPA conjugate were evaluated by inhibition ELISA to determine the percentage of antibodies generated by the vaccine to the backbone and the O-acetyl moieties. Data presented in Table 1 show that, even though all vaccinees received identical vaccines, their immune responses were varied. The immune response was mainly to the O-acetyl moiety in the majority of the vaccinees (6 of 10 [60%]). Four of the vaccinees responded mainly to backbone epitopes (4 of 10). The data also show that while three of the volunteers responded with <10% of their antibodies to the backbone, all volunteers responded with ≥30% of their antibodies to the O-acetyl moiety. The distribution of antibodies between the backbone and the O-acetyl moiety was not related to the initial level of antibodies achieved by vaccination. Among O-acetyl high responders, a wide range of total levels of CP-specific antibody was achieved—90 to 942 μg of specific IgG per ml. Similar data were obtained with sera from vaccinees receiving CP 8-rEPA vaccines (data not shown).
TABLE 1.
Distribution of antibodies elicited by S. aureus CP 5 conjugate vaccine in adult volunteers
| Volunteer | Concn (μg/ml) of IgG to CP 5 in neat serum | % of IgG specific for:
|
|
|---|---|---|---|
| O-acetyl group | Backbone | ||
| 1 | 942 | 89 | 11 |
| 2 | 827 | 100 | 0 |
| 3 | 707 | 35 | 65 |
| 4 | 665 | 41 | 59 |
| 5 | 246 | 61 | 39 |
| 6 | 391 | 40 | 60 |
| 7 | 275 | 94 | 6 |
| 8 | 281 | 30 | 70 |
| 9 | 120 | 100 | 0 |
| 10 | 90 | 86 | 14 |
Opsonophagocytosis of S. aureus isolates of different degrees of O acetylation.
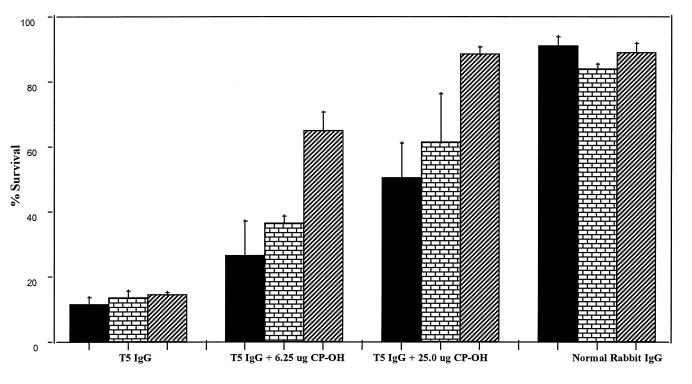
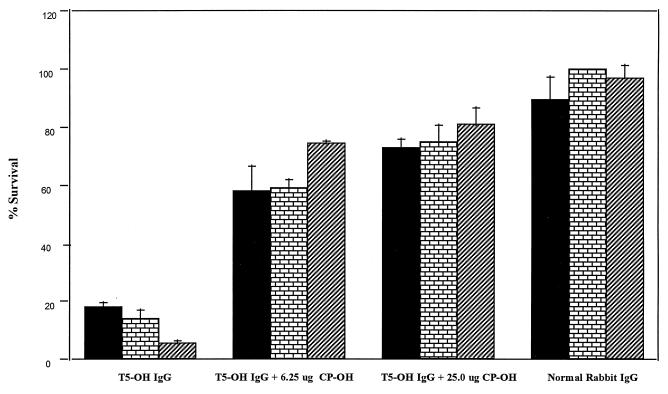
Rabbit antibodies generated by native CP 5 conjugate were efficient in killing all strains of S. aureus used, including the non-O-acetylated strain, JL232 (Fig. 2). When CP 5-OH was used to absorb the antibodies to native vaccine, the opsonophagocytic killing of JL232 was dramatically reduced (from 85 to 35%) with as little as 6.25 μg of CP 5-OH and brought to background levels (<10%) with the addition of 25 μg of the same CP. The efficiency of CP 5-OH in absorbing out the opsonic activity against strain Reynolds or strain 7007 was limited even at a concentration of 25 μg/ml. As shown in Fig. 3, antibodies to the de-O-acetylated vaccine killed the three isolates at an efficiency comparable to that of antibodies to the native vaccine. When de-O-acetylated CP was used for absorption of antibodies to de-O-acetylated vaccine, the opsonic activity against all three strains was absorbed out equally with all concentrations of CP 5-OH.
FIG. 2.
Opsonophagocytosis of three S. aureus strains varying in their degree of O acetylation by unabsorbed or CP 5-OH-absorbed IgG generated in rabbits against CP 5-rEPA conjugate vaccines. The test mixture contained, in 200 μl, 105 CFU of the appropriate S. aureus strain (strain Reynolds [solid], strain 7007 [bricks], or strain JL232 [stripes]) and 5 × 105 HL60 cells in 10% fetal calf serum in RPMI 1640 medium. The appropriate sera were added and the numbers of organisms were counted by direct plating on TSA plates at 0 and 60 min. Results are expressed as percent CFU at 60 min compared to time zero value (means plus standard deviations).
FIG. 3.
Opsonophagocytosis of three S. aureus strains varying in their degree of O-acetylation by unabsorbed or CP 5-OH-absorbed IgG generated in rabbits against CP 5-OH-rEPA conjugate vaccines. Experiments, symbols, expression of data, and strains are as described for Fig. 2.
DISCUSSION
Bacterial polysaccharides are composed of saccharide repeat units each of which is either a mono- or a multisaccharide. In many cases, bacterial species may have capsules which are structurally varied and which allow the classification of bacteria into different serotypes. For example, Streptococcus pneumoniae has >80 serotypes, Escherichia coli has >100 K antigens, and Klebsiella pneumoniae has >26 K antigens (15). The antigen specificity of these polysaccharides is dependent on immunodeterminants present on their structures and the accessibility of these immunodeterminants to the immune system. Bacterial polysaccharides may contain an array of acid- or base-sensitive side groups, including O-acetyl (22, 25), phosphate (27), and sialic acid (34) residues. These substituent side chains may constitute an important part of the immunodominant epitopes of their respective polysaccharides.
The role of O-acetylation in the immunogenicity and stability of these polysaccharides and in the pathogenicity of other microorganisms was previously evaluated (1, 13, 25). The O-acetyl groups play important and different roles in the immunology and pathogenesis of different organisms. While O-acetyl-positive Neisseria meningitidis group C CP was less immunogenic than its O-acetyl-negative variant, an opposite phenomenon was observed with E. coli K1 capsules (13, 25); i.e., O-acetyl-positive K1 CP was more immunogenic than its O-acetyl-negative variant. Furthermore, N. meningitidis group C with O-acetyl-positive capsules was more virulent than its O-acetyl-negative variant. It was also found that asymptomatic carriers of this organism have high titers of antibody to the O-acetyl-negative variant, which might explain the fact that >85% of N. meningitidis group C clinical isolates are O-acetyl positive (1, 13).
O-acetyl groups may be important to the survival of the microorganism as well. E. coli K1 O-acetyl-negative capsules are highly sensitive to depolymerization by the enzyme neuraminidase, whereas the O-acetyl-positive capsules are highly resistant (5). These shifts between the different variants may contribute to the survival of the O-acetyl-positive strains in the intestine and the O-acetyl-negative strains in the bloodstream (5). In all of these cases, antibodies generated against O-acetyl-positive or -negative forms were protective against both forms of organisms (25). Conversely, it was previously reported that only antibodies specific to the O-acetyl moiety of Salmonella paratyphi A lipopolysaccharide were bactericidal in an in vitro assay. The de-O-acetylated lipopolysaccharide conjugate vaccine was immunogenic in animals, but those antibodies failed to exhibit bactericidal activity in that assay (19).
In nature, 85 to 90% of all type 5 or type 8 clinical S. aureus isolates possess a highly O-acetylated CP. The degree of CP O acetylation on S. aureus clinical isolates varies but remains at relatively high levels (>60%) (unpublished data). Evaluation of the accessibility of the backbone as indicated by reactivity of the carboxylic groups with ONPH revealed that the availability of these groups for chemical reactions was limited on highly acetylated (71%) CP 5 and CP 8. For both CP types, removal of the O-acetyl groups was expected to result in a 2.3-fold increase in the number of carboxyl groups accessible for derivatization. Our data show an increase of 6.5-fold for CP 5 and 5.09-fold for CP 8. These data indicate that the O-acetyl groups on the native CP sterically hindered the availability of carboxyl groups, and thus the backbone portion of the native CP, for chemical reactions. This steric hindrance was similar to that observed with the Salmonella typhi Vi antigen (4). A Courtauld-Koltun space-filling atomic model of the S. typhi Vi antigen showed that the backbone components, i.e., the carboxyl groups, were deeply buried inside the polysaccharide structure while the O-acetyl groups on the surface of that structure were readily available to interact with antibodies and other chemical agents (32). Since S. typhi Vi backbone immunodeterminants are not accessible to the immune system, the immune response is directed almost totally toward the O-acetyl moieties. This blocking effect of O-acetyl groups on the backbone, rendering it inaccessible for interaction with the immune system, has also been shown with other microbial CP such as N. meningitidis group C and E. coli K1 (13, 25). It was also suggested that the sialic acid present on group B streptococcus (GBS) type III CP plays a role similar to that of the main immunodeterminant for that CP. Immunization with type III CP conjugate vaccines generated antibodies to GBS type III with no cross-reactivity to S. pneumoniae type 14 even though the latter CP is identical to that of GBS type III except that it lacks the sialic acid portion (20).
Adult human vaccinees immunized with highly O-acetylated type 5 or type 8 S. aureus CP conjugate vaccines produced two major populations of antibodies: one specific to the O-acetyl and the other to backbone immunodeterminants. Although the immune response to each specificity varied among the different vaccinated individuals, the O-acetyl determinant appeared to be the dominant immunodeterminant among those tested. This trend was not related to the levels of S. aureus CP-specific IgG achieved in these individuals following vaccination with the conjugate vaccine. The immune response levels and the dominant immunodeterminant seem to be determined by the immune system of the individual vaccinee, presumably due to differences in the processing and presentation of the antigen.
The role of O acetylation in the virulence of S. aureus type 5 was previously explored. S. aureus mutants with de-O-acetylated capsules were less virulent in a mouse challenge model and more sensitive to opsonophagocytosis (3). In this study, we evaluated antibodies to the CP backbone and to the native S. aureus CP in the opsonophagocytosis assay. Our data suggest that native CP 5-rEPA vaccine induced antibodies which were opsonic against different strains with varying degrees of O acetylation. In absorption studies, de-O-acetylated CP was partially effective in removing the opsonic antibodies against the native type 5 and the partially acetylated strain 7007. However, the opsonic activity against the de-O-acetylated strain was totally removed by absorption with the de-O-acetylated CP. These data indicate that backbone as well as O-acetyl antibodies participate in opsonophagocytic killing of acetylated strains while only the backbone-specific antibodies are efficient in killing O-acetyl-negative strains. This conclusion was supported by the data generated by using only antibodies specific to the backbone. These antibodies were efficient in opsonophagocytic killing of highly acetylated, moderately acetylated, and nonacetylated strains, indicating that backbone epitopes are accessible on all three strains. Moreover, de-O-acetylated CP was equally efficient in removing the opsonic activity against the three strains from the backbone-specific IgG. Our data suggest that (i) the highly O-acetylated CP in S. aureus conjugate vaccines contains stretches of non-O-acetylated backbone that are capable of eliciting backbone-specific antibodies and (ii) backbone portions of the highly O-acetylated S. aureus strains are available for binding backbone-specific antibodies that consequently mediate the opsonophagocytic killing of these organisms. We are currently evaluating monoclonal antibodies specific to these antigenic determinants in in vitro opsonophagocytosis functional assays and in vivo animal protection models.
ACKNOWLEDGMENTS
We thank Jean Lee from Channing Labs for providing the JL232 strain, Yun Hee Cho for making the de-O-acetylated conjugates, Judit Milstein for performing the ONPH experiments, and Betto Ortiz for drawing the figures.
REFERENCES
- 1.Arakere G, Frasch C E. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun. 1991;59:4349–4356. doi: 10.1128/iai.59.12.4349-4356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeit R, Karakawa W W, Vann W F, Robbins J B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin N, Albus A, Michon F, Livolsi P J, Park J-S, Lee J C. Identification of a gene essential for O-acetylation of Staphylococcus aureus type 5 capsular polysaccharides. Mol Microbiol. 1998;27:9–21. doi: 10.1046/j.1365-2958.1998.00646.x. [DOI] [PubMed] [Google Scholar]
- 4.Bystricky S, Szu S C. O-acetylation affects the binding properties of the carboxyl group of Vi bacterial polysaccharide. Biophys Chem. 1994;51:1–7. doi: 10.1016/0301-4622(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 5.Dickson J J, Messer M. Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to sialic acid content of milk. Biochem J. 1978;170:407–412. doi: 10.1042/bj1700407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattom A, Schneerson R, Watson D C, Karakawa W W, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla D A, Robbins J B. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993;61:1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fattom A, Schneerson R, Szu S C, Vann W F, Shiloach J, Karakawa W W, Robins J B. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990;58:2367–2374. doi: 10.1128/iai.58.7.2367-2374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattom A, Shiloach J, Bryla D, D, Fitzgerald D, Pastan I, Karakawa W W, Robbins J B, Schneerson R. Comparative immunogenicity of conjugates composed of the Staphylococcus aureus type 8 capsular polysaccharide bound to carrier proteins by adipic acid dihydrazide or N-succinimidyl-3-(2-pyridyldithio)propionate. Infect Immun. 1992;60:584–589. doi: 10.1128/iai.60.2.584-589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier J-M, Vann W F, Karakawa W W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984;45:87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier J M, Bouvet A, Boutonnier A, Audurier A, Goldstein F, Pierre J, Bure A, Lebrun L, Hochkeppel H K. Predominance of capsular polysaccharide type 5 among oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1987;25:1932–1933. doi: 10.1128/jcm.25.10.1932-1933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier J-M, Hannon K, Moreau M, Karakawa W W, Vann W F. Isolation of type 5 capsular polysaccharide from Staphylococcus aureus. Ann Inst Pasteur/Microbiol. 1987;138:561–567. doi: 10.1016/0769-2609(87)90041-x. [DOI] [PubMed] [Google Scholar]
- 13.Glode M P, Lewin E B, Sutton A, Le C T, Gotschlich E C, Robbins J B. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J Infect Dis. 1979;139:52–59. doi: 10.1093/infdis/139.1.52. [DOI] [PubMed] [Google Scholar]
- 14.Herstrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem. 1949;180:249–261. [PubMed] [Google Scholar]
- 15.Jennings H J, Pon P A. Polysaccharides and glycoconjugates as human vaccines. In: Dumitriu S, editor. Polysaccharides in medical applications. New York, N.Y: Marcel Dekker, Inc.; 1997. p. 443. [Google Scholar]
- 16.Karakawa W W, Vann W F. Capsular polysaccharides of Staphylococcus aureus. In: Weinstein L, Fields B N, editors. Seminars in infectious disease. IV. Bacterial vaccines. New York, N.Y: Thieme-Stratton; 1982. p. 285. [Google Scholar]
- 17.Karakawa W W, Fournier J-M, Vann W F, Arbeit R, Schneerson R S, Robbins J B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985;22:445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konadu E, Shiloach J, Bryla D A, Robbins J B, Szu S C. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid, with emphasis on the role of O acetyls. Infect Immun. 1996;64:2709–2715. doi: 10.1128/iai.64.7.2709-2715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagergard T, Shiloach J, Robbins J B, Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990;58:687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J C, Park J-S, Shepherd S E, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau M, Richards J C, Fournier J-M, Byrd R A, Karakawa W W, Vann W F. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 1990;201:285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- 23.Murata Y, Kawashima S, Miyamoto E, Masauji N, Honda A. Colorimetric determination of alginates and fragments thereof as the 2-nitrophenylhydrazides. Carbohydr Res. 1990;208:289–292. [Google Scholar]
- 24.Musher D M, McKenzie S O. Infections due to S. aureus. Medicine. 1977;56:383–409. doi: 10.1097/00005792-197709000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Orskov F, Orskov I, Sutton A, Schneerson R, Lin W, Egan W, Hoff G E, Robbins J B. Form variation in E. coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson P K, Wilkinson B J, Kim Y, Schmeling D, Quie P G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978;19:943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards J C, Perry M B. Structure of specific capsular polysaccharide of Streptococcus pneumoniae type 23F (American type 23) Biochem Cell Biol. 1988;66:758–771. doi: 10.1139/o88-087. [DOI] [PubMed] [Google Scholar]
- 28.Robbins J B, Schneerson R. Polysaccharide-protein conjugates. A new generation of vaccines. J Infect Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 29.Robbins J B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978;15:839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- 30.Sheagren J N. Staphylococcus aureus: the persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- 31.Sompolinsky D, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szu S C, Li X-R, Stone A L, Robbins J B. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991;59:4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vann W F, Moreau M, Sutton A, Byrd R A, Karakawa W W. Structure and immunochemistry of Staphylococcus aureus capsular polysaccharides. In: Horowitz M, editor. Bacteria-host cell interaction. New York, N.Y: Alan R. Liss, Inc.; 1988. p. 187. [Google Scholar]
- 34.Wessels M R, Pozsgay V, Kasper D L, Jennings H J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of the type III group B Streptococcus. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]