Abstract
Antiphospholipid antibody syndrome (APS) is an autoimmune disorder characterised by thrombosis and the presence of antiphospholipid antibodies (aPL): lupus anticoagulant and/or IgG/IgM anti-β2-glycoprotein I and anticardiolipin antibodies. APS carries significant morbidity for a relatively young patient population from recurrent thrombosis in any vascular bed (arterial, venous, or microvascular), often despite current standard of care, which is anticoagulation with vitamin K antagonists (VKA). Platelets have established roles in thrombosis at any site, and platelet hyperreactivity is clearly demonstrated in the pathophysiology of APS. Together with excess thrombin generation, platelet activation and aggregation are the common end result of all the pathophysiological pathways leading to thrombosis in APS. However, antiplatelet therapies play little role in APS, reserved as a possible option of low dose aspirin in addition to VKA in arterial or refractory thrombosis. This review outlines the current evidence and mechanisms for excessive platelet activation in APS, how it plays a central role in APS-related thrombosis, what evidence for antiplatelets is available in clinical outcomes studies, and potential future avenues to define how to target platelet hyperreactivity better with minimal impact on haemostasis.
Keywords: antiphospholipid syndrome, antiphospholipid antibodies, thrombosis, platelets, antiplatelets
1. Introduction
Thrombotic antiphospholipid syndrome (APS) is a complex immune-mediated thrombotic disorder, classified by laboratory features: persistent antiphospholipid antibodies (aPL), namely lupus anticoagulant (LA) and/or IgG/IgM anti-β2-glycoprotein I (aβ2GPI) and IgG/IgM anticardiolipin antibodies (aCL), together with thrombosis [1], with thrombocytopenia recently included again as a clinical criterion [2]. APS confers significant morbidity on a relatively young population, with a mean age of 45 years at diagnosis [3]. It has an incidence of 2 to 5 per 100,000 persons/year and prevalence of approximately 50 per 100,000 [4]. In patients under 50 years of age, persistent aPL are present in 17% of patients with ischaemic stroke and approximately 10% of those with unprovoked venous thromboembolism (VTE) [5,6]. A small proportion of APS patients can develop catastrophic thrombotic crises termed as ‘catastrophic APS’ (CAPS), which carries a mortality rate of 30% despite current standard of care, triple therapy with anticoagulation, corticosteroids and plasma exchange/intravenous immunoglobulin [7]. The current standard of care for APS is primarily anticoagulation with vitamin K antagonists (VKA), with consideration for addition of an antiplatelet agent for arterial thrombosis [8,9]. High rates of recurrent thrombosis remain despite optimal anticoagulation, with one meta-analysis finding 5.4% recurrence within two years after venous thrombosis, and concerningly, 22% following arterial thrombosis [10]. Improving these poor outcomes in APS patients represents a significant area of need and including appropriate antiplatelet therapy may address this, as suggested by a recent meta-analysis of arterial thrombosis in APS [11].
Many pathophysiological processes have been shown to act in concert to cause APS-related thrombosis, including activation and recruitment of multiple cell types—endothelial cells, platelets, leukocytes—as well as the coagulation and complement systems (Figure 1). This complex interplay manifests in heterogeneous thrombosis, which can occur in any vascular bed; venous, arterial, or microvascular. While the concept of targeting the many pathophysiological processes is overwhelming and would potentially have substantial adverse effects, clinical focus has mainly been on targeting coagulation factors. However, platelets have been shown to have a significant role in thromboses within any vascular bed, raising the consideration that platelets could have a central role in APS [12,13]. Thromboses within arteries are known to be mainly driven by platelets, typically initiated by adhesion to pathologically exposed subendothelial collagen, followed by platelet activation and aggregation [14]. Venous thrombosis has traditionally been considered to be fibrin and red cell rich, with intact endothelium and a lesser role for platelets. However, more recently, in vitro models have demonstrated venous thrombus extension driven by a procoagulant subpopulation of platelets in a glycoprotein (GP)VI-dependent manner [15]. This procoagulant platelet subpopulation forms after strong stimulation, most potently with thrombin and collagen, which causes sustained raised cytosolic calcium, leading to translocation of phosphatidylserine (PS) from the inner to the outer platelet membrane, providing a negatively charged surface for coagulation factor complexes to form and generate a burst of thrombin to drive fibrin formation [16]. This platelet subpopulation is more implicated in thrombosis than haemostasis, making it an attractive target for novel therapeutics. Microvascular thrombosis is characterised by fibrin and platelet-rich thrombi, with a critical role for the procoagulant properties of platelets, and their interaction with neutrophils, now apparent [17].
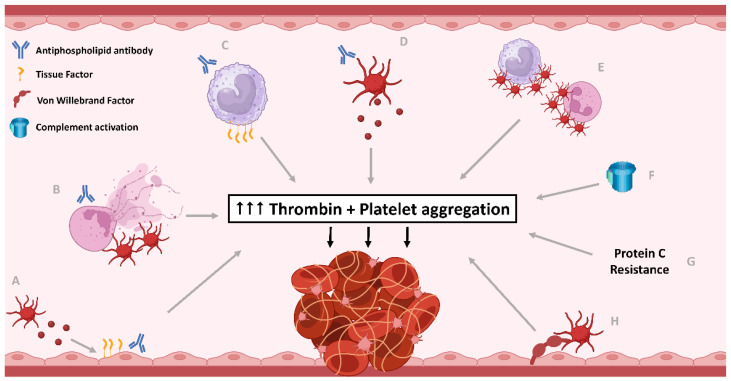
Figure 1.
The central role of excess thrombin generation and platelet activation in pathophysiological mechanisms contributing to thrombosis in antiphospholipid syndrome (APS). Antiphospholipid antibodies (aPL) have impact on many different cells and pathways leading to thrombosis. (A) Endothelial cells are directly impacted by aPL causing increased tissue factor (TF) expression, as well as indirectly with aPL-related platelet microparticle-induced endothelial pyroptosis, resulting in thrombin generation. (B) aPL induce neutrophils to release extracellular traps (NETs), as well as reduce NETs clearance and activate platelets which also lead to NET formation, which in turn activates platelets. Neutrophils also expose phosphatidylserine (PS) to increase thrombin generation. (C) aPL lead to increased tissue factor expression by monocytes, causing increased thrombin generation. (D) aPL induce platelet activation, aggregation, and PS exposure, releasing platelet microparticles, generating bursts of thrombin for fibrin clot stabilisation. (E) aPL-related platelet and leukocyte activation leads to platelet-leukocyte aggregate formation, which potentiates further platelet activation and thrombin generation. (F) The classical and alternative pathways of complement activation are upregulated in APS, with some evidence of lectin pathway also being involved. Complement activation contributes to platelet activation and thrombin generation. (G) Protein C cleaves and inactivates coagulation factors V and VIII, necessary for thrombin generation. Protein C resistance is evident in APS, with anti-Protein C antibodies in APS observed in some studies. (H) The quantity and activity of von Willebrand Factor (vWF) is increased in APS, resulting in increased platelet adhesion, activation, and aggregation. Created with Biorender.com.
This review explores the increased platelet activation observed in APS and the mechanisms driving it. It outlines how platelets interact with the various cells and complement factors involved in APS pathophysiology, and also assesses a possibly greater role of antiplatelets in APS management.
2. Platelet Activation in Antiphospholipid Syndrome
2.1. Platelet Activation
Platelets can be activated through a number of signalling pathways. Signalling can be initiated by either fixed agonists, such as exposed subendothelial collagen, or by soluble agonists such as thrombin. These pathways have the purpose of increasing cytosolic calcium concentration, which is a prerequisite for achieving an activated state. Platelet receptors typically involved include G-protein coupled receptors (GPCR): protease-activated receptors (PAR) 1 and 4 (thrombin receptors), adenosine diphosphate (ADP) receptors P2Y1 and P2Y12, the thromboxane receptor and the adrenoreceptor; immunoreceptor tyrosine-based activation motif (ITAM) linked receptors (ILR): GPVI and FcɣRIIa, as well as GPIbα (major ligand von Willebrand Factor-vWF). The majority of platelet signalling pathways result in phospholipase C (PLC: β and ɣ) activation, a key step for liberating calcium from organelle stores such as the endoplasmic reticulum [18]. This increased calcium drives granule release, thromboxane (TXA) production, and together with the PLC-generated diacylglycerol (DAG), activates calcium and DAG-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) [19], which results in integrin αIIbβ3 changing conformation to one that can bind fibrinogen for platelet aggregation [19,20]. If this increase in calcium is sustained, typically through extracellular calcium entry, a subpopulation of platelets will become procoagulant [16].
2.2. The Relationship between Antiphospholipid Antibodies and Platelet Activation
There appears to be an interdependent relationship between aPL and activated platelets. As described, activated procoagulant platelets expose anionic PS on their surface. β2GPI binds to PS [21], including on activated platelet membranes [22], altering its conformational structure to allow for exposure of the antigenic epitope on domain I of β2GPI, resulting in β2GPI-specific antibody production [23,24,25]. Persistent exposure to anionic surfaces allows for sustained antigenicity and ongoing autoantibody production [26]. Thus, the presence of persistent or recurrent platelet activation may play a significant role in APS pathogenesis. Furthermore, platelets release platelet factor 4 (PF4) from their alpha granules upon activation, tetramers of which bind to β2GPI, creating β2GPI dimers [27]. Both purified β2GPI dimers and dimers within the PF4-β2GPI complex cause platelet activation, with PF4-complexed β2GPI having greater antigenicity/greater aβ2GPI binding than the non-complexed form [27,28]. Together, these studies show that platelet activation during triggering events such as surgery and infections can be a driver in APS pathogenesis and the second hit mechanism required for an APS-related thrombotic event.
Persistent low-level activation of platelets is seen in APS patients. When measuring thromboxane B2, a metabolite of TXA and a marker of platelet activation through the arachidonic acid (AA) pathway, in blood and urine, patients with APS have higher levels than controls [29,30]. In one study, there was a dose dependent increase in thromboxane B2 with increasing titres of aβ2GPI [29]. This increased platelet activation at steady state, i.e., separate from thrombosis or acute phase events, is supported by studies of other activation markers. APS patients have higher levels of circulating platelet-derived microparticles, which have procoagulant properties [31,32]. Plasma markers of platelet activation, such as soluble P-selectin and soluble CD40 ligand, and platelet activation markers CD63 expression (dense granule release) and PAC-1 binding (activated integrin αIIbβ3) have also been shown to be increased compared to controls [33,34]. Linking this activation to an aPL effect, aCL complexed to β2GPI obtained from APS patients was shown to induce increased thromboxane B2 secretion from healthy donor platelets [30]. Furthermore, heat-inactivated (which removes complement and coagulation proteins) APS patient serum was seen to increase platelet-derived prothrombinase activity, implicating aPL-induced procoagulant platelet formation [35]. This was supported by another study that demonstrated greater PS exposure and prothrombinase activity following incubation with aPL, an effect that reversed the anticoagulant effect of aPL in a thrombin generation system, thus indicating that platelets are required for the procoagulant effect of aPL [36]. Finally, platelets incubated with aPL exhibited substantially more coverage and thrombi formation on collagen and subendothelium in ex vivo models than controls [37,38], giving this increased activation functional relevance.
2.3. Mechanisms of aPL-Induced Platelet Activation
Many studies have been published evaluating the mechanism of platelet activation in APS. While there are indirect platelet activation pathways involved through the effect of aPL on other cells complement, and coagulation factors, this section will focus on the direct aPL-dependent platelet activation (Figure 2).
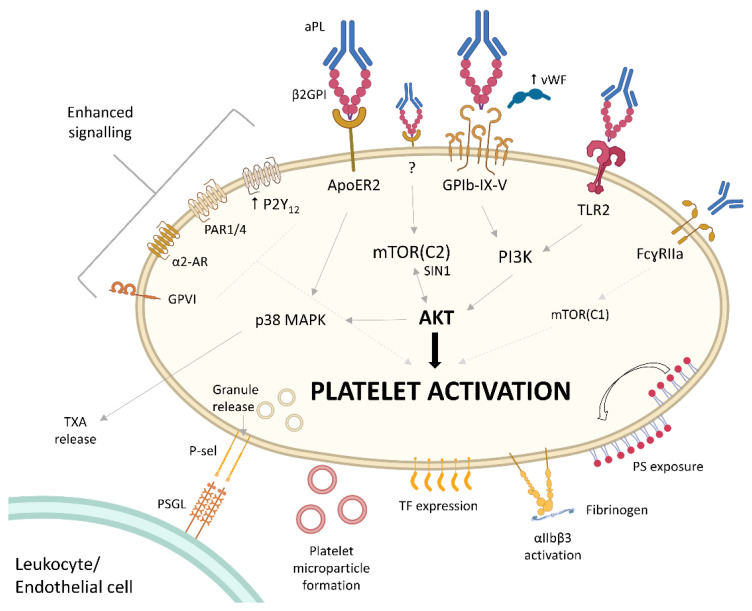
Figure 2.
Platelets exhibit hyperreactivity through many pathways in antiphospholipid syndrome (APS). A large number of platelet receptor agonist stimulation pathways result in greater platelet activation in APS with lower concentrations of agonist. This is due to the “priming” by antiphospholipid antibodies (aPL) complexed to dimerised open-conformation β2 glycoprotein binding to Apolipoprotein E receptor 2 (ApoER2), glycoprotein (GP)Ib-IX-V receptor, and toll-like receptor 2 (TLR2). There is evidence that the Fc portion of some aPL can also prime platelets to hyperreactivity through the FcɣRIIa receptor and mTOR cluster 1 (mTORC1) signalling. The activity of AKT appears central to many of the priming pathways, most critically with the mTORC2-SIN1-AKT axis, although the upstream receptor for this pathway (shown as “?”) has not yet been identified. Hyperreactivity has been identified with ADP agonist stimulation through the P2Y12 receptor, which is overexpressed in APS patient platelets. Protease-Activated Receptor (PAR) 1 and 4 signalling triggered by thrombin, α2-adrenoreceptor (AR) stimulated by adrenaline, and collagen signalling (presumably through GPVI receptor and α2β1 integrin) have all been shown to be hyperreactive. The platelet activation resulting from these pathways are illustrated. TXA—thromboxane, P-sel—P-selectin, TF—tissue factor, PS—phosphatidylserine. Created with Biorender.com.
One of the main platelet receptor targets of aPL is apolipoprotein E receptor 2 (ApoER2). In a murine model of thrombosis, aβ2GPI and β2GPI dimers exacerbated thrombosis, an effect lost in ApoER2 knockout mice [39]. Dimerisation of β2GPI by aPL substantially increases the phospholipid binding of β2GPI and the lupus anticoagulant activity of aPL [40,41], with β2GPI dimers mimicking the in vitro activity of aβ2GPI-β2GPI complexes [42]. Β2GPI dimers increase the adhesion of platelets to collagen and fibronectin under venous and arterial shear stress, an effect shown to be ApoER2-dependent, with binding of β2GPI dimers to ApoER2 confirmed with co-immunoprecipitation [28,43,44]. Binding of aPL/β2GPI dimers to ApoER2 has also been shown to increase platelet aggregation, TXA production, granule release, and integrin αIIbβ3 activation [43,45]. The effect of the ApoER2 pathway depends on concurrent major platelet glycoprotein, GPIbα, signalling. β2GPI dimers also bind GPIbα, GPIbα and ApoER2 are complexed on the platelet surface, and inhibition of GPIbα through a variety of means abrogates the effect of aβ2GPI/β2GPI dimers on platelet activation and adhesion [43,44,45]. Both pathways appear to be required to drive platelet activation through p38 MAPK phosphorylation, as blockade of either ApoER2 or GPIbα completely reverses the aβ2GPI-induced p38 MAPK phosphorylation [45]. Indeed, p38 MAPK phosphorylation is an important part of aPL-induced platelet activation seen in other studies, increasing the potency of very low levels of platelet agonists [27,46]. GPIbα-mediated platelet activation by aPL has also been shown to involve enhanced phosphatidylinositol 3-kinase (PI3K) signalling, which is seen in aPL-induced platelet activation through toll-like receptor 2 (TLR2) as well [47,48].
The GPIbα pathway of platelet activation is also important with regards to the altered secretion and function of vWF seen in patients with APS. vWF is critical to the adhesion of platelets to a site of injury, binding to exposed subendothelium and capturing platelets through the binding of its A1 domain to platelet GPIbα [49]. This initial capture triggers GPIbα signalling, which results in increased cytosolic calcium and granule release [50], as well as allowing for binding of collagen to integrin α2β1 and GPVI for further platelet activation and recruitment [49]. vWF is regulated by ADAMTS13, which cleaves ultra-large multimers of vWF to reduce the degree of platelet binding. When ADAMTS13 activity is impaired, excessive platelet activation and consumption occurs, the severe form of which is the life-threatening disorder thrombotic thrombocytopenic purpura [49]. Immunoglobulin G from patients with APS has been shown to stimulate vWF release from endothelial cells ex vivo [51,52], and excess vWF levels were confirmed in vivo in APS patients [53]. There is also evidence that aPL increase the platelet-binding activity of vWF. A subset of aβ2GPI isolated from APS patients was also found to inhibit the activity of ADAMTS13 [52]. Furthermore, β2GPI can inhibit binding of platelets to the A1 domain of vWF and the presence of aβ2GPI was shown to neutralise this [54]. Primary (not associated with other autoimmune disorders) APS patients have higher levels of tyrosine nitrated β2GPI, which cannot inhibit vWF-platelet binding compared to the non-nitrated form [55]. Together, these studies demonstrate increased vWF quantity and function in APS, providing another mechanism for excess platelet activation in APS.
Multiple platelet activation pathways, through GPCR and ILR, appear upregulated in APS. The ADP receptor P2Y12 is overexpressed in APS patients, with concurrent reduction in downstream inhibitory molecules, cyclic AMP, and GMP [56]. The same study showed hyperreactivity of APS-patient platelets to ADP, as well as healthy donor platelets incubated with IgG from APS patients [56]. Stimulation of other GPCR pathways is also potentiated in the presence of aPL, including PAR1 and 4 and the adrenoreceptor, as well as the ILR pathways stimulated by collagen [33,57,58,59,60]. One mechanism for the hyperreactivity to agonist stimulation is the aβ2GPI-induced upregulation of the mammalian target of rapamycin (mTOR) cluster 2 (mTORC2)-AKT pathway [13]. Tang and colleagues performed RNA sequencing of APS patient-platelets and found 4399 genes differentially expressed compared to healthy controls. Gene-set expression analysis found upregulated platelet activation pathways, as well as the mTOR pathway, with the authors reporting the hyperreactivity to ADP, thrombin, and collagen in the presence of aβ2GPI reversed with disruption of the mTORC2-AKT axis. This assessment was made by using an AKT inhibitor (MK2206) and with platelet-specific Sin1 knockout mouse models. SIN1 is a subunit of mTORC2, essential for its function. The upregulation of the mTORC2-AKT pathway caused by aβ2GPI was not inhibited by FcɣRIIa-blockade. Importantly, the study further demonstrated that SIN1 deficiency in platelets reversed the aβ2GPI-induced increased arterial, venous, and microvascular thrombosis, without prolonging tail bleeding times. This highlights the importance of platelets in all forms of thrombotic APS.
There also appears to be a role for FcɣRIIa, the ILR for the Fc portion of IgG, in excessive platelet activation seen in APS. Arvieux and colleagues [60] demonstrated that aβ2GPI potentiated subthreshold concentrations of platelet agonists ADP and adrenaline in aggregating platelets, an effect inhibited by adding F(ab’)2 fragments of the antibody or IV.3, which is an FcɣRIIa antagonist. Fab fragments alone did not have any effect [60]. This suggested that aPL bind to platelets by the Fab portion and activate platelets via the Fc portion, triggering the FcɣRIIa pathway. Other studies have supported this, with one showing β2GPI immune complexes induced thrombosis and thrombocytopenia in a transgenic mouse model with humanised FcɣRIIa [61], as well as FcɣRIIa-dependence of platelet hyperreactivity demonstrated ex vivo using both aβ2GPI and anti-prothrombin antibodies (a non-criteria aPL) [62,63]. Downstream inhibition of mTOR or knockout of CalDAG-GEFI reversed the effect of aPL [61,62], highlighting their importance in this pathway. The involvement of FcɣRIIa in enhanced platelet activation, however, may be related to the specific aPL used in the experimental model, given other studies with synthesised monoclonal antibodies have demonstrated Fab fragments to be sufficient [64], or have demonstrated no effect of IV.3 [28].
Tissue factor (TF) is an initiator of the coagulation cascade, forming thrombin and fibrin. While there is some controversy over the source and measurement of TF expression in and on platelets, particularly with potential monocyte contamination, appropriately conducted studies indicate a potential role of platelet-expressed TF in APS. Platelets from APS patients (with <1 leukocyte/107 platelets), and healthy platelets incubated with aPL, have been shown to have increased TF expression [65]. aPL appear to cause IRAK phosphorylation and NF-κB activation, resulting in the increased TF expression [65]. This activity is reversed upon inhibition of platelet heparanase activity, an enzyme causing degradation of heparan sulphates [66]. Platelet levels and activity of heparanase increase in stress situations such as sepsis [67], providing another potential mechanism for the “second hit” that is often seen in APS-related thrombosis.
This work collectively provides ample evidence that, regardless of which receptor or signalling pathway is triggered, the presence of aPL leads to platelet hyperreactivity.
2.4. Thrombocytopenia in APS
Thrombocytopenia is commonly seen in APS, typically in 20–30% of patients [68,69,70,71]. It has been associated as an independent risk factor for recurrent thrombosis in numerous studies [68,72,73,74,75]. One study reported that development of thrombocytopenia occurred in all patients that had catastrophic APS (CAPS) episodes [76], while a large registry of CAPS patients found that ~75% had thrombocytopenia [77]. While anti-platelet antibodies can frequently be seen in APS [78,79], the exact mechanism leading to thrombocytopenia has not been clarified. One study of over 200 patients found that platelet differential width and mean platelet volume were associated with thrombosis in APS, suggesting increased platelet activation and turnover (i.e., consumption), respectively [80]. In a murine model of thrombocytopenia induced by infusion of aβ2GPI/β2GPI complexes, thrombocytopenia was demonstrated to result from platelet activation and consumption in thrombosis [61]. These studies collectively suggest that a consumptive process from excess platelet activation plays a significant role in APS-related thrombosis, particularly in the more severe manifestations.
3. The Prothrombotic Interaction of Platelets with Other Cells in APS
Other cell types have been implicated in the pathogenesis of APS-related thrombosis. One of the most important is endothelial cells. Normal endothelial homeostasis maintains anticoagulant properties to prevent spontaneous thrombosis and aPL have been shown to cause endothelial activation/dysfunction, with reduced production of anticoagulant factors such as nitric oxide, increased TF expression, and release of procoagulant microparticles [81]. However, some studies in primary APS patients with no previous vascular risk factors have shown no difference in many markers of endothelial activation compared to normal controls, including soluble E-selectin, soluble VCAM-1, and endothelin-1 [82,83,84]. Thus, endothelial dysfunction may not be the initiating, or parallel, mechanism for thrombosis in APS. Evidence for this concept was provided in an important study by Proulle and colleagues, which concluded that platelets were likely the initial target of aPL, going on to drive the involvement of endothelial cells [12]. In a murine cremasteric arteriole and venule laser-injury model of thrombosis in APS, they only found aβ2GPI-β2GPI complex on the platelet surface within the thrombus, but none was found on the endothelial cell surface within the thrombus [12]. In this in vivo study, aPL were able to induce enhanced endothelial (intercellular adhesion molecule-1 expression) activation, platelet activation and fibrin formation. However, when using eptifibatide to inhibit platelet aggregation specifically via integrin αIIbβ3, the enhancement of endothelial activation and fibrin formation by aPL was completely reversed [12]. This work strongly implicated platelets as the key driver of APS thrombosis in this microvasculature model, and this mechanism will likely hold in platelet-rich thrombi in large artery thrombosis, although this remains to be seen. Further support for the idea is provided by a study describing excess NLRP3 levels in platelet microparticles derived from platelets stimulated by aβ2GPI-β2GPI complexes. The microparticles were internalised by endothelial cells in vitro, leading to prothrombotic endothelial pyroptosis [85].
Platelets have several co-stimulatory pathways with leukocytes that are also evident in APS. Given that APS patients have increased circulating platelet-leukocyte and platelet-monocyte aggregates [34], this cross-talk likely plays a role in APS thrombosis. Monocytes in APS have been well characterised, with demonstration of increased monocyte TF expression driven by aPL [86,87,88]. While this aPL effect can be direct, there is likely contribution from platelets. Monocyte TF expression has been shown to be driven rapidly by platelet P-selectin, expressed upon platelet activation [89]. Monocytes from APS patients express higher levels of PAR2, the inhibition of which has been shown to reduce TF expression [90]. Platelet-dependent thrombin generation seen in patients with APS can, therefore, be a key trigger for monocyte TF expression and procoagulant activity. There is a similar interplay between platelet and neutrophils. Neutrophils are being identified as important contributors to thrombosis in APS through the release of their highly prothrombotic cell contents (primarily free DNA and histones) in neutrophil extracellular traps (NETs) following activation [91,92]. APS patients have higher levels of circulating NETs, with healthy neutrophil incubation with aPL shown to result in NET release [92,93]. These released NETs promote thrombin generation in vitro, confirming their procoagulant nature [92]. The presence of anti-NET antibodies that reduce NET degradation has also been identified in APS [94]. Activated platelets cause neutrophil activation and NET release [95], while histones from NETs can induce procoagulant platelet formation [96]. NET formation is highly dependent on this interaction, with one study demonstrating loss of NET formation after platelet depletion in a sepsis model [97]. The interaction of neutrophil P-selectin glycoprotein ligand 1 (PSGL-1) with P-selectin is important in thrombosis, with PSGL-1 inhibition demonstrating attenuation of venous and arterial thrombosis in a murine model of APS [98]. Looking at the platelet-neutrophil interaction more closely, platelets aggregated to neutrophils all express P-selectin and PS in both healthy controls and APS patients; however, only the APS patient neutrophils went to also express PS, thus significantly increasing generation of thrombin by the aggregated cells [99]. This increased procoagulant activity occurred despite the patients being on low molecular weight heparin and aspirin therapy, indicating that alternative targeting of procoagulant platelets, rather than targeting aggregating platelets only with aspirin, may have an additional benefit in APS.
4. Platelets and Complement in APS
The presence of increased complement activation is well recognised in APS [100,101,102,103], with a possible key role in APS thrombosis. Murine model genetic knockouts or with inhibited components of the complement system, namely C3, C5 and C6, have shown reversal of aPL-enhanced thrombosis and endothelial activation [104,105,106]. Complement activation is known to be procoagulant through interaction with the coagulation system [107]. However, anticoagulation with VKA has not completely overcome recurrent thrombosis in APS, especially APS-related arterial thrombosis [10]. This problem may be due to contribution of the mutually activating interaction of complement components and platelets. Platelets are known to drive complement activation through P-selectin, which facilitates C3b binding, with subsequent generation of the complement activation product, anaphylatoxin C3a, and the terminal complement complex, C5b-9 [108]. Complement can also activate platelets and is associated with thrombosis. Several studies have demonstrated a correlation between deposition of complement activation products on platelets, most commonly C4d, in systemic lupus erythematosus (SLE) patients with or without aPL with venous and arterial thrombosis [109,110,111,112]. Deposition of complement on platelets was enhanced by aPL [109,110] and was associated with higher levels of platelet activation (granule release measured by P-selectin) and aggregation responses [110,111]. The correlation with arterial thrombosis was particularly evident with higher C4d levels deposited on healthy donor platelets following addition of aPL-containing serum from SLE patients [109]. These studies suggest a link between complement, platelet activation, and thrombosis in APS. Potential mechanisms for this link are seen in studies demonstrating that complement activation products C3a and C5b-9 can directly activate platelets. At high concentrations, C3a can induce platelet aggregation, with lower concentrations causing sensitisation of aggregation response to platelet agonist ADP [113]. In vivo studies with C3 knockout models confirmed reduced platelet aggregation and less venous and arterial thrombosis in mice [114,115], with the platelet activation mechanism subsequently demonstrated to be due to binding of C3a to the platelet C3a receptor (C3aR) [116]. Platelets treated with purified C5b-9 have a large influx of calcium, a marked increase in coagulation factor V binding, and a 10-fold increase in platelet-mediated prothrombinase activity, generating thrombin and fibrin [117,118]. C5b-9 also results in the release of platelet microparticles with prothrombinase activity [119]. Collectively, these studies demonstrate the interplay of complement and platelet activation, providing another indication that procoagulant platelets play a significant role in APS and may be a therapeutic target.
5. The Role of Antiplatelets in the Management of Thrombosis in APS
While APS is a heterogeneous disease with many contributing factors to thrombosis, making a one size fits all single modality treatment unrealistic, there is ample evidence that platelets play a central role and are an attractive target. Yet, antiplatelet agents do not have a clearly defined role in APS management. This is likely due to a paucity of randomised controlled trials (RCTs) specifically assessing the impact of the different antiplatelet agents. Current guidelines only recommend low-dose aspirin (LDA) as primary prophylaxis in high-risk aPL carriers, or in combination with VKA (international normalised ratio, INR target range 2.0–3.0) as one of the secondary prophylaxis options for arterial thrombosis, which also include VKA alone at INR target range 2.0–3.0 or 3.0–4.0 [8,9]. The recommendation for venous thrombosis is use of VKA with INR target range 2.0–3.0, with consideration of the addition of LDA in refractory cases [120].
5.1. Antiplatelets as Primary Prophylaxis for Thrombosis in APS
Given that the presence of aPL clearly creates a prothrombotic tendency, primary prophylaxis in asymptomatic carriers would be ideal, especially in high-risk patients, such as those who are triple aPL positive, or with other cardiovascular risk factors. Indeed, meta-analyses evaluating LDA as primary prophylaxis found a benefit [121,122], with an individual patient data meta-analysis of ~500 patients finding a hazard ratio of 0.43 (0.25–0.75 95% CI) with LDA compared to control [122]. Most patients had higher risk aPL profiles (persistent LA, multiple aPL, or high aPL-titre positivity), resulting in recommendations for LDA being restricted to these patients. Other antiplatelets have not been evaluated.
5.2. Antiplatelets as Secondary Prophylaxis for Thrombosis in APS
As mentioned in the introduction, recurrent APS-related thrombosis standard-of-care treatment remains a problem, particularly in APS patients with arterial thrombosis [10]. Two RCTs with >100 patients each explored increasing the intensity of VKA (2.0–3.0 vs. 3.0–4.0 or 4.5) to reduce recurrence and failed to show improvement, with a significant increase in bleeding complications [123,124]. Patients with initial arterial thrombosis were underrepresented in both studies, however, and most of the thromboses in the higher intensity arms occurred when INR was less than 3.0. A systematic review which included these studies identified INR > 3.0 as ideal for patients with arterial or recurrent thrombosis [125]. There is far less available data regarding the benefit of incorporating single or multiple antiplatelets in secondary prophylaxis, with studies limited to arterial thrombosis. A multicentre, international study including retrospective and prospective data on 139 APS patients with initial arterial thrombosis found antiplatelet alone to be inferior to anticoagulant alone, with 37.2% of the antiplatelet group having recurrent thrombosis after median 4.24 year follow-up [126]. Combined antiplatelet and anticoagulant treatment was far superior to either agent alone. One large RCT (APASS-WARSS) compared LDA to VKA in 1770 ischaemic stroke patients tested for aPL (LA and aCL only) at baseline, demonstrating no difference in outcomes between the two treatments in aPL-positive patients (n = 720, 41%) [127]. However, the INR target was only 1.4–2.8 and no repeat aPL testing was routinely performed to confirm APS diagnosis, which is seen in far fewer (17% under age 50) ischaemic stroke patients [6]. As such, it is difficult to draw conclusions from this study. A small RCT of 20 patients compared LDA to VKA (INR 2.0–3.0) plus LDA and reported a statistically significant reduction in recurrent thrombosis with combination therapy [128]. A review in 2021 focussing on arterial thrombosis in APS explored the role of antiplatelets, finding that VKA plus antiplatelet was superior to standard-intensity VKA alone (RR 0.43, 95% CI 0.22–0.85) [11]. However, this was based on a single retrospective study with 34 patients in these cohorts [129]. The review also found that dual antiplatelets (LDA plus P2Y12 antagonist or cilostazol) were superior to LDA alone, with RR 0.29 (0.09–0.99 95% CI) [11]. While the strength of evidence is weak, it appears that combination treatments including an antiplatelet drug may be effective in APS with arterial thrombosis.
5.3. How Can Platelets Be Targeted Better to Improve Outcomes in APS?
Despite clear pathophysiological research indicating the value of targeting platelets, the most studied antiplatelet treatment, LDA, cannot prevent recurrent thrombosis. Aspirin only inhibits thromboxane-driven secondary platelet aggregation [130], and does not inhibit procoagulant platelet formation [131]. As described, the ADP-P2Y12 axis of platelet aggregation is upregulated in APS [56]. This platelet hyperreactivity was resistant to aspirin, but responsive to P2Y12 antagonist ticagrelor, potentiated further by alternative antiplatelet cilostazol, a PDE3 inhibitor. This work highlights the need to better evaluate the readily available P2Y12 inhibitors—clopidogrel, prasugrel, and ticagrelor—in APS.
Promising antiplatelet treatment strategies requiring further research are listed in Table 1. One antiplatelet agent of increasing interest is dipyridamole. Predominantly used in combination with aspirin for secondary stroke prevention [132], there may be potential for including it in both arterial and venous thrombosis management in patients with APS. Dipyridamole exerts antithrombotic properties, similar to cilostazol, by increasing the availability of intracellular cyclic AMP, which inhibits protein kinase A and platelet activation/aggregation [133]. Furthermore, dipyridamole has been shown to (directly and probably indirectly through platelets) reduce aPL-induced NET formation from neutrophils and increased venous thrombosis in mice, suggesting a potential role for management of APS with venous thrombosis.
Table 1.
Potential therapeutic strategies against platelet hyperreactivity for further research in thrombotic antiphospholipid syndrome.
| Therapeutic Strategy | Available Agents | Potential Benefits/Limitations |
|---|---|---|
| Inhibiting P2Y12 receptor | Clopidogrel, prasugrel, ticagrelor, etc. | Benefits: |
| ||
| Limitations: | ||
| ||
| Increasing cyclic AMP | Cilastazol, dipyridamole | Benefits: |
| ||
| Limitations: | ||
| ||
| Reducing procoagulant platelet formation | Ciclosporin, acetazolamide | Benefits: |
| ||
| Limitations: | ||
| ||
| mTOR inhibition | Everolimus, sirolimus | Benefits: |
| ||
| Limitations: | ||
| ||
| Inhibition of mTORC2 (SIN1)-AKT axis | ? | Benefits: |
| ||
| Limitations: | ||
| ||
| Reducing Neutrophil Extracellular Traps (NETs) | Dipyridamole, ? | Benefits: |
| ||
| Limitations: | ||
| ||
| Inhibiting excessive complement activation | Eculizumab (C5), ravalizumab (C5), sutimlimab (C1s), pegcetacoplan (C3), etc. | Benefits: |
| ||
| Limitations: | ||
|
Refocussing antiplatelet treatment to target procoagulant platelets may be another avenue to explore. Loss of procoagulant platelets reduces thrombosis (in mice) and has a minor impact on haemostasis in mouse models and in Scott’s syndrome, an inherited genetic disorder with loss of the gene encoding the phospholipid scramblase TMEM16F required for platelet PS exposure [134,135]. This platelet subpopulation was demonstrated to be increased by aPL, tipping the balance towards excess thrombin generation by reversing the anticoagulant effect of aPL, while preserving the procoagulant effect of activated protein C resistance [35,36]. Given its role in thrombosis at any vascular site and its interactions with other cells and complement described above, procoagulant platelets provide a potential new antiplatelet target that would minimise additional compromise of haemostasis.
Other agents with antiplatelet properties have also been shown to have utility in APS and warrant further investigation. Hydroxychloroquine, an antimalarial with great clinical utility in SLE, has been shown to reduce aPL-enhanced thrombosis in mice [136]. Subsequent in vitro studies demonstrated hydroxychloroquine reversed the platelet hyperreactivity caused by aPL [137]. Statins also appear protective in APS and have multiple mechanisms of reducing platelet activation [138,139]. Complement activation appears to be a marker of more severe APS [140], and, given the known impact it has on platelet activation, is another potential target. With the mTOR pathway, especially mTORC2-AKT, having an important role in platelet hyperreactivity, mTOR, SIN1, and AKT inhibition represents exciting potential targets that could have minimal impact on haemostasis.
6. Conclusions
Recurrent thrombosis in APS patients represents an area of unmet need and is the leading cause of mortality in this patient population. Platelets play a central role in thrombosis and there is extensive research confirming that platelets are not only key players in APS-related thrombosis, but also have a role in the formation and persistence of aPL. Much further research is needed to delineate the best way to apply antiplatelet agents in APS. Several established antiplatelet agents are available for further evaluation, either in combination with aspirin or VKA. There are also exciting novel approaches targeting the platelet-neutrophil interactions and NETs, and the mTORC2-AKT axis, as well as the potential for utilising complement activation as a biomarker for patients who would benefit from complement inhibition to reduce platelet activation. Finally, as procoagulant platelets are the prothrombotic product of many of the pathophysiological processes in APS, they represent an important new specific antiplatelet target.
Author Contributions
Conceptualization, all authors; writing—original draft preparation, I.T.-E.; writing—review and editing, all authors; supervision, M.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding. I.T.-E. was supported by a Haematology Society of Australia and New Zealand New Investigator Clinical Fellowship.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., Derksen R.H.W.M., De Groot P.G., Koike T., Meroni P.L., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Barbhaiya M., Zuily S., Naden R., Hendry A., Manneville F., Amigo M.C., Amoura Z., Andrade D., Andreoli L., Artim-Esen B., et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023;75:1687–1702. doi: 10.1002/art.42624. [DOI] [PubMed] [Google Scholar]
- 3.Sevim E., Zisa D., Andrade D., Sciascia S., Pengo V., Tektonidou M.G., Ugarte A., Gerosa M., Belmont H.M., Zamorano M.A.A., et al. Characteristics of Patients with Antiphospholipid Antibody Positivity in the APS ACTION International Clinical Database and Repository. Arthritis Care Res. 2022;74:324–335. doi: 10.1002/acr.24468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duarte-García A., Pham M.M., Crowson C.S., Amin S., Moder K.G., Pruthi R.K., Warrington K.J., Matteson E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019;71:1545–1552. doi: 10.1002/art.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miranda S., Park J., Le Gal G., Piran S., Kherani S., Rodger M.A., Delluc A. Prevalence of confirmed antiphospholipid syndrome in 18–50 years unselected patients with first unprovoked venous thromboembolism. J. Thromb. Haemost. 2020;18:926–930. doi: 10.1111/jth.14720. [DOI] [PubMed] [Google Scholar]
- 6.Sciascia S., Sanna G., Khamashta M.A., Cuadrado M.J., Erkan D., Andreoli L., Bertolaccini M.L. The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: A systematic review. Ann. Rheum. Dis. 2015;74:2028–2033. doi: 10.1136/annrheumdis-2014-205663. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Pintó I., Espinosa G., Erkan D., Shoenfeld Y., Cervera R. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatology. 2018;57:1264–1270. doi: 10.1093/rheumatology/key082. [DOI] [PubMed] [Google Scholar]
- 8.Cohen H., Cuadrado M.J., Erkan D., Duarte-Garcia A., Isenberg D.A., Knight J.S., Ortel T.L., Rahman A., Salmon J.E., Tektonidou M.G., et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus. 2020;29:1571–1593. doi: 10.1177/0961203320950461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., Cuadrado M.J., Dörner T., Ferrer-Oliveras R., Hambly K., et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019;78:1296. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortel T.L., Meleth S., Catellier D., Crowther M., Erkan D., Fortin P.R., Garcia D., Haywood N., Kosinski A.S., Levine S.R., et al. Recurrent thrombosis in patients with antiphospholipid antibodies and an initial venous or arterial thromboembolic event: A systematic review and meta-analysis. J. Thromb. Haemost. 2020;18:2274–2286. doi: 10.1111/jth.14936. [DOI] [PubMed] [Google Scholar]
- 11.Aibar J., Schulman S. Arterial Thrombosis in Patients with Antiphospholipid Syndrome: A Review and Meta-Analysis. Semin. Thromb. Hemost. 2021;47:709–723. doi: 10.1055/s-0041-1725057. [DOI] [PubMed] [Google Scholar]
- 12.Proulle V., Furie R.A., Merrill-Skoloff G., Furie B.C., Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. 2014;124:611–622. doi: 10.1182/blood-2014-02-554980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Z., Shi H., Chen C., Teng J., Dai J., Ouyang X., Liu H., Hu Q., Cheng X., Ye J., et al. Activation of Platelet mTORC2/Akt Pathway by Anti-β2GP1 Antibody Promotes Thrombosis in Antiphospholipid Syndrome. Arter. Thromb. Vasc. Biol. 2023;43:1818–1832. doi: 10.1161/ATVBAHA.123.318978. [DOI] [PubMed] [Google Scholar]
- 14.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017;38:785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann M., Schoeman R.M., Krohl P.J., Wallbank A.M., Samaniuk J.R., Jandrot-Perrus M., Neeves K.B. Platelets Drive Thrombus Propagation in a Hematocrit and Glycoprotein VI–Dependent Manner in an In Vitro Venous Thrombosis Model. Arterioscler. Thromb. Vasc. Biol. 2018;38:1052–1062. doi: 10.1161/ATVBAHA.118.310731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohidi-Esfahani I., Lee C.S.M., Liang H.P.H., Chen V.M.Y. Procoagulant platelets: Laboratory detection and clinical significance. Int. J. Lab. Hematol. 2020;42:59–67. doi: 10.1111/ijlh.13197. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiler S., Massberg S., Engelmann B. Biological basis and pathological relevance of microvascular thrombosis. Thromb. Res. 2014;133((Suppl. 1)):S35–S37. doi: 10.1016/j.thromres.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Fernández D.I., Kuijpers M.J.E., Heemskerk J.W.M. Platelet calcium signaling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity. Platelets. 2021;32:863–871. doi: 10.1080/09537104.2020.1859103. [DOI] [PubMed] [Google Scholar]
- 19.Stefanini L., Roden R.C., Bergmeier W. CalDAG-GEFI is at the nexus of calcium-dependent platelet activation. Blood. 2009;114:2506–2514. doi: 10.1182/blood-2009-04-218768. [DOI] [PubMed] [Google Scholar]
- 20.Bertoni A., Tadokoro S., Eto K., Pampori N., Parise L.V., White G.C., Shattil S.J. Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J. Biol. Chem. 2002;277:25715–25721. doi: 10.1074/jbc.M202791200. [DOI] [PubMed] [Google Scholar]
- 21.Wurm H. beta 2-Glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int. J. Biochem. 1984;16:511–515. doi: 10.1016/0020-711X(84)90168-X. [DOI] [PubMed] [Google Scholar]
- 22.Khamashta M.A., Harris E.N., Gharavi A.E., Derue G., Gil A., Vázquez J.J., Hughes G.R. Immune mediated mechanism for thrombosis: Antiphospholipid antibody binding to platelet membranes. Ann. Rheum. Dis. 1988;47:849–854. doi: 10.1136/ard.47.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agar C., van Os G.M., Mörgelin M., Sprenger R.R., Marquart J.A., Urbanus R.T., Derksen R.H., Meijers J.C., de Groot P.G. Beta2-glycoprotein I can exist in 2 conformations: Implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 24.de Laat B., Derksen R.H., van Lummel M., Pennings M.T., de Groot P.G. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 25.de Laat B., van Berkel M., Urbanus R.T., Siregar B., de Groot P.G., Gebbink M.F., Maas C. Immune responses against domain I of β(2)-glycoprotein I are driven by conformational changes: Domain I of β(2)-glycoprotein I harbors a cryptic immunogenic epitope. Arthritis Rheum. 2011;63:3960–3968. doi: 10.1002/art.30633. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi Y., Seta N., Kaburaki J., Kobayashi K., Matsuura E., Kuwana M. Excessive exposure to anionic surfaces maintains autoantibody response to beta(2)-glycoprotein I in patients with antiphospholipid syndrome. Blood. 2007;110:4312–4318. doi: 10.1182/blood-2007-07-100008. [DOI] [PubMed] [Google Scholar]
- 27.Sikara M.P., Routsias J.G., Samiotaki M., Panayotou G., Moutsopoulos H.M., Vlachoyiannopoulos P.G. {beta}2 Glycoprotein I ({beta}2GPI) binds platelet factor 4 (PF4): Implications for the pathogenesis of antiphospholipid syndrome. Blood. 2010;115:713–723. doi: 10.1182/blood-2009-03-206367. [DOI] [PubMed] [Google Scholar]
- 28.Lutters B.C., Derksen R.H., Tekelenburg W.L., Lenting P.J., Arnout J., de Groot P.G. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2′. J. Biol. Chem. 2003;278:33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 29.Forastiero R., Martinuzzo M., Carreras L.O., Maclouf J. Anti-beta2 glycoprotein I antibodies and platelet activation in patients with antiphospholipid antibodies: Association with increased excretion of platelet-derived thromboxane urinary metabolites. Thromb. Haemost. 1998;79:42–45. doi: 10.1055/s-0037-1614216. [DOI] [PubMed] [Google Scholar]
- 30.Robbins D.L., Leung S., Miller-Blair D.J., Ziboh V. Effect of anticardiolipin/beta2-glycoprotein I complexes on production of thromboxane A2 by platelets from patients with the antiphospholipid syndrome. J. Rheumatol. 1998;25:51–56. [PubMed] [Google Scholar]
- 31.Breen K.A., Sanchez K., Kirkman N., Seed P.T., Parmar K., Moore G.W., Hunt B.J. Endothelial and platelet microparticles in patients with antiphospholipid antibodies. Thromb. Res. 2015;135:368–374. doi: 10.1016/j.thromres.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Štok U., Blokar E., Lenassi M., Holcar M., Frank-Bertoncelj M., Erman A., Resnik N., Sodin-Šemrl S., Čučnik S., Pirkmajer K.P., et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells. 2020;9:1211. doi: 10.3390/cells9051211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bontadi A., Ruffatti A., Falcinelli E., Giannini S., Marturano A., Tonello M., Hoxha A., Pengo V., Punzi L., Momi S., et al. Platelet and endothelial activation in catastrophic and quiescent antiphospholipid syndrome. Thromb. Haemost. 2013;109:901–908. doi: 10.1160/TH12-03-0212. [DOI] [PubMed] [Google Scholar]
- 34.Joseph J.E., Harrison P., Mackie I.J., Isenberg D.A., Machin S.J. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 2001;115:451–459. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- 35.Warner M.N., Pavord S., Moore J.C., Warkentin T.E., Hayward C.P., Kelton J.G. Serum-induced platelet procoagulant activity: An assay for the characterization of prothrombotic disorders. J. Lab. Clin. Med. 1999;133:129–133. doi: 10.1016/S0022-2143(99)90005-7. [DOI] [PubMed] [Google Scholar]
- 36.Membre A., Wahl D., Latger-Cannard V., Max J.P., Lacolley P., Lecompte T., Regnault V. The effect of platelet activation on the hypercoagulability induced by murine monoclonal antiphospholipid antibodies. Haematologica. 2008;93:566–573. doi: 10.3324/haematol.12364. [DOI] [PubMed] [Google Scholar]
- 37.Reverter J.C., Tàssies D., Font J., Khamashta M.A., Ichikawa K., Cervera R., Escolar G., Hughes G.R., Ingelmo M., Ordinas A. Effects of human monoclonal anticardiolipin antibodies on platelet function and on tissue factor expression on monocytes. Arthritis Rheum. 1998;41:1420–1427. doi: 10.1002/1529-0131(199808)41:8<1420::AID-ART11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Reverter J.C., Tàssies D., Escolar G., Font J., López Soto A., Ingelmo M., Ordinas A. Effect of plasma from patients with primary antiphospholipid syndrome on platelet function in a collagen rich perfusion system. Thromb. Haemost. 1995;73:132–137. doi: 10.1055/s-0038-1653738. [DOI] [PubMed] [Google Scholar]
- 39.Romay-Penabad Z., Aguilar-Valenzuela R., Urbanus R.T., Derksen R.H., Pennings M.T., Papalardo E., Shilagard T., Vargas G., Hwang Y., de Groot P.G., et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–1414. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willems G.M., Janssen M.P., Pelsers M.M., Comfurius P., Galli M., Zwaal R.F., Bevers E.M. Role of divalency in the high-affinity binding of anticardiolipin antibody-beta 2-glycoprotein I complexes to lipid membranes. Biochemistry. 1996;35:13833–13842. doi: 10.1021/bi960657q. [DOI] [PubMed] [Google Scholar]
- 41.Arnout J., Wittevrongel C., Vanrusselt M., Hoylaerts M., Vermylen J. Beta-2-glycoprotein I dependent lupus anticoagulants form stable bivalent antibody beta-2-glycoprotein I complexes on phospholipid surfaces. Thromb. Haemost. 1998;79:79–86. [PubMed] [Google Scholar]
- 42.Lutters B.C., Meijers J.C., Derksen R.H., Arnout J., de Groot P.G. Dimers of beta 2-glycoprotein I mimic the in vitro effects of beta 2-glycoprotein I-anti-beta 2-glycoprotein I antibody complexes. J. Biol. Chem. 2001;276:3060–3067. doi: 10.1074/jbc.M008224200. [DOI] [PubMed] [Google Scholar]
- 43.Urbanus R.T., Pennings M.T., Derksen R.H., de Groot P.G. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J. Thromb. Haemost. 2008;6:1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 44.Pennings M.T., Derksen R.H., van Lummel M., Adelmeijer J., VanHoorelbeke K., Urbanus R.T., Lisman T., de Groot P.G. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: Glycoprotein Ibalpha and apolipoprotein E receptor 2′. J. Thromb. Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W., Gao F., Lu D., Sun N., Yin X., Jin M., Liu Y. Anti-β2 glycoprotein I antibodies in complex with β2 glycoprotein I induce platelet activation via two receptors: Apolipoprotein E receptor 2′ and glycoprotein I bα. Front. Med. 2016;10:76–84. doi: 10.1007/s11684-015-0426-7. [DOI] [PubMed] [Google Scholar]
- 46.Vega-Ostertag M., Harris E.N., Pierangeli S.S. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50:2911–2919. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 47.Shi T., Giannakopoulos B., Yan X., Yu P., Berndt M.C., Andrews R.K., Rivera J., Iverson G.M., Cockerill K.A., Linnik M.D., et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 48.Terrisse A.D., Laurent P.A., Garcia C., Gratacap M.P., Vanhaesebroeck B., Sié P., Payrastre B. The class I phosphoinositide 3-kinases α and β control antiphospholipid antibodies-induced platelet activation. Thromb. Haemost. 2016;115:1138–1146. doi: 10.1160/TH15-08-0661. [DOI] [PubMed] [Google Scholar]
- 49.Bryckaert M., Rosa J.P., Denis C.V., Lenting P.J. Of von Willebrand factor and platelets. Cell Mol. Life Sci. 2015;72:307–326. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falati S., Edmead C.E., Poole A.W. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, Fyn, and Lyn to activate human platelets. Blood. 1999;94:1648–1656. doi: 10.1182/blood.V94.5.1648. [DOI] [PubMed] [Google Scholar]
- 51.Lindsey N.J., Dawson R.A., Henderson F.I., Greaves M., Hughes P. Stimulation of von Willebrand factor antigen release by immunoglobulin from thrombosis prone patients with systemic lupus erythematosus and the anti-phospholipid syndrome. Br. J. Rheumatol. 1993;32:123–126. doi: 10.1093/rheumatology/32.2.123. [DOI] [PubMed] [Google Scholar]
- 52.Ng C.J., McCrae K.R., Ashworth K., Sosa L.J., Betapudi V., Manco-Johnson M.J., Liu A., Dong J.F., Chung D., White-Adams T.C., et al. Effects of anti-β2GPI antibodies on VWF release from human umbilical vein endothelial cells and ADAMTS13 activity. Res. Pr. Thromb. Haemost. 2018;2:380–389. doi: 10.1002/rth2.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Der H., Kerekes G., Veres K., Szodoray P., Toth J., Lakos G., Szegedi G., Soltesz P. Impaired endothelial function and increased carotid intima-media thickness in association with elevated von Willebrand antigen level in primary antiphospholipid syndrome. Lupus. 2007;16:497–503. doi: 10.1177/0961203307080224. [DOI] [PubMed] [Google Scholar]
- 54.Hulstein J.J., Lenting P.J., de Laat B., Derksen R.H., Fijnheer R., de Groot P.G. beta2-Glycoprotein I inhibits von Willebrand factor dependent platelet adhesion and aggregation. Blood. 2007;110:1483–1491. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 55.Krilis M., Qi M., Ioannou Y., Zhang J.Y., Ahmadi Z., Wong J.W.H., Vlachoyiannopoulos P.G., Moutsopoulos H.M., Koike T., Sturgess A.D., et al. Clinical relevance of nitrated beta 2-glycoprotein I in antiphospholipid syndrome: Implications for thrombosis risk. J. Autoimmun. 2021;122:102675. doi: 10.1016/j.jaut.2021.102675. [DOI] [PubMed] [Google Scholar]
- 56.Leonardi G.R., Lescano C.H., Costa J.L., Mazetto B., Orsi F.A., Monica F.Z. Adenosine diphosphate-induced aggregation is enhanced in platelets obtained from patients with thrombotic primary antiphospholipid syndrome (t-PAPS): Role of P2Y(12) -cAMP signaling pathway. J. Thromb. Haemost. 2022;20:1699–1711. doi: 10.1111/jth.15724. [DOI] [PubMed] [Google Scholar]
- 57.Lin Y.L., Wang C.T. Activation of human platelets by the rabbit anticardiolipin antibodies. Blood. 1992;80:3135–3143. doi: 10.1182/blood.V80.12.3135.3135. [DOI] [PubMed] [Google Scholar]
- 58.Martinuzzo M.E., Maclouf J., Carreras L.O., Lévy-Toledano S. Antiphospholipid antibodies enhance thrombin-induced platelet activation and thromboxane formation. Thromb. Haemost. 1993;70:667–671. doi: 10.1055/s-0038-1649646. [DOI] [PubMed] [Google Scholar]
- 59.Campbell A.L., Pierangeli S.S., Wellhausen S., Harris E.N. Comparison of the effects of anticardiolipin antibodies from patients with the antiphospholipid syndrome and with syphilis on platelet activation and aggregation. Thromb. Haemost. 1995;73:529–534. doi: 10.1055/s-0038-1653808. [DOI] [PubMed] [Google Scholar]
- 60.Arvieux J., Roussel B., Pouzol P., Colomb M.G. Platelet activating properties of murine monoclonal antibodies to beta 2-glycoprotein I. Thromb. Haemost. 1993;70:336–341. [PubMed] [Google Scholar]
- 61.Amirkhosravi A., Boulaftali Y., Robles-Carrillo L., Meyer T., McKenzie S.E., Francis J.L., Bergmeier W. CalDAG-GEFI deficiency protects mice from FcγRIIa-mediated thrombotic thrombocytopenia induced by CD40L and β2GPI immune complexes. J. Thromb. Haemost. 2014;12:2113–2119. doi: 10.1111/jth.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hollerbach A., Müller-Calleja N., Ritter S., Häuser F., Canisius A., Orning C., Jurk K., Lackner K.J. Platelet Activation by Antiphospholipid Antibodies Depends on Epitope Specificity and is Prevented by mTOR Inhibitors. Thromb. Haemost. 2019;119:1147–1153. doi: 10.1055/s-0039-1685453. [DOI] [PubMed] [Google Scholar]
- 63.Chayoua W., Nicolson P.L.R., Meijers J.C.M., Kardeby C., Garcia-Quintanilla L., Devreese K.M.J., de Laat B., Watson S.P., de Groot P.G. Antiprothrombin antibodies induce platelet activation: A possible explanation for anti-FXa therapy failure in patients with antiphospholipid syndrome? J. Thromb. Haemost. 2021;19:1776–1782. doi: 10.1111/jth.15320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jankowski M., Vreys I., Wittevrongel C., Boon D., Vermylen J., Hoylaerts M.F., Arnout J. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101:157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 65.Capozzi A., Manganelli V., Riitano G., Recalchi S., Truglia S., Alessandri C., Longo A., Garofalo T., Misasi R., Valesini G., et al. Tissue factor over-expression in platelets of patients with anti-phospholipid syndrome: Induction role of anti-β2-GPI antibodies. Clin. Exp. Immunol. 2019;196:59–66. doi: 10.1111/cei.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capozzi A., Riitano G., Recalchi S., Manganelli V., Costi R., Saccoliti F., Pulcinelli F., Garofalo T., Misasi R., Longo A., et al. Effect of heparanase inhibitor on tissue factor overexpression in platelets and endothelial cells induced by anti-β2-GPI antibodies. J. Thromb. Haemost. 2021;19:2302–2313. doi: 10.1111/jth.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eustes A.S., Campbell R.A., Middleton E.A., Tolley N.D., Manne B.K., Montenont E., Rowley J.W., Krauel K., Blair A., Guo L., et al. Heparanase expression and activity are increased in platelets during clinical sepsis. J. Thromb. Haemost. 2021;19:1319–1330. doi: 10.1111/jth.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y., Zhao J., Jiang H., Huang C., Qi W., Song Y., Wang Q., Li M., Tian X., Zhao Y., et al. Thrombocytopenia in primary antiphospholipid syndrome: Association with prognosis and clinical implications. Rheumatology. 2022;62:256–263. doi: 10.1093/rheumatology/keac264. [DOI] [PubMed] [Google Scholar]
- 69.Cuadrado M.J., Mujic F., Muñoz E., Khamashta M.A., Hughes G.R. Thrombocytopenia in the antiphospholipid syndrome. Ann. Rheum. Dis. 1997;56:194–196. doi: 10.1136/ard.56.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unlu O., Erkan D., Barbhaiya M., Andrade D., Nascimento I., Rosa R., Banzato A., Pengo V., Ugarte A., Gerosa M., et al. The Impact of Systemic Lupus Erythematosus on the Clinical Phenotype of Antiphospholipid Antibody-Positive Patients: Results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Clinical Database and Repository. Arthritis Care Res. 2019;71:134–141. doi: 10.1002/acr.23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barbui T., Berrettini M., Ferrini P.L.R., Finazzi G., Galli M., Ciavarella N., Schiavoni M., Palareti G., Marongiu F., Muleo G., et al. Thrombosis and thrombocytopenia in antiphospholipid syndrome (idiopathic and secondary to SLE): First report from the Italian Registry. Italian Registry of Antiphospholipid Antibodies (IR-APA) Haematologica. 1993;78:313–318. [PubMed] [Google Scholar]
- 72.Hisada R., Kato M., Sugawara E., Fujieda Y., Oku K., Bohgaki T., Amengual O., Yasuda S., Atsumi T. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: A longitudinal study. J. Thromb. Haemost. 2017;15:1782–1787. doi: 10.1111/jth.13763. [DOI] [PubMed] [Google Scholar]
- 73.Song X., Fan Y., Jia Y., Li G., Liu M., Xu Y., Zhang J., Li C. A novel aGAPSS-based nomogram for the prediction of ischemic stroke in patients with antiphospholipid syndrome. Front. Immunol. 2022;13:930087. doi: 10.3389/fimmu.2022.930087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naranjo L., Stojanovich L., Djokovic A., Andreoli L., Tincani A., Maślińska M., Sciascia S., Infantino M., Garcinuño S., Kostyra-Grabczak K., et al. Circulating immune-complexes of IgG/IgM bound to B2-glycoprotein-I associated with complement consumption and thrombocytopenia in antiphospholipid syndrome. Front. Immunol. 2022;13:957201. doi: 10.3389/fimmu.2022.957201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardos-Gea J., Marques-Soares J.R., Buján S., Ordi-Ros J., Alijotas-Reig J. Persistent thrombocytopenia predicts poor long-term survival in patients with antiphospholipid syndrome: A 38-year follow-up study. Rheumatology. 2022;61:1053–1061. doi: 10.1093/rheumatology/keab475. [DOI] [PubMed] [Google Scholar]
- 76.Pontara E., Banzato A., Bison E., Cattini M.G., Baroni G., Denas G., Calligaro A., Marson P., Tison T., Ruffatti A., et al. Thrombocytopenia in high-risk patients with antiphospholipid syndrome. J. Thromb. Haemost. 2018;16:529–532. doi: 10.1111/jth.13947. [DOI] [PubMed] [Google Scholar]
- 77.López-Benjume B., Rodríguez-Pintó I., Amigo M.C., Erkan D., Shoenfeld Y., Cervera R., Espinosa G. Eculizumab use in catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis from the “CAPS Registry”. Autoimmun. Rev. 2022;21:103055. doi: 10.1016/j.autrev.2022.103055. [DOI] [PubMed] [Google Scholar]
- 78.Galli M., Daldossi M., Barbui T. Anti-glycoprotein Ib/IX and IIb/IIIa antibodies in patients with antiphospholipid antibodies. Thromb. Haemost. 1994;71:571–575. [PubMed] [Google Scholar]
- 79.Panzer S., Gschwandtner M.E., Hütter D., Spitzauer S., Pabinger I. Specificities of platelet autoantibodies in patients with lupus anticoagulants in primary antiphospholipid syndrome. Ann. Hematol. 1997;74:239–242. doi: 10.1007/s002770050291. [DOI] [PubMed] [Google Scholar]
- 80.Shi Y., Jiang H., Huang C., Hu C., Zhao J., Li M., Zeng X. Platelet distribution width is highly associated with thrombotic events in primary antiphospholipid syndrome. Clin. Rheumatol. 2021;40:4581–4588. doi: 10.1007/s10067-021-05843-z. [DOI] [PubMed] [Google Scholar]
- 81.Velásquez M., Rojas M., Abrahams V.M., Escudero C., Cadavid Á.P. Mechanisms of Endothelial Dysfunction in Antiphospholipid Syndrome: Association with Clinical Manifestations. Front. Physiol. 2018;9:1840. doi: 10.3389/fphys.2018.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gresele P., Migliacci R., Vedovati M.C., Ruffatti A., Becattini C., Facco M., Guglielmini G., Boscaro E., Mezzasoma A.M., Momi S., et al. Patients with primary antiphospholipid antibody syndrome and without associated vascular risk factors present a normal endothelial function. Thromb. Res. 2009;123:444–451. doi: 10.1016/j.thromres.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Cugno M., Borghi M.O., Lonati L.M., Ghiadoni L., Gerosa M., Grossi C., De Angelis V., Magnaghi G., Tincani A., Mari D., et al. Patients with antiphospholipid syndrome display endothelial perturbation. J. Autoimmun. 2010;34:105–110. doi: 10.1016/j.jaut.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Williams F.M., Parmar K., Hughes G.R., Hunt B.J. Systemic endothelial cell markers in primary antiphospholipid syndrome. Thromb. Haemost. 2000;84:742–746. doi: 10.1055/s-0037-1614108. [DOI] [PubMed] [Google Scholar]
- 85.Di L., Zha C., Liu Y. Platelet-derived microparticles stimulated by anti-β(2)GPI/β(2)GPI complexes induce pyroptosis of endothelial cells in antiphospholipid syndrome. Platelets. 2023;34:2156492. doi: 10.1080/09537104.2022.2156492. [DOI] [PubMed] [Google Scholar]
- 86.Sorice M., Longo A., Capozzi A., Garofalo T., Misasi R., Alessandri C., Conti F., Buttari B., Riganò R., Ortona E., et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 87.Zhou H., Wolberg A.S., Roubey R.A. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood. 2004;104:2353–2358. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- 88.Cuadrado M.J., López-Pedrera C., Khamashta M.A., Camps M.T., Tinahones F., Torres A., Hughes G.R., Velasco F. Thrombosis in primary antiphospholipid syndrome: A pivotal role for monocyte tissue factor expression. Arthritis Rheum. 1997;40:834–841. doi: 10.1002/art.1780400509. [DOI] [PubMed] [Google Scholar]
- 89.Ivanov I.I., Apta B.H.R., Bonna A.M., Harper M.T. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019;9:13397. doi: 10.1038/s41598-019-49635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.López-Pedrera C., Aguirre M.A., Buendía P., Barbarroja N., Ruiz-Limón P., Collantes-Estevez E., Velasco F., Khamashta M., Cuadrado M.J. Differential expression of protease-activated receptors in monocytes from patients with primary antiphospholipid syndrome. Arthritis Rheum. 2010;62:869–877. doi: 10.1002/art.27299. [DOI] [PubMed] [Google Scholar]
- 91.Martinod K., Wagner D.D. Thrombosis: Tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yalavarthi S., Gould T.J., Rao A.N., Mazza L.F., Morris A.E., Núñez-Álvarez C., Hernández-Ramírez D., Bockenstedt P.L., Liaw P.C., Cabral A.R., et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zha C., Zhang W., Gao F., Xu J., Jia R., Cai J., Liu Y. Anti-β(2)GPI/β(2)GPI induces neutrophil extracellular traps formation to promote thrombogenesis via the TLR4/MyD88/MAPKs axis activation. Neuropharmacology. 2018;138:140–150. doi: 10.1016/j.neuropharm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Zuo Y., Yalavarthi S., Gockman K., Madison J.A., Gudjonsson J.E., Kahlenberg J.M., Joseph McCune W., Bockenstedt P.L., Karp D.R., Knight J.S. Anti-Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol. 2020;72:2130–2135. doi: 10.1002/art.41460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wienkamp A.K., Erpenbeck L., Rossaint J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022;13:953129. doi: 10.3389/fimmu.2022.953129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vulliamy P., Gillespie S., Armstrong P.C., Allan H.E., Warner T.D., Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc. Natl. Acad. Sci. USA. 2019;116:17444–17449. doi: 10.1073/pnas.1904978116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McDonald B., Urrutia R., Yipp B.G., Jenne C.N., Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 98.Nayak L., Sweet D.R., Thomas A., Lapping S.D., Kalikasingh K., Madera A., Vinayachandran V., Padmanabhan R., Vasudevan N.T., Myers J.T., et al. A targetable pathway in neutrophils mitigates both arterial and venous thrombosis. Sci. Transl. Med. 2022;14:eabj7465. doi: 10.1126/scitranslmed.abj7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agbani E.O., Mahe E., Chaturvedi S., Yamaura L., Schneider P., Barber M.R.W., Choi M., Lee A., Skeith L. Platelets and neutrophils co-drive procoagulant potential in secondary antiphospholipid syndrome during pregnancy. Thromb. Res. 2022;220:141–144. doi: 10.1016/j.thromres.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 100.Breen K.A., Seed P., Parmar K., Moore G.W., Stuart-Smith S.E., Hunt B.J. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb. Haemost. 2012;107:423–429. doi: 10.1182/blood.V116.21.4216.4216. [DOI] [PubMed] [Google Scholar]
- 101.Davis W.D., Brey R.L. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin. Exp. Rheumatol. 1992;10:455–460. [PubMed] [Google Scholar]
- 102.Oku K., Atsumi T., Bohgaki M., Amengual O., Kataoka H., Horita T., Yasuda S., Koike T. Complement activation in patients with primary antiphospholipid syndrome. Ann. Rheum. Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 103.Arachchillage D.R., Mackie I.J., Efthymiou M., Chitolie A., Hunt B.J., Isenberg D.A., Khamashta M., Machin S.J., Cohen H. Rivaroxaban limits complement activation compared with warfarin in antiphospholipid syndrome patients with venous thromboembolism. J. Thromb. Haemost. 2016;14:2177–2186. doi: 10.1111/jth.13475. [DOI] [PubMed] [Google Scholar]
- 104.Pierangeli S.S., Vega-Ostertag M., Liu X., Girardi G. Complement activation: A novel pathogenic mechanism in the antiphospholipid syndrome. Ann. N. Y. Acad. Sci. 2005;1051:413–420. doi: 10.1196/annals.1361.083. [DOI] [PubMed] [Google Scholar]
- 105.Fischetti F., Durigutto P., Pellis V., Debeus A., Macor P., Bulla R., Bossi F., Ziller F., Sblattero D., Meroni P., et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 106.Carrera-Marín A., Romay-Penabad Z., Papalardo E., Reyes-Maldonado E., García-Latorre E., Vargas G., Shilagard T., Pierangeli S. C6 knock-out mice are protected from thrombophilia mediated by antiphospholipid antibodies. Lupus. 2012;21:1497–1505. doi: 10.1177/0961203312458839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dzik S. Complement and Coagulation: Cross Talk Through Time. Transfus. Med. Rev. 2019;33:199–206. doi: 10.1016/j.tmrv.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Del Conde I., Crúz M.A., Zhang H., López J.A., Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peerschke E.I., Yin W., Alpert D.R., Roubey R.A., Salmon J.E., Ghebrehiwet B. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus. 2009;18:530–538. doi: 10.1177/0961203308099974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lood C., Tydén H., Gullstrand B., Sturfelt G., Jönsen A., Truedsson L., Bengtsson A.A. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS ONE. 2014;9:e99386. doi: 10.1371/journal.pone.0099386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gartshteyn Y., Mor A., Shimbo D., Khalili L., Kapoor T., Geraldino-Pardilla L., Alexander R.V., Conklin J., Dervieux T., Askanase A.D. Platelet bound complement split product (PC4d) is a marker of platelet activation and arterial vascular events in Systemic Lupus Erythematosus. Clin. Immunol. 2021;228:108755. doi: 10.1016/j.clim.2021.108755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Svenungsson E., Gustafsson J.T., Grosso G., Rossides M., Gunnarsson I., Jensen-Urstad K., Larsson A., Ekdahl K.N., Nilsson B., Bengtsson A.A., et al. Complement deposition, C4d, on platelets is associated with vascular events in systemic lupus erythematosus. Rheumatology. 2020;59:3264–3274. doi: 10.1093/rheumatology/keaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polley M.J., Nachman R.L. Human platelet activation by C3a and C3a des-arg. J. Exp. Med. 1983;158:603–615. doi: 10.1084/jem.158.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gushiken F.C., Han H., Li J., Rumbaut R.E., Afshar-Kharghan V. Abnormal platelet function in C3-deficient mice. J. Thromb. Haemost. 2009;7:865–870. doi: 10.1111/j.1538-7836.2009.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Subramaniam S., Jurk K., Hobohm L., Jäckel S., Saffarzadeh M., Schwierczek K., Wenzel P., Langer F., Reinhardt C., Ruf W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129:2291–2302. doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sauter R.J., Sauter M., Reis E.S., Emschermann F.N., Nording H., Ebenhöch S., Kraft P., Münzer P., Mauler M., Rheinlaender J., et al. Functional Relevance of the Anaphylatoxin Receptor C3aR for Platelet Function and Arterial Thrombus Formation Marks an Intersection Point Between Innate Immunity and Thrombosis. Circulation. 2018;138:1720–1735. doi: 10.1161/CIRCULATIONAHA.118.034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. doi: 10.1182/blood.V68.4.875.875. [DOI] [PubMed] [Google Scholar]
- 118.Wiedmer T., Esmon C.T., Sims P.J. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J. Biol. Chem. 1986;261:14587–14592. doi: 10.1016/S0021-9258(18)66911-X. [DOI] [PubMed] [Google Scholar]
- 119.Sims P.J., Faioni E.M., Wiedmer T., Shattil S.J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988;263:18205–18212. doi: 10.1016/S0021-9258(19)81346-7. [DOI] [PubMed] [Google Scholar]
- 120.Cohen H., Isenberg D.A. How I treat anticoagulant-refractory thrombotic antiphospholipid syndrome. Blood. 2021;137:299–309. doi: 10.1182/blood.2020004942. [DOI] [PubMed] [Google Scholar]
- 121.Arnaud L., Mathian A., Ruffatti A., Erkan D., Tektonidou M., Cervera R., Forastiero R., Pengo V., Lambert M., Martinez-Zamora M.A., et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: An international and collaborative meta-analysis. Autoimmun. Rev. 2014;13:281–291. doi: 10.1016/j.autrev.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 122.Arnaud L., Mathian A., Devilliers H., Ruffatti A., Tektonidou M., Forastiero R., Pengo V., Lambert M., Lefevre G., Martinez-Zamora M.A., et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun. Rev. 2015;14:192–200. doi: 10.1016/j.autrev.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 123.Crowther M.A., Ginsberg J.S., Julian J., Denburg J., Hirsh J., Douketis J., Laskin C., Fortin P., Anderson D., Kearon C., et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N. Engl. J. Med. 2003;349:1133–1138. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 124.Finazzi G., Marchioli R., Brancaccio V., Schinco P., Wisloff F., Musial J., Baudo F., Berrettini M., Testa S., D’Angelo A., et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS) J. Thromb. Haemost. 2005;3:848–853. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 125.Ruiz-Irastorza G., Hunt B.J., Khamashta M.A. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;57:1487–1495. doi: 10.1002/art.23109. [DOI] [PubMed] [Google Scholar]
- 126.Jackson W.G., Oromendia C., Unlu O., Erkan D., DeSancho M.T. Recurrent thrombosis in patients with antiphospholipid antibodies and arterial thrombosis on antithrombotic therapy. Blood Adv. 2017;1:2320–2324. doi: 10.1182/bloodadvances.2017008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levine S.R., Brey R.L., Tilley B.C., Thompson J.L., Sacco R.L., Sciacca R.R., Murphy A., Lu Y., Costigan T.M., Rhine C., et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA. 2004;291:576–584. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 128.Okuma H., Kitagawa Y., Yasuda T., Tokuoka K., Takagi S. Comparison between single antiplatelet therapy and combination of antiplatelet and anticoagulation therapy for secondary prevention in ischemic stroke patients with antiphospholipid syndrome. Int. J. Med. Sci. 2009;7:15–18. doi: 10.7150/ijms.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ohnishi N., Fujieda Y., Hisada R., Nakamura H., Kato M., Oku K., Bohgaki T., Amengual O., Yasuda S., Atsumi T. Efficacy of dual antiplatelet therapy for preventing recurrence of arterial thrombosis in patients with antiphospholipid syndrome. Rheumatology. 2019;58:969–974. doi: 10.1093/rheumatology/key340. [DOI] [PubMed] [Google Scholar]
- 130.Warner T.D., Nylander S., Whatling C. Anti-platelet therapy: Cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br. J. Clin. Pharmacol. 2011;72:619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pasalic L., Wing-Lun E., Lau J.K., Campbell H., Pennings G.J., Lau E., Connor D., Liang H.P., Muller D., Kritharides L., et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J. Thromb. Haemost. 2018;16:1198–1210. doi: 10.1111/jth.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verro P., Gorelick P.B., Nguyen D. Aspirin plus dipyridamole versus aspirin for prevention of vascular events after stroke or TIA: A meta-analysis. Stroke. 2008;39:1358–1363. doi: 10.1161/STROKEAHA.107.496281. [DOI] [PubMed] [Google Scholar]
- 133.Harker L.A., Kadatz R.A. Mechanism of action of dipyridamole. Thromb. Res. Suppl. 1983;4:39–46. doi: 10.1016/0049-3848(83)90356-0. [DOI] [PubMed] [Google Scholar]
- 134.Toti F., Satta N., Fressinaud E., Meyer D., Freyssinet J.M. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–1415. doi: 10.1182/blood.V87.4.1409.bloodjournal8741409. [DOI] [PubMed] [Google Scholar]
- 135.Hua V.M., Abeynaike L., Glaros E., Campbell H., Pasalic L., Hogg P.J., Chen V.M. Necrotic platelets provide a procoagulant surface during thrombosis. Blood. 2015;126:2852–2862. doi: 10.1182/blood-2015-08-663005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Edwards M.H., Pierangeli S., Liu X., Barker J.H., Anderson G., Harris E.N. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96:4380–4384. doi: 10.1161/01.CIR.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 137.Espinola R.G., Pierangeli S.S., Gharavi A.E., Harris E.N. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb. Haemost. 2002;87:518–522. doi: 10.1055/s-0037-1613033. [DOI] [PubMed] [Google Scholar]
- 138.Lopez-Pedrera C., Ruiz-Limon P., Aguirre M.A., Rodriguez-Ariza A., Cuadrado M.J. Potential use of statins in the treatment of antiphospholipid syndrome. Curr. Rheumatol. Rep. 2012;14:87–94. doi: 10.1007/s11926-011-0222-6. [DOI] [PubMed] [Google Scholar]
- 139.Nenna A., Nappi F., Lusini M., Satriano U.M., Schilirò D., Spadaccio C., Chello M. Effect of Statins on Platelet Activation and Function: From Molecular Pathways to Clinical Effects. Biomed. Res. Int. 2021;2021:6661847. doi: 10.1155/2021/6661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaturvedi S., Braunstein E.M., Yuan X., Yu J., Alexander A., Chen H., Gavriilaki E., Alluri R., Streiff M.B., Petri M., et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135:239–251. doi: 10.1182/blood.2019003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.