Abstract
We have analyzed proteasomal adaptation and associated changes in the B27-bound peptide repertoire in response to cellular invasion with Salmonella. The peptide repertoire of HLA-B27 complexes was analyzed by two different methods: (i) high-pressure liquid chromatography (HPLC) profiles of newly synthesized peptides eluted from B27 following metabolic labeling with arginine and (ii) reactivities with two B27 monoclonal antibodies, Ye-2 and B27.M2, sensitive to peptide-induced conformational changes. LMP, MECL, and PA28 expression was analyzed by reverse transcription-PCR (RT-PCR) of mRNA and by Western blot analysis for LMP2. Invasion of HLA-B27-transfected HeLa cells by Salmonella typhimurium induced significant changes in the reactivities of HLA-B27 with these two antibodies, which was accompanied by significant quantitative and qualitative changes in the HPLC profile of peptides eluted from HLA-B27. We also observed increases in the RT-PCR values for the LMP2, LMP7, and MECL proteasome subunit genes, as well as the proteasomal activator PA28α and -β genes, and increased expression of the LMP2 protein by Western blotting. Upregulation of LMP2, but not LMP7, gene expression showed a close correlation with the changes in antibody reactivities observed upon bacterial invasion. We observed similar changes in reactivity with the Ye-2 or the B27.M2 antibody of lymphoblastoid cells upon gamma interferon treatment, which significantly correlated with the increased RT-PCR values for the LMP2 gene. This was accompanied by consistent HPLC profile changes for eluted peptides. Thus, Salmonella invasion leads to serologically recognizable changes in the B27-bound peptide repertoire, which may include peptides of host origin potentially through modulation of proteasome LMP2 subunit expression and, as a consequence, proteasomal activities.
The class I major histocompatibility complex (MHC) consists of the polymorphic heavy chain, β2-microglobulin, and antigenic peptides. Antigenic peptides are derived from endogenous proteins as well as from the proteins of intracellular pathogens such as viruses and invasive bacteria. To provide antigenic peptides, these proteins are first degraded by the proteolytic multicatalytic proteasome complex (24) and then transported into the endoplasmic reticulum, where they associate with the class I molecule to form the class I MHC. One of the physiological functions of class I MHCs is recognition by peptide-specific T-cell receptors of cytolytic T lymphocytes (CTL). In the case of virally or bacterially infected cells, cytolysis serves to eliminate cells harboring pathogens. It has been demonstrated that for some intracellular bacteria, CTL activity against bacterium-infected cells not only is induced (29) but also plays a role in host defense (5). It has also been postulated that similar CTL activities play a role in autoimmune disease, such as Salmonella typhimurium-induced HLA-B27-related arthritis (12).
Studies of the immune response towards bacterium-infected cells have so far been limited to the recognition of peptides generated from bacterial proteins. One study has shown that class I presentation of bacterium-derived peptides is decreased by inhibitors of the proteasome (17). It is known that the proteolytic specificity of an uninfected cell can change by modulation of the relative contributions of the various components to the entire proteasome complex. The genes of two components, the LMP2 and LMP7 genes, reside in the MHC class II region (11, 16). Their transcription is modulated by activators such as gamma interferon (IFN-γ) (31). A change in the LMP2-to-LMP7 ratio in the complex has been shown to induce changes in the carboxyl termini of the peptides which are generated (10). We have reported previously that in the case of HLA-B27, these IFN-γ-induced changes can be detected by a monoclonal antibody, Ye-2 (32), which demonstrates specificity for peptide C-terminal residues and is sensitive to peptide-induced conformational changes of the heavy chain (9). We later reported on the reactivity of another monoclonal antibody, B27.M2, which is also sensitive to peptide-induced conformational change (15). Proteasomal activity and proteolytic specificity are also influenced by the binding of an IFN-γ-inducible activator protein, PA28α and -β (18), which generates peptides with C termini tailored for binding to class I molecules (7). A further IFN-γ-inducible, though non-MHC-encoded, proteasome subunit, MECL, has recently been identified (21) and may be functional in MHC class I-restricted antigen presentation.
In this work, we have studied the effect of Salmonella invasion on the peptide-sensitive antibody reactivity of HLA-B27 which has been transfected into HeLa cells and have compared the results to the effects of IFN-γ. We found that Salmonella invasion could induce changes in peptide-sensitive antibody reactivities parallel to those induced by IFN-γ and changes in expression of LMP2, LMP7, MECL, and PA28α and -β genes, as evaluated by reverse transcription-PCR (RT-PCR). Using regression analysis, we were able to pinpoint the LMP2 gene as the LMP gene whose expression correlated with the observed changes in peptide-sensitive antibody reactivities. In addition, we have taken advantage of the fact that nearly all peptides that bind HLA-B27 contain an arginine at position 2 (20), and we therefore labeled cells with a [3H]arginine amino acid precursor for incorporation into proteins. Following degradation, these proteins provide labeled peptides which are eventually loaded into class I molecules, such as HLA-B27, and can then be analyzed by high-pressure liquid chromatography (HPLC). This approach increases the sensitivity of the assay and greatly diminishes the quantity of cells necessary for these analyses. HPLC analysis of newly synthesized peptides eluted from B27 revealed significant qualitative and quantitative differences following either bacterial invasion or incubation with IFN-γ. Hence, invasion by Salmonella can lead to recognizable changes in the B27-bound peptide repertoire, possibly through modulation of LMP2 subunit expression.
MATERIALS AND METHODS
Cell lines and culture conditions.
The HeLa cell line was purchased from the American Type Culture Collection, Rockville, Md. We obtained the cDNA for the B*2705 gene, which was already inserted into the RSV5.neo vector, from Beatrice Carreno (University of Washington, Seattle). The vector was transfected into HeLa cells with Lipofectin by a procedure provided by the manufacturer (Gibco BRL, Gaithersburg, Md.). G418-resistant clones were screened by immunofluorescence for the expression of HLA-B27. This transfectant was designated HLA-B27-HeLa. It was cultured in medium supplemented with 0.5 mg of G418 per ml.
All other cell lines were Epstein-Barr virus-transformed human lymphoblastoid cells. They were maintained in RPMI 1640 containing penicillin, streptomycin, l-glutamine, HEPES, and 10% fetal calf serum. The following cell lines were HLA-B27 positive: SEG, GOODGAI, GLEN, SHAWNA, DENISE, DIANE, JES612, WT623, MWI735, 4F4, and HOM2. The following cell lines did not express HLA-B27: LAS, DAD, BOS, JOK, and T1V. The presence of HLA-B27 was determined by the standard microcytotoxicity method with multiple HLA typing sera.
To test the effect of IFN-γ, cells were cultured at 1 ml per well in 24-well plates at 37°C in a humidified 5% CO2 incubator. In preliminary experiments, we determined that the optimum conditions for inducing changes in expression of HLA-B27 were 105 cells per ml, 600 U of IFN-γ (Biosource International, Camarillo, Calif.) per ml, and a total culture period of 72 h.
Bacterial strain and invasion procedure.
The Salmonella wild-type invasive strain used was S. typhimurium SL1344, provided by Brett Finlay, University of British Columbia, Vancouver, Canada. A noninvasive strain (SB111) was also provided. An inoculum of each bacterial strain in a 10-ml aliquot of Luria broth was cultured overnight at 37°C without shaking the culture flask. HeLa or HLA-B27-HeLa cells were seeded at a density of 2 × 106 cells per 100-mm-diameter culture dish. The next day, salmonellae were added to achieve a bacterium/human cell ratio of 25:1. The preparations were then cultured at 37°C for 1 h. Afterwards, they were washed four times with phosphate-buffered saline and incubated in the same volume of RPMI–10% fetal calf serum containing 5 μg of gentamicin sulfate per ml. The culture was continued for another 24 h before the cells were harvested for assay. Control mock-invaded samples were tested in parallel and were the same except that no bacteria were added.
Monoclonal antibodies and immunofluorescence assay.
The anti-HLA-B27 antibodies were ME1, B27.M2, and Ye2. The first was immunoglobulin G (IgG), and the latter two were IgM. The method for indirect immunofluorescence analysis with flow cytometry (fluorescence-activated cell sorting [FACS]; Becton Dickinson, San Jose, Calif.) followed previously described procedures (9). Briefly, cells were incubated first with saturating amounts of first antibodies and then with phycoerythrin-conjugated goat anti-mouse IgG or IgM (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The degree of reactivity was computed as the mean intensity of fluorescence expressed on an arithmetic scale.
Total RNA extraction, reverse transcription, and adjustment of sample dilutions.
Total RNA was extracted by the guanidium thiocyanate procedure according to the protocol provided by the manufacturer (Micro RNA isolation kit; Stratagene, La Jolla, Calif.). The concentration of RNA was assessed by spectrophotometry with GenQuant II (Pharmacia, Piscataway, N.J.). Reverse transcription was carried out in 20-μl reaction volumes. Besides total RNA, each sample contained the following: 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL), 2 U of RNase inhibitor (Promega, Madison, Wis.), 2 μl of 10× PCR buffer II, 5 mM MgCl2 (Perkin-Elmer, Foster City, Calif.), 5 pmol of oligo(dT) (Promega), and 2 mM each dATP, dCTP, dGTP, and dTTP. This mixture was incubated at 42°C for 40 min. The reaction was terminated by incubation at 99°C for 5 min. In preliminary experiments, we tested 10-fold-increasing amounts of total RNA from 0.001 to 1.0 μg per sample. The single-stranded cDNA generated by each sample was then subjected to PCR with primers for β-actin by a PCR method described below. The optimum amount of total RNA for each reverse transcription experiment was determined to be 1.0 μg. This was the amount used in all experiments reported in this paper.
PCR amplification.
PCR amplification of a targeted gene sequence was carried out with an automated thermocycler (RoboCycler 40; Stratagene). Each 20-μl reaction volume consisted of water, 2 μl of 10× PCR buffer, 0.2 μl of 10 mM deoxynucleoside triphosphate mix (Boehringer, Indianopolis, Ind.), 0.2 μl of Taq polymerase (Perkin-Elmer), 2 μl of first-strand cDNA, and 20 pmol of each primer. The reaction mixture was subjected to 30 amplification cycles, each consisting of 96°C for 1 min, 57°C for 30 s, and 72°C for 1 min. The PCR products were separated by electrophoresis in a 2% agarose gel. To assess the amount of amplified product, the DNA was stained with ethidium bromide and photographed with Polaroid (Cambridge, Mass.) type 55 films. The negatives of the films were then subjected to densitometry measurements (UltroScan XL; Pharmacia). All of the densitometry measurements obtained in this study fell in a linear relationship with the amount of DNA. Primer sequences were as follows: for β-actin, sense 5′-AAC TGG GAC GAC ATG GAG AA-3′ and antisense 5′-CCACGT CGC AGCCAT ACA TAT-3′; for HLA-B27, sense 5′-GAC GAC ACG CTG TTC GTG-3′ and antisense 5′-CCA CGT CGC AGC CAT ACA TAT-3′; for LMP2, sense 5′-GGC GTT GTG ATG GGT TCT GAT TCC-3′ and antisense 5′-AAG ATG ACT CGA TGG TCC ACA CCG-3′; for LMP7, sense 5′-CCC TGT TTC CAG CGG ATG C-3′ and antisense 5′-GCA GCA GGT CAC TGA CAT CTG-3′; for PA28β, sense 5′-GCA AAC AGG TGG AGG TCT TCAGG-3′ and antisense 5′-CAT TACATG AGT CTCCTT GGAGG-3′; for PA28α, sense 5′-GTG GAT GTG TTT CGT GAA GACCT-3′ and antisense 5′-GCT GCT TGG CTG CTT TAG TCACT-3′; and for MECL, sense 5′-CGA ACATGACGCTGGAGG CTG-3′ and antisense 5′-CTG GGTCAGGACAGC TGT GGT-3′.
The sequences of the primers for β-actin were those previously published by other investigators (8). The primers for LMP2 and LMP7 were minor modifications of those published by other investigators (22). These two pairs of primers were designed to generate PCR products of 536 and 690 bp, respectively. The validity of these primers was verified by sequencing the PCR products (data not shown). The PA28α and PA28β primers were designed from published sequence data (1, 23) and were verified by sequencing the PCR products (data not shown). These primers generated PCR products of 535 and 515 bp, respectively. The primers to amplify human MECL were based on the published genomic DNA sequence (19). The antisense primer for MECL spanned two intron-exon junctions and generated a PCR product of 212 bp; the validity of the primers was verified by sequencing the PCR products (data not shown). The primers to amplify HLA-B27 were based on published sequence data. The antisense primer in exon 3 is specific for HLA-B27, while the sense primer is in exon 2, generating a PCR product of 266 bp, which was verified by sequencing (data not shown).
Semiquantitative analysis of proteasome subunit gene expression following Salmonella invasion or IFN-γ treatment.
To allow comparison of RT-PCR values for LMP2, LMP7, MECL, HLA-B27, and PA28α and -β genes derived from different samples, the concentration of cDNA in each sample was adjusted so that they would all yield similar amounts of PCR product when amplified by primers for β-actin. In our experiments, the variation of β-actin PCR products between adjusted samples of cDNA did not exceed 5%. Quantitation was computed as a ratio of the densitometry reading for the test gene to that for β-actin. In addition, cDNAs were serially diluted and PCR amplified with specific primers and β-actin primers; test gene/β-actin ratios were calculated by determining the linear range for each sample, plotting the best-fit line, and determining the test gene/β-actin ratio at the x intercept. Samples from control cells and cells invaded by Salmonella or incubated with IFN-γ were analyzed on the same day and run on adjacent lanes. The changes induced by Salmonella or IFN-γ were expressed as a percentage of the values for the control samples. In preliminary experiments, we assayed gene expression at various time points (4, 8, 16, and 24 h) following bacterial invasion (data not shown). Maximal expression was observed at 16 h after invasion, and these data are presented below.
Preparation of LMP2 and anti-human MHC class I antisera.
Rabbit antiserum was generated against the C-terminal peptide of LMP2 (HRVILLNELPKFYDE) (2). Rabbits were primed and challenged repeatedly with peptide conjugated to keyhole limpet hemocyanin emulsified in complete Freund’s adjuvant and incomplete Freund’s adjuvant. Prebleed sera lacked reactivity to proteasome components. The murine H-2Db molecule was purified from EL4 tumor cells grown as ascites fluid in 6- to 8-month old mice as previously described (28). Detergent lysates from 1010 EL4 cells were passaged over a Sepharose 4B precolumn followed by a B22.249 monoclonal antibody column (4). Columns were washed with 0.1% deoxycholate (DOC)–40 mM NaCl–10 mM Tris (pH 8.2) and 0.5% DOC–0.65 M NaCl–10 mM Tris (pH 8.5). The bound H-2Db was eluted with 0.5% DOC–0.15 M NaCl–15 mM Na2CO3 (pH 10.5). Solid-phase enzyme-linked immunosorbent assay with the B22.249 monoclonal antibody was performed to identify fractions containing H-2Db. Purity of the fractions was assessed by silver staining. Protein quantitation was determined by a micro-bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.). Peak fractions, determined by enzyme-linked immunosorbent assay, from each preparation were pooled. Approximately 0.8 ml containing 50 to 150 μg of H-2Db was mixed with an equal volume of complete Freund’s adjuvant and injected into a rabbit. Three additional injections were performed at 1-month intervals with 0.8 ml of the H-2Db samples mixed with incomplete Freund’s adjuvant. The rabbit was bled, and the serum was found to immunoblot several murine class I heavy-chain molecules, including H-2Kb, Db, Kk, Dk, and Dd, and to cross-react with human class I MHC molecules, including HLA-B27, but it did not immunoblot control proteins such as murine class II molecules or antibodies. No reactivity to the class I MHC molecules was observed with the serum from the rabbit prebleed.
Western blot analysis.
Twenty million cells were lysed at 4°C for 30 min in 0.5% Nonidet P-40–150 mM NaCl–50 mM Tris HCl–1 mM phenylmethylsulfonyl fluoride–10 mM iodoacetamide–5 mM EDTA–10 μg of aprotinin per ml. Equivalents of 5 × 105 cells were boiled for 3 min in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer, and the proteins were separated by SDS–10.5% polyacrylamide gel electrophoresis. The separated proteins were electrophoretically transferred onto a nitrocellulose membrane. The membrane was incubated with rabbit anti-LMP2 serum at a 1:100 dilution. Bound antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, Calif.) by using the supersignal CL-HRP substrate system (Pierce, Rockford, Ill.).
In other experiments, cell lysates from 20 × 106 cells were immunoprecipitated with 250 μl (500 μg) of ME1 (HLA-B27-specific) antibody on beads for 24 h. The beads were washed three times in 20 mM Tris (pH 7.5)–150 mM NaCl and then once in 50 mM ammonium acetate (pH 7). Beads were resuspended in reducing buffer, and serial dilutions from salmonella-invaded and control cells were run on an SDS–12.5% acrylamide gel. Proteins were transferred onto an Immobilon-P membrane (Millipore Corporation) over 3 h at 400 mA and blocked overnight with 4% bovine serum albumin. The membrane was incubated with the rabbit anti-human MHC class I serum at a 1:500 dilution. Bound antibodies were detected with HRP-conjugated goat anti-rabbit IgG by using enzyme chemiluminescence (New England Life Science Products).
Isolation and analysis of metabolically labeled B27-bound peptides.
Following incubation with the two S. typhimurium strains, HeLa cells were incubated for 16 h at 37°C at 2 × 106 cells per ml in medium containing 90 μCi of [3H]arginine (Amersham, Oakville, Ontario, Canada) per ml. Following metabolic labeling, the cells were solubilized in lysis buffer composed of 0.5% Triton X-100, 10 mM Tris, and 50 mM NaCl (pH 8.0). The HLA-B27 molecules were then immunoprecipitated with ME1 monoclonal antibody-coupled Sepharose beads by rotation for 4 h at 4°C in the presence of protease inhibitors, aprotinin, and phenylmethylsulfonyl fluoride (Sigma, St. Louis, Mo.). The beads were acid eluted with 10% (vol/vol) acetic acid in water, and low-molecular-weight material corresponding to eluted peptides was recovered after Centricon 10 (Amicon, Beverly, Mass.) filtration by previously described procedures (27). Eluted peptides were washed twice with distilled water and dried with a speed vacuum device. Isolated peptides were resuspended in 0.1% trifluoracetic acid (TFA) in water and separated on a narrow-bore C18 (2 mm by 25 cm) column set up on a Beckman System Gold HPLC with solution A consisting of 0.1% TFA in water and solution B consisting of 0.1%TFA, 80% acetonitrile, and 20% H2O. The flow rate (0.15 to 0.2 ml/min) and rate of increase of the acetonitrile gradient (15 to 50%) were adjusted to maximize resolution of the labeled peptides. Collected fractions (200 μl each) were dried with the speed vacuum device, and radioactivity was determined by scintillation counting.
Statistics.
Statistical comparisons were made only for the percent changes in values for antibody fluorescence intensity and RT-PCR values for gene expression as the methods used were semiquantitative. To compare two parameters, the percent changes in each parameter were entered into separate columns in the Instat software (Graph Pad, San Diego, Calif.). The linear regression figures and the r2 values were generated by the software.
RESULTS
The IFN-γ-induced increase in reactivity with the peptide-sensitive anti-HLA-B27 Ye-2 and B27.M2 antibodies correlates with LMP2 gene expression.
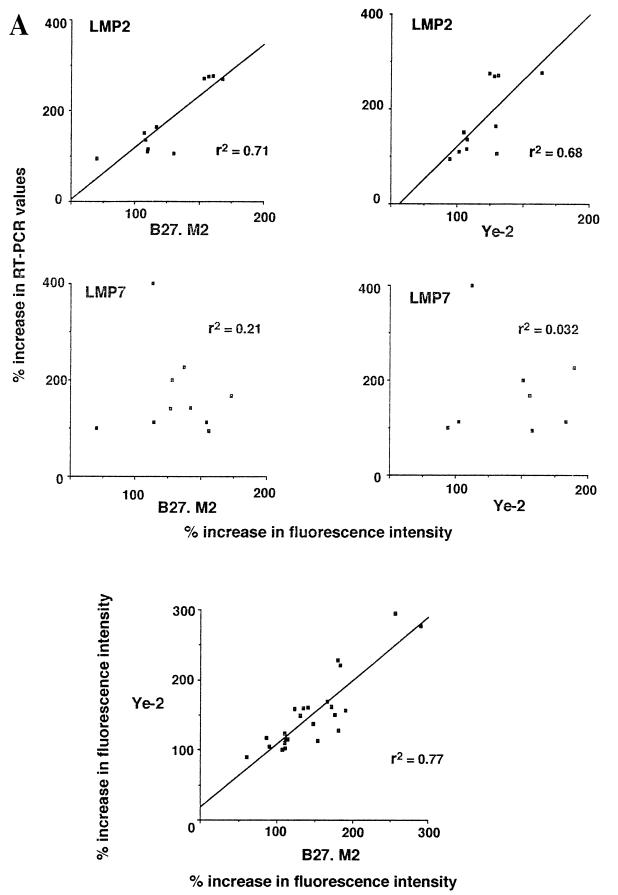
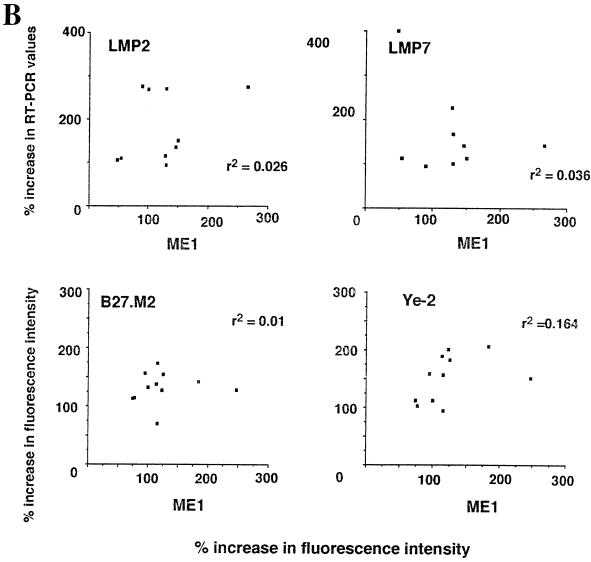
The parallel use of antibodies sensitive to peptide-induced conformational change of the B27 heavy chain and RT-PCR assays of LMP mRNA constitutes a novel approach. To assess the possibility of data correlations with these assays, we tested the effect of IFN-γ on 11 HLA-B27-positive lymphoblastoid cell lines. Depending on the particular cell line, there was considerable variation in changes in RT-PCR values of LMP2 or LMP7, as well as the reactivities with each of the three antibodies B27.M2, Ye-2, and ME1. Strikingly, the variations were not random. The increases in reactivity with either the B27.M2 or the Ye-2 peptide-sensitive antibody correlated remarkably well with increases in LMP2 RT-PCR values (r2 = 0.71 and 0.68, respectively). In contrast, the correlation with increases in LMP7 RT-PCR values was poor (r2 = 0.21 and 0.03, respectively). The results of these experiments are shown in Fig. 1. That these antibody assays were reliable was demonstrated by the finding that none of the HLA-B27-negative lymphoblastoid cell lines reacted positively with B27.M2 or Ye-2, and the IFN-γ-induced increases in reactivities with these two particular peptide-sensitive antibodies in the HLA-B27-positive cells correlated with one another (r2 = 0.77) (Fig. 1). Changes in reactivities with B27.M2 and Ye-2 were not secondary to changes in expression of HLA-B27, because there was no correlation between these changes in B27.M2 and Ye-2 reactivities and changes in reactivities with the non-peptide-sensitive anti-HLA-B27 ME1 antibody (Fig. 1). An increase in the LMP2 protein was also documented by Western blot analysis following incubation with IFN-γ (Fig. 2).
FIG. 1.
Comparison of changes in reactivities of ME1, Ye-2, and B27.M2 monoclonal antibodies to changes in LMP2 and LMP7 RT-PCR values when 11 HLA-B27-positive lymphoblastoid cell lines were incubated with IFN-γ. Reactivities with antibodies were assayed by FACS, while LMP2 and LMP7 mRNA expression was assayed by semiquantitative PCR. Values after IFN-γ incubation are expressed as percent increases over control values. Linear regression figures and r2 values were derived by using Instat software. (A) Comparisons using the peptide-sensitive Ye-2 and B27.M2 antibodies. (B) Comparisons using the non-peptide-sensitive ME1 antibody.
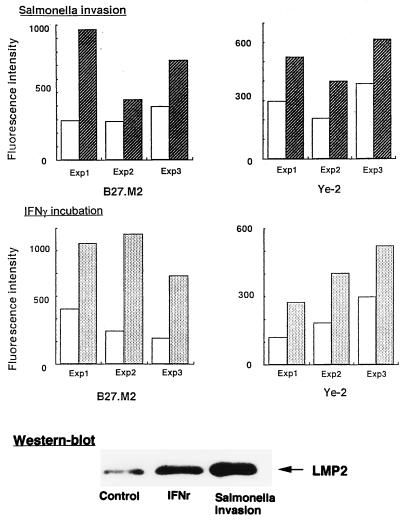
FIG. 2.
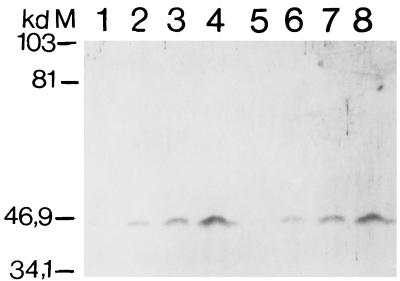
Effect of Salmonella invasion and IFN-γ activation on Ye-2 and B27.M2 antibody reactivities of HLA-B27-HeLa cells in three separate experiments (Exp). Open bars, data from uninvaded controls; hatched and filled bars, data from samples analyzed following Salmonella invasion and IFN-γ incubation, respectively. The lower portion shows a Western blot of HLA-B27-HeLa cell lysates following Salmonella invasion or IFN-γ incubation with LMP2 antiserum.
As a negative control, poor correlation was observed between increases in reactivity with the non-peptide-sensitive anti-HLA-B27 ME1 antibody and increases in RT-PCR values for either LMP2 or LMP7 (r2 = 0.1 and 0, respectively) (Fig. 1).
Salmonella invasion into HLA-B27 HeLa cells induces increased reactivities with the peptide-sensitive Ye-2 and B27.M2 antibodies.
HLA-B27 HeLa cells were subjected to Salmonella invasion, and the antibody reactivities were compared to those for a sample which was mock invaded and a sample which was cultured with the noninvasive mutant SB111. Compared to the mock-invaded sample, significant increases in reactivities were observed with the peptide-sensitive antibodies Ye-2 and B27.M2 (Fig. 2), which was consistently observed in three separate assays (Fig. 2). There was no increase in reactivity with the non-peptide-sensitive ME1 antibody (data not shown). With the parent HeLa cell line, which carries the HLA-A3 and -B5 alleles, no reactivity was observed with anti-HLA-B27 antibodies regardless of invasion. Data from the sample cultured with the noninvasive mutant were similar to those for uninvaded controls (data not shown). As a positive control, increases were also observed with IFN-γ incubation compared to control uninfected HeLa cells (Fig. 2).
Salmonella invasion into HLA-B27-HeLa cells induces increased LMP, MECL, and PA28, but not HLA-B27, expression.
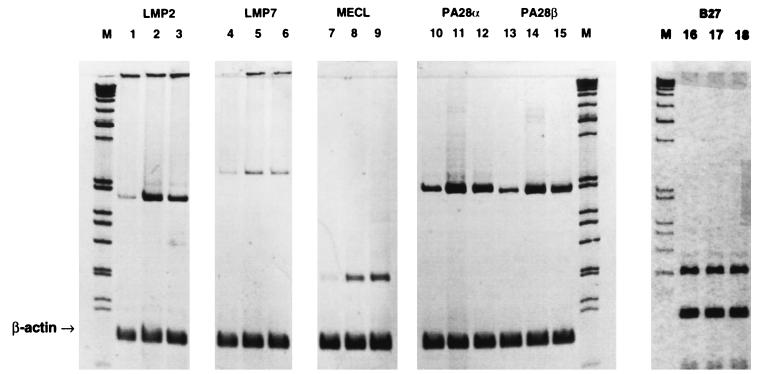
In parallel with increases in reactivities with the peptide-sensitive Ye-2 and B27.M2 antibodies, an increase in RT-PCR values was observed for the LMP2 gene in HLA-B27-HeLa cells following Salmonella invasion (Fig. 3). Increased RT-PCR values for LMP7, MECL, and PA28α and -β, but not HLA-B27, were also observed in cDNA samples where the concentration was adjusted to yield similar amounts of β-actin after PCR amplification (Fig. 3). Incubation with IFN-γ served as a positive control. Test gene/β-actin ratios following PCR amplification of serially diluted template were consistent with the data summarized in Fig. 3 (data not shown). An increase in the LMP2 protein was also documented by Western blot analysis following Salmonella invasion as well as incubation with IFN-γ (Fig. 2). That the increased reactivity of the Ye-2 and B27.M2 antibodies was not due to upregulation of B27 expression was confirmed in immunoprecipitation experiments demonstrating no change in the amount of the HLA-B27 protein following Salmonella invasion (Fig. 4). Incubation of HeLa cells with supernatant from cell cultures with invasive bacteria did not induce LMP2 gene expression, indicating that such expression was unlikely to be secondary to autocrine stimulation with IFN-γ following bacterial invasion (data not shown).
FIG. 3.
Effect of Salmonella invasion and IFN-γ activation on HLA-B27, LMP2, LMP7, MECL, and PA28α and -β gene expression in HLA-B27-HeLa cells as determined by RT-PCR. Lanes 1, 4, 7, 10, 13, and 16, control; lanes 2, 5, 8, 11, 14, and 17, IFN-γ incubation; lanes 3, 6, 9, 12, 15, and 18, Salmonella invasion; lanes M, markers.
FIG. 4.
Western blot analysis with rabbit anti-class I antibody of HLA-B27 immunoprecipitated with the B27-specific ME1 antibody from HeLa cells. Lanes 1 to 4, progressive doubling of immunoprecipitate from Salmonella-invaded HeLa cells; lanes 5 to 8, progressive doubling of immunoprecipitate from control, uninvaded HeLa cells; lane M, markers.
HPLC profiles of newly synthesized HLA-B27-bound peptides.
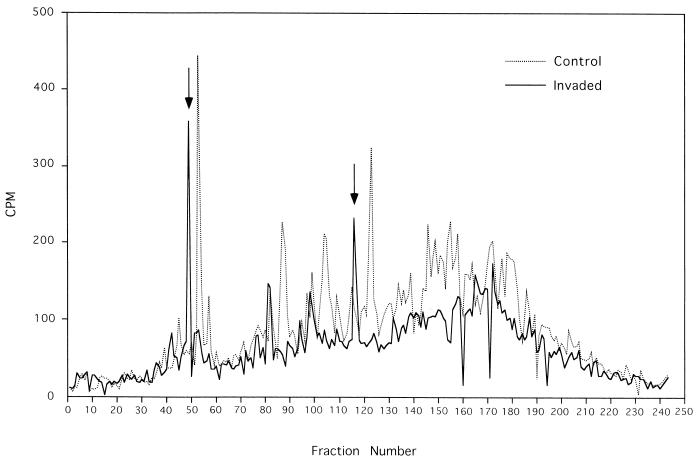
The preceding results indicate that Salmonella invasion of HeLa cells results in the induction of LMP2 expression, a change associated with alteration of the binding characteristics of monoclonal antibodies that recognize peptide-induced conformational change of the B27 heavy chain, suggesting that invasion may alter the peptides bound by HLA-B27 in the invaded cell. To address this possibility more directly, we analyzed the repertoire of B27-bound peptides of B27-transfected HeLa cells following bacterial invasion and compared this with the peptide profile of B27-bound peptides from the same HeLa cells in the absence of invasion. The HPLC profile of [3H]arginine-labeled peptides extracted from HLA-B27 molecules from noninvaded cells shows a diverse profile of peptides that distinguishes at least 40 peptide peaks, including approximately 20 major peptide peaks (Fig. 5). Salmonella invasion substantially alters the profile of bound peptides found in HLA-B27. Qualitative changes include both the appearance of prominent new peptide peaks and the reduction in yield or disappearance of others, particularly in the late elution fractions. Quantitative changes include an overall diminution in [3H]arginine-labeled peptides eluted from B27. These conclusions are based on reproducible changes in HPLC peptide profiles observed in four separate experiments. In particular, repeated analyses demonstrated that the prominent new peaks following bacterial invasion highlighted in Fig. 5 were consistently observed (data not shown). Thus, despite the fact that overall B27 expression as measured by ME1 FACS and immunoprecipitation of HLA-B27 is unchanged following Salmonella invasion (Fig. 4), significant changes are occurring in the repertoire of arginine-containing peptides bound by B27.
FIG. 5.
HPLC profiles of newly synthesized HLA-B27-associated peptides in the HLA-B27-HeLa cell line and the effect of Salmonella invasion. HPLC profiles shown are from one of four separate experiments demonstrating reproducible changes. The arrows indicate prominent new peptide peaks.
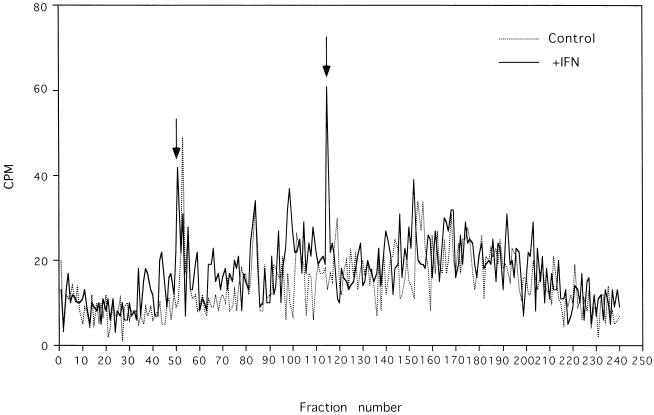
We also evaluated HPLC profiles of B27-eluted peptides following HeLa cell incubation with and without IFN-γ. Again, significant HPLC profile differences were observed (Fig. 6), and certain prominent new peaks after stimulation with IFN-γ (Fig. 6) were consistently detected in repeated analyses (data not shown). Despite some differences in the overall yield of [3H]arginine-labeled peptides between experiments, superimposition of HPLC profiles following either Salmonella invasion or incubation with IFN-γ revealed that two induced peptide peaks appeared to correspond in retention time (Fig. 5 and 6). The radioactivity in HPLC profiles of acid-eluted peptides from antibody isotype control beads incubated with lysates from S. typhimurium-invaded HeLa cells and HeLa cells incubated with and without IFN-γ was less than 25 cpm per fraction (data not shown).
FIG. 6.
HPLC profiles of newly synthesized HLA-B27-associated peptides in the HLA-B27-HeLa cell line and the effect of IFN-γ. HPLC profiles shown are from one of four separate experiments demonstrating reproducible changes. The arrows indicate prominent new peaks.
DISCUSSION
While the effectiveness of CTL in defense against viral infection has been known and manipulated for decades, study of their role in bacterial infection is more recent. Nevertheless, multiple investigators have succeeded in generating MHC class I-restricted CTL against target cells infected in vitro with, for example, S. typhimurium, Yersinia eneterocolitica, and Chlamydia trachomatis (12, 29, 30). Only a small number of bacterial proteins which provide peptides for T-cell recognition have been identified (29, 30). Mounting evidence suggests that pathogen-derived proteins are processed by the same cytosolic pathway utilized by host proteins (17). Not surprisingly, certain viruses have developed subversive strategies to evade class I-restricted antigen processing and presentation (13). Strategies by which intracellular bacteria might attempt to evade or modify antigen processing have not yet been reported. Understanding the interplay between host cells and invading gram-negative bacteria with respect to class I-restricted antigen processing and presentation may yield insights toward defining the molecular basis of HLA-B27-associated arthritides induced by disease-associated bacteria.
The major contribution of the present study is that it shows that invasion by S. typhimurium induces transcription of the LMP2, LMP7, MECL, and PA28α and -β genes and expression of the LMP2 protein, a subunit of the cytosolic proteasome complex responsible for class I-restricted antigen processing. These changes parallel those reported with IFN-γ. In the case of IFN-γ, the LMP2, LMP7, and recently described MECL1 (21) subunits become integrated into the 20S complex of the proteasome, displacing the respective constituents Y, X, and Z (3, 14). As a result, there is modification of the terminal cleavage specificity of the catalytic core. The results of our experiments with IFN-γ and HLA-B27-transfected HeLa cells are consistent with the above-described general principle and indicate that cellular stimuli resulting in modification of proteasome subunit expression can be extended to include encounters with invasive bacteria (26). Furthermore, we were able to identify LMP2 expression in particular as correlating with changes to the B27-bound peptide repertoire as detected by peptide-sensitive antibodies. However, this type of correlation analysis alone offers only indirect evidence for the role of LMP2 in inducing changes in peptide repertoire. Direct evidence requires the analysis of the peptide repertoire.
A previous study has demonstrated alterations in the HPLC profiles of HLA-B27-bound peptides following Salmonella infection of CIR B27-transfected cells, although the changes observed were primarily quantitative rather than qualitative as they appear in our study (25). A distinction from the previous study with respect to B27-bound peptide analysis is that we performed metabolic labeling of peptides with radiolabeled arginine, a nearly canonical B27 peptide anchor residue for the B27 peptide binding groove. This approach can provide greater sensitivity for peptide repertoire changes subsequent to or as a consequence of bacterial invasion or IFN-γ treatment, since it identifies newly generated peptides that are not displayed against a potentially large background of previously processed B27-bound peptides.
A recent study has shown altered expression of serologic HLA-B27 epitopes on human monocytes following exposure to Yersinia or invasion by Salmonella enteritidis, although in contrast with our data, ME1 epitope expression was decreased (33). This could reflect differences in signal transduction and/or antigen processing associated with bacterial invasion. In addition, similar Salmonella invasion assays with the U937 cell line in our lab resulted in toxicity, consistent with other data indicating that Salmonella invasion of phagocytic cell lines results in cell death (15a), suggesting that decreased ME1 epitope expression may reflect a toxicity phenomenon.
Based on previous findings with IFN-γ (10), it is likely that activation of the LMP2 gene induced by S. typhimurium invasion will alter antigen processing and consequently the HLA-B27 peptide repertoire. Indeed, our results from experiments using peptide-sensitive antibodies as well as HPLC analysis of newly synthesized peptides are consistent with this hypothesis. It seems unlikely that LMP2 gene activation following bacterial invasion is secondary to autocrine stimulation with IFN-γ. Incubation of HeLa cells with supernatant from cell cultures with invasive bacteria did not induce LMP2 gene expression. Furthermore, we did not observe increased expression of the IFN-γ-inducible HLA-B27 gene following bacterial invasion.
An intriguing consequence of the changes in LMP2 expression induced by Salmonella invasion is that they may result in changes in the processing of self proteins to yield autoantigens. We have reported previously that activation of synovial T lymphocytes by bacterium-invaded stimulator cells can lead to generation of “autoreactive” CD8+ CTL (12). The findings in this paper provide a potential mechanism for generation of immunogenic self peptides.
Finally, the present findings are also important in clearly emphasizing that invasion of bacteria into host cells is associated with complex events. The view that they merely provide target peptides is likely an oversimplification. A major clue as to how Salmonella might induce multiple cellular events is provided by recent findings that bacterial invasion activates signal transduction systems of host cells (6). This activation probably leads to generation of transcription factors such as AP1 and NF-κB. Since a particular transcription factor is utilized by enhancer regions of multiple genes, several unrelated events will be triggered. In addition, if cytokine genes are triggered, the cytokines can in turn exert an autocrine effect and recruit additional gene activation. These events might hold the key to defense or generation of autoimmunity.
ACKNOWLEDGMENTS
We thank Elizabeth Hermann for review of the project.
Walter P. Maksymowych and Takashi Ikawa contributed equally to this work.
This work was supported by the Medical Research Council (MRC) of Canada, the Nora Eccles Treadwell Foundation, and the U.S. Department of Agriculture. W.P.M. is a Scholar with the Alberta Heritage Foundation for Medical Research (AHFMR), and K.P.K. is an AHFMR Senior Scholar and MRC Scholar.
REFERENCES
- 1.Ahn J Y, Tanahashi N, Akiyama K, Hisamatsu H, Noda C, Tanaka K, Chung C H, Shibmara N, Willy P J, Mott J D, Slaughter C A, DeMartino G N. Primary structures of two homologous subunits of PA28, a γ-interferon-inducible protein activator of the 20S proteasome. FEBS Lett. 1995;366:37–42. doi: 10.1016/0014-5793(95)00492-r. [DOI] [PubMed] [Google Scholar]
- 2.Aki M, Shimbara N, Takashina M, Akiyama K, Kagawa S, Tamura T, Tanahashi N, Yoshimura T, Tanaka K, Ichihara A. Interferon-γ induces different subunit organizations and functional diversity of proteasomes. J Biochem. 1994;115:247–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama K, Yokota K, Kagawa S, Shimbara N, Tamura T, Akioka H, Nothwang H G, Noda C, Tanaka K, Ichihara A. cDNA cloning and interferon γ down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- 4.Allen H, Wraith D, Pala P, Askonas B, Flavell R A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1948;309:279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth I B, Beer M, Bohn E, Kaufmann S H, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliska J B, Galán J E, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 7.Dick P T, Ruppert T, Groettrup M, Kloetzel P M, Kuehn L, Koszinowski U H, Stevanovic S, Schild H, Rammensee H G. Coordinated dual cleavages induced by the proteasome regulator PA28 lead to dominant MHC ligands. Cell. 1996;86:253–262. doi: 10.1016/s0092-8674(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez O, Coto E, Martinez-Naves E, Choo S Y, Lopez-Larrea C. Molecular typing of HLA-B27 alleles. Immunogenetics. 1992;36:277–282. doi: 10.1007/BF00215655. [DOI] [PubMed] [Google Scholar]
- 9.Fukazawa T, Wang J, Huang F, Wen J, Tyan D, Williams K M, Raybourne R B, Yu D T Y. Testing the importance of each residue in a HLA-B27 binding peptide using monoclonal antibodies. J Immunol. 1994;152:1190–1196. [PubMed] [Google Scholar]
- 10.Gaczynska M, Rock K L, Spies T, Goldberg A L. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci USA. 1994;91:8213–8217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glynne R, Powis S H, Beck S, Kelly A, Kerr L A, Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991;353:357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- 12.Hermann E, Yu D T Y, Meyerzum-Buschenfelde K H, Fleischer B. HLA-B27 restricted CD8 T cells derived from the synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 13.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 14.Hisamatsu H, Shimbara N, Saito Y, Kristensen P, Hendil K B, Fujiwara T, Takahashi E I, Tanahashi N, Tamura T, Ichihara A, Tanaka K. Newly identified pair of proteasomal subunits regulated reciprocally by interferonγ. J Exp Med. 1996;183:1807–1816. doi: 10.1084/jem.183.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang F, Hermann E, Wang J, Cheng X K, Tsai W C, Wen J, Kuipers J G, Kellner H, Ackermann B, Roth G, Yu D T Y, Raybourne R B. A patient-derived cytotoxic T-lymphocyte clone and two peptide-dependent monoclonal antibodies recognize HLA-B27 peptide complexes with low stringency for peptide sequences. Infect Immun. 1996;64:120–127. doi: 10.1128/iai.64.1.120-127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Jones, B. Personal communication.
- 16.Kelly A, Powis S H, Glynne R, Radley E, Beck S, Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991;353:667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- 17.Kovascovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 18.Kuehn L, Dahlmann B. Proteasome activator PA28 and its interaction with 20S proteasomes. Arch Biochem Biophys. 1996;329:87–96. doi: 10.1006/abbi.1996.0195. [DOI] [PubMed] [Google Scholar]
- 19.Larsen F, Soheim J, Kristensen T, Kolsto A B, Prydz H. A tight cluster of 5 unrelated human genes on chromosome 16q22.1. Hum Mol Genet. 1993;2:1589–1592. doi: 10.1093/hmg/2.10.1589. [DOI] [PubMed] [Google Scholar]
- 20.Madden D R, Gorga J C, Strominger J L, Wiley D C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991;353:321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- 21.Nandi D, Jiang H, Monaco J J. Identification of MECL1-1 (LMP-10) as the third IFNγ-inducible proteasome subunit. J Immunol. 1996;156:2361–2364. [PubMed] [Google Scholar]
- 22.Nocera A, Barocci S, Gorski J. Transcription analysis of non-HLA genes within or flanking the class II region in a B cell line from an HLA-SCID patient. Tissue Antigens. 1993;41:94–96. doi: 10.1111/j.1399-0039.1993.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 23.Realini C, Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Molecular cloning and expression of a γ-interferon-inducible activator of the multicatalytic protease. J Biol Chem. 1994;269:20727–20732. [PubMed] [Google Scholar]
- 24.Rechsteiner M, Hoffman L, Dubiel W. The multicatalytic and 26S proteases. J Biol Chem. 1993;268:6066–6068. [PubMed] [Google Scholar]
- 25.Ringrose, J. H., B. A. Yard, A. Muijsers, C. J. P. Boog, and T. E. W. Feltkamp. 1996. Comparison of peptides eluted from the groove of HLA-B27 from Salmonella infected and non-infected cells. Clin. Rheumatol. 15(Suppl. 1):74–78. [DOI] [PubMed]
- 26.Rock K L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Kane K P. Differential ability of isolated H-2Kb subsets to serve as TCR ligands for allo-specific CTL clones: potential role for N-linked glycosylation. J Exp Med. 1995;181:1773–1783. doi: 10.1084/jem.181.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen L, Potter T, Kane K P. Glu 227 Lys substitution in the acidic loop of major histocompatibility complex class I α3 domain distinguishes low avidity CD8 coreceptor and avidity-enhanced CD8 accessory function. J Exp Med. 1996;184:1671–1683. doi: 10.1084/jem.184.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starnbach M N, Bevan M J. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J Immunol. 1994;152:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 30.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 31.Tanaka K. Role of proteasomes modified by interferon-gamma in antigen processing. J Leukoc Biol. 1994;56:571–575. doi: 10.1002/jlb.56.5.571. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yu D T Y, Fukazawa T, Kellner H, Wen J, Cheng X K, Roth G, Williams K M, Raybourne R B. A monoclonal antibody that recognizes HLA-B27 in the context of peptides. J Immunol. 1994;152:1197–1205. [PubMed] [Google Scholar]
- 33.Wuorela M, Jalkanen S, Kirveskari J, Laitio P, Granfors K. Yersinia enterocolitica serotype O:3 alters the expression of serologic HLA-B27 epitopes on human monocytes. Infect Immun. 1997;65:2060–2066. doi: 10.1128/iai.65.6.2060-2066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]