Abstract
Spiders, abundant and diverse arthropods which occur in vegetation, have received little attention in studies investigating spider–plant interactions, especially in plants which have extrafloral nectaries (EFNs). This study examines whether spiders attracted to EFNs on the plant Heteropterys pteropetala (Malpighiaceae) function as biological protectors, mitigating leaf herbivory and positively impacting plant fitness, through manipulative experiments. Spiders are attracted to EFNs because, in addition to consuming the resource offered by these structures, they also consume the herbivores that are attracted by the nectar. At the same time, we documented the reproductive phenology of the plant studied and the abundance of spiders over time. Our results revealed that the plant’s reproductive period begins in December with the emergence of flower buds and ends in April with the production of samarids, fruits which are morphologically adapted for wind dispersal, aligning with the peak abundance of spiders. Furthermore, our results demonstrated that spiders are attracted to plants that exude EFNs, resulting in a positive impact on reducing leaf area loss but with a neutral effect on protecting reproductive structures. By revealing the protective function of spiders’ vegetative structures on plants, this research highlights the ecological importance of elucidating the dynamics between spiders and plants, contributing to a deeper understanding of ecosystems.
Keywords: Heteropoterys pteropetala, biotic defence, Cerrado, plant fitness, facultative mutualism, neutral effect
1. Introduction
Interactions between spiders and plants can provide evidence of the existence of facultative mutualistic relationships, influencing the structure of ecological communities and the fitness of plants [1,2,3,4]. Understanding the function of each organism in this interaction will allow for a better understanding of the evolutionary paths that lead to mutualism. Spiders are considered excellent predators, and, when they live on plants, they forage for their prey. In addition, spiders occasionally use plant resources to supplement their insect-based diet [5,6,7,8]. Among these plant-supplied foods are extrafloral nectaries (EFNs) [8,9]. Thus, spiders provide various services to plants, including protection against leaf and flower herbivores, consequently reducing leaf herbivory and/or increasing seed production [1,10,11,12], acting as important biological defenders [13]. Although spiders are traditionally considered predators in ecosystems, these organisms also frequently feed on the products of extrafloral nectaries [14]. Biotic defences have a mutualistic character whereby resources such as plant EFNs are exchanged for spider services and are mediated by the interests, costs, and benefits for both groups [8]. The costs and benefits of this association can vary depending on various factors, such as the identity of the spider family [15], the season of EFN activity, and even the presence of competitors such as ants [1,2,16]. However, the relationship between spiders and plants can also be negative, as spiders consume or interfere with pollinators, leading to a reduction in plant fitness [17,18].
EFNs are nectar-producing structures not associated with pollination and found in various parts of plants, such as leaves, stems, and flower bud calyxes [19,20]. These nectaries produce a solution rich in water, sugars, amino acids, proteins, and lipids [21,22]. EFN-bearing plants are common in the Cerrado biome [23,24]. A study by Nahas et al. [14] found the presence of fructose from EFNs from eight different plant species in 39 spider species from seven families. This indicates that feeding on EFNs is advantageous for spiders, as nectar is an excellent source of energy [1,25,26,27].
Although spiders are among the most abundant and diverse arthropods in vegetation, studies on their interactions with plants are relatively scarce, and the literature on integrative studies on the relationship between spiders and extrafloral nectaries is still limited [28,29]. In this context, the aim of this study is to determine whether or not the presence of extrafloral nectaries on Heteropterys pteropetala A. Juss. (Malpighiaceae) is an attractive factor for spiders and whether these animals act as biological protectors. Our main hypothesis is that spiders are attracted to the extrafloral nectaries on this plant and, consequently, that their presence reduces leaf damage and increases the reproductive success of H. pteropetala (Table 1). Spiders can reduce damage to leaves and increase the reproductive success of a plant by consuming the herbivores present on it. In addition, we describe the phenology of the different reproductive phases of H. pteropetala and the abundance of spiders found over time.
Table 1.
Overview of the hypotheses (H) and predictions tested in this study. EFNsinactive are the nectaries that were obstructed with enamel, while EFNsactive are the nectaries without manipulation (see methodology).
| Overview | Prediction | Approach | Resource |
|---|---|---|---|
| H1: EFNs attract spiders. | EFNsactive plants exhibit a higher abundance of spiders compared to EFNsinactive plants. | Evaluation of spider abundance between EFNsactive and EFNsinactive plants. |
Figure 1 and Figure 2
Table 2 |
| H2: Spiders act as protectors against leaf damage. | EFNsactive plants have lower herbivory rates than EFNsinactive plants. | Analysis of herbivory rates throughout the year between EFNsactive and EFNsinactive plants. |
Figure 3
Table 3 |
| H3: Positive impact of spiders on plant reproductive success. | EFNsactive plants show a higher reproductive rate than EFNsinactive plants. | Evaluation of the ratio between samarids/buds and samarids/flowers as well as the total seed weight and fruiting rate between EFNsactive and EFNsinactive plants. | Table 4 |
2. Results
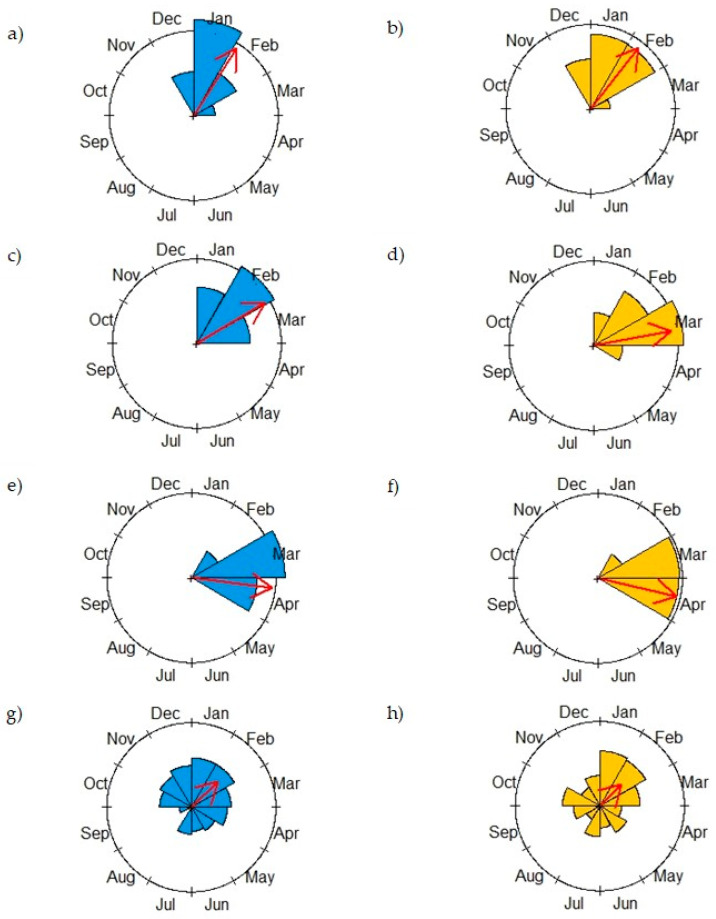
The reproductive period of the H. pteropetala in our study began in December 2021 with the emergence of the first floral buds and ended in May 2022 with the collection of the samarids. The reproductive peak for the floral buds in both groups (EFNsactive and EFNsinactive) was in February (Rayleigh test for buds with EFNsactive z = 0.94; p < 0.001; buds with EFNsinactive z = 0.91; p < 0.001) (Table 2, Figure 1a,b). The inflorescences on the plants EFNsactive reached their peak in February (Rayleigh test: z = 0.943; p < 0.01), while the EFNsinactive plants reached their inflorescence peak in March (Rayleigh test: z = 0.932; p < 0.01) (Table 1, Figure 1c,d). The peak of samarid production occurred in April for both manipulations (Rayleigh test for samarids with EFNsactive z = 0.957; p < 0.01; samarids with EFNsinactive z = 0.952; p < 0.01) (Table 1, Figure 1e,f). Spider abundance was higher in January and February, with peak abundance being observed in February in both manipulations (EFNsactive z = 0.436; p < 0.01; and EFNsinactive z = 0.366; p < 0.03) (Table 1, Figure 1g,h). There was an absence of spiders on the EFNsactive plants in August, and the lowest abundance of spiders was also recorded in August for the EFNsinactive plants.
Table 2.
Circular statistics applied to reproductive phenophases and spider abundance in Heteropoterys pteropetala with EFNsactive (n = 20) and EFNsinactive (n = 20) in a Cerrado area at the Ecological Reserve of Clube Caça e Pesca Itororó in Uberlândia, Minas Gerais, Brazil. The Rayleigh test was conducted with a significance level of 0.05.
| Reproductive Phenophases | Abundance | |||||||
|---|---|---|---|---|---|---|---|---|
| Floral Buttons EFNsactive |
Floral Buttons EFNsinactive |
Flowers EFNsactive | Flowers EFNsinactive | Samarids EFNsactive |
Samarids EFNsinactive |
Spiders EFNsactive |
Spiders EFNsinactive |
|
| Abundance total | 15,368 | 14,734 | 7183 | 13,140 | 24,105 | 24,620 | 97 | 60 |
| Length of mean vector (r) | 0.49 | 0.44 | 0.51 | 0.85 | 0.77 | 0.84 | 0.43 | 0.37 |
| Mean vector (µ) | 32.15 | 34.1 | 59.4 | 71.3 | 97.8 | 102.2 | 42.7 | 41.65 |
| Month | February | February | February | March | April | April | February | February |
| Rayleigh test (Z) | 0.94 | 0.91 | 0.943 | 0.932 | 0.957 | 0.952 | 0.436 | 0.366 |
| Rayleigh test (p) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.01 | 0.03 |
Figure 1.
Number of reproductive structures produced by Heteropoterys pteropetala and spider abundance between December 2021 and January 2022 for the EFNsactive (blue, left) and EFNsinactive (yellow, right) groups: (a,b) floral buds; (c,d) inflorescences; (e,f) samarids; and (g,h) spider abundance. Arrow position represents the mean vector (µ), and arrow length represents the length of the mean vector (r).
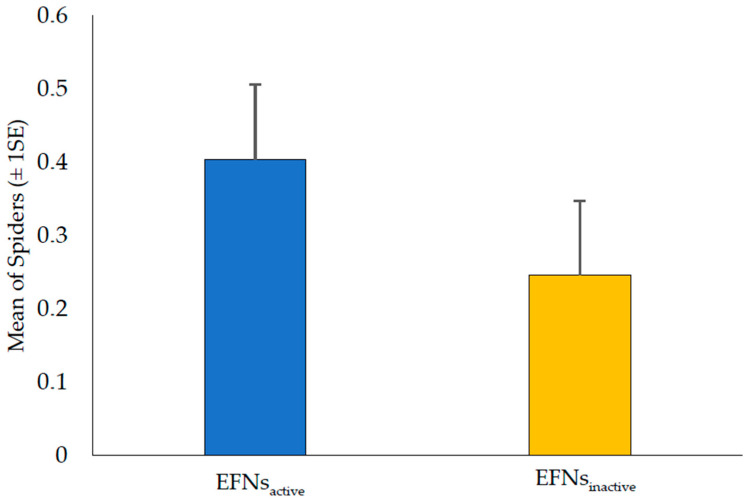
Spider abundance was higher in the EFNsactive plants than in the EFNsinactive plants (χ2 = 9.0681; df = 1; p = 0.0026, Figure 2). A total of 157 spider specimens were counted, with 97 individuals found on the EFNsactive plants and 60 found on the EFNsinactive plants (Table 3). The EFNsactive plants had a higher number of spiders from the families Thomisidae, Araneidae, and Salticidae compared to the EFNsinactive plants (Table 3). However, only the Cheiracanthiidae family had a higher number of representatives in the EFNsinactive plants compared to the EFNsactive plants (Table 3). The families Theridiidae and Oxyopidae showed no significant difference in the number of individuals between the treatment and control plants. A total of 470 insects were seen to be associated with H. pteropetala in both treatments, with the orders Lepidoptera (larval stage) and Hemiptera being the most abundant, especially in plants with EFNsinactive (Table A1).
Figure 2.
Mean number of spiders (±1 SE/standard error) per plant of Heteropoterys pteropetala with and without active EFNs (Extrafloral Nectaries).
Table 3.
Abundance of spider families found in different manipulations of the plant Heteropoterys pteropetala.
| Family | Number of Individuals on Plants with EFNsactive | Number of Individuals on Plants with EFNsinactive | X2 | p |
|---|---|---|---|---|
| Thomisidae | 47 | 27 | 7.33 | 0.026 |
| Araneidae | 22 | 7 | 9.88 | 0.014 |
| Salticidae | 13 | 7 | 8.87 | 0.042 |
| Theridiidae | 9 | 6 | 2.21 | 0.652 |
| Cheiracanthiidae | 4 | 12 | 8.21 | 0.041 |
| Oxyopidae | 2 | 1 | 0.24 | 0.423 |
| Total | 97 | 60 |
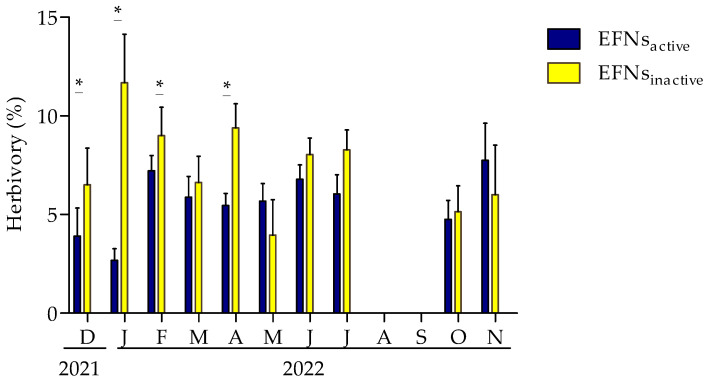
The EFNsinactive plants showed less loss of leaf area (χ2 = 35.646; df = 1; p = 0.0019) and less variation in herbivory within the manipulated groups (χ2 = 11.609; df = 1; p = 0.0065), and this variation persisted over the months studied (χ2 = 13.206; df = 1; p = 0.0001) compared to the EFNsinactive plants. There was also variation in the herbivory between different plant manipulations in December (2021), January, February, and April (2022) (Figure 3). In August and September, the plants were leafless, which resulted in a lack of herbivory, and they only began to sprout again in September (Figure 3). The samarid/bud ratios, samarid/flower ratios, and seed weight did not differ significantly between the EFNsactive and EFNsinactive plants (Table 4).
Figure 3.
Foliar area loss (mean ± SE/standard error) in Heteropoterys pteropetala from December 2021 to November 2022. The response variable was herbivory; plant type (EFNsactive and EFNsinactive) was the predictor variable; months of the year and plant identification were the random variables. * = significant difference between the manipulations (Tukey’s post hoc: p < 0.05).
Table 4.
Productivity of Heteropoterys pteropetala in the presence and absence of spiders indicated by the ratio of samarids/buds, samarids/flowers, and seed weight. Values represent mean ± SE (standard error).
| EFNsactive | EFNsinactive | X2 | p | |
|---|---|---|---|---|
| Samarids/Buds (Gamma) | 1.7 ± 0.13 | 2 ± 0.13 | 0.957 | 0.328 |
| Samarids/Flowers (Gamma) | 0.384 ± 0.132 | 0.563 ± 0.132 | 2.36 | 0.12 |
| Seed Weight (Gaussian) | 23.08 ± 2.28 | 26.16 ± 2.28 | 0.0672 | 0.795 |
3. Discussion
Our study suggests that spiders are attracted to plants that exude EFNs and that this attraction has a positive effect on reducing leaf area loss for H. pteropetala plants in the Brazilian Cerrado, confirming the first and second hypotheses. Thus, we have demonstrated that the presence of active nectaries acts as a source of attraction for spiders that can act as efficient biological protectors. In other words, the nectar produced in EFNs can complement the diet of arthropod predators [26,27] and consequently attract these organisms, leading to a reduction in herbivory [30].
In particular, we found that active EFNs more efficiently attract spiders belonging to the Thomisidae, Araneidae, and Salticidae families. Similar results were obtained by Stefani et al. [31], who, after isolating shrubs of Palicourea rigida Kunth (Rubiaceae) from ants and measuring the recruitment of spiders visiting post-floral nectaries, found representatives of the Thomisidae and Salticidae families to be among those most abundant. This abundance of spiders may be associated with both the attractiveness of the nectar and the absence of ants. Supposedly, the great challenge faced by different species of spiders that use EFNs as a source of complementary food is to break through the defenses promoted by ants [2,16]. The greatest abundance of spiders in our study was recorded during the reproductive period of H. pteropetala in both manipulations (Figure 1), although the greatest abundance was observed in the EFNsactive plants (Figure 1 and Figure 2; Table 2). This increase in abundance (in both manipulations) may have influenced neutral protection rates during this period, refuting our third hypothesis. The presence of reproductive structures, such as buds, flowers, and fruits (forming samarids), can provide spiders with a wide variety of shelters, opportunities to find conspecifics, anchoring points for webs, and opportunities to use different foraging methods, even on plants with inactive EFNs [11,32].
Of all the spider families found, the Thomisidae family was the most abundant (Table 3), found on the vegetative and reproductive parts of H. pteropetala. Thomisidae spiders are known to be flower spiders, as they often camouflage themselves in flower petals or structures, waiting for pollinating prey to arrive [33]. Thus, spiders from the Thomisidae family are strongly associated with the reproductive period of their host plants. Studies have shown that, when individuals from this family are present in the reproductive parts of a plant, they can have positive, neutral, or negative effects on its reproduction. For example, Romero and Vasconcellos-Neto (2003) demonstrated positive effects on the reproduction of Trichogoniopsis adenantha (DC) (Asteraceae) in the presence of Thomisidae spiders, as the plants with the presence of these spiders produced more seeds compared to the plants without them [34]. Neutral effects, for example, were presented by Gavini et al. (2019), who studied interactions between the flowers of Anemone multifida (Ranunculaceae), their floral visitors, and Misumenops pallidus (Thomisidae). The authors observed that the presence of spiders did not reduce the number of floral visitors or the quantity and quality of the fruit and seeds formed [12]. Finally, there is ample evidence of the negative effects that spiders have on the reproduction of their host plants. For example, in another study, the presence of Thomisidae spiders in Leucanthemum vulgare (Vaill.) Lam. (Asteraceae) flowers reduced the number of floral visitors and the time pollinators spent in the flowers, generating a cascade effect which resulted in a 17% reduction in fruit and seed formation [35].
Spiders from the Salticidae and Araneidae families were also more abundant on the EFNsactive plants in our study. According to Jackson et al. (2001), Salticidae spiders may have the habit of feeding on nectar, indicating that nectar feeding is possibly a common behaviour in this family [27]. Orb-weaving spiders, such as Araneidae (Table 3), may also have the habit of feeding on nectar from EFNs (as well as dismantling and rebuilding their webs at regular intervals, allowing them to build their webs where resources are most abundant) [36]. For example, Nahas et al. [14] investigated the presence of fructose in the bodies of spiders that visit plants with EFNs in a neotropical savannah environment. In their study, the species Araneus venatrix (Araneidae), collected at night from Qualea grandiflora (Vochysiaceae) plants, showed the highest concentrations of fructose [14]. Thus, araneids build their webs to capture their prey on plants with EFNs and supplement their diet with nectar. The arrival of new herbivores on the plant can occur by air, meaning that the webs built on the plant capture these herbivores before they even reach the plant, reducing the damage caused by herbivory.
Unlike the other spider families found on H. pteropetala, the Cheiracanthiidae family was more abundant on the EFNsinactive plants than on the EFNsactive plants, despite the fact that this family is known for its nectar consumption [36]. As the representatives of the Cheiracanthiidae family were adults and in the oviposition period, they were possibly found in greater numbers on the EFNsinactive plants because these locations allowed them to avoid the presence of competing spiders and probable predators of their eggs and young. In addition, spiders during egg sac care reduce their consumption of prey [37], thus affecting any positive interaction with the plant.
In summary, spiders are attracted to EFN nectar, confirming the existence of mutualism in the form biotic protection between spiders and H. pteropetala. However, the positive relationship is limited to leaf structures, while, in the reproductive parts, the association found was neutral. Thus, the predatory activity of spiders on reproductive structures suggests a commensal role in which one species (spider) benefits from the interaction, but the other (plant) is neither benefited nor harmed.
4. Materials and Methods
4.1. The Study Site and Species of Plant
This study was conducted from December 2021 to November 2022 at the Ecological Reserve of the Clube de Caça e Pesca Itororó de Uberlândia (18°59′ S and 48°18′ W, WGS84 Datum, ~640 ha), in the state of Minas Gerais (MG), Brazil. The reserve’s vegetation comprises various savanna physiognomies, with trees reaching up to 8 m in height [38]. The mean monthly rainfall ranges between 0 and 360 mm, and the mean monthly temperature is between 20.0 and 25.5 °C, with a dry season between May and September and a rainy season between October and April [3,39].
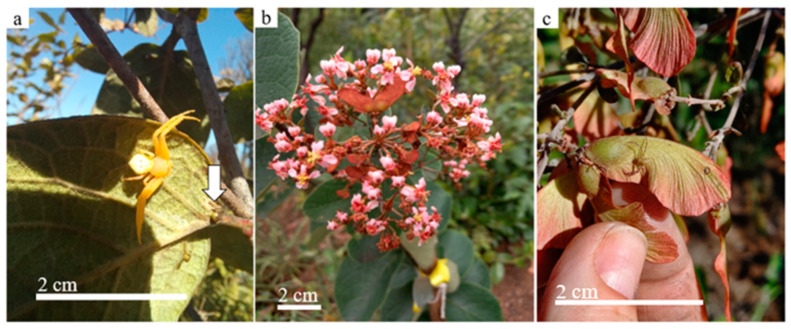
The plant species studied, Heteropterys pteropetala, is a shrub approximately 2 m tall, with two extrafloral nectaries (EFNs) at the base of each leaf (Figure 4a), at the base of the pedicel of the flower buds, and on the bracts of the inflorescences [40]. The inflorescences are terminal panicles with pink flowers (Figure 4b) and are zygomorphic, with five petals and five sepals, and, at the base of each sepal, there are two elaiophores (oil glands), totalling between eight and ten glands per flower [41]. Each flower can produce up to three samarids (a fruit morphologically adapted for wind dispersal) (Figure 4c) [42]. H. pteropetala is dependent on cross-pollination for fruiting and is an important species for studies of ecological interactions due to the diversity in its guild of floral visitors. The presence of organisms that take part in pollen transport increases the fruiting and reproductive success of the species [42].
Figure 4.
The studied plant, Heteropterys pteropetala, in the Cerrado sensu stricto at the Ecological Reserve of Clube Caça e Pesca Itororó in Uberlândia, Minas Gerais, Brazil. (a) Thomisidae spider on the abaxial surface of the leaf—note the white arrow indicating the pair of EFNs at the base of the abaxial region of the leaf. (b) Inflorescences of a studied plant. (c) Samarids with the presence of a juvenile Thomisidae spider.
4.2. Experimental Design
To test hypotheses I, II, and III (see Table 1), we isolated the plant against the presence of ants. It is known that ants are also attracted to EFNs, making them important competitors for spiders. According to a study carried out by Lange et al. [16] with nine different plant species with EFNs in a neotropical savannah area, a negative spatial/temporal effect of spider abundance was observed in the presence of ants. In addition, Stefani et al. [2] observed that spider species’ richness was significantly higher in the absence of ants, although the reverse was not true, possibly due to the different species composition of the ants and spiders found and, consequently, the different types of interactions between them. Thus, the absence of ants in this study was necessary so that these organisms would not influence our results. Non-toxic resin (entomological glue—Tanglefoot®) was added to the base of the trunk of all the plants to prevent ants from accessing the plant. All the structures, such as grasses, that could serve as a bridge for the ants to access the plants were removed. We then carried out two different manipulations on the H. pteropetala plants in a natural environment: (I) EFNsactive plants were individuals with active extrafloral nectaries (n= 20); and (II) EFNsinactive plants were individuals with inactive extrafloral nectaries (n = 20). The plants in the EFNsinactive group underwent a process of enamelling all the nectaries, blocking them, and preventing the release of nectar—in other words, making them inactive. In the plants in the EFNsactive group, glaze was also applied to the abaxial part of the leaf, next to the extrafloral nectary, allowing the normal release of nectar. Weekly inspections were carried out on all the plants to check the integrity of the nectary obstructions in the EFNsinactive plants, as well as the entomological resin at the base of the trunk in both manipulations, to prevent ant access. To describe the reproductive phenology of H. pteropetala, all flower buds, inflorescences, and samarids were quantified weekly during the plants’ reproductive period.
To test hypothesis I, all the experimental plants were inspected weekly; the spiders found were photographed and quantified after a visual search of the entire bush. The branches were also shaken over a white tray, so that, if any animals were not found during the visual sweep, they would be on the tray for quantification. After the procedure, all the spiders were placed back on the plant.
To test hypothesis II, herbivory rates were measured monthly on five leaves of each plant in both manipulations. Initially, the five leaves of each plant were marked at the initial stage of expansion to monitor and record the loss of leaf area throughout the leaves’ ontogeny, i.e., from budding to senescence. Herbivory was calculated from digital images analysed using the ImageJ software version 1.53, as performed by Calixto et al. [43].
To test hypothesis III, flower buds, flowers, and samarids were quantified weekly during the reproductive period for both manipulations. Around 30 days after flowering, the samarids were harvested, dried in the sun for a fortnight, and then weighed separately, depending on the plant manipulation, using a precision electronic analytical balance.
4.3. Data Analysis
All statistical analyses were performed in R version 4.2.2 (R CoreTeam, 2022). Below, we describe the packages used in each analysis.
4.3.1. Phenology of Heteropoterys pteropetala
The data for calculating the phenology of the H. pteropetala plant were observed using circular statistical analyses. These analyses served to verify the occurrence of seasonality between different reproductive phenophases (presence of floral buds, flowers, and fruits) throughout the year, as well as to analyse spider abundance on plants with and without active EFNs. For the circular analyses, we divided the 360° range into 12 groups. Each group represents a month of the year, with each month corresponding to a 30° angle and the mean vector (μ) being indicative of the direction (month) where the data are possibly more concentrated (reproductive phenophases and spider abundance). Subsequently, to evaluate different plant phenophases and whether spider abundance showed a non-random distribution throughout the year, we used the Rayleigh test for uniformity after confirming the normality of the circular data [44]. In the Rayleigh test, p-values below 0.05 and a mean vector length (r) close to 1 indicated seasonality in the data, i.e., phenological activities were concentrated around a single period or mean angle [44]. The mean month for each variable was obtained by converting the angular mean of the corresponding mean months [44,45].
4.3.2. Hypothesis I
We used “glmmTMB” [46] and “Dharma” [47] with a “Poisson” distribution to answer whether spiders are attracted to EFNs. We compared differences in spider abundance (response variable) between the plants with EFNsinactive and EFNsactive (predictor variables), considering the months of the year and plant identification as random variables.
4.3.3. Hypothesis II
To answer the hypothesis that spiders act in a mutualistic relationship as protectors, we analysed variations in herbivory (response variable) between the EFNsinactive and EFNsactive plants (predictor variables), considering the months of the year and plant identification as random variables. We used GLMM (binomial distribution), with Tukey’s post hoc tests being performed between the manipulations. The GLMM was conducted with the “glmer” function from the “lme4” package [47], followed by “Dharma” [48] to fit and check the residuals. Model significance was analysed with the Wald χ2 test through the Anova function, using the “car” package [49].
4.3.4. Hypothesis III
To verify whether the presence of spiders impacted the reproductive success of the plants, we used glmmTMB with a Gamma distribution to check whether EFNsinactive and EFNsactive (predictor variables) influenced the proportion between samarids/buds and samarids/flowers (response variables). Plant ID was considered a random effect [49]. To analyse if there was variation in the total weight of seeds produced between the EFNsinactive and EFNsactive plants, we used glmmTMB with a Gaussian distribution with the “identity” link. Individual plant identity was considered a random factor (Table 1).
Acknowledgments
We thank the Clube de Caça e Pesca Itororó de Uberlândia for having made available the reserve for the development of this study and the Universidade Federal de Uberlândia (UFU).
Appendix A
Table A1.
Main insect orders found on Heteropoterys pteropetala in each of the manipulations.
| Order | Number of Individuals on Plants with EFNactive | Number of Individuals on Plants with EFNsinactive |
|---|---|---|
| Hemiptera | 16 | 64 |
| Diptera | 35 | 18 |
| Lepdoptera (larval stage) |
134 | 167 |
| Coleoptera | 10 | 10 |
| Hymenoptera | 4 | 4 |
| Orthoptera | 3 | 5 |
| Total | 202 | 268 |
Author Contributions
Conceptualization, K.P.d.O.D. and V.S.; methodology, K.P.d.O.D. and V.S.; formal analysis, V.S.; writing—original draft preparation, K.P.d.O.D. and V.S.; writing—review and editing, K.P.d.O.D. and V.S.; visualization, K.P.d.O.D. and V.S.; supervision, V.S.; project administration, V.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data underlying our study was deposited in the Dryad Digital Repository https://doi.org/10.5061/dryad.v15dv423k.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was financed in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) with grant number 403647/2021-5 (V.S.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) with grant number 88887.666322/2022-00 (K.P.O.D.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nahas L., Gonzaga M.O., Del-Claro K. Emergent Impacts of Ant and Spider Interactions: Herbivory Reduction in a Tropical Savanna Tree. Trop. Biol. Conserv. 2012;44:498–505. doi: 10.1111/j.1744-7429.2011.00850.x. [DOI] [Google Scholar]
- 2.Stefani V., Pires T.L., Torezan-Silingardi H.M., Del-Claro K. Beneficial Effects of Ants and Spiders on the Reproductive Value of Eriotheca gracilipes (Malvaceae) in a Tropical Savanna. Public Libr. Sci. 2015;10:e0131843. doi: 10.1371/journal.pone.0131843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva M.O., Santos M.P., Sousa A.C., Silva R.L., Moura I.A., Silva R.S., Costa K.D. Soil Quality: Biological Indicators for Sustainable Management. Braz. J. Dev. 2021;7:6853–6875. doi: 10.34117/bjdv7n1-463. [DOI] [Google Scholar]
- 4.Bronstein J.L. Plant-Animal Interactions. Springer International Publishing; New York, NY, USA: 2021. The Gift That Keeps on Giving: Why Does Biological Diversity Accumulate Around Mutualisms? pp. 283–306. [Google Scholar]
- 5.Smith R.B., Mommsen T.P. Pollen Feeding in an Orb-Weaving Spider. Science. 1984;226:1330–1332. doi: 10.1126/science.226.4680.1330. [DOI] [PubMed] [Google Scholar]
- 6.Diniz S., Tizo-Pedroso E., Lange D., Andrade Vilela A., Justino D.G., Alves Martins F., Germanos E., Arruda R., Stefani V. Might Heterostyly Underlie Spider Occurrence on Inflorescences? A Case Study of Palicourea rigida (Rubiaceae), a Common Shrub from Brazilian Cerrado. Psyche. 2012;2012:791395. [Google Scholar]
- 7.Nyffeler M., Olson E.J., Symondson W.O. Plant-eating by spiders. J. Arachnol. 2016;44:15–27. doi: 10.1636/P15-45.1. [DOI] [Google Scholar]
- 8.Del-Claro K., Stefani V., Nahas L., Torezan-Silingardi H.M. Behaviour and Ecology of Spiders. Springer International Publishing; New York, NY, USA: 2017. Spiders as Plant Partners: Complementing Ant Services to Plants with Extrafloral Nectaries; pp. 215–226. [Google Scholar]
- 9.Cross F.R., Jackson R.R. Odour-mediated response to plants by evarcha culicivora, a blood-feeding jumping spider from East Africa. N. Z. J. Zool. 2009;36:75–80. doi: 10.1080/03014220909510142. [DOI] [Google Scholar]
- 10.Wise D.H. Spiders in Ecological Webs. Cambridge University Press; Cambridge, UK: 1993. [Google Scholar]
- 11.Romero G.Q., Souza J.C., Vasconcellos-Neto J. Anti-herbivore protection by mutualistic spiders and the role of plant glandular trichomes. Ecology. 2008;89:3105–3115. doi: 10.1890/08-0267.1. [DOI] [PubMed] [Google Scholar]
- 12.Gavini S.S., Quintero C., Tadey M. Ecological role of a flower-dwelling predator in a tri-trophic interaction in northwestern Patagonia. Acta Oecologica. 2019;95:100–107. doi: 10.1016/j.actao.2018.12.001. [DOI] [Google Scholar]
- 13.Moura R.F., Colberg E., Alves-Silva E., Mendes-Silva I., Fagundes R., Stefani V., Del-Claro K. Plant-Animal Interactions. Springer International Publishing; New York, NY, USA: 2021. Biotic Defenses Against Herbivory; pp. 93–118. [Google Scholar]
- 14.Nahas L., Gonzaga M.O., Del-Claro K. Wandering and web spiders feeding on the nectar from extrafloral nectaries in neotropical savanna. J. Zool. 2017;301:125–132. doi: 10.1111/jzo.12400. [DOI] [Google Scholar]
- 15.Whitney K.D. Experimental Evidence That Both Parties Benefit in a Facultative Plant–Spider Mutualism. Ecology. 2004;85:1642–1650. doi: 10.1890/03-0282. [DOI] [Google Scholar]
- 16.Lange D., Calixto E.S., Del-Claro K., Stefani V. Spatiotemporal niche-based mechanisms support a stable coexistence of ants and spiders in an extrafloral nectary-bearing plant community. J. Anim. Ecol. 2021;90:1570–1582. doi: 10.1111/1365-2656.13477. [DOI] [PubMed] [Google Scholar]
- 17.Heiling A.M., Herberstein M.E. Predator-prey coevolution: Australian native bees avoid their spider predators. Proc. R. Soc. London Ser. B Biol. Sci. 2004;271:S196–S198. doi: 10.1098/rsbl.2003.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ings T.C., Chittka L. Predator crypsis enhances behaviourally mediated indirect effects on plants by altering bumblebee foraging preferences. Proc. R. Soc. London Ser. B Biol. Sci. 2009;276:2031–2036. doi: 10.1098/rspb.2008.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado S.R., Morellato L.P., Sajo M.G., Oliveira P.S. Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado. Plant Biol. 2008;10:660–673. doi: 10.1111/j.1438-8677.2008.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Marazzi B., Bronstein J.L., Koptur S. The diversity, ecology and evolution of extrafloral nectaries: Current perspectives and future challenges. Ann. Bot. 2013;111:1243–1250. doi: 10.1093/aob/mct109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heil M., Koch T., Hilpert A., Fiala B., Boland W., Linsenmair K.E. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc. Natl. Acad. Sci. USA. 2001;98:1083–1088. doi: 10.1073/pnas.98.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del-Claro K., Rico-Gray V., Torezan-Silingardi H.M., Alves-Silva E., Fagundes R., Lange D., Dáttilo W., Vilela A.A., Aguirre A., Rodriguez-Morales D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Sociaux. 2016;63:207–221. doi: 10.1007/s00040-016-0466-2. [DOI] [Google Scholar]
- 23.Oliveira P.S., Leitao-Filho H.F. Extrafloral Nectaries: Their Taxonomic Distribution and Abundance in the Woody Flora of Cerrado Vegetation in Southeast Brazil. Trop. Biol. Conserv. 1987;19:140. doi: 10.2307/2388736. [DOI] [Google Scholar]
- 24.Rico-Gray V., Oliveira P.S. The Ecology and Evolution of Ant-Plant Interactions. University of Chicago Press; Chicago, IL, USA: 2007. [Google Scholar]
- 25.Taylor R.M., Bradley R.A. Plant nectar increases survival, molting, and foraging in two foliage wandering spiders. J. Arachnol. 2009;37:232–237. doi: 10.1636/Sh07-69.1. [DOI] [Google Scholar]
- 26.Sanders D. Spider Ecophysiology. Springer; Berlin/Heidelberg, Germany: 2013. Herbivory in Spiders; pp. 385–391. [Google Scholar]
- 27.Jackson R.R., Pollard S.D., Nelson X.J., Edwards G.B., Barrion A.T. Jumping spiders (Araneae: Salticidae) that feed on nectar. J. Zool. 2001;255:25–29. doi: 10.1017/S095283690100108X. [DOI] [Google Scholar]
- 28.Halaj J., Ross D.W., Moldenke A.R. Importance of habitat structure to the arthropod food-web in Douglas-fir canopies. Oikos J. 2000;90:139–152. doi: 10.1034/j.1600-0706.2000.900114.x. [DOI] [Google Scholar]
- 29.Souza A.L., Martins R.P. Foliage Density of Branches and Distribution of Plant-Dwelling Spiders. Trop. Biol. Conserv. 2005;37:416–420. [Google Scholar]
- 30.Bucher R., Menzel F., Entling M.H. Risk of spider predation alters food web structure and reduces local herbivory in the field. Oecologia. 2015;178:571–577. doi: 10.1007/s00442-015-3226-5. [DOI] [PubMed] [Google Scholar]
- 31.Stefani V., Alves V.N., Lange D. Induced indirect defence in a spider–plant system mediated by pericarpial nectaries. Austral Ecol. 2019;44:1005–1012. doi: 10.1111/aec.12766. [DOI] [Google Scholar]
- 32.Jiménez-Valverde A., Lobo J.M. Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: Climate and altitude vs. habitat structure. Ecol. Entomol. 2007;32:113–122. doi: 10.1111/j.1365-2311.2006.00848.x. [DOI] [Google Scholar]
- 33.Bhaskara R.M., Brijesh C.M., Ahmed S., Borges R.M. Perception of ultraviolet light by crab spiders and its role in selection of hunting sites. J. Comp. Physiol. A. 2009;195:409–417. doi: 10.1007/s00359-009-0419-6. [DOI] [PubMed] [Google Scholar]
- 34.Romero G.Q., Vasconcellos-Neto J. Natural history of Misumenops argenteus (Thomisidae): Seasonality and diet on Trichogoniopsis adenantha (Asteraceae) J. Arachnol. 2003;31:297–304. doi: 10.1636/02-19. [DOI] [Google Scholar]
- 35.Suttle K.B. Pollinators as mediators of top-down effects on plants. Ecol. Lett. 2003;6:688–694. doi: 10.1046/j.1461-0248.2003.00490.x. [DOI] [Google Scholar]
- 36.Taylor R.M., Pfannenstiel R.S. How Dietary Plant Nectar Affects the Survival, Growth, and Fecundity of a Cursorial Spider Cheiracanthium inclusum (Araneae: Miturgidae) Environmetal Entomol. 2009;38:1379–1386. doi: 10.1603/022.038.0505. [DOI] [PubMed] [Google Scholar]
- 37.Foelix R.F. Biology of Spiders. 3rd ed. Oxford University Press; New York, NY, USA: 2011. pp. 1–432. [Google Scholar]
- 38.Bacci L.F., Versiane A.F., Oliveira A.L., Romero R. Melastomataceae na RPPN do Clube Caça e Pesca Itororó, Uberlândia, MG, Brasil. Hoehnea. 2016;43:541–556. doi: 10.1590/2236-8906-27/2016. [DOI] [Google Scholar]
- 39.Silva C.P., Rocha G.F., Silva F.A. Trabalhadores Rurais e Acesso à Renda: Estudo Sobre a Agricultura Familiar Orgânica em Pernambuco (Brasil). Meio Ambiente (Brasil) 2020. [(accessed on 20 November 2021)]. Available online: https://meioambientebrasil.com.br/index.php/MABRA/article/view/25.
- 40.Reu W.F., Jr., Del-Claro K. Natural history and biology of Chlamisus minax Lacordaire (Chrysomelidae: Chlamisinae) Neotrop. Entomol. 2005;34:357–362. [Google Scholar]
- 41.Assunção M.A., Torezan-Silingardi H.M., Del-Claro K. Do ant visitors to extrafloral nectaries of plants repel pollinators and cause an indirect cost of mutualism? Flora Morphol. Distrib. Funct. Ecol. Plants. 2014;209:244–249. doi: 10.1016/j.flora.2014.03.003. [DOI] [Google Scholar]
- 42.Schmidt I.B., Sampaio A.B., Borghetti F. Efeitos da época de queima sobre a reprodução sexuada e estrutura populacional de Heteropterys pteropetala (Adr. Juss.), Malpighiaceae, em áreas de Cerrado sensu stricto submetidas a queimas bienais. Acta Bot. Bras. 2005;19:927–934. doi: 10.1590/S0102-33062005000400027. [DOI] [Google Scholar]
- 43.Calixto E.S., Lange D., Del-Claro K. Foliar anti-herbivore defenses in Qualea multiflora Mart. (Vochysiaceae): Changing strategy according to leaf development. Flora Morphol. Distrib. Funct. Ecol. Plants. 2015;212:19–23. doi: 10.1016/j.flora.2015.02.001. [DOI] [Google Scholar]
- 44.Morellato L.P., Alberti L.F., Hudson I.L. Phenological Research. Springer; Dordrecht, The Netherlands: 2010. Applications of Circular Statistics in Plant Phenology: A Case Studies Approach; pp. 339–359. [Google Scholar]
- 45.Vilela A.A., Del Claro V.T., Torezan-Silingardi H.M., Del-Claro K. Climate changes affecting biotic interactions, phenology, and reproductive success in a savanna community over a 10-year period. Arthropod Plant Interact. 2018;12:215–227. doi: 10.1007/s11829-017-9572-y. [DOI] [Google Scholar]
- 46.Brooks M.E., Kristensen K., Van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Machler M., Bolker B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017;9:378. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- 47.Bates D., Mächler M., Bolker B., Walker S. lme4: Linear Mixed-Effects Models using ‘Eigen’ and S4 (Version 1.1-27) [R Package] [(accessed on 11 December 2023)]. Available online: https://cran.r-project.org/package=lme4.
- 48.Hartig F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models (Version 0.4.1) [R Package] [(accessed on 11 December 2023)]. Available online: https://cran.r-project.org/package=DHARMa.
- 49.Fox J. Applied Regression Analysis and Generalized Linear Models. 3rd ed. SAGE Publications; Thousand Oaks, CA, USA: 2015. pp. 1–816. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying our study was deposited in the Dryad Digital Repository https://doi.org/10.5061/dryad.v15dv423k.