Abstract
Tumefactive multiple sclerosis (MS) is a subtype of atypical and rare MS that presents with tumor-like lesions in the central nervous system. The lesions may demonstrate a mass effect, edema, with ring enhancement. They can be mistaken for brain tumors or brain abscesses radiologically and clinically. Here we describe an instructive case of a 55-year-old woman with tumefactive MS who presented with occasional numbness in her right arm and leg, headache, thought confusion, and blurred vision for 2 years.
Keywords: Abscess, demyelinating, tumor
CASE SUMMARY
A55-year-old right-handed woman presented with right-sided headache, blurred vision, occasional numbness, and loss of strength in her right arm and leg with onset 2 years prior. She had no known prior medical condition and was on no medication. On physical examination, she was afebrile with stable vital signs.
Upon neurological examination, right upper and lower extremity strength was 4/5 and right hypoesthesia was confirmed; deep tendon reflexes were normoactive. Brain computed tomography (CT) was unremarkable. Subsequent magnetic resonance imaging (MRI) revealed diffusion-limiting lesions in the right periventricular region, the left half of the pons, and with well-circumscribed, edematous lesions in the left parietal region and the left parietal region (Figure 1). CT angiography of the brain and neck was negative for obstructive cerebrovascular stenosis. MRI venography was normal. The patient was admitted to the neurology service for further examination, diagnostic evaluation, and treatment.
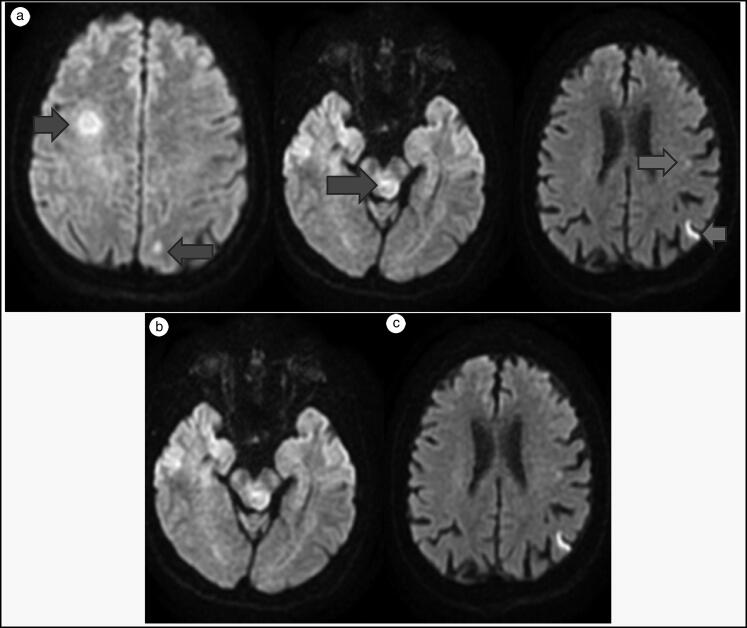
Figure 1.
Lesions consistent with tumefactive multiple sclerosis were noted on MRI diffusion sequence of the (a) right periventricular region and (b) left half of the mesencephalon. (c) Diffusion restricted lesions in the left mid and poterior parietal regions.
Serial brain MRI 12 hours after admission demonstrated widespread, hyperintense lesions in the cerebral white matter and brainstem in T2-weighted, fluid-attenuated inversion recovery sequences, reaching a diameter of approximately 2 cm in the subcortical white matter of the right frontal lobe, prominently hyperintense and nodular in central T2-weighted sequences, and lower hyperintensity surrounded by a halo. The large lesions had generally lobulated contours. Gadolinium MRI contrast neuroimaging sequences revealed a lesion with annular, intermediate enhancement. In addition, a small juxtacortical lesion with an open ring-enhancing lesion facing the cortical surface was noted in the left posterior parietal region. MRI findings were suggestive of active demyelinating lesions, including relatively large 13 to 14 mm diameter lesions involving the periventricular white matter in the right frontal lobe and 12 mm diameter lesions in the left semiposterior mesencephalon and surrounding the aquaductus cerebri, as well as near the trigone formed by the occipital and temporal horns of both lateral ventricles. Heterogenous periventricular lesions including those with diffuse and nodular features were observed in the cerebral white matter. The septal interface in the corpus callosum was similarly involved. These nonenhancing lesions harbor imaging characteristics of demyelinating plaques. A millimetric lesion with radiographic features consistent with demyelinating plaque was noted at the level of the C4 vertebra, with a short segment involving the left half of the spinal cord (Figure 2).
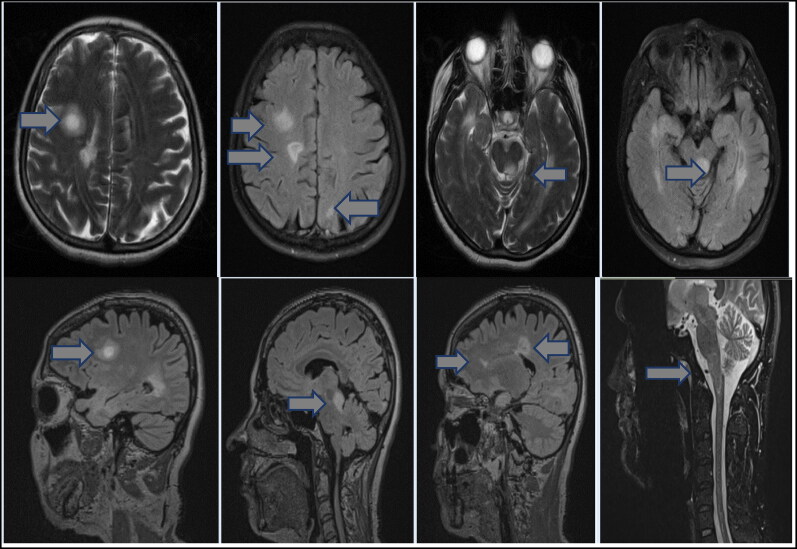
Figure 2.
Intermediate ring-enhancing lesions denoted by arrows in the right frontal lobe and the left half of the mesencephalon demonstrate mass effect. A short segment lesion in the left half of the cervical C4 spine is also highlighted.
Normal laboratory tests included complete blood count and peripheral smear, platelet function, coagulation studies, routine chemistry panels, complete urinalysis, serum and urine protein electrophoresis, antiphospholipid, antinuclear, antithyroid, anti-SSA and anti-SSB antibodies, vitamin B12, thyroid function, hemoglobin A1c, erythrocyte sedimentation rate, Schirmer test, homocysteine, anticardiolipin, rheumatoid factor, protein C and protein S, anti-streptolysin O, rheumatoid factor, and immunoglobin A, G, and M levels. Serology for brucellosis, hepatitis A, B, and C viruses, human immunodeficiency virus, and syphilis (fluorescent treponemal antibody) were also negative. Investigations for herpes simplex virus type 1 and 2, varicella zoster virus, enterovirus, parechovirus, Ebstein-Barr virus, cytomegalovirus, and adenovirus by both serum and cerebrospinal fluid (CSF) polymerase chain reaction assays were also negative. Pathergy test for Behçet was negative. CSF examination results included negative cytology and positive oligoclonal band type 2. Anti-aquaporin-4 antibody and anti-myelin oligodendrocyte glycoprotein were negative from both serum and CSF.
Visual evoked potentials were consistent with bilateral visual pathway dysfunction. Thorax and abdominal CT were negative for tumors. Tumefactive MS was considered the most likely diagnosis, and 1 g of methylprednisolone was administered intravenously for 10 days, correlating with a regression in symptoms. Subcutaneous interferon beta-1a was prescribed for maintenance. Unfortunately, long-term status is unknown, as the patient did not return for recommended outpatient neurology clinic follow-up.
CLINICAL QUESTIONS
- Which of the following is not included in the differential diagnosis of tumefactive MS?
- Tumors
- Other demyelinating disease
- Arnold-Chiari malformation
- Abscess
- None of the above
- A 21-year-old woman presents to the hospital with numbness in the left arm and leg that lasts more than 24 hours. You suspect an MS attack after examination and brain imaging. What are the primary initial therapies for MS attack?
- Plasma exchange and intravenous immunoglobulin
- Rituximab
- Cyclophosphamide
- Glucocorticoids
- None of the above
Answers are provided at the end of the article.
DISCUSSION
MS is an autoimmune, inflammatory, and neurodegenerative demyelinating disease of the central nervous system characterized by multifocal demyelinating lesions. MS lesions are typically small and well circumscribed. Tumefactive MS or tumefactive demyelinating disease is a rare variant of MS that poses a diagnostic challenge. Tumefactive lesions are defined as areas of cerebral focal demyelination >2 cm1–3 and may be seen any time during the disease course of MS. Concentric sclerosis of Baló lesions is characterized histopathologically by concentric, lamellar demyelinating and accompanying remyelinating areas, correlating with concentric rings on MRI or spiral-shaped lesions on T2-weighted images; such lesions were not observed in this case. Acute disseminated encephalomyelitis (ADEM) is a disease that involves damage to the myelin sheath around nerve cells, especially affecting the white matter part of the brain. It usually occurs in children and young adults and sometimes follows vaccination or infection. White matter lesions seen in ADEM tend to be more widespread, large and symmetrical, as compared to lesions seen in MS. Lesions with a similar contrast enhancement pattern are suggestive of lesions that are of comparable age. Although oligoclonal bands can be detected occasionally in the CSF of patients with ADEM, they are transient, whereas they persist in MS.
It is difficult to distinguish tumor from brain abscess on MRI. When the diagnosis is in doubt, visual evoked potentials and CSF analysis are recommended before invasive procedures, along with empiric high-dose corticosteroid therapy.4–6 Tumefactive MS lesions may mimic tumors and manifest mass effect, edema, and an open ring enhancement.
The prevalence of tumefactive MS is estimated to be 1 to 3/1000 cases of MS with an annual incidence of 0.3/100,000. Although it can manifest at any age, peak onset is between 20 to 40 years, and it occurs more often in women. Its etiopathogenesis is not fully understood; antibody-mediated and B-cell–mediated immunological mechanisms may play a role. Motor, sensory, cognitive, and cerebellar symptoms predominate.5–9
Our patient presented indolently with complaints of 2 years of right-sided headache, numbness in the right arm and leg, difficulty walking, blurred vision, and confusion. MRI revealed open-ring enhancement of the lesions and mass effect. Additional features to suggest malignancy and vasculitic and infectious diseases were absent. CSF revealed type 2 oligoclonal bands. Visual evoked potential was compatible with bilateral visual pathway dysfunction, suggesting demyelinating disease, and the patient was responsive to conventional immunomodulation with high-dose corticosteroid induction followed by interferon beta-1a maintenance, Although tumefactive MS and brain tumors and brain abscesses may mimic each other radiographically, workup was negative for any supportive evidence of tumor or infectious pathologies, and her empiric response to immunomodulation is consistent with the diagnosis of tumefactive MS.
The differential diagnoses of brain tumor, brain abscess, or tumefactive MS should all be considered in patients presenting with similar lesions. When tumefactive MS is diagnosed accompanied by appropriate radiographic and clinical regression, brain biopsy may sometimes be avoided.
ANSWERS FOR CLINICAL QUESTIONS
Question 1, c. The differential diagnoses usually considered are brain tumors (such as multifocal glioma, metastasis, central nervous system lymphoma), brain abscess, tuberculoma, and other inflammatory entities such as sarcoidosis and Sjogren’s syndrome.10
Question 2, d. The use of steroids for treatment of MS exacerbations has been endorsed by the National MS Society and the American Academy of Neurology. Level I data support the beneficial effect of corticosteroids on speed of recovery after MS relapses. Initial data from the optic neuritis treatment trial showed that treatment with intravenous methylprednisolone 1 g daily for 3 days followed by a 21-day tapering oral steroid treatment hastened recovery compared to placebo and oral prednisone.11
Disclosure Statement
The authors report no funding or conflicts of interest. The patient provided consent for this case report to be published.
References
- 1.Dagher AP, Smirniotopoulos J.. Tumefactive demyelinating lesions. Neuroradiology. 1996;38(6):560–565. doi: 10.1007/BF00626098. [DOI] [PubMed] [Google Scholar]
- 2.Ayrignac X, Carra-Dallière C, Labauge P.. Atypical inflammatory demyelinating lesions and atypical multiple sclerosis. Rev Neurol (Paris). 2018;174(6):408–418. doi: 10.1016/j.neurol.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Frederick MC, Cameron MH.. Tumefactive demyelinating lesions in multiple sclerosis and associated disorders. Curr Neurol Neurosci Rep. 2016;16(3):26. doi: 10.1007/s11910-016-0626-9. [DOI] [PubMed] [Google Scholar]
- 4.Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG.. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 2016;15(9):967–981. doi: 10.1016/S1474-4422(16)30043-6. [DOI] [PubMed] [Google Scholar]
- 5.Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48-49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Stadelmann C, Brück W.. Lessons from the neuropathology of atypical forms of multiple sclerosis. Neurol Sci. 2004;25(S4):S319–322. doi: 10.1007/s10072004-0333-1. [DOI] [PubMed] [Google Scholar]
- 7.Pilz G, Harrer A, Wipfler P, et al. Tumefactive MS lesions under fingolimod: a case report and literature review. Neurology. 2013;81(19):1654–1658. Erratum in: Neurology. 2014;82(17):1569. doi: 10.1212/01.wnl.0000435293.34351.11. [DOI] [PubMed] [Google Scholar]
- 8.Omerhodžić I, Džurlić A, Lisica D, et al. Relapsing tumefactive demyelination: a case report. Acta Med Acad. 2018;47(2):193–198. doi: 10.5644/ama2006-124.231. [DOI] [PubMed] [Google Scholar]
- 9.Mamilly A, Aslan A, Adeeb N, Al Asfari A, Cuellar H.. Tumefactive multiple sclerosis of the cervical spinal cord: a rare case report. Cureus. 2020;12(1):e6754. doi: 10.7759/cureus.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George T, Cicilet S, Hoisala R, Rout P.. Multifocal tumefactive demyelination mimicking intracranial neoplasm. JCDR. 2016;10(3):TD10–1. doi: 10.7860/JCDR/2016/15589.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ontaneda D, Rae-Grant AD.. Management of acute exacerbations in multiple sclerosis. Ann Indian Acad Neurol. 2009;12(4):264–272. doi: 10.4103/0972-2327.58283. [DOI] [PMC free article] [PubMed] [Google Scholar]