Abstract
Background
Agomelatine (AGO) is an antidepressant with unique pharmacological effects; however, its underlying mechanisms remain unknown. In this study, we examined agomelatine’s effects on catalase activity, oxidative stress, and inflammation.
Methods
Chronic restraint stress (CRS) model mice were established over 4 weeks, and AGO 50 mg/kg was administered to different groups alongside a deferasirox (DFX) 10 mg/kg gavage treatment. Behavioral tests were performed to assess the effect of AGO on the remission of depression-like behaviors. Meanwhile, the expression of CAT, the oxidative stress signaling pathway and inflammatory protein markers were assessed using ELISA, qRT-PCR, Western blot, and immunohistochemistry.
Results
Four weeks of AGO treatment significantly improved depression-like behavior in mice through the activation of catalase in the hippocampus and serum of the model mice, increased superoxide dismutase expression, reduced malondialdehyde expression, and reduced oxidative stress damage. Deferasirox was found to offset this therapeutic effect partially. In addition, the inflammatory pathway (including nuclear factor-κB and nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha) was not significantly altered.
Conclusions
AGO can exert antidepressant effects by altering oxidative stress by modulating catalase activity.
Background
Depression (major depressive disorder, MDD) is one of the most common clinical psychiatric disorders, affecting nearly 350 million people worldwide [1]. Epidemiological surveys have shown that the lifetime prevalence of mood disorders has reached 7.37% [2], with depression accounting for 4.7% [3]. However, because of the enormous disease burden of MDD, the current scientific understanding of its occurrence and progression remains inadequate.
Currently, most antidepressants in clinical use, such as selective serotonin reuptake inhibitors, work by selectively inhibiting serotonin reuptake (5-hydroxytryptamine, 5-HT) in the brain. Agomelatine (AGO), an alternative antidepressant, may exert its anxiolytic effects through an agonist effect on MT1/MT2 receptors and an antagonist effect on 5-HT2C receptors. Lu et al. [4] showed that AGO could improve depression symptoms in mice with chronic unpredictable mild stress (CUMS) by regulating the level of a brain-derived neurotrophic factor in the hippocampus. Previous studies have shown that the antidepressant effects of AGO are not entirely mediated through the same mechanisms as other conventional antidepressants [5–9].
Oxidative stress is an important factor in MDD pathogenesis [10] and is considered one of the main causes of MDD [11]. Maes et al. [12] found that plasma peroxide levels in MDD patients significantly increased compared to normal controls. Moreover, the peroxide levels were significantly higher in patients in the acute phase of the disease than in those with chronic depression (defined as depression lasting more than 2 years). As an important site of reactive oxygen species (ROS) production, the peroxisome plays a crucial role in maintaining the dynamic redox balance in cells and participates in various oxidative stress processes [13]. Catalase (CAT), such as superoxide dismutase (SOD) and glutathione peroxidase, are antioxidative enzymes that can overcome the role of oxidative stress [14]. CAT comprises approximately 40% of all peroxisomal enzymes and is involved in cell proliferation-associated transduction pathways, apoptosis, carbohydrate metabolism, and platelet activation [15]. CAT acts as an antioxidant enzyme by removing excess intracellular hydrogen peroxide (H2O2) and ROS [16]. Moreover, CAT activity can be significantly suppressed under oxidative stress conditions [17]. Ding et al. [18] conducted an in vivo study of depression using constructed fluorescent probe techniques in mouse models. They revealed the excess of peroxisomes and intracellular H2O2 in the mouse brain, leading to CAT inactivation, dysfunction of the 5-HT system, and, ultimately, depression-related behaviors in mice.
Studies have shown that cysteine and vitamin C can enhance CAT activity and remove excess H2O2 [19]. In contrast, deferasirox (DFX) can inhibit CAT activity and improve H2O2 levels [18]. The results of several clinical studies identified elevated CAT activity in the acute and chronic phases of MDD [20–22]. Increased CAT activity may reflect a compensatory mechanism reducing the effects of oxidative stress. However, some studies have presented the opposite conclusion. Ozcan et al. [23] found significantly lower CAT activity in depressed patients than in healthy controls, with no significant change in CAT activity after treatment. However, their study did not describe a specific drug treatment regimen. Bhatt et al. [24] found decreased CAT levels in the brains of mice with CUMS. Olsen et al. [25] found that antidepressants improved memory and reduced depression and anxiety symptoms by increasing CAT levels in mouse brain tissue, even without increased oxidative stress.
The activation of the oxidative stress pathway is an important pathophysiological factor in depression, based on the proposed hypothesis of the involvement of oxidative stress in the pathogenesis of MDD. AGO may improve depression symptoms, anxiety symptoms, and biochemical indicators of depression by affecting oxidative stress and inflammation.
This study mainly examined the changes in CAT levels and oxidative stress in the brain tissue during AGO treatment in a depression mouse model, determined the presence or absence of a correlation between this change and depression and anxious behavior in mice, and investigated whether this change would be affected by DFX. It was found that AGO can modulate oxidative stress in depression model mice by regulating CAT, thus highlighting new ideas for treating depression.
Results
AGO attenuated depressive behavior in depression model mice
The effect of AGO on depression-like behavior was investigated, first analyzing baseline body weight in the six groups of mice (Fig 1A). Secondly, body weight after 28 days of chronic restraint stress (CRS) and drug intervention (Fig 1B) and body weight gain values in the same groups of mice (Fig 1C). Statistical analysis showed that the CRS group lost significantly more body weight than the control group. AGO and AGO + DFX treatment partially offset this weight loss. The therapeutic effect of AGO was partially weakened by DFX, while the DFX treatment group and CRS group did not show any significant differences. At the end of the chronic stress period, a series of well-established behavioral tests that were designed to measure anxiety-like Open Field Test (OFT) and depression-like Sugar-water preference test (SPT), and Forced swimming test (FST) behaviors were conducted in different groups of mice. CRS mice showed lower sucrose preference (Fig 1D), significantly prolonged rest in the FST (Fig 1E), and a significant decrease in total travel in the central region of the OFT (Fig 1F) compared with control mice. These findings were significantly recovered in mice treated with AGO and AGO + DFX compared with those in CRS mice. However, the DFX treatment and CRS groups did not show significant differences in the tests mentioned above. Unfortunately, no significant effects were observed in the total travel time and central region movement time in the OFT tests (Fig 1G–1H). These experiments demonstrated that AGO treatment could restore depressive-like behavior after CRS. However, the therapeutic effect of AGO would be partially attenuated by DFX, indicating that AGO partially improves depressive-like behavior in mice.
Fig 1. Effects of the CRS, AGO, and DFX intervention on body weight and behavioral tests.
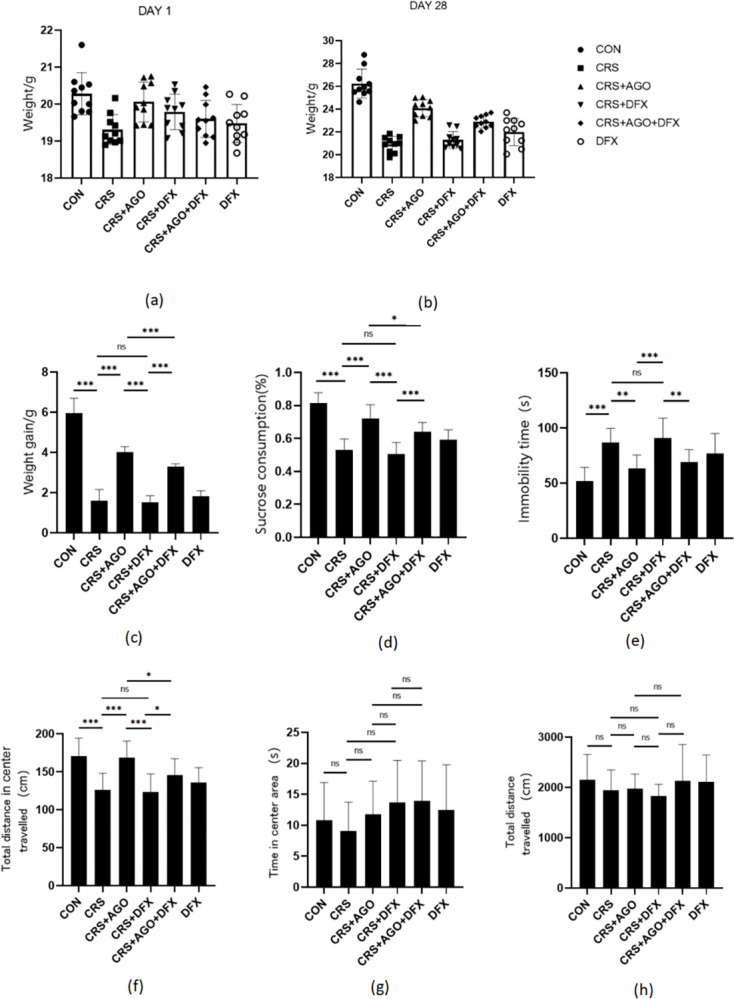
Depression-like behavior in mice induced by CRS. (a) Initial body weight of the mice. (b) Body weight of the mice after different treatments. (c) Weight gain of the mice. (d) Sucrose preference rate in mice. (e) Forced swimming rest time in mice. (f) The total distance of mice in the central region of the open field test. (g) Total time of mice in the central region in the open field test. (h) The total distance of mice in the open field test. *P<0.05; ** P<0.01; *** P<0.001; CRS, chronic restraint stress; AGO, agomelatine; DFX, deferasirox; ns, no significant difference. Data are expressed as the mean ± standard deviation and were analyzed by one-way ANOVA, followed by post hoc multiple comparisons (LSD method). n = 10.
Effect of AGO on CAT levels in mice
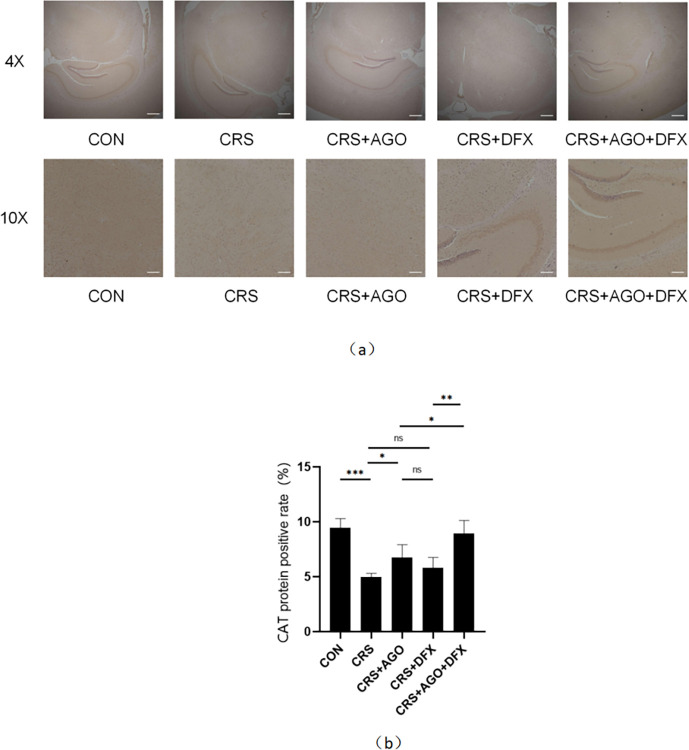
The hippocampal sections were immunohistochemically stained with CAT as a specific marker to investigate the effect of AGO on CAT protein expression in the hippocampus of the model mice (Fig 2A). The expression of CAT protein in the CRS group decreased significantly relative to that in the normal control group, and CAT protein expression in the AGO treatment group was significantly restored compared with that in the CRS group (Fig 2B).
Fig 2. Effects of the CRS, AGO, and DFX intervention on immunohistochemistry of CAT protein in the hippocampus of mice.
Immunohistochemistry shows the changes in CAT protein expression in the hippocampus of mice after CRS, AGO, and DFX intervention. (a) Staining of CAT protein. (B) Positive rate of CAT protein expression in the mouse hippocampus. *P<0.05; **P<0.01; ***P<0.001; CAT, catalase; CRS, chronic restraint stress; AGO, agomelatine; DFX, deferasirox; ns, no significant difference. Data are expressed as the mean ± standard deviation and were analyzed by one-way ANOVA, followed by post hoc multiple comparisons (LSD method). n = 3.
Meanwhile, we detected CAT activity in mouse serum (Fig 3A) and found that CAT activity was significantly lower in the CRS group than in the control group. CAT activity increased significantly after AGO treatment, and the DFX had a clear inhibitory effect.
Fig 3. ELISA, Western blot, and PCR results.
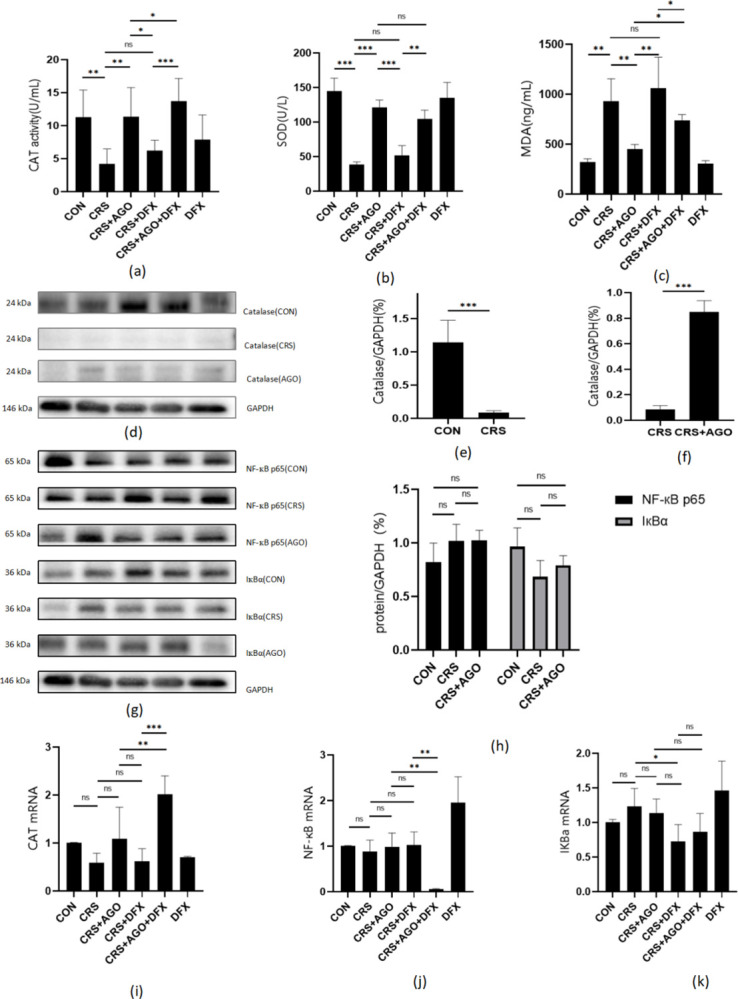
Effect of agomelatine on oxidative stress and the NF-кB pathway in CRS mice. (a) CAT activity in the serum of mice under CRS, AGO, and DFX intervention. (b-c) SOD and MDA concentrations in the serum of mice under CRS, AGO, and DFX intervention. (d, g) Representative protein blot results of CAT, NF-кB p65, and IкBα in the mouse hippocampus under CRS and AGO intervention, showing the quantitative density analysis of CAT in the hippocampus (e, f, h). (i-k) mRNA expression levels of CAT, NF-кB, and IкBα in the mouse hippocampus. *P<0.05; **P<0.01; ***P<0.001; CAT, catalase; CRS, chronic restraint stress; AGO, agomelatine; DFX, deferasirox; SOD, superoxide dismutase; MDA, malondialdehyde; ns, no significant difference. Data are expressed as the mean ± standard deviation and were analyzed by one-way ANOVA, followed by post hoc multiple comparisons (LSD method). n = 3–5.
In addition, analysis of CAT protein expression levels by protein immunoblotting (Fig 3D) confirmed that CAT protein expression was significantly lower in the CRS group than in the control group and that AGO treatment effectively restored CAT protein expression (Fig 3E and 3F).
We observed differences in CAT expression by measuring the mRNA expression of CAT in each group through quantitative real-time polymerase chain reaction (PCR), although these were not statistically significant.
In conclusion, we showed that AGO could effectively increase the CAT expression in depressed model mice with CRS.
Effects of AGO on oxidative stress in depression model mice
The serum concentrations of SOD and malondialdehyde (MDA) were measured using enzyme-linked immunosorbent assay (ELISA) to confirm the effect of AGO on oxidative stress in depression model mice (Fig 3B and 3C). The results showed that the CRS group had significantly lower serum MDA concentrations after AGO treatment, while serum SOD increased significantly with AGO treatment. Meanwhile, this therapeutic effect was partially offset by DFX.
Effect of AGO treatment on the NF-кB pathway in mouse models of depression
Extensive preclinical and clinical evidence shows that external stress is accompanied by increased proinflammatory cytokines and their downstream regulators. Nuclear factor (NF)-кB is a transcription factor reported to regulate an excess of proinflammatory cytokines. The expression levels of the NF-кB protein and IкB-α were analyzed by protein immunoblot (Fig 3G and 3H). Meanwhile, we detected the mRNA expression levels of NF-кB and IкBα by quantitative and real-time PCR (Fig 3I–3K). Unfortunately, our experiments did not reveal significant differences in the expressions of NF-кB and IкBα in each group.
Discussion
We hypothesized that the effect of AGO in treating depression is associated with reducing the oxidative stress process in vivo, given the role of oxidative stress in the pathogenesis of depression. CRS induced depression-like behavior in mice, leading to increased MDA and decreased SOD and CAT levels, showing a redox imbalance and oxidative stress state in mice, consistent with previous studies [26]. The marked improvement in depression-like behavior and decreased MDA in depression model mice treated with AGO may be related to increased antioxidant enzymes. In this study, we found that AGO treatment for 4 weeks could activate CAT in the hippocampus of CRS mice, while the CAT inhibitor DFX could partially counteract this therapeutic effect. These findings indicate that AGO can alleviate depression-like behavior in mice by reducing oxidative stress. In addition, inflammation (as determined by NF-κB and IкBα levels) was not significantly altered, meaning that regulating oxidative stress may be another mechanism of action for depression of AGO [27].
The brain uses aerobic respiration to meet its high energy demand. Although the brain is only 2% of the body’s body weight, it consumes approximately 20% oxygen and 25% glucose [28]. Therefore, the central nervous system is very susceptible to oxidative stress, high levels of which can eventually cause oxidative damage, which is mainly manifested as an imbalance of pro-oxidants and antioxidants [29]. SOD converts superoxide anion radicals into H2O2; CAT, which reduces hydrogen to H2O2; and glutathione peroxidase are the main enzymatic antioxidants [30]. Previously, it was shown that oxidative stress plays a critical role in the pathophysiology of MDD [31]. Patients with MDD had a lower total antioxidant status and higher MDA levels in their blood than the controls [32]. However, the initially reduced SOD activity and elevated MDA levels in the serum of patients with MDD have been shown to normalize after long-term antidepressant treatment [33,34]. Oxidative stress is an imbalance between ROS and antioxidant enzymes that may cause biomolecular damage. Excess ROS eventually leads to the formation of proinflammatory factors, such as MDA and 8-hydroxy-2-deoxyguanosine, promoting the damage-related molecular patterns of immune responses [35]. MDA inhibits nucleotide excision repair systems and sensitization mutagenesis; disrupts DNA; and causes mitochondrial damage by increasing mitochondrial ROS, inhibiting mitochondrial respiration processes, and reducing mitochondrial membrane potential and antioxidant levels [36]. Superoxide production is increased in the brains of rats with CUMS [37], alongside increased protein and lipid peroxidation and unbalanced SOD and CAT activity [38]. Studies show that elevated oxidative stress has also been found in the brains of adult rats after maternal care deprivation [39]. Our data showed that CRS led to a decreased sugar water preference rate, prolonged forced swimming rest time, and depression-like behaviors such as decreased central total distance in the open field in mice. Meanwhile, CRS also leads to increased MDA expression in the serum, decreased CAT activity in the mouse hippocampus, and decreased SOD expression in the serum. The results of this study are consistent with those of previous studies.
AGO acts as a potent agonist of melatoninergic MT1 and MT2 receptors and as a 5-HT2C receptor antagonist. Evidence accumulated over the extensive course of a previous study[40,41] supports the idea that the antidepressant effect of AGO is because of its synergistic melatoninergic and serotonergic effects [27]. The antidepressant effects of AGO have been illustrated in several mature rodent models reflecting the core clinical features of depression [27]. Chronic (3-week) AGO (40 mg/kg, intraperitoneal injection) has been shown to increase cell proliferation, neurogenesis, and cell survival in the rat hippocampus [39]. Furthermore, AGO improves cell maturity and survival throughout the hippocampus [42]. It has been reported that AGO increases SOD activity in the rat striatum and posterior cortex at 10 mg/kg and 30 mg/kg, respectively [43]. Our results showed that AGO significantly attenuated depressive-like behavior in CRS mice; by increasing CAT activity in the hippocampus and serum, it increased SOD and decreased MDA expression, thus reducing oxidative stress. Meanwhile, we administered the CAT-specific inhibitor DFX to the CRS model mice. We observed that the depression-like behavior and oxidative stress levels in this group were not significantly different from those in the CRS model group. It may be that CAT inhibition has participated in the pathological mechanism of the CRS model. In addition, we concurrently administered DFX and AGO treatment, which showed that the depression-like behavior and oxidative stress levels in the mice were significantly different from those in the AGO treatment group and the CRS group alone. This finding indicated that the therapeutic effect of AGO was produced through multiple different mechanisms and was partly achieved by enhancing CAT expression and activity, thus reducing oxidative stress.
The NF-κB protein family plays an important role in the expression of several proinflammatory genes as a key transcription factor. Regulation of this pathway by bioactive substances may be a potential solution for treating inflammatory diseases [44]. NF-κB is a key mediator of chronic stress-induced depression-like behaviors [45]. Moreover, it has been shown that cellular damage can be induced by the activation of the NF-κB signaling pathway, which is heavily involved in inflammation through ROS and proinflammatory cytokines. Research shows that AGO blocked this activation and the above effects, showing that antidepressant treatment may have broad neuroprotective effects [46]. AGO treatment has been shown to significantly inhibit NF-κB phosphorylation without changing the basal levels of the NF-κB/p65 protein. Because of the inhibition of NF-κB phosphorylation, NF-κ cannot be activated and transported into the nucleus, thus preventing cytokine synthesis [47]. Our experiments showed that NF-κB expression varied within different groups. However, there were no significant differences between groups, possibly because AGO affects NF-κB phosphorylation rather than the basal amount of NF-κB expression.
Conclusions
In conclusion, our study demonstrated that AGO has a clear antidepressant-like effect in a CRS model, consistent with previous studies. AGO can also reduce oxidative stress in mice by activating CAT, which we believe is one of the underlying antidepressant mechanisms.
Methods
Study animals
This study used 6–8-week-old specific pathogen-free male C57BL/6 mice weighing 22±3 g provided by Beijing Weitong Lihua Experimental Animal Company (experimental animal production license no. SCXK [Beijing] 2019–0011) and raised by the Experimental Animal Center of Zhejiang University of Traditional Chinese Medicine (experimental animal use license no. SYXK [Zhejiang] 2019–0022). The animals were adaptively maintained for 1 week. The mice were given a standard pelleted diet and free water, and they were housed at a controlled temperature (23°C±2°C) with 60% relative humidity and a 12 h light/dark cycle.
Model construction and grouping
Establishment of the CRS model [47,48]: the mice were placed in a transparent cylindrical tube with a length of approximately 10 cm and an internal diameter of approximately 3 cm. Therefore, this restricted the head and limb movement so that the head could only be moved slightly. Around the bound tube were eight ventilated holes approximately 0.6 cm in diameter for the mice to breathe. The binding time was from 10:00 to 16:00 (6 h). Each mouse was fed water ad libitum, although the water was not given during the binding process. We have considered the following aspects to ensure that the CRS model has been successfully built as much as possible: The weight of the mice after CRS modeling decreased significantly, with statistical significance.Through behavioral tests, it proved that mice after CRS modeling had depressive behavior. In this experiment, 1) the decreased sugar water preference rate in the sugar-water preference test, 2) the prolonged forced swimming rest time in the forced swimming test, 3) the decreased central total distance in the open field tests.[49,50] Successful mice were studied after 4 weeks of CRS molding.
Group: Sixty mice were randomly divided into six groups of 10 mice each: normal control (CON), CAT inhibitor (DFX), model (CRS), model + AGO (CON + AGO), model + CAT inhibitor (deferasirox) (CRS + DFX), and model + AGO + CAT inhibitor (CRS + AGO + DFX).
Drug treatment: Animals were treated for 4 weeks through intragastric administration. CRS + AGO mice, CRS + DFX mice, and CRS + AGO + DFX mice were treated with AGO (Jiangsu Hausen Pharmaceutical Company) 50 mg/kg, DFX, or both (Shanghai Bide Medical Technology Company) 10 mg/kg once a day, all drugs were dissolved in normal saline and administered as described in previous studies [5,51]. Mice from the CRS group were given equal normal saline from the first day until the fourth weekend. Mice in the CON and DFX groups were not treated with CRS stimulation. The CON group was administered equal amounts of normal saline (10 mg/kg) once a day; meanwhile, the DFX group was administered DFX (10 mg/kg) once a day.
Behavior index detection
Sugar-water preference test (SPT)
This Tang et al. [52] test is divided into adaptation and experimental stages. Adaptation stage: Single-cage feeding, with two water bottles placed simultaneously. Both bottles were filled with 1% sucrose water Within the first 24 h. One bottle contained pure water for the subsequent 24 h, and the other contained 1% sucrose water. Experimental stage: one bottle of 1% sucrose water and one bottle of pure water were given after 24 h of the SPT, and the overall weights of the bottles were recorded. The positions of the two water bottles were exchanged after 12 h, and their overall weight was re-recorded after 24 h. The sugar water preference rate was calculated as the indicator of the SPT (sugar water preference rate = sugar water consumption/[sugar water consumption + pure water consumption] × 100%).
Open Field Test (OFT)
SMART 3.0 virtually divided the absent field analysis box (40 cm × 40 cm × 40 cm) into central and peripheral parts using the Song et al. [53] OFT. Experiments were performed in a darker observation box. First, the mice were placed in the central grid to be observed for 5 min. After the experiment, the field box was wiped to remove the residual odor, and the next animal was tested. The main observation indicators were the central crossing time (s) and the total distance (cm).
Forced swimming test (FST)
Using the Lin et al. [54] FST, mice were placed in a circular transparent swimming bucket, 30 cm high, 12 cm in diameter, and 18 cm deep (i.e., that the tail and hind limbs could not touch the bottom), with a water temperature of 25°C±1°C. Each mouse first swam for 1 min to adapt to the environment. The behavioral indicators of the mice were recorded with a camera for 5 min, and their stationary time was recorded. The mice were dried with a towel after the experiment.
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde (PFA) after being perfused with 50 mL (±) of saline through the heart for the immunohistochemistry study, and their brains were removed. The hippocampus was isolated and fixed in 4% paraformaldehyde for 3 days at 4 °C after that. Fixed Hippocampal tissue was embedded in paraffin and sectioned to 10μm thickness. Paraffin tissue sections were taken and baked at 60°C for 1 h; dried in three cylinders of xylene for 10 min; dehydrated by 95%, 80%, and 75% gradient alcohol for 1 min; immersed in tap water for 1 min; soaked in 3% H2O2 at 37°C for 30 min, phosphate-buffered saline (PBS) for 3 min, and 0.01 M citrate buffer for 20 min at 95°C; cooled to room temperature; and washed with PBS. Normal sheep serum working fluid was blocked at 37°C for 10 min. One-drop antibody plus rabbit anti-rat CAT (ab209211, Abcam) was added at 4°C overnight and rinsed in PBS. It was incubated with horseradish-labeled secondary antibody for 30 min in DAB. The section was re-stained with hematoxylin and sealed. PBS was used instead of the primary antibody as a negative control, and the normal mucosa was used as a positive control. The positive cell rate was calculated by randomly selecting five high-power fields (400X) and counting 100 cells per field [5,55].
ELISA experiments
The mice were anesthetized with 0.3% (0.15 ml/10 g) pentobarbital sodium by intraperitoneal injection. Blood was collected through a puncture of the retrobulbar venous plexus after anesthesia was deemed effective. Approximately 0.2 ml of blood was collected in a labeled centrifuge tube and kept at 20°C for 1–2 h. The blood was precooled to 4°C and centrifuged at 3000 rpm/min for 10 min. The serum was pipetted into a newly labeled centrifuge tube, frozen, and stored at -80°C for backup. Mouse catalase (CAT) ELISA Kit (ml037752, Enzyme-Linked Biotechnology), mouse superoxide dismutase (SOD) ELISA kit (ml643059, Enzyme-Linked Biotechnology), and MDA (Malondialdehyde) ELISA Kit (E-EL-0060c, Elabscience) content were determined according to the manufacturer’s instructions.
CAT activity detection
The methodology was carried out according to the manufacturer’s instructions (BC0200, Solarbio) [56–58]. Ice bath homogenate at a ratio of tissue mass (g): extract volume (mL) of 1:5°C–10.4°C was centrifuged at 8000 g for 10 min. The supernatant was then removed and placed on ice for testing. The working fluid was then configured according to the instructions. The spectrophotometer was preheated for more than 30 min, the wavelength was adjusted to 240 nm, and the distilled water was adjusted to 0. The CAT working fluid was detected for 10 min before determination in a 25°C water bath. Then, 1 mL of CAT detection solution was added to a 1 mL quartz plate before 35 L samples were added and mixed for 5 s. The initial light absorption at 240 nm and light absorption after 1 min were measured immediately at room temperature.
Quantitative real-time-PCR
Primers for CAT, GAPDH, NF-κB, and IкBα were designed and submitted to TaKaRa for synthesis. Total RNA was extracted with the RNA extraction kit. The reverse transcription system was 20 μL, which was performed according to the reagent instructions. The reaction solution was taken for quantitative PCR according to the kit’s instructions. GAPDH was used as the internal reference control, and 2-ΔΔCt calculated the relative expression content of the target gene expression. Each group of samples was tested more than three times.
Western blot
Each group of mice was killed by cervical dislocation after completing the last behavioral test. Each subject’s brain was dissected and quickly placed on the ice surface. The hippocampal tissue was quickly isolated according to the stereotactic map of the mouse brain, immediately placed in liquid nitrogen, and then moved to an -80°C refrigerator for storage. The brain tissue was ground to a homogenate for the subsequent experimental testing, and a radioimmunoprecipitation assay lysate was added on ice for 30 min. The solution was centrifuged at 4°C at 14400 rpm for 15 min. The supernatant was removed and quantified with a bicinchoninic acid assay kit using a 5-protein loading buffer at 95°C for 10 min. SDS-PAGE electrophoresis was used to separate the protein, transferred to a polyvinylidene fluoride membrane, and then blocked for 1 h at room temperature with 5% skim milk powder. The membrane was then washed three times in TBST plus CAT (ab209211, Abcam), NF-κB (ab207297, Abcam), IкBα (ab32518, Abcam), and GAPDH (ab9484, Abcam). The primary antibody was incubated overnight at 4°C and then washed away with TBST. Then the corresponding secondary antibody was added for 1 h. The membrane was washed in TBST three times and swept with the ODYSSEY two-color infrared laser imaging system. The experimental results were processed with Image Studio Ver 2.0 software.
Statistical analysis
SPSS 25.0 software (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. All data are presented as mean ± standard deviation. Multi-group overall comparisons were analyzed using one-way ANOVA and post hoc multiple comparisons (LSD method). A P-value of <0.05 was considered statistically significant.
Supporting information
(ZIP)
Acknowledgments
We appreciate the Experimental Animal Center of Zhejiang Chinese Medical University for the technical assistance they provided.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by The National Natural Science Foundation of China (grant number 8177051246).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310: 591–608. doi: 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6: 211–224. doi: 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- 3.Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43: 471–481. doi: 10.1017/S0033291712001511 [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Ho CS, McIntyre RS, Wang W, Ho RC. Agomelatine-induced modulation of brain-derived neurotrophic factor (BDNF) in the rat hippocampus. Life Sci. 2018;210: 177–184. doi: 10.1016/j.lfs.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Chen S, Liu J, Amin N, Jin W, Fang M. Agomelatine softens depressive-like behavior through the regulation of autophagy and apoptosis. BioMed Res Int. 2021;2021: 6664591. doi: 10.1155/2021/6664591 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lan T, Wu Y, Zhang Y, Li S, Zhu Z, Wang L, et al. Agomelatine rescues lipopolysaccharide-induced neural injury and depression-like behaviors via suppression of the Gαi-2-PKA-ASK1 signaling pathway. J Neuroinflammation. 2022;19: 117. doi: 10.1186/s12974-022-02479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebai R, Jasmin L, Boudah A. Agomelatine effects on fat-enriched diet induced neuroinflammation and depression-like behavior in rats. Biomed Pharmacother. 2021;135: 111246. doi: 10.1016/j.biopha.2021.111246 [DOI] [PubMed] [Google Scholar]
- 8.Ozdamar Unal G, Demirdas A, Nazıroglu M, Ovey IS. Agomelatine attenuates calcium signaling and apoptosis via the inhibition of TRPV1 channel in the hippocampal neurons of rats with chronic mild stress depression model. Behav Brain Res. 2022;434: 114033. doi: 10.1016/j.bbr.2022.114033 [DOI] [PubMed] [Google Scholar]
- 9.Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaër E, Hen R, et al. Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol. 2012;15: 321–335. doi: 10.1017/S1461145711000356 [DOI] [PubMed] [Google Scholar]
- 10.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7: 137–151. doi: 10.1038/nrn1846 [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76: 197–205. doi: 10.1016/j.psyneuen.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125: 287–294. doi: 10.1016/j.jad.2009.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Yano T, Oku M, Akeyama N, Itoyama A, Yurimoto H, Kuge S, et al. A novel fluorescent sensor protein for visualization of redox states in the cytoplasm and in peroxisomes. Mol Cell Biol. 2010;30: 3758–3766. doi: 10.1128/MCB.00121-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15: 71. doi: 10.1186/s12937-016-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61: 192–208. doi: 10.1007/s00018-003-3206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonenkov VD, Grunau S, Ohlmeier S, Hiltunen JK. Peroxisomes are oxidative organelles. Antioxid Redox Signal. 2010;13: 525–537. doi: 10.1089/ars.2009.2996 [DOI] [PubMed] [Google Scholar]
- 17.Rai A, Gill M, Kinra M, Shetty R, Krishnadas N, Rao CM, et al. Catechin ameliorates depressive symptoms in Sprague Dawley rats subjected to chronic unpredictable mild stress by decreasing oxidative stress. Biomed Rep. 2019;11: 79–84. doi: 10.3892/br.2019.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Q, Tian Y, Wang X, Li P, Su D, Wu C, et al. Oxidative damage of tryptophan hydroxylase-2 mediated by peroxisomal superoxide anion radical in brains of mouse with depression. J Am Chem Soc. 2020;142: 20735–20743. doi: 10.1021/jacs.0c09576 [DOI] [PubMed] [Google Scholar]
- 19.Lyons JE, Ellis PE, Myers HK. Halogenated metalloporphyrin complexes as catalysts for selective reactions of acyclic alkanes with molecular oxygen. J Catal. 1995;155: 59–73. doi: 10.1006/jcat.1995.1188 [DOI] [Google Scholar]
- 20.Szuster-Ciesielska A, Słotwińska M, Stachura A, Marmurowska-Michałowska H, Dubas-Slemp H, Bojarska-Junak A, et al. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32: 686–694. doi: 10.1016/j.pnpbp.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 21.Vaváková M, Ďuračková Z, Trebatická J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid Med Cell Longev. 2015;2015: 898393. doi: 10.1155/2015/898393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camkurt MA, Fındıklı E, Bakacak M, Tolun Fİ, Karaaslan MF. Evaluation of malondialdehyde, superoxide dismutase and catalase activity in fetal cord blood of depressed mothers. Clin Psychopharmacol Neurosci. 2017;15: 35–39. doi: 10.9758/cpn.2017.15.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19: 89–95. doi: 10.1097/00004850-200403000-00006 [DOI] [PubMed] [Google Scholar]
- 24.Bhatt S, Mahesh R, Jindal A, Devadoss T. Neuropharmacological effect of novel 5-HT3 receptor antagonist, N-n-propyl-3-ethoxyquinoxaline-2-carboxamide (6n) on chronic unpredictable mild stress-induced molecular and cellular response: behavioural and biochemical evidences. Pharmacol Rep. 2014;66: 804–810. doi: 10.1016/j.pharep.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 25.Olsen RH, Johnson LA, Zuloaga DG, Limoli CL, Raber J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem. 2013;125: 303–313. doi: 10.1111/jnc.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JH, Lee MJ, Chang Y, Lee S, Kim HJ, Lee SW, et al. Valeriana fauriei exerts antidepressant-like effects through anti-inflammatory and antioxidant activities by inhibiting brain-derived neurotrophic factor associated with chronic restraint stress. Rejuvenation Res. 2020;23: 245–255. doi: 10.1089/rej.2018.2157 [DOI] [PubMed] [Google Scholar]
- 27.Guardiola-Lemaitre B, De Bodinat C, Delagrange P, Millan MJ, Munoz C, Mocaër E. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171: 3604–3619. doi: 10.1111/bph.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SY, Lee SJ, Han C, Patkar AA, Masand PS, Pae CU. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46: 224–235. doi: 10.1016/j.pnpbp.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 29.Balmus IM, Ciobica A, Antioch I, Dobrin R, Timofte D. Oxidative stress implications in the affective disorders: main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxid Med Cell Longev. 2016;2016: 3975101. doi: 10.1155/2016/3975101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev. 2012;2012: 609421. doi: 10.1155/2012/609421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel TM, Pülschen D, Thome J. The role of oxidative stress in depressive disorders. Curr Pharm Des. 2012;18: 5890–5899. doi: 10.2174/138161212803523554 [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Zhong S, Liao X, Chen J, He T, Lai S, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10: e0138904. doi: 10.1371/journal.pone.0138904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38: 247–252. doi: 10.1016/j.arcmed.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 34.Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76: 1658–1667. doi: 10.4088/JCP.14r09179 [DOI] [PubMed] [Google Scholar]
- 35.Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144: 365–373. doi: 10.1111/imm.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18: 595–606. doi: 10.1038/mp.2012.33 [DOI] [PubMed] [Google Scholar]
- 37.Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 2009;54: 358–362. doi: 10.1016/j.neuint.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 38.Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res. 2009;43: 864–869. doi: 10.1016/j.jpsychires.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 39.Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, et al. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci Lett. 2015;584: 83–87. doi: 10.1016/j.neulet.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 40.Millan MJ, Marin P, Kamal M, Jockers R, Chanrion B, Labasque M, et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT(2C) receptors. Int J Neuropsychopharmacol. 2011;14(6):768–83. doi: 10.1017/S1461145710001045 [DOI] [PubMed] [Google Scholar]
- 41.Norman TR, Cranston I, Irons JA, Gabriel C, Dekeyne A, Millan MJ, et al. Agomelatine suppresses locomotor hyperactivity in olfactory bulbectomised rats: a comparison to melatonin and to the 5-HT(2c) antagonist, S32006. European journal of pharmacology. 2012;674(1):27–32. doi: 10.1016/j.ejphar.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 42.Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L, et al. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology. 2009;34: 2390–2403. doi: 10.1038/npp.2009.72 [DOI] [PubMed] [Google Scholar]
- 43.de Mello AH, Souza Lda R, Cereja AC, Schraiber Rde B, Florentino D, Martins MM, et al. Effect of subchronic administration of agomelatine on brain energy metabolism and oxidative stress parameters in rats. Psychiatry Clin Neurosci. 2016;70: 159–66 doi: 10.1111/pcn.12371 [DOI] [PubMed] [Google Scholar]
- 44.Hyeon JY, Choi EY, Choe SH, Park HR, Choi JI, Choi IS, et al. Agomelatine, a MT1/MT2 melatonergic receptor agonist with serotonin 5-HT2C receptor antagonistic properties, suppresses Prevotella intermedia lipopolysaccharide-induced production of proinflammatory mediators in murine macrophages. Arch Oral Biol. 2017;82: 11–18. doi: 10.1016/j.archoralbio.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 45.Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, et al. Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int J Neuropsychopharmacol. 2014;18. doi: 10.1093/ijnp/pyu076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asci H, Ozmen O, Erzurumlu Y, Sofu A, Icten P, Kaynak M. Agomelatine protects heart and aorta against lipopolysaccharide-induced cardiovascular toxicity via inhibition of NF-kbeta phosphorylation. Drug Chem Toxicol. 2022;45: 133–142. doi: 10.1080/01480545.2019.1663209 [DOI] [PubMed] [Google Scholar]
- 47.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39: 112–119. doi: 10.1016/j.pnpbp.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 48.Jung J, Lee SM, Lee MJ, Ryu JS, Song JH, Lee JE, et al. Lipidomics reveals that acupuncture modulates the lipid metabolism and inflammatory interaction in a mouse model of depression. Brain Behav Immun. 2021;94: 424–436. doi: 10.1016/j.bbi.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 49.Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull. 2010;26(4):327–37. doi: 10.1007/s12264-010-0323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. Animal models of anxiety disorders and stress. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999). 2013;35 Suppl 2:S101–11. doi: 10.1590/1516-4446-2013-1139 [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Ran L, Yang Y, Gao X, Peng M, Liu S, et al. Deferasirox alleviates dss-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. 2023;314: 121312. doi: 10.1016/j.lfs.2022.121312 [DOI] [PubMed] [Google Scholar]
- 52.Tang J, Liang X, Dou X, Qi Y, Yang C, Luo Y, et al. Exercise rather than fluoxetine promotes oligodendrocyte differentiation and myelination in the hippocampus in a male mouse model of depression. Transl Psychiatry. 2021;11: 622. doi: 10.1038/s41398-021-01747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, et al. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation. 2020;17: 178. doi: 10.1186/s12974-020-01848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J, Song Z, Chen X, Zhao R, Chen J, Chen H, et al. Trans-cinnamaldehyde shows anti-depression effect in the forced swimming test and possible involvement of the endocannabinoid system. Biochem Biophys Res Commun. 2019;518: 351–356. doi: 10.1016/j.bbrc.2019.08.061 [DOI] [PubMed] [Google Scholar]
- 55.Cai M, Lee JH, Yang EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J Neuroinflammation. 2019;16: 264. doi: 10.1186/s12974-019-1665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Jing S, Lin H, Sun W, Jiang W, Yu C, et al. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019;10: 7755–7766. doi: 10.1039/c9fo01182j [DOI] [PubMed] [Google Scholar]
- 57.Kong D, Yan Y, He XY, Yang H, Liang B, Wang J, et al. Effects of resveratrol on the mechanisms of antioxidants and estrogen in Alzheimer’s disease. Biomed Res Int. 2019;2019: 8983752. doi: 10.1155/2019/8983752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang ZX, Lian WW, He J, He XL, Wang YM, Pan CH, et al. Cornuside ameliorates cognitive impairments in scopolamine induced ad mice: involvement of neurotransmitter and oxidative stress. J Ethnopharmacol. 2022;293: 115252. doi: 10.1016/j.jep.2022.115252 [DOI] [PubMed] [Google Scholar]
- 59.Yuan YH, Sun JD, Wu MM, Hu JF, Peng SY, Chen NH. Rotenone could activate microglia through nfκb associated pathway. Neurochem Res. 2013;38: 1553–1560. doi: 10.1007/s11064-013-1055-7 [DOI] [PubMed] [Google Scholar]
- 60.El Mouatassim S, Guérin P, Ménézo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod. 1999;5: 720–725. doi: 10.1093/molehr/5.8.720 [DOI] [PubMed] [Google Scholar]
- 61.Feng X, Wang H, Ye S, Guan J, Tan W, Cheng S, et al. Up-regulation of microRNA-126 may contribute to pathogenesis of ulcerative colitis via regulating NF-kappaB inhibitor IκBα. PloS One. 2012;7: e52782. doi: 10.1371/journal.pone.0052782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zha H, Miao W, Rong W, Wang A, Jiang W, Liu R, et al. Remote ischaemic perconditioning reduces the infarct volume and improves the neurological function of acute ischaemic stroke partially through the miR-153-5p/TLR4/p65/IkBa signalling pathway. Folia Neuropathol. 2021;59: 335–349. doi: 10.5114/fn.2021.112127 [DOI] [PubMed] [Google Scholar]