Abstract
Purpose
We conducted a comprehensive meta-analysis to compare the effects of balanced crystalloids (BC) and isotonic saline (IS) in pediatric sepsis.
Methods
A systematic search was performed for studies comparing BC and IS in pediatric sepsis. Outcomes included mortality, acute kidney injury (AKI), need for renal replacement therapy (RRT), hospital length of stay (LOS), and pediatric intensive care unit (PICU) LOS. A random-effect models was used to calculated pooled odds ratios (OR) and mean differences (MD) with 95% confidence intervals (CIs).
Results
The analysis included six studies with 8753 children. BC demonstrated significant reductions in overall mortality (OR 0.84, 95% CI 0.71 to 0.98, P = 0.03, I2 = 0%) and AKI (OR 0.74, 95% CI 0.57 to 0.96, P = 0.03, I2 = 37%) compared to IS. RRT need was similar between the BC and IS groups (OR 0.79, 95% CI 0.60 to 1.02, P = 0.07, I2 = 0%). Hospital and PICU LOS did not differ significantly. However, subgroup analysis of randomized controlled trials revealed significantly shorter hospital LOS in the BC group (mean difference −0.66 days, 95% CI −1.10 to −0.23, P = 0.003, I2 = 0%).
Conclusion
Our meta-analysis demonstrates that using BC in pediatric sepsis is associated with reduced mortality, AKI, and hyperchloremia rates compared to IS, while maintaining similar hospital and PICU LOS. Large-scale randomized controlled trials are needed to validate these findings.
Keywords: Balanced crystalloids, isotonic saline, lactated ringer, pediatric sepsis
Pediatric sepsis continues to be a significant cause of child mortality worldwide.1 Recent epidemiological studies have uncovered an evolving landscape. In 1995, the incidence was 0.56 cases per 1000, with a 10.3% in-hospital mortality rate. From 1995 to 2005, the incidence rose to 0.89 cases per 1000, with mortality declining to 8.9%, signaling changing trends in pediatric sepsis dynamics.2 In the United States alone, sepsis accounts for a significant portion of pediatric intensive care unit (PICU) admissions and remains a leading cause of mortality among pediatric patients. In the study by Baker et al, among 54,129 weighted pediatric ED visits for severe sepsis or septic shock, emergency department mortality was 0.58%, and hospital stay mortality was 9.8%.3 The disease burden of pediatric sepsis extends beyond individual patients, impacting families, healthcare systems, and economies on a global scale.4 Addressing sepsis in pediatric patients is a pressing concern, necessitating research that explores optimal treatment strategies.
Fluid resuscitation stands as a pivotal initial intervention in the management of pediatric sepsis. Crystalloid fluids are the preferred type of fluids in the resuscitation of septic patients.5 Crystalloid fluids are classified to either nonbalanced fluids, such as 0.9% isotonic saline (IS), or balanced fluids, such as lactated Ringer (LR) or PlasmaLyte.6 In comparison to IS, balanced fluids contain an electrolyte content that is closer to plasma.6 IS has been linked to acute kidney injury (AKI) and hyperchloremic metabolic acidosis.7,8
BC may be superior to normal saline in adult populations, according to mounting research. In adults with sepsis, a recent meta-analysis found that BC was related to lower mortality and AKI benefits when compared to IS.9 Similarly, the Surviving Sepsis Campaign international guidelines in 2020 suggested a weak recommendation to use BC over IS for fluid resuscitation of children with sepsis or septic shock.10 However, the recommendation was based on a very low quality of evidence from two observational studies11,12 in children with sepsis and indirect evidence from adult sepsis.10 A recent meta-analysis by Lehr et al13 was published comparing balanced versus unbalanced fluid in critically ill children, which showed no difference in clinical outcomes such as mortality, AKI, or need for renal replacement therapy (RRT). However, this analysis included all critically ill children, whether septic or nonseptic, and did not include a subgroup analysis for children with sepsis alone. The crystalloid fluid of choice in pediatric sepsis remains unclear. Therefore, we performed this comprehensive systematic review and metaanalysis to evaluate the effects of BC versus IS on the clinical outcomes of pediatric patients with sepsis or septic shock.
METHODS
Data sources and search strategies
We conducted a systematic literature search that adhered to the Peer Review of Electronic Search Strategies (PRESS) guidelines.14 We incorporated appropriate search terms, Boolean operators, and MeSH terms in our comprehensive search strategy. Our search strategy targeted PubMed, Embase, Web of Science, and Cochrane Central databases from inception to April 22, 2022, to include all published observational studies and randomized controlled trials (RCTs) that performed a head-to-head comparison between BC and IS. We further enhanced our search by manually examining references from included studies to identify any additional relevant articles that might have been missed in the initial electronic search. The following search terms were used: (“pediatric sepsis”), (“normal saline” or “isotonic saline”), and (“balanced crystalloids” or “lactated Ringer” or “PlasmaLyte”). The search was not limited by language, study design, or country of origin. Supplementary Table 1 describes the full search terms used in each database searched.
Eligibility criteria
All studies that compared BC versus IS in pediatric patients (≥1 month to 18 years) with sepsis and reported the clinical outcomes of mortality, AKI, need for RRT, and hospital length of stay (LOS) were eligible for inclusion. We excluded studies that included patients without sepsis to ensure the homogeneity of our analysis and to maintain the focus on the specific population of interest, namely pediatric patients with sepsis. Combining patients with and without sepsis could introduce confounding factors and compromise the internal validity of our results. This methodological choice enhances the robustness and clinical relevance of our findings within the targeted patient population. Furthermore, we excluded single-arm studies, case reports, or case series to ensure the comparability and rigor of the included studies. Additionally, studies involving the neonatal population (age <1 month old) were excluded to maintain a cohesive age range within the pediatric population under study.
Data extraction
The following data were extracted from the studies: first author name, publication year, country of origin, study design, sample size, gender and age of patients, location of patients, severity of sepsis, type of BC used, fluid volume, and follow-up duration. Outcome measures were retrieved, including mortality, AKI, need for RRT, LOS, and hyperchloremia. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines to select the final studies.15 Two investigators (AS and AB) independently performed the search and shortlisted the studies for final review. Discrepancies were resolved by a third reviewer (OS).
Outcomes of interest
The primary outcome of our study was mortality between BC and IS. The secondary outcomes were AKI, need for RRT, hospital and PICU LOS, and hyperchloremia.
Statistical analysis
We performed a metaanalysis of the included studies using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Nordic Cochrane Centre) and Comprehensive Meta-Analysis (Biostat, Englewood, NJ, USA). The median and interquartile range were converted to mean and standard deviation (SD) where applicable.16 Due to the marked heterogeneity in patients with sepsis,17 we used a random-effects model to analyze our data. The Mantel-Haenszel method was used to calculate the pooled odds ratio (OR) and mean difference (MD) with the corresponding confidence intervals (CI) for proportional and continuous variables, respectively. A P value <0.05 was considered statistically significant. The heterogeneity was evaluated using the I2 statistic as defined by the Cochrane Handbook for Systematic Reviews. An I2 value of 50% was considered substantial heterogeneity for all outcomes.18
Sensitivity and subgroup analyses
We performed subgroup analysis based on the study design (RCTs vs observational studies) and the type of BC (LR) if at least two studies reported the outcome. We also performed a subgroup analysis of full-text studies by exclusion of abstracts. We performed sensitivity analyses for outcomes reported by ≥5 studies using leave-one-out metaanalysis to see if it significantly influenced the metaanalysis result (i.e., jackknife sensitivity analysis) to confirm that our results were robust.
Bias assessment
The Jadad composite scale was used to assess the methodological quality of the clinical trials based on randomization, blinding, and withdrawals.19 The scale ranged from 0 to 5 points.19 Studies with a total score of ≥3 were considered to have a low risk of bias. The Newcastle Ottawa Quality Assessment Scale was used to assess the quality of the observational studies based on the selection of the study groups, comparability of study groups, and ascertainment of exposure/outcome.20 Studies with total scores of ≥6 were considered to have a low risk of bias. Two authors (AM and AB) independently assessed each study for bias. Discrepancies were resolved by a third reviewer (OS). We could not evaluate for publication bias given the limited number of included studies (<10 studies).
RESULTS
Study selection
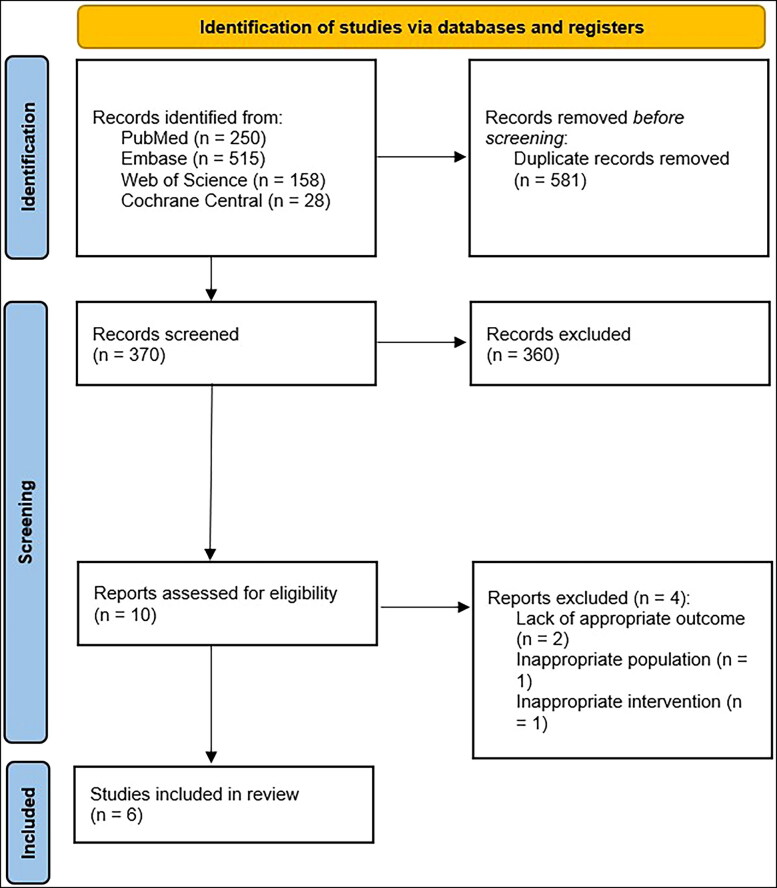
Our search strategy retrieved a total of 951 studies. Among these studies, 10 were eligible for systematic review. Subsequently, we excluded four studies because of the lack of appropriate population, intervention, or outcome. Eventually, six studies11,12,21–24 met our inclusion criteria and were included in the metaanalysis. Figure 1 shows the PRISMA flow chart that illustrates how the final studies were selected.
Figure 1.
PRISMA flow diagram for the selection of studies.
Study and patient characteristics
Table 1 shows the study and patient characteristics of the studies included in the metaanalysis. All the included studies were published between 2017 and 2021 and included patients with sepsis or severe sepsis/septic shock. Three studies11,12,22 originated from the USA, two studies21,23 from Thailand, and one study24 from India. Of the six included studies, five11,12,21–23 were full-text publications, and one24 was an abstract. Regarding the design of studies, four21–24 were RCTs and two11,12 were observational studies. A total of 8753 children with sepsis were included; 1869 children received BC while 6884 children received IS.
Table 1.
Study and patient characteristics of the studies included in the metaanalysis
| Study, year | Publication form | Study design | Country | Total n (BC/IS) | Male, n | Age, mean ± SD or median (IQR), years | Severity of sepsis (BC/IS) | Location | Type of BC | Fluid volume (BC/IS) (mL), mean ± SD or median (IQR) | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anantasit, 2020 | Full text | RCT | Thailand | 61 (30/31) | 36 | 5.2 (1.1–11.8)/9.7 (2.6–16.3) | PRISM III: 7.5 (3–13)/9 (3–17) | ICU | Ringer’s acetate | 24 h: 480 (285–1272)/ 845 (50–1370) | NR |
| Balamuth, 2019 | Full text | RCT | USA | 50 (24/26) | 27 | 3.8 (2.0–10.4)/9.7 (2.6–16.3) | NR | ED | LR | Total: 2000 (1000–4500)/ 2500 (1500–4500) | 90 days |
| Emrath, 2017 | Full text | RC | USA | 7000 (1000/6000) | NR | NR | NR | NR | NR | NR | 3 days |
| Sankar, 2021 | Abstract | RCT | India | 708 (351/357) | NR | NR | NR | NR | PlasmaLyte | First 6 h: 50 (37–64)/ 49 (39/68) | 7 days |
| Trepatchayakorn, 2021 | Full text | RCT | Thailand | 16 (5/11) | NR | NR | NR | ICU | LR | NR | NR |
| Weis, 2017 | Full text | RC | USA | 918 (459/459) | NR | NR | NR | ICU and ward | LR | NR | NR |
BC indicates balanced crystalloids; ED, emergency department; ICU, intensive care unit; IQR, interquartile range; IS, isotonic saline; LR, lactated Ringer; NR, not reported; RC, retrospective cohort; RCT, randomized controlled trials; SD, standard deviation.
Outcomes of interest
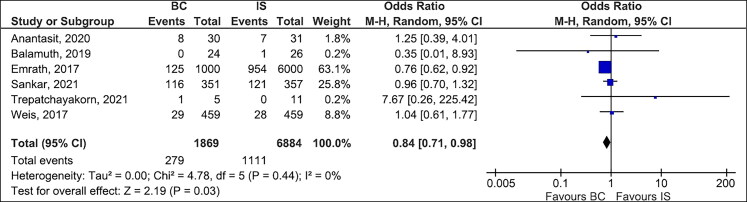
Mortality. Table 2 summarizes the outcomes of the individual studies included in the metaanalysis. Table 3 shows the detailed analysis of the outcomes of the metaanalysis with subgroup analysis based on the study design (RCTs). All six studies,11,12,21–24 which included 8753 children with sepsis, reported the mortality rate (14.9% in the BC group and 16.1% in the IS group). The overall mortality rate was lower in the BC group than in the IS group (OR 0.84, 95% CI 0.71–0.98, P = 0.03, I2 = 0%, Figure 2). However, the overall mortality rate was not significant in the subgroup of LR only (OR 1.06, 95% CI 0.63–1.79, P = 0.83, I2 = 0%, Supplementary Figure 1). The subgroup analysis based on the study design (observational studies vs RCTs) showed lower mortality among those who received BC compared to IS, regardless of the study design. The test for subgroup differences was not significant (I2 = 12.8%, chi-square = 1.15, degrees of freedom = 1, P value = 0.28, Supplementary Figure 2). However, the subgroup analysis for RCTs revealed no difference in mortality between the two groups (OR 0.99, 95% CI 0.73–1.33, P = 0.93, I2 = 0%, Supplementary Figure 2). Furthermore, the subgroup analysis of full-text publications demonstrated lower mortality in the BC group compared to the IS group (OR 0.80, 95% CI 0.66–0.96, P = 0.02, I2 = 0%, Supplementary Figure 3).
Table 2.
Outcomes of the studies included in the metaanalysis
| Study, year | Mortality, n (BC/IS) | AKI, n (BC/IS) | Need for RRT, n (BC/IS) | Hospital LOS, mean ± SD or median (IQR), days (BC/IS) | ICU LOS, mean ± SD, days (BC/IS) | Hyperchloremia, n (BC/IS) |
|---|---|---|---|---|---|---|
| Anantasit, 2020 | 8/7 | 6/5 | 1/1 | 33.7 (12.1–67.4)/31.6 (17.9–62) | 2.9 (1.7–11)/3.5 (1.5–4.7) | 10/17 |
| Balamuth, 2019 | 0/1 | 0/1 | 0/0 | 5 (3–8)/3 (2–9) | NR | 3/7 |
| Emrath, 2017 | 125/954 | 160/1153 | 54/433 | 21 (19.1–23)/18.1 (16–19.3) (in 95% CI) | 18.9 (14–25.4)/14.2 (12–16.8) (in 95% CI) | NR |
| Sankar, 2021 | 116/121 | 73/119 | (33/48)/(66/99) | 6 (4–8)/6 (5–9) | 4 (3–6)/4 (3–6) | (103/229)/(141/225) |
| Trepatchayakorn, 2021 | 1/0 | 1/1 | NR | NR | NR | NR |
| Weis, 2017 | 29/28 | 45/49 | 5/5 | 11.9 (5–18)/10.5 (4–14) | 5.8 (1–10)/5.5 (1–9) | NR |
BC indicates balanced crystalloids; ICU, intensive care unit; IQR, interquartile range; IS, isotonic saline; NR, not reported; SD, standard deviation.
Table 3.
Detailed analysis of the outcomes of the metaanalysis with subgroup analysis based on the study design
| Outcome measures (n) | Overall effect (OR, 95% CI, P value, I2) | RCTs only, n (OR, 95% CI, P value, I2) |
|---|---|---|
| Mortality (6) | OR 0.84, 95% CI 0.71–0.98, P = 0.03, I2 = 0% | n = 4 (OR 0.99, 95% CI 0.73–1.33, P = 0.93, I2 = 0%) |
| AKI (6) | OR 0.74, 95% CI 0.57–0.96, P = 0.03, I2 = 37% | n = 4 (OR 0.54, 95% CI 0.39–0.75, P = 0.0003, I2 = 0%) |
| Need for RRT (5) | OR 0.79, 95% CI 0.60–1.02, P = 0.07, I2 = 0% | n = 3 (OR 1.10, 95% CI 0.54–2.24, P = 0.80, I2 = 0%) |
| Hyperchloremia (3) | OR 0.47, 95% CI 0.34–0.67, P < 0.0001, I2 = 0% | n = 3 (OR 0.47, 95% CI 0.34–0.67, P < 0.0001, I2 = 0%) |
| Hospital LOS (4) | MD 0.67 days, 95% CI −1.51, 2.84, P = 0.55, I2 = 87% | n = 3 (MD −0.66 days, 95% CI −1.10, −0.23, P = 0.003, I2 = 0%) |
| ICU LOS (3) | MD 0.27 days, 95% CI −0.36, 0.90, P = 0.40, I2 = 47% | n = 2 (MD 0.66 days, 95% CI −1.32, 2.64, P = 0.51, I2 = 60%) |
AKI indicates acute kidney injury; CI, confidence interval; ICU, intensive care unit; IS, isotonic saline; LOS, length of stay; MD, mean difference; N/A, not applicable for outcomes that were reported by ≤1 study; NR, not reported; OR, odds ratio; RCTs, randomized controlled trials; RRT, renal replacement therapy.
Figure 2.
Forest plots comparing balanced crystalloids to isotonic saline regarding overall mortality.
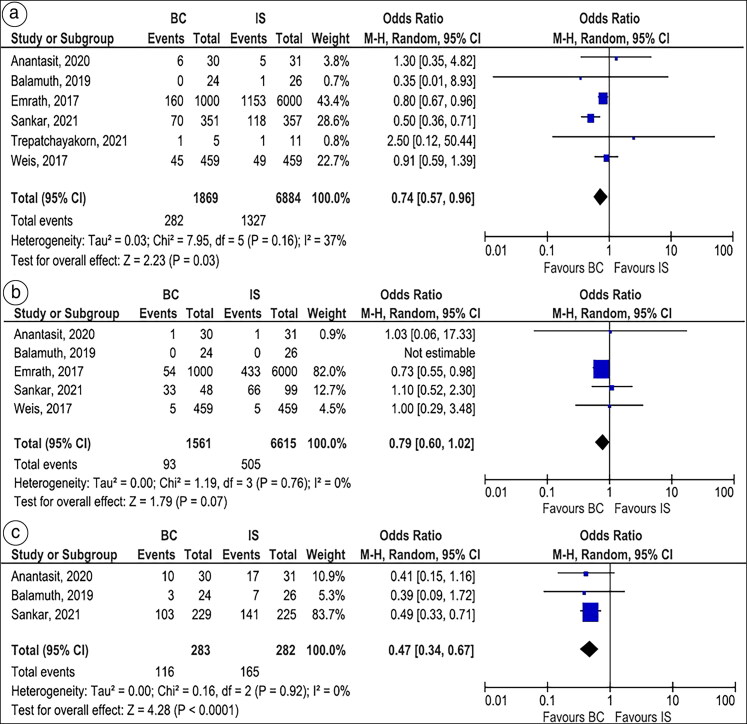
Acute kidney injury. All six studies,11,12,21–24 which included 8753 children with sepsis, reported the rate of AKI (15.1% in the BC group and 19.3% in the IS group). Overall, BC was associated with lower AKI compared to IS (OR 0.74, 95% CI 0.57–0.96, P = 0.03, I2 = 37%, Figure 3A). The results were consistent on the subgroup analysis based on the study design, whether RCTs (OR 0.54, 95% CI 0.39–0.75, P = 0.0003, I2 = 0%) or observational studies (OR 0.82, 95% CI 0.69–0.96, P = 0.02, I2 = 0%) (Supplementary Figure 4). The subgroup of full-text publications also demonstrated lower rates of AKI in the BC group compared to the IS group (OR 0.82, 95% CI 0.70–0.97, P = 0.02, I2 = 0%, Supplementary Figure 5). However, there was no significant difference in AKI between the two groups when the analysis was restricted to LR only (OR 0.91, 95% CI 0.60–1.39, P = 0.67, I2 = 0%, Supplementary Figure 6).
Figure 3.
Forest plots comparing balanced crystalloids to isotonic saline regarding (a) acute kidney injury, (b) need for renal replacement therapy, and (c) hyperchloremia.
Need for renal replacement therapy. Five studies11,12,21,22,24 (three RCTs and two cohort studies), which included 8176 children with sepsis, reported the need for RRT. The need for RRT was similar between BC and IS groups (OR 0.79, 95% CI 0.60–1.02, P = 0.07, I2 = 0%, Figure 3B). The results remained consistent on the subgroup analysis of RCTs (OR 1.10, 95% CI 0.54–2.24, P = 0.80, I2 = 0%, Figure 4A). However, the subgroup analysis of observational studies showed a lower need for RRT with BC compared to IS (OR 0.75, 95% CI 0.56–0.99, P = 0.04, I2 = 0%) (Supplementary Figure 7). Furthermore, the subgroup analysis of full-text publications demonstrated a lower need for RRT with BC than IS (OR 0.75, 95% CI 0.56–0.99, P = 0.04, I2 = 0%, Supplementary Figure 8).
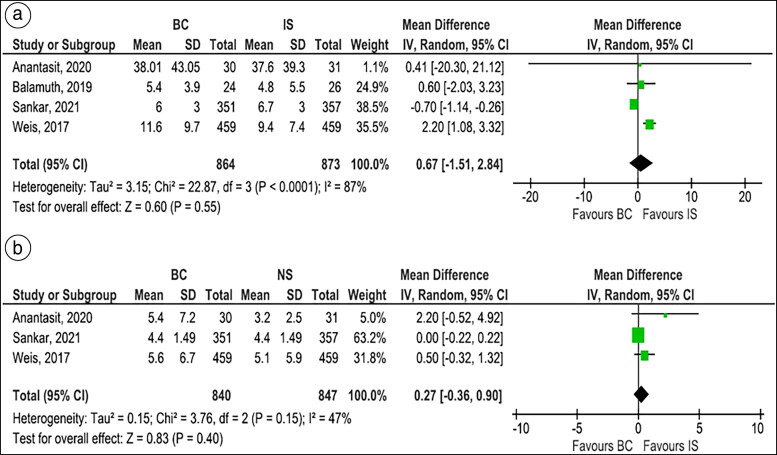
Figure 4.
Forest plots comparing balanced crystalloids to isotonic saline regarding (a) hospital length of stay and (b) pediatric intensive care unit length of stay.
Hyperchloremia. Three studies,21,22,24 which included 565 children with sepsis, reported hyperchloremia. BC was significantly associated with a lower incidence of hyperchloremia compared to IS (OR 0.47, 95% CI 0.34–0.67, P < 0.0001, I2 = 0%, Figure 3C).
Hospital and PICU length of stay. Four studies,11,21,22,24 which included 1737 children with sepsis, reported the hospital LOS. There was no significant difference between BC and IS groups with regard to the hospital LOS (MD 0.67 days, 95% CI −1.51 to 2.84, P = 0.55, I2 = 87%, Figure 4A). However, subgroup analysis of RCTs showed significantly lower hospital LOS in the BC group compared to the IS group (MD −0.66 days, 95% CI −1.10 to −0.23, P = 0.003, I2 = 0%, Supplementary Figure 9).
Three studies, which included 1687 children with sepsis, reported the PICU LOS. The PICU LOS was similar between BC and IS groups (MD 0.27 days, 95% CI −0.36 to 0.90, P = 0.40, I2 = 47%, Figure 4B). The results for PICU LOS were consistent on subgroup analysis of RCTs (MD 0.66 days, 95% CI −1.32 to 2.64, P = 0.51, I2 = 60%, Supplementary Figure 10).
Sensitivity analysis for mortality and AKI
A leave-one-out sensitivity analysis for both in-hospital mortality and AKI showed consistent results, as shown in Supplementary Figures 11 and 12, respectively.
Quality and publication bias assessment
Quality assessment scores of the RCTs and observational studies are summarized in Supplementary Table 2. There was a low risk of bias for all five full-text studies.11,12,21–23 Given the constrained number of studies that fulfilled our inclusion criteria, conducting a substantial subgroup analysis centered on the risk of bias was not feasible. The modest sample size within each subgroup, compounded by the omission of Sankar’s study,24 “as it was published as a conference abstract,” from this evaluation, hampers the attainment of robust statistical comparisons. Nevertheless, our sensitivity analysis, employing a leave-one-out approach, consistently yielded outcomes that bolstered the robustness of our findings and enhanced the assurance in the conclusions drawn from our study. Finally, we couldn’t assess for publication bias due to the limited number of included studies in our analysis.
DISCUSSION
To the best of our knowledge, this is the first systematic review and metaanalysis to assess the effect of BC versus IS on the clinical outcomes of sepsis in the pediatric population. We identified six studies, including four RCTs with a total of 8753 children. Overall, BC was associated with lower in-hospital mortality, AKI, and hyperchloremia when compared to IS with similar in-hospital and PICU LOS. The subgroup of RCTs demonstrated no mortality difference between the two groups, but the superiority of BC on the rates of AKI and hyperchloremia was consistent. Furthermore, the hospital LOS was shorter with BC compared to IS in the subgroup of RCTs.
In 2020, the Surviving Sepsis Campaign international guidelines recommended that children with septic shock or other sepsis-related organ dysfunction receive balanced/buffered crystalloid rather than IS for initial resuscitation. However, this weak recommendation was based on evidence of poor quality, with only two large observational studies in children with sepsis and indirect evidence from adult patients supporting it.10 Recently, adult studies are increasingly supporting this recommendation, with a recent metaanalysis of eight RCTs and seven cohort studies involving 20,329 adults with sepsis finding that BC was linked with lower overall mortality, 28/30-day mortality, and AKI than IS.9 However, due to differences in pediatric and adult sepsis pathophysiology in terms of the maturation of pediatric organ systems, more direct evidence based solely on studies conducted among children with sepsis is required, and the best management of sepsis among this delicate group of patients should be directed considering these differences.25 As a result, the goal of our study was to compile information from all relevant studies conducted among the pediatric population while avoiding the overwhelming evidence focused on the adult population.
In our study, children who received BC had a higher chance of surviving than those who received IS. Although the RCT subgroup analysis failed to find such a difference, most of the available RCTs were underpowered due to small sample sizes and low event rates; therefore, type II error should not be dismissed, as the observed difference may have been underestimated in the RCT subgroups. The observed survival advantage could be explained by our study’s consistent findings of lower rates of hyperchloremia and its associated acidosis. Hyperchloremic metabolic acidosis was linked to the type of fluid given to children with septic shock, and hyperchloremia was found to be an independent risk factor for 28-day mortality or organ failure persistence.26 Furthermore, our findings consistently demonstrated that BC was associated with a decreased risk of AKI, even though the difference in RRT demand between the two groups was not significant. Nonetheless, a subgroup analysis of observational studies revealed that BC patients required less RRT than IS patients. Again, the small sample size of the included RCTs may have understated the trend of lower RRT odds in the BC group. Some adult studies have also revealed that using IS increases the risk of developing AKI.27,28 Furthermore, many animal and human investigations have indicated that IS-induced hyperchloremia causes renal hypoperfusion and AKI.29,30 As a result, AKI is significantly linked to a higher risk of death among critically ill patients.31,32 Finally, according to our findings, the LOS in the hospital and in the PICU was similar in the BC and IS groups. The subgroup analysis of RCTs showed a statistically significant lower hospital LOS in the BC group compared to the IS group. However, the clinical relevance of such a difference (0.66 day) may be negligible.
Lehr et al13 conducted a metaanalysis evaluating balanced versus imbalanced fluid in critically ill children, using three RCTs with a total of 162 patients. In terms of pH and serum bicarbonate changes, BC was found to be superior to IS. The study, however, found no difference in clinical outcomes, including death, AKI, or the requirement for RRT. However, this study included all critically ill children, whether septic or not, and there was no subgroup analysis for children with sepsis alone. In addition, their research comprised a variety of diagnoses, severity levels of sickness, outcome measures, and time periods, all of which could compromise the internal validity of their findings.
Several limitations of this study should be acknowledged. First, among the six studies included in our analysis, four were rigorous RCTs, where the definitions of mortality and other outcomes were uniform and well defined. However, it is important to acknowledge that the remaining two studies were retrospective in nature. In these retrospective studies, the outcomes were established through the utilization of International Classification of Diseases, 9th Revision (ICD-9) codes. While this coding approach facilitated the inclusion of a larger patient cohort, it could introduce some potential variability in reporting due to differences in coding practices and documentation. Despite this limitation, our sensitivity analysis using a leave-one-out approach consistently supported the strength of our findings, bolstering the reliability of the results and the associated conclusions. Further large-scale RCTs are necessary to confirm our findings. Second, the sepsis cohort is heterogeneous, and controlling the source and severity of sepsis was not feasible in our study. Third, even though the random-effects model was used in our analysis, moderate to significant heterogeneity was noted in the measurement of some outcomes, such as hospital LOS. This might be driven by differences in patient characteristics, sepsis severity, and variable follow-up duration across the included studies. Lastly, we could not evaluate publication bias due to the limited number of included studies.
Despite the limitations, our study has significant strengths. First, we included a total of six studies (four RCTs) with over 8700 children with sepsis. To our knowledge, this is the first comprehensive systematic review and metaanalysis to compare the impact of BC versus IS on different clinical outcomes of sepsis among the pediatric population. Second, we performed a subgroup analysis based on the design of studies (RCTs vs observational studies) to evaluate the robustness of our results. In addition, our results remained consistent on sensitivity analysis of mortality and AKI. Lastly, all the included studies were of high quality based on quality assessment.
In conclusion, our metaanalysis demonstrated that BC was associated with lower mortality, AKI, and hyperchloremia rates compared to IS with similar hospital and PICU LOS. The subgroup analysis of RCTs showed lower rates of AKI and shorter hospital LOS in the BC group without significant differences in mortality and PICU LOS between the two groups. Future large-scale RCTs are warranted to validate our findings.
Supplementary Material
Funding Statement
The authors report no funding or conflicts of interest.
Disclosure statement
The authors report no funding or conflicts of interest.
References
- 1.Watson RS, Carcillo JA.. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–S5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 2.Prusakowski MK, Chen AP.. Pediatric sepsis. Emerg Med Clin North Am. 2017;35(1):123–138. doi: 10.1016/j.emc.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Baker AH, Monuteaux MC, Eisenberg MA, Hudgins JD.. Pediatric sepsis survival in pediatric and general emergency departments. Am J Emerg Med. 2022;51:53–57. doi: 10.1016/j.ajem.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JC. Understanding the global burden of pediatric sepsis. Am J Respir Crit Care Med. 2015;191(10):1096–1098. doi: 10.1164/rccm.201503-0594ED. [DOI] [PubMed] [Google Scholar]
- 5.Karakala N, Raghunathan K, Shaw AD.. Intravenous fluids in sepsis: what to use and what to avoid. Curr Opin Crit Care. 2013;19(6):537–543. doi: 10.1097/MCC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 6.Chang R, Holcomb JB.. Choice of fluid therapy in the initial management of sepsis, severe sepsis, and septic shock. Shock. 2016;46(1):17–26. doi: 10.1097/SHK.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes W. Abnormal saline in abnormal kidney function: risks and alternatives. Pediatr Nephrol. 2019;34(7):1191–1199. doi: 10.1007/s00467-018-4008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beran A, Altorok N, Srour O, et al. Balanced crystalloids versus normal saline in adults with sepsis: a comprehensive systematic review and meta-analysis. J Clin Med. 2022;11(7):1971. doi: 10.3390/jcm11071971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(Suppl 1):10–67. doi: 10.1007/s00134-019-05878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss SL, Keele L, Balamuth F, et al. Crystalloid fluid choice and clinical outcomes in pediatric sepsis: a matched retrospective cohort study. J Pediatr. 2017;182:304–310.e10. doi: 10.1016/j.jpeds.2016.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB.. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177–1183. doi: 10.1097/CCM.0000000000002365. [DOI] [PubMed] [Google Scholar]
- 13.Lehr AR, Rached-d’Astous S, Barrowman N, et al. Balanced versus unbalanced fluid in critically ill children: systematic review and meta-analysis. Pediatr Crit Care Med. 2022;23(3):181–191. doi: 10.1097/PCC.0000000000002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C.. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo D, Wan X, Liu J, Tong T.. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 17.Fohner AE, Greene JD, Lawson BL, et al. Assessing clinical heterogeneity in sepsis through treatment patterns and machine learning. J Am Med Inform Assoc. 2019;26(12):1466–1477. doi: 10.1093/jamia/ocz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane; 2021. [Google Scholar]
- 19.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Anantasit N, Thasanthiah S, Lertbunrian R.. Balanced salt solution versus normal saline solution as initial fluid re-suscitation in pediatric septic shock: a randomized, double-blind controlled trial. Critical Care and Shock. 2020;23:158–168. [Google Scholar]
- 22.Balamuth F, Kittick M, McBride P, et al. Pragmatic Pediatric Trial of Balanced Versus Normal Saline Fluid in Sepsis: the PRoMPT BOLUS randomized controlled trial pilot feasibility study. Acad Emerg Med. 2019;26(12):1346–1356. doi: 10.1111/acem.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepatchayakorn S, Sakunpunphuk M, Samransamruajkit R.. Balanced salt solution versus normal saline in resuscitation of pediatric sepsis: a randomized, controlled trial. Indian J Pediatr. 2021;88(9):921–924. doi: 10.1007/s12098-021-03808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankar J, Muralidharan J, Av L, et al. 33: Balanced crystalloids versus saline for initial fluid resuscitation in children with septic shock. Crit Care Med. 2021;49:17. [Google Scholar]
- 25.Emr BM, Alcamo AM, Carcillo JA, Aneja RK, Mollen KP.. Pediatric sepsis update: how are children different? Surg Infect (Larchmt). 2018;19(2):176–183. doi: 10.1089/sur.2017.316. [DOI] [PubMed] [Google Scholar]
- 26.Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155–160. doi: 10.1097/PCC.0000000000001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M.. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 28.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255(5):821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Huh K, Lee J, Kim S, Jeong S, Choi Y.. Comparison of the effects of normal saline versus Plasmalyte on acid-base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplant Proc. 2013;45(6):2191–2196. doi: 10.1016/j.transproceed.2013.02.124. [DOI] [PubMed] [Google Scholar]
- 30.Hansen PB, Jensen BL, Skott O.. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32(6):1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 31.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW.. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 32.Mandelbaum T, Lee J, Scott DJ, et al. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med. 2013;39(3):414–419. doi: 10.1007/s00134-012-2767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.