Abstract
Low-dose methotrexate has several known side effects associated with mild toxicity including nausea, oral ulcers, and anemia. However, it is rare for patients taking low-dose methotrexate to present with symptoms of severe toxicity including pancytopenia, macular punctate rash, and severe stomatitis. Here we present an 83-year-old patient with a history of rheumatoid arthritis on low-dose methotrexate for 1 year presenting with 5 days of worsening facial swelling, oral lesions, and a macular rash to the extremities. Initial workup revealed severe leukopenia, thrombocytopenia, and previously undiagnosed chronic kidney injury. Computed tomography showed edema surrounding a left maxillary dental implant suggestive of infection. The patient was admitted for suspected methotrexate toxicity complicated by possible dental infection. Methotrexate was withheld. The patient’s stomatitis and facial swelling improved with administration of folate, leucovorin, and piperacillin/tazobactam. The patient’s severe neutropenia gradually resolved following administration of granulocyte colony-stimulating factor. Infectious workup was negative throughout admission. This case report details factors that precipitate severe methotrexate toxicity at low doses.
Keywords: Leucovorin, methotrexate, pancytopenia, rheumatoid arthritis, toxicity
CASE SUMMARY
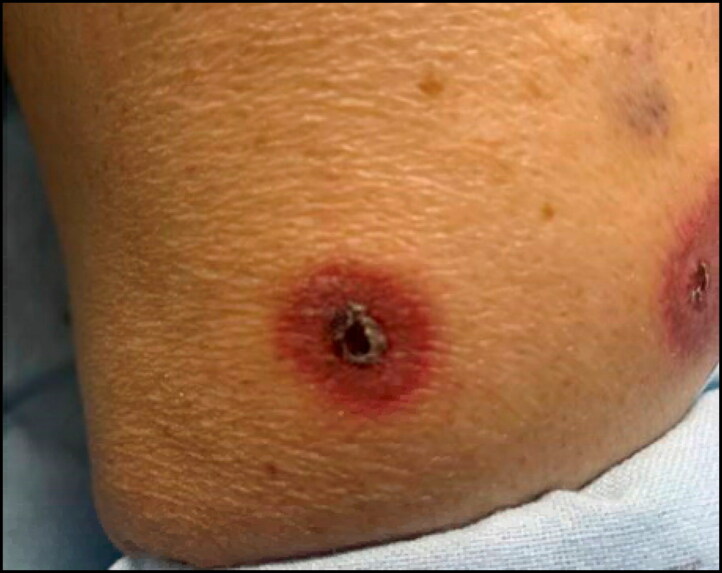
An 83-year-old man with a past medical history significant for rheumatoid arthritis on low-dose methotrexate 10 mg weekly for the past 16 months presented to the emergency department for evaluation of 5 days of oral sores, dysphagia, and worsening bilateral arm rash as well as 2 days of worsening left-sided facial swelling. The patient had been followed by rheumatology for the past 1.5 years with routine monitoring of his blood chemistry profile and cell counts (Table 1), which showed a gradual decline in estimated glomerular filtration rate (eGFR) and a corresponding increase in creatinine consistent with stage 3A chronic kidney disease. The patient was afebrile and hemodynamically stable at the time of admission. Physical examination was remarkable for significant swelling to the left mandibular region, multiple white oropharyngeal sores, and tender punctate circular lesions with a necrotic center on both the upper and lower extremities (Figure 1). The patient’s last dose of methotrexate was 5 days prior to presentation.
Table 1.
The patient’s renal function and complete blood count measurements prior to presentation
| Test (reference range) | 17 months prior | 15 months prior | 12 months prior | 7 months prior | 2 months prior | On presentation |
|---|---|---|---|---|---|---|
| Blood chemistry profile | ||||||
| Creatinine (0.60–1.30 mg/dL) | 1.06 | 1.04 | 1.47 (H) | 1.31 (H) | 1.32 (H) | 1.50 (H) |
| eGFR (≥90 mL/min/1.73 m2) | 65 (L) | 67 (L) | 47 (L) | 54 (L) | 54 (L) | 46 (L) |
| Complete blood count | ||||||
| WBC (4.0–12.0 k/µL) | 5.9 | 5.9 | 6.4 | – | 7.3 | 1.6 (L) |
| RBC (4.30–5.90 m/µL) | 4.11 (L) | 4.03 (L) | 3.81 (L) | – | 3.56 (L) | 4.15 (L) |
| Hemoglobin (13.5–17.5 g/dL) | 11.7 (L) | 11.7 (L) | 11.5 (L) | – | 11.0 (L) | 12.9 (L) |
| MCV (80–100 fl) | 87 | 90 | 91 | – | 93 | 89 |
| Platelets (140–440 k/µL) | 203 | 268 | 211 | – | 232 | 82 (L) |
eGFR indicates estimated glomerular filtration rate; MCV, mean corpuscular volume; RBC, red blood cell count; WBC, white blood cell count.
Figure 1.
Patient’s skin with punctate circular lesions involving a necrotic center.
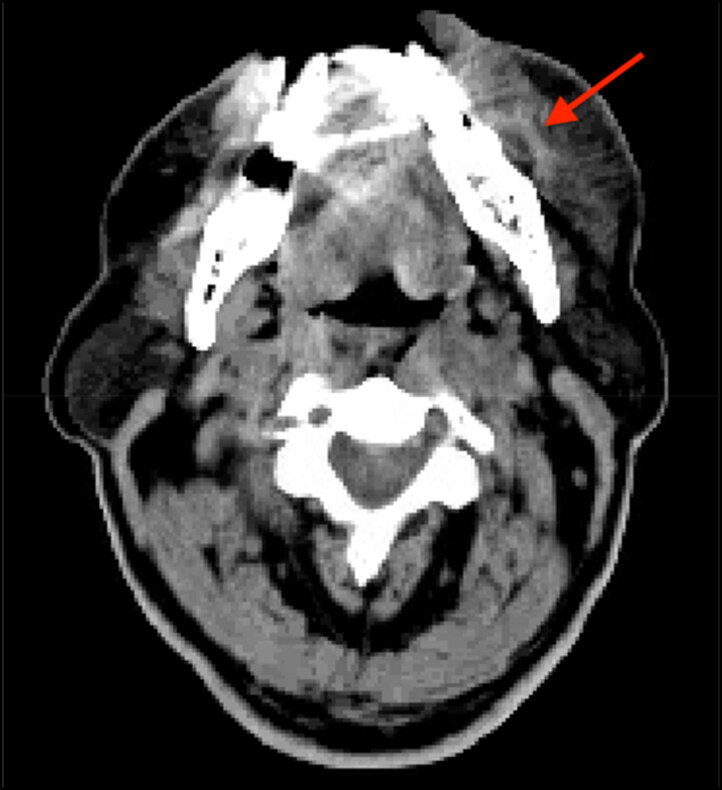
Laboratory investigation showed a hemoglobin of 12.9 g/dL, white blood count of 1.6 k/µL, and platelet count of 82 k/µL. Computed tomography (CT) scan of the head and neck showed ill-defined edema to the subcutaneous adipose tissue of the left lower face with possible infection around a posterior left maxillary dental implant without signs of abscess (Figure 2). The patient was started on intravenous vancomycin and piperacillin/tazobactam and was admitted for further evaluation and management. Vancomycin was discontinued on day 2 after further imaging showed no signs of abscess. Rheumatology was consulted and the patient was started on intravenous leucovorin 15 mg every 6 hours along with folate and vitamin B12 supplementation. Flexible laryngoscopy showed moderate arytenoid and interarytenoid mucosal edema without signs of airway compromise.
Figure 2.
CT scan without contrast showing ill-defined edema of subcutaneous tissue overlying the patient’s left mandible without signs of abscess.
Infectious screenings for HIV, Epstein-Barr virus IgM, mumps, cytomegalovirus, respiratory syncytial virus, herpes simplex virus, varicella-zoster virus, Aspergillus, Cryptococcus, and Histoplasma antigen came back negative. Two blood cultures obtained on day 1 of admission showed no signs of bacterial growth, and an additional blood culture obtained on day 5 showed no signs of fungal growth at 5 days. The patient had been on intravenous antibiotics prior to obtaining these cultures. On day 2 of hospitalization, the patient remained thrombocytopenic with his platelet count dropping from 59 k/µL to 26 k/µL. Hematology/oncology was consulted, and the patient was started on subcutaneous filgrastim granulocyte colony-stimulating factor (G-CSF) 480 mg. Dermatology decided against performing a biopsy of the patient’s rash given the risk of introducing infection. The patient continued a regimen of filgrastim G-CSF, intravenous leucovorin, vitamin B12, and folic acid supplementation. On day 8, white blood cell count and platelet count improved to 32.2 k/µL and 117 k/µL, respectively. The patient’s swelling improved significantly. Filgrastim G-CSF was discontinued given the patient’s leukocytosis. Intravenous piperacillin/tazobactam was discontinued on day 8.
After 48 hours of observation, the patient showed continued improvement without infectious signs. He was discharged home with a diagnosis of pancytopenia secondary to low-dose methotrexate toxicity. At 1-week follow-up, the patient reported significant improvement in his symptoms with normalization of white blood cell count and platelet count. The patient’s prescription of methotrexate was discontinued following a discussion with Rheumatology.
CLINICAL QUESTIONS
1. A 48-year-old woman with a past medical history of rheumatoid arthritis, type 2 diabetes mellitus, and depression presents with painful white oral lesions for 3 days. She denies nausea, vomiting, and joint pain. The patient takes her prescriptions of weekly low-dose methotrexate with folate supplementation, metformin, and citalopram as instructed and reports no recent changes in medication dosage. Her temperature is 99.3°F; blood pressure, 133/85 mm Hg; heart rate, 77 beats/min; respiratory rate, 14 breaths/min; and oxygen saturation, 98% on room air. Laboratory workup is notable for a complete blood count revealing pancytopenia, glucose of 150 mg/dL, and liver function tests showing elevated concentrations of aspartate transaminase and alanine transaminase. Complete metabolic panel is unremarkable. The patient reports that 1 week prior to the onset of her symptoms, she had accidentally started taking medication from a bottle prescribed to her friend with HIV, believing that it was her citalopram prescription. She realized this mistake 2 days earlier and stopped. Which medication did this patient most likely start taking?
a. Trimethoprim/sulfamethoxazole
b. Elfavirenz
c. Didanosine
d. Ibuprofen
2. An 85-year-old man with a past medical history of rheumatoid arthritis on low-dose methotrexate, diabetes mellitus, and a recent diagnosis of chronic kidney disease presents with 4 days of gradually worsening painful sores throughout his oral cavity with new onset bleeding from his mouth while attempting to brush his teeth. He has had difficulty controlling his bleeding, prompting his visit to the emergency department. The patient denies nausea, vomiting, and shortness of breath but notes that he has had a burning pain with urination and frequency for the last week. Complete blood count is notable for pancytopenia including leukopenia, macrocytic anemia, and severe thrombocytopenia. A complete metabolic panel is notable for a creatinine of 2.1 mg/dL (elevated from a baseline of 1.5 mg/dL measured 5 days prior) and blood urea nitrogen of 45 mg/dL. What is the most likely cause of this patient’s presentation?
a. Urinary tract infection
b. Overdose of medication
c. Progression of chronic kidney disease
d. Undiagnosed hemophilia
DISCUSSION
There are a handful of cases describing severe mucositis, cutaneous ulcers, necrotic rashes, or pancytopenia in patients on low-dose methotrexate.1–5 While the symptoms and patient demographics differ, cases generally describe one of two main methods by which patients taking low-dose methotrexate end up presenting with severe toxicity: excessive drug intake or reduced renal excretion.
Overdosage of methotrexate has been described by several case reports where the patient either is prescribed too high a dose due to logistical error or the patient accidentally takes too much of the medication.3,4 One case reported a patient presenting with large necrotic skin lesions and painful oral ulcers after a simple misunderstanding of instructions led to daily intake of methotrexate 7.5 mg for 15 days.4 However, in our case, the patient’s family reported that the patient had been taking methotrexate as prescribed weekly for 16 months alongside daily folate supplementation with no recent adjustments to the dose.
Reduced glomerular filtration rate is a more common reported cause of toxicity in patients on low-dose methotrexate. There are several reported cases of patients on low-dose methotrexate taking nonsteroidal antiinflammatory drugs prior to the onset of severe methotrexate toxicity. One case reported a patient with a history of chronic kidney disease on low-dose methotrexate for rheumatoid arthritis presenting in acute kidney injury with a creatinine of 1.65 mg/dL and symptoms of severe mucositis and pancytopenia.1 Our patient had a 17-month history of mildly elevated creatinine concentrations and gradual reduction in eGFR consistent with previously undiagnosed stage 3A chronic kidney disease (Table 1). Continued deficits in eGFR following recovery and discharge further support this diagnosis (Table 2). The intracellular elimination half-life of methotrexate in red blood cells has been measured between 1.2 and 4.3 weeks, with a suggested correlation found between decreased renal function and increasing intracellular concentrations.6 Decreased serum clearance of methotrexate secondary to undiagnosed chronic kidney disease may have caused a gradual increase in intracellular methotrexate levels as additional weekly doses of methotrexate were taken by the patient. This increase could have set the stage for acute onset of severe methotrexate toxicity following the patient’s final dose prior to presentation. His last dose of methotrexate and date of symptom onset were both 5 days before presentation.
Table 2.
The patient’s renal function and complete blood count measurements following discharge
| Test (reference range) | 1 week postdischarge | 5 weeks postdischarge |
|---|---|---|
| Blood chemistry profile | ||
| Creatinine (0.60–1.30 mg/dL) | 1.32 (H) | 1.31 (H) |
| eGFR (≥90 mL/min/1.73 m2) | 52 (L) | 52 (L) |
| Complete blood count | ||
| WBC (4.0–12.0 k/µL) | 9 | 10.2 |
| RBC (4.30–5.90 m/µL) | 2.87 (L) | 3.85 (L) |
| Hemoglobin (13.5–17.5 g/dL) | 9.2 (L) | 11.9 (L) |
| MCV (80–100 fl) | 95 | 92 |
| Platelets (140–440 k/µL) | 386 | 206 |
eGFR indicates estimated glomerular filtration rate; MCV, mean corpuscular volume; RBC, red blood cell count; WBC, white blood cell count.
However, this patient’s case also has evidence of an unproven yet presumptive orofacial infection, which raises the possibility of acute on chronic kidney failure secondary to infection as the explanation for our patient’s methotrexate toxicity. Orofacial infection is consistent with the patient’s unilateral left-sided facial swelling, CT findings suggestive of posterior left maxillary dental implant infection, and a positive response to a therapy regimen including intravenous antibiotics. Chronic kidney disease is considered a risk factor for acute kidney injury, and an eGFR of 54 mL/min/1.73 m2 seen in this patient carries an estimated 37% increased risk for infection.7,8 Infection could acutely worsen the patient’s eGFR to a point where a once therapeutic weekly dose of methotrexate results in cytotoxic intracellular elevations.7 The resulting pancytopenia would mask leukocytosis otherwise induced by infection. Empirical antibiotic therapy provided to this patient could possibly mask otherwise positive blood cultures, and negative blood cultures do not rule out the possibility of local orofacial infection.
There is a question of whether the patient’s laboratory values reflect acute on chronic kidney injury. The patient’s presenting creatinine level of 1.50 mg/dL does not show an absolute elevation of 0.3 mg/dL in 48 hours from his baseline around 1.30 mg/dL required by Acute Kidney Injury Network guidelines for acute kidney injury.9 However, his last creatinine measurement was 2 months prior to presentation, and given the constellation of findings consistent with infection, acute on chronic kidney injury secondary to infection remains a promising explanation for our patient’s presentation.
Symptoms of methotrexate toxicity in our patient improved significantly by day 8 of admission after 5 days of intravenous leucovorin 15 mg every 6 hours alongside folate/vitamin B12 supplementation and G-CSF administration. Intravenous leucovorin rescue and folate supplementation are routinely used in the inpatient management of cases of severe methotrexate toxicity. There is no established dosage regimen for leucovorin rescue in management of severe low-dose methotrexate toxicity. One of the first randomized controlled trials comparing leucovorin dosages in severe low-dose methotrexate toxicity was published in the current year. Among the 38 patients included, the trial found no significant difference in mortality or time to recovery for blood counts or stomatitis when comparing doses of 15 mg and 25 mg every 6 hours.8 This result may suggest there is no added benefit to leucovorin regimens higher than 15 mg every 6 hours. However, additional research is needed on the efficacy of doses lower than 15 mg every 6 hours as well. Leucovorin regimens (dosed every 6 hours) varied significantly among cases reviewed, with a reported regimen of 10 mg in one case,1 15 mg in two cases,2,4 and 50 mg in two other cases.3,5 In our case, given that leucovorin rescue was provided alongside several other interventions, it is difficult to say to what degree our specific dosage regimen was responsible for the positive outcome observed.
ANSWERS TO CLINICAL QUESTIONS
Question 1, a. Methotrexate shares the same antifolate mechanism of action as trimethoprim/sulfamethoxazole. Simultaneous use of both of these medications therefore carries a risk of severe antifolate toxicity, which can present as pancytopenia, severe stomatitis, and liver toxicity. Trimethoprim/sulfamethoxazole is prescribed for HIV patients as prophylaxis against opportunistic infections such as Toxoplasmosis. Of note, penicillin-related antibiotics should also be avoided in patients on methotrexate; however, the reason for this is different from that for trimethoprim/sulfamethoxazole. Penicillin competes with methotrexate for renal secretion via organic anion transporters, decreasing methotrexate’s elimination. A large dose of a nonsteroidal antiinflammatory drugs such as (d) ibuprofen can cause constriction of the afferent arteriole reducing GFR and elimination of methotrexate. However, this patient’s complete metabolic panel is unremarkable, suggesting both a normal creatinine and blood urea nitrogen, which would both be elevated in patients with acute kidney injury, making this possible cause of methotrexate toxicity less likely. (b) Elfavirenz is a nonnucleoside reverse transcriptase inhibitor that can be part of highly active antiretroviral therapy for HIV. Side effects include hepatic toxicity (seen in this patient) as well as insomnia, dizziness, headache, and psychosis. However, accidental efavirenz intake would not explain the patient’s oral lesions or severe pancytopenia. (c) Didanosine is a nucleoside reverse transcriptase inhibitor which can be part of highly active antiretroviral therapy for HIV. Its side effects include pancreatitis, peripheral neuropathy, and lactic acidosis, none of which are described in this patient.
Question 2, a. Patients on methotrexate with a history of chronic kidney disease require careful monitoring of renal function to ensure prompt adjustment of methotrexate concentrations when indicated. Methotrexate is primarily eliminated through the kidneys. Discontinuation of methotrexate should be considered if the patient experiences severe methotrexate toxicity as a complication of impaired renal function. Patients with chronic renal disease are sensitive to acute on chronic kidney injury secondary to infections such as a urinary tract infection (UTI) (a). This patient shows signs of a UTI with a >20 blood urea nitrogen/creatinine ratio consistent with prerenal acute on chronic kidney injury secondary to dehydration associated with his UTI. (b) Medication overdose of methotrexate would explain the patient’s oral sores and pancytopenia with thrombocytopenic-related bleeding, but would not explain the patient’s acute on chronic kidney injury. Progression of chronic kidney disease (c) would lead to a reduction in glomerular filtration rate and could cause a previously therapeutic dose of methotrexate to become hazardous, but a creatinine increase of 0.6 within 1 week is more consistent with acute on chronic kidney injury than natural progression of chronic kidney disease. Undiagnosed hemophilia (D) may explain the patient’s mucosal bleeding but does not explain his pancytopenia. It is also more commonly diagnosed in younger patients and less likely to be initially diagnosed at 85 years of age.
DISCLOSURE STATEMENT
The authors report no funding or conflicts of interest. The patient gave permission for this case to be published.
References
- 1.Jara-Palacios MA, Chun W, Traub NL.. Potential contributors to low dose methotrexate toxicity in a patient with rheumatoid arthritis and pernicious anemia: case report. BMC Rheumatol. 2021;5(1):5. doi: 10.1186/s41927-020-00175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manappallil RG, Prasan D, Peringat J, Biju IK.. Severe bone marrow suppression due to methotrexate toxicity following aceclofenac-induced acute kidney injury. BMJ Case Rep. 2018;2018:bcr-2018-224722. doi: 10.1136/bcr-2018-224722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asghar M, Shoaib H, Kang W, Tariq I, Chatterjee T.. Methotrexate toxicity: a simple solution to a complex problem. Cureus. 2021;13(4):e14364. doi: 10.7759/cureus.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimkar SV, Yelne P, Gaidhane SA, Acharya S, Kumar S.. Fatal manifestations of methotrexate overdose in case of psoriasis due to dosing error. Cureus. 2022;14(10):e30041. doi: 10.7759/cureus.30041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouellette S, Shah R, Razi S, Ashforth G, Wassef C.. Fatal low-dose methotrexate toxicity: a case report and literature review. Dermatol Ther. 2022;35(12):e15945. doi: 10.1111/dth.15945. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Katz R, Kestenbaum B, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2012;59(3):356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhargava M, Kopp CR, Naidu S, et al. Comparison of two doses of leucovorin in severe low-dose methotrexate toxicity—a randomized controlled trial. Arthritis Res Ther. 2023;25(1):82. doi: 10.1186/s13075-023-03054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]