Abstract
Animal studies have demonstrated that noise exposure can lead to the loss of the synapses between the inner hair cells and their afferent auditory nerve fiber targets without impacting auditory thresholds. Although several non-invasive physiological measures appear to be sensitive to cochlear synaptopathy in animal models, including auditory brainstem response (ABR) wave I amplitude, the envelope following response (EFR), and the middle ear muscle reflex (MEMR), human studies of these measures in samples that are expected to vary in terms of the degree of noise-induced synaptopathy have resulted in mixed findings. One possible explanation for the differing results is that synaptopathy risk is lower for recreational noise exposure than for occupational or military noise exposure. The goal of this analysis was to determine if EFR magnitude and ABR wave I amplitude are reduced among young Veterans with a history of military noise exposure compared with non-Veteran controls with minimal noise exposure. EFRs and ABRs were obtained in a sample of young (19-35 years) Veterans and non-Veterans with normal audiograms and robust distortion product otoacoustic emissions (DPOAEs). The statistical analysis is consistent with a reduction in mean EFR magnitude and ABR wave I amplitude (at 90 dB peSPL) for Veterans with a significant history of noise exposure compared with non-Veteran controls. These findings are in agreement with previous ABR wave I amplitude findings in young Veterans and are consistent with animal models of noise-induced cochlear synaptopathy.
Keywords: cochlear synaptopathy, hidden hearing loss, envelope following response, auditory brainstem response, noise-induced hearing loss, frequency following response
1. Introduction1
Noise-induced cochlear synaptopathy, the loss of the synaptic connections between the inner hair cells (IHCs) and their afferent auditory nerve fiber targets, has been demonstrated in a number of mammalian species, including non-human primates (Kujawa & Liberman 2009; Lin et al. 2011; Hickox et al. 2017; Valero et al. 2017). Animal studies suggest that a single noise exposure can result in ~50% loss of synapses (Kujawa & Liberman 2009) and accelerate age-related synapse loss (Fernandez et al. 2015).
Human temporal bones indicate that cochlear synapses are lost at a rate of ~7.5% per decade of life (Wu et al. 2019). However, despite the high predicted prevalence of synaptopathy among older adults and individuals with a history of noise exposure, there is currently no diagnostic test for this condition. Synaptopathy is particularly difficult to detect clinically because it has little impact on auditory thresholds (e.g., Kujawa & Liberman 2009) and therefore remains “hidden” from the standard audiogram. Although synaptopathy can only be confirmed through post-mortem temporal bone analysis, several physiological measures of auditory function (auditory brainstem response (ABR) wave I amplitude, the envelope following response (EFR), and the middle ear muscle reflex (MEMR)) are sensitive to synaptopathy in animal models (Kujawa & Liberman 2009; Shaheen et al. 2015; Valero et al. 2018).
A number of human studies of synaptopathy have investigated the relationship between these three physiological metrics and risk factors for synaptopathy (age and noise exposure history) among individuals with normal to near-normal audiograms, with mixed results. Studies of young people reporting high levels of recreational noise exposure (e.g., from concerts, night clubs, bars, and personal music players) have generally failed to find a clear relationship between self-reported noise exposure and ABR wave I amplitude (Fulbright et al. 2017; Grinn et al. 2017; Grose et al. 2017; Guest et al. 2017; Prendergast et al. 2017; Prendergast et al. 2018), EFR magnitude (Grose et al. 2017; Guest et al. 2017; Paul et al. 2017b; Prendergast et al. 2017; Paul et al. 2018), or MEMR thresholds (Guest et al. 2019). In contrast, studies investigating young military Veterans (Bramhall et al. 2017), marching band members (Suresh & Krishnan 2020), non-Veterans who have used firearms (Bramhall et al. 2017), and adults that span a wide age range (Konrad-Martin et al. 2012; Bramhall et al. 2015; Grose et al. 2017; Valderrama et al. 2018; Shehorn et al. 2020) have identified relationships between physiological indicators of synaptopathy (ABR wave I amplitude and MEMR magnitude) and risk factors for synaptopathy (age and noise exposure history) that are consistent with animal models. This suggests that ABR wave I amplitude and MEMR magnitude may be useful for detecting synaptopathy in humans, but that common sources of recreational noise exposure may not reach intensity levels that are synaptopathic.
The potential for the EFR as a tool for diagnosing synaptopathy in humans is less clear. Reductions in EFR magnitude have been demonstrated in mouse models of age- (Parthasarathy & Kujawa 2018) and noise exposure-related (Shaheen et al. 2015) synaptopathy. However, the modulation frequencies used for the EFR in these studies, designed to target the auditory nerve, are very high (700-1000 Hz) compared to the modulation frequencies typically used in human EFR studies (80-120 Hz), which target the auditory brainstem (Kiren et al. 1994; Herdman et al. 2002; Kuwada et al. 2002). This makes it difficult to predict whether the EFR will be impacted by synaptopathy in humans. However, given that there is currently no diagnostic test for synaptopathy in living humans and the relative merits of the different physiological indicators of synaptopathy (ABR, MEMR, and EFR) are unclear, further exploration of the EFR’s potential for differentiating groups based on synaptopathy risk factors such as noise exposure is warranted.
One potential advantage of using the EFR as an indicator of synaptopathy is that at the stimulus levels commonly used to measure the EFR (70-80 dB sound pressure level (SPL)), computational models suggest that subclinical outer hair cell (OHC) dysfunction (i.e. OHC dysfunction in individuals with normal pure tone thresholds) will not impact EFR magnitude (Encina-Llamas et al. 2019). As a result, lower EFR magnitudes observed in a high noise exposure group compared to a low noise exposure group are unlikely to be due to greater levels of noise-related OHC damage in the high exposure group.
Stimulus characteristics may play an important role in the ability of the EFR to identify synaptopathy. Animal studies suggest that low spontaneous rate (SR), high threshold, auditory nerve fibers are particularly vulnerable to noise- (Furman et al. 2013) and age-related synaptopathy (Schmiedt et al. 1996). Bharadwaj et al. (2014) hypothesized that the ability of high SR, low threshold fibers to encode the envelope of an amplitude modulated signal at higher stimulus levels (e.g. 70-80 dB SPL) would be poor, particularly for shallow modulation depths where the crests and troughs of the envelope are similar in intensity. Low SR fibers are better suited for coding these types of stimuli due to higher thresholds and saturation levels. For this reason, it was predicted that EFR stimuli with smaller modulation depths would be the most sensitive to synaptopathy (Bharadwaj et al. 2014; Bharadwaj et al. 2015). However, more recent computational modeling suggests that at these intensity levels, the EFR is dominated by more basally located off-frequency high SR fibers that have not yet reached saturation, even at shallow modulation depths (Encina-Llamas et al. 2019).
Relatively few human studies have investigated the relationship between the EFR and noise exposure. In a small sample of young people with high self-reported recreational noise exposure, Bharadwaj et al. (2015) found a steeper EFR slope (i.e., the rate of decline in EFR magnitude as the modulation depth decreases) compared to a less exposed group. Three additional studies of young people reporting high levels of recreational noise exposure have failed to find statistically significant noise exposure-related changes in EFR magnitude (Grose et al. 2017; Guest et al. 2017; Prendergast et al. 2017), although Prendergast et al. did find a significant reduction in EFR magnitude for males as self-reported noise exposure increased. Another study observed a trend towards a reduction in EFR magnitude among their high noise group, but this was later found to be statistically significant only when the EFR stimulus was presented in 40 dB SPL spectrum level narrowband noise (Paul et al. 2017b; Paul et al. 2018). The authors suggested that narrowband noise at this particular intensity level saturated the response of the high SR fibers, but not the low SR fibers, making it easier to detect differences between the high and low noise groups. To date, the EFR has not been measured in a sample with military noise exposure.
The objectives of this analysis were to determine: 1) the magnitude of the difference in mean EFR measurements taken from young military Veterans versus non-Veteran controls with minimal noise exposure history and 2) if ABR wave I amplitudes were reduced in Veterans compared with non-Veteran controls. The hypotheses were that EFR magnitude would be reduced among Veterans with high levels of self-reported noise exposure compared with controls, particularly for low modulation depths, and that ABR wave I amplitude would also be reduced among Veterans with high noise exposure compared with controls, confirming previous findings (Bramhall et al. 2017).
2. Material and methods
2.1. Participants
Seventy-nine young adults (48 military Veterans and 31 non-Veterans) aged 19-35 years who participated in a hidden hearing loss study at the VA National Center for Rehabilitative Auditory Research (NCRAR) were included in this analysis. Inclusion criteria included: pure tone air conduction thresholds ≤20 dB HL from 0.25-8 kHz, normal tympanogram (226 Hz tympanogram, compliance 0.3-1.9 ml, and peak pressure between ±50 dPa), no air-bone gaps >15 dB and no more than one 15 dB air-bone gap, normal distortion-product otoacoustic emissions (DPOAEs, criteria described below), and no history of otologic or neurologic disorder (including traumatic brain injury or concussion). Non-Veteran controls were required to have minimal noise exposure, including no self-reported firearm use, and no self-report of frequent or constant tinnitus or decreased sound tolerance (DST) as these perceptual deficits could indicate auditory dysfunction. Fifty-four potential participants were excluded because they did not meet these criteria (abnormal audiogram or DPOAEs: 39, history of concussion: 2, abnormal tympanogram: 1, non-Veterans reporting tinnitus and/or DST: 7, non-Veterans reporting a history of loud noise exposure: 5). If only one ear met the inclusion criteria, all test measures were collected in that ear. If both ears met the inclusion criteria, test measures were collected in the ear with the better DPOAEs to minimize OHC dysfunction. All participants provided written informed consent and were paid for their participation. All study procedures were approved by the VA Portland Health Care System Institutional Review Board. Data from a subset of the study participants were described in Bramhall et al. (2020a).
2.2. Procedures
2.2.1. Audiometry
Pure tone air conduction thresholds were measured from 0.25-8 kHz and bone conduction thresholds were measured at 0.5, 1, 2 and 4 kHz. Extended high frequency air conduction thresholds were obtained from 9-16 kHz using Sennheiser HDA 200 headphones (Old Lyme, CT).
2.2.2. Distortion Product Otoacoustic Emissions
DPOAE testing was conducted using a custom system that includes an ER-10 B+ probe microphone and EMAV software from Boys Town National Research Hospital (Neely & Liu 1993). DPOAE stimuli were presented in a primary frequency sweep from 1-8 kHz in 1/6-octave increments at stimulus frequency levels of and dB SPL and a fixed primary frequency ratio of to generate a DP-gram. Responses were compared to the DPOAE levels from a distribution of individuals with abnormal pure tone thresholds (Gorga et al. 1997, table A1). Only individuals at or above the 90th percentile from 1.5-6 kHz were included. Measurement based stopping rules were employed in which averaging continued until 30 seconds of artifact-free data were collected or until the noise floor was below −15 dB SPL.
2.2.3. Envelope Following Response (EFR)
Intelligent Hearing Systems (Miami, FL) SmartEP-CAM was used to record the EFR with an acquisition sampling rate of 10,000 Hz and a bandpass filter setting of 100–4000 Hz at 6 dB/octave. Electrodes were placed at Cz (active), Fpz (ground), and the mastoid of the test ear (reference). Electrode impedance was less than or equal to 5.0 kΩ in all but four participants. Three of these participants had impedances less than or equal to 5.6 kΩ and the fourth had an impedance of 8.5 kΩ. Stimuli consisted of 80 dB SPL sinusiodally amplitude modulated (SAM) 4 kHz tones. A 4 kHz carrier was chosen because this is the audiometric frequency most likely to be impacted by noise exposure in Veterans (Wilson & McArdle 2013). The 200 ms carrier tone was modulated at 110 Hz, similar to previous human EFR studies (Bharadwaj et al. 2015; Garrett & Verhulst 2019; Billings et al. 2020), with varying modulation depths (100%, 63%, and 40%). Each stimulus was presented 3100 times, with the stimulus polarity alternating for each sweep. Pairs of epochs of alternating polarities were averaged together ((rarefaction + condensation)/2) to emphasize the response to the stimulus envelope and reduce the contribution from artifacts such as the cochlear microphonic (Aiken & Picton 2008). Three inter-stimulus intervals (offset to onset) were randomly presented in equal proportions within each block: 66.67, 83.33, and 100 ms. Stimuli were presented through an electrostatically shielded Etymotic ER3 insert transducer with shielded cables. Participants were comfortably seated in a recliner with the lights off and were encouraged to sleep during testing.
Continuous electroencephalography was epoched and processed in MATLAB (MathWorks, Natick, MA) as described in Billings et al. (2020). Specifically, using the MATLAB fir1 function, each epoch was bandpass filtered using a zero-phase 800th order finite impulse response filter from 80-4140 Hz and any energy at 60 Hz was filtered out. The noisiest epochs were removed using an absolute value of 60 μV as the artifact rejection criterion. The magnitude of the response at 110 Hz was calculated as a ratio of the signal and noise voltages (i.e. a signal-to-noise ratio (SNR)), expressed in dB. The noise level was calculated by taking the average voltage of the five frequency bins above and the five frequency bins below 110 Hz, skipping the frequency bins at 105 and 115 Hz to avoid spectral leakage and the bin at 120 Hz because this is a multiple of the 60 Hz powerline frequency.
2.2.4. Auditory Brainstem Response (ABR)
ABR testing was completed using an Intelligent Hearing Systems SmartEP system (Miami, FL) during a separate test session from the EFR. As with the EFR testing, participants were seated in a recliner in a dark room and were encouraged to sleep during testing. Etymotic Research gold foil ER3-26A tiptrode electrodes (Elk Grove Village, IL) were placed in the ear canal, the reference electrode was placed on the high forehead, and the ground on the low forehead. Stimuli consisted of alternating polarity 4 kHz tonebursts presented at 90, 100, and 110 dB peak equivalent sound pressure level (peSPL), with a rise/fall time of 0.5 ms and a Blackman envelope. Stimuli were presented at a rate of 11.1/s. The ABR response was band-pass filtered from 10-1500 Hz and averaged across 2048 stimulus presentations for the 90 and 100 dB stimuli and 1024 presentations for the 110 dB stimuli to limit exposure to the higher intensity level. For each participant, at least two replications were obtained. Electrode impedance was less than or equal to 5.0 kΩ in all but four participants; these participants had impedances less than or equal to 7.0 kΩ. The positive peak and the following negative trough for wave I was initially identified with an automated Python-based peak picking program (adapted from Buran 2015). Peaks and troughs were then evaluated by two independent raters and reassigned if both were in agreement. In cases of disagreement, a third rater made a final assignment. Wave I amplitudes were defined as the difference between the voltage at the positive peak and the voltage at the following negative trough. Wave I amplitude was determined to be unscoreable in one participant at 90 dB peSPL. All participants had scoreable wave I amplitudes at 100 and 110 dB peSPL.
2.2.5. Hearing Questionnaire
All participants completed a hearing questionnaire including the questions, “Do you have constant or frequent ringing in the ears?” and “Over the last week, were sounds too loud or uncomfortable for you when they seemed normal to others around you?”. The responses were used to assess the presence of tinnitus and decreased sound tolerance respectively. Non-Veterans who reported auditory perceptual deficits (i.e., tinnitus in the test ear or DST) were excluded from the analysis to limit the likelihood of individuals with auditory dysfunction being included in the control group. Six Veterans reporting DST were also excluded because we previously found physiological evidence of hyperactivity early in the auditory pathway among individuals reporting DST (Bramhall et al. 2020b), which could complicate the interpretation of the EFR data. Relationships between physiological measurements and report of tinnitus and/or DST will be addressed in a separate paper.
2.2.6. Noise Exposure History
Noise exposure history was assessed in all participants using the Lifetime Exposure to Noise and Solvents Questionnaire (LENSQ; Bramhall et al. 2017; Gordon et al. 2017). The LENS-Q was scored as described in Bramhall et al. (2017), except that scoring of the non-occupational section was modified. Previous experience with the LENS-Q has demonstrated that in this section, participants tend to report similar patterns of hearing protection use and frequency/duration of exposure for items within a particular category (Gunfire, Transportation, Music Attended, Music Played, Woodworking/Power tools, Attended Sports Games, Attended Motor Sports Events, and Yard and Garden Power Equipment). This may reflect instances where several similar exposures occurred within the same time frame. For example, when target shooting, several different types of firearms (e.g. pistol, rifle, and shotgun) may be discharged in a single session or when woodworking, several types of power tools (e.g., power saw, lathe, sander, router, electric drill, pneumatic drill) may be used within the same time period. To avoid over-estimation of non-occupational exposures, only the noise item with the highest exposure score within each category was included in the calculation of the final non-occupational noise exposure score. All but two Veterans reported firearm use on the LENS-Q. For these Veterans, this was most likely a reporting error because almost all military Service Members complete firearms training during their basic training. Non-Veterans who reported ever using a firearm on the LENS-Q were excluded from the study. Veteran participants were also asked to report their military occupational specialty (MOS), a descriptor of the job that they performed during their military service. The Veterans Benefits Administration has rated each MOS based on the probability of experiencing hazardous noise exposure while performing job duties (low, medium, or high; VBA 2010). Examples of MOS designations in the present dataset that are rated as having a high probability of hazardous noise exposure include: Infantry, Combat Engineer, Cannon Crew, and Fire Support Specialist. Veteran participants were divided into two groups (Veteran High Noise and Veteran Medium Noise) based on their reported MOS and their overall LENS-Q score. Veterans with an MOS rated as having a high probability of hazardous noise exposure or a LENS-Q score in the top 25th percentile for all Veterans in the sample were assigned to the Veteran High Noise group. All other Veterans were assigned to the Veteran Medium Noise group.
2.3. Statistical analysis
2.3.1. EFR Model
When analyzing the relationship between noise exposure and EFR magnitude, possible confounding factors need to be considered. A recent study showed sex differences in the magnitude of the frequency following response (Krizman et al. 2015), with smaller magnitudes in males. The Veteran High Noise group in this study is dominated by males, therefore, it’s important to confirm that any observed noise exposure effects are not due to sex differences between the groups. In addition, although computational modeling suggests the EFR is relatively resistant to OHC dysfunction (Encina-Llamas et al. 2019), it is still possible that OHC dysfunction could impact EFR magnitudes by altering auditory input. If OHC dysfunction is greater in groups with more noise exposure, this could bias the estimated effect of noise exposure group on EFR magnitude.
To account for the confounding effects of sex and OHC dysfunction, sex and average DPOAE level from 3-8 kHz were included as predictors in a Bayesian multilevel linear regression model where the mean EFR magnitude was estimated for each noise exposure group and modulation depth. For an introduction to Bayesian analysis and how it can be applied to auditory research, see McMillan and Cannon (2019).
Although age-related loss of cochlear synapses has been demonstrated in both animal models and human temporal bone studies (Sergeyenko et al. 2013; Wu et al. 2019), potentially confounding the analysis of noise exposure group as a predictor of EFR magnitude, the age range in this study is limited (19-35 years) and mean age differs across the noise exposure groups by less than four years. Lifetime noise exposure is expected to be positively correlated with age because as a person ages, they will accumulate more noise exposure, particularly if they are serving in the military. Therefore, adjusting for age would adjust out some of the noise exposure effect on mean EFR magnitude. For this reason, age was not included in the model. However, results for an alternate model that includes age as a predictor can be found in Tables A1 and A2.
Let be the observed EFR measurement (), assumed Gaussian, with parameters consisting of the population mean EFR response and residual variance .
where is the linear relationship between the participant’s mean EFR magnitude and the EFR modulation depth . In vector notation, are participant-specific random effects describing each participant’s intercept and rate of change with modulation depth . These are assumed to be bivariate normal random variables i.e.
where and are the overall intercept and slope relating the population mean EFR magnitude to modulation depth. The vector is a participant-specific vector of binary sex and noise exposure group indicators, and their interactions, and and are vectors of regression coefficients for these effects. The parameter is the covariance matrix for the random effects. This model represents each participant’s linear EFR by modulation depth function, with the effect of sex and noise exposure history described through the and coefficient vectors. The parameters , , , are categorical effects describing deviations from the linear model that are specific to modulation depth , noise group , sex , and their interactions. This model structure allows for non-linear relationships between modulation depth and mean EFR magnitude that are specific to noise exposure group and sex to be considered in the analysis. Finally, the are modulation depth-specific DPOAE effects on the mean EFR magnitude.
The parameter vectors and were given weakly informative multivariate normal priors with a mean vector of 0 and a covariance matrix equal to , were is the identity matrix. The , , , , were given independent normal priors with mean vector 0 and with variances given weakly informative half-normal(1) priors. The overall DPOAE effect was given a Normal(0,1) prior. The intercept was given a Normal(13.25, 102) prior reflecting the observation that the mean EFR magnitude at a modulation depth of 100% (0 dB) observed by Paul et al. (2017b) across their two noise exposure groups is approximately 13.25 dB SNR, and is given a normal(−5, 2.52) prior reflecting the assumption that EFR magnitude will decrease with decreasing modulation depth. The among-subject covariance matrix was given a hierarchical half-t prior (Huang & Wand 2013), which induces weak regularization towards common participant-specific effects. Finally, the residual standard deviation was given a weakly informative half-Normal(5) prior. These priors center the modulation depth, sex, and noise exposure effects and their interactions at zero, which corresponds to a prior belief of no effect of these parameters on EFR magnitude.
The model was run using SAS software, version 9.4 PROC MCMC for 200,000 iterations following a 200,000 sample burn-in period thinned to every 200th iteration. The model was run three times with different initial values, resulting in Gelman-Rubin diagnostics all below 1.02.
2.3.2. ABR model
When analyzing the relationship between noise exposure and ABR magnitude, the same confounding factors that were addressed with the EFR model need to be considered for the ABR model. Previous studies have demonstrated sex-related differences in ABR wave I amplitude, with smaller amplitudes for males (Trune et al. 1988; Mitchell et al. 1989). In addition, OHC function could impact ABR wave I amplitudes for the same reasons they may impact EFR magnitudes. In a previous study (Bramhall et al. 2017), we estimated the effect of noise exposure group on ABR wave I amplitude after adjusting for maximum DPOAE level at 1, 3, 4, and 6 kHz (measured from input-output functions) and sex. The analysis presented here is similar to that model, with the replacement of maximum DPOAE level with average DPOAE level from 3-8 kHz (measured with a DP-gram), as used in more recent publications (Bramhall et al. 2018; Bramhall et al. 2020a).
A lognormal likelihood, with parameters and , was assumed for the wave I amplitude measures, reflecting the increase in variability that occurs as mean wave I amplitude increases and the fact that wave I amplitude cannot be negative. The population mean wave I amplitude is equal to . Note that some of the notation from the EFR model is reused here, but these are two distinct models, datasets, and results.
The parameters describe the linear relationship between mean log(wave I amplitude) and stimulus level . is transformed by subtracting 100 and dividing by 10 to adjust the intercept. allows each stimulus level to deviate from the linear relationship between level and mean log(wave I amplitude). The remaining parts of the equation follow a similar format and modify the linear relationship between mean log(wave I amplitude) and stimulus levels based on female sex (the parameters), average DPOAE level (the parameters), and noise exposure group (the parameters). Finally, models participant-specific random intercepts. The , , , , , parameters were given weakly informative normal(0, 1) prior distributions, and the remaining parameters were given normal hierarchical priors with separate variance components for each parameter. These variance components were in turn given weakly informative half-normal(1) priors. These priors center the non-linear level effects and all the noise exposure effects at zero, which corresponds to a prior belief of no effect of these parameters on ABR wave I amplitude.
The model was run using SAS software, version 9.4 PROC MCMC with the No-U-Turn sampler for 1,000 iterations following a 1,000 sample burn-in period. The model was run three times with different initial values, resulting in Gelman-Rubin diagnostics all below 1.03.
2.3.3. Why p-values and statistical significance testing are not presented in this report
The American Statistical Association Statement on Statistical Significance and P-Values lists several important principles concerning the use of p-values (Wasserstein & Lazar 2016). These include: “p-values do not measure the probability that the studied hypothesis is true, or the probability that the data were produced by random chance alone”, “scientific conclusions and business or policy decisions should not be based only on whether a p-value passes a specific threshold”, “a p-value, or statistical significance, does not measure the size of an effect or the importance of a result”, and “by itself, a p-value does not provide a good measure of evidence regarding a model or hypothesis”. Unfortunately, these principles are not broadly understood in the scientific community, leading to frequent misinterpretation of p-values and statistical significance testing (Szucs & Ioannidis 2017; Amrhein et al. 2019; McShane et al. 2019). To avoid the common pitfalls of p-values and significance testing, Bayesian or otherwise, in this report and many of our previous papers (e.g., Bramhall et al. 2017; Bramhall et al. 2018; Bramhall et al. 2019; Bramhall et al. 2020a), we have chosen to investigate our hypotheses by reporting Bayesian posterior probability distributions of parameters and contrasts. We feel this approach provides a better description of the data’s support for a particular hypothesis than a binary classification of “significant” or “not significant”.
An alternative to p-values when using Bayesian analysis is to calculate a Bayes Factor (Spiegelhalter et al. 2003), which is the ratio of the likelihoods of two hypotheses, often the null hypothesis (H0) and an alternative hypothesis (HA). This ratio is interpreted as the strength of evidence for H0. The problem with this approach is that if H0 is very unlikely, for instance in the case of the EFR analysis described here, H0 says that the difference in mean EFR magnitude between the Non-Veteran Control group and the Veteran High Noise group is exactly zero. This H0 is highly unlikely given the measurement error associated with the EFR, leading to a Bayes Factor that indicates that HA is infinitely more likely than H0. Because this result is not particularly helpful in terms of determining the strength of the evidence for the HA, we have chosen not to use Bayes Factor analysis in this report.
Results
3.1. Noise exposure group characteristics
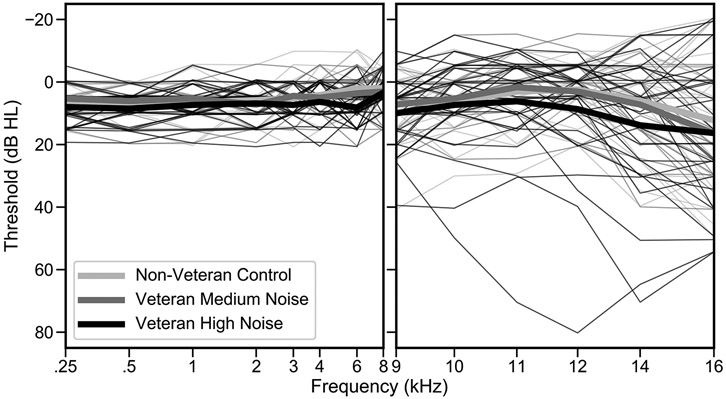
Table 1 summarizes the characteristics of the three noise exposure groups (Non-Veteran Control, Veteran Medium Noise, and Veteran High Noise). Mean age is similar across the groups. The Non-Veteran Control and Veteran Medium Noise groups consist of roughly equal numbers of males and females, while the Veteran High Noise group is predominantly male. Mean LENS-Q scores are lowest (indicating less noise exposure) for the Non-Veteran Control group and highest for the Veteran High Noise group. A one point increase in LENS-Q score represents a 10-fold increase in noise exposure, suggesting that, on average, the noise exposure history of the Veteran High Noise group is more than 10 times the exposure of the Veteran Medium Noise group. Mean pure tone averages (PTAs), in the conventional and extended high frequencies (EHFs), differ by less than 5 dB across groups. Twenty-three Veterans reported an MOS that is rated as having a high probability of hazardous noise exposure. Sixteen Veterans (two Medium Noise and 14 High Noise) reported tinnitus in the test ear. Audiometric thresholds from 0.25 to 16 kHz, color-coded by noise exposure group, are plotted in Figure 1. Mean pure tone thresholds are 6-7 dB poorer for the Veteran High Noise group compared with the Non-Veteran Control group at 6, 12.5, and 14 kHz. All other group contrasts are less than 4 dB. Individual and group mean DPOAE levels (from the DP-gram) are plotted in Figure 2. Average DPOAE levels from 1-8 kHz differ by no more than 4 dB across groups.
Table 1.
Participant Characteristics by Noise Exposure Group
| Non-Veteran Control |
Veteran Medium Noise |
Veteran High Noise |
|
|---|---|---|---|
| Age (years) | 26.7 (4.5) | 30.2 (3.8) | 30.0 (3.0) |
| Number of males | 14 | 8 | 24 |
| LENS-Q score | 4.1 (0.8) | 8.4 (1.2) | 9.6 (0.8) |
| Number with high MOS rating | N/A | 0 | 23 |
| Report tinnitus in the test ear | 0 | 2 | 14 |
| PTA (0.5, 1, & 2 kHz; dB HL) | 6.5 (4.4) | 6.2 (4.8) | 7.6 (4.4) |
| High freq. PTA (3, 4, & 6 kHz; dB HL) | 3.8 (4.8) | 4.4 (4.6) | 7.3 (4.4) |
| EHF PTA (9-16 kHz; dB HL) | 5.8 (8.4) | 7.0 (9.1) | 10.2 (14.5) |
| Average DPOAE (3-8 kHz; dB SPL) | 4.2 (4.8) | 3.1 (4.1) | 1.8 (3.9) |
| Total Participants | 31 | 18 | 30 |
For mean values, standard deviations are shown in (). PTA – pure tone average, EHF – extended high frequency, LENS-Q – Lifetime Exposure to Noise and Solvents – Questionnaire, MOS – Military Occupational Specialty. High MOS rating refers to a high probability of hazardous noise exposure associated with that MOS.
Figure 1. Audiograms are similar across noise exposure groups.
Mean audiometric thresholds from 0.25-16 kHz are plotted for each noise exposure group (thick lines) and for individual participants (thin lines). Color-coding indicates the noise exposure group.
Figure 2. DP-grams are similar across noise exposure groups.
Mean DPOAE levels from 1-8 kHz obtained from the DP-gram are plotted for each noise exposure group (thick lines). DP-grams for individual participants are shown with the thin lines and are color-coded by group.
3.2. Mean EFR magnitudes are smaller for the group with the most noise exposure
Mean EFR magnitudes are 2.7, 2.5, and 3.4 dB smaller for the Veteran High Noise group than for the Non-Veteran Control group at modulation depths of 100%, 63% and 40%, respectively (Figure 3). For the Veteran Medium Noise group, mean EFR magnitudes are only reduced compared with the control group at a modulation depth of 63%, with a reduction of 2.2 dB.
Figure 3. Mean EFR magnitude is reduced for the Veteran High Noise group compared with controls.
Mean EFR magnitudes are plotted by noise exposure group for each modulation depth with thick color-coded lines. Thin lines show EFR magnitudes for individual participants.
3.3. Model-based mean EFR magnitudes are smallest for the population with the most noise exposure after statistical adjustment for sex and average DPOAE levels
Bayesian regression analysis was used to evaluate noise exposure population differences in EFR magnitude for each modulation depth while adjusting for sex and average DPOAE level from 3-8 kHz. The model fit to the data is shown in Figure A1. The model indicated an interaction effect of noise exposure category and sex on EFR magnitude, so the results are presented separately for males and females. Given the fitted model, sex-adjusted EFR magnitude contrasts between noise exposure categories were computed at each modulation depth, along with 90% Bayesian confidence intervals (CI, also known as a credible interval) reflecting the range of population contrast values that the model predicts to include the true contrast (shown in Table 2). The CI represents the interval over which an unobserved parameter falls within the given probability. In Bayesian analysis, the choice of a 90% vs. a 95% CI is arbitrary and does not change the conclusion when correctly interpreted (Kruschke 2015).
Table 2.
Fitted mean EFR magnitude contrasts (in dB) between noise exposure categories.
| Non-Veteran Control – Veteran High Noise |
Non-Veteran Control – Veteran Medium Noise |
Veteran Medium Noise – Veteran High Noise |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Mod. Depth |
.05 | .25 | .50 | .75 | .95 | .05 | .25 | .50 | .75 | .95 | .05 | .25 | .50 | .75 | .95 |
| Male | 100% | 0.0 | 1.3 | 2.1 | 3.0 | 4.3 | −3.2 | −1.7 | −0.8 | 0.1 | 1.7 | 0.3 | 1.9 | 2.9 | 3.9 | 5.4 |
| 63% | −0.5 | 0.7 | 1.6 | 2.5 | 3.8 | −2.3 | −0.7 | 0.3 | 1.4 | 3.2 | −1.4 | 0.2 | 1.3 | 2.3 | 3.8 | |
| 40% | −0.9 | 0.7 | 1.7 | 2.8 | 4.3 | −3.6 | −1.6 | −0.3 | 1.1 | 3.0 | −1.0 | 0.8 | 2.0 | 3.3 | 5.0 | |
| Female | 100% | −2.0 | −0.1 | 1.0 | 2.2 | 3.8 | −2.9 | −1.4 | −0.3 | 0.8 | 2.3 | −2.1 | −0.1 | 1.3 | 2.6 | 4.8 |
| 63% | −1.0 | 0.8 | 1.9 | 3.1 | 4.8 | −0.8 | 0.8 | 1.9 | 3.0 | 4.7 | −3.5 | −1.4 | 0.1 | 1.5 | 3.3 | |
| 40% | −0.5 | 1.5 | 2.9 | 4.4 | 6.6 | −2.0 | −0.3 | 1.0 | 2.3 | 4.0 | −2.0 | 0.3 | 1.9 | 3.6 | 6.0 | |
Columns show the percentiles of the probability distribution of the mean EFR magnitudes for the difference between the two indicated noise exposure categories (the first category minus the second category). The mean contrast is indicated by the 50th percentile (.50), while the 90% confidence interval is indicated by the 5th and 95th percentiles.
These contrasts are plotted by noise exposure category and sex in Figure 4. At all modulation depths, modeled mean EFR magnitudes are larger for male Non-Veteran Controls than for male High Noise Veterans. This contrast is largest for a modulation depth of 100% (2.1 dB, CI = 0 to 4.3 dB). Modeled mean EFR magnitudes are also larger for female Non-Veteran Controls than for female High Noise Veterans across all modulation depths, however the biggest contrasts are for the shallower modulations (1.9 dB, CI = −1.0 to 4.8 dB and 2.9 dB, CI = −0.5 to 6.6 dB for 63% and 40% modulations, respectively). Modeled mean EFR magnitudes for Medium Noise Veterans are similar to those for Non-Veteran Controls, except for females at a 63% modulation depth, where the modeled mean is similar to the High Noise Veterans. Many of the group contrasts have Bayesian CIs that include negative values, indicating the possibility of a smaller mean EFR magnitude for the Non-Veteran Control group than for the Veteran group.
Figure 4. Modeled mean EFR magnitude by noise exposure category and sex.
Model fitted mean EFR magnitudes are plotted by noise exposure category and sex for each modulation depth. Thick lines show the interquartile range and thin lines show the 90% Bayesian confidence interval.
The Bayesian regression analysis results in posterior probability distributions for each noise exposure category and modulation depth, broken out by sex. These probability distributions can be used to evaluate the posterior probability that the mean EFR magnitude for the Non-Veteran Control population is greater than the mean magnitude for the Veteran High Noise population by calculating the mean EFR magnitude posterior probability distribution for Non-Veteran Control–Veteran High Noise. The integral of the portion of the distribution that is greater than zero represents the probability that the Non-Veteran Control population has a bigger mean EFR magnitude than the Veteran High Noise population (see Figure 5 for an example for males at a modulation depth of 100%). Posterior probabilities for this contrast are presented for males and females in Table 3. These posterior probabilities represent the level of certainty that the Non-Veteran Control population’s distribution of mean EFR magnitudes is greater than the mean EFR magnitude distribution for the Veteran High Noise population. The posterior probability that mean EFR magnitude for Non-Veteran Controls (at a modulation depth of 100%) is greater than the mean EFR magnitude for High Noise Veterans is 95% for males and 73% for females. For a modulation depth of 40%, the posterior probability that mean EFR magnitude is greater for Non-Veteran Controls than for High Noise Veterans is 86% for males and 92% for females.
Figure 5. Histogram illustrating the calculation of posterior probabilities for group contrasts.
The histogram shows the posterior probability distribution for Non-Veteran Control mean EFR magnitude minus High Noise Veteran mean EFR magnitude (for males at a modulation depth of 100%). The portion of the distribution that is greater than zero, indicating higher mean EFR magnitudes for Non-Veteran Controls than for High Noise Veterans, is shaded dark gray and represents 95% of the distribution. The portion of the distribution that is less than zero, indicating lower mean EFR magnitudes for Non-Veteran Controls, is shaded light gray and represents 5% of the distribution. Therefore, the posterior probability that male Non-Veteran Controls have a mean higher EFR magnitude than High Noise Veterans at 100% modulation depth is 95%, given the data and the fitted model.
Table 3.
Posterior probabilities of fitted mean EFR magnitude contrasts between the Non-Veteran Control and Veteran High Noise populations.
| Non-Veteran Control > Veteran High Noise | ||
|---|---|---|
| Sex | Mod. Depth |
Posterior Probability |
| Male | 100% | 95% |
| 63% | 89% | |
| 40% | 86% | |
| Female | 100% | 73% |
| 63% | 87% | |
| 40% | 92% | |
Values indicate the posterior probability that the mean EFR magnitude for the Non-Veteran Controls is greater than the mean EFR magnitude for High Noise Veterans.
Similar results are observed if age is included as a predictor in the EFR model, but the effect sizes and posterior probabilities are smaller (see Tables A1 and A2).
3.4. Mean ABR wave I amplitudes are smaller for the group with the most noise exposure
Mean ABR wave I amplitudes are smaller for the Veteran High Noise group than for the Non-Veteran Control group by 0.04-0.05 μV across stimulus level (Figure 6). For the Veteran Medium Noise group, mean ABR wave I amplitudes are only reduced compared with the control group at the highest stimulus level (110 dB peSPL), with a reduction of 0.04 μV.
Figure 6. Mean ABR wave I amplitude is reduced for the Veteran High Noise group compared with controls.
Mean ABR wave I amplitudes are plotted by noise exposure group for each ABR stimulus level with thick color-coded lines. Thin lines show wave I amplitudes for individual participants.
3.5. After statistical adjustment for sex and average DPOAE level, model-based mean ABR wave I amplitudes at 90 dB peSPL are smallest for the population with the most noise exposure
Bayesian regression analysis was used to evaluate noise exposure population differences in ABR wave I amplitude for each stimulus level while adjusting for sex and average DPOAE level from 3-8 kHz. The model fit to the data is shown in Figure A2. Model-based mean ABR wave I amplitudes are shown for each noise exposure category, sex, and stimulus level in Table 4 and are plotted in Figure 7. Posterior probabilities for the noise exposure contrasts are presented in Table 5. Note that the fitted ABR model did not indicate any sex-specific noise exposure effects on wave I amplitude, so the contrasts between noise exposure categories are identical for males and females. For this reason, posterior probabilities for the noise exposure population contrasts are not reported separately for males and females. The posterior probability that mean ABR wave I amplitude is greater for Non-Veteran Controls than for High Noise Veterans at stimulus levels of 90, 100, and 110 dB peSPL is 94%, 71%, and 51%, respectively.
Table 4.
Fitted mean ABR wave I amplitudes (in μV) by noise exposure category, sex, and stimulus level
| Non-Veteran Control | Veteran High Noise | Veteran Medium Noise | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Level (dB peSPL) |
Percentile | ||||||||
| .05 | .50 | .95 | .05 | .50 | .95 | .05 | .50 | .95 | ||
| Male | 90 | 0.10 | 0.11 | 0.13 | 0.09 | 0.10 | 0.12 | 0.08 | 0.09 | 0.11 |
| 100 | 0.19 | 0.23 | 0.27 | 0.19 | 0.22 | 0.25 | 0.18 | 0.21 | 0.26 | |
| 110 | 0.30 | 0.36 | 0.42 | 0.28 | 0.33 | 0.38 | 0.30 | 0.37 | 0.44 | |
| Female | 90 | 0.13 | 0.15 | 0.17 | 0.11 | 0.13 | 0.16 | 0.10 | 0.12 | 0.15 |
| 100 | 0.23 | 0.27 | 0.32 | 0.22 | 0.26 | 0.31 | 0.21 | 0.26 | 0.31 | |
| 110 | 0.34 | 0.40 | 0.47 | 0.30 | 0.36 | 0.44 | 0.33 | 0.41 | 0.49 | |
Columns show the percentiles of the ABR wave I amplitude probability distributions for each noise exposure category. The mean contrast is indicated by the 50th percentile (.50), while the 90% confidence interval is indicated by the 5th and 95th percentiles.
Figure 7. Modeled mean ABR wave I amplitude by noise exposure category and sex.
Model fitted mean ABR wave I amplitudes are plotted by noise exposure category and sex for each stimulus level. Thick lines show the interquartile range and thin lines show the 90% Bayesian confidence interval.
Table 5.
Posterior probabilities of fitted mean ABR wave I amplitude contrasts between the Non-Veteran Control and Veteran High Noise populations.
| Non-Veteran Control > Veteran High Noise | |
|---|---|
| Level (dB peSPL) |
Posterior Probability |
| 90 | 94% |
| 100 | 71% |
| 110 | 51% |
Values indicate the posterior probability that the mean ABR wave I amplitude for Non-Veteran Controls is greater than the mean wave I amplitude for High Noise Veterans.
3. Discussion
4.1. Greater noise exposure history is associated with lower EFR magnitudes
Across all measured modulation depths, both raw and modeled mean EFR magnitudes were lower for the Veteran High Noise group than for the Non-Veteran Control group, although the size and certainty of this contrast varied by modulation depth and sex. The mean EFR magnitude reduction of 1.8 dB observed in the raw data for the Veteran High Noise group compared to the Non-Veteran Control group for a modulation depth of 100% (as shown in Figure 3) is similar to the difference in EFR magnitudes (a little less than 2 dB) observed between the lower and higher noise exposure groups in Paul et al. for a 5 kHz carrier tone 100% modulated at 86 Hz (Paul et al. 2017a, Figure 2b). In addition, a reduction in modeled mean EFR magnitude in High Noise Veterans compared with Non-Veterans Controls is qualitatively consistent with a mouse study that showed a reduction in EFR magnitude in mice with histologically confirmed noise-induced synaptopathy (Shaheen et al. 2015).
4.2. Greater noise exposure history is associated with lower ABR wave I amplitudes at 90 dB peSPL
We previously reported reductions in ABR wave I amplitudes for a 110 dB peSPL 4 kHz toneburst in a similar population of young Veterans with normal audiograms who reported high levels of noise exposure when compared with non-Veteran controls who denied ever using firearms (Bramhall et al. 2017). Here, in a separate sample, modeled mean ABR wave I amplitudes for a 4 kHz toneburst are consistent with a reduction in wave I amplitude for Veterans reporting high levels of noise exposure compared with non-Veteran controls for a 90 dB peSPL stimulus, but not 100 and 110 dB peSPL stimuli. These differences could be related to the slightly relaxed DPOAE inclusion criteria for the present study compared to the previous study, which could result in a study population with more cochlear gain loss. Computational modeling suggests that cochlear gain loss can steepen the ABR wave I amplitude growth curve, resulting in increased wave I amplitudes for stimulus levels greater than 90 dB peSPL (Verhulst et al. 2018). Consistent with this view, when the ABR model was re-run without the seven participants (two from the Veteran High Noise group, two from the Veteran Medium Noise group, and three from the Non-Veteran Control group) who did meet the DPOAE inclusion criteria from Bramhall et al. (2017), the posterior probability that mean ABR wave I amplitude is greater for Non-Veteran Controls than for High Noise Veterans increased to 97%, 80%, and 65% at stimulus levels of 90, 100, and 110 dB peSPL, respectively.
4.3. Human synaptopathy may not be confined to low SR auditory nerve fibers
Computational modeling of the EFR by Encina-Llamas et al. (2019) suggests that even at shallow modulation depths, high SR auditory nerve fibers dominate the EFR. In fact, simulated loss of 100% of low and medium SR fibers produced reductions in EFR magnitude of less than 1.5 dB across a wide range of stimulus levels for a 2000 Hz carrier tone modulated at 93 Hz with modulation depths of 85% and 25%. Therefore, the modeled mean EFR magnitude reduction for the 100% modulated condition of 2.1 dB for the male Veteran High Noise population compared with the Non-Veteran Control population suggests a loss of high SR auditory nerve fibers. This is not the first EFR study to suggest that synaptopathy in humans may impact high SR fibers. Paul et al. (2017a) found that the addition of 40 dB spectrum level 1/3-octave narrowband masking noise centered around the EFR carrier frequency resulted in a smaller reduction in EFR magnitude for individuals with tinnitus than for control participants without tinnitus. At this low intensity level, the masking should primarily impact high SR fibers because these fibers have the lowest threshold sensitivity (Young & Barta 1986). As such, the finding that the masking had less impact on EFR magnitude among participants with tinnitus suggests a loss of high SR fibers among these individuals. This interpretation was supported by their computational modeling results that indicated a loss of high SR fibers was necessary to produce the EFR magnitudes measured in the tinnitus participants. This is perhaps not surprising given that a reanalysis of the guinea pig data from Furman et al. (2013) suggests that although low and medium SR fibers were preferentially impacted by synaptopathy, high SR fibers made up 26% of the lost fibers (Marmel et al. 2015). These observations from an animal model as well as the findings from both simulated and measured human EFR data suggest that we should reconsider the commonly held assumption that human synaptopathy predominately impacts low and medium SR auditory nerve fibers.
Although the largest contrast between male High Noise Veterans and Non-Veteran Controls was observed at the 100% modulation depth, this was not the case with females, where the largest contrast was observed at the 40% modulation depth and the 100% modulation contrast was modest. This suggests that, on average, loss of high SR fibers is greater for male High Noise Veterans than for female High Noise Veterans. This may be a consequence of differences in MOS between males and females because until recently the military prohibited female Service members from many front-line combat jobs, such as the Infantry. This could result in different types and quantities of noise exposure experienced by men versus women during their military service. Women may also be more likely to consistently use hearing protection or less likely to have an MOS that puts them in situations where it is difficult to predict and prepare for sudden noise exposures, such as explosions. Alternatively, biological differences between males and females, such as estrogen levels (Shuster et al. 2019), may confer differences in susceptibility to this type of noise damage.
4.4. Synaptopathy risk may depend on the intensity level of the noise exposure
This is one of the first studies to show evidence of a noise exposure-related reduction in EFR magnitude in humans and the first study to measure the EFR in a sample with a history of military noise exposure. In a sample of 26 subjects, Bharadwaj et al. (2015) found a steeper decrease in EFR magnitude associated with reducing the EFR modulation depth among young people with greater reported recreational noise exposure versus those reporting less noise exposure. However, several other studies have contrasted EFR magnitudes and EFR slopes as modulation depth decreases between groups with low and high histories of recreational noise exposure without finding any clear group differences (Grose et al. 2017; Guest et al. 2017; Prendergast et al. 2017). A number of human studies have also failed to observe an effect of common sources of recreational noise exposure on ABR wave I amplitude (Fulbright et al. 2017; Grinn et al. 2017; Grose et al. 2017; Guest et al. 2017; Prendergast et al. 2017; Prendergast et al. 2018). In contrast, this study (at a stimulus level of 90 dB peSPL) and our previous study (Bramhall et al. 2017) show reductions in wave I amplitude among individuals with a history of military noise exposure or recreational firearm use. One interpretation of the findings across these EFR and ABR studies is that very high intensity noise exposures, such as those experienced during military service and when using firearms, are associated with a high risk of synaptopathy, while the risk of synaptopathy from recreational noise exposures such as concert attendance, visiting night clubs and bars, and the use of personal music players may be lower. This interpretation is supported by studies indicating that humans are less susceptible to temporary threshold shifts than mice (Dobie & Humes 2017) and higher intensity noise exposures are required to induce synaptopathy in non-human primates compared with mice (Valero et al. 2017). It is also possible that ABR wave I amplitude and EFR magnitude can only detect relatively large degrees of synaptopathy in humans and the necessary degrees of synaptopathy are more likely to occur among individuals with a history of military service or firearm use rather than among individuals with a history of recreational noise exposure.
4.5. Limitations
Studies of the impacts of noise exposure on the human auditory system are limited by two main factors: the imprecise nature of evaluating noise exposure history and variability in susceptibility to noise damage. In this study, noise exposure history was evaluated based on a lifetime noise exposure history questionnaire that included questions about occupational, military, and recreational noise exposure, including firearm use. However, a relatively high degree of measurement error is expected with this type of assessment due to individual variability in the ability to recall events over a lifetime. In addition, the scoring system for this questionnaire weights impulse/impact noise exposure from firearms, military weapons, and explosions more highly than continuous noise exposure (Bramhall et al. 2017), which could bias noise exposure scores. Finally, even when comparing individuals with exactly the same noise exposure history, differences in auditory function are expected based on genetic differences in the susceptibility to noise-related damage (Sliwinska-Kowalska & Pawelczyk 2013; Miao et al. 2019). These factors will make it difficult to detect physiological differences between high and low noise exposure groups even if the mean number of synapses differ between groups. This should be considered when interpreting the results of this and other human studies of noise-induced cochlear synaptopathy.
Another limitation is the potential impact of noise exposure on OHC function and the extent to which EFR or ABR measurements are affected by OHC function. In a regression analysis one typically interprets the individual regression coefficients as the effect of a unit change in the predictor on the mean outcome at fixed levels of the other predictors. This interpretation allows us to state, for example, that there is a 2.1 dB difference in mean EFR magnitude between male Veterans with high noise exposure and non-Veteran controls that have the exact same average DPOAE level (for a modulation depth of 100%, from Table 2). However, noise exposure can damage OHC function and impact DPOAEs. This creates a possible contradiction - we are simultaneously recognizing that noise exposure can damage OHCs, while claiming that there are populations of differentially noise exposed people with identical OHC function. This phenomenon is called post treatment bias (Montgomery et al. 2018) and is an important caveat when moving from the mathematical and probabilistic basis for regression analysis to the more qualitative interpretations of causal effects. The extent to which post-treatment bias occurs depends on the extent to which noise exposure at the level identified in the study sample damages OHCs. The degree of noise exposure-related OHC damage in this sample is expected to be minimal given that the study target population required a clinically normal audiogram and DPOAE levels that met the screening criteria. Computational modeling of the EFR suggests that OHC dysfunction has little impact on EFR magnitudes because the loss of gain resulting from OHC dysfunction will only effect the on-frequency auditory nerve fibers (i.e. fibers whose characteristic frequency is close to the stimulus frequency), while the EFR in response to stimulus levels of 60-90 dB SPL is dominated by off-frequency fibers (Encina-Llamas et al. 2019). Similarly, computational modeling of the ABR suggests that OHC damage has limited impact on wave I amplitude at 90 dB peSPL and that OHC damage results in slightly enhanced (as opposed to reduced) wave I amplitudes at 100 dB peSPL (Verhulst et al. 2018; Figure 6C). This suggests that the observed reductions in ABR wave I amplitude at 90 dB peSPL and EFR magnitudes across modulation depths among High Noise Veterans are not due to OHC dysfunction and that a DPOAE adjustment may not be necessary. For comparison, the EFR analysis was also completed without the DPOAE adjustment (Table A3).
A final limitation is that the EFR for a modulation frequency of 110 Hz is generated by multiple subcortical sources (Kuwada et al. 2002). Amplitude modulation coding in the midbrain is a complex interaction between populations of neurons that enhance the response and populations that suppress the response (Kim et al. 2020). Therefore, central gain at the level of the midbrain resulting from decreased peripheral auditory input could potentially complicate the interpretation of EFR strength.
4. Conclusions
The EFR and ABR analyses presented here are consistent with a reduction in EFR magnitude across multiple modulation depths and ABR wave I amplitude at 90 dB peSPL among Veterans with a significant history of noise exposure compared with non-Veterans with minimal noise exposure. These results parallel observations from animal models of noise-induced cochlear synaptopathy and suggest that military noise exposure may lead to noise-induced synaptopathy in humans. The findings of this study are also consistent with previous results showing decreased ABR wave I amplitude in a separate sample of young military Veterans with normal audiograms (Bramhall et al. 2017). In addition, the observation that modeled mean EFR magnitudes are lower in male Veterans reporting high noise exposure compared with non-Veteran controls, even at modulation depths thought to be dominated by high SR auditory nerve fibers, suggests that noise-related synaptopathy may impact high SR fibers in humans.
Supplementary Material
Figure A2. Model fit to ABR data. Measured ABR data (black line) for each stimulus level is plotted for individual participants along with the modeled posterior prediction interval (blue shaded region). If the model is well fit, at least half of the ABR observations should fall within this interval. Note that the identification numbers shown at the top of each panel do not match those shown in Figure A1.
Figure A1. Model fit to EFR data. Measured EFR data (black line) at each modulation depth is plotted for individual participants along with the interquartile range of the predictive distribution (blue shaded region). If the model is well fit, at least half of the EFR observations should fall within this interval.
Acknowledgements
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service - Award #C2104-W (to N.F.B.) and #C9230-C (to NCRAR). Research audiologist support was also provided by the Department of Defense Hearing Center of Excellence and zCore Business Solutions, Inc.. The opinions and assertions presented are private views of the authors and are not to be construed as official or as necessarily reflecting the views of the VA or the Department of Defense.
Footnotes
Abbreviations: ABR – Auditory brainstem response; DPOAE – Distortion product otoacoustic emission; EFR – Envelope following response; IHC – Inner hair cell; LENS-Q – Lifetime exposure to noise and solvents questionnaire; MEMR – Middle ear muscle reflex; NCRAR – National Center for Rehabilitative Auditory Research; OHC – Outer hair cell; peSPL – peak equivalent sound pressure level; SAM – Sinusoidally amplitude modulated; SPL – sound pressure level; SR – Spontaneous rate
Declarations of interest: None
References
- Aiken SJ, Picton TW (2008). Envelope and spectral frequency-following responses to vowel sounds. Hear Res, 245, 35–47. [DOI] [PubMed] [Google Scholar]
- Amrhein V, Greenland S, McShane B (2019). Scientists rise up against statistical significance. Nature, 567, 305–307. [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG (2015). Individual differences reveal correlates of hidden hearing deficits. J Neurosci, 35, 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG (2014). Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Gordon SY, McMillan GP, Gallun FJ, Molis MR, Konrad-Martin D (2020). Noise-induced enhancement of envelope following responses in normal-hearing adults. J Acoust Soc Am, 147, EL201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J, Parker M (2015). Speech Perception Ability in Noise is Correlated with Auditory Brainstem Response Wave I Amplitude. J Am Acad Audiol, 26, 509–517. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP (2018). Tinnitus and Auditory Perception After a History of Noise Exposure: Relationship to Auditory Brainstem Response Measures. Ear Hear, 39, 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP, Griest SE (2017). Auditory Brainstem Response Altered in Humans With Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear, 38, e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, McMillan GP, Gallun FJ, Konrad-Martin D (2019). Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus. J Acoust Soc Am, 146, 3849. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Niemczak CE, Kampel SD, Billings CJ, McMillan GP (2020a). Evoked Potentials Reveal Noise Exposure-Related Central Auditory Changes Despite Normal Audiograms. Am J Audiol, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Theodoroff SM, Kampel SD (2020b). Measures of Synaptopathy Linked with Tinnitus and Hyperacusis. In Association for Research in Otolaryngology MidWinter Meeting. San Jose, CA. [Google Scholar]
- Buran B. (2015). Auditory-wave-analysis: v1.1. Retrieved April 14, 2017, from http://zenodo.org/record/17365#.VoMCrjbUi70. [Google Scholar]
- Dobie RA, Humes LE (2017). Commentary on the regulatory implications of noise-induced cochlear neuropathy. Int J Audiol, 56, 74–78. [DOI] [PubMed] [Google Scholar]
- Encina-Llamas G, Harte JM, Dau T, Shinn-Cunningham B, Epp B (2019). Investigating the Effect of Cochlear Synaptopathy on Envelope Following Responses Using a Model of the Auditory Nerve. J Assoc Res Otolaryngol, 20, 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG (2015). Aging after noise exposure: acceleration of cochlear synaptopathy in "recovered" ears. J Neurosci, 35, 7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulbright ANC, Le Prell CG, Griffiths SK, Lobarinas E (2017). Effects of Recreational Noise on Threshold and Suprathreshold Measures of Auditory Function. Semin Hear, 38, 298–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol, 110, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M, Verhulst S (2019). Applicability of subcortical EEG metrics of synaptopathy to older listeners with impaired audiograms. Hear Res, 380, 150–165. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Griest SE, Thielman EJ, Carlson KF, Helt WJ, Lewis MS, Blankenship C, Austin D, Theodoroff SM, Henry JA (2017). Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hear Res, 349, 21–30. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, Peters J (1997). From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear, 18, 440–455. [DOI] [PubMed] [Google Scholar]
- Grinn SK, Wiseman KB, Baker JA, Le Prell CG (2017). Hidden Hearing Loss? No Effect of Common Recreational Noise Exposure on Cochlear Nerve Response Amplitude in Humans. Front Neurosci, 11, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Buss E, Hall JW 3rd. (2017). Loud Music Exposure and Cochlear Synaptopathy in Young Adults: Isolated Auditory Brainstem Response Effects but No Perceptual Consequences. Trends Hear, 21, 2331216517737417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Plack CJ (2019). Acoustic Middle-Ear-Muscle-Reflex Thresholds in Humans with Normal Audiograms: No Relations to Tinnitus, Speech Perception in Noise, or Noise Exposure. Neuroscience, 407, 75–82. [DOI] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ (2017). Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res, 344, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW (2002). Intracerebral sources of human auditory steady-state responses. Brain Topogr, 15, 69–86. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP (2017). Translational issues in cochlear synaptopathy. Hear Res, 349, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Wand MP (2013). Simple Marginally Noninformative Prior Distributions for Covariance Matrices. Bayesian Anal, 8, 439–452. [Google Scholar]
- Kim DO, Carney L, Kuwada S (2020). Amplitude modulation transfer functions reveal opposing populations within both the inferior colliculus and medial geniculate body. J Neurophysiol, 124, 1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiren T, Aoyagi M, Furuse H, Koike Y (1994). An experimental study on the generator of amplitude-modulation following response. Acta Otolaryngol Suppl, 511, 28–33. [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF (2012). Age-related changes in the auditory brainstem response. J Am Acad Audiol, 23, 18–35; quiz 74-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizman J, Tierney A, Fitzroy AB, Skoe E, Amar J, Kraus N (2015). Continued maturation of auditory brainstem function during adolescence: A longitudinal approach. Clin Neurophysiol, 126, 2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK (2015). Doing Bayesian Analysis: A Tutorial with R, JAGS, and Stan. (2nd ed.). San Diego, CA. [Google Scholar]
- Kujawa SG, Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci, 29, 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D'Angelo WR (2002). Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol, 13, 188–204. [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC (2011). Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol, 12, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Rodríguez-Mendoza MA, Lopez-Poveda EA (2015). Stochastic undersampling steepens auditory threshold/duration functions: implications for understanding auditory deafferentation and aging. Front Aging Neurosci, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan GP, Cannon JB (2019). Bayesian Applications in Auditory Research. J Speech Lang Hear Res, 62, 577–586. [DOI] [PubMed] [Google Scholar]
- McShane B, Gal D, Gelman A, Robert C, Tackett JL (2019). Abandon statistical signficance. Am Stat, 73, 235–245. [Google Scholar]
- Miao L, Ji J, Wan L, Zhang J, Yin L, Pu Y (2019). An overview of research trends and genetic polymorphisms for noise-induced hearing loss from 2009 to 2018. Environ Sci Pollut Res Int, 26, 34754–34774. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Phillips DS, Trune DR (1989). Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear Res, 40, 75–85. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Nyhan B, Torres M (2018). How conditioning on posttreatment variables can ruin your experiment and what to do about it. Am J Pol Sci, 62, 760–775. [Google Scholar]
- Neely S, Liu Z (1993). EMAV: Otoacoustic emission averager. In Tech Memo No. 17: Boys Town National Research Hospital Omaha. [Google Scholar]
- Parthasarathy A, Kujawa SG (2018). Synaptopathy in the Aging Cochlea: Characterizing Early-Neural Deficits in Auditory Temporal Envelope Processing. J Neurosci, 38, 7108–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BT, Bruce IC, Roberts LE (2017a). Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear Res, 344, 170–182. [DOI] [PubMed] [Google Scholar]
- Paul BT, Bruce IC, Roberts LE (2018). Envelope following responses, noise exposure, and evidence of cochlear synaptopathy in humans: Correction and comment. J Acoust Soc Am, 143, EL487. [DOI] [PubMed] [Google Scholar]
- Paul BT, Waheed S, Bruce IC, Roberts LE (2017b). Subcortical amplitude modulation encoding deficits suggest evidence of cochlear synaptopathy in normal-hearing 18-19 year olds with higher lifetime noise exposure. J Acoust Soc Am, 142, EL434. [DOI] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Leger A, Hall DA, Heinz MG, Plack CJ (2017). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear Res, 344, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Tu W, Guest H, Millman RE, Kluk K, Couth S, Munro KJ, Plack CJ (2018). Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA (1996). Age-related loss of activity of auditory-nerve fibers. J Neurophysiol, 76, 2799–2803. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013). Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci, 33, 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen LA, Valero MD, Liberman MC (2015). Towards a Diagnosis of Cochlear Neuropathy with Envelope Following Responses. J Assoc Res Otolaryngol, 16, 727–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehorn J, Strelcyk O, Zahorik P (2020). Associations between speech recognition at high levels, the middle ear muscle reflex and noise exposure in individuals with normal audiograms. Hear Res, 392, 107982. [DOI] [PubMed] [Google Scholar]
- Shuster BZ, Depireux DA, Mong JA, Hertzano R (2019). Sex differences in hearing: Probing the role of estrogen signaling. J Acoust Soc Am, 145, 3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska-Kowalska M, Pawelczyk M (2013). Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutat Res, 752, 61–65. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Abrams KR, Myles JP (2003). Bayesian approaches to clinical trials and health-care evaluation. New York, NY: John Wiley & Sons. Ltd. [Google Scholar]
- Suresh CH, Krishnan A (2020). Search for Electrophysiological Indices of Hidden Hearing Loss in Humans: Click Auditory Brainstem Response Across Sound Levels and in Background Noise. Ear Hear. [DOI] [PubMed] [Google Scholar]
- Szucs D, Ioannidis JPA (2017). When Null Hypothesis Significance Testing Is Unsuitable for Research: A Reassessment. Front Hum Neurosci, 11, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS (1988). The relative importance of head size, gender and age on the auditory brainstem response. Hear Res, 32, 165–174. [DOI] [PubMed] [Google Scholar]
- Valderrama JT, Beach EF, Yeend I, Sharma M, Van Dun B, Dillon H (2018). Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hear Res, 365, 36–48. [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC (2017). Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear Res, 353, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero MD, Hancock KE, Maison SF, Liberman MC (2018). Effects of cochlear synaptopathy on middle-ear muscle reflexes in unanesthetized mice. Hear Res, 363, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VBA VBA (2010). The Duty MOS Noise Exposure Listing. In. [Google Scholar]
- Verhulst S, Altoe A, Vasilkov V (2018). Computational modeling of the human auditory periphery: Auditory-nerve responses, evoked potentials and hearing loss. Hear Res, 360, 55–75. [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA (2016). The ASA Statement on p-values: Context, process, and purpose. Am Stat, 70, 129–133. [Google Scholar]
- Wilson RH, McArdle R (2013). Characteristics of the audiometric 4,000 Hz notch (744,553 veterans) and the 3,000, 4,000, and 6,000 Hz notches (539,932 veterans). J Rehabil Res Dev, 50, 111–132. [DOI] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O'Malley JT, Liberman MC (2019). Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience, 407, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ED, Barta PE (1986). Rate responses of auditory nerve fibers to tones in noise near masked threshold. J Acoust Soc Am, 79, 426–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A2. Model fit to ABR data. Measured ABR data (black line) for each stimulus level is plotted for individual participants along with the modeled posterior prediction interval (blue shaded region). If the model is well fit, at least half of the ABR observations should fall within this interval. Note that the identification numbers shown at the top of each panel do not match those shown in Figure A1.
Figure A1. Model fit to EFR data. Measured EFR data (black line) at each modulation depth is plotted for individual participants along with the interquartile range of the predictive distribution (blue shaded region). If the model is well fit, at least half of the EFR observations should fall within this interval.