Abstract
Introduction
The incidence of myelodysplastic syndrome and acute myeloid leukemia is significantly increased in children with Down syndrome (DS). Within the revised 2016 WHO edition, these entities are jointly classified as myeloid leukemia associated with DS (ML-DS). Additionally, infants with DS may develop transient abnormal myelopoiesis (TAM) which is histomorphologically similar to ML-DS. While TAM is self-limiting, it is associated with an increased risk of subsequently developing ML-DS. Differentiating TAM and ML-DS is challenging but clinically critical.
Methods
We performed a retrospective review of ML-DS and TAM cases collected from five large academic institutions in the USA. We assessed clinical, pathological, immunophenotypical, and molecular features to identify differentiating criteria.
Results
Forty cases were identified: 28 ML-DS and 12 TAM. Several features were diagnostically distinct, including younger age in TAM (p < 0.05), as well as presentation with clinically significant anemia and thrombocytopenia in ML-DS (p < 0.001). Dyserythropoiesis was unique to ML-DS, as well as structural cytogenetic abnormalities aside from the constitutional trisomy 21. Immunophenotypic characteristics of TAM and ML-DS were indistinguishable, including the aberrant expression of CD7 and CD56 by the myeloid blasts.
Discussion
The findings of the study confirm marked biological similarities between TAM and ML-DS. At the same time, several significant clinical, morphological, and genetic differences were observed between TAM and ML-DS. The clinical approach and the differential diagnosis between these entities are discussed in detail.
Keywords: Down syndrome, Myeloid leukemia, Trisomy 21, Transient abnormal myelopoiesis, GATA1 mutation, Immunophenotype
Introduction
Compared to children without Down syndrome (DS), individuals with DS have a 50-fold increased incidence of acute leukemia in the first 5 years of life [1]. Although many children with DS will go on to develop acute lymphoblastic leukemia, the most common pediatric malignancy overall, the predisposition to developing myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) in young children with DS is also much higher. AML in DS often follows a prolonged MDS-like phase, without a significant biological difference recognized between the MDS/AML entities [1]. Therefore, the revised 4th edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues, as well as the recently published 5th edition of WHO Classification and the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemia, classify MDS and AML in children with DS (including mosaicism of trisomy 21) together in one unique category: myeloid leukemia associated with DS (ML-DS) [1–3]. ML-DS is categorized apart from other childhood myeloid leukemias due to a unique propensity for megakaryoblastic differentiation, better prognosis, better induction therapy remission rates, and reduced relapse rates, although patients with ML-DS are still at risk for treatment-related deaths [4–7]. Younger children with ML-DS often have a more impressive response to chemotherapy with a favorable prognosis, while older children (>3 years) with ML-DS demonstrate poorer outcomes, each when compared to age-matched children without DS [1, 8]. A white blood cell (WBC) count of >20 k/µL at diagnosis and a normal karyotype outside the constitutional trisomy 21 have also been found to be independent predictors of poor event-free survival in patients with ML-DS [8].
Approximately 10% of newborns with DS (or trisomy 21 mosaicism) may present in the neonatal period with a distinct, but related entity, called transient abnormal myelopoiesis (TAM) [1–3]. TAM presents with clinical and morphological findings that may be indistinguishable from AML [1–3]. TAM is a disease of neonates, most commonly presenting at 3–7 days of life with spontaneous remission [1–3]. However, 20–30% of patients with TAM will go on to develop ML-DS within 1–3 years following resolution of TAM [1, 3, 9]. ML-DS and TAM are distinct from leukemias seen in children without DS, from a cytogenetic and molecular standpoint. The extra chromosome 21 in DS development (or mosaicism) has been attributed to playing an initiating role in leukemogenesis of these disorders [5]. ML-DS and TAM children have also been shown to acquire pathognomonic somatic mutations in the GATA1 gene, a key factor in hematopoiesis involved in regulation of megakaryocytic and erythroid differentiation [1, 5]. Additionally, trisomy 8 is a common cytogenetic abnormality seen in ML-DS, occurring in 13–36% of patients, while being absent in TAM [4].
Although these entities typically occur in distinct age-groups, ML-DS and TAM can be clinically and histopathologically identical. The differentiation remains imperative since TAM follows a self-limiting and transient disease course in most cases, without the requirement of therapy, as opposed to overt ML-DS which requires aggressive chemotherapy [1–3].
Materials and Methods
The pathology laboratory information system archives of five major academic institutions (Weill Cornell Medicine, University of Pittsburgh Medical Center, Boston Children’s Hospital, University of Miami, and Nationwide Children’s Hospital) were investigated for cases of ML-DS and TAM. Each case was re-reviewed for the purpose of this study and classified as ML-DS or TAM according to the revised 4th edition of WHO classification, the most recent classification at the time of review. Clinical and pathological information was collected including patient age, WBC count at diagnosis, hemoglobin, mean corpuscular volume (MCV), platelet count, circulating peripheral blood blast percentage (%), bone marrow aspirate blast percentage (%), blast differentiation, presence of dysplasia (and which cell lineage), and the presence or absence of bone marrow fibrosis. The CBC values were adjusted for the patient’s age [10]. If available, cytogenetic and molecular genetic information was recorded. Molecular testing was performed by Sanger sequencing and/or next-generation sequencing, based on each institution’s protocols. These parameters were compared between the ML-DS and TAM groups using Fisher’s exact test. Statistical significance was determined at p < 0.05. Patient outcomes were documented, whenever possible.
Results
Clinical Information
A total of 40 cases of myeloid proliferations associated with Down syndrome (MP-DS) were identified, including 28 ML-DS and 12 TAM. Three patients (7.5%) had no clinical diagnosis of DS at presentation, and they showed mosaicism for trisomy 21; two patients had ML-DS, and one presented with TAM. The median ages at presentation were 4 days (range 1–35 days) for TAM and 18 months (range 6–33 months) for ML-DS. One patient was a 3-year-old male with an abnormal myeloid proliferation with 15% blasts and an RSV infection that rapidly regressed with the resolution of the viral infection. Given the temporal relationship with the infection, this unusual case was deemed to be reactive in nature and, therefore, not included in the study.
The average WBC count, hemoglobin, MCV, and platelet count differed significantly between the TAM group (27.8 k/µL, 14.0 g/dL, 107.5 fL, and 177 k/µL) and the ML-DS group (7.4 k/µL [p < 0.05], 9.8 g/dL [p < 0.05], 89.1 fL [p < 0.05], and 53.3 k/µL [p < 0.05]). Thus, patients with TAM had no significant CBC abnormalities, while patients with ML-DS tended to present with anemia and thrombocytopenia, as corrected for age (Table 1).
Table 1.
Comparison of CBC data at presentation in patients with myeloid proliferations associated with DS
| ML-DS | TAM | Significance (p) | |
|---|---|---|---|
| WBC, k/µL | 7.4 | 27.8 | <0.05 |
| Hemoglobin, g/dL | 9.8 | 14.0 | <0.05 |
| MCV, fL | 89.1 | 107.5 | <0.05 |
| Platelet count, k/µL | 53.3 | 177 | <0.05 |
ML-DS, myeloid leukemia associated with DS; TAM, transient abnormal myelopoiesis.
The median time of follow-up after initial presentation was 2.9 years (range: 118 days to 10.7 years). Of the 12 TAM patients, three had available clinical follow-up information to evaluate the duration between diagnosis and complete blood count recovery. The counts of the three patients returned to normal values with the absence of circulating peripheral blasts in 42, 49, and 57 days (average: 49.3 days), following supportive care. Of the 28 patients with ML-DS, 12 patients (43%) had a documented history of TAM. During the follow-up period of this investigation, 2/12 (17%) of the TAM patients went on to develop ML-DS, one at 12 months and the second at 20 months after TAM diagnosis. Both patients had interval resolution of TAM symptoms and normal CBC values up until development of ML-DS, when they were diagnosed with neutropenia, thrombocytopenia, and pancytopenia, respectively. The remaining 10/12 (83%) patients with TAM were alive with no evidence of disease at the last follow-up. Of the TAM patients, following diagnosis, 11/12 underwent supportive treatment modalities, while 1/12 (8.3%) initially underwent a Dana Farber Clinical Institute (DFCI) AML protocol. Of the ML-DS group, the clinical course and follow-up data were available for 26/28 cases (93%) following treatment. Treatment modalities included children’s oncology group AML protocols and DFCI pediatric leukemia protocols. In examining overall outcomes, 25/26 (96%) ML-DS achieved complete remission following treatment, while 1/26 (4%) continued to demonstrate residual disease with an increase in marrow blasts. 3/25 (12%) patients, ages 12, 21, and 33 months, eventually relapsed and subsequently underwent stem cell transplant. At the last follow-up, at the time of this publication, 24/26 (92%) of the ML-DS patients were alive with no residual evidence of disease. The remaining 2 patients died, one secondary to complications of stem cell transplant and the second due to complications of constitutional DS. There were no deaths attributed to myeloid leukemia itself.
Peripheral Blood and Bone Marrow Morphology
Morphologic examination showed clinically significant dyserythropoiesis in 18/28 (64%) of ML-DS cases, compared to no such cases (0%) in TAM (p < 0.05) (Table 2). In several cases of ML-DS that had an evaluable bone marrow aspirate, megakaryocytes showed peculiar morphologic features including peripherally located nuclei, either multiple or signet-like, surrounding a central eosinophilic inclusion (Fig. 1). Notably, however, dysgranulopoiesis was minimal to absent in both groups. Bone marrow fibrosis was interpreted on reticulin staining and showed increased fibrosis (MF1–MF3) in a great majority of ML-DS cases (22/28 [79%]) compared to a smaller number in TAM (3/10 [30%, p < 0.05]).
Table 2.
Summary of histomorphologic features in patients with myeloid proliferations associated with DS
| ML-DS, % | TAM, % | Significance (p) | |
|---|---|---|---|
| Blasts Peripheral blood (mean % per case) |
12.3 | 30 | <0.05 |
| Blasts Bone marrow aspirate (mean % per case) |
24 | 12.1 | 0.08 |
| Erythroid dysplasia (% of cases) | 64 | 0 | <0.05 |
| Fibrosis (% of cases) | 79 | 30 | <0.05 |
| Megakaryocytic differentiation (% of cases) | 64 | 33 | 0.4 |
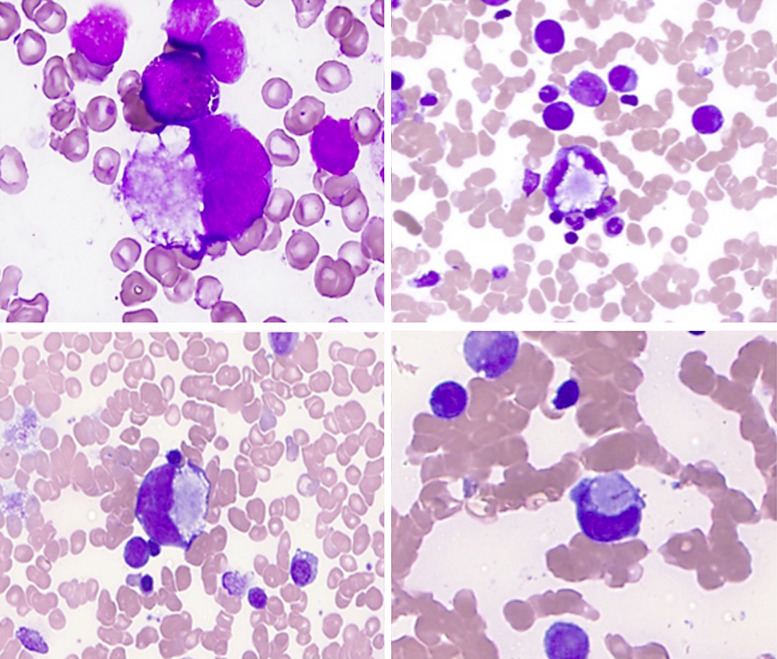
Fig. 1.
Abnormal megakaryocyte morphology, bone marrow aspirate, ML-DS. A subset of the ML-DS cases showed evidence of megakaryocytes with peripherally located nuclei, surrounding central cytoplasmic inclusions. Some atypical megakaryocytes had signet-ring morphology.
The average number of peripheral blood blasts was significantly higher in TAM compared to ML-DS (30 vs. 12.3%, respectively; p < 0.05), while the bone marrow mean blast percentages were not significantly different with a trend for lower number in TAM (24 vs. 12%, respectively). ML-DS cases with increased blasts by bone marrow aspirate differential comprised 25/28 (89%) of ML-DS cases. Of these cases, 13/25 (52%) satisfied criteria for AML (>20% blasts) and 12/25 (48%) satisfied criteria for MDS with excess blasts. The remaining three cases (11%) showed significant erythroid and megakaryocytic dysplasia without increased blasts. Megakaryoblastic differentiation was evident in 33% of TAM cases and 64% of ML-DS cases, respectively. Overall, bone marrow and peripheral blood examination of individual cases showed significant morphologic overlap between ML-DS and DS (Fig. 2).
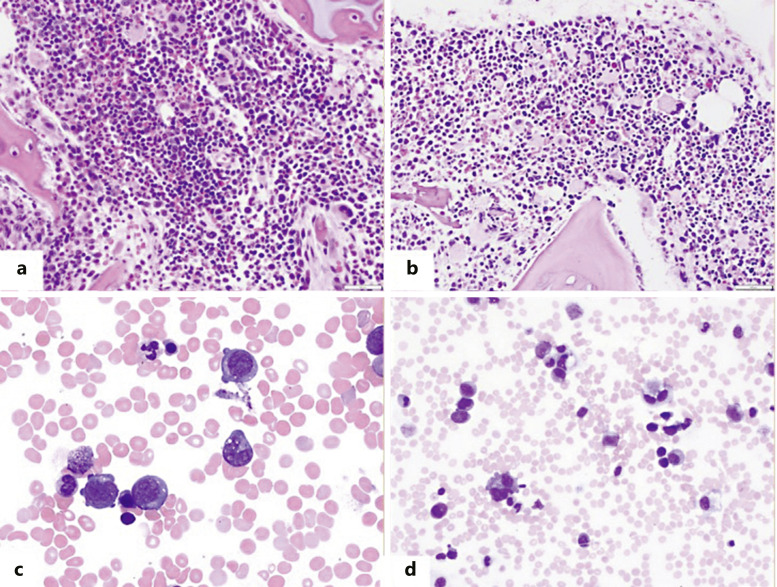
Fig. 2.
Bone marrow (a) and peripheral blood findings (c) of a representative patient with TAM. Bone marrow (b) and peripheral blood (d) findings of a representative patient with ML-DS. Both patients have a hypercellular bone marrow with increased dysplastic megakaryocytes. Increased blasts with cytoplasmic blebbing, suggesting megakaryoblasts are identified on bone marrow aspirate smears in both TAM and ML-DS.
Immunophenotyping
Flow cytometry analysis was available in 39/40 cases and showed very similar blast immunophenotypes between the TAM and ML-DS groups. None of the markers reached statistically significant difference to aid in the differential diagnosis. The most frequently expressed markers on the neoplastic myeloid blasts included CD34 (100% of TAM vs. 79% of ML-DS), CD117 (100% of TAM vs. 89% of ML-DS), and aberrant CD7 (91% of TAM vs. 71% of ML-DS). There was substantially common expression of aberrant CD56 (55% TAM vs. 50% ML-DS), HLA-DR (73% TAM vs. 52% ML-DS), CD36 (55% TAM vs. 36% ML-DS), and CD38 (88% TAM vs. 42% ML-DS) (Fig. 3). Expression of megakaryocytic markers (CD41 and/or CD61) was seen in 50% and 65% of the TAM and ML-DS cases, respectively (Table 3). Post-therapy flow cytometry follow-up samples were available in two ML-DS patients who had CD56 expression in the diagnostic sample(s). One patient had evidence of persistent expression of CD56 in the myeloid blasts (as well as granulocytes and monocytes) but had no other immunophenotypic abnormalities detected in the diagnostic samples (Fig. 4). The other patient had persistent expression of CD56 in the granulocytes and monocytes but not blasts.
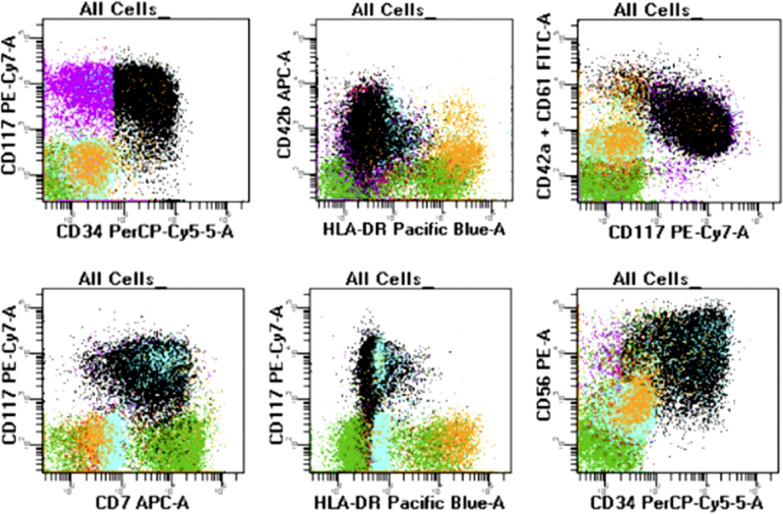
Fig. 3.
Flow cytometry evaluation of a representative ML-DS patient. The neoplastic cells (in black and purple, respectively) express CD34 and CD117. They have megakaryoblastic differentiation with evidence of CD42a, CD42b, and CD61 positivity. The neoplastic blasts have aberrant expression of CD7 and CD56 (bright). They are negative for HLA-DR.
Table 3.
Summary of the most frequent immunophenotypic markers expressed by the neoplastic blasts based on the flow cytometry
| Diagnosis | CD34 (% cases) | CD117 (% cases) | CD7 (% cases) | CD56 (% cases) | HLA-DR (% cases) | CD38 (% cases) | Mk (% cases) | CD36 (% cases) |
|---|---|---|---|---|---|---|---|---|
| TAM | 11/11 (100) | 11/11 (100) | 10/11 (91) | 6/11 (55) | 8/11 (73) | 7/8 (88) | 5/10 (50) | 6/11 (55) |
| ML-DS | 22/28 (79) | 24/27 (89) | 20/28 (71) | 14/28 (50) | 13/25 (52) | 8/19 (42) | 17/26 (65) | 10/28 (36) |
Mk, megakaryocyte markers (CD41, CD61, and CD42b).
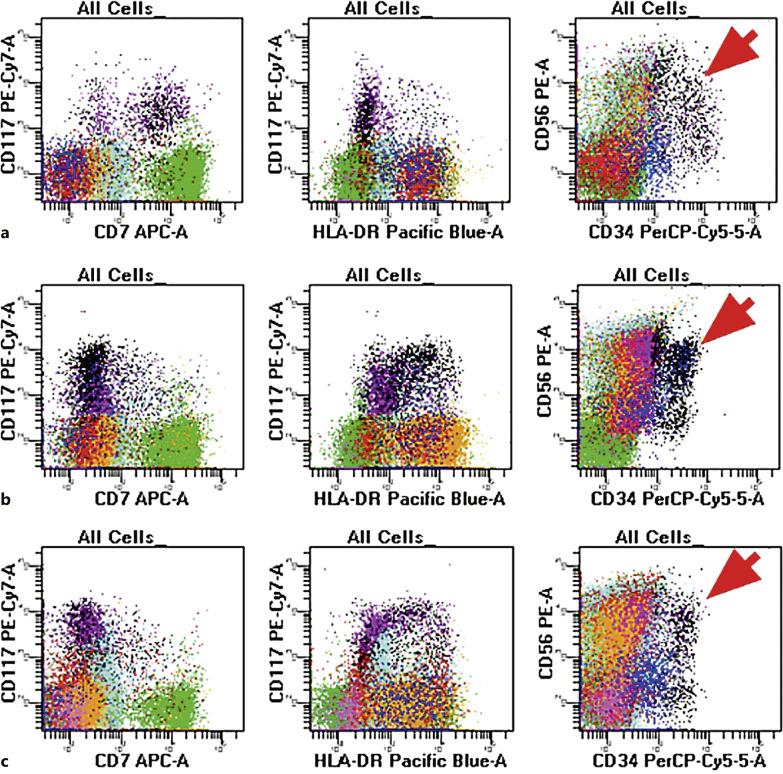
Fig. 4.
Flow cytometry evaluation of the diagnostic sample (top panel), 3 months post induction therapy (mid-panel) and 10 months post induction therapy (lower panel) in a patient with ML-DS who achieved and maintained complete remission after induction. The diagnostic sample (top panel, a) shows the presence of myeloid blasts with expression of CD7 and CD56 with the absence of HLA-DR. The post-therapy samples (mid-panel b, lower panel, c) show similar findings with persistent CD56, absent CD7, and variable HLA-DR. Aberrant CD56 expression (as indicated by the arrow) is an inherent characteristic of many DS patients and is not, in isolation, diagnostic of residual myeloid leukemia.
Cytogenetic and Molecular Data
Cytogenetic studies were available in 35/40 patients (TAM = 10, ML-DS = 25). In ML-DS, 20/25 (80%) showed additional structural cytogenetic abnormalities aside from the trisomy 21, compared to none (0%) in the TAM group (p < 0.05). Among ML-DS, 16/25 cases had one abnormality in addition to the constitutional trisomy 21, and 4/25 cases showed a complex karyotype. Cytogenetic abnormalities detected included trisomy 8 (11/25, 44%), abnormal 7p (7/25, 28%), and deletion 7q (4/25, 16%).
Molecular studies have been performed in 8 ML-DS and 4 TAM patients. A GATA1 mutation was detected in 3/8 (38%) ML-DS cases (variant allele frequencies [VAF] of 3%, 32%, and 35%, respectively) and 2/4 (50%) TAM cases (VAF of 29% and 87%). Among our institutions, a combination of Sanger sequencing and next-generation sequencing (NGS) was utilized in the molecular analysis of GATA1 mutation detection. Interestingly, of the two TAM cases in which a GATA1 mutation was identified, one patient also had an ANKRD2 mutation with VAF of 46%. Otherwise, no additional genetic mutations were detected in the remaining patients.
Discussion
This large study of carefully annotated TAM and ML-DS cases collected from academic hematopathology departments provides data that are useful in the clinicopathological distinction of TAM versus ML-DS. The study confirmed that patient’s age alone allows for differentiation, as TAM occurs exclusively in newborns (median age 4 days, range 1–35 days), as compared to ML-DS (median age 17 months, range 6–33 months), with 75% of ML-DS presenting at >1 year of age. The CBC parameters prove another useful tool in the differentiation, as TAM demonstrated no significant abnormalities (accounted for normal ranges in this age-group), whereas ML-DS patients had clinically significant anemia and thrombocytopenia. It is worth reinforcing that the normal peripheral blood CBC values are dynamic and change with pediatric development. Most notably, normal WBC count, hemoglobin, and MCV values decrease as neonates’ age from infancy to 2 years old; therefore, the apparent macrocytosis and leukocytosis observed in TAM babies are likely due to physiologic CBC values rather than the myeloid pathology [10]. Prior studies have evaluated TAM CBC values compared to non-TAM patients with DS and showed no statistically significant difference with the exception of lymphocytosis identified in TAM [11]. Conversely, ML-DS was characterized by presentation at older age and with median hemoglobin and platelet values below what is considered normal for age, indicating that the anemia and thrombocytopenia seen in these patients was secondary to the underlying myeloid neoplasm.
Another useful finding of this study is the demonstration of morphologic dysplasia in the erythroid lineage that appeared to be unique to ML-DS and absent in all studied cases of TAM. On the other hand, there was minimal to absent evidence of dysgranulopoiesis in all cases. Some patients with ML-DS showed evidence of characteristic dysplastic features in the megakaryoblasts which has been documented in a prior study [12]. This kind of megakaryocyte dysplasia apparently unique to ML-DS cases consists of central eosinophilic cytoplasmic inclusions with peripherally displaced multiple or signet-like nuclei. Given the prior reporting of these features, their presence, thus, supported the diagnosis of ML-DS [12].
By definition, both ML-DS and TAM are characterized by increased myeloid blasts, either in the bone marrow or peripheral blood. As previously reported, TAM patients had significantly higher mean peripheral blood blast counts (30% circulating blasts on average) than did ML-DS patients (12% blasts). This contrasts with the mean bone marrow aspirate blast differential counts, which were slightly higher on average in the ML-DS group (24% blasts) than the TAM group (12.1% blasts). In attempts to explain this finding, we investigated the spleen size at diagnosis; however, no significant splenomegaly was observed in either cohort at diagnosis. TAM does not have established peripheral circulating blast count cutoffs for diagnosis. As a result, circulating blast cutoff percentages have ranged from 1% or more [13–15] to 10% or more [16–18] in the literature. We hypothesize that the higher peripheral blood blast counts in TAM may be related to young age, potential effects of recent birth, or endogenous cytokine stimulation. Variably increased marrow fibrosis also showed statistical significance between the two groups, being significantly more often identified in the ML-DS cohort (Table 2). The frequent megakaryoblastic myeloid blast differentiation likely contributes to the increased reticulin fibrosis in the marrow, as seen in other types of megakaryoblastic AML. This finding is similarly evidenced in rare cases of disseminated leukemic infiltration of the liver, where hepatic fibrosis subsequently develops [19].
Flow cytometric analysis demonstrated that the presence of an aberrant immunophenotype is not a feature unique to ML-DS, as the blast immunophenotype was overall nearly identical between the TAM and ML-DS cohorts. Aberrant expression of CD56 in the myeloid blasts, in particular, was observed in 55% and 50% of cases with TAM and ML-DS, respectively. CD56, typically expressed by the natural killer cells and a subset of CD4+ T-helper cells, is often an aberrant marker in AMLs [20]. In AML, CD56 has been considered a promoter of leukemogenesis associated with lower overall survival, a higher incidence of central nervous system involvement, disease progression, and relapse [20, 21]. In DS, however, CD56 appears to be a unique inherent characteristic identified in blasts of TAM and ML-DS, an expression which was also observed in our study [20]. Moreover, CD56 has been identified in monocytes and granulocytes of healthy individuals with DS [22]. It has been hypothesized that the increased CD56 expression on monocytes and granulocytes in hematologically normal patients with DS could be multifactorial and does not play a role in leukemogenesis, in contrast to non-DS AML [20]. Understanding the presence of CD56 in DS even in the absence of MP-DS is imperative when evaluating the samples of ML-DS post-chemotherapy. Residual CD56 expression, taken in isolation, should not be considered diagnostic of residual disease but rather a DS-associated feature of regenerating monocytes and granulocytes.
Cytogenetic abnormalities (outside of the constitutional trisomy 21 or mosaicism) are a helpful differentiating characteristic, as they were observed in 80% of ML-DS but were not seen in any TAM cases, supporting that these diseases are genetically distinct despite overlapping histopathologic and immunophenotypic features. Based on the prior literature, approximately 13–36% of patients with ML-DS demonstrate trisomy 8 in addition to the gain of chromosome 21, while loss of chromosome 5 and/or loss of chromosome 7 material is present in ∼23% of ML-DS [4, 7, 8]. These findings are confirmed by the results of this study.
GATA1 mutations are well documented in the pathophysiology of both TAM and ML-DS, however, at different frequencies. GATA1 is an essential regulator of the erythroid and megakaryocytic hematopoietic lineages in normal hematopoiesis [23]. Mutations in this hematopoietic transcription factor result in a truncated protein which is believed to cooperate with trisomy 21 to drive the development of ML-DS [23]. Studies using highly sensitive GATA1 detection methods have been published, emphasizing the importance of GATA1 detection in MP-DS, including the application of advanced neonatal screening to identify neonates at risk for myeloid leukemia development [24, 25]. In fact, studies have investigated various levels of sensitivity in GATA1 mutation detection, where higher sensitivity combination methods of Sanger screening followed by NGS were deemed superior in identifying the actual incidence of GATA1 mutations in ML-DS [24]. GATA1 mutations have been documented in ∼3.8–59% of neonates with DS [4, 16, 17]. Particularly in TAM, GATA1 mutations have been detected in 10–15% of cases, where variation in blast percentage cutoffs constitute the TAM diagnosis [5, 11, 25]. In contrast, GATA1 mutations appear more frequent in ML-DS and have been detected in 56–90% of cases [24]. Our study showed frequencies higher in TAM (50%) and lower in ML-DS (38%) than those aforementioned documented in the literature; however, the overall number of positive cases was small. The 5th edition of WHO includes detection of exon 2/3 GATA1 mutation as an essential diagnostic criterion for both TAM and ML-DS. The results of this study indicate that the strict application of these criteria may be problematic in clinical practice since the molecular testing is performed infrequently and, when done, the sensitivity of some GATA1 testing is not high enough to detect the mutation in a large subset of cases. We therefore suggest that the presence of a GATA1 mutation is desirable but not an essential criterion for clinical diagnosis of MP-DS.
One TAM patient also carried an ANKRD26 mutation with a VAF of 46%. ANKRD26 mutations have been associated with an inherited form of thrombocytopenia [26–28]. While TAM cases overall showed significantly higher platelet counts compared to ML-DS, this patient with the ANKRD26 mutation did have a lower platelet count (85 k/µL) compared to other patients in the cohort. Therefore, the patient may have a very rare presentation of a concomitant DS with germline ANKRD21 mutation.
A strength of this study is that clinical follow-up was available for most (26/28) ML-DS cases. All underwent chemotherapy regimens, with an overall excellent clinical outcome. The relapse rate in ML-DS has significantly decreased in the last few decades; however, patients who do relapse appear to have limited treatment options [23]. Studies have shown that the overall survival after relapse is worse than the generally optimistic prognosis for ML-DS without relapse. A Japanese group found a 3-year overall survival of 26% following relapse, while the Center for International Blood and Marrow Transplant Research found a 3-year overall survival of 19% [29, 30]. Each study emphasized the poorer outcomes in these patient cohorts.
Our study incorporated three patients who were mosaics for DS and thus presented an additional diagnostic challenge. In phenotypically normal infants, the development of leukocytosis with large percentages of blasts can prompt the diagnosis of AML. One informative case in the literature describes a phenotypically normal male, with normal prenatal cell-free DNA screening, who was diagnosed with AML demonstrating partial megakaryoblastic differentiation at 24 hours of life [31]. Given the rapidly rising WBC count, he was started on cytarabine therapy. With the integration of fluorescence in situ hybridization (FISH) which in this case showed 3 copies of RUNX1, raising suspicion of trisomy 21, and targeted NGS, revealing a GATA1 frameshift mutation and subsequently confirming a gain of chromosome 21, the diagnosis was revised to that of TAM. This case report together with our findings indicates that a high index of suspicion is required in evaluating cases of neonatal AML. Integration of FISH, cytogenetics, and rapid NGS testing is critical in rapid and accurate evaluation and appropriate therapeutic approach to these babies.
In summary, the findings of this large multi-institutional study showed that there are significant differences in age, CBC values at presentation, as well as diagnostic morphologic and cytogenetic parameters that allow for accurate distinction between cases of ML-DS and TAM. Furthermore, the study served to show that the findings, in TAM and ML-DS patients with trisomy 21 patients, were also seen in those with mosaicism, which are otherwise phenotypically normal, thus emphasizing the necessity of maintaining a high index of suspicion in all pediatric patients presenting with an AML-like picture. One limitation of this study was limited molecular evaluation of the patients, most significantly lack of GATA1 testing in most patients. However, since this study summarizes a real-life approach to pediatric patients in several major academic institutions in the USA, we believe that accurate diagnosis can be achieved even in these cases, based on the parameters evaluated.
Statement of Ethics
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Specifically, approval for the clinical study was obtained from the Institutional and/or National Research Committee of each participating institution. The Institutional Review Board of Weill Cornell Medicine approved this work under protocol number 1007011151. Written informed consent from participants was not required, in accordance with local/national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no external funding.
Author Contributions
Material preparation and data collection and analysis were performed by Tayler van den Akker, Yen-Chun Liu, Huifei Liu, Jennifer Chapman, Jennifer M. Levine, Olga K. Weinberg, and Julia T. Geyer. The first draft of the manuscript was written by Tayler van den Akker and reviewed by Julia T. Geyer. All authors contributed to the study conception and design, commented on previous versions of the manuscript, and have read and approved the final manuscript.
Funding Statement
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. J (Eds) . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th Edition). Lyon: IARC; 2017. [Google Scholar]
- 2. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health organization classification of haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022 Jul;36(7):1703–19. 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arber DA, Orazi A, Hasserjian RP, et al. International Consensus classification of myeloid neoplasms and acute leukemia: integrating morphological, clinical, and genomic data. Blood. 2022 Sep 15;140(11):1200–1228. 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chisholm KM. Myeloid proliferations in Down syndrome. Atlas Genet Cytogenet Oncol Haematol. 2018(2). 10.4267/2042/68762. [DOI] [Google Scholar]
- 5. Bhatnagar N, Nizery L, Tunstall O, Vyas P, Roberts I. Transient abnormal myelopoiesis and AML in down syndrome: an update. Curr Hematol Malig Rep. 2016 Oct;11(5):333–41. 10.1007/s11899-016-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao A, Hills RK, Stiller C, Gibson BE, de Graaf SSN, Hann IM, et al. Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol. 2006 Mar;132(5):576–83. 10.1111/j.1365-2141.2005.05906.x. [DOI] [PubMed] [Google Scholar]
- 7. Gamis AS, Woods WG, Alonzo TA, Buxton A, Lange B, Barnard DR, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children's Cancer Group Study 2891. J Clin Oncol. 2003 Sep 15;21(18):3415–22. 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 8. Blink M, Zimmermann M, von Neuhoff C, Reinhardt D, de Haas V, Hasle H, et al. Normal karyotype is a poor prognostic factor in myeloid leukemia of Down syndrome: a retrospective, international study. Haematologica. 2014 Feb;99(2):299–307. 10.3324/haematol.2013.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massey GV, Zipursky A, Chang MN, Doyle JJ, Nasim S, Taub JW, et al. A prospective study of the natural history of transient leukemia (TL) in neonates with Down syndrome (DS): children’s Oncology Group (COG) study POG-9481. Blood. 2006 Jun 15;107(12):4606–13. 10.1182/blood-2005-06-2448. [DOI] [PubMed] [Google Scholar]
- 10. Medical Laboratories Mayo . Complete blood count normal pediatric values. Retrieved from: http://a1.mayomedicallaboratories.com/webjc/attachments/110/30a2131-complete-blood-count-normal-pediatric-values.pdf. [Google Scholar]
- 11. Orozco-Vela M, Corona-Rivera A, Cruz-Osorio RM, Mendoza-Maldonado L, Márquez-Mora A, Barba-Barba CC, et al. Complete blood count differences in a cohort of Down syndrome neonates with transient abnormal myelopoiesis screened for GATA1 pathogenic variants. Am J Med Genet A. 2020 Sep;182(9):2085–93. 10.1002/ajmg.a.61748. [DOI] [PubMed] [Google Scholar]
- 12. Mast KJ, Taub JW, Alonzo TA, Gamis AS, Mosse CA, Mathew P, et al. Pathologic features of down syndrome myelodysplastic syndrome and acute myeloid leukemia: a report from the children’s oncology group protocol AAML0431. Arch Pathol Lab Med. 2020;144(4):466–72. 10.5858/arpa.2018-0526-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gamis AS, Alonzo TA, Gerbing RB, Hilden JM, Sorrell AD, Sharma M, et al. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children’s Oncology Group Study A2971. Blood. 2011 Dec 22;118(26):6752–9; quiz 6996. 10.1182/blood-2011-04-350017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim DW, Kim HR, Shin MG, Baek HJ, Kook H, Hwang TJ, et al. Distinctive hematological abnormalities in east Asian neonates and children with down syndrome. Int J Lab Hematol. 2011;33(4):369–77. 10.1111/j.1751-553X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 15. Jackson GL, Sendelbach DM, Rambally B, Manning MD, Engle WD. Circulating blasts and associated hematologic disorders in neonates with down syndrome. Am J Perinatol. 2012;29(4):259–66. 10.1055/s-0031-1285103. [DOI] [PubMed] [Google Scholar]
- 16. Pine SR, Guo Q, Yin C, Jayabose S, Druschel CM, Sandoval C. Incidence and clinical implications of GATA1 mutations in newborns with down syndrome. Blood. 2007;110(6):2128–31. 10.1182/blood-2007-01-069542. [DOI] [PubMed] [Google Scholar]
- 17. Roberts I, Alford K, Hall G, Juban G, Richmond H, Norton A, et al. GATA1-mutant clones are frequent and often unsuspected in babies with Down syndrome: identification of a population at risk of leukemia. Blood. 2013 Dec 5;122(24):3908–17. 10.1182/blood-2013-07-515148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tunstall O, Bhatnagar N, James B, Norton A, O'Marcaigh AS, Watts T, et al. Guidelines for the investigation and management of transient leukaemia of down syndrome. Br J Haematol. 2018 Jul;182(2):200–11. 10.1111/bjh.15390. [DOI] [PubMed] [Google Scholar]
- 19. Roy A, Roberts I, Vyas P. Biology and management of transient abnormal myelopoiesis (TAM) in children with Down syndrome. Semin Fetal Neonatal Med. 2012 Aug;17(4):196–201. 10.1016/j.siny.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 20. Gadgeel M, AlQanber B, Buck S, Taub JW, Ravindranath Y, Savaşan S. Aberrant myelomonocytic CD56 expression in Down syndrome is frequent and not associated with leukemogenesis. Ann Hematol. 2021 Jul;100(7):1695–700. 10.1007/s00277-021-04531-x. [DOI] [PubMed] [Google Scholar]
- 21. Sasca D, Szybinski J, Schüler A, Shah V, Heidelberger J, Haehnel PS, et al. NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood. 2019 May 23;133(21):2305–19. 10.1182/blood-2018-12-889725. [DOI] [PubMed] [Google Scholar]
- 22. Karandikar NJ, Aquino DB, McKenna RW, Kroft SH. Transient myeloproliferative disorder and acute myeloid leukemia in Down syndrome. An immunophenotypic analysis. Am J Clin Pathol. 2001 Aug;116(2):204–10. 10.1309/XREF-C9T2-6U0A-4EDT. [DOI] [PubMed] [Google Scholar]
- 23. Boucher AC, Caldwell KJ, Crispino JD, Flerlage JE. Clinical and biological aspects of myeloid leukemia in Down syndrome. Leukemia. 2021 Dec;35(12):3352–60. 10.1038/s41375-021-01414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terui K, Toki T, Taga T, Iwamoto S, Miyamura T, Hasegawa D, et al. Highly sensitive detection of GATA1 mutations in patients with myeloid leukemia associated with Down syndrome by combining Sanger and targeted next generation sequencing. Genes Chromosomes Cancer. 2020;59(3):160–7. 10.1002/gcc.22816. AL. [DOI] [PubMed] [Google Scholar]
- 25. Goemans BF, Noort S, Blink M, Wang YD, Peters STCJ, van Wouwe JP, et al. Sensitive GATA1 mutation screening reliably identifies neonates with Down syndrome at risk for myeloid leukemia. Leukemia. 2021 Aug;35(8):2403–6. 10.1038/s41375-021-01128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noris P, Perrotta S, Seri M, Pecci A, Gnan C, Loffredo G, et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011 Jun 16;117(24):6673–80. 10.1182/blood-2011-02-336537. [DOI] [PubMed] [Google Scholar]
- 27. Perez Botero J, Dugan SN, Anderson MW. ANKRD26-Related thrombocytopenia. 2018 Jun 21. In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews® [internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507664/. [PubMed] [Google Scholar]
- 28. Zidan NI, AbdElmonem DM, Elsheikh HM, Metwally EA, Mokhtar WA, Osman GM. Relation between mutations in the 5' UTR of ANKRD26 gene and inherited thrombocytopenia with predisposition to myeloid malignancies. An Egyptian study. Platelets. 2021 Jul 4;32(5):642–50. 10.1080/09537104.2020.1790512. [DOI] [PubMed] [Google Scholar]
- 29. Taga T, Saito AM, Kudo K, Tomizawa D, Terui K, Moritake H, et al. Clinical characteristics and outcome of refractory/relapsed myeloid leukemia in children with Down syndrome. Blood. 2012 Aug 30;120(9):1810–5. 10.1182/blood-2012-03-414755. [DOI] [PubMed] [Google Scholar]
- 30. Hitzler JK, He W, Doyle J, Cairo M, Camitta BM, Chan KW, et al. Outcome of transplantation for acute myelogenous leukemia in children with Down syndrome. Biol Blood Marrow Transplant. 2013 Jun;19(6):893–7. 10.1016/j.bbmt.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aziz-Bose R, Wachter F, Chiarle R, Lindeman NI, Kim AS, Degar BA, et al. Rapid next-generation sequencing aids in diagnosis of transient abnormal myelopoiesis in a phenotypically normal newborn. Blood Adv. 2022 May 10;6(9):2893–6. 10.1182/bloodadvances.2021006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.