Abstract
The ability of Shigella flexneri to multiply within colonic epithelial cells and spread to adjacent cells is essential for production of dysentery. Two S. flexneri chromosomal loci that are required for these processes were identified by screening a pool of TnphoA insertion mutants. These mutants were able to invade cultured epithelial cells but could not form wild-type plaques. Analysis of the nucleotide sequence indicated that the sites of TnphoA insertion were within two different regions that are almost identical to Escherichia coli K-12 chromosomal sequences of unknown functions. One region is located at 70 min on the E. coli chromosome, upstream of murZ, while the other is at 28 min, downstream of tonB. The mutant with the insertion at 70 min was named vpsC because it showed an altered pattern of virulence protein secretion. The vpsC mutant formed pinpoint-sized plaques, was defective in recovery from infected tissue culture cells, and was sensitive to lysis by the detergent sodium dodecyl sulfate. Recombinant plasmids carrying the S. flexneri vpsA, -B, and -C genes complemented all of the phenotypes of the vpsC mutant. A mutation in vpsA resulted in the same phenotype as the vpsC mutation, suggesting that these two genes are part of a virulence operon in S. flexneri. The mutant with the insertion at 28 min was interrupted in the same open reading frame as S. flexneri ispA. This ispA mutant could not form plaques and was defective in bacterial septation inside tissue culture cells.
Shigella species, causative agents of dysentery in humans, initiate disease by invading colonic epithelial cells (17, 32, 44, 56). The bacteria multiply within the cells and spread to adjacent cells. The molecular and genetic mechanisms of pathogenesis have been intensively studied in Shigella flexneri. The ability of shigellae to infect and provoke disease is a relatively complex process and requires the products of a number of plasmid and chromosomal genes.
Most of the genes involved in invasion and intercellular spread are located on a large virulence plasmid (180 to 230 kb) that is conserved among the Shigella species and enteroinvasive Escherichia coli (57). Proteins encoded by the S. flexneri virulence plasmid include IpaB, IpaC, and IpaD, which are required for bacterial entry into host cells and for escape from endocytic vesicles, and Mxi-Spa, consisting of about 20 gene products that are involved in a type III secretory pathway for secretion of Ipa proteins. Other plasmid-encoded virulence proteins that are required for invasion or intercellular spread include IcsA (VirG), IcsB, IpgC, VirK, and SopA. VirF and VirB, which positively regulate transcription of ipa, mxi, and icsA, also are encoded on the virulence plasmid (13, 32, 44, 57, 66). A small plasmid, pHS-2, has been implicated in S. flexneri virulence. An O-antigen chain length determinant gene, cldpHS-2, on this plasmid is required for full resistance to complement-mediated serum killing and for induction of a strong inflammatory response in the guinea pig Serény test (21).
Chromosomal loci also have been implicated in S. flexneri virulence, either as virulence factors or as regulators of virulence gene expression. The lipopolysaccharide (LPS) synthesis loci galU, rfa, rfb, and rfc and the O-antigen chain length regulator rol are required for correct surface presentation or distribution of IcsA and thus are important for intercellular spreading (21, 41, 42, 53–55, 67). An S. flexneri ispA mutant was found to be defective in intercellular spreading due to abnormal septation when growing within cultured epithelial cells (27). Another gene, vacJ, is required for intercellular spread, but its function is not known (62). The aerobactin synthesis and transport genes iuc and iut are important for iron acquisition and bacterial growth in extracellular environments but do not appear to be required for intracellular multiplication (25, 37). Chromosomally encoded regulatory factors include the thermo-osmotic regulator VirR, an H-NS-like protein whose product binds the virB promoter and represses its expression at temperatures lower than 37°C (22, 49, 65); the two-component osmolarity-responsive regulatory system OmpR-EnvZ (2, 3); and VacB and VacC, which are involved in regulating the expression of Ipa proteins, either at the transcriptional level (VacC) or posttranscriptionally (VacB) (11, 64).
This study was undertaken to identify additional genes that are involved in S. flexneri virulence, especially those required for intracellular multiplication and spread to adjacent cells. Two mutants that retained the ability to invade tissue culture cells but failed to form normal plaques were characterized.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains, plasmids, and their sources are listed in Table 1. Bacteria were cultured at 37°C in Luria-Bertani broth (LB) or on L agar for general purposes. S. flexneri strains were routinely streaked on Congo red agar (tryptic soy broth with 1.5% agar containing 0.01% [wt/vol] Congo red dye [Sigma Chemical Co., St. Louis, Mo.]) to differentiate the colonies that bind Congo red (Crb+) from those that do not (Crb−) (45). The intracellular salts medium (ISM), a medium mimicking the intracellular conditions, was described previously (20). The concentrations of antibiotics used were as follows: carbenicillin, used for ampicillin-resistant strains, 250 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 50 μg/ml; tetracycline, 12.5 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| SA100 | S. flexneri wild type, serotype 2a, Crb+, virulent | 46 |
| SA101 | SA100 derivative, deletion in the large virulence plasmid, Crb−, noninvasive | 9 |
| SA511 | SA100 Cmr, invasive, formed normal plaques | 21 |
| SA2122 | SA511 vpsC::TnphoA, Kmr | This study |
| SA2054 | SA511 ispA::TnphoA, Kmr | This study |
| SA4100 | SA100 vpsA::aphA-3, Kmr | This study |
| SA5122 | SA100 vpsC::TnphoA, Kmr | This study |
| SA5122W | SA5122 Crb− | This study |
| W3110 | E. coli K-12 | I. J. Molineux |
| SB221 | E. coli lpp | 36 |
| SM10λpir | E. coli pirR6K | 63 |
| Plasmids | ||
| pACYC177 | cloning vector, Apr Kmr | 7 |
| pACYC184 | cloning vector, Tcr Cmr | 7 |
| pBR322 | cloning vector, Apr Tcr | 4 |
| pWSc-1 | cloning vector, Sucs | 40 |
| pMQA | SA100 vpsA-yrbA cloned in pBR322, Tcr | This study |
| pMQB | SA100 vpsC-yrbA cloned in pBR322, Tcr | This study |
| pMQC | SA100 vpsA-C cloned in pBR322, Tcr | This study |
| pMQD | SA100 vpsA-yrbC cloned in pBR322, Tcr | This study |
| pMQE | SA100 vpsA-yrbB cloned in pBR322, Tcr | This study |
| pMQF | SA100 vpsC cloned in pBR322, Tcr | This study |
| pMQG | SA100 vpsA-B cloned in pBR322, Tcr | This study |
| pYG400 | vpsA::aphA-3 cloned in pWSc-1 | This study |
| pRZ526 | E. coli yciC-tonB cloned in pRZ112, Kmr | 51 |
| pML14 | pRZ526 with bla from pACYC177 inserted at the HindIII site, Apr Kmr | This study |
| pRT733 | oriR6K mob+ TnphoA Apr Kmr | 63 |
Construction of S. flexneri mutants.
Random TnphoA mutagenesis of S. flexneri was performed as previously described (21) to generate insertional mutations in genes encoding secreted or membrane proteins. Briefly, SA511, previously described as SA514(pMA9) (21), a chloramphenicol-resistant (Cmr) derivative of SA100 that invades tissue culture cells and spreads normally, was mated with SM10λpir(pRT733), which carries TnphoA (28) on a suicide vector (63). Crb+ colonies that were Kmr and Cmr but sensitive to ampicillin (Aps) and had alkaline phosphatase activity (PhoA+) were tested in Henle cell invasion and plaque assays to identify the mutants that retained invasiveness but were defective in cell-to-cell spread. Southern hybridization confirmed that each mutant had a single insertion. The TnphoA insertion in the vpsC mutant SA2122 was moved to the wild-type SA100 background by P1 transduction (35), yielding SA5122. The phenotype of SA5122 was the same as that of the donor strain, SA2122.
The mutation in vpsA was constructed by insertion of a nonpolar kanamycin cassette (aphA-3) (34) in frame at the BsiWI site in pMQE. The PstI fragment containing vpsA::aphA-3 was ligated into the PstI site of pWSc-1 to yield pYG400. SA100 was transformed with pYG400, and the mutation was transferred to the chromosome of SA100 by allelic exchange, yielding SA4100. The chromosomal DNA was amplified by PCR to verify the mutation.
Tissue culture cell invasion, plaque assays, and recovery of intracellular bacteria.
The ability of S. flexneri to invade Henle cells was determined by the procedure of Hale and Formal (18) as follows. Subconfluent cell monolayers in 35-mm-diameter plates were infected with 2 × 108 CFU. After 1 h, extracellular bacteria were removed from the monolayer by washing, and medium containing gentamicin (20 μg/ml) was added to kill any remaining extracellular bacteria. This concentration of gentamicin was above the MIC (3.25 to 6.5 μg/ml) determined for each of the strains used in this study. The infected monolayers were incubated for an additional 1 to 2 h. At least 300 cells on each plate were observed by microscopy after staining with Wright-Giemsa stain (Baxter Scientific Products, McGaw Park, Ill.), and those containing 3 or more bacteria per cell were considered infected. To differentiate intracellular bacteria from those associated with the surface of the Henle cells, indirect immunofluorescence labeling was performed as follows. The monolayers were fixed with 1% paraformaldehyde. The primary antibody was polyclonal rabbit anti-S. flexneri group B (Difco Laboratories, Detroit, Mich.), and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (Organon Teknika Corp., West Chester, Pa.) was the secondary antibody. The slides were mounted and observed with a Zeiss Axioskop fluorescence microscope. The images of the phase-contrast field for total bacteria and those of the fluorescence field were compared. Only the extracellular bacteria were stained with the antibody, which did not penetrate the Henle cells.
To recover the intracellular bacteria, the cell monolayers were detached with trypsin (0.025% [wt/vol]) and lysed with 0.5% (wt/vol) sodium deoxycholate (DOC) as described previously (20). The lysate was plated on agar medium to determine bacterial CFU. The number of Henle cells harvested was determined with a hemocytometer prior to lysis. CFU recovered per infected Henle cell was calculated as number of CFU per (total number of Henle cells harvested × percentage of Henle cells infected).
Plaque assays were performed as described by Oaks et al. (39) as follows. Confluent Henle cell monolayers in 35-mm-diameter plates were infected with 102 to 105 bacteria. After 90 min of incubation, the Henle cells were overlaid with fresh medium plus 0.45% (wt/vol) glucose, 0.5% (wt/vol) agarose, and 20 μg of gentamicin per ml and incubated for 72 h.
Sequence analysis of the site of TnphoA insertion.
The SA2122 gene interrupted by TnphoA was identified by cloning the BamHI fragment containing the kanamycin resistance gene and upstream sequence into pACYC184, and sequence was obtained by using a primer located within phoA, namely, 5′-ATATCGCCCTGAGCAG-3′ (primer A). For SA2054, inverse PCR was performed as previously described (21). Briefly, total DNA was digested with TaqI and religated. Two primers derived from phoA sequence, primer A and primer B (5′-CAACCGGTGTCAAAACC-3′), were used to amplify the appropriate product. The purified PCR fragments were sequenced with either primer A or primer B with an ABI Prism 377 DNA sequencer (Perkin-Elmer Co. Applied Biosystem Division, Foster City, Calif.). The sequences of portions of the vpsA and vpsB regions and of the entire vpsC-yrbA region (Fig. 1A) (GenBank accession no. AF053073) were obtained by direct sequencing of the PCR products of amplified S. flexneri chromosomal DNA and by sequencing the recombinant plasmid pMQB (Fig. 1), as described below. Analyses of the sequences and homology searches were performed with the IntelliGenetics (Mountain View, Calif.) Suite program (release 5.4) and the Blast network service at the National Center for Biotechnology Information (1).
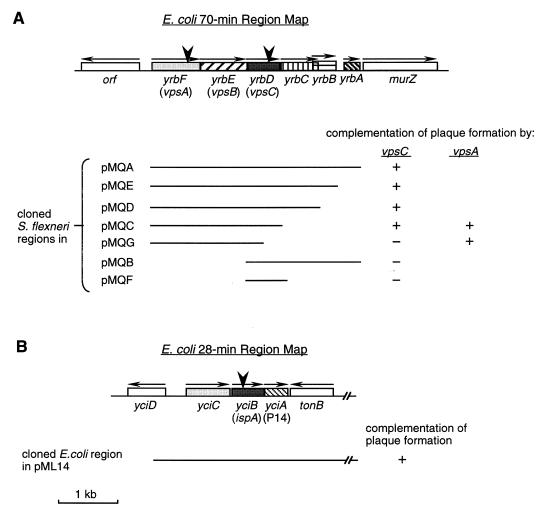
FIG. 1.
Maps of the 70- and 28-min regions of E. coli and S. flexneri. These maps are in accordance with E. coli chromosomal sequences at the 70-min (A) and 28-min (B) regions to which S. flexneri sequences are highly homologous (60; also this study) (GenBank accession no. U18997). Boxes with different patterns represent ORFs or genes that were characterized. Horizontal arrows above ORFs indicate the putative directions of transcription. yrb and yci designations are those of Plunkett (47) and Stoltzfus et al. (59). vps genes were named in this study, and ispA was identified by Mac Síomóin et al. (27), while P14 was named by Postle and Good (50). SA2122 had the TnphoA inserted after the codon for isoleucine at amino acid position 127 of VpsC, VpsA is interrupted in SA4100 by insertion of aphA-3 after the codon for arginine at amino acid position 241, and SA2054 had TnphoA inserted after the codon for threonine at amino acid position 65 of IspA. Insertions are indicated by large arrowheads. Lines beneath the maps represent the wild-type regions cloned in the indicated recombinant plasmids. All pMQ plasmids carried wild-type S. flexneri sequences, while pML14 had the wild-type E. coli sequence. The complementation results in the plaque assays are shown.
Construction of recombinant plasmids and strains.
To clone various portions of the wild-type S. flexneri yrb region, PCR was carried out with SA100 chromosomal DNA by using the Pfu DNA polymerase (Stratagene, La Jolla, Calif.) with the following primers designed from E. coli sequences: primer C (5′-AACTGCAGTCAACGCAAGACGAAGGG-3′), which hybridizes upstream of vpsA (yrbF) (Fig. 1); primer D (5′-AACTGCAGCCCATAGCTCAAAAGCCG-3′), which hybridizes downstream of yrbA; primer E (5′-TTGGGAATTGAGTTCATGC-3′), which hybridizes upstream of vpsC; primer F (5′-AACTGCAGATCACCAGCAAAGCGACC-3′), which binds downstream of vpsC; primer G (5′-AACTGCAGATCCAGCTCAGTGACTCG-3′), which binds downstream of yrbC; primer H (5′-AACTGCAGGGTTAACGCCATATCCGG-3′), which binds downstream of yrbB. All primers except primer E contained a 5′ PstI site. PCR was carried out with C-D, C-F, C-G, C-H, E-D, or E-F primer combinations. The PCR products were digested with PstI and cloned into pBR322 at the PstI site, except the one obtained from primer pair E-D, which was cloned into pBR322 digested with PstI and ScaI. The open reading frames (ORFs) were inserted in the orientation of bla transcription and designated the pMQ series, as shown in Fig. 1A. pMQF was generated by deleting the 1.3-kb SacII-PstI fragment from pMQB, while pMQG was obtained by deleting the 472-bp SnaBI-PmeI fragment from pMQC. Because pMQB failed to complement the vpsC mutant, sequence analysis of pMQB was performed to confirm that the sequence matched the chromosomal DNA sequence. The vpsC mutant SA5122 was transformed with each of the pMQ plasmids by using the method of Dagert and Ehrlich (8) as modified by Hromockyj et al. (22).
To generate pML14, the bla gene was inserted into pRZ526 (51), which contains the E. coli yciC-tonB region, as follows. A 1.47-kb BamHI-BstBI fragment of pACYC177 that carries the bla gene was filled in with the DNA polymerase I Klenow fragment (New England Biolabs, Inc., Beverly, Mass.) and ligated to pRZ526 that was digested with HindIII and also filled in.
Protein analyses.
The cytosol, cytoplasmic membrane, and outer membrane were isolated by the methods of Inouye and Guthrie (23) and of Filip et al. (14), and the proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with 12% polyacrylamide (24). Proteins in the supernatants of exponential-phase LB or ISM cultures were precipitated with 10% (wt/vol) trichloroacetic acid and solubilized in Tris-SDS (1 M Tris-HCl [pH 8.0], 2% [wt/vol] SDS) as described previously (48). Samples of supernatant proteins corresponding to 400 μl of bacterial culture (A650 = 1.0) were separated by SDS-PAGE (12%) and detected by silver stain or by immunoblotting. The S. flexneri antisera used for immunoblotting analyses were convalescent-phase sera from monkeys infected with S. flexneri, rabbit polyclonal antibodies against IcsA (VirG) or IpaD, and mouse monoclonal antibodies recognizing IpaB (2F1) or IpaC (2G2). All S. flexneri antisera were provided by Edwin Oaks (Walter Reed Army Institute of Research, Washington, D.C.). Polyclonal (5 Prime → 3 Prime, Boulder, Colo.) and monoclonal (Chemicon, Inc., Temecula, Calif.) antibodies were used to detect alkaline phosphatase (PhoA) fusion proteins.
To determine PhoA activity, bacteria were grown to exponential phase in LB (in vitro sample) or harvested from infected Henle cells (intracellular sample) and enzyme activity was determined by the method of Brickman and Beckwith (5).
In vitro translations were performed by use of an E. coli in vitro transcription-translation kit (Promega) by following the protocol provided by the supplier. Proteins synthesized in the reaction were labeled with [35S]methionine (NEN, Boston, Mass.) and analyzed by SDS-PAGE.
LPS analysis.
LPS was isolated, and its structure was analyzed by SDS-PAGE as described previously (21). Two indirect measures of LPS structure, serum sensitivity and crystal violet sensitivity, were also measured as described previously (21).
SDS sensitivity assay.
Bacterial sensitivity to SDS lysis was measured as described by Stathopoulos et al. (58) with the following modifications. Cultures were grown in LB for 1 h and then divided, and where indicated, 0.01% Congo red, 0.01% hemin, or 0.1% (wt/vol) DOC was added. Cultures were grown to early stationary phase, centrifuged at 14,000 × g for 3 min, and resuspended in 25 mM Tris-HCl (pH 7.5) to an A595 of 1.2. SDS (0.1%) was then added to one set of samples of each strain, while the other set of samples was left untreated. Samples were incubated at 37°C without aeration. The ratio of A595 at each time point (Tn) to A595 at time zero (T0) was expressed as a percentage (% relative A595). Strains SA5122(pMQC) and SA5122(pMQB) grown in the presence of tetracycline were extremely sensitive to SDS, probably due to the membrane instability caused by induction of tetracycline pumps (12), so these strains were grown in the absence of antibiotic, and maintenance of the recombinant plasmids was determined by plating on media with or without tetracycline following incubation.
RNase I leakage and antibiotic sensitivity assays.
The leakage of the periplasmic protein RNase I from bacterial cells was assayed by using plate cultures grown at 37°C as described by Lopes et al. (26). Bacterial sensitivity to antibiotics was tested by overlaying L agar plates with soft L agar containing 100 μl of an overnight culture and measuring the zones of inhibition around disks containing the following antibiotics or dye: tetracycline, 30 μg; gentamicin, 10 μg; chloramphenicol, 30 μg; carbenicillin, 30 μg; crystal violet, 10 μg.
Indirect immunofluorescence labeling of IcsA (VirG) on the bacterial surface.
To determine the presence and distribution of IcsA (VirG) on the bacterial cell surface, indirect immunofluorescence labeling was performed on bacterial suspensions and the bacteria were air dried on slides as described by Van Den Bosch et al. (67). Labeling and observations were done as described above, except that a polyclonal rabbit anti-IcsA antiserum was used as the primary antibody. The location of IcsA was determined by comparing the phase-contrast field for bacteria and those of the fluorescence field for IcsA staining.
RESULTS
Generation of TnphoA mutants defective in plaque formation in cultured cells.
We previously constructed a collection of S. flexneri TnPhoA insertion mutants that invaded cultured epithelial cells but failed to form plaques in confluent cell monolayers, indicating defects in intracellular survival, multiplication, or cell-to-cell spread (21). Analysis of two additional mutants, SA2122 and SA2054 (Table 2), is reported here. SA2122 had an insertion in a gene not previously identified as involved in Shigella virulence, and it has been designated a vpsC mutant for its altered virulence protein secretion phenotype (Vps−), as described below. SA2054 had an insertion in ispA, a gene involved in intracellular septation. The ispA insertion mutant is similar, but not identical, to that described by Mac Síomóin et al. (27).
TABLE 2.
Characteristics of S. flexneri mutants
| Strain | Genotype | % Invasiona | Plaque formationb |
|---|---|---|---|
| SA511 | Parental strain | 92.0 ± 4.1 | + |
| SA2122 | vpsC::TnphoA | 88.2 ± 2.5 | P |
| SA5122 | vpsC::TnphoA | 83.0 ± 5.6 | P |
| SA4100 | vpsA::aphA-3 | 79.3 ± 3.8 | P |
| SA2054 | ispA::TnphoA | 63.3 ± 18.9 | − |
Percentage of Henle cells infected with 3 or more bacteria. At least 300 cells were observed per experiment, and means ± standard deviations of three experiments are shown.
+, 1.5- to 2.0-mm-diameter plaques; P, pinpoint (<0.2-mm-diameter) plaques; −, no plaques.
Henle cell invasion and intracellular multiplication of the vpsC mutant.
The vpsC mutant SA2122 produced plaques that were less than 0.2 mm in diameter, as compared to wild-type plaques of 1.5 to 2.0 mm (Table 2). There was no increase in the size of the plaques produced by the mutant, even after prolonged incubation. The vpsC mutation in SA2122 was transduced into a wild-type SA100 background to ensure that the strain contained no additional, uncharacterized mutations. The resulting strain, SA5122, had the same defect in plaque formation (Table 2).
Although the mutant failed to form normal plaques, it was similar to the wild-type parent strain in an invasion assay (Table 2). Invasion was verified at 2 h postinfection by indirect immunofluorescence labeling of intact, infected Henle cells with an antibody against S. flexneri. The number of both SA100 and SA5122 bacteria detected by this procedure, which labels extracellular but not intracellular bacteria, was found to be less than 5% of the total number of bacteria visualized by phase-contrast microscopy (data not shown), indicating that the majority of bacteria were intracellular.
Defects in IcsA localization, LPS synthesis, peptidoglycan synthesis, and intracellular septation all have been associated with failure to form plaques. Results indicating that SA5122 is unaffected in these functions are summarized in Table 3, along with results of other assays described in more detail below.
TABLE 3.
Phenotypes of Vps strains
| Strain | Phenotype | IcsA localizationa | Intracellular septationb | Colony morphologyc | SDS sensitivityd | Serum sensitivitye | Carbenicillin MIC (μg/ml) | Antibiotic and dye sensitivityf | LPS structureg |
|---|---|---|---|---|---|---|---|---|---|
| SA100 | Wild type | Polar | WT | S | R | 0.51 | 20 | WT | WT |
| SA5122 | VpsC− | Polar | WT | W | S | 0.76 | 20 | WT | WT |
| SA5122(pMQA) | VpsC+ | Polar | WT | S | R | NDh | 20 | ND | ND |
| SA5122(pMQB) | VpsC− | Polar | WT | W | S | ND | 20 | ND | ND |
| SA4100 | VpsA− | ND | WT | W | S | ND | ND | ND | ND |
Cells were observed by microscopy after indirect immunofluorescence labeling with anti-IcsA antiserum. Polar, the strain displayed equivalent amounts of IcsA as the wild-type strain did at one pole of each cell.
WT, wild-type Shigella cell morphology and septation during growth within Henle cells.
S, smooth and glossy; W, wrinkled, rough.
R, the A550 in the presence of SDS was ≥75% of the A550 in the absence of SDS; S, the A550 was reduced by more than 25% after 1 h of incubation.
Serum sensitivity expressed as log10 kill = (log10 CFU per ml after 2 h in the absence of serum) − (log10 CFU per ml after 2 h in the presence of 10% normal human serum) (21).
Sensitivities to gentamicin, tetracycline, chloramphenicol, and crystal violet were compared to those of the wild type.
WT, wild-type amount and chain length distribution of LPS.
ND, not determined.
Survival and multiplication of the vpsC mutants within Henle cells were assessed by infecting the monolayer and determining the number of intracellular bacteria at various times after infection (Table 4). The average numbers of intracellular bacteria observed in stained monolayers were equivalent in the vpsC mutant and the parental strain, and there was an increase with time in the number of vpsC bacteria inside the infected Henle cells, suggesting that the mutant was not defective in survival or multiplication inside tissue culture cells during the first 3 h after invasion (Table 4). However, there was a difference between the mutant and wild type in the number of the intracellular bacteria recovered by harvesting and plating on agar medium; the number of vpsC mutant CFU recovered was less than 3% of the intracellular bacteria counted by microscopic observation, while approximately 80% of the intracellular SA511 (parental strain) bacteria were recovered under the same conditions (Table 4). The difference between the number of vpsC CFU recovered per infected cell and that of the wild-type parental strain was significant (P < 0.02) in each experiment, although the range of numbers varied among experiments (Table 4 and data not shown). Failure to recover the vpsC bacteria did not appear to be associated with the manipulations involved in harvesting the bacteria. A mock infection in which the bacteria were exposed to the same conditions used to harvest bacteria from the infected Henle cells had no significant effect on the viability of the vpsC mutant (data not shown).
TABLE 4.
Bacterial invasion of Henle cells and recovery of bacteria from cells
| Expt and strain | Time postinfection (h) | Invasion ratioa | No. of bacteria counted/ infected Henle cellb | Bacterial CFU recovered/infected Henle cellc |
|---|---|---|---|---|
| Expt 1 | ||||
| SA511 (WTd) | 2 | 1.00 | 13.8 ± 0.7 | 10.7 ± 4.5 |
| 3 | 1.08 | 43.8 ± 7.4 | 33.9 ± 8.0 | |
| SA2122 | 2 | 1.01 | 11.7 ± 2.4 | 0.2 ± 0.1 |
| 3 | 1.09 | 34.4 ± 4.5 | 0.9 ± 0.9 | |
| Expt 2 | ||||
| SA100 (WTd) | 2 | 1.00 | ND | 31.3 ± 4.8 |
| SA5122 | 2 | 0.81 | ND | 3.2 ± 0.3 |
| SA5122(pMQA) | 2 | 0.83 | ND | 30.2 ± 5.1 |
| SA5122(pMQB) | 2 | 0.82 | ND | 6.7 ± 1.4 |
Invasion ratio = percentage of Henle cells invaded by the mutant/percentage of cells invaded by the parental strain.
Values are average numbers of intracellular bacteria counted in three separate fields of at least 20 infected Henle cells per field ± standard deviations.
Bacterial CFU recovered per infected Henle cell = (total bacterial CFU recovered per plate)/(total number of Henle cells harvested per plate × percentage of Henle cells infected).
WT, wild type.
Sequence analysis of DNA flanking the TnphoA insertion in vpsC and cloning of the wild-type Shigella genes.
The DNA sequence upstream of phoA in the vpsC mutant SA2122 revealed that TnphoA was inserted into a gene with 98% nucleotide identity to an ORF of unknown function located at 70 min on the E. coli K-12 chromosome (yrbD) (GenBank accession no. U18997; GenPept accession no. P45391) (47). In E. coli, yrbD is one of six ORFs (yrbABCDEF) (Fig. 1A) contained on the same strand immediately upstream of murZ, a peptidoglycan synthesis gene (29). The wild-type S. flexneri chromosomal DNA was amplified by PCR by using primers designed with the E. coli sequence, and the PCR fragments were cloned into the β-lactamase gene of pBR322 in the same orientation as bla transcription, yielding pMQA to -G (Fig. 1A). The genetic organization of the region was the same in S. flexneri and E. coli, and the DNA sequences of the regions were almost identical. The only amino acid difference in the VpsC and YrbD sequences was the serine at position 38 of VpsC in S. flexneri instead of the proline at the same position in E. coli YrbD (GenPept accession no. P45391). vpsC and the two ORFs immediately upstream are closely linked, potentially forming an operon. No sequence with homology to the E. coli ς70 consensus promoter was found upstream of any of these three genes nor were there obvious terminator-like sequences at the end of any of these genes.
If vpsC and the two upstream ORFs form an operon, the three gene products might function together or in the same pathway in the formation of plaques by S. flexneri. To test this, a nonpolar aphA-3 cassette (34) insertion mutation in vpsA (yrbF) was constructed. The mutant, SA4100, had the same phenotype as the vpsC mutants (Table 2). This suggests that the products of the upstream genes function in concert with VpsC in Shigella plaque formation. Therefore, we have designated these genes vpsA and vpsB.
Complementation of the vps mutations.
Plasmids containing vps sequences were tested for complementation of the vpsA and vpsC mutations. The results indicated that sequences in addition to the vpsC sequence were required for complementation of the vpsC mutations (Fig. 1A; Table 4). For example, a plasmid containing the entire Shigella vps (yrbA to -F) region (pMQA) complemented the defect in plaque formation, but a plasmid containing only the vpsC gene (pMQF) did not complement the defect (Fig. 1A). Analysis of additional clones showed that it was the upstream genes, vpsA and vpsB, that were required along with vpsC for complementation of the vpsC mutation; truncation of vpsAB (in pMQB and pMQF) or vpsC (in pMQG) abolished the complementation (Fig. 1A). Similar results were obtained in testing for complementation of the reduced recovery from Henle cells; pMQA (carrying vpsABC) complemented the low-recovery phenotype of the vpsC mutant, while pMQB, which lacked vpsA and vpsB, did not (Table 4). None of the downstream genes, yrbCBA, were required for complementation of the vpsC mutation (Fig. 1A). This result indicates that the defect in plaque formation of the vpsC mutant was not due to a polar effect of the mutation on the downstream genes.
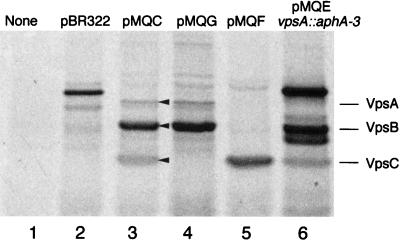
The requirement of the upstream genes for complementation suggested that expression of vpsC in these clones may be dependent upon upstream sequences. However, in vitro transcription and translation of pMQF indicates that this plasmid expresses vpsC (Fig. 2, lane 5), although in vitro transcription does not necessarily imply in vivo expression.
FIG. 2.
In vitro translation of proteins encoded by plasmids from the vpsABC region. [35S]methionine-labeled proteins produced in an E. coli extract were separated on an SDS–12.5% polyacrylamide gel and visualized by autoradiography. Plasmid DNA templates were as follows: none (lane 1), pBR322 (lane 2), pMQC (vpsABC) (lane 3), pMQG (vpsAB) (lane 4), pMQF (vpsC) (lane 5), pMQEvpsA::aphA-3 (vpsBC) (lane 6). The arrowheads show the positions of the VpsA, VpsB, and VpsC proteins.
Both pMQC and pMQG complemented the vpsA mutation in the plaque assay (Fig. 1A). The vpsA mutant containing pMQC produced smaller plaques than that containing pMQG, however. It is not clear why the full-length clone would be less effective in complementing this mutation than in complementing the vpsC mutation, unless the increased amount of VpsC contributed by the plasmid is detrimental to the vpsA mutant.
Structure of Vps proteins and cellular location of VpsC.
The predicted protein sequence of VpsA has extensive homology to the sequence of many ABC transporter proteins (GenPept accession no. P45393) and has a conserved ATP-binding site (GXXGXGKX), suggesting that vpsA, the first ORF in this region, encodes a member of an ABC transporter family. VpsB has four stretches of hydrophobic residues which may be transmembrane domains, suggesting that VpsB is an integral membrane protein. VpsC appears to have a leader peptide, since the amino terminus has positively charged residues followed by a hydrophobic region and a consensus leader peptidase cleavage site. The remainder of the VpsC protein is relatively neutral, with no obvious transmembrane domains or highly charged regions. The proteins encoded by vpsABC were synthesized by in vitro transcription and translation, separated by SDS-PAGE, and visualized by autoradiography (Fig. 2). Proteins of the predicted sizes for VpsA (29 kDa), VpsB (27.8 kDa), and VpsC (19.5 kDa) were produced when pMQC was the template (Fig. 2, lane 3). The identity of each band was confirmed by comparison with the proteins produced from templates lacking one or more of the genes (Fig. 2). Interestingly, the amount of VpsA produced relative to that of VpsB and VpsC was low. VpsA and VpsB have similar numbers of methionines (13 and 12, respectively), yet the amount of [35S]methionine in the band corresponding to VpsA was much less than that in VpsB and VpsC. The lower intensity of the VpsC band relative to the VpsB band was expected, given that VpsC has only two methionines. It is not known whether VpsA is produced in lower amounts than VpsB and VpsC within the bacterial cell or if this is an artifact of the in vitro system.
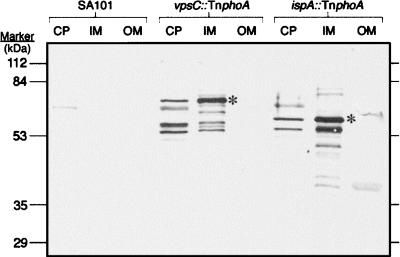
The characteristics of the amino acid sequence and the fact that the TnPhoA insertion in vpsC had a PhoA-positive phenotype suggests that VpsC is a periplasmic protein or a membrane protein with a region exposed to the periplasm. To determine the location of VpsC within the bacterial cell, cell fractions were separated and the VpsC-PhoA fusion protein was detected by immunoblotting with a monoclonal antibody against PhoA (Fig. 3). The results indicated that the VpsC-PhoA fusion protein was located in the S. flexneri cytoplasmic membrane fraction. Bands were also detected in the fraction containing the cytoplasmic and periplasmic proteins; these may represent cytoplasmic VpsC that has not been exported to the membrane as well as degradation products. Additional bands that also may represent degradation products were observed in the cytoplasmic membrane fraction. No bands were seen in the outer membrane fractions (Fig. 3). Thus, it is likely that VpsC is a cytoplasmic membrane protein, although it is possible that the VpsC-PhoA fusion protein does not have the same cellular location as VpsC.
FIG. 3.
Localization of PhoA fusion proteins by immunoblot analysis. Cells were fractionated, and samples of the cytosol and periplasm (CP), inner membrane (IM), and outer membrane (OM) were analyzed by SDS-PAGE. The proteins were transferred to nitrocellulose, and the PhoA fusion proteins were detected with a monoclonal antibody against PhoA. Lanes 1 to 3, SA101 (VpsC+ IspA+ PhoA−); lanes 4 to 6, SA2122 (vpsC::TnphoA); lanes 7 to 9, SA2054 (ispA::TnphoA). Asterisks indicate the bands of the expected size of each PhoA fusion protein.
Phenotypes associated with vps mutations: altered colony morphology and hypersecretion of virulence proteins.
In comparing the vps mutants to the wild-type strain, it was noted that the mutant strains had an altered colony morphology on Congo red agar. The wild-type colonies were smooth and glossy, while both the vpsA and vpsC mutants formed colonies that had a rough, wrinkled appearance (Table 3). The complementing plasmids that restored plaque formation also restored the wild-type colony morphology, whereas those that did not complement had no effect on the wrinkled colony morphology (Table 3).
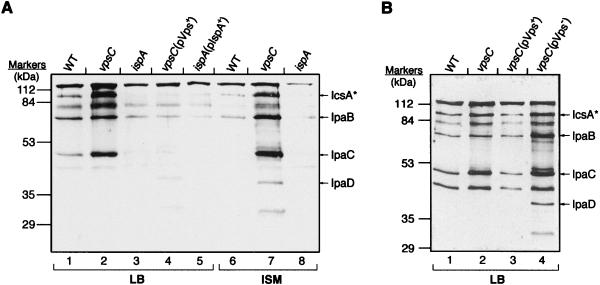
The altered colony morphology of the vps mutants suggested a cell wall or cell surface alteration. Because vpsA encodes a potential ABC transporter, it was possible that the colony morphology, as well as the defect in plaque formation, was associated with an effect on protein translocation or secretion. To test this, proteins in the culture supernatants of various strains were analyzed by immunoblotting with a monkey convalescent-phase antiserum that detects the S. flexneri major virulence antigens, IpaB, IpaC, IpaD (19, 38), IcsA, and IcsA* (the cleaved and secreted form of IcsA) (16) (Fig. 4). The identity of each band in the immunoblots was confirmed by using antisera against the individual proteins (data not shown). Analysis of the secreted proteins showed that the vpsC mutant secreted larger amounts of the virulence proteins than did the wild-type strain (Fig. 4). The altered virulence protein secretion phenotype (Vps−) was the basis for designating the interrupted gene, vpsC. The increased amounts of the virulence proteins in the supernatants were due to increased secretion rather than increased synthesis of these proteins; the total amounts of virulence proteins were similar in whole-cell lysates of the wild-type and the vpsC mutant strains (data not shown). Because induction of tetracycline resistance can cause instability of the bacterial outer membrane (12), antibiotics used to maintain the plasmids were omitted from one set of cultures to show that hypersecretion was not associated with the presence of antibiotics (Fig. 4B).
FIG. 4.
Immunoblot analysis of proteins in culture supernatants. Cultures were grown either in the presence (A) or in the absence (B) of the antibiotics to which the strains are resistant, and proteins were precipitated from culture supernatants. Proteins from equivalent numbers of cells were separated on an SDS–12% PAGE gel and then analyzed by immunoblotting with monkey convalescent-phase antiserum. Lanes containing strains grown in LB or ISM are indicated at the bottom of the lanes. The positions of the Ipas and IcsA* are indicated to the right of the gels. (A) Lanes 1 and 6, wild-type SA100; lanes 2 and 7, SA5122; lanes 3 and 8, SA2054; lane 4, SA5122(pMQC); lane 5, SA2054(pML14); (B) lane 1, SA100; lane 2, SA5122; lane 3, SA5122(pMQC); lane 4, SA5122(pMQB).
The hypersecretion phenotype was complemented by the plasmid that restored plaque formation ability, pMQC (pVps+) (Fig. 4A, lane 4; Fig. 4B, lane 3), but not by pMQB, a plasmid that did not restore plaque formation (pVps−) (Fig. 4B, lane 4). The vpsA mutant also hypersecreted the virulence proteins (data not shown). Thus, there was a correlation between protein hypersecretion and the defective cell-to-cell spread phenotype in the vps mutants. The Vps− hypersecretion phenotype was observed both in rich medium (LB) and in ISM (Fig. 4A), suggesting that hypersecretion of virulence proteins may occur during growth within cultured cells as well as in vitro.
Effect of vps mutations on membrane permeability.
The proteins that were secreted in the vpsC mutant included both IcsA, which is transported via the general secretory pathway, and Ipa proteins, which are specifically exported through a type III secretory apparatus (56). This fact, coupled with the observations that SA5122 was not readily recovered from the intracellular environment and that it had an altered colony morphology, suggested the possibility that the vpsC mutation had an effect on S. flexneri membrane permeability or cell wall integrity. Assays used to detect potential defects included measuring penicillin sensitivity as an indicator of peptidoglycan function (43); analysis of expression and distribution of the major membrane proteins by SDS-PAGE; analysis of LPS structure; measurement of sensitivity to antibiotics, dyes, and detergents; and screening for leakage of RNase I from the periplasm (Table 3 and data not shown). The vpsC mutants were indistinguishable from the wild type in all of these assays except sensitivity to lysis by SDS (Table 3). The vps mutants were more sensitive to SDS than the wild type (Fig. 5). This did not reflect a general detergent sensitivity, since exposure to DOC did not result in cell lysis or loss of viability (data not shown). The increased SDS sensitivity of SA5122 was abrogated by introduction of pMQC, which also complemented the other phenotypes (Vps+), but not by pMQB, which failed to complement the other phenotypes (Vps−) (Fig. 5A). Increased SDS sensitivity indicated that the vpsC mutation altered S. flexneri membrane function but did not increase outer membrane permeability to the extent that antibiotics were able to enter the cell more freely or that proteins of the size of RNase I (31 kDa) (31) could leak out. Because the Ipa and IcsA proteins are larger than RNase I, it is unlikely that the increased amount of these proteins in the supernatant was due to increased leakage.
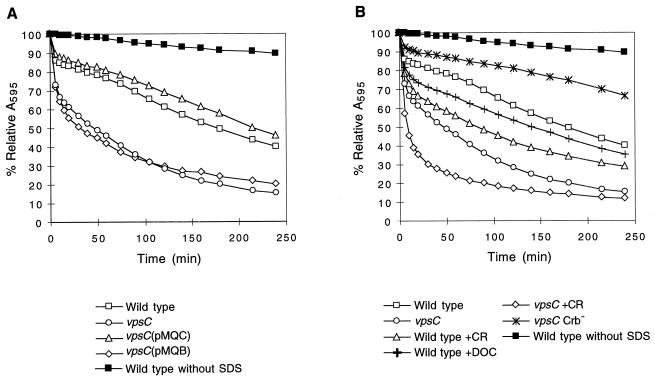
FIG. 5.
Effect of vpsC and protein secretion on SDS sensitivity of S. flexneri. Bacteria were grown to early stationary phase in the absence of antibiotics. Congo red dye or DOC was added to certain samples, as indicated below the graphs, at 1 h after inoculation. The cells were washed, and 0.1% SDS was added (T0). A595 was determined at various time points. % Relative A595 = A595 at Tn/A595 at T0. Three or more samples of each strain and each condition were tested, and the means of the results were plotted. Standard deviations are ≤1.7%. The relative absorbance of the wild type, SA100, incubated in the absence of SDS is shown in each panel for comparison. Results for the following strains are shown: (A) Wild type, SA100; vpsC mutant, SA5122; vpsC(pMQC), SA5122(pMQC); vpsC(pMQB), SA5122(pMQB); (B) wild type, SA100; vpsC mutant, SA5122; wild type +CR, SA100 grown with 0.01% Congo red; wild type +DOC, SA100 grown with 0.1% DOC; vpsC +CR, SA5122 grown with 0.01% Congo red; vpsC Crb−, SA5122W.
Effect of Congo red, DOC, and hemin on SDS sensitivity.
It was possible that the increased SDS sensitivity in the vps mutants was a direct result of hypersecretion of the Ipa proteins. To determine this, the wild-type strain was tested for SDS sensitivity following growth under conditions that enhance Ipa secretion (Fig. 5B). SA100 was grown in the presence of DOC (48), Congo red (44), or hemin prior to testing SDS sensitivity. The result showed that growth in medium containing Congo red or DOC (Fig. 5B) or hemin (data not shown) increased SDS sensitivity of the wild-type strain. These data indicate that enhancement of protein secretion by mechanisms other than mutation of vpsC increased SDS sensitivity. However, the effects of these compounds were not as great as the effect of the vpsC mutation in causing increased lysis by SDS (Fig. 5B), and growth in the presence of Congo red further increased the SDS sensitivity of the vpsC mutant (Fig. 5B). This suggested that the mechanisms for the increase in SDS sensitivity by Congo red and the vpsC mutation are not identical, although there may be some overlap in the targets or mechanisms through which the vpsC mutation and Congo red induce SDS sensitivity.
Congo red or hemin binding (Crb+) is a characteristic of virulent Shigella, and Crb− strains are avirulent (9, 45). To test whether the ability of S. flexneri to bind these compounds is correlated with SDS sensitivity, a Crb− virulence plasmid deletion derivative of the vpsC mutant was isolated and assayed. The result showed that the Crb− vpsC strain was more resistant to SDS than the Crb+ vpsC strain, indicating that loss of Congo red-binding ability suppressed the SDS sensitivity phenotype of the vpsC mutant (Fig. 5B). This may reflect reduced virulence protein secretion due to the deletion in the plasmid or other changes in cell wall structure and function.
Isolation of a putative ispA mutant.
SA2054, a second TnphoA insertion mutant, invaded Henle cells but did not form plaques (Table 2). This mutant appeared to multiply as well as the wild type during the first 3 h postinfection, as determined by counting bacteria in stained monolayers and by harvesting and plating the intracellular bacteria (data not shown). Between 3 and 6 h postinfection, however, SA2054 formed long filaments inside the Henle cells (data not shown), indicating defective bacterial septation. After 6 h postinfection, bacteria again appeared normal (data not shown), but invasion of adjacent cells did not occur (Table 2). The failure to form plaques despite the apparent recovery of normal septation after 6 h suggests that there may be cell changes in addition to the septation defect that lead to the loss of intercellular spread.
The DNA sequence upstream of phoA in SA2054 has very high homology to an E. coli K-12 ORF at 28 min, yciB (60) (GenPept accession no. P21366) (Fig. 1B). A recombinant plasmid carrying the wild-type E. coli yciC-tonB region, pML14, restored the plaque-forming ability in the mutant (Fig. 1B). Both sequence and phenotypic analyses suggested that SA2054 was interrupted in ispA (defective intracellular septation), a gene described previously by Mac Síomóin et al. (27).
Sequence homology searches did not reveal any other genes of known functions with strong homologies to ispA or the two closely linked ORFs, yciA and yciC. ORFs homologous to yciABC were found in Haemophilus influenzae. tonB is closely linked to yci in E. coli and S. flexneri, but a tonB-homologous ORF is not present in the same region of the H. influenzae chromosome (15).
Localization of IspA.
The putative location of IspA in the bacterial cell was determined by detecting the IspA-PhoA fusion protein in immunoblots of cell fractions. A fusion protein of the predicted size (56 kDa) was found in the S. flexneri cytoplasmic membrane, with smaller amounts found in the cytosol fraction (Fig. 3). Additional bands in the inner membrane and cytosol fractions may represent degradation products and cross-reacting proteins. The minor bands detected in some outer membrane fractions with this monoclonal antibody were not observed when polyclonal antiserum against PhoA was used (data not shown), suggesting that these are not IspA-PhoA fusion proteins (Fig. 3).
Effect of the ispA mutation on protein secretion and IcsA localization.
Protein secretion by the ispA mutant was studied (Fig. 4A). The ispA mutant secreted slightly less protein than the wild type (Fig. 4A, lanes 4 and 8), but the amount was similar to that secreted by the ispA mutant containing pML14, which has the wild-type ispA sequence (pIspA+) (Fig. 4A, lane 5). All of the membrane permeability assays described above were performed on the ispA mutant, and they did not demonstrate any difference between the ispA mutant and the wild-type strain, suggesting normal membrane structure of the ispA mutant (data not shown).
The ispA mutant also was analyzed by indirect immunofluorescence for the presence and localization of IcsA on the cell surface. The results showed that the ispA mutant SA2054 had normal polar localization of IcsA, although the levels of fluorescence observed with SA2054 and SA2054(pML14) were slightly less than that observed with the wild type (data not shown).
Expression of vpsC and ispA under different environmental conditions.
Environmental conditions such as temperature and osmolarity have been shown to influence the expression of S. flexneri virulence genes (2, 30). To determine whether vpsC and ispA might be regulated by some of these same conditions, the vpsC::TnphoA and ispA::TnphoA fusions were used as reporter genes to study the regulation of vpsC and ispA expression. Among the environmental conditions tested were temperature (37 versus 30°C), pH (pH 5.5 to 7.5), iron (iron rich or iron depleted), DOC, O2 (aerobic versus anaerobic), osmolarity (0 to 10% sucrose), nutrients, reducing agents, and extracellular versus intracellular environment. None of these conditions affected the expression of vpsC::TnphoA or ispA::TnphoA (data not shown). Additionally, the vpsC and ispA mutants grew as well as the wild type did under all the conditions tested, indicating that neither VpsC nor IspA was required for survival under any of these conditions. It was reported that the ispA mutant was defective in growth under reducing conditions in ISM (27). We measured the MIC of the reducing reagent 2-mercaptoethanol for the wild-type and ispA mutant strains grown in ISM and found that it was 50 mM for both strains.
DISCUSSION
In this study, we identified two S. flexneri chromosomal loci involved in intercellular spread. A mutation in vpsC resulted in reduced recovery of bacteria from infected tissue culture cells, increased bacterial protein secretion, and increased sensitivity to detergent lysis. These phenotypes have not been reported for other Shigella virulence genes, suggesting that the vpsC gene acts through a novel virulence mechanism. Another gene, yciB, was required for normal septation inside tissue culture cells and was found to be the same gene as ispA, which was reported by Mac Síomóin et al. (27).
The vpsC mutant strains were as invasive as the wild type but formed extremely small plaques. The number of vpsC CFU recovered from infected tissue culture cells was only 1 to 10% of those of the wild type, although the average number of intracellular mutant bacteria observed by microscopy was equivalent to the wild type. The vpsC mutant was probably not defective in survival inside cells but was unable to survive isolation from infected cells. Decreased viability of the vpsC mutant could not be detected under any of the in vitro growth conditions tested, suggesting that the effect was specific to bacteria passaged through the cytoplasm of cultured cells. The vpsC mutant, however, did show an altered colony morphology. On Congo red plates, the mutant colonies appeared rough and wrinkled, as compared to the glossy and smooth wild-type colonies. This rough colony morphology was similar to that of the mutants lacking LPS O-antigen side chains, but the LPS distribution pattern and serum resistance of the vpsC mutant were normal.
The vpsC mutant secreted larger amounts of virulence proteins than the wild type did. The hypersecreted proteins include the Ipa proteins, which are exported via a type III secretory pathway, and IcsA, which is secreted through the cytoplasmic membrane by the general secretory pathway (61). Therefore, the vpsC mutation had a general effect on protein translocation rather than a specific effect on a single secretory pathway. This effect was probably different from the increased protein secretion specifically through the type III secretory pathway Mxi-Spa that has been observed in S. flexneri grown in the presence of epithelial cells (34), fetal bovine serum (33), Congo red (44), or bile salts (48). The increased protein secretion phenotype of the vpsC mutant was observed with bacteria grown in ISM, the medium mimicking the intracellular condition, indicating that increased protein secretion also is likely to occur during intracellular growth. Our previous studies (20) indicated that production of at least two virulence proteins, IpaB and IpaC, was reduced during intracellular growth of the wild-type strain, suggesting that synthesis or secretion of the virulence proteins is regulated within the host cell. Failure to control Ipa or IcsA secretion could result in premature lysis of the host cell or otherwise reduce the efficiency of the spread of shigellae to adjacent cells.
The low recovery rate and the protein hypersecretion phenotypes of the vpsC mutant, along with its altered colony morphology, suggested that the defect could be in a bacterial surface structure. The increased sensitivity of the vpsC mutant to SDS lysis also suggested a membrane defect, since increased SDS sensitivity has been correlated with increased outer membrane permeability in other studies (58). However, the RNase I leakage and the antibiotic sensitivity experiments did not detect any difference between the vpsC mutant and the wild type, indicating that the increased membrane permeability in the vpsC mutant was not sufficient to cause leakage of proteins of the size of RNase I or to allow increased entrance of antibiotics (31). This made it unlikely that Ipa and IcsA proteins leaked out, because these proteins are larger than RNase I. In addition, the amounts of both major outer membrane porins OmpC and OmpF in the vpsC mutant and the wild type were similar, suggesting that the increased outer membrane permeability of the vpsC mutant was not due to changes in the amounts or ratio of porins.
Experiments to determine the effects of the vps mutations on detergent sensitivity led to the observation that Congo red and DOC, both of which enhanced Ipa secretion, increased sensitivity of wild-type S. flexneri to SDS lysis. This result indicated that there is a correlation between enhancement of protein secretion and increased sensitivity to detergents.
Complementation of the vpsC mutant with cloned wild-type S. flexneri chromosomal regions showed that DNA sequences downstream of vpsC were not required but that two upstream genes, vpsA and vpsB, together with vpsC, were required for complementation of the vpsC mutation. vpsABC are closely linked, with only 7 bases between the stop codon of vpsA and the start codon of vpsB and 4 bases between vpsB and vpsC. These observations suggest that the three genes are likely to form an operon, with transcription starting upstream of vpsA, and that expression of vpsC may rely on upstream sequences. However, pMQF, which contains only the vpsC ORF and did not complement the vpsC mutation, does appear to produce VpsC when analyzed by an in vitro transcription-translation system. This suggested that the products of these genes might function together and that the ratio of the VpsABC proteins is important. Overproduction of VpsC from the plasmid relative to the amounts of VpsA and VpsB produced from the chromosomal genes could be detrimental. If these genes do form an operon and the gene products have a common function, we anticipated that a mutant defective in vpsA would have the same phenotype as a vpsC mutant. Although the vpsA mutant has not been characterized as extensively as the vpsC mutant, it appeared to have the same phenotype; it failed to form plaques in Henle cell monolayers, it hypersecreted the virulence proteins, it was sensitive to SDS, and it produced rough, wrinkled colonies on agar medium. Plasmids carrying vpsA complemented the mutation, but it was noted that pMQG, which encodes only VpsA and VpsB, complemented better than pMQC, which encodes VpsA, -B, and -C. This supports the idea that the relative amounts of these proteins, particularly VpsA and VpsC, were critical for efficient complementation. The VpsA protein sequence is homologous to a family of ATP-binding transporters, and the predicted protein has a highly conserved ATP-binding motif (68). The vpsB sequence encodes a probable integral membrane protein because of its predicted multiple membrane-spanning domains, while vpsC was found in this study to encode a cytoplasmic membrane protein. The three gene products may form a complex in the cytoplasmic membrane and either export or import certain molecules.
Sequences highly homologous to vpsA, -B, and -C were found in both E. coli (yrbDEF) (GenBank accession no. U18997) and H. influenzae (gene identification no. HI1085), and the organization of the entire vpsA (yrbF)-murZ region is conserved between E. coli and H. influenzae (15). In both E. coli and H. influenzae, the location of yrb is upstream of murZ, an essential gene involved in bacterial peptidoglycan synthesis (6). The fact that the vpsC mutation in S. flexneri was not lethal in vitro and that the downstream sequences were not required for complementation of the mutation ruled out the possibility that the vpsC mutation was polar on murZ. However, a subtle change in peptidoglycan structure could explain the protein hypersecretion phenotype, as it has been suggested that the peptidoglycan layer can serve as a barrier for protein translocation (10). Although the carbenicillin sensitivity experiments indicated that there was no major defect in the peptidoglycan structure of the vpsC mutant in vitro, it is possible that there is a defect that does not increase sensitivity to penicillins or that vpsC had an altered peptidoglycan layer in vivo. Recent studies by Quintela et al. (52) showed that the peptidoglycan structure of S. typhimurium grown within tissue culture cells was changed from that in vitro. Whether the cell wall structure of intracellular S. flexneri vpsC bacteria is different from extracellular bacteria is a question that requires more study.
Taken together, the data suggest that vpsA, -B, and -C encode cytoplasmic membrane proteins whose normal function is required for Shigella to progress from the initial step of invasion to infection of neighboring cells. Disruption of vpsA or vpsC decreases the stability of the cytoplasmic membrane, as measured by susceptibility to SDS lysis, and causes increased secretion of virulence proteins. Failure to control the amount and timing of secretion of these proteins could disrupt the normal sequence of events involved in intracellular growth and intercellular trafficking of the bacteria.
The other mutant characterized in this study (SA2054) was defective in bacterial septation in the intracellular environment. DNA sequence analysis indicated that the mutation was in an ORF highly homologous to E. coli yciB (60), a gene that is designated ispA in S. flexneri (27). A recombinant plasmid that carried the wild-type E. coli yciB gene was shown to complement the plaque formation defect of SA2054. The amino acid sequence analysis showed multiple potential membrane-spanning regions; thus, IspA was predicted to be a membrane protein (27) (GenPept accession no. P21366). This study localized the IspA-PhoA fusion protein to the cytoplasmic membrane of SA2054, confirming the prediction. The phenotype of the ispA mutant constructed in this study was slightly different from the one reported by Mac Síomóin et al. (V3404) (27). SA2054 failed to produce plaques and showed a shorter period of defective septation, which was 3 to 6 h postinfection, as compared to 2 to 8 h for V3404. The difference might be due to the cell lines used: we used Henle 407, an intestinal epithelial cell line, while Mac Síomóin et al. (27) used MK2, a monkey kidney cell line. While SA2054 had a TnphoA insertion after the amino acid at position 65, V3404 was reported to have a Tn10 insertion after the amino acid at position 127; thus, SA2054 should have a larger truncation of the gene product than V3404. SA2054 also grew to the same extent as the wild type in ISM containing reducing agents. It was reported that V3404 did not grow in ISM supplemented with 100 mM 2-mercaptoethanol, but a comparison to the wild type was not indicated (27). Nonetheless, the ispA mutant SA2054 showed the same major phenotype as that of V3404, which was defective intracellular septation. Whether IspA is involved in bacterial septation directly or indirectly, what intracellular signal(s) induces the mutant phenotype, and whether IspA plays the same role in S. flexneri and E. coli remain to be determined.
ACKNOWLEDGMENTS
We gratefully thank the following people for their generous help: Edwin Oaks for providing the various antisera against S. flexneri and against individual S. flexneri antigens; Charles Earhart for discussion of the results; Douglas Henderson for critical reading of the manuscript; Kathleen Postle for providing plasmids, including pRZ526; Leodocia Pope, Guang-Chao Chen, and Clarence Chan for expert technical assistance with immunofluorescence labeling and microscopy; Roger Milkman for discussion of yciB and ispA; and the DNA core facility of the Institute for Cellular and Molecular Biology for DNA sequencing.
This work was supported by Public Health Service grant AI 16935 from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini M L, Fontaine A, Sansonetti P J. The two-component regulatory system OmpR-EnvZ controls the virulence of Shigella flexneri. J Bacteriol. 1990;172:6274–6281. doi: 10.1128/jb.172.11.6274-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardini M L, Sanna M G, Fontaine A, Sansonetti P J. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect Immun. 1993;61:3625–3635. doi: 10.1128/iai.61.9.3625-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 5.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown E D, Vivas E I, Walsh C T, Kolter R. MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J Bacteriol. 1995;177:4194–4197. doi: 10.1128/jb.177.14.4194-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagert M, Ehrlich S D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 9.Daskaleros P A, Payne S M. Congo red binding phenotype is associated with hemin binding and increased infectivity of Shigella flexneri in the HeLa cell model. Infect Immun. 1987;55:1393–1398. doi: 10.1128/iai.55.6.1393-1398.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand J M, Okada N, Tobe T, Watarai M, Fukuda I, Suzuki T, Nakata N, Komatsu K, Yoshikawa M, Sasakawa C. vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J Bacteriol. 1994;176:4627–4634. doi: 10.1128/jb.176.15.4627-4634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert B, Beck C F. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J Bacteriol. 1989;171:3557–3559. doi: 10.1128/jb.171.6.3557-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egile C, d’Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 14.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg M B, Bârzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale T L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991;55:206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale T L, Formal S B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981;32:137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale T L, Oaks E V, Formal S B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985;50:620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Headley V L, Payne S M. Differential protein expression by Shigella flexneri in intracellular and extracellular environments. Proc Natl Acad Sci USA. 1990;87:4179–4183. doi: 10.1073/pnas.87.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong M, Payne S M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 22.Hromockyj A E, Tucker S C, Maurelli A T. Temperature regulation of Shigella virulence: identification of the repressor gene virR, an analogue of hns, and partial complementation by tyrosyl transfer RNA (tRNA1Tyr) Mol Microbiol. 1992;6:2113–2124. doi: 10.1111/j.1365-2958.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 23.Inouye M, Guthrie J P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci USA. 1969;64:957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor K M, Daskaleros P A, Robinson R E, Payne S M. Virulence of iron transport mutants of Shigella flexneri and utilization of host iron compounds. Infect Immun. 1987;55:594–599. doi: 10.1128/iai.55.3.594-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes J, Gottfried S, Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of “periplasmic leaky” mutants. J Bacteriol. 1972;109:520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mac Síomóin R A, Nakata N, Murai T, Yoshikawa M, Tsuji H, Sasakawa C. Identification and characterization of ispA, a Shigella flexneri chromosomal gene essential for normal in vivo cell division and intercellular spreading. Mol Microbiol. 1996;19:599–609. doi: 10.1046/j.1365-2958.1996.405941.x. [DOI] [PubMed] [Google Scholar]
- 28.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquardt J L, Siegele D A, Kolter R, Walsh C T. Cloning and sequencing of Escherichia coli murZ and purification of its product, a UDP-N-acetylglucosamine enolpyruvyl transferase. J Bacteriol. 1992;174:5748–5752. doi: 10.1128/jb.174.17.5748-5752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurelli A T, Blackmon B, Curtiss R I. Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meador J I, Kennel D. Cloning and sequencing the gene encoding Escherichia coli ribonuclease I: exact physical mapping using the genome library. Gene. 1990;95:1–7. doi: 10.1016/0378-1119(90)90406-h. [DOI] [PubMed] [Google Scholar]
- 32.Ménard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–226. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 33.Ménard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 36.Nakamura K, Masui Y, Inouye M. Use of a lac promoter-operator fragment as a transcriptional control switch for expression of the constitutive lpp gene in Escherichia coli. J Mol Appl Genet. 1982;1:289–299. [PubMed] [Google Scholar]
- 37.Nassif X, Mazert M-C, Mounier J, Sansonetti P J. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect Immun. 1987;55:1963–1969. doi: 10.1128/iai.55.9.1963-1969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oaks E V, Hale T L, Formal S B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Occhino, D. A., E. E. Wyckoff, D. P. Henderson, T. J. Wrona, and S. M. Payne. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol. Microbiol., in press. [DOI] [PubMed]
- 41.Okada N, Sasakawa C, Tobe T, Talukder K A, Komatsu K, Yoshikawa M. Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Mol Microbiol. 1991;5:2171–2180. doi: 10.1111/j.1365-2958.1991.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 42.Okada N, Sasakawa C, Tobe T, Yamada M, Nagai S, Talukder K A, Komatsu K, Kanegasaki S, Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991;5:187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 43.Olden K, Ito S, Wilson T H. d-Alanine-requiring cell wall mutant of Escherichia coli. J Bacteriol. 1975;122:1310–1321. doi: 10.1128/jb.122.3.1310-1321.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsot C. Shigella flexneri: genetics of entry and intercellular dissemination in epithelial cells. Curr Top Microbiol Immunol. 1994;192:217–241. doi: 10.1007/978-3-642-78624-2_10. [DOI] [PubMed] [Google Scholar]
- 45.Payne S M, Finkelstein R A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977;18:94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne S M, Niesel D W, Peixotto S S, Lawlor K M. Expression of hydroxamate and phenolate siderophores by Shigella flexneri. J Bacteriol. 1983;155:949–955. doi: 10.1128/jb.155.3.949-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plunkett, G. GenPept accession no. P45391.
- 48.Pope L M, Reed K E, Payne S M. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun. 1995;63:3642–3648. doi: 10.1128/iai.63.9.3642-3648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter M E, Dorman C J. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol. 1994;176:4187–4191. doi: 10.1128/jb.176.13.4187-4191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postle K, Good R F. A bidirectional rho-independent transcription terminator between the E. coli tonB gene and an opposing gene. Cell. 1985;41:577–585. doi: 10.1016/s0092-8674(85)80030-1. [DOI] [PubMed] [Google Scholar]
- 51.Postle K, Reznikoff W S. HindII and HindIII restriction maps of the attφ80-tonB-trp region of the Escherichia coli genome, and location of the tonB gene. J Bacteriol. 1978;136:1165–1173. doi: 10.1128/jb.136.3.1165-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintela J C, de Pedro M A, Zîllner P, Allmaier G, Garcia-del Portillo F. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol Microbiol. 1997;23:693–704. doi: 10.1046/j.1365-2958.1997.2561621.x. [DOI] [PubMed] [Google Scholar]
- 53.Rajakumar K, Jost B H, Sasakawa C, Okada N, Yoshikawa M, Adler B. Nucleotide sequence of the rhamnose biosynthetic operon of Shigella flexneri 2a and role of lipopolysaccharide in virulence. J Bacteriol. 1994;176:2362–2373. doi: 10.1128/jb.176.8.2362-2373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 55.Sandlin R C, Lampel K A, Keasler S P, Goldberg M B, Stolzer A L, Maurelli A T. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sansonetti P J. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr Top Microbiol Immunol. 1992;180:1–19. doi: 10.1007/978-3-642-77238-2_1. [DOI] [PubMed] [Google Scholar]
- 57.Sasakawa C, Buysse J M, Watanabe H. The large virulence plasmid of Shigella. Curr Top Microbiol Immunol. 1992;180:21–44. doi: 10.1007/978-3-642-77238-2_2. [DOI] [PubMed] [Google Scholar]
- 58.Stathopoulos C, Georgiou G, Earhart C F. Characterization of Escherichia coli expressing an Lpp′OmpA(46-159)-PhoA fusion protein localized in the outer membrane. Appl Microbiol Biotechnol. 1996;45:112–119. doi: 10.1007/s002530050657. [DOI] [PubMed] [Google Scholar]
- 59.Stoltzfus, A., J. F. Leslie, and R. Milkman. GenPept accession no. P21366.
- 60.Stoltzfus A, Leslie J F, Milkman R. Molecular evolution of the Escherichia coli chromosome. I. Analysis of structure and natural variation in a previously uncharacterized region between trp and tonB. Genetics. 1988;120:345–358. doi: 10.1093/genetics/120.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki T, Lett M-C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki T, Murai T, Fukuda I, Tobe T, Yoshikawa M, Sasakawa C. Identification and characterization of a chromosomal virulence gene, vacJ, required for intercellular spreading of Shigella flexneri. Mol Microbiol. 1994;11:31–41. doi: 10.1111/j.1365-2958.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 63.Taylor R K, Manoil C, Mekalanos J J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989;171:1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tobe T, Sasakawa C, Okada N, Honma Y, Yoshikawa M. vacB, a novel chromosomal gene required for expression of virulence genes on the large plasmid of Shigella flexneri. J Bacteriol. 1992;174:6359–6367. doi: 10.1128/jb.174.20.6359-6367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tobe T, Yoshikawa M, Mizuno T, Sasakawa C. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by VirF and repression by H-NS. J Bacteriol. 1993;175:6142–6149. doi: 10.1128/jb.175.19.6142-6149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uchiya K-I, Tobe T, Komatsu K, Suzuki T, Watarai M, Fukuda I, Yoshikawa M, Sasakawa C. Identification of a novel virulence gene, virA, on the large plasmid of Shigella, involved in invasion and intercellular spreading. Mol Microbiol. 1995;17:241–250. doi: 10.1111/j.1365-2958.1995.mmi_17020241.x. [DOI] [PubMed] [Google Scholar]
- 67.Van Den Bosch L, Manning P A, Morona R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- 68.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]