Abstract
Although social-communication difficulties and repetitive behaviors are hallmark features of autism spectrum disorder (ASD) and persist across the lifespan, very few studies have compared age-related differences in these behaviors between youth with ASD and same-age typically developing (TD) peers. We examined this issue using SRS-2 (Social Responsiveness Scale-Second Edition) measures of social-communicative functioning and repetitive behaviors in a stratified cross-sectional sample of 324 youth with ASD in the absence of intellectual disability, and 438 TD youth (aged 4–29 years). An age-by-group interaction emerged indicating that TD youth exhibited age-related improvements in social-communication scores while the ASD group demonstrated age-related declines in these scores. This suggests that adolescents/adults with ASD may fall increasingly behind their same-age peers in social-communicative skills.
Keywords: Autism, Social, Communication, Repetitive behavior, Age, Social Responsiveness Scale
Introduction
Difficulties in social-communication and the presence of restricted and repetitive behaviors (RRB) are hallmark features of autism spectrum disorder (ASD; APA 2013). Of the increasing number of children identified with an ASD, the fastest growing sub-group is those without co-occurring intellectual disability (ID) (Wingate et al. 2014). Although there is an expectation of positive outcome for these individuals based on relatively higher cognitive and language abilities, longitudinal studies have found that even those without ID do not necessarily have better employment and independent living outcomes (Howlin 2003), for example. In addition, research that has specifically investigated changes in the quintessential features of ASD across development, namely social impairments and RRB, has yielded mixed findings and varying rates of developmental change (McGovern and Sigman 2005; Shattuck et al. 2007; Taylor and Seltzer 2010). The purpose of the present study is to investigate differences in the association between age (using a stratified cross-sectional design), IQ, and sex with social-communicative functioning, and RRB among youth with ASD without ID and typically developing (TD) youth on a commonly used measure, the Social Responsiveness Scale (SRS).
Examination of age-related differences using the Autism Diagnostic Interview-Revised (ADI-R), a structured parent interview that provides historical and current information regarding autism symptoms (Lord et al. 1994), has generally revealed modest improvement or relative stability in social functioning in ASD during childhood, adolescence, and into young adulthood. A variety of longitudinal investigations have found improvements in parent reported social functioning in ASD as measured by the ADI-R from early childhood to late adolescence (McGovern and Sigman 2005; Taylor and Seltzer 2010). However, some studies have noted that the improvement of social reciprocity in ASD noticeably slows after high school exit, indicating that the rate of change in social functioning varies across development (Taylor and Seltzer 2010). Also utilizing the ADI-R, Shattuck et al. (2007) found evidence for both improvement and stability in social-communication interaction (SCI) in ASD, with half of their adolescent and adult-aged sample remaining stable in SCI throughout the 4.5 year study period. It is important to note, however, that the ADI-R was constructed as a categorical measure of diagnosis, not a continuous measure of symptomatology and therefore may not be ideally suited for assessing age-related changes in symptom expression.
The SRS is a continuous measure commonly used to assess autistic symptoms throughout development (Constantino and Gruber 2005). Studies utilizing the SRS have demonstrated mixed results regarding change in social symptoms with age. At least one study found modest longitudinal improvements in social functioning in males with ASD (Constantino et al. 2009), while the Social Responsiveness Scale-Second Edition (SRS-2; Constantino and Gruber 2012) normative sample indicated that age was not an important factor in social impairment. These changes could result from the age-related differences in observed behavior and/or changes in parental perceptions of their children’s behavior over time. Other investigations have used the Vineland Adaptive Behavior Scales (VABS), a standardized, structured parent/caregiver interview that assesses adaptive abilities and includes a Socialization domain (Sparrow et al. 1984, 2005). Pugliese et al. (2015) found that standard scores of socialization skills decreased with increasing age in a cross-sectional sample of children and adolescents with ASD without ID, suggesting they are falling further behind chronological age expectations (based on the standardization sample) and reflecting a different social functioning trajectory than studies tracking social problems or symptoms.
Similar to studies of age-related differences in social functioning, investigations of developmental differences in non-social ASD RRB symptoms are characterized by mixed findings. A variety of longitudinal studies using the ADI-R have demonstrated small improvements of RRB with age (McGovern and Sigman 2005; Taylor and Seltzer 2010). Similarly, a cross-sectional investigation utilizing the Repetitive Behavior Scale-Revised (Bodfish et al. 2000), found that overall RRB and specific subtypes of RRB, such as stereotyped movements, self-injurious behaviors, compulsive behaviors, ritualistic/sameness behaviors, and restricted interests, were reported less frequently and were less severe in older individuals with ASD, indicating a negative relationship between age and RRB symptoms (Esbensen et al. 2009). While SRS scores, which include one subscale measuring RRB, have shown modest improvements longitudinally in two school-age samples of children with ASD (Constantino et al. 2009), no such relationship was found within the (cross-sectional) SRS-2 standardization sample (Constantino and Gruber 2012). The discrepant results regarding the change in RRB throughout development suggest further investigation with a broader age range and independent sample is warranted.
Taken as a whole, the majority of studies examining social and RRB symptoms in ASD without ID indicate slight improvements or stability in symptoms across age groups/over time in both longitudinal (e.g., McGovern and Sigman 2005; Taylor and Seltzer 2010; Constantino et al. 2009) and cross-sectional (Constantino and Gruber 2012) explorations. This lack of change or small amount of change is hard to interpret, however, because little is known about how changes in these domains manifest across development in TD children. The few results reported thus far suggest that TD children are rated as having fewer social impairments and RRB (as measured by the SRS) with increasing age (Wallace et al. 2012), though neither the original SRS (Constantino and Gruber 2005) nor subsequent SRS-2 (Constantino and Gruber 2012) are age-normed, suggesting that age was not found to be an important factor in the accompanying standardization samples. More work is needed to clarify this relationship in TD samples.
The literature on sex differences in symptomatology in ASD is rife with inconsistency and concerns over possible ascertainment bias and under-representation of females with ASD, particularly those without ID. Nevertheless, a recent meta-analysis of 20 studies found no sex differences in core ASD social and communication deficits (Van Wijngaarden-Cremers et al. 2014). However, there was evidence for more restricted interests and stereotyped behaviors among females than among males with ASD. Among TD groups, there is also mixed evidence for sex differences in so-called autistic traits. The instrument used most commonly with adults in prior studies, the Autism spectrum Quotient (AQ), consistently finds sex differences; TD males self-report more autistic traits than TD females (Ruzich et al. 2015). Among children and adolescents, one of the most frequently employed instruments, the SRS, has revealed mixed evidence of sex differences in TD populations. Whereas within the original and more recent standardization samples more autistic traits were found among males than females based on parent ratings (Constantino and Gruber 2005, 2012), no sex differences in parent ratings of autistic traits using the SRS were reported in an independent community-based sample (Wallace et al. 2012).
To date, there is limited research comparing the associations between demographic variables (i.e., age and sex) and both social functioning and RRB, using the same measure, in ASD vs. TD groups. The SRS, unlike for example the ADI-R, is ideal for such a comparison as it provides a continuous measure of both social functioning and RRB wherein variance in scores within both TD and ASD populations is expected. Therefore, the present stratified cross-sectional study utilizes a robust sample to investigate differences in the association between age and sex with social-communicative functioning, and RRB among youth with ASD without ID and TD controls. We hypothesized that TD youth would have more pronounced negative associations between age and both social and RRB symptoms than youth with ASD without ID and that TD males would have higher SCI and RRB scores (indicating more autistic traits) than TD females.
Methods
Participants
Participants were 324 children, adolescents, and young adults diagnosed with ASD without intellectual disability (268 males) and 438 TD youth (215 males) between 4 and 29 years of age. Subjects were placed into one of four age groupings [4–8.99 (ASD n = 91; TD n = 95) vs. 9–11.99 (ASD n = 96; TD n = 84) vs. 12–16.99 (ASD n = 85; TD n = 150) vs. 17+ year olds (ASD n = 52; TD n = 109)], the number and range of which were determined by balancing group size with statistical power. In addition, age was treated continuously in follow-up regression analyses. Comparison of demographic characteristics across age groupings within each diagnostic group (ASD vs. TD) did not reveal significant differences in level of mother’s educational attainment (categorized as either some college/ higher education or greater vs. no higher education) or sex ratio (see Table 1). ASD participants were recruited through two clinical and/or research centers specializing in ASD and neuropsychological assessment. All ASD participants received an ASD diagnosis from a trained and experienced clinician based on DSM-IV-TR (APA 2000) or DSM-5 criteria (APA 2013). In addition, ASD participants met criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (Lainhart et al. 2006) using the Autism Diagnostic Interview (ADI; Le Couteur et al. 1989) or Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994; see Table 1 for further detail) and/or the first or second edition of the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000, 2012). 258 ASD participants received the first edition of the ADOS (Module 2: n = 18; Module 3: n = 171; Module 4: n = 69) administered by research reliable clinicians. In addition, 50 participants received the second edition of the ADOS (Module 3: n = 50). In total, 94.1% of participants met the ADOS module cutoffs for ‘ASD,’ and 74.3% of total participants met the more narrow ADOS module cutoffs for ‘Autism’ (Lord et al. 2000, 2012). TD participants were recruited from the community and completed a telephone screening prior to inclusion in the study to exclude participants with psychiatric, learning or neurological disorders. All participants received a Full Scale IQ score (FSIQ) score of 70 or above as measured by the Wechsler Intelligence Scales or the Differential Ability Scales. Informed assent and consent were obtained from all participants and/or their parent/guardian when appropriate. See Tables 1 and 2 for further information regarding demographic characteristics and diagnostic scores for these groups.
Table 1.
Demographic characteristics and diagnostic scores for the complete sample of individuals with autism spectrum disorder and typically developing controls
| Autism spectrum disorder | Typically developing controls | Statistic (χ2/t), p value | |
|---|---|---|---|
| (n = 324) | (n = 438) | ||
| Sex (% male) | 82.7 | 49.1 | χ2 = 90.75, p < .001 |
| Age | 12.04 (4.39) | 13.72 (5.28) | t = 4.80, p < .001 |
| FSIQ | 106.45 (18.21) | 113.09 (12.51) | t = 5.63, p < .001 |
| Maternal education (% with some college or more) | 75.9 | 66.6 | χ2 = 6.20, p = .01 |
| SRS-2 SCIa | 73.86 (21.40) | 19.48 (12.15) | t = 41.10, p < .001 |
| SRS-2 RRBa | 17.02 (6.64) | 2.15 (2.82) | t = 37.84, p < .001 |
| ADI/-R RSI | 18.56 (6.14) | – | – |
| ADI/-R Comm. | 15.17 (4.64) | – | – |
| ADI/-R Rep. Beh. | 5.57 (2.50) | – | – |
FSIQ full scale intelligence quotient, SRS-2 Social Responsiveness Scale-Second Edition, SCI social communication index, RRB restricted repetitive behaviors, ADI/-R autism diagnostic interview/-revised, RSI reciprocal social interaction, Comm. communication (verbal), Rep. Beh. repetitive and stereotyped patterns of behavior
Raw/unstandardized scores
Table 2.
Demographic characteristics and Social Responsiveness Scale-2 scores for the individuals with autism spectrum disorder and typically developing controls by age group
| Autism spectrum disorder | Typically developing controls | |||||||
|---|---|---|---|---|---|---|---|---|
| 4–8.99 (n = 91) | 9–11.99 (n = 96) | 12–16.99 (n = 85) | 17+ (n = 52) | 4–8.99 (n = 95) | 9–11.99 (n = 84) | 12–16.99 (n = 150) | 17+ (n = 109) | |
| Sex (% male) | 82.4 | 78.1 | 85.9 | 86.5 | 37.9 | 51.2 | 51.3 | 54.1 |
| Age | 7.60 (1.07) | 10.43 (0.85) | 13.83 (1.35) | 19.86 (2.83) | 6.99 (1.14) | 10.63 (0.88) | 14.60 (1.44) | 20.77 (3.09) |
| FSIQb | 101.12 (18.96) | 109.14 (19.05) | 106.53 (17.24) | 110.69 (14.78) | 109.68 (13.44) | 115.46 (13.62) | 114.03 (11.58) | 112.75 (11.58) |
| Maternal education (% with some college or more) | 84.1 | 76.67 | 67.1 | 77.1 | 69.6 | 73.2 | 64.3 | 62.5 |
| SRS-2 SCIa | 68.59 (20.26) | 75.48 (19.86) | 76.20 (22.95) | 76.27 (22.48) | 23.59 (12.51) | 22.07 (12.44) | 17.31 (11.30) | 16.88 (11.51) |
| SRS-2 RRBa | 16.93 (6.66) | 17.84 (6.58) | 16.73 (7.11) | 16.12 (5.93) | 2.55 (3.13) | 2.46 (2.97) | 2.02 (2.70) | 1.73 (2.53) |
FSIQ full scale intelligence quotient, SRS Social Responsiveness Scale-Second Edition, SCI social communication index, RRB restricted repetitive behaviors
Raw/unstandardized scores
There were significant differences in FSIQ across the age groups for both individuals with autism spectrum disorder and typically developing controls
Measures
Autism Diagnostic Interview/Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule/Autism Diagnostic Observation Schedule-2nd Edition (ADI/ADI-R; ADOS/ADOS-2)
The ADI is a structured parent interview about the child’s developmental history with an emphasis on communication, social development, and repetitive and restricted behaviors. The ADOS is a semi-structured, play interview that scores a participant’s response to social presses for communication, reciprocal social behavior, and repetitive behaviors and stereotyped interest patterns. Scores on both the ADOS and ADI are aggregated into symptom clusters that correspond with a DSM-IV or DSM-5 diagnosis of ASD.
Social Responsiveness Scale—Second Edition (SRS-2)
The SRS-2 (Constantino and Gruber 2012) is a 65-item informant report of autistic traits rated on a 4-point Likert Scale (0–3 points). Higher scores indicate more autistic traits; T-scores ≥ 65 (i.e., 1.5 SDs ≥ the population mean of 50) suggest clinically significant autistic traits. The SRS-2 scoring is aligned with DSM-5 criteria for diagnosis of an ASD. The update includes the creation of two higher order indices that correspond to the two symptom domains of ASD: Social Communication and Interaction (SCI) and Restricted Interests and Repetitive Behavior (RRB). Informants provided a single SRS-2 rating for each child in this cross-sectional study.
Data Analysis
Before undertaking primary analyses the best fit for the relationship between age and SRS scores was examined. Because linear fits were better than any higher order (e.g., quadratic) associations (SCI F = 18.41, p < .001; RRB F = 21.43, p < .001), the most parsimonious approach was to examine linear effects. Because it is meaningful and informative to observe these data across various periods of childhood and adolescence, an analysis of variance (ANOVA) was run to examine the effects of age (categorized as 4–8.99 vs. 9–11.99 vs. 12–16.99 vs. 17+ year olds), diagnosis (ASD vs. TD controls), and sex (male vs. female) as between group variables, and their interactions on SCI and RRB scores (i.e., the dependent variables) from the SRS-2. Initial analyses of demographic variables indicated that the ASD and TD groups were characterized by significant differences in age, FSIQ, and sex ratio (see Table 1). Therefore, two approaches were utilized to address this issue. First, similar analyses were run again; however, analysis of covariance (ANCOVA) in which FSIQ was entered as a covariate, was utilized to examine whether IQ exerted an influence over results obtained initially using ANOVA. Second, because a fuzzy matching procedure proved too conservative and discarded data from too many participants, subgroups of individuals with ASD (n = 247) and TD controls (n = 258) were selected to maximize sample size while creating groups that no longer differ in terms of sex ratio and FSIQ (i.e., from the original sample, older TD participants and TD females were removed individually until the ASD and TD groups no longer differed in terms of sex ratio and FSIQ score). The original ANOVA was then re-run using these subgroups to examine the effects of diagnosis (ASD vs. TD controls), age (4–8.99 vs. 9–11.99 vs. 12–16.99 vs. 17+ year olds), sex (male vs. female), and their interactions on SCI and RRB scores. Finally, complementary and confirmatory regression-based analyses were run to examine how well diagnosis (ASD vs. TD controls), age (treated continuously), sex (male vs. female), IQ, and the age by diagnosis interaction term predicted SCI and RRB scores, respectively, from the SRS-2.
Results
The ANOVA for SCI total raw score revealed a main effect of diagnosis (F = 1224, p < .001, ), but no main effects of age (ns) or sex (ns); however, this was qualified by an interaction between diagnosis and age group (F = 5.73, p = .001, ). Follow-up t tests revealed that in the TD group, older groups received lower SCI scores (denoting fewer traits) than the younger groups. More specifically, 12–16.99 (t = 4.07, p < .001, Cohen’s d = 0.53) and 17+ (t = 3.99, p < .001, Cohen’s d = 0.56) year olds were rated as having fewer SCI traits than 4–8.99 year olds; 12–16.99 (t = 2.98, p = .003, Cohen’s d = 0.40) and 17+ (t = 3.00, p = .003, Cohen’s d = 0.43) year olds were rated as having fewer SCI traits than 9–11.99 year olds. In contrast, for the ASD group the three oldest groups received higher SCI scores (denoting more traits) than the youngest group: 9–11.99 (t = −2.35, p = .02, Cohen’s d = 0.34), 12–16.99 (t = −2.34, p = .02, Cohen’s d = 0.35), and 17+ (t = −2.10, p = .04, Cohen’s d = 0.36) year olds were rated as having more SCI traits than 4–8.99 year olds. See Fig. 1. Entering IQ scores into the ANCOVA as a covariate did not change the pattern of findings. There remained a main effect of diagnosis (F = 1176, p < .001, ) qualified by an interaction between diagnosis and age group (F = 5.78, p = .001, ) for SCI scores. Utilizing subgroups (total n = 505) of individuals with ASD and TD controls that did not differ in terms of sex ratio or FSIQ, results were highly similar to those reported above in the original ANOVA. There was a main effect of diagnosis (F = 681, p < .001, ), which was qualified by a trend for a significant interaction between diagnosis and age group (F = 2.38, p = .07, ), but there were no main effects of age (ns) or sex (ns). Although this interaction did not reach the threshold for statistical significance, it was clearly affected by the loss in power from a diminished sample size during the group matching procedure. More specifically, 12–16.99 (t = 2.25, p = .03, Cohen’s d = 0.37) and 17+ (t = 2.66, p < .01, Cohen’s d = 0.48) year olds were rated as having fewer SCI traits than 4–8.99 year olds. In contrast, for the ASD group there were no differences in SCI scores across age groups (ns).
Fig. 1.
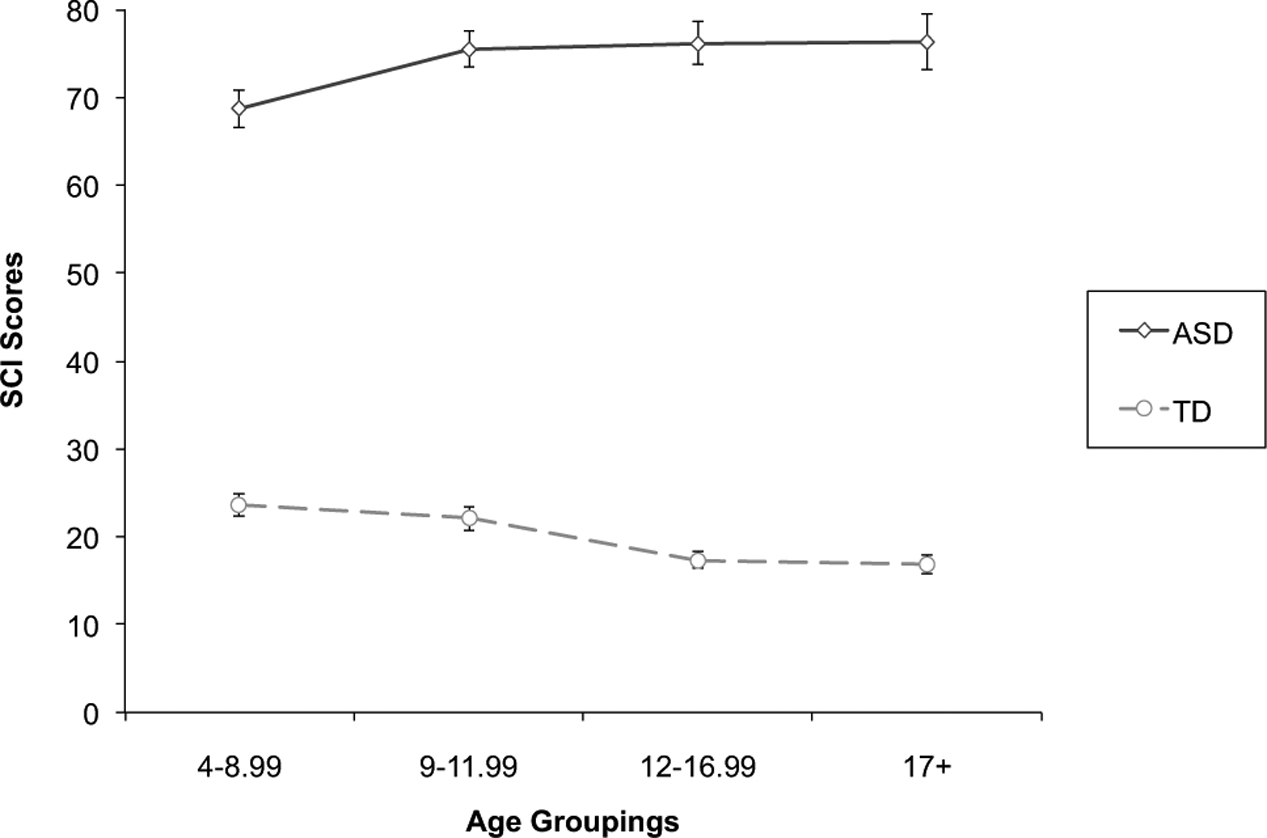
Age-related differences in Social Communication and Interaction (SCI) scores for the autism spectrum disorder (ASD) and typically developing (TD) groups
The ANOVA for RRB total raw score from the SRS revealed only a main effect of diagnosis (F = 1035, p < .001, ), but no main effects of age (ns) or sex (ns) or any interactions amongst these factors (ns). This same pattern of results [i.e., only a main effect of diagnosis (F = 987, p < .001, )] was found after entering IQ as a covariate into an ANCOVA. Utilizing subgroups (total n = 505) of individuals with ASD and TD controls that did not differ in terms of sex ratio or FSIQ, results were highly similar to those reported above in the original ANOVA examining RRB scores. There was a main effect of diagnosis (F = 584, p < .001, ), but no main effects of age (ns) or sex (ns) or any interactions amongst these factors (ns) on RRB scores.
Finally, approaching the age-related data continuously using regression-based analyses revealed that diagnostic group and IQ, unlike sex, were significant predictors of both SCI and RRB scores from the SRS-2 while age and the diagnosis by age interaction term were uniquely predictive of SCI (unlike RRB) scores in the full sample (see Table 3). Moreover, when utilizing regressions to examine these (non-group-related) predictors in the ASD and TD groups separately, notably, age was significantly associated with SCI scores in each group, but in opposite directions (ASD t = 2.68, p < .01; TD t = −4.08, p < .001).
Table 3.
Social Responsiveness Scale-2 scores regressed onto age, diagnostic group, IQ, sex, and the age by diagnostic group interaction term in the combined sample of individuals with autism spectrum disorder and typically developing controls
| Predictor | Social communication index | Restricted repetitive behaviors | ||||
|---|---|---|---|---|---|---|
| B | SE B | t | B | SE B | t | |
| Age | −1.62 | 0.37 | −4.34** | −0.07 | 0.11 | −0.56 |
| Diagnostic group | 38.61 | 3.56 | 10.84** | 14.32 | 1.05 | 13.71** |
| Sex | −0.10 | 1.34 | −0.08 | −0.07 | 0.39 | −0.19 |
| IQ | −0.14 | 0.04 | −3.60** | −0.04 | 0.01 | −3.01* |
| Diagnostic group × age | 1.18 | 0.26 | 4.54** | 0.02 | 0.08 | 0.24 |
p < .01
p < .001
Discussion
Utilizing data from a relatively large sample, we found that whereas TD children, adolescents, and young adults exhibited age-related improvements in parent-reported social-communicative functioning, children, adolescents, and young adults with ASD demonstrated a general pattern of age-related declines in these scores. In addition, sex differences in social-communicative and repetitive behaviors were not found in either the ASD or TD group.
It is perhaps unsurprising to find that social-communicative (ASD) traits reduce with increasing age during childhood and from childhood into adolescence/young adulthood in the context of typical development. There is an extensive literature on the explosive growth of social cognitive skills during childhood and adolescence in typical development, although most of the existing literature has relied upon performance-based tasks of social cognition (e.g., see review by Burnett et al. 2011). Complementing this approach, at least two studies utilizing a different measure of autistic trait ratings [i.e., the Childhood Autism Spectrum Test/Childhood Asperger Syndrome Test (CAST)] in a general population sample during middle to late childhood have found longitudinal improvements in scores (Robinson et al. 2011; Holmboe et al. 2014) consistent with the present findings. However, it is interesting to note that the SRS and SRS-2 are not age-normed, which implies that social-communicative (ASD) traits are developmentally invariant. The present findings and those from at least one longitudinal study showing, if anything, an improvement of symptoms on the SRS for a group of children with ASD suggest more work is needed in this area (Constantino et al. 2009). More specifically, additional longitudinal studies are required to assess trajectories of these behaviors in the context of both TD and ASD youth.
These findings, if anything, contradict the broader literature in showing early increases then relatively stable SCI symptoms and no age-related differences in RRB symptoms in ASD during childhood, adolescence, and into young adulthood. Unlike prior research, which relied primarily upon the ADI as the ASD symptom measure, the present study finds higher, not lower, degrees of impairment with age in ASD. And even more striking, the current study finds higher SCI scores on the SRS in older age groups, whereas the previous longitudinal paper (Constantino et al. 2009) showed decreasing symptoms over time using the total score from the SRS. Nevertheless, at least one study has shown age-related differences in adaptive social abilities (using the Vineland Adaptive Behavior Scales) in ASD (Pugliese et al. 2015), which mirrors the present findings of age-related differences in social disabilities. Although we documented the mildest SCI symptoms in the youngest ASD group, this finding might reflect increasing expectations at later developmental periods and/or cohort effects tied to potential differences in type and access to early intervention, rather than an actual worsening of SCI symptoms in the transition from middle to late childhood and beyond. However, the fact that lower SCI scores are not observed in older individuals with ASD across these age bands in spite of extensive, albeit variable, interventions implemented for these youth with ASD is concerning and should be investigated further in future research. Consideration should also be given to age norming the SRS, given the modest yet significant age-related differences observed across childhood and adolescence in the TD group.
Our findings partially support a recent meta-analysis documenting no sex differences in social behaviors in males relative to females with ASD (Van Wijngaarden-Cremers et al. 2014) given that sex differences in SCI scores were not found in the ASD group. However, unlike Van Wijngaarden-Cremers and colleagues’ results, wherein males with ASD had elevated RRB compared to females, we did not did not find sex differences in RRB scores in the ASD group. This could be a product of the SRS’ relative focus on the social and communication features of the ASD phenotype; therefore, it might not be as sensitive in measuring potential sex differences in RRB. As in the ASD sample, sex differences in the SCI and RRB scores were not found in the TD group, which is discrepant from some prior findings (e.g., Holmboe et al. 2014; Ruzich et al. 2015) and from the SRS standardization samples (Constantino and Gruber 2005, 2012).
Given that youth with ASD without ID do not exhibit the same maturational gains in social-communicative functioning observed in typical development during this age range, adolescents and adults with ASD might fall increasingly behind their same-age peers in this domain. This developmental lag would in turn significantly impact their daily lives and functioning in the academic and vocational arenas. Future research should investigate these possibilities more closely by utilizing longitudinal designs to examine trajectories of social-communicative functioning in both ASD and TD youth and the downstream consequences of these varying trajectories on daily living skills.
This study had several limitations to consider. Perhaps most salient is that a stratified cross-sectional design was utilized to examine age-related differences. Although our confidence in the age-related findings is bolstered by comparable demographic features (i.e., sex ratio, maternal educational levels) across age groups within each diagnostic group, the most robust test of developmental effects is a longitudinal design. Future longitudinal studies will be needed in order to evaluate the robustness of the findings reported here. Additionally, we cannot rule out the possibility that age-related improvements in SCI scores in the TD group are not at least partially a measurement feature of the SRS-2. It is conceivable that parents reporting on their children’s behavior become increasingly confident in the absence of these (ASD-like) social-communicative traits at older ages. However, the present study did have several other strengths as well. For example, it included a relatively large sample size and rigorously evaluated individuals with ASD, and it included both broader unmatched and matched (e.g., on sex ratio and IQ) subgroups. The ASD group did not include individuals with co-morbid ID, which prevents potential confounds where differences could be attributed to the presence of ID. Nevertheless, future research should examine age and sex differences in social-communication and RRB among ASD + ID groups and both syndromic and non-syndromic ID groups to assess potential discrepancies in developmental trajectories.
Acknowledgments
This work was supported by the Intramural Research Program at NIMH, NIH under Grants 1-ZIA-MH002794, 1-ZIA-MH002920. LA, CEP, and LK were supported by The Gudel-sky Family Foundation and an IDDRC Grant HD046388-01A2. CEP was supported by a T32 Grant 5P30HD040677-15. We would like to express our gratitude to the individuals and families who volunteered their time to contribute to this research.
Footnotes
Conflict of interest All authors declare no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, & Lewis MH (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30, 237–243. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, & Blakemore SJ (2011). The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews, 35, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Abbachi AM, Lavesser PD, Reed H, Givens L, Chiang L, Gray T, Gross M, Zhang Y, & Todd RD (2009). Developmental course of autistic social impairment in males. Developmental and Psychopathology, 21, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2005). Social responsiveness scale (SRS). Los Angelos, CA: Western Psychological Services. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale, second edition (SRS-2). Torrence, CA: Western Psychological Services. [Google Scholar]
- Esbensen AJ, Mailick Seltzer M, Lam SL, & Bodfish JW (2009). Age-related differences in restricted and repetitive behaviors in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe K, Rijsdijk FV, Hallett V, Happe F, Plomin R, & Ronald A (2014). Strong genetic influences on the stability of autistic traits in childhood. Journal of the American Academy of Child and Adolescent Psychiatry, 53(2), 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P (2003). The outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders, 33, 3–13. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. (2006). Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics, 140, 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Hold-grafer M, et al. (1989). Autism diagnostic interview—A standardized investigator-based instrument. Journal of Autism and Developmental Disorders, 19, 363–387. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). ADOS-2: Autism diagnostic observation schedule, second edition (ADOS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- McGovern CW, & Sigman M (2005). Continuity and change from early childhood to adolescence in autism. Journal of Child Psychology and Psychiatry, 46, 401–408. [DOI] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, & Kenworthy L (2015). Increasing adaptive behavior skill deficits from childhood to adolescence in autism spectrum disorder: Role of executive function. Journal of Autism and Developmental Disorders, 45, 1579–1587. doi: 10.1007/s10803-014-2309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Munir K, Munafò MR, Hughes M, McCormick MC, & Koenen KC (2011). Stability of autistic traits in the general population: further evidence for a continuum of impairment. Journal of the American Academy of Child and Adolescent Psychiatry, 50(4), 376–384. doi: 10.1016/j.jaac.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, & Baron-Cohen S (2015). Measuring autistic traits in the general population: A systematic review of autism-spectrum quotient (AQ) in a nonclincal population sample of 6,900 typical adult males and females. Molecular Autism, 6(2), 1–12. doi: 10.1186/2040-2392-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, et al. (2007). Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 37(9), 1735–1747. doi: 10.1007/s10803-006-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla DA, & Cicchetti D (1984). Vineland adaptive behavior scales (expanded form). Circle Pine, MN: American Guidance Service. [Google Scholar]
- Sparrow SS, Cicchetti D, & Balla DA (2005). Vineland adaptive behavior scales-2nd edition manual. Minneapolis, MN: NCS Pearson, Inc. [Google Scholar]
- Taylor JL, & Seltzer MM (2010). Changes in the autism behavioral phenotype during the transition to adulthood. Journal of Autism and Developmental Disorders, 40(12), 1431–1446. doi: 10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers JM, van Eeten E, Groen WB, Van Deurzen V, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44, 627–635. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Raitano Lee N, Clasen LS, Raznahan A, Lenroot R, et al. (2012). Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. Journal of Neuroscience, 32(14), 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate M, Kirby RS, Pettygrove S, Cunniff C, Schulz E, Ghosh T, et al. (2014). Prevalence of autism spectrum disorder among children aged 8 years—Autism developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summary, 63(2), 1–21. [PubMed] [Google Scholar]


