Abstract
Introduction
Atezolizumab and bevacizumab (Ate/Bev) combination has become the new first-line systemic therapy for unresectable hepatocellular carcinoma (HCC). Although several studies reported thyroid dysfunction after treatment with immune checkpoint inhibitors, the clinical and immunological significance of thyroid dysfunction in patients treated with Ate/Bev has not been comprehensively addressed. We aimed to comprehensively evaluate the clinical and immunological implications of thyroid dysfunction in unresectable HCC patients treated with Ate/Bev.
Methods
We enrolled 208 patients with unresectable HCC treated with Ate/Bev from three Korean cancer centers. Thyroid adverse events (AEs) were reviewed, and cytokines and T cells in the blood samples were analyzed at baseline. For external validation, we analyzed clinical outcomes according to thyroid AEs in patients treated with Ate/Bev in the IMbrave150 study.
Results
Forty-one (19.7%) out of 208 patients experienced thyroid dysfunction (hypothyroidism [17.3%] and thyrotoxicosis [5.8%]) after Ate/Bev treatment. Median time to onset of hypothyroidism and thyrotoxicosis after Ate/Bev treatment was 3.5 and 1.3 months, respectively. Patients with thyroid AEs demonstrated significantly better progression-free survival, overall survival, and objective response rate than those without thyroid AEs. These findings were still consistent even after adjusting for confounding factors. Furthermore, favorable survival outcomes in patients with thyroid AEs were also validated in a cohort of IMbrave150 patients. While patients with thyrotoxicosis showed a significantly lower level of baseline IL-6, those with hypothyroidism did not show significant differences in circulating cytokine levels and CD8+ T-cell fractions.
Conclusions
A fraction of patients with HCC treated with Ate/Bev experienced thyroid dysfunction, and the development of thyroid AEs was associated with favorable clinical outcomes.
Keywords: Hepatocellular carcinoma, Thyroid adverse events, Atezolizumab, Bevacizumab
Introduction
In the IMbrave150 phase III trial, atezolizumab, an anti-programmed death-1 (PD-1) ligand 1 (PD-L1) antibody, and bevacizumab, an anti-vascular endothelial growth factor antibody, demonstrated statistically significant survival benefit over sorafenib, thus becoming the new first-line systemic therapy for unresectable hepatocellular carcinoma (HCC) [1–3]. Although immune checkpoint inhibitor (ICI) therapy is generally well tolerated, it can induce immune-related adverse events (irAEs) [4]. The exact mechanisms of irAEs have not yet been fully elucidated; however, they are assumed to be caused by the bystander effects of ICI-induced T-cell activation [5, 6]. In addition, the association between irAEs and various circulating cytokines has also been suggested in recent studies [7, 8]. In some reports, the occurrence of irAEs after ICI therapy was associated with improved survival outcomes in various types of solid tumors [9, 10]. Thyroid dysfunction is one of the most common adverse events (AEs) after ICI treatment and can be easily monitored using a thyroid function test in clinical practice [11]. In the IMbrave150 trial, 15.5% of patients have been reported to develop thyroid dysfunction after atezolizumab and bevacizumab (Ate/Bev) treatment. However, the clinical and immunological significance of thyroid AEs in patients treated with Ate/Bev has not yet been comprehensively addressed. Therefore, this study aimed to comprehensively evaluate the clinical and immunological implications of thyroid AEs in unresectable HCC patients treated with Ate/Bev.
Materials and Methods
Study Design and Patients
This study prospectively enrolled patients with unresectable HCC who were treated with Ate/Bev between June 2020 and December 2021 at three tertiary cancer centers in Korea for the analysis of cytokines and T cells in the peripheral blood (n = 276; CONSORT diagram shown in online suppl. Fig. 1 left; for all online suppl. material, see https://doi.org/10.1159/000531182). We retrospectively reviewed treatment-related AEs, including thyroid AEs. The exclusion criteria were as follows: Eastern Cooperative Group (ECOG) performance status of 2 (n = 8), Child-Pugh score of C (n = 1), patients who received Ate/Bev treatment as second-line therapy (n = 11), patients who had no follow-up thyroid function test data after initiation of treatment (n = 21), and those who had thyroid dysfunction before initiation of treatment (n = 27). Finally, 208 HCC patients were eligible for analysis in this study. This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the participating hospitals’ Institutional Review Boards (CHA Bundang Medical Center, CHA-2017-11-052, CHA-2017-11-054; Ulsan University Hospital, 2020-12-006; Haeundae Paik Hospital, 2020-12-019-001). Written informed consent was obtained from all the patients. As an external validation cohort, we analyzed clinical outcomes according to thyroid AEs in patients treated with Ate/Bev in the IMbrave150 study (ClinicalTrials.gov, NCT03434379), a prospective, randomized, phase III trial (CONSORT diagram shown in online suppl. Fig. 1 right).
Treatment and Outcome Evaluation
All patients were treated with atezolizumab (1,200 mg fixed dose) and bevacizumab (15 mg/kg) every 3 weeks following the IMbrave150 protocol [12]. Treatment was continued until disease progression, intolerable toxicities, or withdrawal of consent. Tumor response was evaluated every 6 or 9 weeks using computed tomography or magnetic resonance imaging, following the Response Evaluation Criteria in Solid Tumors version 1.1. AEs were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Definitions
Thyroid AE was defined as a newly developed thyroid dysfunction after the initiation of Ate/Bev treatment. Overt hypothyroidism was defined as a thyroid-stimulating hormone (TSH) level above the upper reference limit with a free thyroxine (T4) level below the lower reference limit or by TSH ≥10.0 µIU/mL regardless of the free T4 level. Overt thyrotoxicosis was defined as a TSH level below the lower reference limit with a free T4 level above the upper reference limit. Subclinical hypothyroidism was defined as normal free T4 levels with elevated TSH levels above the upper reference limit and <10 µIU/mL, and subclinical thyrotoxicosis was defined as normal free T4 levels with reduced TSH levels below the lower reference limit. Isolated hypo- and hyperthyroxinemia were defined as normal TSH levels with free T4 levels below or above the reference interval, respectively [13, 14]. Normal TSH and free T4 levels were determined based on the reference ranges of each hospital.
Blood Sample Collection and Measurement of Serum Cytokines
Blood samples were obtained immediately before the first administration of Ate/Bev. The blood was centrifuged at 1,000 g for 5 min to isolate the serum, which was stored at −80°C. Serum cytokine levels were analyzed using a cytometric bead array (560484, BD Biosciences), according to the manufacturer’s instructions.
T-Cell Proliferation and Cytokine Production Assay
Peripheral blood mononuclear cells were isolated using the density gradient centrifugation with Ficoll-Paque PLUS (GE Healthcare). T-cell proliferation and cytokine production were analyzed as previously described [15, 16].
Flow Cytometry Analysis
Flow cytometry analysis was performed as previously described [16, 17]. Briefly, single-cell suspensions were stained with the following fluorochrome-conjugated antibodies: anti-human CD3 (clone SK7, eBioscience), anti-human CD8 (clone RPA-T8, eBioscience), anti-human PD-1 (clone EH12.2H7, BioLegend), anti-human TIM-3 (clone F38-2E2, BioLegend), and anti-human TIGIT (clone A15153G,. BioLegend). For intracellular staining, cells were fixed and permeabilized using a FoxP3 staining buffer kit (Thermo Fisher Scientific) and stained with the following antibodies: anti-human Ki67 (clone Ki67, BioLegend), anti-human interferon-γ antibody (clone B27, BD Bioscience), and anti-human tumor necrosis factor (TNF)-α antibody (clone Mab11, BD Bioscience). All flow data were acquired using a CytoFLEX flow cytometer (Beckman Coulter) and analyzed using FlowJo software (Tree Star Inc.).
Statistical Analysis
Categorical variables were compared using either Pearson’s χ2 test or Fisher’s exact test. For continuous variables, the Kolmogorov-Smirnov test was used to test the normality of data, and either the independent t test or Wilcoxon-Mann-Whitney test was used for comparisons. Survival analysis was performed using the Kaplan-Meier method, and the subgroups were compared using log-rank statistics. Cox proportional hazards regression was used to perform univariate and multivariate analyses of the survival outcomes. In time-dependent Cox regression model, the development of thyroid AEs was used as a time-dependent exposure variable. All statistical analyses were performed using SPSS Statistics software version 26 (IBM, Armonk, NY, USA). Statistical significance was defined as two-sided p values of <0.05.
Results
Patient Characteristics and Treatment Outcomes
The baseline patient characteristics are presented in Table 1. The median patient age was 61.0 (interquartile range [IQR]: 54.3–68.0) years, and 86.1% of the patients were male. Most patients had Child-Pugh class A (81.3%) and Barcelona clinical liver cancer stage C (81.7%). Hepatitis B (68.8%) was the most common cause of HCC. Most patients (69.7%) had received at least one prior local therapy for HCC. The median follow-up duration was 11.0 (IQR, 8.2–15.9) months. The objective response rate (ORR) was 31.3% (95% confidence interval [CI], 25.0–37.6%), and the median progression-free survival (PFS) and overall survival (OS) were 7.6 (95% CI, 6.0–9.3) and 17.2 (95% CI, 14.9–19.5) months, respectively.
Table 1.
Baseline clinical characteristics
| All patients (n = 208) | Thyroid AE (n = 41) | No thyroid AE (n = 167) | p value | ||
|---|---|---|---|---|---|
| Age, median (IQR), years | 61.0 (54.3–68.0) | 62.0 (54.0–67.5) | 61.0 (54.0–68.0) | 0.822 | |
| Male sex, n (%) | 179 (86.1) | 33 (80.5) | 146 (87.4) | 0.251 | |
| ECOG performance status, n (%) | |||||
| 0 | 97 (46.6) | 19 (46.3) | 78 (46.7) | 0.967 | |
| 1 | 111 (53.4) | 22 (53.7) | 89 (53.3) | ||
| Child-Pugh classification, n (%) | |||||
| A | 169 (81.3) | 35 (85.4) | 134 (80.2) | 0.451 | |
| B | 39 (18.8) | 6 (14.6) | 33 (19.8) | ||
| Barcelona clinical liver cancer stage, n (%) | |||||
| B | 38 (18.3) | 9 (22.0) | 29 (17.4) | 0.496 | |
| C | 170 (81.7) | 32 (78.0) | 138 (82.6) | ||
| AFP ≥400 ng/mL, n (%) | 72 (34.6) | 9 (22.0) | 63 (37.7) | 0.057 | |
| NLR, median (IQR) | 2.6 (1.7–4.2) | 2.1 (1.6–3.5) | 2.8 (1.8–4.2) | 0.040 | |
| Presence of MVI, n (%) | 84 (40.4) | 17 (41.5) | 67 (40.1) | 0.875 | |
| Presence of extrahepatic spread, n (%) | 127 (61.1) | 23 (56.1) | 104 (62.3) | 0.467 | |
| Etiology of HCC, n (%) | |||||
| Hepatitis B | 143 (68.8) | 28 (68.3) | 115 (68.9) | 0.663 | |
| Hepatitis C | 13 (6.3) | 1 (2.4) | 12 (7.2) | ||
| Alcohol | 25 (12.0) | 6 (14.6) | 19 (11.4) | ||
| Other or unknown | 27 (13.0) | 6 (14.6) | 21 (12.6) | ||
| Prior local therapy for HCC, n (%) | 145 (69.7) | 32 (78.0) | 113 (67.7) | 0.195 | |
Significant p values in bold.
AE, adverse event; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; MVI, macrovascular invasion.
Characteristics of Thyroid AEs after Ate/Bev in HCC
The Ate/Bev-associated thyroid AEs occurred in 41 (19.7%) patients during the study period, and the median time to onset of thyroid dysfunction was 2.7 (IQR, 1.3–4.5) months. Primary hypothyroidism was the most common thyroid AE, affecting 36 (17.3%) patients (Table 2). Overt and subclinical hypothyroidism occurred in 29 (13.9%) and seven (3.4%) patients, respectively. Primary thyrotoxicosis occurred in 12 (5.8%) patients, six of whom were overt, and six were subclinical. There were three (1.4%) patients with isolated hypothyroxinemia and no patients with isolated hyperthyroxinemia. The median time to onset of hypothyroidism and thyrotoxicosis after Ate/Bev treatment was 3.5 (IQR, 2.6–6.0) and 1.3 (IQR, 1.2–2.7) months, respectively (Table 2). In addition, the median duration of hypothyroidism and thyrotoxicosis after Ate/Bev treatment was 8.4 (IQR, 4.0–14.4) and 2.4 (IQR, 1.8–4.2) months, respectively. Of the 36 patients who experienced hypothyroidism, four (11.1%) recovered to normal thyroid function, while the remaining patients had permanent hypothyroidism. In accordance with clinical practice guidelines [18, 19], thyroid hormone supplementation was prescribed for patients with permanent hypothyroidism who had symptoms or a sustained TSH level over 10 μIU/mL. None of the patients who experienced thyrotoxicosis continued to have thyrotoxicosis or required antithyroid drug treatment during the follow-up period. While 3/12 (25.0%) patients recovered to normal thyroid function, 9/12 (75.0%) patients progressed to hypofunction of the thyroid (seven overt hypothyroidism, one subclinical hypothyroidism, and one isolated hypothyroxinemia). All cases were classified as grade 1 or 2 by CTCAE 4.0 criteria. Table 1 shows the baseline characteristics according to the thyroid AEs. Notably, the baseline neutrophil-lymphocyte ratio (NLR) was significantly lower in patients with thyroid AEs than in those without thyroid AEs. In addition, among the Ate/Bev treatment-related AEs, dermatological AEs and adrenal insufficiency were significantly more common in patients with thyroid AEs than in those without thyroid AEs (online suppl. Table 1).
Table 2.
Characteristics of thyroid AEs
| Type of thyroid dysfunction, n (%) | |
| Hypothyroidism | 36 (17.3) |
| Overt hypothyroidism | 29 (13.9) |
| Subclinical hypothyroidism | 7 (3.4) |
| Thyrotoxicosis | 12 (5.8) |
| Overt thyrotoxicosis | 6 (2.9) |
| Subclinical thyrotoxicosis | 6 (2.9) |
| Isolated hypothyroxinemia | 3 (1.4) |
| Isolated hyperthyroxinemia | 0 (0.0) |
| Time to onset of thyroid dysfunction, median (IQR), months | |
| Hypothyroidism | 3.5 (2.6–6.0) |
| Thyrotoxicosis | 1.3 (1.2–2.7) |
| Isolated hypothyroxinemia | 3.1 (2.2–5.0) |
| Duration of thyroid dysfunction, median (IQR), months | |
| Hypothyroidism | 8.4 (4.0–14.4) |
| Thyrotoxicosis | 2.4 (1.8–4.2) |
| Isolated hypothyroxinemia | 10.7 (10.1–12.7) |
| Resolution of thyroid dysfunction, n (%) | |
| Hypothyroidism | 4/36 (11.1) |
| Thyrotoxicosis | 12/12 (100.0) |
| Isolated hypothyroxinemia | 1/3 (33.3) |
| CTCAE grade, n (%) | |
| Grade 1 | 25 (49.0) |
| Grade 2 | 26 (51.0) |
IQR, interquartile range; CTCAE, Common Terminology Criteria for Adverse Events.
Association of Thyroid AEs with Favorable Clinical Outcomes of Ate/Bev in HCC
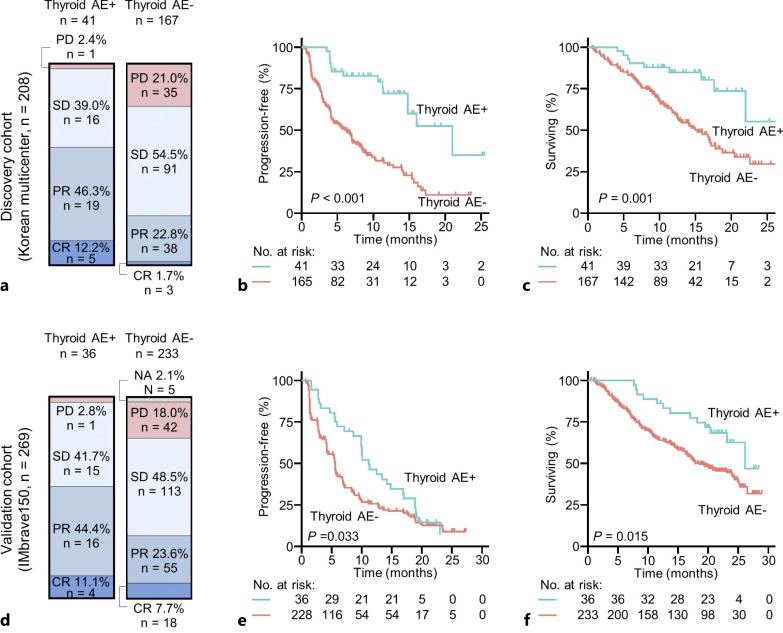
We examined whether the development of thyroid AEs was associated with the clinical outcomes of the Ate/Bev treatment. Patients with thyroid AEs had higher ORR compared to those without thyroid AEs (58.5 vs. 24.6%, respectively, p < 0.001; Fig. 1a). Moreover, PFS and OS were significantly better in patients with thyroid AEs than in those without thyroid AEs (median PFS, 21.0 vs. 6.3 months, respectively, p < 0.001; median OS, not reached vs. 15.3 months, respectively, p = 0.001; Fig. 1b, c). Furthermore, among irAEs, thyroid AEs had the greatest impact on both DFS and OS (online suppl. Table 2). Because the incidence of thyroid dysfunction increased with longer duration of Ate/Bev treatment and survival of patients, we further performed a time-dependent analysis using a time-dependent Cox regression model in which the development of thyroid AEs was used as a time-dependent variable. Even in this analysis, PFS and OS were consistently longer in patients with thyroid AEs compared with those without thyroid AEs (p = 0.024 and p = 0.027, respectively; online suppl. Fig. 2a, b).
Fig. 1.
Best response to therapy, PFS, and OS according to thyroid AEs. a–c Discovery cohort. d–f Validation cohort. AE, adverse event; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
To minimize false-negatives from patients with short-term Ate/Bev exposure, we excluded patients with progressive disease at the first response evaluation and performed a subgroup analysis in the remaining patients. Consistently, patients with thyroid AEs showed better PFS and OS than those without thyroid AEs (p < 0.001 and p = 0.035, respectively; online suppl. Fig. 2c, d).
On categorizing the patients into overt and subclinical thyroid AE groups, patients in both the overt and subclinical thyroid dysfunction groups were strongly associated with better PFS than those without thyroid AEs (online suppl. Fig. 2e). However, OS was significantly better in patients in the overt thyroid dysfunction group than in those without thyroid AEs, but not in patients in the subclinical thyroid dysfunction group (online suppl. Fig. 2f). In particular, the median OS in the overt thyroid dysfunction group was significantly longer than that in the subclinical thyroid dysfunction group (p = 0.005). In addition, when patients with thyroid AEs were categorized into early- and late-onset groups according to the median time to onset of thyroid dysfunction, they were strongly associated with better PFS and OS than patients without thyroid AEs (online suppl. Fig. 2g, h). There was no difference in PFS and OS between the early- and late-onset groups.
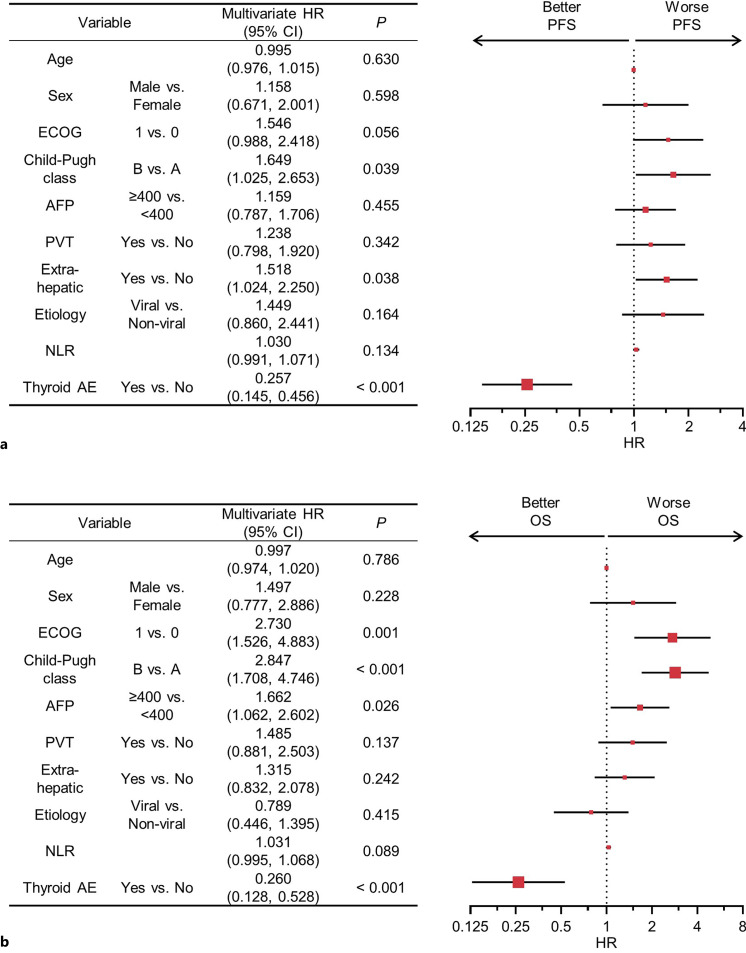
Because various factors could confound the association of thyroid AEs with a favorable prognosis of HCC, we further validated the clinical implications using the multivariate Cox proportional hazard model. Even after adjusting for age, sex, ECOG performance status, Child-Pugh score, alpha-fetoprotein level, macroscopic vascular invasion, extrahepatic spread, etiology of HCC, and NLR, the development of thyroid AEs during Ate/Bev treatment remained the most significant factor associated with better PFS and OS (hazard ratios, 0.3 [95% CI, 0.1–0.4; p < 0.001] and 0.3 [95% CI, 0.1–0.5; p < 0.001], respectively; Figure 2a, b).
Fig. 2.
Multivariable analysis of PFS and OS. Forest plots showing multivariate HRs and 95% CIs for PFS (a) and OS (b). HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group performance status; AFP, alpha-fetoprotein; MVI, macrovascular invasion; NLR, neutrophil-lymphocyte ratio; AE, adverse event; PFS, progression-free survival; OS, overall survival.
Validation of Thyroid AEs with Favorable Clinical Outcomes of Ate/Bev in HCC
To externally validate our finding, we analyzed clinical outcomes according to thyroid AEs in patients treated with Ate/Bev in the IMbrave150 study. Of a total of 336 patients, 269 were included in the analysis, excluding 64 with data sharing prohibited due to consent, legal, regulatory, or contractual constraints and three with thyroid dysfunction before initiation of treatment or related to other medical conditions. The baseline patient characteristics of the IMbrave150 study cohort are presented in online supplementary Table 3. In the IMbrave150 study cohort, 13.4% (36/269) of patients had thyroid AEs following Ate/Bev treatment, of whom 26 were hypothyroidism and 10 were hyperthyroidism. Patients with thyroid AEs had higher ORR than those without thyroid AEs (55.6 vs. 31.3%, p = 0.004, Fig. 1d). Moreover, patients with thyroid AEs exhibited better PFS and OS than those without thyroid AEs (p = 0.033 and 0.015, respectively, Fig. 1e, f). Therefore, we confirmed the association between thyroid AEs and favorable clinical outcomes of Ate/Bev in our multicenter cohort and the IMbrave150 study cohort.
Association of the Development of Thyrotoxicosis with Baseline IL-6 Levels
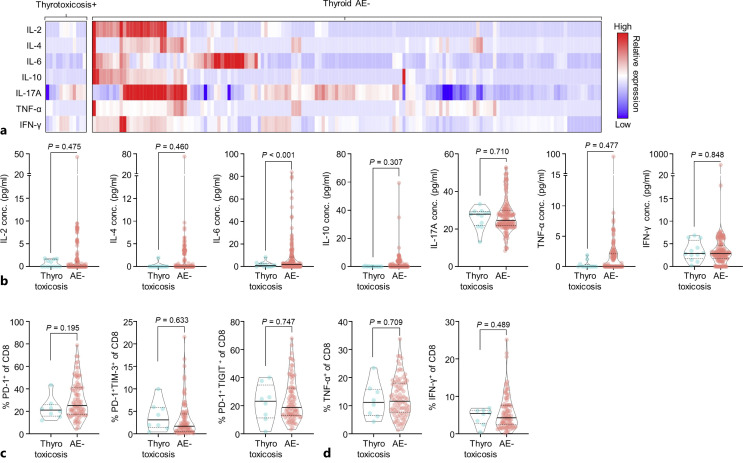
As ICI-induced immune responses may mediate thyroid AEs, we compared circulating cytokine levels and early T-cell responses in patients with and without thyroid AEs. When peripheral blood cytokine levels at baseline were compared, baseline IL-6 levels were significantly lower in patients with thyrotoxicosis. However, there were no significant differences in the levels of IL-2, IL-4, IL-10, IL-17A, TNF-α, and IFN-γ (Fig. 3a, b). Next, we compared the proportion of CD8+ T cells and their effector cytokine production. At baseline, the fractions of PD-1+CD8+ T cells, TIM3+PD1+CD8+ T cells, and TIGIT+PD1+CD8+ T cells did not differ between the two groups (Fig. 3c). Moreover, the production of effector cytokines (TNF-α and IFN-γ) in CD8+ T cells was comparable between the two groups (Fig. 3d). However, patients with hypothyroidism did not show significant differences in circulating cytokine levels and T-cell subsets (online suppl. Fig. 3a–c). Overall, the development of thyrotoxicosis was significantly associated with peripheral IL-6 levels at baseline, but not with other cytokines and T-cell subsets.
Fig. 3.
Comparison of baseline cytokine levels and T-cell fractions between patients with thyrotoxicosis and those without thyroid AEs. Baseline cytokine levels (a, b), CD8+ cell subset fractions (c), and effector cytokines in CD8+ T cells (d).
Discussion
In this study, we found that thyroid AEs occurred in 19.7% of patients (17.3% with hypothyroidism and 5.8% with thyrotoxicosis) after Ate/Bev treatment, with a median follow-up of 11.0 months. Patients with thyroid AEs exhibited favorable clinical outcomes with Ate/Bev treatment compared with those without thyroid AEs. Through the analysis of circulating cytokine levels and early T-cell responses in patients with and without thyroid AEs, we demonstrated that patients with thyrotoxicosis showed a significantly lower level of baseline IL-6.
In a systematic review and meta-analysis of patients treated with cytotoxic T-lymphocyte-associated protein-4 inhibitors (ipilimumab), PD-1 inhibitors (nivolumab or pembrolizumab), or PD-L1 inhibitors (atezolizumab), the incidence of hypothyroidism was 3.9%, 7.0%, and 3.9%, and that of thyrotoxicosis was 1.7%, 3.2%, and 0.6%, respectively. Although the specific tumor type was not significantly associated with the incidence of hypothyroidism or thyrotoxicosis, patients treated with combination immunotherapy were significantly more likely to experience thyroid dysfunction [11]. In the IMbrave150 trial with a median follow-up of 8.9 months, 10.9% and 4.6% of patients treated with Ate/Bev experienced hypothyroidism and thyrotoxicosis, respectively [12], and this incidence was higher than atezolizumab alone in a previous meta-analysis. In the present study, the incidence of thyroid dysfunction, especially hypothyroidism, was higher than that reported in the IMbrave150 trial. A possible explanation is that we included patients with subclinical thyroid dysfunction and had a longer median follow-up duration.
The median time to onset of hypo- and hyperthyroidism after Ate/Bev treatment was 3.5 and 1.3 months, respectively. This finding is consistent with that reported by Ikeda et al. in the IMbrave150 analysis (3.5 months of hypothyroidism and 2.7 months of hyperthyroidism) [12]. These results indicate that thyroid AEs are relatively early events after Ate/Bev treatment rather than a consequence of chronic exposure to Ate/Bev treatment. The clinical course of thyroid AEs in the present study was consistent with that of previous ICI treatments for other solid tumors [14, 20, 21]. Most patients with thyroid AEs progressed to hypothyroidism with or without a prior hyperthyroidism period.
Patients with thyroid AEs demonstrated significantly better clinical outcomes in terms of PFS, OS, and ORR than those without thyroid AEs. These findings were consistent even after adjusting for confounding factors, such as age, sex, ECOG performance status, Child-Pugh class, alpha-fetoprotein level, macroscopic vascular invasion, extrahepatic spread, etiology of HCC, and NLR. Furthermore, favorable survival outcomes in patients with thyroid AEs were also externally validated in a cohort of IMbrave150 patients treated with Ate/Bev. In a further evaluation conducted after excluding patients with progressive disease to minimize short-term exposure to Ate/Bev, thyroid AEs still showed favorable survival outcomes, indicating that this subset of patients received significant clinical benefit from Ate/Bev. Furthermore, both overt and subclinical thyroid dysfunction were associated with prolonged PFS; however, only overt thyroid dysfunction was confirmed as a prognostic factor for OS. This result was consistent with a previous study on thyroid AEs after nivolumab treatment, which found that better OS was only associated with overt thyroid dysfunction and not subclinical thyroid dysfunction [22]. Meanwhile, the survival outcomes of patients with early- and late-onset thyroid AEs were comparable. This indicates that the favorable prognostic impact of thyroid AEs was maintained regardless of the time of onset.
Many studies have reported the association between thyroid irAEs and improved outcomes in other cancer types [10, 23, 24]. A recent meta-analysis showed that thyroid irAE development during ICI therapy was associated with improved PFS and OS (hazard ratios, 0.58 and 0.52, respectively) [24]. However, the majority of the study population consisted of patients with non-small cell lung cancer, and the vast majority of patients received PD-1 inhibitors, making it difficult to extrapolate the study’s results to the broader cancer population treated with any ICIs. Therefore, our study stands out from previous studies as it involves the concurrent administration of bevacizumab, an angiogenesis inhibitor, along with ICI. Furthermore, our study has the strength of being the first to report the correlation between thyroid irAEs and Ate/Bev treatment in patients with HCC.
Notably, this immunological side effect of immunotherapy is closely related to the antitumor mechanism. In this study, patients with thyroid AEs had a lower NLR and a higher incidence of dermatological AEs and adrenal insufficiency, suggesting that T-lymphocyte activation might be involved in the occurrence of thyroid AEs and a favorable response to immunotherapy. Thus, we attempted to elucidate the association between thyroid AEs, T-cell immunity, and various cytokines using multiplex flow cytometric analyses. We were able to identify that patients with thyrotoxicosis had a lower level of baseline IL-6. This is consistent with our previous finding that high baseline IL-6 levels can be associated with reduced clinical outcomes and impaired T-cell function in patients with unresectable HCC after Ate/Bev treatment [25].
We found a favorable survival outcome in patients with thyroid AEs through univariate and multivariate analyses in a large multicenter cohort and IMbrave150 validation cohort. Therefore, thyroid AEs after Ate/Bev treatment may provide a clue for favorable efficacy as an early on-treatment pharmacodynamic marker.
However, this study had some limitations. As Ate/Bev treatment is a combination therapy of anti-PD-L1 and anti-vascular endothelial growth factor, bevacizumab may affect the development of thyroid AEs through nonimmune mechanisms. However, we were unable to separately evaluate the effects of Ate/Bev on thyroid dysfunction. Therefore, further research is required to elucidate the precise mechanisms underlying Ate/Bev-induced thyroid dysfunction. In addition, although we elucidated the association between thyrotoxicosis and low level of IL-6, we were unable to identify the association between specific immunological phenotypes and hypothyroidism. In the present study, we only examined the immune profiles at baseline, but long-term serial monitoring of peripheral blood cytokines may be required to further elucidate immunologic changes that precede or accompany thyroid dysfunctions during Ate/Bev treatment. Moreover, there is a possibility that the background liver function might affect the baseline immune profile, but the significance was unclear because of the limited number of enrolled patients.
This study demonstrated that a fraction of patients with HCC experienced thyroid dysfunction relatively early after Ate/Bev treatment. Patients with thyroid AEs during Ate/Bev treatment demonstrated favorable clinical outcomes compared with those without thyroid AEs. Therefore, thyroid AEs may provide early clinical clues for treatment outcomes in patients with unresectable HCC treated with Ate/Bev.
Acknowledgments
We thank So Jung Kong and Seung Joon Lee for their technical support. This publication is based on research using data from data contributors Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Statement of Ethics
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the participating hospitals’ Institutional Review Boards (CHA Bundang Medical Center, CHA-2017-11-052, CHA-2017-11-054; Ulsan University Hospital, 2020-12-006; Haeundae Paik Hospital, 2020-12-019-001). Written informed consent was obtained from all the patients.
Conflict of Interest Statement
Hong Jae Chon has a consulting or advisory role at Eisai, Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, SillaJen, Menarini, and GreenCross Cell and has received research grants from Roche, Dong-A ST, and Boryung Pharmaceuticals. Chan Kim has a consulting or advisory role at Roche, ONO, MSD, BMS, Oncocross, and Virocure and has received research grants from Boryung Pharmaceuticals, Oncocross, SillaJen, and Virocure. Ho Yeong Lim has a consulting or advisory role at Eisai, Roche, Bayer, ONO, MSD, BMS, and AstraZeneca. Vincent E. Gaillard is a full-time employee of F. Hoffmann-La Roche Ltd. The other authors have no potential conflicts of interest to disclose.
Funding Sources
This work was supported by the National Research Foundation of Korea [NRF] grants funded by the Korean government [MSIT] [NRF-2023R1A2C2004339 to Hong Jae Chon, NRF-2023R1A2C2006375 to Chan Kim, and NRF-2020R1C1C1003924 to Young Shin Song].
Author Contributions
Conceptualization: Chan Kim and Hong Jae Chon; methodology and formal analysis: Young Shin Song and Hannah Yang; investigation: Young Shin Song, Hannah Yang, Beodeul Kang, Jaekyung Cheon, Ilhwan Kim, Hyeyeong Kim, Won Suk Lee, Yun Beom Sang, Sanghoon Jung, Ho Yeong Lim, Vincent E. Gaillard, Chan Kim, and Hong Jae Chon; resources: Ilhwan Kim, Hyeyeong Kim, Vincent E. Gaillard, Chan Kim, and Hong Jae Chon; data curation: Young Shin Song and Hong Jae Chon; writing – original draft: Young Shin Song, Hannah Yang, Chan Kim, and Hong Jae Chon; writing – review and editing: Beodeul Kang, Jaekyung Cheon, Ilhwan Kim, Hyeyeong Kim, Won Suk Lee, Yun Beom Sang, Sanghoon Jung, Ho Yeong Lim, and Vincent E. Gaillard.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.
Funding Statement
This work was supported by the National Research Foundation of Korea [NRF] grants funded by the Korean government [MSIT] [NRF-2023R1A2C2004339 to Hong Jae Chon, NRF-2023R1A2C2006375 to Chan Kim, and NRF-2020R1C1C1003924 to Young Shin Song].
Supplementary Material
References
- 1. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382(20):1894–905. 10.1056/nejmoa1915745. [DOI] [PubMed] [Google Scholar]
- 2. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022 Apr;76(4):862–73. 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 3. Fulgenzi CAM, Cheon J, D'Alessio A, Nishida N, Ang C, Marron TU, et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: results of the AB-real study. Eur J Cancer. 2022 Nov;175:204–13. 10.1016/j.ejca.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 4. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73–82. 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. [Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment]. Bull Cancer. 2018 Nov;105(11):1033–41. 10.1016/j.bulcan.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Zhai X, Li J, Guan J, Xu S, Li Y, et al. The role of cytokines in predicting the response and adverse events related to immune checkpoint inhibitors. Front Immunol. 2021;12:670391. 10.3389/fimmu.2021.670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lechner MG, Cheng MI, Patel AY, Hoang AT, Yakobian N, Astourian M, et al. Inhibition of IL-17a protects against thyroid immune-related adverse events while preserving checkpoint inhibitor antitumor efficacy. J Immunol. 2022 Aug 15;209(4):696–709. 10.4049/jimmunol.2200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306. 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lima Ferreira J, Costa C, Marques B, Castro S, Victor M, Oliveira J, et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother. 2021 Feb;70(2):299–309. 10.1007/s00262-020-02664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018 Feb 1;4(2):173–82. 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kudo M, Ikeda M, Zhu AX, Qin S, Kim T-Y, Lim HY, et al. 169P IMbrave150: management of adverse events of special interest (AESIs) for atezolizumab (atezo) and bevacizumab (bev) in unresectable HCC. Ann Oncol. 2020;31(6):S1304–05. 10.1016/j.annonc.2020.10.190. [DOI] [Google Scholar]
- 13. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. 2021 Aug 18;106(9):e3704–13. 10.1210/clinem/dgab263. [DOI] [PubMed] [Google Scholar]
- 14. Yoon JH, Hong AR, Kim HK, Kang HC. Characteristics of immune-related thyroid adverse events in patients treated with PD-1/PD-L1 inhibitors. Endocrinol Metab. 2021 Apr;36(2):413–23. 10.3803/EnM.2020.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH, et al. The first-week proliferative response of peripheral blood PD-1(+)cd8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019 Apr 1;25(7):2144–54. 10.1158/1078-0432.ccr-18-1449. [DOI] [PubMed] [Google Scholar]
- 16. Kim C, Yang H, Kim I, Kang B, Kim H, Kim H, et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 2022 Oct 20;8(12):1825. 10.1001/jamaoncol.2022.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest. 2019 Jul 25;129(10):4350–64. 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021 Dec 20;39(36):4073–126. 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 19. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Dec;33(12):1217–38. 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 20. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018 Oct;28(10):1243–51. 10.1089/thy.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, Wada E, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. 2020 Mar;122(6):771–7. 10.1038/s41416-020-0736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14(5):e0216954. 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotwal A, Ryder M. Survival benefit of endocrine dysfunction following immune checkpoint inhibitors for nonthyroidal cancers. Curr Opin Endocrinol Diabetes Obes. 2021 Oct 1;28(5):517–24. 10.1097/MED.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 24. Cheung YM, Wang W, McGregor B, Hamnvik OPR. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2022 Aug;71(8):1795–812. 10.1007/s00262-021-03128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H, Kang B, Ha Y, Lee SH, Kim I, Kim H, et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 2023 Apr;5(4):100672. 10.1016/j.jhepr.2023.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.