Abstract
In recent years, a substantial amount of data have supported an active role of gut microbiota in mediating mammalian brain function and health. Mining gut microbiota and their metabolites for neuroprotection is enticing but requires that the fundamental biochemical details underlying such microbiota–brain crosstalk be deciphered. While a neuronal gut–brain axis (through the vagus nerve) is not disputable, accumulating studies also point to a humoral route (via blood/lymphatic circulation) by which innumerable microbial molecular cues translocate from local gut epithelia to circulation with potentials to further cross the blood–brain barrier and reach the brain. In this Perspective, we review a realm of gut microbial molecules to evaluate their fate, function, and neuroactivities in vivo as mediated by microbiota. We turn to seminal studies of neurophysiology and neurologic disease models for the elucidation of biochemical pathways that link microbiota to gut–brain signaling. In addition, we discuss opportunities and challenges for advancing the microbiota–brain axis field while calling for high-throughput discovery of microbial molecules and studies for resolving the interspecies, interorgan, and interclass interaction among these neuroactive microbial molecules.
Graphical Abstract
1. MICROBIOTA, GUT–BRAIN AXIS, AND MOLECULES
The advancement of next-generation sequencing (NGS) technologies over the past decade has led to explosive growth in the knowledge of the trillions of microorganisms colonizing our gastrointestinal (GI) tract, collectively known as gut microbiota.1 These resident microbes have been demonstrated to mediate important physiological processes in mammalian host bodies, ranging from energy metabolism,2 vitamin biosynthesis,3 and immune cell development4 to hormonal regulation5 and epithelial homeostasis,6 and are appealing in new therapeutic venues such as countermeasure against radiation-induced injuries.7 In turn, when perturbed or in an imbalanced state (i.e., dysbiotic), gut microbial communities have been linked to a long list of diseases and conditions, including metabolic syndrome (e.g., obesity and type II diabetes), autoimmune conditions (e.g., inflammatory bowel disease), and cancer (e.g., colon cancer). Notably, emerging studies on neurologic pathologies such as autism,8–10 Alzheimer’s disease,11–13 amyotrophic lateral sclerosis (ALS),14 and multiple sclerosis15 collectively support the idea that microbiota also actively interacts with the mammalian brain. This raises interesting questions about gut microbiota’s neuroactivity and the associated effects on the gut–brain axis, i.e., the bidirectional communication between the central nervous system (CNS, the “first brain”) and the enteric nervous system (ENS, the “second brain”).16
Accumulating research, mostly in vivo model-based, has explored microbiota’s roles in the neuronal, immune, and endocrine aspects of the two-way gut–brain crosstalk.17–19 Sudo and colleagues first reported that commensal microbiota in mice perturbed stress responses through the hypothalamic–pituitary–adrenal (HPA) axis.20 Compared with their conventional wild-type counterparts, pathophenotypes observed in germ-free (GF) mice such as heightened stress responses and low brain-derived neurotrophic factor (BDNF) levels in cortex and hippocampus were substantially restored through select microbial reconstitution during a critical developmental window. This indicated a causal but conditional role of microbiota in stress response regulation. Likewise, mice regularly fed Lactobacillus rhamnosus exhibited improved emotional behavior likely owing to altered neurotransmission in the central GABAergic system mediated through the vagus nerve.21 In a seminal work probing the effects of gut bacteria on autism spectrum disorder (ASD) pathogenesis,8 maternal immune activation (MIA) during pregnancy successfully induced murine offspring ASD-mimicking defects in the GI barrier and behaviors. These defects were dramatically restored by inoculating human Bacteroidetes fragilis, suggesting for autism a molecular mechanism through effects of gut bacterial products on the host metabolome. More recently, fecal microbiota transplantation (FMT) from young mice (3–4 months old) reversed aging-associated cognitive behavioral deficits (e.g., long-term spatial memory) in aged recipient mice (19–20 months old) while offsetting immunological, transcriptome, and metabolome differences, providing fundamental evidence for using FMT as a therapeutic approach toward healthy aging.22 Conversely, improved neurogenesis and prolongevity signaling were observed in FMT on germ-free mouse models, as well (from old to young).23 All of these pioneering studies indicate an active role of gut microbiota in brain development and function, opening doors of microbiome-based therapies for improved neurologic/mental health outcomes.
While the idea of targeting microbes for neuroprotection is enticing, controversies regarding how a microbiota–gut–brain axis can be defined and determined for human populations remain. Not until recently, however, has the first population-scale evidence linking microbiota to mental health been published,24,25 moving beyond the limitations of mainstream evidence mostly derived from animal models. By correlating fecal metagenomic features with quality of life (QoL) and depression indicators for the large Flemish Gut Flora Project (FGFP) cohort, Valles-Colomer and colleagues associated butyrate-producing Faecalibacterium and Coprococcus with higher QoL scores while identifying considerably lower levels of Dialister and Coprococcus spp. in patients diagnosed with depression. Gut–brain module analyses singled out the microbial synthetic potential of 3,4-dihydroxyphenylacetic acid (DOPAC), a dopamine metabolite, as being positively correlated with QoL scores alongside altered γ-aminobutyric acid (GABA) pathways in depression. In another cohort study, metagenome-wide association of gut microbial features in schizophrenia patients identified microbial short-chain fatty acid (SCFA) synthesis, tryptophan metabolism, and synthesis/degradation of neurotransmitters as contributors to schizophrenia pathogenesis.26 Further transplantation of schizophrenia fecal-enriched Streptococcus vestibularis in mice induced deficits in social behavior and perturbation of peripheral neurotransmitter profiles, while microbiota-related metabolite trajectories were examined across the perinatal period associated with mental health outcomes, overall revealing substantial temporal variation in tryptophan and bile acid metabolism with marked interindividual variability.27 Although these human cohort studies are by nature descriptive, they strengthen the notion that the gut microbiota harbors neuroactive potentials that can be harnessed for improved gut–brain signaling, physiologies, and neural health outcomes.
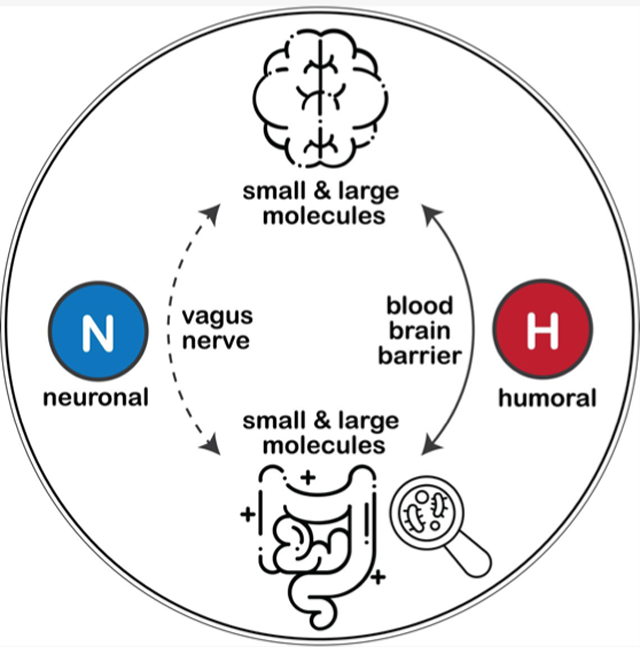
In the targeting of microbiota for neuroprotection, the biochemical underpinnings linking microbiota to the gut–brain axis remain elusive.28,29 While the neuronal pathway (through the vagus nerve connecting the CNS to the ENS) is not disputable, a growing body of evidence also points to a humoral route (via blood and lymphatic circulation)30 given the massive enzymatic activities and high turnover rates of microbiota-driven metabolism, especially in neurotransmitter production.19,31 Due to the relatively small sizes and/or ease of interorgan transportation, innumerable microbial molecular cues may likely translocate from local gut epithelia to the circulating blood and further cross the blood–brain barrier (BBB) to reach the brain while harboring potent signaling/regulatory potentials.32,33 A growing number of studies showed that gut microbe-related molecules modulate host homeostasis,34,35 alter barrier permeability,36 and induce neuroimmune responses,37 with multifaceted functional roles, for example, as neurotransmitters,38 as reactive oxygen species (ROS) scavengers,39 and/or as ligands for activating immune signal transductions.40
In this Perspective, we conduct a comprehensive and up-to-date review of the reported molecules of gut microbial sources, including both metabolites and peptides, to evaluate their biochemical fate and functional roles in host–microbe interaction. Importantly, we turn to seminal models of neurophysiology, neurologic diseases, and/or mental disorders to elucidate specific biochemical pathways of the microbiota–gut–brain axis in health and disease. Lastly, we discuss the implications of existing studies, especially the major challenges and opportunities for advancing microbiota–brain research for health sciences spanning the biomedical, nutrition, and public health arenas.
2. SMALL MOLECULES
2.1. Short-Chain Fatty Acids (SCFAs).
SCFAs are saturated fatty acids consisting of one to six carbon atoms. In mammalian colon, acetate, propionate, and butyrate are produced in large quantities through microbial fermentation of indigestible dietary fibers, comprising >95% of total SCFAs with a typical molar ratio of 6:2:2.41 The specific biosynthesis, uptake, and distribution details in vivo have been summarized recently.42–44 Acetate can be broadly transformed by gut bacteria from acetyl-CoA, which is derived from pyruvate, a key intermediate of the glycolysis–citric acid cycle.41 More substrate-specific and species-conserved, propionate is synthesized through multistep reduction of lactate or succinate or depends on substrates of deoxyhexose sugars (e.g., fucose and rhamnose),43 whereas butyrate can be formed either from downstream acetyl-CoA products such as butyryl-CoA and butyryl-phosphate, from lactate and acetate by certain gut bacterial species (e.g., Eubacterium hallii and Anaerostipes caccae), or from protein through the lysine pathway, as indicated by emerging metagenomics data.45 The concentration profile of SCFAs varies significantly along the GI tract but typically peaks at cecum and proximal colon, followed by a decline toward distal colon.42 Such a luminal SCFA decline can be partially explained by the transport of acetate and propionate into the circulating blood and local consumption of butyrate by colonocytes.42
SCFAs have been intensely studied as key bacterial metabolites conferring a multitude of benefits for host health, with confirmed SCFA producers such as Bacteroides spp., Clostridium spp., and Streptococcus spp.42 Because SCFAs act as an important energy source with signaling activities, a large proportion of the existing studies targeted SCFAs for linking microbiota to metabolic syndromes such as diabetes and obesity. Interestingly, although SCFAs manifest metabolic benefits like improved glucose tolerance46 and increased thermogenesis,47 fecal SCFA levels in obese patients are higher than in the lean controls, which warrants elucidation in the future.48 SCFAs also contribute to gut integrity and immune homeostasis; inside gut lumen, SCFAs modulate the pH, balance redox equivalent production, and fortify the gut lining.41,43,49 In immune regulation, SCFAs activate G protein-coupled receptors (GPRs), inhibit histone deacetylases (HDACs),42 control regulatory T cell (Tregs) expansion,50 and regulate local synthesis and release of key endocrine agents, including glucagon-like peptide 1 (GLP-1)41 and serotonin.51
Mounting studies show profound effects of gut microbial SCFAs on host brain function and/or CNS-mediated pathogenesis through, for example, regulating host neurodevelopment (e.g., microglial maturation),52 modulating BBB permeability,53 and acting on the ENS for appetite control.54 Erny and colleagues reported that eradicating a complex host microbiota, either constitutionally (as found in GF mice) or in an induced fashion (e.g., by antibiotics, or abx), led to severe defects in the density, morphology, and maturation of microglia, the resident macrophages in mammalian brain.52 In turn, a four-week administration of SCFAs (cocktail mix of acetate, propionate, and butyrate) markedly rectified the microglial defects in GF mice. Interestingly, while the Ffar2−/− mice (i.e., double knockout of genes encoding free fatty acid receptor 2) exhibited substantial microglial compromises, the FFAR2 protein (also known as GPR43) was not detected in CNS tissues, leaving how SCFAs communicated with the CNS incompletely elucidated. In another work employing mouse models of Parkinson’s disease (PD), Sampson et al. identified gut microbiota as a causal contributor to the hallmark motor and GI dysfunction of PD.55 One mechanism established is postnatal gut–brain signaling by microbial SCFAs to mediate neuroinflammatory responses and aggregation of α-synuclein (αSyn) protein. In addition, Perry and colleagues identified acetate as a microbiome–brain–β-cell axis mediator for promoting metabolic syndromes.56 Using whole-body turnover rate determination and stable-isotope tracer analysis in vivo, the authors observed in high-fat diet (HFD)-fed rats a marked increase in the rate of acetate turnover, for which the gut microbiome was confirmed as arguably the main source. Strikingly, such surging acetate levels promoted glucose-stimulated insulin secretion (GSIS) via activating the parasympathetic nervous system, resulting in hyperphagia, obesity, and related sequelae, all of which were prevented by severing the vagus nerve. In a new work, gut bacteria-derived acetate was discovered to also drive microglial maturation and functions during neurodegeneration, specifically the microglial phagocytosis of amyloid-β and disease progression in a mouse model of AD.13 Beyond these seminal studies, research also showed that SCFAs modulate BBB,57 regulate satiety,41 and interact extensively with the ENS, where local SCFA receptor GPR41 is strongly expressed.42,58
2.2. Bile Acids (BAs).
BAs are hepatically synthesized steroid acids (de novo, from cholesterol) that are subsequently conjugated to taurine, glycine, phenylalanine, tyrosine, and leucine (collectively termed primary BAs).59,60 Upon food intake, primary BAs are discharged from the gallbladder into the gut lumen, where they emulsify dietary fats and experience massive microbial transformation into bioactive products known as secondary BAs.59 With amphipathic functional groups, BAs exhibit detergent-like properties that facilitate lipid uptake, transport, and metabolism, with the enterohepatic circulatory pool cycle occurring at a frequency that depends on dietary patterns. Interestingly, more recent work revealed BAs as signaling molecules, as well. For example, BAs activate the nuclear receptor farnesoid X receptor (FXR) and Takeda G protein-coupled bile acid receptor (TGR5), both of which, although working seemingly independently, have been shown to be potent regulators of BA synthesis, energy metabolism, and immune cell homeostasis.61–63
Understanding gut microbiota’s control over BA synthesis and signaling will benefit the fundamental design of therapeutic solutions for diseases ranging from diabetes64 and nonalcoholic fatty liver disease (NAFLD)65,66 to colon cancer.67 On one hand, BAs curtail gut bacterial growth toward a more balanced ecological state that contributes to gut epithelial homeostasis. On the other hand, gut bacteria harbor a versatile enzymatic toolkit (e.g., glucuronidase and dehydroxylase) that drives biotransformation of primary BAs into secondary BAs that typically embrace increased chemical structural diversity and signaling potency.68,69 Targeted profiling of BAs in GF-treated, abx-treated, and conventionally raised rats showed that the absence of microbes (as found in both GF- and abx-treated mice) led to less diverse BA profiles dominated by taurine-conjugated BAs at extrahepatic sites of kidney, heart, and blood relative to those in conventional counterparts.70 In addition, Sayin et al. identified for conventional wild-type C57BL/6 mice decreased enterohepatic levels of muricholic acid (MCA) but not cholic acid (CA) compared with those of GF individuals.71 Using Fxr–/– murine strains, the study demonstrated gut microbiota’s control over the expression of ileum fibroblast growth factor 15 (FGF15) and hepatic 7α-cholesterol hydrolase in a farnesoid X receptor (FXR)-dependent manner, specifically through reducing tauro-β-muricholic acid (TβMCA), a natural FXR antagonist. Remarkably, Quinn and colleagues recently discovered new BA conjugates (in addition to tauro and glyco species) with massive chemical impacts from microbiota, improving our fundamental understanding of the complex and diverse bile acid chemistry.60 Comparative microbial (16s rRNA amplicon sequencing) and metabolite profiling (molecular networking-based annotation) across 29 organs (and/or matrices) of GF versus colonized mice identified a unique panoply of contrasting chemical signatures in the GI tract owing to microbiota, later structurally revealed as amide BA conjugates, including phenylalanocholate, tyrosocholate, and leucocholate, that have never before been reported; with much human health relevance, their functional roles related to FXR signaling were further confirmed in vitro and in vivo.
To date, a growing number of studies have been reported linking BAs to the evolving definition of the microbiota–gut–brain axis. Liu and colleagues showed that BAs induced antidiabetic effects through signaling FGF receptor 1 (FGFR1) in hypothalamic agouti-related protein (AgRP)-producing neurons.72 In ob/ob obese mice, oral gavage of taurocholic acid markedly improved glucose tolerance by upregulating the level of ileum expression of FGF15 (murine counterpart of human FGF19), likely through an FXR-specific pathway. Other studies suggest that bacterially derived BAs trigger a gut–liver–brain axis for satiety control,73 for improved glucose metabolism (as found for bariatric surgery),74,75 and to remedy alcohol-associated liver injuries even in cases of cirrhosis and alcoholic hepatitis.76
BAs are linked to altered brain function and behaviors, as well. One work employing rodent ASD models associated decreased abundances of gut bacterial species involved in BA metabolism (e.g., Bifidobacterium and Blautia spp.) with increased GI dysfunctional and autism-like behavioral scores.77 Another work reported that a gut-based bariatric surgery led to larger bile acid pools, an attenuated cocaine-induced increase in dopamine levels in nucleus accumbens, and reduced addiction-related behaviors; using knockout mouse models, the study further identified TGR5 and bile acid signaling as the key mediator.78 In a recent study, a humoral route via which the circulating BAs reach the brain and control the satiety was confirmed, where BA administration, either peripheral or central, led to an anorexigenic effect in a TGR5-specific manner.79
Large human cohort studies (n > 1400) of Alzheimer’s disease (AD) associated altered serum BA profiles with markers of cognitive decline and AD pathophysiology, supporting the hypothesis that circulating BAs contribute to AD pathogenesis in which gut microbiota plays a role.80,81 Notably, significantly lower levels of circulating CA (a primary BA) and higher levels of deoxycholic acid (or DCA, a secondary BA converted from CA by bacteria), alongside their glycine and/or taurine conjugates, were observed in AD patients compared to their age-matched normal controls. A strong positive association was identified and validated among the surging DCA:CA ratios and markers of cognitive impairment, indicating gut bacterial 7α-dehydroxylation of CA as one mechanistic route of AD pathogenesis. For specific modes of action, questions remain about whether BAs act locally on TGR5-expressing ENS to signal the CNS61 or originating from peripheral circulation, they cross the BBB (in unconjugated nonpolar forms), reach the CNS, and act in situ, as indicated by previous data.82–86 In addition, the functional roles of BAs in AD pathogenesis exhibited gender specificity, as revealed in a targeted metabolomics study examining 28 BAs in enterohepatic circulation of AD transgenic (PP/PS1 induced) and wild-type colonized mice. Compared with conventional controls, female AD transgenic mice had decreased levels of primary BAs (taurine-conjugated) and increased levels of secondary BAs in plasma and liver, while their male counterparts exhibited the opposite trend.87
2.3. Tryptophan Catabolites.
l-Tryptophan (Trp) is an essential aromatic amino acid exclusively obtained from the diet via eggs, fish, and milk, with a required daily intake of 3.5 mg/kg of body weight.88 Trp is actively involved in protein biosynthesis and cell growth while serving as a substrate in producing metabolites that are crucial for host physiological processes spanning immune cell homeostasis, energy balance, and neurotransmission.88 In mammals, Trp is transported throughout the body (except across the BBB) in albumin-bound forms by large neutral amino acid transporters (LATs).89 Trp catabolism (only in free form) occurs mostly inside the GI tract via three distinct pathways, namely, the kynurenine pathway (KP), the serotonin pathway, and production of indoles.90 In the human body, >95% of Trp is metabolized through KP, generating a realm of kynurenine (Kyn) derivatives with wide-ranging neuroactivities, including the end product nicotinamide adenine dinucleotide (NAD+), a cofactor essential for cellular energy metabolism.88,91 The Trp–Kyn pathway has been detailed from the perspective of both evolution and drug development.88,92 Smaller portions of Trp are converted into serotonin (5-HT) and other downstream neurotransmitters such as N-acetylserotonin (NAS), melatonin, and 5-hydroxyindole acetate (5-HIAA) that are potentially implicated in microbe–brain crosstalk.93,94 In addition, gut microbiota transforms Trp into indole and/or indolyl derivatives, among which many have been identified as key regulators of inflammation and mucosal homeostasis.95,96
The crucial role of all three Trp catabolic fluxes in orchestrating the gut–brain signaling has been increasingly identified, particularly in cases associating gut microbiota and neuropathologies.97,98 The notion of a microbiota–brain axis has been recently buttressed by the aforementioned FGFP cohort study,25 in which gut–brain module (GBM) analyses identified the presence of animal-like GBM (5-HT synthesis I) rather than the plant-like GBM (5-HT synthesis II) in roughly 20% of gut-associated genomes from the Integrated Microbial Genomes (IMG); microbial genera, including Akkermansia and Alistipes, were discovered as potential 5-HT producers. Such findings may open doors for targeting gut microbiota-regulated Trp pathways for improved mental health outcomes.
2.3.1. Serotonin.
Perhaps the best known biochemical details of microbiota–Trp interaction revolve around serotonin [or 5-hydroxytryptamine (5-HT)], a monoamine neurotransmitter that regulates mood control, appetite, and cognitive behaviors in brain as well as motility99 and hemostasis100 in the GI tract. Strikingly, >90% of 5-HT is synthesized de novo by gut endocrine cells such as enterochromaffin cells (ECs) and myenteric neurons, where commensal microbes are actively involved. Novel signaling cascades by which the microbiota acts on local 5-HT biosynthesis were recently reported; one is through direct signaling of colonic ECs to promote gene expression of tryptophan hydrolase 1 (TPH1).101 The gut microbiota was also discovered to constantly regulate ENS maturation even until adulthood via an enteric 5-HT network.102 Note that reportedly gut-derived 5-HT cannot cross the BBB, and 5-HT levels in brain would thus solely depend on its CNS synthesis in situ;28 how microbe-mediated 5-HT metabolism in the ENS (and the extended circulatory or peripheral systems) mediates CNS activities and leads to neuro-related changes constitutes a critical direction of future research.
2.3.2. Kynurenines.
Kynurenines, including Kyn and the downstream catabolites, are increasingly recognized to regulate brain health. In CNS, 40% of the Kyn pool is synthesized de novo, whereas the remaining 60% comes from peripheral sites through crossing the BBB. For the Trp–Kyn pathway occurring in peripheral tissues where enzyme levels are much higher than in brain, the first step is the rate-limiting one. Both extrahepatic indoleamine 2,3-dioxygenases (IDOs) and hepatic tryptophan 2,3-dioxygenase (TDO) catalyze the conversion from Trp to N-formylkynurenine, the immediate precursor of Kyn.103 Kyn metabolism in brain yields disparate metabolite sets with cell-specific effects. One is microglial generation of 3-hydroxykynurenine (3-HK) and its immediate derivative, quinolinic acid (Quin); 3-HK is linked to oxidative stress and apoptosis inside the CNS, while Quin exhibits neuronal excitotoxicity through activating the N-methyl-D-aspartate receptor (NMDARs).104–106 The other is kynurenic acid (Kyna), a CNS astrocyte-producing Kyn derivative that exerts neuroprotective effects by serving as an antagonist to both NMDARs and α7-nicotinic acetylcholine receptors (α7 nAChRs)107 and as an agonist for the aryl hydrocarbon receptor (AhR),108 a master immune regulator and an important transcription factor in xenobiotic metabolism. Measuring Quin:Kyna ratios in the CNS, therefore, will help monitor the status of CNS excitation and neuroinflammation.88,109 Overall, accumulation of Kyn in the CNS due to peripheral inflammation has been generally associated with mental disorders such as depression and schizophrenia;110 it would thus be beneficial to dissect the role of gut microbiota in controlling peripheral Kyn availability and to systematically identify microbial Trp metabolites that can be transported across the BBB alongside the associated transporters and cellular receptors.
2.3.3. Indoles.
Bacterial Trp products, notably indole and its indolyl derivatives, have been identified as potent regulators that sustain mucosal homeostasis while eliciting proper immune responses.111 One striking example is indole-3-propionic acid (IPA), an indolyl metabolite exclusively from Clostridiales family bacteria such as Clostridium sporogenes.34 IPA plays an active role of the ROS scavenger itself (i.e., an antioxidant), activates the AhR receptor to promote the release of anti-inflammatory cytokines [e.g., interleukin-10 (IL-10)], and suppresses proinflammatory cytokines such as tumor necrosis factor-α (TNF-α).112 Likewise, indole-3-acrylic acid (IAcrA), produced by Peptostreptococcus russellii (a mucin user), is an AhR agonist leading to suppressed inflammation. Indole-3-acrylic acid (IAcrA) also binds to the pregnane X receptor (PXR) to promote gene expression of muc2 that encodes mucin 2 to enhance the integrity of the intestinal epithelial barrier (IEB).113
Reported evidence has linked microbial indoles to CNS function and health. One in vivo study of multiple sclerosis (MS) demonstrated that bacterially Trp-derived indole, indole 3-sulfate (I3S), IPA, and indole 3-carboxaldehyde (I3A), can modulate CNS inflammation directly by activating the AhR receptor expressed in astrocytes.114 Another study of the human cohort correlated indole, indole-3-acetic acid, and skatole levels with quantitative readouts of functional and anatomical connectivity for the extended central reward network that incorporates amygdala, nucleus accumbens, and anterior insula. Association between these indoles and body mass index (BMI), food addiction, and anxiety symptoms was also discovered.115 Note that microbiota-derived indoles can easily enter mammalian circulation and reshape host blood metabolomes.30,34,111 Further research should thus be conducted to identify and track peripheral indole species that can reach the brain and to examine how they mediate CNS-resident immune homeostasis and neurological functions.
2.4. Neurotransmitters.
Neurotransmitters are presynaptic compounds that evoke postsynaptic electrical signaling (through binding to receptors in situ) to fulfill neurophysiological functions, typically with a fast complete cycle of synthesis, release, and reuptake.116 In the human GI tract, multiple neurotransmitters, including GABA, 5-HT, and histamine, are synthesized in large quantities by gut bacteria. This has led to an increasing number of inquiries about the involvement of neurotransmitters in microbe–brain crosstalk and regulation of local gut microbial growth and ecology.19,117,118 Microbial endocrinology theories are continuously developing for neurotransmitters that embrace evolutionarily shared synthetic pathways, receptor families, and neuroendocrine effects among mammals and microbes.119–121 Such interkingdom crosstalk in the language of neurotransmitters was supported by the landmark FGFP cohort study.25 The results associated perturbed GABA synthetic pathways, 5-HT metabolism, and dopamine derivatives with quality of life (QoL) proxies and depression status. Here, we focus on amino acids (e.g., glutamate, GABA, and 5-HT) and biogenic amines (e.g., dopamine, norepinephrine, and epinephrine).
2.4.1. Glutamate and γ-Aminobutyric Acid.
l-Glutamate (Glu) and γ-aminobutyric acid (GABA) are the major excitatory and inhibitory neurotransmitters, respectively, in the human CNS, and interestingly, they are biochemically interconvertible via the two-way (glutamine)Glu–GABA cycle.116,122 Both glutamatergic and GABAergic loops incorporate the fate of Glu/GABA, neurotransmission through activating widespread receptors in the host, and inactivation by high-affinity transport systems in glial cells (mainly astrocytes), with the machineries detailed elsewhere.123 For Glu–GABA metabolism, it is generally agreed that Glu cannot pass the BBB124,125 and, therefore, CNS Glu originates from only in situ synthesis, relying on glutamine (Gln) as the precursor or through transamination of 2-oxoglutarate from the citric acid cycle.123,126 On the contrary, evidence supported the idea that GABA could cross the BBB.127,128 In the CNS, GABA can be derived from glycolysis or from Glu as catalyzed by l-glutamic acid decarboxylase (GAD), an enzyme almost exclusively present in GABAergic neurons. In parallel, innumerable bacterial strains have been discovered to be producers of Glu (e.g., Bifidobacterium spp., Lactobacillus spp., and Corynebacterium glutamycum) and GABA (e.g., Lactobacillus spp., Escherichia coli, and Pseudomonas spp.) for functional purposes, including interspecies interaction.123 Studies revealed that both Glu and GABA serve as energy substrates for gut microbes, balance luminal pH, modulate pathogen virulence, regulate gut motility, etc.117,123 In this light, the microbe–brain axis through the glutamatergic and/or GABAergic system may be a viable pathway to explore.
Studies linking gut bacteria-derived Glu and GABA to neurological health phenotypes are emerging. In a landmark study,21 the lactic acid bacterial strain L. rhamnosus has been demonstrated to exert a direct effect on murine CNS GABA receptor expression while conferring anxiolytic benefits in mice, as shown in elevated plus maze (EPM) behavioral test and stress hormone analyses. Through vagomization trials, the authors further identified the vagus nerve as a constitutive pathway of communication between gut-resident microbes and brain. More recently, Pokusaeva and colleagues showed in a rat model that oral administration of Bifidobacterium breve NCIMB8807 pESHgadB, an engineered GABA-producing strain with overexpressed GadB genes encoding the GAB protein, led to reduced sensitivity to visceral pain compared to wild-type strains.38 In another work, Zheng and colleagues combined fecal transplant trials and revealed that gut microbiota of schizophrenia patients could modulate the Gln–Glu–GABA cycle and induced schizophrenia-relevant behaviors in mice.129
2.4.2. Catecholamines: Dopamine, Norepinephrine, and Epinephrine.
Catecholamines, including dopamine, norepinephrine, and epinephrine, are biogenic amine neurotransmitters that carry crucial roles in a wide range of neurophysiological and behavioral processes, spanning CNS homeostasis, attention, emotional control, and coordination of body movement.116,130 In mammalian brain, dopamine is produced in the cytoplasm of presynaptic terminals through decarboxylation of its immediate precursor, l-3,4-dihydroxyphenylala-nine (L-DOPA), that is derived from tyrosine (Tyr) as a rate-limiting step. In downstream reactions, dopamine serves as a substrate for producing norepinephrine and epinephrine.116 Although the three compounds are biochemically interconvertible, they carry distinct activities and functions in the CNS. More specifically, dopamine, mainly located in corpus striatum (an area for motor control), carries essential roles in motivation, reinforcement, reward, and hedonistic regulation.131 Many substances of abuse (e.g., cocaine and amphetamine) work by altering dopaminergic synapses in the CNS. Moreover, drug design for psychotherapeutic use typically mimics dopamine’s structure to fulfill antianxiety, antidepressant, and antipsychotic benefits. Norepinephrine is known to regulate attention, sleep/wakefulness, and feeding behavior, whereas epinephrine, at much lower CNS levels, may have an impact on memory and learning.116
The microorganisms have long been recognized as catecholamine producers. For example, Bacillus, Serratia, and Proteus spp. synthesize dopamine, while Escherichia, Bacillus, and Saccharomyces spp. are known to produce norepinephrine.118,119,132 Although how gut commensal bacteria contribute to host catecholamine metabolism remains to be revealed, accumulating data conferred such insights.133–136 Heijtz et al. discovered contrasting neurophenotypes between GF and specific pathogen-free (SPF) conventional mice based on behavioral test scores, neurochemical concentration profiles, and patterns of gene expression. Increased turnover ratios of dopamine and norepinephrine were found in GF mice, suggesting a role of microbiota.134 In addition, Kiraly and colleagues revealed in a mouse model that antibiotic knockdown of gut microbiota, without affecting cocaine metabolism and behavior-modulating stress hormones, resulted in altered cocaine-mediated behaviors, as characterized by heightened sensitivity to cocaine reward in conjunction with changes to the dopaminergic system such as increased activity of CNS D1 dopamine receptor Drd1.135 More recently, human cohort studies associated gut microbial compositions and functional genes with altered dopaminergic systems in disease models such as depression and PD.25,137 However, as all three polar catecholamines cannot cross the BBB,116 how bacterially mediated catecholamine metabolism interacts with gut–brain crosstalk remains elusive and warrants elucidation.
2.4.3. Other Small-Molecule Neurotransmitters.
Aside from 5-HT, glutamate/GABA, and catecholamines, many other neurotransmitters of confirmed microbial sources may also mediate gut–brain signaling and alter paths of associated psychopathologies, although the current evidence is only emerging or at best suggestive. These span histamine, trace amines (β-phenylethylamine, tyramine, and tryptamine), glycine, acetylcholine (ACh), serine, and taurine. For instance, histamine is an organic nitrogenous mediator of inflammatory responses and itching; histamine contributes to tissue swelling during inflammation by increasing the permeability of capillaries to white blood cells and proteins. One study showed the probiotic strain Lactobacillus reuteri (with histidine decarboxylase) converts histidine to histamine, which suppresses TNF through modulation of protein kinase (PKA) and extracellular signal-regulated kinase (ERK) signaling.138 Likewise, trace amines tryptamine and β-phenylethylamine are known to play significant roles in appetite control, attention, and emotional processing. A range of lactic acid bacteria have been confirmed as their synthesizers, with Lactobacillus bulgaricus and C. sporogenes for producing tryptamine and Enterococcus faecalis and Leuconostoc strains for producing both β-phenylethylamine and tyramine.139,140 In a recent work, two gut bacteria-derived carnitine analogues, 3-methyl-4-(trimethylammonio)butanoate and 4-(trimethylammonio)pentanoate, were newly identified as mediators of gut–brain communication, specifically through inhibition of carnitine-mediated fatty acid oxidation in brain white matter.141 Future studies should examine neurotransmitters for which kinetics of microbial synthesis in gut are better modeled, the gut microbial proportion to the CNS levels is differentiated, and their functional roles in modulating gut–brain signaling are delineated.
3. LARGE MOLECULES
3.1. Microorganism-Associated Molecular Patterns.
On both molecular and ecological levels, microorganisms are in constant flux of biochemical signaling for intra- and/or interspecies communication, inhabitant assessment, trigger of immune responses, formation of symbiotic biofilm, etc.142,143 At the center are the microorganism-associated molecular patterns (MAMPs),144 highly class-specific and evolutionally conserved microbial protein or peptide molecular motifs through which microbial species identify one another. As evidenced, MAMPs, of which many are pathogens to mammals, can translocate from the gut to the remote peripheral blood system to induce immune responses, raising the possibility that they are involved in the microbiome–gut–brain axis,145 the two most notable examples being lipopolysaccharides (LPS) and bacterial peptidoglycans (PNGs).
3.1.1. Lipopolysaccharides.
Lipopolysaccharide (LPS) (or endotoxin) is a major component of Gram-negative gut bacteria and can translocate from the gut lumen to the systemic circulation through leaky mucosal linings. Once sensed by pattern recognition receptors (PRRs) [e.g., Toll-like receptor 4 (TLR4)] of the host innate immune system, cytokines are produced that can either signal directly to the CNS or excite vagal and spinal afferent neurons, given that PRRs of LPS (e.g., TLRs) are prevalently expressed in GI epithelial cells, ENS neurons, and the rest anatomical makeup of the gut–brain axis.146 There have been multiple studies linking intestinal microbial LPS to brain health. Wu and colleagues observed significantly higher circulating and brain levels of LPS in aged mice compared with young controls while associating age with gut dysbiosis and markers of neuroinflammation.147 Maes and colleagues reported for patients with both chronic fatigue syndrome148 and chronic depression149 increased circulating immunoglobulin levels (e.g., IgA and IgM) in response to propagated gut bacterial LPS, suggesting a role for LPS in the microbe–brain interplay.
In parallel, administration of LPS, through injection or oral gavage, can lead to exacerbated neurological pathophenotypes in mice such as depression-mimicking behaviors150 and cognitive impairment.151 These are collectively demonstrated for LPS as potent molecular cues of microbiota to influence host brain function and behaviors.
3.1.2. Peptidoglycans.
Peptidoglycans (PGNs) constitute another type of (Gram-positive) bacterial peptide with effects on brain function. A seminal study by Arentsen and colleagues reported for GF mice (compared with normal mice) extremely low or below-detection-limit serum PGN levels using ultra-sensitive mass spectrometry platforms, suggesting gut microbiota as a major source for peripheral PGNs.152 Microbial PGNs, once relocated to circulating blood (including under normal physiological conditions) and recognized by cytosolic nucleotide-binding and leucine-rich repeat-containing receptors (NLRs) (the major PRRs of PGNs), trigger downstream immune responses and regulate CNS function. Recent evidence supports such a link between microbial PGNs and CNS health.153 On one hand, the NLRs play an active role in shaping and controlling gut microbiota, with notable examples of NLRP6 and NLRP12 demonstrated to regulate intestinal inflammation, gut microbial composition, and metabolism.154 Pusceddu and colleagues also revealed a novel role of NLRs in gut–brain signaling.155 The authors observed for knockout mouse models (NodDKO) deficient in both Nod1 and Nod2 exacerbated stress-induced behaviors, an impaired cerebral serotonergic system, a decline in hippocampal neurogenesis, and increased GI permeability. In turn, administration of fluoxetine, a selective 5-HT reuptake inhibitor, corrected these behavioral deficits while restoring 5-HT signaling. As for PGNs, the MIA ASD model has been established as maternal immune activation itself, through infecting pregnant mice with proinflammatory MAMPs (e.g., PGNs), which induces ASD-like behaviors in mouse offspring. Such effects of PGNs at the maternal–fetal interface suggest a critical role of maternal microbiota in shaping fetal brain health. This was supported by the latest studies examining the placental mobility of the PGN–teichoic acid complex (a cell wall component of pathogen Streptococcus pneumoniae) in parallel with fetal outcomes of neuro-proliferation and cognitive development;156 the details are reviewed elsewhere with critical questions proposed.157
3.2. Peptides of Neurodegenerative Hallmarks: α-Synuclein and β-Amyloid.
Much interest has been stimulated in researching the relationship between gut microbiota and pathogenesis of neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). For AD and PD, aggregated CNS levels of β-amyloid (Aβ) and α-synuclein (α-syn), respectively, are the two main associated pathological hallmark proteins.158,159 A recent work discovered the capability of microbes to produce extracellular amyloid-like proteins that in turn exacerbate in aged rats the pathogenic course of α-syn, indicating multifaceted effects of microbiota on neurodegeneration.160 Note that α-syn, once in the pathologic state, has a prion-like activity to propagate and translocate through the vagus nerve of the gut–brain axis and leads to impaired CNS physiology and behavioral deficits.161 This hypothesis was supported by a recent report by Sangjune and colleagues.162 The authors performed gut injection of α-syn fibrils and discovered that murine endogenous α-syn was converted to a pathologic species that could spread from the gut to the brain, resulting in pathological traits of PD, including degeneration of dopamine neurons and motor (and non-motor) deficits. Strikingly, these traits were prevented via truncal vagotomy or α-syn deficiency (by knockout of the Snca gene), supporting the vagus nerve as an essential conduit for the α-syn pathology of PD. This neuronal route was further supported by epidemiological studies (e.g., Danish cohort163 and Swedish cohort164) that aimed to dissect the effects of truncal vagotomy procedures on PD risks. Because the mucosal microbiota is in the vicinity of the ENS, whether and how the gut microbiota contributes to α-syn pathogenesis in PD through the vagus nerve remains to be resolved.165
3.3. Neuropeptides in Appetite Control.
The GI tract, with tubular ENS tissue lining all over, is home to innumerable neuroendocrine cells as well as their neuropeptide products spanning neuropeptide tyrosine (NPY), calcitonin gene-related peptide (CGRP), peptide YY (PYY), and GLP-1. NPY and CGRP are expressed at all levels throughout the gut–brain axis, while PYY and GLP-1 are exclusively produced in distal ileum and colon by local enteroendocrine L cells. Due to their proximity to the intestinal mucosa where the microbiota resides, a question about whether the gut microbiota interacts with these neuropeptides to further modulate gut–brain signaling arises. One relevant topic of research is gut microbial regulation of mammalian appetite control, focusing on SCFAs as a proof of principle. Through in vivo and in vitro models, one study reported that colonic microbial propionate stimulated local release of PYY and GLP-1 from L cells in wild-type mice, but not in in Ffa2–/– mice deficient in expression of FFA2, a receptor for SCFAs. The results suggest that the gut microbiota harbors great potential to modulate neuropeptide profiles, associated satiety perception, and food intake behaviors.166
A recent review article underscores the role of the microbiota in appetite control.131 GABAergic and glutamatergic neurons, both belonging to the hippocampal arcuate nucleus (ARC) family and closely related to the Glu/GABA cycle, are key regulators of appetite status. GABAergic neurons express the orexigenic NPY and agouti-related protein (AgRP), whereas glutamatergic neurons express pro-opiomelanocortin (POMC), a precursor of α-melanocyte-stimulating hormone (αMSH) and an anorexigenic neuropeptide. Often, the NPY, AgRP, and POMC hormone populations are viewed as the “first-order” neurons in satiety and hunger pathways. Most interestingly, recent studies together suggest a causal role of gut bacteria in influencing host energy metabolism and feed behaviors, with colonic bacterial growth profiles overlapping much with patterns of satiety hormone release (e.g., GLP-1 and PYY) and satiety perception, especially during first 20 min upon meal induction.131 The author also speculated bacterial metabolites (e.g., quorum-sensing acylhomoserine lactones), if detected in portal circulation, might act directly on hypothalamic neurons.
4. IMPLICATIONS
4.1. Discovering Novel Molecules of the Microbiota.
Complex and dynamic in constant metabolic fluxes, the gut microbiota harbors innumerable novel molecules yet to be discovered and mined for neuroactive effects. Identifying those through which our commensal microbiota mediates the CNS function thus is a pressing issue to resolve. One example involves bacterial quorum-sensing molecules N-acylhomoserine lactones (AHLs) for interspecies communication regarding local population growth and nutrient use. Previous studies showed an effect of gut-derived AHLs on the function of neurons,167 but whether and how they may reach humoral circulation has only been recently discussed.168 In addition, the gut microbiota may be a source of steroid hormones; one example is gut bacterial strain Clostridium scindens performing such glucocorticoid-to-androgen conversion.169 Moreover, some bacterially derived molecules, even at low levels, might be implicated in a wide range of host neurophysiological processes. One example is tetrahydrobiopterin (BH4) that is a cofactor that is essential for normal CNS dopamine synthesis and function. A recent work confirmed gut-residing Adler-creutzia equolifaciens and Microbacterium schleiferi as BH4 producers, leading to the question of whether they contribute to the CNS pool of BH4.170 Another example is vitamin B6, which can be bacterially derived in the gut.171 Vitamin B6 is the precursor of pyridoxal phosphate, a cofactor essential for GAD enzymatic conversion of glutamate to GABA. The lack of vitamin B6 can result in a massive decrease in CNS GABA levels, subsequent loss of synaptic inhibition, and seizures.116 Also, amines of confirmed bacterial sources, including putrescine, spermidine, spermine, and cadaverine, have been shown to be implicated in the responses of the CNS to stress.172 It should be noted that the authors have recently published a high-coverage metabolomics study comparing GF and wild-type colonized mice, reporting as many as 701 unique differential metabolites owing to the presence of the microbiota.30 Presenting a valuable data set for future research probing microbial neuroactive potentials, this study points to not only a need to mind tissue/organ specificity when studying microbiota’s effects but also the importance of using novel and powerful analytical platforms, including high-resolution mass spectrometry (HRMS) and integrated cheminformatics, to provide hard chemical evidence when examining the microbiota–brain crosstalk. Here, we therefore present a summary of select metabolites of microbiota–brain links for tissue/organ specificity and analytical detectability (Table 1).
Table 1.
Reported Tissue/Organ Specificity and Analytical Detection for Representative Molecules (or turnover) of Potential Microbiota–Brain Links from Select Reviewed Research Articles in This Perspectivea
| molecule (or turnover) | abbreviation | type | group | subgroup | organism | tissue/organs | platform | context | ref |
|---|---|---|---|---|---|---|---|---|---|
| acetate | AA | small molecules | fatty acids | SCFAs | mice and cells | brain, colon, liver, and serum; cell culture (primary glial astrocytes, neurons, and microglia) | GC-MS | Alzheimer’s disease | 13 |
| taurocholate | TCA | small molecules | bile acids | primary, conjugated | mice | hypothalamus | LC-HRMS | TGR5-specific anorexigenic effects | 79 |
| deoxycholate | DCA | small molecules | bile acids | secondary, free | mice | hypothalamus | LC-HRMS | TGR5-specific anorexigenic effects | 79 |
| ω-muricholate | ωMCA | small molecules | bile acids | primary, free | mice | hypothalamus | LC-HRMS | TGR5-specific anorexigenic effects | 79 |
| 5-hydroxyindoleacetate/serotonin | 5-HIAA/5-HT | small molecules | aromatic amino acids | tryptophan: serotonin | mice | striatum | LC-EC | germ-free mousebased comparison | 64 |
| dihydroxyphenylacetate/dopamine | DOPAC/DA | small molecules | neuro transmitters | catecholamines | mice | striatum | LC-HRMS | germ-free mousebased comparison | 64 |
| 3-methoxy-4-hydroxyphenylglycol/noradrenaline | MHPG/NA | small molecules | neuro transmitters | catecholamines | mice | striatum | LC-HRMS | germ-free mousebased comparison | 64 |
| indoxyl sulfate | IS | small molecules | aromatic amino acids | tryptophan: indoles | mice | feces, serum, and cortical brain tissues | LC-HRMS | germ-free mousebased comparison | 30 |
| 4-ethylphenylsulfate | 4EPS | small molecules | aromatic amino acids | phenols | mice | feces and serum | GC/LC-MS | autism spectrum disorder | 8 |
| p-cresol | small molecules | aromatic amino acids | phenols | mice | feces | GC-MS | autism spectrum disorder | 9 | |
| nicotinamide | NAM | small molecules | vitamins | vitamin B | humans | serum and CSF | UHPLC-MS/MS, GC-MS | amyotrophic lateral sclerosis | 14 |
| γ-aminobutyrate | GABA | small molecules | neurotransmitters | glutamate–glutamine–GABA cycle | mice | hypothalamus | LC-HRMS | aging-related FMT | 22 |
| glutamate | Glu | small molecules | neurotransmitters | glutamate–glutamine–GABA cycle | mice | LC-QTOF | schizophrenia-related FMT | 129 | |
| trimethylamine N-oxide | TMAO | small molecules | choline | amine oxides | mice | feces, serum, and cortical brain tissues | LC-HRMS | germ-free mousebased comparison | 30 |
| peptidoglycan | PGN | large molecules | biopolymers | sugar-amino acid polymer | mice | brain tissues (prefrontal cortex, striatum, and cerebellum) and serum | SLP | Pglyrp2 KO mice | 152 |
Abbreviations: SCFA, short-chain fatty acids; CSF, cerebrospinal fluid; GC, gas chromatography; HRMS, high-resolution mass spectrometry; QTOF, quadrupole time-of-flight; EC, electrochemical (detection); SLP, silkworm larval plasma detection; FMT, fecal microbiota transplant; TGR5, G protein-coupled bile acid receptor-1; Pglyrp2 KO, peptidoglycan recognition protein 2 knockout.
4.2. Unraveling Interclass Interaction.
The microbiota–gut–brain axis field is currently undergoing a paradigm shift from asking (i) what microbes are to (ii) what they do and, eventually, (iii) how we could leverage them for health causes. Measures should be taken to go beyond curating gut microbial neurochemicals and dissecting their role (in a reductionist manner) toward gaining an integrated perspective of them together. In other words, it is important to identify the interaction between seemingly distinct compound classes by structures, sources, and biochemical pathways for systems biology-level insights. There is no lack of data supporting such cross-class interaction and/or interspecies communication. Notable examples included interaction between bacterial SCFAs and tryptophan metabolites; for example, Clostridium bacterially derived SCFAs have been shown to modulate microbial 5-HT biosynthesis in gut enterochromaffin cells, specifically through promoting gene expression of Tph1.51 Likewise, a new study suggested that cortisol regulates portal 5-HT levels.173 Moreover, factors associated with altered gut permeability or worsening of the gut–brain axis structure (e.g., leaky gut or leaky BBB) may converge as a systemic process, in which many compounds spanning disparate chemical pools are likely to be involved.36,112
4.3. Opportunities and Challenges.
With recent data collectively supporting an active microbiota–brain axis, targeting gut microbiota and the neuroactive metabolites naturally arises as an appealing approach for achieving improved neurophysiological states, cognitive functions, emotional states, and behaviors.174–176 In this light, frameworks for drug development are transformed to incorporate NGS-driven data mining efforts of the microbiome for novel discovery, compound screening, and the design of synthetic biology strategies, with the goal of counteracting neurological disorders32,139 or bacterially mediated side effects of drug treatment.68,177 In parallel, the nutrition and food industries have started leveraging the effects and use of gut microbiota to design novel and healthier diets,178 with applications ranging from development of neuroprotective prebiotics and probiotics (together coined psychobiotics) for achieving improved psychological states even in healthy normal individuals19 to the design of a ketogenic diet for antiseizure benefits;179 in parallel, a dynamic mouse peptidome landscape was constructed in response to probiotic modulation of the gut–brain axis and could provide value for future probiotics research toward better mental health.180 In the public health arena, environmental toxicological research may target gut microbiota as one functional entity (or “organ”) in mediating the fate and neurotoxic effects of ever-prevailing environmental toxicants, including pesticides,181 bisphenol A,182 and nanoparticles.183
Challenges will be encountered in advancing the microbiota–gut–brain axis field. To date, the molecular underpinnings of such crosstalk remain largely unelucidated and are hindered by methodological limitations in the standardization of neuro-phenotyping,24 development of relevant in vivo models,184 microbiota characterization,185 microbial source apportionment (for example, from host or direct food intake),186 confounder adjustment,25 neurochemistry analysis,187 etc. Given the bidirectional, dynamic, and elastic nature of the gut microbiota, it is inherently challenging to define their neuroactive potential in quantitative proxies. To resolve this, the 56 gut–brain biochemical modules curated in the recent FGFP cohort study25 may be a valuable reference, with each module referring to a biochemical step (for production or degradation) of a singular neurochemical. Beyond that, sequencing-based approaches, on which most existing microbiome studies heavily relied, encounter both experimental and computational challenges to achieve accurate and definitive characterization of the gut microbiome;188 best practices, protocols, and standards were proposed.188,189
Importantly, given that current insights are mostly derived from animal studies, result extrapolation from animals to the human population can be problematic. Specifically, fundamental gaps remain in terms of whether mice are good models for human microbiome alongside other emerging animal models190 and to what extent the mouse-to-human inference can be made that establishes in humans valid and meaningful cause–effect relationships of the microbiota without overextrapolation, which is surprisingly common (95%; 36 of 38) as assessed by a recent systematic review article.191 Through systematic analysis of existing translational studies using human microbiota-associated rodent models, Walters and colleagues identified most critical aspects for advancing interspecies inference as (i) insufficient rigor in experimental design and statistics and (ii) a paucity of negative results in the scientific community where oftentimes only positive findings are published.191 New machine learning models have thus been proposed for advancing mouse-to-human inference,192,193 and more large-scale human epidemiological studies are needed to test and validate findings based on mouse models, especially those coupled with high-throughput omics screening approaches (e.g., metabolomics, metaproteomics, and metagenomics).187,194 Even for compounds with well-validated neuroactive effects (e.g., SCFAs), caution needs to be taken concerning dose levels and routes of delivery when testing in both experimental settings and clinical trials to ensure safety and efficacy. This entails knowledge of pharmacokinetic/toxicokinetic modeling, the critical time window for dosage, individual disease susceptibility and comorbidity, intra- and interindividual variability of gut microbial dynamics, etc. In a nutshell, to advance the microbiota and gut–brain axis field, future work needs to be undertaken to examine (i) our fundamental understanding and definition of the gut microbiome, gut–brain axis, human health, and related neurologic/mental diseases,195 (ii) developing comprehensive, unbiased, and definitive approaches in characterizing the gut microbiome and health proxies,188 (iii) enhancing rigor in experimental design and statistics while promoting reporting of negative results for valid and unbiased animal-to-human inference,191 and (iv) multiomics integration across multiple cohorts and experimental models for biomarker discovery and validation.196
5. CONCLUDING REMARKS
Recent studies collectively support an active functional role of the gut microbiota in modulating human neurophysiological states, emotions, and behaviors, opening doors of targeting microbiota and their neuroactive metabolites for improved neurologic/mental health. In this Perspective, we focus on the biochemical aspects of the microbiota–brain axis by reviewing microbial molecules spanning small-molecule and larger neuropeptides. We summarized for these molecules the biosynthesis, uptake, activities, and functions as mediated by the gut microbiota, turned to disease models and cohort studies to carefully evaluate their neuroactive roles, and discussed opportunities and challenges toward mechanistic elucidation and therapeutic application. Future studies revolving around finding novel microbial molecules of gut–brain signaling may entail multiomics mining efforts in parallel with hypothesis-driven research on interclass compound interaction.
ACKNOWLEDGMENTS
The authors thank all of the scientists who have inspired and contributed to this fledging field linking microbiota to the gut-brain axis.
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) via Grants R01ES024950, R03ES032067, P30ES010126, and P42ES031007. M.J.Z. is supported by NIEHS Grant R35ES028366. Y.L. also acknowledges partial funding from the Chen-Yu Yen and Whay-Ray C. Yen Graduate Fellowship of UNC Gillings School of Global Public Health (2018-2019).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.1c00656
The authors declare no competing financial interest.
Contributor Information
Yunjia Lai, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States;.
Radhika Dhingra, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States; Institute of Environmental Health Solutions, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Zhenfa Zhang, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Louise M. Ball, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Mark J. Zylka, UNC Neuroscience Center and Department of Cell and Molecular Physiology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States; Carolina Institute for Developmental Disabilities, The University of North Carolina at Chapel Hill, Carrboro, North Carolina 27510, United States
Kun Lu, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States; Curriculum in Toxicology and Environmental Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States;.
REFERENCES
- (1).Turnbaugh PJ; Ley RE; Hamady M; Fraser-Liggett CM; Knight R; Gordon JI The human microbiome project. Nature 2007, 449 (7164), 804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Turnbaugh PJ; Ley RE; Mahowald MA; Magrini V; Mardis ER; Gordon JI An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444 (7122), 1027–1031. [DOI] [PubMed] [Google Scholar]
- (3).Ramakrishna BS Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol 2013, 28 (Suppl. 4), 9–17. [DOI] [PubMed] [Google Scholar]
- (4).Hooper LV; Littman DR; Macpherson AJ Interactions Between the Microbiota and the Immune System. Science 2012, 336 (6086), 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Neuman H; Debelius JW; Knight R; Koren O Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiology Reviews 2015, 39 (4), 509–521. [DOI] [PubMed] [Google Scholar]
- (6).Wells JM; Rossi O; Meijerink M; van Baarlen P Epithelial crosstalk at the microbiota–mucosal interface. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (Suppl. 1), 4607–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Guo H; Chou W-C; Lai Y; Liang K; Tam JW; Brickey WJ; Chen L; Montgomery ND; Li X; Bohannon LM; Sung AD; Chao NJ; Peled JU; Gomes ALC; van den Brink MRM; French MJ; Macintyre AN; Sempowski GD; Tan X; Sartor RB; Lu K; Ting JPY Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370 (6516), eaay9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hsiao EY; McBride SW; Hsien S; Sharon G; Hyde ER; McCue T; Codelli JA; Chow J; Reisman SE; Petrosino JF; Patterson PH; Mazmanian SK Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155 (7), 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bermudez-Martin P; Becker JAJ; Caramello N; Fernandez SP; Costa-Campos R; Canaguier J; Barbosa S; Martinez-Gili L; Myridakis A; Dumas M-E; Bruneau A; Cherbuy C; Langella P; Callebert J; Launay J-M; Chabry J; Barik J; Le Merrer J; Glaichenhaus N; Davidovic L The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 2021, 9 (1), 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Peralta-Marzal LN; Prince N; Bajic D; Roussin L; Naudon L; Rabot S; Garssen J; Kraneveld AD; Perez-Pardo P The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci 2021, 22 (18), 10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wang H; Yang F; Zhang S; Xin R; Sun Y Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. npj Parkinson’s Dis. 2021, 7 (1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chen C; Ahn EH; Kang SS; Liu X; Alam A; Ye K Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv 2020, 6 (31), eaba0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Erny D; Dokalis N; Mezo C; Castoldi A; Mossad O; Staszewski O; Frosch M; Villa M; Fuchs V; Mayer A; Neuber J; Sosat J; Tholen S; Schilling O; Vlachos A; Blank T; Gomez de Agüero M; Macpherson AJ; Pearce EJ; Prinz M Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33 (11), 2260–2276.e7. [DOI] [PubMed] [Google Scholar]
- (14).Blacher E; Bashiardes S; Shapiro H; Rothschild D; Mor U; Dori-Bachash M; Kleimeyer C; Moresi C; Harnik Y; Zur M; Zabari M; Brik RB-Z; Kviatcovsky D; Zmora N; Cohen Y; Bar N; Levi I; Amar N; Mehlman T; Brandis A; Biton I; Kuperman Y; Tsoory M; Alfahel L; Harmelin A; Schwartz M; Israelson A; Arike L; Johansson MEV; Hansson GC; Gotkine M; Segal E; Elinav E Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572 (7770), 474–480. [DOI] [PubMed] [Google Scholar]
- (15).Ghezzi L; Cantoni C; Pinget GV; Zhou Y; Piccio L Targeting the gut to treat multiple sclerosis. J. Clin. Invest 2021, 131 (13), n/a DOI: 10.1172/JCI143774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Foster JA; McVey Neufeld K-A Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36 (5), 305–312. [DOI] [PubMed] [Google Scholar]
- (17).Powell N; Walker MM; Talley NJ The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol 2017, 14 (3), 143–159. [DOI] [PubMed] [Google Scholar]
- (18).Fung TC; Olson CA; Hsiao EY Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 2017, 20 (2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dinan TG; Cryan JF The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017, 46 (1), 77–89. [DOI] [PubMed] [Google Scholar]
- (20).Sudo N; Chida Y; Aiba Y; Sonoda J; Oyama N; Yu XN; Kubo C; Koga Y Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 2004, 558 (1), 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bravo JA; Forsythe P; Chew MV; Escaravage E; Savignac HM; Dinan TG; Bienenstock J; Cryan JF Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (38), 16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Boehme M; Guzzetta KE; Bastiaanssen TFS; van de Wouw M; Moloney GM; Gual-Grau A; Spichak S; Olavarría-Ramírez L; Fitzgerald P; Morillas E; Ritz NL; Jaggar M; Cowan CSM; Crispie F; Donoso F; Halitzki E; Neto MC; Sichetti M; Golubeva AV; Fitzgerald RS; Claesson MJ; Cotter PD; O’Leary OF; Dinan TG; Cryan JF Microbiota from young mice counteracts selective age-associated behavioral deficits. Nature Aging 2021, 1 (8), 666–676. [DOI] [PubMed] [Google Scholar]
- (23).Kundu P; Lee HU; Garcia-Perez I; Tay EXY; Kim H; Faylon LE; Martin KA; Purbojati R; Drautz-Moses DI; Ghosh S; Nicholson JK; Schuster S; Holmes E; Pettersson S Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med 2019, 11 (518), eaau4760. [DOI] [PubMed] [Google Scholar]
- (24).Hooks KB; Konsman JP; O’Malley MA Microbiota-gut-brain research: a critical analysis. Behav Brain Sci. 2019, 42, 1–40. [DOI] [PubMed] [Google Scholar]
- (25).Valles-Colomer M; Falony G; Darzi Y; Tigchelaar EF; Wang J; Tito RY; Schiweck C; Kurilshikov A; Joossens M; Wijmenga C; Claes S; Van Oudenhove L; Zhernakova A; Vieira-Silva S; Raes J The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol 2019, 4 (4), 623–632. [DOI] [PubMed] [Google Scholar]
- (26).Zhu F; Ju Y; Wang W; Wang Q; Guo R; Ma Q; Sun Q; Fan Y; Xie Y; Yang Z; Jie Z; Zhao B; Xiao L; Yang L; Zhang T; Feng J; Guo L; He X; Chen Y; Chen C; Gao C; Xu X; Yang H; Wang J; Dang Y; Madsen L; Brix S; Kristiansen K; Jia H; Ma X Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun 2020, 11 (1), 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kimmel M; Jin W; Xia K; Lun K; Azcarate-Peril A; Plantinga A; Wu M; Ataei S; Rackers H; Carroll I; Meltzer-Brody S; Fransson E; Knickmeyer R Metabolite trajectories across the perinatal period and mental health: A preliminary study of tryptophan-related metabolites, bile acids and microbial composition. Behav. Brain Res 2022, 418, 113635. [DOI] [PubMed] [Google Scholar]
- (28).Rogers GB; Keating DJ; Young RL; Wong ML; Licinio J; Wesselingh S From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 2016, 21 (6), 738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Agirman G; Hsiao EY SnapShot: The microbiota-gut-brain axis. Cell 2021, 184 (9), 2524–2524.e1. [DOI] [PubMed] [Google Scholar]
- (30).Lai Y; Liu CW; Yang Y; Hsiao YC; Ru H; Lu K High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat. Commun 2021, 12 (1), 6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).de la Fuente-Nunez C; Meneguetti BT; Franco OL; Lu TK Neuromicrobiology: How Microbes Influence the Brain. ACS Chem. Neurosci 2018, 9 (2), 141–150. [DOI] [PubMed] [Google Scholar]
- (32).Donia MS; Fischbach MA Small molecules from the human microbiota. Science 2015, 349 (6246), 1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sharon G; Garg N; Debelius J; Knight R; Dorrestein PC; Mazmanian SK Specialized Metabolites from the Microbiome in Health and Disease. Cell Metab. 2014, 20 (5), 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wikoff WR; Anfora AT; Liu J; Schultz PG; Lesley SA; Peters EC; Siuzdak G Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (10), 3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Nicholson JK; Holmes E; Kinross J; Burcelin R; Gibson G; Jia W; Pettersson S Host-gut microbiota metabolic interactions. Science 2012, 336 (6086), 1262–7. [DOI] [PubMed] [Google Scholar]
- (36).Kelly J; Kennedy P; Cryan J; Dinan T; Clarke G; Hyland N Breaking Down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-related Psychiatric Disorders. Front. Cell. Neurosci 2015, 9 (392), 392 DOI: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Veiga-Fernandes H; Pachnis V Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol 2017, 18 (2), 116–122. [DOI] [PubMed] [Google Scholar]
- (38).Pokusaeva K; Johnson C; Luk B; Uribe G; Fu Y; Oezguen N; Matsunami RK; Lugo M; Major A; Mori-Akiyama Y; Hollister EB; Dann SM; Shi XZ; Engler DA; Savidge T; Versalovic J GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil 2017, 29 (1), e12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Wu S; Bekhit AE-DA; Wu Q; Chen M; Liao X; Wang J; Ding Y Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol 2021, 108, 164–176. [Google Scholar]
- (40).Meng D; Sommella E; Salviati E; Campiglia P; Ganguli K; Djebali K; Zhu W; Walker WA Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res 2020, 88 (2), 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).den Besten G; van Eunen K; Groen AK; Venema K; Reijngoud DJ; Bakker BM The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res 2013, 54 (9), 2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Koh A; De Vadder F; Kovatcheva-Datchary P; Backhed F From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165 (6), 1332–1345. [DOI] [PubMed] [Google Scholar]
- (43).Morrison DJ; Preston T Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7 (3), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Flint HJ; Duncan SH; Scott KP; Louis P Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc 2015, 74 (1), 13–22. [DOI] [PubMed] [Google Scholar]
- (45).Vital M; Howe AC; Tiedje JM Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. mBio 2014, 5 (2), e00889–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bjursell M; Admyre T; Göransson M; Marley AE; Smith DM; Oscarsson J; Bohlooly-Y M Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. American Journal of Physiology-Endocrinology and Metabolism 2011, 300 (1), E211–E220. [DOI] [PubMed] [Google Scholar]
- (47).Kovatcheva-Datchary P; Nilsson A; Akrami R; Lee YS; De Vadder F; Arora T; Hallen A; Martens E; Björck I; Bäckhed F Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22 (6), 971–982. [DOI] [PubMed] [Google Scholar]
- (48).Schwiertz A; Taras D; Schäfer K; Beijer S; Bos NA; Donus C; Hardt PD Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18 (1), 190–195. [DOI] [PubMed] [Google Scholar]
- (49).Ríos-Covián D; Ruas-Madiedo P; Margolles A; Gueimonde M; de los Reyes-Gavilán CG; Salazar N Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol 2016, 7, 185 DOI: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Smith PM; Howitt MR; Panikov N; Michaud M; Gallini CA; Bohlooly-Y M; Glickman JN; Garrett WS The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341 (6145), 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Reigstad CS; Salmonson CE; Rainey JF III; Szurszewski JH; Linden DR; Sonnenburg JL; Farrugia G; Kashyap PC Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015, 29 (4), 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Erny D; Hrabe de Angelis AL; Jaitin D; Wieghofer P; Staszewski O; David E; Keren-Shaul H; Mahlakoiv T; Jakobshagen K; Buch T; Schwierzeck V; Utermohlen O; Chun E; Garrett WS; McCoy KD; Diefenbach A; Staeheli P; Stecher B; Amit I; Prinz M Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 2015, 18 (7), 965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Braniste V; Al-Asmakh M; Kowal C; Anuar F; Abbaspour A; Tóth M; Korecka A; Bakocevic N; Ng LG; Kundu P; Gulyás B; Halldin C; Hultenby K; Nilsson H; Hebert H; Volpe BT; Diamond B; Pettersson S The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med 2014, 6 (263), 263ra158–263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Frost G; Sleeth ML; Sahuri-Arisoylu M; Lizarbe B; Cerdan S; Brody L; Anastasovska J; Ghourab S; Hankir M; Zhang S; Carling D; Swann JR; Gibson G; Viardot A; Morrison D; Louise Thomas E; Bell JD The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun 2014, 5, 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sampson TR; Debelius JW; Thron T; Janssen S; Shastri GG; Ilhan ZE; Challis C; Schretter CE; Rocha S; Gradinaru V; Chesselet MF; Keshavarzian A; Shannon KM; Krajmalnik-Brown R; Wittung-Stafshede P; Knight R; Mazmanian SK Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167 (6), 1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Perry RJ; Peng L; Barry NA; Cline GW; Zhang D; Cardone RL; Petersen KF; Kibbey RG; Goodman AL; Shulman GI Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534 (7606), 213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Al-Asmakh M; Hedin L Microbiota and the control of blood-tissue barriers. Tissue Barriers 2015, 3 (3), e1039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Nøhr MK; Pedersen MH; Gille A; Egerod KL; Engelstoft MS; Husted AS; Sichlau RM; Grunddal KV; Seier Poulsen S; Han S; Jones RM; Offermanns S; Schwartz TW GPR41/FFAR3 and GPR43/FFAR2 as Cosensors for Short-Chain Fatty Acids in Enteroendocrine Cells vs FFAR3 in Enteric Neurons and FFAR2 in Enteric Leukocytes. Endocrinology 2013, 154 (10), 3552–3564. [DOI] [PubMed] [Google Scholar]
- (59).Hofmann AF The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med 1999, 159 (22), 2647–2658. [DOI] [PubMed] [Google Scholar]
- (60).Quinn RA; Melnik AV; Vrbanac A; Fu T; Patras KA; Christy MP; Bodai Z; Belda-Ferre P; Tripathi A; Chung LK; Downes M; Welch RD; Quinn M; Humphrey G; Panitchpakdi M; Weldon KC; Aksenov A; da Silva R; Avila-Pacheco J; Clish C; Bae S; Mallick H; Franzosa EA; Lloyd-Price J; Bussell R; Thron T; Nelson AT; Wang M; Leszczynski E; Vargas F; Gauglitz JM; Meehan MJ; Gentry E; Arthur TD; Komor AC; Poulsen O; Boland BS; Chang JT; Sandborn WJ; Lim M; Garg N; Lumeng JC; Xavier RJ; Kazmierczak BI; Jain R; Egan M; Rhee KE; Ferguson D; Raffatellu M; Vlamakis H; Haddad GG; Siegel D; Huttenhower C; Mazmanian SK; Evans RM; Nizet V; Knight R; Dorrestein PC Global chemical effects of the microbiome include new bile-acid conjugations. Nature 2020, 579 (7797), 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kuipers F; Bloks VW; Groen AK Beyond intestinal soap–bile acids in metabolic control. Nat. Rev. Endocrinol 2014, 10 (8), 488–98. [DOI] [PubMed] [Google Scholar]
- (62).Sun J; Chang EB Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis 2014, 1 (2), 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wahlstrom A; Sayin SI; Marschall HU; Backhed F Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- (64).Lefebvre P; Cariou B; Lien F; Kuipers F; Staels B Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol. Rev 2009, 89 (1), 147–191. [DOI] [PubMed] [Google Scholar]
- (65).Arab JP; Karpen SJ; Dawson PA; Arrese M; Trauner M Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017, 65 (1), 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Schroeder BO; Backhed F Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med 2016, 22 (10), 1079–1089. [DOI] [PubMed] [Google Scholar]
- (67).Bernstein H; Bernstein C; Payne CM; Dvorakova K; Garewal H Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res., Rev. Mutat. Res 2005, 589 (1), 47–65. [DOI] [PubMed] [Google Scholar]
- (68).Bhatt AP; Redinbo MR; Bultman SJ The role of the microbiome in cancer development and therapy. Ca-Cancer J. Clin 2017, 67 (4), 326–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Guzior DV; Quinn RA Review: microbial transformations of human bile acids. Microbiome 2021, 9 (1), 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Swann JR; Want EJ; Geier FM; Spagou K; Wilson ID; Sidaway JE; Nicholson JK; Holmes E Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (Suppl. 1), 4523–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Sayin SI; Wahlstrom A; Felin J; Jantti S; Marschall HU; Bamberg K; Angelin B; Hyotylainen T; Oresic M; Backhed F Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17 (2), 225–35. [DOI] [PubMed] [Google Scholar]
- (72).Liu S; Marcelin G; Blouet C; Jeong JH; Jo YH; Schwartz GJ; Chua S Jr. A gut-brain axis regulating glucose metabolism mediated by bile acids and competitive fibroblast growth factor actions at the hypothalamus. Mol. Metab 2018, 8, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Parséus A; Sommer N; Sommer F; Caesar R; Molinaro A; Ståhlman M; Greiner TU; Perkins R; Bäckhed F Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66 (3), 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Li JV; Ashrafian H; Bueter M; Kinross J; Sands C; le Roux CW; Bloom SR; Darzi A; Athanasiou T; Marchesi JR; Nicholson JK; Holmes E Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011, 60 (9), 1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Dixon JB; Lambert EA; Lambert GW Neuroendocrine adaptations to bariatric surgery. Mol. Cell. Endocrinol 2015, 418 (2), 143–152. [DOI] [PubMed] [Google Scholar]
- (76).Bajaj JS Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol 2019, 16 (4), 235–246. [DOI] [PubMed] [Google Scholar]
- (77).Golubeva AV; Joyce SA; Moloney G; Burokas A; Sherwin E; Arboleya S; Flynn I; Khochanskiy D; Moya-Perez A; Peterson V; Rea K; Murphy K; Makarova O; Buravkov S; Hyland NP; Stanton C; Clarke G; Gahan CGM; Dinan TG; Cryan JF Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine 2017, 24, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Reddy IA; Smith NK; Erreger K; Ghose D; Saunders C; Foster DJ; Turner B; Poe A; Albaugh VL; McGuinness O; Hackett TA; Grueter BA; Abumrad NN; Flynn CR; Galli A Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol. 2018, 16 (7), e2006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Perino A; Velazquez-Villegas LA; Bresciani N; Sun Y; Huang Q; Fenelon VS; Castellanos-Jankiewicz A; Zizzari P; Bruschetta G; Jin S; Baleisyte A; Gioiello A; Pellicciari R; Ivanisevic J; Schneider BL; Diano S; Cota D; Schoonjans K Central anorexigenic actions of bile acids are mediated by TGR5. Nat. Metab 2021, 3 (5), 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).MahmoudianDehkordi S; Arnold M; Nho K; Ahmad S; Jia W; Xie G; Louie G; Kueider-Paisley A; Moseley MA; Thompson JW; St John Williams L; Tenenbaum JD; Blach C; Baillie R; Han X; Bhattacharyya S; Toledo JB; Schafferer S; Klein S; Koal T; Risacher SL; Kling MA; Motsinger-Reif A; Rotroff DM; Jack J; Hankemeier T; Bennett DA; De Jager PL; Trojanowski JQ; Shaw LM; Weiner MW; Doraiswamy PM; van Duijn CM; Saykin AJ; Kastenmuller G; Kaddurah-Daouk R Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimer’s Dementia 2019, 15 (1), 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Nho K; Kueider-Paisley A; MahmoudianDehkordi S; Arnold M; Risacher SL; Louie G; Blach C; Baillie R; Han X; Kastenmuller G; Jia W; Xie G; Ahmad S; Hankemeier T; van Duijn CM; Trojanowski JQ; Shaw LM; Weiner MW; Doraiswamy PM; Saykin AJ; Kaddurah-Daouk R Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dementia 2019, 15 (2), 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Kamp F; Hamilton JA Movement of fatty acids, fatty acid analogs, and bile acids across phospholipid bilayers. Biochemistry 1993, 32 (41), 11074–11085. [DOI] [PubMed] [Google Scholar]
- (83).Higashi T; Watanabe S; Tomaru K; Yamazaki W; Yoshizawa K; Ogawa S; Nagao H; Minato K; Maekawa M; Mano N Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids 2017, 125, 107–113. [DOI] [PubMed] [Google Scholar]
- (84).Parry GJ; Rodrigues CM; Aranha MM; Hilbert SJ; Davey C; Kelkar P; Low WC; Steer CJ Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol 2010, 33 (1), 17–21. [DOI] [PubMed] [Google Scholar]
- (85).Mertens KL; Kalsbeek A; Soeters MR; Eggink HM Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci 2017, 11, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Quinn M; DeMorrow S Bile in the Brain? A Role for Bile Acids in the Central Nervous System. J. Cell Sci. Ther 2012, 3, 7 DOI: 10.4172/2157-7013.1000e113. [DOI] [Google Scholar]
- (87).Wu J; Zhu X; Lin H; Chen Z; Tang H; Wang Y Gender differences in the bile acid profiles of APP/PS1 transgenic AD mice. Brain Res. Bull 2020, 161, 116–126. [DOI] [PubMed] [Google Scholar]
- (88).Cervenka I; Agudelo LZ; Ruas JL Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357 (6349), eaaf9794 DOI: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- (89).Ruddick JP; Evans AK; Nutt DJ; Lightman SL; Rook GAW; Lowry CA Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med 2006, 8 (20), 1–27. [DOI] [PubMed] [Google Scholar]
- (90).McMenamy RH; Oncley JL The Specific Binding of l-Tryptophan to Serum Albumin. J. Biol. Chem 1958, 233 (6), 1436–1447. [PubMed] [Google Scholar]
- (91).Bender DA Effects of a dietary excess of leucine on the metabolism of tryptophan in the rat: a mechanism for the pellagragenic action of leucine. Br. J. Nutr 1983, 50 (1), 25–32. [DOI] [PubMed] [Google Scholar]
- (92).Platten M; Nollen EAA; Rohrig UF; Fallarino F; Opitz CA Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discovery 2019, 18 (5), 379–401. [DOI] [PubMed] [Google Scholar]
- (93).Clarke G; Grenham S; Scully P; Fitzgerald P; Moloney RD; Shanahan F; Dinan TG; Cryan JF The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18 (6), 666–73. [DOI] [PubMed] [Google Scholar]
- (94).Li Y; Hao Y; Fan F; Zhang B The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Frontiers in Psychiatry 2018, 9, 669 DOI: 10.3389/fpsyt.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Hubbard TD; Murray IA; Perdew GH Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos 2015, 43, 1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Arora PK; Sharma A; Bae H Microbial Degradation of Indole and Its Derivatives. J. Chem 2015, 2015, 1–13. [Google Scholar]
- (97).Roager HM; Licht TR Microbial tryptophan catabolites in health and disease. Nat. Commun 2018, 9 (1), 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Agus A; Planchais J; Sokol H Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23 (6), 716–724. [DOI] [PubMed] [Google Scholar]
- (99).Gershon MD; Tack J The Serotonin Signaling System: From Basic Understanding To Drug Development for Functional GI Disorders. Gastroenterology 2007, 132 (1), 397–414. [DOI] [PubMed] [Google Scholar]
- (100).Mercado CP; Quintero MV; Li Y; Singh P; Byrd AK; Talabnin K; Ishihara M; Azadi P; Rusch NJ; Kuberan B; Maroteaux L; Kilic F A serotonin-induced N-glycan switch regulates platelet aggregation. Sci. Rep 2013, 3, 2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Yano JM; Yu K; Donaldson GP; Shastri GG; Ann P; Ma L; Nagler CR; Ismagilov RF; Mazmanian SK; Hsiao EY Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161 (2), 264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).De Vadder F; Grasset E; Manneras Holm L; Karsenty G; Macpherson AJ; Olofsson LE; Backhed F Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (25), 6458–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Kanai M; Funakoshi H; Takahashi H; Hayakawa T; Mizuno S; Matsumoto K; Nakamura T Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain 2009, 2 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Guillemin GJ; Smythe G; Takikawa O; Brew BJ Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49 (1), 15–23. [DOI] [PubMed] [Google Scholar]
- (105).Müller N; Schwarz MJ The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988. [DOI] [PubMed] [Google Scholar]
- (106).Amori L; Guidetti P; Pellicciari R; Kajii Y; Schwarcz R On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem 2009, 109 (2), 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Albuquerque EX; Schwarcz R Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: Facts and challenges. Biochem. Pharmacol 2013, 85 (8), 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).DiNatale BC; Murray IA; Schroeder JC; Flaveny CA; Lahoti TS; Laurenzana EM; Omiecinski CJ; Perdew GH Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci 2010, 115 (1), 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).O’Mahony SM; Clarke G; Borre YE; Dinan TG; Cryan JF Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res 2015, 277, 32–48. [DOI] [PubMed] [Google Scholar]
- (110).Chen Y; Guillemin GJ Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int. J. Tryptophan Res 2009, 2, IJTR.S2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Gao J; Xu K; Liu H; Liu G; Bai M; Peng C; Li T; Yin Y Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol 2018, 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Venkatesh M; Mukherjee S; Wang H; Li H; Sun K; Benechet AP; Qiu Z; Maher L; Redinbo MR; Phillips RS; Fleet JC; Kortagere S; Mukherjee P; Fasano A; Le Ven J; Nicholson JK; Dumas ME; Khanna KM; Mani S Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41 (2), 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Wlodarska M; Luo C; Kolde R; d’Hennezel E; Annand JW; Heim CE; Krastel P; Schmitt EK; Omar AS; Creasey EA; Garner AL; Mohammadi S; O’Connell DJ; Abubucker S; Arthur TD; Franzosa EA; Huttenhower C; Murphy LO; Haiser HJ; Vlamakis H; Porter JA; Xavier RJ Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22 (1), 25–37.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Rothhammer V; Mascanfroni ID; Bunse L; Takenaka MC; Kenison JE; Mayo L; Chao CC; Patel B; Yan R; Blain M; Alvarez JI; Kebir H; Anandasabapathy N; Izquierdo G; Jung S; Obholzer N; Pochet N; Clish CB; Prinz M; Prat A; Antel J; Quintana FJ Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 2016, 22 (6), 586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Osadchiy V; Labus JS; Gupta A; Jacobs J; Ashe-McNalley C; Hsiao EY; Mayer EA Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS One 2018, 13 (8), e0201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Purves D Neuroscience; Sinauer Associates: Sunderland, MA, 2012. [Google Scholar]
- (117).Strandwitz P Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Mittal R; Debs LH; Patel AP; Nguyen D; Patel K; O’Connor G; Grati M; Mittal J; Yan D; Eshraghi AA; Deo SK; Daunert S; Liu XZ Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol 2017, 232 (9), 2359–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Lyte M Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 2011, 33 (8), 574–81. [DOI] [PubMed] [Google Scholar]
- (120).Villageliu DN; Rasmussen S; Lyte M A microbial endocrinology-based simulated small intestinal medium for the evaluation of neurochemical production by gut microbiota. FEMS Microbiol. Ecol 2018, 94 (7), n/a DOI: 10.1093/femsec/fiy096. [DOI] [PubMed] [Google Scholar]
- (121).Lyte M Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004, 12 (1), 14–20. [DOI] [PubMed] [Google Scholar]
- (122).Hertz L The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. (Lausanne, Switz.) 2013, 4, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Mazzoli R; Pessione E The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol 2016, 7, 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Janeczko MJ; Stoll B; Chang X; Guan X; Burrin DG Extensive Gut Metabolism Limits the Intestinal Absorption of Excessive Supplemental Dietary Glutamate Loads in Infant Pigs. J. Nutr 2007, 137 (11), 2384–2390. [DOI] [PubMed] [Google Scholar]
- (125).Hawkins RA The blood-brain barrier and glutamate. Am. J. Clin. Nutr 2009, 90 (3), 867S–874S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Cherlyn SYT; Woon PS; Liu JJ; Ong WY; Tsai GC; Sim K Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: A decade of advance. Neurosci. Biobehav. Rev 2010, 34 (6), 958–977. [DOI] [PubMed] [Google Scholar]
- (127).Boonstra E; de Kleijn R; Colzato LS; Alkemade A; Forstmann BU; Nieuwenhuis S Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Frontiers in Psychology 2015, 6, 1520 DOI: 10.3389/fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Kakee A; Takanaga H; Terasaki T; Naito M; Tsuruo T; Sugiyama Y Efflux of a suppressive neurotransmitter, GABA, across the blood–brain barrier. J. Neurochem 2001, 79 (1), 110–118. [DOI] [PubMed] [Google Scholar]
- (129).Zheng P; Zeng B; Liu M; Chen J; Pan J; Han Y; Liu Y; Cheng K; Zhou C; Wang H; Zhou X; Gui S; Perry SW; Wong M-L; Licinio J; Wei H; Xie P The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science Advances 2019, 5 (2), eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Volkow ND; Wise RA; Baler R The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci 2017, 18 (12), 741–752. [DOI] [PubMed] [Google Scholar]
- (131).Fetissov SO Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol 2017, 13 (1), 11–25. [DOI] [PubMed] [Google Scholar]
- (132).Roshchina VV Evolutionary Considerations of Neurotransmitters in Microbial, Plant, and Animal Cells. Microbial Endocrinology 2010, 17–52. [Google Scholar]
- (133).Liu WH; Chuang HL; Huang YT; Wu CC; Chou GT; Wang S; Tsai YC Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res 2016, 298, 202–209. [DOI] [PubMed] [Google Scholar]
- (134).Diaz Heijtz R; Wang S; Anuar F; Qian Y; Bjorkholm B; Samuelsson A; Hibberd ML; Forssberg H; Pettersson S Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (7), 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Kiraly DD; Walker DM; Calipari ES; Labonte B; Issler O; Pena CJ; Ribeiro EA; Russo SJ; Nestler EJ Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep 2016, 6, 35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Moya-Perez A; Perez-Villalba A; Benítez-Páez A; Campillo I; Sanz Y Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain, Behav., Immun 2017, 65, 43–56. [DOI] [PubMed] [Google Scholar]
- (137).Bedarf JR; Hildebrand F; Coelho LP; Sunagawa S; Bahram M; Goeser F; Bork P; Wullner U Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017, 9 (1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Thomas CM; Hong T; van Pijkeren JP; Hemarajata P; Trinh DV; Hu W; Britton RA; Kalkum M; Versalovic J Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012, 7 (2), e31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Williams BB; Van Benschoten AH; Cimermancic P; Donia MS; Zimmermann M; Taketani M; Ishihara A; Kashyap PC; Fraser JS; Fischbach MA Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16 (4), 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Pessione E; Pessione A; Lamberti C; Coïsson DJ; Riedel K; Mazzoli R; Bonetta S; Eberl L; Giunta C First evidence of a membrane-bound, tyramine and β-phenylethylamine producing, tyrosine decarboxylase in Enterococcus faecalis: A two-dimensional electrophoresis proteomic study. Proteomics 2009, 9 (10), 2695–2710. [DOI] [PubMed] [Google Scholar]
- (141).Hulme H; Meikle LM; Strittmatter N; van der Hooft JJJ; Swales J; Bragg RA; Villar VH; Ormsby MJ; Barnes S; Brown SL; Dexter A; Kamat MT; Komen JC; Walker D; Milling S; Osterweil EK; MacDonald AS; Schofield CJ; Tardito S; Bunch J; Douce G; Edgar JM; Edrada-Ebel R; Goodwin RJA; Burchmore R; Wall DM Microbiome-derived carnitine mimics as previously unknown mediators of gut-brain axis communication. Sci. Adv 2020, 6 (11), eaax6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Motta J-P; Wallace JL; Buret AG; Deraison C; Vergnolle N Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol 2021, 18 (5), 314–334. [DOI] [PubMed] [Google Scholar]
- (143).Goyal A; Wang T; Dubinkina V; Maslov S Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat. Commun 2021, 12 (1), 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Negi S; Das DK; Pahari S; Nadeem S; Agrewala JN Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol 2019, 10, 2441–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Mu C; Yang Y; Zhu W Gut Microbiota: The Brain Peacekeeper. Front. Microbiol 2016, 7, 345–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Yoo BB; Mazmanian SK The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46 (6), 910–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Wu ML; Yang XQ; Xue L; Duan W; Du JR Age-related cognitive decline is associated with microbiota-gut-brain axis disorders and neuroinflammation in mice. Behav. Brain Res 2021, 402, 113125. [DOI] [PubMed] [Google Scholar]
- (148).Maes M; Kubera M; Leunis JC; Berk M Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affective Disord 2012, 141 (1), 55–62. [DOI] [PubMed] [Google Scholar]
- (149).Maes M; Twisk FNM; Kubera M; Ringel K; Leunis JC; Geffard M Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J. Affective Disord 2012, 136 (3), 909–917. [DOI] [PubMed] [Google Scholar]
- (150).O’Connor J; Lawson M; Andre C; Moreau M; Lestage J; Castanon N; Kelley K; Dantzer R Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14 (5), 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Zhao J; Bi W; Xiao S; Lan X; Cheng X; Zhang J; Lu D; Wei W; Wang Y; Li H; Fu Y; Zhu L Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep 2019, 9, 5790 DOI: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Arentsen T; Qian Y; Gkotzis S; Femenia T; Wang T; Udekwu K; Forssberg H; Diaz Heijtz R The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 2017, 22 (2), 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Tosoni G; Conti M; Diaz Heijtz R Bacterial peptidoglycans as novel signaling molecules from microbiota to brain. Curr. Opin. Pharmacol 2019, 48, 107–113. [DOI] [PubMed] [Google Scholar]
- (154).Guo H; Gibson SA; Ting JPY Gut microbiota, NLR proteins, and intestinal homeostasis. J. Exp. Med 2020, 217 (10), e20181832 DOI: 10.1084/jem.20181832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Pusceddu MM; Barboza M; Keogh CE; Schneider M; Stokes P; Sladek JA; Kim HJD; Torres-Fuentes C; Goldfild LR; Gillis SE; Brust-Mascher I; Rabasa G; Wong KA; Lebrilla C; Byndloss MX; Maisonneuve C; Bäumler AJ; Philpott DJ; Ferrero RL; Barrett KE; Reardon C; Gareau MG Nod-like receptors are critical for gut-brain axis signalling in mice. J. Physiol 2019, 597 (24), 5777–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Humann J; Mann B; Gao G; Moresco P; Ramahi J; Loh LN; Farr A; Hu Y; Durick-Eder K; Fillon SA; Smeyne RJ; Tuomanen EI Bacterial Peptidoglycan Traverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe 2016, 19 (3), 388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Gonzalez-Santana A; Diaz Heijtz R Bacterial Peptidoglycans from Microbiota in Neurodevelopment and Behavior. Trends Mol. Med 2020, 26 (8), 729–743. [DOI] [PubMed] [Google Scholar]
- (158).Murphy MP; LeVine H 3rd Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis 2010, 19 (1), 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Stefanis L α-Synuclein in Parkinson’s disease. Cold Spring Harbor Perspect. Med 2012, 2 (2), a009399–a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Friedland RP Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis 2015, 45 (2), 349–62. [DOI] [PubMed] [Google Scholar]
- (161).Cryan JF; O’Riordan KJ; Sandhu K; Peterson V; Dinan TG The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19 (2), 179–194. [DOI] [PubMed] [Google Scholar]
- (162).Kim S; Kwon SH; Kam TI; Panicker N; Karuppagounder SS; Lee S; Lee JH; Kim WR; Kook M; Foss CA; Shen C; Lee H; Kulkarni S; Pasricha PJ; Lee G; Pomper MG; Dawson VL; Dawson TM; Ko HS Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103 (4), 627–641.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Svensson E; Horváth-Puhó E; Thomsen RW; Djurhuus JC; Pedersen L; Borghammer P; Sørensen HT Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol 2015, 78 (4), 522–9. [DOI] [PubMed] [Google Scholar]
- (164).Liu B; Fang F; Pedersen NL; Tillander A; Ludvigsson JF; Ekbom A; Svenningsson P; Chen H; Wirdefeldt K Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 2017, 88 (21), 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Fitzgerald E; Murphy S; Martinson HA Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci 2019, 13, 369–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Psichas A; Sleeth ML; Murphy KG; Brooks L; Bewick GA; Hanyaloglu AC; Ghatei MA; Bloom SR; Frost G The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes 2015, 39 (3), 424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Hughes DT; Sperandio V Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol 2008, 6 (2), 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Xue J; Chi L; Tu P; Lai Y; Liu CW; Ru H; Lu K Detection of gut microbiota and pathogen produced N-acyl homoserine in host circulation and tissues. NPJ. Biofilms Microbiomes 2021, 7 (1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Ridlon JM; Ikegawa S; Alves JM; Zhou B; Kobayashi A; Iida T; Mitamura K; Tanabe G; Serrano M; De Guzman A; Cooper P; Buck GA; Hylemon PB Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res 2013, 54 (9), 2437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Belik J; Shifrin Y; Arning E; Bottiglieri T; Pan J; Daigneault MC; Allen-Vercoe E Intestinal microbiota as a tetrahydrobiopterin exogenous source in hph-1 mice. Sci. Rep 2017, 7, 39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Kau AL; Ahern PP; Griffin NW; Goodman AL; Gordon JI Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Bienenstock J; Collins S 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Psycho-neuroimmunology and the intestinal microbiota: clinical observations and basic mechanisms. Clin. Exp. Immunol 2010, 160 (1), 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Mudd AT; Berding K; Wang M; Donovan SM; Dilger RN Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 2017, 8 (6), 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Naveed M; Zhou QG; Xu C; Taleb A; Meng F; Ahmed B; Zhang Y; Fukunaga K; Han F Gut-brain axis: A matter of concern in neuropsychiatric disorders···! Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110051. [DOI] [PubMed] [Google Scholar]
- (175).Sun J; Li H; Jin Y; Yu J; Mao S; Su KP; Ling Z; Liu J Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain, Behav., Immun 2021, 91, 703–715. [DOI] [PubMed] [Google Scholar]
- (176).Generoso JS; Giridharan VV; Lee J; Macedo D; Barichello T The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J. Psychiatry 2021, 43 (3), 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Wallace BD; Wang H; Lane KT; Scott JE; Orans J; Koo JS; Venkatesh M; Jobin C; Yeh L-A; Mani S; Redinbo MR Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science 2010, 330 (6005), 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Valdes AM; Walter J; Segal E; Spector TD Role of the gut microbiota in nutrition and health. BMJ. 2018, 361, k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Olson CA; Vuong HE; Yano JM; Liang QY; Nusbaum DJ; Hsiao EY The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173 (7), 1728–1741.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Zhang P; Wu X; Liang S; Shao X; Wang Q; Chen R; Zhu W; Shao C; Jin F; Jia C A dynamic mouse peptidome landscape reveals probiotic modulation of the gut-brain axis. Sci. Signaling 2020, 13 (642), n/a DOI: 10.1126/scisignal.abb0443. [DOI] [PubMed] [Google Scholar]
- (181).Gao B; Bian X; Mahbub R; Lu K Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect 2017, 125 (2), 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Ni Y; Hu L; Yang S; Ni L; Ma L; Zhao Y; Zheng A; Jin Y; Fu Z Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [DOI] [PubMed] [Google Scholar]
- (183).Diao J; Xia Y; Jiang X; Qiu J; Cheng S; Su J; Duan X; Gao M; Qin X; Zhang J; Fan J; Zou Z; Chen C Silicon dioxide nanoparticles induced neurobehavioral impairments by disrupting microbiota–gut–brain axis. J. Nanobiotechnol 2021, 19 (1), 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Lyte JM; Proctor A; Phillips GJ; Lyte M; Wannemuehler M Altered Schaedler flora mice: A defined microbiota animal model to study the microbiota-gut-brain axis. Behav. Brain Res 2019, 356, 221–226. [DOI] [PubMed] [Google Scholar]
- (185).Hamady M; Knight R Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009, 19 (7), 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Zimmermann M; Zimmermann-Kogadeeva M; Wegmann R; Goodman AL Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363 (6427), eaat9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Vernocchi P; Del Chierico F; Putignani L Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol 2016, 7, 1144 DOI: 10.3389/fmicb.2016.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Bharti R; Grimm DG Current challenges and best-practice protocols for microbiome analysis. Briefings in bioinformatics 2021, 22 (1), 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Knight R; Vrbanac A; Taylor BC; Aksenov A; Callewaert C; Debelius J; Gonzalez A; Kosciolek T; McCall LI; McDonald D; Melnik AV; Morton JT; Navas J; Quinn RA; Sanders JG; Swafford AD; Thompson LR; Tripathi A; Xu ZZ; Zaneveld JR; Zhu Q; Caporaso JG; Dorrestein PC Best practices for analysing microbiomes. Nat. Rev. Microbiol 2018, 16 (7), 410–422. [DOI] [PubMed] [Google Scholar]
- (190).Nagpal J; Cryan JF Microbiota-brain interactions: Moving toward mechanisms in model organisms. Neuron 2021, DOI: 10.1016/j.neuron.2021.09.036. [DOI] [PubMed] [Google Scholar]
- (191).Walter J; Armet AM; Finlay BB; Shanahan F Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180 (2), 221–232. [DOI] [PubMed] [Google Scholar]
- (192).Normand R; Du W; Briller M; Gaujoux R; Starosvetsky E; Ziv-Kenet A; Shalev-Malul G; Tibshirani RJ; Shen-Orr SS Found In Translation: a machine learning model for mouse-to-human inference. Nat. Methods 2018, 15 (12), 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Brubaker DK; Proctor EA; Haigis KM; Lauffenburger DA Computational translation of genomic responses from experimental model systems to humans. PLoS Comput. Biol 2019, 15 (1), e1006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Kong G; Ellul S; Narayana VK; Kanojia K; Ha HTT; Li S; Renoir T; Cao K-AL; Hannan AJ An integrated metagenomics and metabolomics approach implicates the microbiota-gut-brain axis in the pathogenesis of Huntington’s disease. Neurobiol. Dis 2021, 148, 105199. [DOI] [PubMed] [Google Scholar]
- (195).Gilbert JA; Blaser MJ; Caporaso JG; Jansson JK; Lynch SV; Knight R Current understanding of the human microbiome. Nat. Med 2018, 24 (4), 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Wood-Charlson EM; Anubhav; Auberry D; Blanco H; Borkum MI; Corilo YE; Davenport KW; Deshpande S; Devarakonda R; Drake M; Duncan WD; Flynn MC; Hays D; Hu B; Huntemann M; Li P-E; Lipton M; Lo C-C; Millard D; Miller K; Piehowski PD; Purvine S; Reddy TBK; Shakya M; Sundaramurthi JC; Vangay P; Wei Y; Wilson BE; Canon S; Chain PSG; Fagnan K; Martin S; McCue LA; Mungall CJ; Mouncey NJ; Maxon ME; Eloe-Fadrosh EA The National Microbiome Data Collaborative: enabling microbiome science. Nat. Rev. Microbiol 2020, 18 (6), 313–314. [DOI] [PubMed] [Google Scholar]


