Abstract
Tissue inhibitors of metalloproteases (TIMPs) have caught the attention of many scientists due to their role in various physiological and pathological processes. TIMP-1, 2, 3, and 4 are known members of the TIMPs family. TIMPs exert their biological effects by, but are not limited to, inhibiting the activity of metalloproteases (MMPs). The balance between MMPs and TIMPs is critical for maintaining homeostasis of the extracellular matrix (ECM), while the imbalance between MMPs and TIMPs can lead to pathological changes, such as cancer. In this review, we summarized the current knowledge of TIMP-1 in several pulmonary diseases namely, acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), pneumonia, asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and pulmonary fibrosis. Considering the potential of TIMP-1 serving as a non-invasive diagnostic and/or prognostic biomarker, we also reviewed the circulating TIMP-1 levels in translational and clinical studies.
Keywords: Metalloproteases, Extracellular matrix, Chronic obstructive pulmonary disease, Idiopathic pulmonary fibrosis, Cystic fibrosis, Lung injury
Introduction
Since their discovery in the 1980s, tissue inhibitors of metalloproteases (TIMPs) have caught the attention of many scientists due to their role in various physiological and pathological reactions. TIMP-1, 2, 3, and 4 are known members of the TIMPs family, where TIMPs exert their biological effects by, but are not limited to, inhibiting the activity of metalloproteases (MMPs).1,2 Due to its bodily omnipresence, TIMPs activity varies depending on the affected tissues, where this activity increases in certain tissues and decreases in others in the pathogenesis of certain diseases. TIMPs are involved in inhibiting metastases and angiogenesis in cancer and supporting neuronal regulation and certain cellular functions.3,4 The balance between MMPs and TIMPs is critical for maintaining homeostasis of the extracellular matrix (ECM), while the imbalance between MMPs and TIMPs can lead to pathological changes, such as cancer.2,5 Among the four members of the TIMPs family, TIMP-1 uniquely exhibits certain effects through ways other than only binding to different forms of MMPs.2,6,7 TIMP-1 can be detected in body fluids and most tissues/organs and promotes cell growth.8,9 These pathways are activated by TIMP-1 via activating p38, mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK).10 Growth factors, such as transforming growth factor β1 (TGF-β1), fibroblast growth factor (FGF), and epidermal growth factor (EGF), along with phorbol ester and some cytokines such as interleukin-1 (IL-1) and interleukin-6 (IL-6), are known inducers of TIMP-1 expression.11 Despite the numerous protective and favorable activities of TIMP-1, its dysregulation has been observed in various disease conditions. For instance, a study reported that TIMP-1 is highly expressed in glioblastoma and is associated with poor prognosis.12 Moreover, the absence of TIMP-1 in immunostaining tests showed a more positive impact on certain types of breast cancer.13 These facts expand our understanding of TIMPs as it clarifies the role of these enzymes by going against the previous concept of the positive impact of TIMPs in tumor prevention.14 Therefore, TIMPs exerted preferable effects on some but not all pathological conditions, where their activities should be investigated independently.
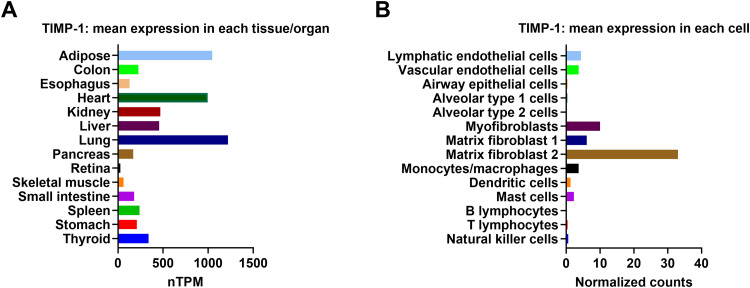
TIMP-1 shows a high expression in the lung [Fig. 1A]. The expression of TIMP-1 is markedly altered in pulmonary diseases due to the remodeling or destruction of the ECM. Elevated levels of TIMP-1 than MMPs may contribute to lung fibrosis whereas lower levels of TIMP-1 than MMPs may enhance the degradation of collagen in the interstitial space causing lung injury. In other lung diseases, several factors including etiology, severity, and duration largely alter TIMP-1 regulation within each disease. Thus, emphasizing the significance of this protein in lung diseases might increase the potential of developing novel therapeutic agents or biomarkers. Therefore, we review the roles of TIMP-1 in pulmonary diseases namely, acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), pneumonia, asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and pulmonary fibrosis. Due to the possibility of TIMP-1 serving as a non-invasive blood marker, we focused on circulating TIMP-1 in clinical studies. Additionally, we also reviewed both in vitro and in vivo studies to provide a better understanding of its function and regulation in lung diseases.
Fig. 1.
TIMP-1 expression profiles. (A) RNA-sequencing tissue data generated by the GTEx project are reported as nTPM, corresponding to mean values of the different individual samples from each tissue. TIMP-1 mRNA expression profile was accessed on 02/28/2023. (B) The lung single-cell RNA-sequencing data generated by LungGENS database show the relative TIMP-1 mRNA expression in various human lung cells (accessed on 02/28/2023). GTEx: Genotype-Tissue Expression; LungGENS: Lung Gene Expression iN Single-cell; mRNA: Messenger RNA; nTPM: Normalized protein-coding transcripts per million; RNA: Ribonucleic acid; TIMP: Tissue inhibitors of metalloprotease.
Expression and regulation of TIMP-1
Gene regulation ensures that cells express the necessary genes to proliferate, differentiate, and maintain their proper function by turning gene transcription on and off. Understanding the regulation of TIMP-1 is critical for the investigation of its involvement in various pulmonary diseases. Single-cell RNA sequencing analysis revealed that fibroblasts have the highest transcriptional level among lung cells, while endothelial cells and myeloid cells have moderate TIMP-1 levels [Fig. 1B].15 The regulation of TIMP-1 by chemicals, inhibitors, cytokines, chemokines, growth factors, etc. has attracted the attention of researchers for decades [Table 1]. Most studies have shown that fibrosis stimulus can trigger TIMP-1 expression in lung fibroblasts, macrophages, and epithelial cells. For instance, TIMP-1 expressions were most prominent in mononuclear inflammatory cells within the regions of tissue damage upon bleomycin stimulation, and TIMP-1 messenger RNA (mRNA) was identified close to areas of inflammatory cell accumulation.16 Consistently, immunoreactive TIMP-l was expressed in alveolar macrophages in both bleomycin-resistant and bleomycin-prone mice.17 Following paraquat and hyperoxia exposure, TIMP-1 was also localized in alveolar macrophages in the lungs of fibrosis rats.18 Similarly, with multi-walled carbon nanotube (MWCNT) model, Mac2+ macrophages were the source of TIMP-1 production, inside the fibrotic foci of the lungs.19 These studies emphasized that the mononuclear inflammatory cells particularly macrophages as a source of TIMP-1.
Table 1.
TIMP-1 regulation in lung cells.
| Cell type | Treatment | Change of TIMP-1 |
|---|---|---|
| Fibroblasts | TGF-β122, 23, 24, 25, 26, 27; CSE28; IL-1327; IL-3329; PI3K inhibitor (LY294002)24,27; Oncostatin M24; WSE30; media of M. tb infected monocytes31 | ↑ |
| IL-1β32,33; TNF-α33; p38 inhibitor (SB203580)24,31; ERK1/2 inhibitor (PD9805)24; Rho/Rock signaling inhibitor (Y-27632) and TGF-β/Smad signaling inhibitor (staurosporine)25; MEK1 inhibitors (U0126 and PD98059)26 | ↓ | |
| TGF-β134; CSE35; HRV36 | → | |
| Monocytes/macrophages | IL-1β37,38; LPS37, 38, 39, 40, 41; nickel nanoparticles42; p38 inhibitor (SB20358)43; CSE37 | ↑ |
| CSE or BCG44; p38 inhibitor (SB203580) and MEK inhibitor (PD98059)45; M. tb43,46; ERK inhibitor (PD9805)43 | → | |
| Epithelial cells | TGF-β147; TNF-α or IL-1β48; Xanthohumol49; Sinomenine50 | ↑ |
| TNF-α51; M. tb52; ERK1/2 inhibitor (PD9805)52; BSE53 | ↓ | |
| TNF-α54; LPS51; IL-1β51,54; HRV55; M. tb56 | → |
Upregulation; ↓ Downregulation; → Unchanged; BCG: Bacille Calmette-Guérin; BSE: Biomass smoke extract; CSE: Cigarette smoke extract; ERK: Extracellular signal-regulated kinase; HRV: Human rhinovirus; IL: Interleukin; LPS: Lipopolysaccharide; MEK: Mitogen-activated protein kinase kinase; M. tb: Mycobacterium tuberculosis; p38: Mitogen-activated protein kinase 14; PI3K: Phosphoinositide 3-kinase; Rho: Rhodopsin; Rock: Rho associated coiled-coil containing protein; SMAD: Suppressor of Mothers against Decapentaplegic; TGF-β: Transforming growth factor-beta; TIMP: Tissue inhibitors of metalloprotease; TNF-α: Tumor necrosis factor-alpha; WSE: Wood smoke extract.
Fibroblasts from the other side appeared to be prominent cells for TIMP-1 expression and production. Hsp47+ fibroblasts were the predominant source of TIMP-1 production inside the fibrotic foci of the lungs in response to MWCNT-induced lung fibrosis.19 Consistently, TIMP-1 mRNA was upregulated in pulmonary fibroblasts derived from fibrosis-sensitive C57BL/6 mice after the stimulation with active TGF-β1 compared to fibroblasts obtained from fibrosis-resistant BALB/c mice.20 Given that Smad-3 is recognized as a major mediator of TGF-β signaling in progressive fibrosis,21 one study has assessed Smad-3 knockout (KO) mice after fibrosis stimulus. These results highlight the importance of fibroblasts as TIMP-1 producers in fibrotic conditions and suggest TIMP-1 as a potential therapy target for pulmonary fibrosis. Other cells including bronchiolar and alveolar epithelial cells were reported to slightly express TIMP-1 in murine fibrotic models.17,18
TIMP-1 in ALI/ARDS
ALI and ARDS are life-threatening diseases in critically ill patients.57, 58, 59 The early stage of ALI and ARDS is identified by exudative alveolar flooding due to a disruption in the air-blood barrier and by extensive alveolar collapse due to surfactant abnormalities.60,61 The heterogeneity in causes of ARDS has resulted in a critical underdiagnosis,62 and to date, no specific pharmacological therapies have shown an improvement in the severe form of lung injury.63,64 Thus, one of the obstacles in ARDS is the identification of a promising biomarker that can be targeted later to enhance drug therapy.65
MMPs and their tissue inhibitors are thought to participate in leukocyte influx and vascular permeability at sites of lung injury.66,67 Particularly, TIMP-1, as a critical protein in ECM turnover,68 has been studied as a biomarker or treatment strategy in lung diseases. Nevertheless, the regulation and function of TIMP-1 in ALI/ARDS are largely unknown.
In a large prospective study of mechanically ventilated patients with acute respiratory failure (ARF), TIMP-1 levels were significantly higher in non-survivors than survivors and were independently associated with 90-day mortality.69 Among different groups of ARF patients, TIMP-1 levels were significantly higher in ARDS subjects than in the entire cohort.69 The high levels of TIMP-1 were associated with the severity of partial pressure of oxygen/fraction of inspired oxygen ratio (PaO2/FiO2) while improving this ratio was associated with reduced TIMP-1 levels.69 In a prospective study of critically ill patients admitted to the intensive care unit (ICU), higher plasma of TIMP-1 concentrations and MMP-9/TIMP-1 ratios were significantly associated with ARDS and 30-day mortality risk.70 Moreover, there was a significant negative correlation between plasma TIMP-1 and MMP-9 levels.70 Recently, our group measured the plasma TIMP-1 level in ARDS patients enrolled in Albuterol to Treat Acute Lung Injury (ALTA) trial.71 Higher plasma TIMP-1 levels were observed in ARDS patients than in normal control subjects.71 Interestingly, circulating TIMP-1 shows an excellent discriminating ability for the prediction of mortality among female ARDS patients.71 These findings highly suggest the potential of circulating TIMP-1 as a prognostic biomarker for ALI/ARDS [Table 2]. Nevertheless, more studies are required from the ARDS biomarker discovery to clinical application, especially as a sex-specific biomarker.
Table 2.
Correlation between circulating TIMP-1 levels and clinical severity of ALI/ARDS.
| Conclusions | P value | Reference |
|---|---|---|
| TIMP-1 levels: non-survivors>survivors | <0.001 | 69 |
| TIMP-1 was independently associated with 90-day mortality | 0.004 | |
| The mortality was significantly higher in patients with TIMP-1 levels exceeding 458.6 ng/mL than in patients with levels below the cutoff | <0.001 | |
| TIMP-1 levels were associated with the severity of hypoxemia | <0.05 | |
| MMP-8/TIMP-1 ratio was not correlated with 90-day mortality | >0.05 | |
| TIMP-1 correlated with MMP-8 (r = 0.247), CRP (r = 0.409), SOFA score (r = 0.323), and SAPS II score (r = 0.162) at 24 h from ICU admission | <0.001 | |
| TIMP-1 negatively correlated with PaO2/FiO2 ratio (r = −0.260) | <0.001 | |
| TIMP-1 levels were associated with ARDS | 0.01 | 70 |
| TIMP-1 levels were associated with 30-day mortality | 0.02 | |
| MMP-9/TIMP-1 ratios were associated with the increased risk of ARDS | 0.02 | |
| MMP-9/TIMP-1 ratios were associated with the increased risk of 30-day mortality | <0.01 | |
| TIMP-1 and MMP-9 show a negative correlation (r = −0.32) | <0.01 | |
| TIMP-1 and MMP-3 show a positive correlation (r = 0.38) | <0.01 | |
| TIMP-1 levels: ARDS patients>normal subjects | <0.001 | 71 |
| TIMP-1 levels: No difference between female and male ARDS patients | 0.481 | |
| TIMP-1 levels: non-survivors > survivors in female patients | <0.001 | |
| TIMP-1 levels: no difference between non-survivors and survivors in male patients | 0.649 | |
| The 90-day mortality was significantly higher in female patients with TIMP-1 levels exceeding 159.7 ng/mL than in patients with levels below the cutoff | <0.001 |
ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome; CRP: C-reactive protein; ICU: Intensive Care Unit; MMP: Metalloprotease; PaO2/FiO2: Partial pressure of oxygen/fraction of inspired oxygen ratio; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; TIMP: Tissue inhibitors of metalloprotease.
The pre-clinical studies on lung injury have focused on various aspects of TIMP-1 by using both wild-type (WT) and Timp-1 deficient mice. These aspects were mainly TIMP-1 expression, MMP-9/TIMP-1 ratio, weight loss, immune cell infiltrations, and lung hemorrhage. Allen et al.67 have shown that influenza infection caused a substantial induction of TIMP-1 in WT mice. Consistently, TIMP-1 expression and MMP-9/TIMP-1 ratio were significantly higher in the ALI group compared with the control group after lipopolysaccharide (LPS) instillation in mice and rats, respectively.72,73 In the latter study, the MMP-9/TIMP-1 ratio was positively associated with the lung wet/dry ratio and the pulmonary permeability index.73 Functionally, Timp-1 deficient mice showed significantly less body weight loss than WT mice after Pseudomonas aeruginosa (P. aeruginosa)74 or influenza infection.67 In addition, Timp-1 deficient mice demonstrated fewer immune cell infiltrates and airway inflammation after influenza infection, suggesting that TIMP-1 promotes lung immune response.67 In line with these findings, the knockdown of TIMP-1 using small interfering RNA (siRNA) leads to a reduced lung inflammatory phenotype during LPS-induced ALI.75 Collectively, TIMP-1 is induced in response to ALI and promotes immune responses. Loss of Timp-1 exerts protective effects by reducing lung inflammation.
TIMP-1 in pneumonia
Pneumonia is defined as an infection that occurs in the lung parenchyma and is described by alveoli filling with inflammatory exudates and ultimately leading to pulmonary tissue solidification.76 In normal physiology, polymorphonuclear neutrophils (PMNs) located in the vascular bed of the lung serve as a powerful host defense barrier.77 In pneumonia, these cells invade the alveolar compartment by secreting enzymes stored in granules and vesicles like MMPs and TIMP, which play crucial roles including ECM turnover, tissue degradation, and repair mechanisms.78
TIMP-1 has been studied in patients with pneumonia including community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) to evaluate whether the levels of TIMP-1 are related to clinical severity of the disease. TIMP-1 concentration was significantly increased in plasma from CAP patients compared to healthy controls79 and patients without lung diseases.80,81 But no significant difference in the MMP-9/TIMP-1 ratio was seen.79 Based on these results, TIMP-1 had a more significant change than MMP-9/TIMP-1 in response to pneumonia. Interestingly, a significant decrease in the TIMP-1 and MMP-9/TIMP-1 ratio was noticed after the CAP patients received antibiotic treatment compared with the pre-treatment level.79,82 Furthermore, both TIMP-1 and MMP-9/TIMP-1 ratios were evaluated in relation to inflammatory cells and pneumonia severity scores. Chiang et al.79 have shown that the plasma MMP-9/TIMP-1 ratio was positively correlated with the number of white blood cells (WBC) and neutrophils. In the same study, the plasma TIMP-1 level was also positively correlated with pneumonia severity scores including Pneumonia Severity Index (PSI), Acute Physiology and Chronic Health Evaluation (APACHE II), and CURB-65 (confusion, uremia, respiratory rate, blood pressure, age ≥65 years) scores.79 Likewise, Bircan et al.82 have reported that TIMP-1 level was correlated with PSI scores as well as oxygenation indices including PaO2 and PaO2/FiO2 ratio. These results suggest that circulating TIMP-1 levels could reflect the clinical severity of pneumonia.
TIMP-1 in asthma
Asthma is a complex disease triggered by some genetic, epigenetic, and environmental factors. It is characterized by lymphocyte and eosinophil infiltrates leading to chronic inflammation, bronchial fibroblast activation, and airway wall remodeling.83,84 ECM production and its degradation are involved in this dynamic process. Likewise, MMPs and their specific inhibitors have been reported to play crucial roles in this process.85 TIMP-1 particularly may contribute to the pathogenesis of exaggerated submucosal ECM accumulation and lack of matrix degradation in asthma.
Overall, circulating TIMP-1 has been studied in clinical cohorts that included asthmatic patients, patients with different stages of asthma, and healthy subjects to assess the variability in TIMP-1 levels between the groups. Although alteration of circulating TIMP-1 levels was seen in patients with asthma, inconsistency in results was reported [Table 3]. For instance, three independent studies have shown that serum TIMP-1 concentrations of asthmatic patients were significantly higher than those of the control subjects.86, 87, 88 However, no difference was seen between asthmatic patients and healthy subjects in another two independent studies.89,90 Furthermore, the TIMP-1 and MMP-9/TIMP-1 ratios were not related to asthma severity as assessed with forced expiratory volume in one second (FEV1).91 Likewise, there were no significant differences in serum TIMP-1, MMP-2/TIMP-1, and MMP-9/TIMP-1 between different groups of asthma.87,92 These studies suggest that TIMP-1 in circulation may not be largely altered due to asthma prognosis or severity.
Table 3.
Circulating TIMP-1 levels and clinical severity of asthma.
| Conclusions | P value | Reference |
|---|---|---|
| Serum TIMP-1 concentrations of asthmatic patients were significantly higher than those of the control subjects | <0.001 | 86 |
| Lower circulating levels of TIMP-1 in patients with asthma than that in controls, but the differences were not statistically significant | 0.27 | 90 |
| Neither TIMP-1 concentration nor MMP-9/TIMP-1 ratio was related to asthma severity | - | 91 |
| Serum TIMP-1 level was higher in asthma patients than in healthy subjects | 0.01 | 87 |
| No difference in the circulating TIMP-1 concentrations between patients with asthma exacerbation or stable asthma | >0.05 | 92 |
| No difference was seen between asthmatic patients and healthy subjects | >0.05 | 89 |
TIMP: Tissue inhibitors of metalloprotease; MMP: Metalloprotease.
The most common preclinical model utilized in asthma studies is ovalbumin (OVA) sensitization.93 The regulation of TIMP-1 has been studied after OVA challenge, and Lin et al.94 have shown that mice sensitized with OVA had significantly higher concentrations of TIMP-1 in bronchoalveolar lavage (BAL) than the control group. Moreover, Sands et al.95 employed Timp‐1 KO mice in an OVA‐induced allergic asthma model (Timp‐1 KO-OVA) to test the hypothesis that the absence of Timp‐1 would increase airway hyperactivity, lung inflammation, and remodeling in asthma. They have shown that Timp‐1 KO-OVA mice were deteriorated based on airway activity, methacholine responsiveness, dynamic lung compliance, and lung histological data in comparison to Timp‐1 KO mice receiving PBS. In addition, Timp‐1 KO-OVA mice showed higher cytokines gene expressions than WT-OVA mice, such as interleukin (IL)-5, IL-6, and IL-10.95 In addition, eosinophil count was significantly higher in Timp‐1 KO-OVA mice than in WT-OVA mice.95 Their findings suggested that TIMP‐1 plays a protective role by modulating inflammatory responses including cytokines expression and eosinophilic inflammation.
In a murine model of toluene diisocyanate (TDI)-induced asthma, TIMP-1 was increased at both mRNA and protein levels in the lung tissues after TDI inhalation in a time-dependent manner.96 Similarly, the concentration of TIMP-1 in the BAL was increased in the TDI-exposed mice at different time points.96 In the asthmatic mice, positive TIMP-1 staining was seen on inflammatory cells around bronchioles and significant correlations between the levels of TIMP-1 and the numbers of lymphocytes, neutrophils, and eosinophils were found in the BAL.96,97
TIMP-1 in COPD
COPD is an inflammatory lung disease affecting the airways, lung parenchyma, and vasculature. It is characterized by slow progressive airflow limitation leading to dyspnea, chest pain, frequent respiratory infections, exercise limitation, and respiratory failure.98 There is a widely accepted theory that ECM remodeling is an important causative factor for COPD, which is mediated by exaggerated inflammation and disruption of the proteinase/anti-proteinase balance. Moreover, both MMPs and TIMPs are believed to play crucial roles in the pathogenesis of COPD.99
Circulating TIMP-1 levels in COPD have been measured in multiple clinical cohorts [Table 4]. In a few clinical studies, the concentration of TIMP-1 in serum was higher in COPD patients than in controls.86,100, 101, 102 However, Shaker et al.103 have identified that patients with COPD had significantly lower levels of plasma TIMP-1 than smokers and non-smokers control. Similarly, D'Armiento et al.104 have shown that plasma TIMP-1 levels were significantly lower in the emphysema cohort compared to both the control and smoker groups. In the affirmative, current smoking, a major cause of COPD, was associated with reduced TIMP-1 levels in COPD patients.105 Different factors including disease severity and duration could explain varying results from previous cohort studies.
Table 4.
Circulating TIMP-1 levels and COPD.
| Conclusions | P value | Reference |
|---|---|---|
| TIMP-1 serum levels were higher in COPD patients than in healthy control | <0.0001 | 86 |
| TIMP-1 serum levels negatively correlated with the FEV1/FVC | <0.05 | |
| The circulating MMP-9/TIMP-1 ratio was significantly lower in COPD than in control subjects | <0.0001 | |
| TIMP-1 increased in COPD compared with that in healthy subjects | <0.001 | 100 |
| No significant difference in TIMP-1 levels between survivors and non-survivors | 0.839 | 114 |
| A significant difference in MMP-9/TIMP-1 ratio between survivors and non-survivors | <0.001 | |
| Patients with COPD had significantly lower levels of plasma TIMP-1 than smokers and non-smokers controls | 0.02 | 103 |
| Plasma TIMP-1 levels were significantly lower in the emphysema cohort compared to both non-smoker and smoker groups | <0.0001 | 104 |
| TIMP-1 in plasma did not correlate with disease parameters and was not predictive of subsequent lung function decline among COPD patients | >0.05 | |
| TIMP-1 did not predict the presence of emphysema in smokers | >0.05 | 115 |
| Circulating MMP-9/TIMP-1 ratio was higher in patients with emphysema than in patients with other phenotypes of COPD | <0.01 | 107 |
| FEV1 was correlated with MMP-9/TIMP-1 ratio in patients with emphysema | <0.001 | |
| No significant differences in the serum TIMP-1 levels between the healthy control group and COPD patients | >0.05 | 116 |
| Increasing age and overweight were significantly correlated to TIMP-1 in COPD | <0.05 | 105 |
| Current smoking was associated with reduced TIMP-1 levels in COPD | 0.013 | |
| Increasing MMP-9/TIMP-1 ratio was associated with current smoking, overweight, and decreasing FEV1% predicted | <0.05 | |
| A significant difference in MMP-9/TIMP-1 ratio between non-smoker subjects and COPD patients | 0.04 | 106 |
| No significant difference in MMP-9/TIMP-1 ratio between control smokers and COPD patients | 0.9 | |
| TIMP-1 was not correlated with annual changes of % predicted FEV1 | 0.961 | 108 |
| A trend of a higher level of TIMP-1 in current smokers than in COPD patients | 0.056 | |
| TIMP-1 concentrations were elevated in COPD than in control subjects | <0.001 | 101 |
| Serum concentrations of TIMP-1 were higher in non-smoking COPD patients as compared with non-smoking control subjects | 0.025 | |
| No significant difference between smokers of COPD and control subjects | >0.05 |
COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; MMP: Metalloprotease; TIMP: Tissue inhibitors of metalloprotease.
The molar ratio of MMP-9/TIMP-1 has been generally considered an important parameter in several COPD studies. Gilowska et al.106 found a significant difference in MMP-9/TIMP-1 ratio between control and COPD patients toward higher levels in COPD patients. Moreover, Uysal and Uzun107 found that the circulating MMP-9/TIMP-1 ratio was higher in patients with emphysema than in patients with other phenotypes of COPD. In contrast, one study has found that the circulating MMP-9/TIMP-1 ratio was significantly lower in COPD than in control subjects.86 The variability in this ratio from previous reports indicates that TIMP-1 level could not easily exhibit MMPs activities and the relationship between these proteins is quite complicated.
Spirometry parameters including FEV1 and forced vital capacity (FVC) are well-known clinical parameters to assess lung function decline in COPD and asthma patients. Among COPD patients, TIMP-1 serum levels negatively correlated with the FEV1/FVC ratio reflecting airway obstruction.86 Yet, two studies have stated that TIMP-1 measurements in plasma were not predictive of subsequent functional decline as assessed by FEV1.104,108 Furthermore, the molar ratio of MMP-9/TIMP-1 has shown a negative correlation with FEV1% predicted in two studies.105,107 These studies indicate that circulating TIMP-1 and MMP-9/TIMP-1 may partially reflect lung function decline among COPD patients as assessed with FEV1 and FVC.
Cigarette smoke (CS) has been widely recognized and utilized as a preclinical model for COPD manifestations.109 Upon CS exposure, mRNA expression of TIMP-1 was highly increased in mice.110,111 In the latter study, the increase of TIMP-1 protein level was also seen in CS-exposed murine lungs.111 These studies indicate the dysregulation of TIMP-1 in response to CS may involve in the pathogenesis of COPD. In addition, the imbalance of TIMP-1 and MMP-9 is believed to be associated with the development of lung emphysema in Klotho mice,112 which exhibit multiple aging-like phenotypes and pulmonary emphysema.113 Although evidence links the alteration of TIMP-1 levels and COPD, the role of TIMP-1 in the development of COPD is not well studied using gain-of-function or loss-of-function strategies.
TIMP-1 in cystic fibrosis
Cystic fibrosis (CF) is a lethal genetic disease caused by several mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CFTR dysfunction leads to chloride channel defects resulting in mucus accumulation, endobronchial infection, and exaggerated pulmonary inflammation.117 One of the hallmarks of CF is the progressive remodeling of tissue and in particular, the accumulation of ECM and the lack of matrix degradation.118 The identification of the mediators involved in CF pathophysiology may provide prognostic markers with the potential to predict disease prognosis and assess response to treatment. Increasing evidence suggests that dysregulated activities of MMPs and their inhibitors lead to scar formation and subsequent tissue fibrosis in CF.118
Both TIMP-1 and its ratios have been assessed in CF patients in relation to pulmonary exacerbations and spirometry parameters including forced expiratory volume and vital capacity. In two independent studies, enhanced TIMP-1 was found in CF patients with pulmonary exacerbations compared to healthy controls and patients without pulmonary exacerbations.119,120 The MMP-9/TIMP-1 ratio was also increased in patients in comparison to healthy controls.120 In relation to spirometry parameters, Rath et al.119 have identified that the serum expression of TIMP-1 was significantly increased in CF adult patients with a declined FEV1 and vital capacity. Similarly, Devereux et al.121 have shown that the plasma MMP-9/TIMP-1 ratio was negatively correlated with FEV1. Higher concentrations of plasma TIMP-1 were associated with increased mortality.121 Altogether, TIMP-1 appears to play a crucial role in CF via controlling ECM homeostasis. The circulating TIMP-1 level may serve as a diagnostic and prognostic blood marker.
TIMP-1 in pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and fibrotic lung disease of unknown etiology characterized by epithelial cell injury, fibroblast proliferation, and excessive accumulation of ECM in the alveolar architecture.122 This disease leads to decreased lung compliance, impaired gas exchange, and eventually lung failure and death despite therapy, with the median survival time being 2–4 years from diagnosis.123 Thus, the identification of host genes that participate in the development of IPF may help uncover novel drug targets.
IPF patients have higher circulating TIMP-1 levels than controls.124, 125, 126 Patients with MMP-9 gene polymorphism showed an elevation of TIMP-1 supporting the importance of MMP-9/TIMP-1 ratio.126 Moreover, TIMP-1 was positively correlated with MMP-9 in another study.127 Thus, altered TIMP-1 levels in IPF patients influence the MMP-9/TIMP-1 ratio, which involves interstitial lung diseases (ILDs).
Pulmonary fibrosis can be induced experimentally using several chemicals including bleomycin, LPS, silica, asbestosis, MWCNT, paraquat and hyperoxia, and cytokine overexpression.128 The most reported method in the literature is bleomycin representing the most applied preclinical model of lung fibrosis.129 TIMP-1 mRNA and protein levels from WT mice were increased after intranasal bleomycin administration in lung tissue and BAL.16,130 Bleomycin-induced TIMP-1 in BAL has been reported in both fibrosis-resistant (BALB/c) and fibrosis-sensitive (C57BL/6) mice.17 However, another study showed that a single intratracheal injection of bleomycin upregulated TIMP-1 levels in the lungs and in the BAL from sensitive C57BL/6 mice but not the bleomycin-resistant BALB/c strain.131 The dose and route of administration could explain the inconsistent findings that were seen in fibrosis-resistant mice. In paraquat and hyperoxia model, fibrosis rats exhibited a significant increase in TIMP-1 mRNA levels from lung homogenates than control.18 In MWCNT-induced fibrosis method, TIMP-1 mRNA lung expression and protein levels in BALF and serum were markedly increased compared with baseline level in a time-and dose-dependent manner.19 These studies emphasize the robust association between TIMP-1 and lung fibrosis.
Although preclinical evidence suggests the altered regulation of TIMP-1 in response to bleomycin administration may play a role in pulmonary fibrosis,17 there was no difference in lung fibrosis between Timp-1 deficient mice and control mice after bleomycin treatment.132,133 In another study, Tang et al.134 repressed TIMP-1 using antisense complementary DNA (cDNA) retroviral vectors. Interestingly, they demonstrated that TIMP-1 knockdown can suppress bleomycin-induced pulmonary fibrosis in the early stages.134 Thus, more studies are needed to address these controversial findings on the role of TIMP-1 in pulmonary fibrosis.
Summary and conclusions
In this review, we summarized the current knowledge of TIMP-1 in pulmonary diseases, including ALI/ARDS, pneumonia, asthma, COPD, cystic fibrosis, and pulmonary fibrosis. We also reviewed the regulation of TIMP-1 in response to in vitro stimulus focusing on various lung cells. These studies mainly focused on fibroblasts, macrophages, and epithelial cells.
TIMP-1 has been reported widely with MMPs denoting the importance of the TIMP-1/MMPs ratios as they may intimately affect each other at tissue and biofluid levels. It is theoretically presumed that TIMP-1 is capable of inhibiting MMPs activities, and this can be simply explained by an inverse relationship. Yet, circulating TIMP-1 was positively correlated with MMP-9 and MMP-3 among IPF and ARDS patients, respectively indicating the complex associations of these proteins.28,71 Generally, it seems that the TIMP-1 level could not easily exhibit MMPs activities and the relationship between these proteins is quite complicated.
Different pathogens or stimulations like P. aeruginosa, influenza, Mycobacterium tuberculosis (M. tb), LPS or bleomycin were applied to induce or suppress TIMP-1 expression in experimental studies. TIMP-1 responds differently to pathogens, which could be caused by several factors including the intensity of each pathogen, the etiology, the molecular pathways, and infectious versus sterile injury. Nevertheless, in most previous reports, Timp-1 KO mice showed less injury in response to ALI, such as H1N1 influenza infection67 and P. aeruginosa.74 These studies not only suggest that the loss of Timp-1 could protect mice from lung infection and injury, but also indicate the potential role of TIMP-1 as an immune modulator.
Fibroblasts appeared to be the most important cells in driving TIMP-1 dysregulation when compared to other cells based on the findings from the current review. For instance, bleomycin administration elicited an increase in the protein levels of TIMP-1 in the BAL and the transcript levels in lung tissue extracts of mice treated with or without anti-PMN antibodies.135 This indicates that polymorphonuclear leukocytes including neutrophils were not enough to be targeted in diminishing TIMP-1 secretion. Similarly, epithelial overexpression of TIMP-1 did not alter lung fibrosis in mice.131 In the study of Smad-3 KO mice, significantly increased expression of TIMP-1 was seen in WT fibroblasts but not in Smad-3 deficient fibroblasts after treatment with recombinant TGF-β1 in vitro. Consistent findings were also seen after TGF-β1 administration in vivo in the same study.136 In another study, the inhibition of TIMP-1 by its neutralizing antibodies in vitro effectively reduced the proliferative effect on fibroblasts.19 Furthermore, TIMP-1 protein secretion was reduced in MRC5 fibroblast cells in response to M. tb infection,31 but was not affected by macrophage infection with the same pathogen.43 Likewise, M. tb infection did not affect TIMP-1 protein secretion in human bronchial epithelial cells (HBECs).56 Overall, these findings indicate that fibroblasts are the most important cells in driving TIMP-1 dysregulation. Thus, further investigation of TIMP-1 in fibroblasts may unveil potential treatment strategies for lung fibrosis.
In clinical cohorts, circulating TIMP-1 has been measured mainly as a diagnostic marker providing a possibility to differentiate between different groups of patients and to reflect the disease severity. The potential of TIMP-1 to serve as a prognostic marker after receiving therapies has not received much attention in the literature so far. In pneumonia, a significant decrease in the MMP-9/TIMP-1 ratio was noticed after the CAP patients received antibiotic treatment in comparison with the pre-treatment level in two independent studies.79,82 Consistently, TIMP-1 levels decreased significantly after glucocorticoid therapy compared with the pre-treatment levels in the IPF patients.126 Thus, TIMP-1 could be investigated as a non-invasive blood marker to evaluate the effectiveness of drug therapies in pulmonary diseases, particularly lung fibrosis.
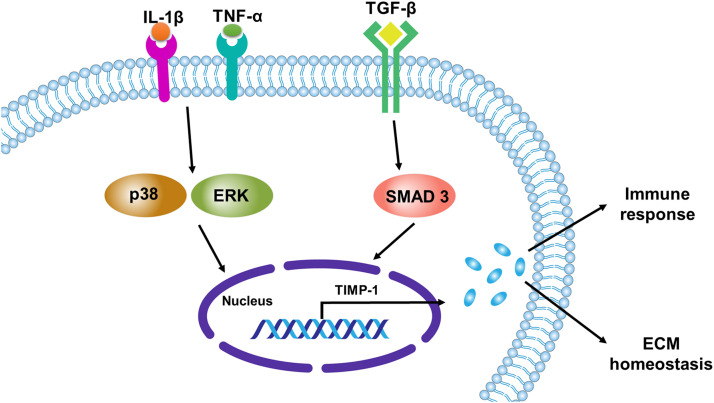
Different mechanisms and pathways have been studied in the regulation of TIMP-1. So far, TGF/Smad pathways are confirmed as upstream modulators of TIMP-1 [Fig. 2]. MWCNTs activated the extracellular regulated protein kinases (ERK) pathway in murine fibroblast cells in a TIMP-1-dependent manner.19 However, inhibition of the ERK pathway using PD980590 had no effect on TIMP-1 secretion on MRC5 cells and normal adult human lung fibroblasts after infection with M. tb.31 Furthermore, inhibition of p38 MAPK pathway using SB203580 reversed infection-induced inhibition of TIMP-1 secretion in the later study.31 Similarly, p38 MAPK inhibitor (SB203580) increased TIMP-1 secretion in a dose-dependent manner after macrophages were infected with M. tb.43 This effect was also seen in normal human bronchial epithelial cells after infection with M. tb and using p38 pathway inhibitor (SB203580).52 Moreover, the upregulation of TIMP-1 by TGF-β1 has been shown in various types of cells.22, 23, 24, 25, 26, 27,47 A significantly increased expression of TIMP-1 in fibroblasts isolated from WT but not in fibroblasts of Smad3 KO mice was seen after treatment with recombinant TGF-β1 in vitro,136 indicating that the TGF-β/Smad signaling pathway plays a role in the regulation of TIMP-1.
Fig. 2.
A schematic representation of TIMP-1 regulation in lung cells. Inflammatory mediators IL-1β, TNF-α, and TGF-β promote TIMP-1 expression via signaling pathways, such as mitogen-activated protein kinases pathways (p38 and ERK) and TGF/SMAD pathway. TIMP-1 may involve in the pathogenesis of pulmonary diseases through the modulation of the immune response and ECM homeostasis. ECM: Extracellular matrix; ERK: Extracellular signal-regulated kinase; IL-1β: Interleukin-1-beta; p38: Mitogen-activated protein kinase 14; SMAD: Suppressor of mothers against decapentaplegic; TGF-β: Transforming growth factor-beta; TIMP: Tissue inhibitors of metalloprotease; TNF-α: Tumor necrosis factor-alpha.
Several other factors may also affect the expression of TIMP-1, including age, weight, and smoking based on the current studies. In COPD, increasing age and overweight were significantly related to increasing TIMP-1 in plasma.105 Moreover, current smoking was associated with reduced TIMP-1 levels in the same study.105 It is well known that TIMP-1 is an adipocyte-secreted protein and can be upregulated by adipokines, which may explain the significant association with overweight patients.137 Similarly, adipokines are well recognized to increase with age and this could be due to increased adipose tissue mass.138 However, lower levels of TIMP-1 among smokers can be explained by the association of smoking with lipolysis and body weight loss.139
Biological sex has been reported to influence susceptibility to infection, immune response, disease severity, and response to therapy.140,141 Sex hormones particularly estradiol plays regulatory roles in immune responses as described by the induction of pro-inflammatory cytokines and macrophage activation.142 Estradiol was also found to upregulate T helper 17 (Th17)-related inflammation and worsen pneumonia in mice.143 TIMP-1 has a genomic location on the X chromosome.144,145 Most genes from the inactivated X-chromosome are silenced, while TIMP-1 may escape X-inactivation. Anderson and Brown146 showed that human TIMP-1 is prone to reactivation and also variable in its inactivation. Under inflammatory conditions, estradiol significantly induced TIMP-1 expression in goat oviductal epithelial cells147 and also in human aortic endothelial cells.148 Thus, there is a possibility that TIMP-1 could be largely regulated by estradiol and may be affected more in females. Our recent clinical study of ALI/ARDS showed that circulating TIMP-1 level was a promising predictor of mortality, ventilator-free days, and ICU-free days among females.71 Nevertheless, no study considered the female sex as a factor that could significantly affect TIMP-1 expression under normal and pathological conditions yet. Most previous studies investigating the role of TIMP-1 in lung injury or fibrosis employed male rodents only.16,19,67,73,74,135,149,150 In addition, some studies only used female rodents20,75,131 and others did not specify the sex of study subjects.72,130,136,151 Therefore, the female sex needs to be considered as a key factor that may critically influence TIMP-1 expression under physiological and pathological conditions.
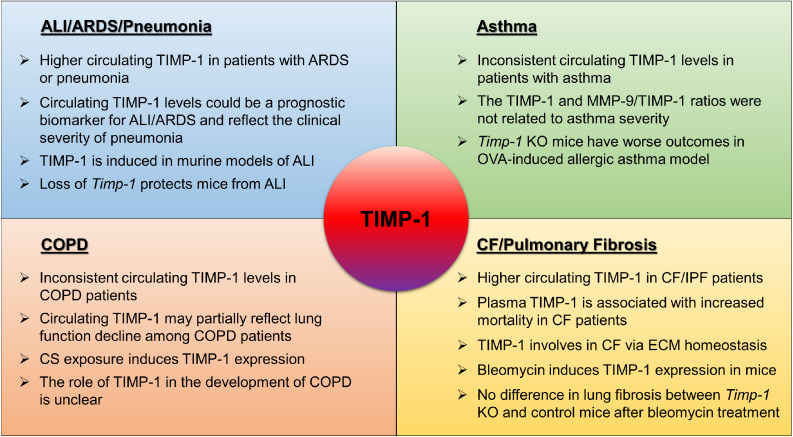
In summary, this review has focused on the dysregulation of TIMP-1 in pulmonary diseases by summarizing the findings in both preclinical and clinical studies [Fig. 3]. It also discussed the heterogeneity and consistency in previous studies. Current findings indicate that TIMP-1 may reflect the pathogenesis of pulmonary diseases, and could serve as a promising targeted therapy for pulmonary fibrosis. In this scenario, neutralizing TIMP-1 using a monoclonal antibody can provide a strategy for inhibiting the abnormally increased TIMP-1 in disease conditions.152 However, preclinical studies are urgently needed to evaluate the therapeutic efficacy and mechanism before its clinical use in treating pulmonary diseases, especially lung fibrosis.
Fig. 3.
A diagram illustrating the regulation and function of TIMP-1 in pulmonary diseases. ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome; CF: Cystic fibrosis; COPD: Chronic obstructive pulmonary disease; ECM: Extracellular matrix; IPF: Idiopathic pulmonary fibrosis; KO: Knockout; MMP: Metalloproteases; OVA: Ovalbumin; TIMP: Tissue inhibitors of metalloprotease.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by National Institutes of Health (NIH) grants NIH/NHLBI R00 HL141685 to D.Z. and NIH/NIAID R03AI169063 to X.W.
Acknowledgment
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health (NIH), and by National Cancer Institute (NCI), National Human Genome Research Institute (NHGRI), National Heart, Lung, and Blood Institute (NHLBI), National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), and National Institute of Neurological Disorders and Stroke (NINDS). The data used for the analyses described in Fig. 1A were obtained from the GTEx Portal of The Human Protein Atlas on 02/28/2023.
Edited by: Peifang Wei
References
- 1.Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989;264:17374–17378. doi: 10.1016/S0021-9258(18)71503-2. [DOI] [PubMed] [Google Scholar]
- 2.Justo BL, Jasiulionis MG. Characteristics of TIMP1, CD63, and beta1-integrin and the functional impact of their interaction in cancer. Int J Mol Sci. 2021;22:9319. doi: 10.3390/ijms22179319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017;17:38–53. doi: 10.1038/nrc.2016.115. [DOI] [PubMed] [Google Scholar]
- 4.Remillard TC, Bratslavsky G, Jensen-Taubman S, Stetler-Stevenson WG, Bourboulia D. Molecular mechanisms of tissue inhibitor of metalloproteinase 2 in the tumor microenvironment. Mol Cell Ther. 2014;2:17. doi: 10.1186/2052-8426-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 6.Triebel S, Bläser J, Gote T, et al. Evidence for the tissue inhibitor of metalloproteinases-1 (TIMP-1) in human polymorphonuclear leukocytes. Eur J Biochem. 1995;231:714–719. doi: 10.1111/j.1432-1033.1995.0714d.x. [DOI] [PubMed] [Google Scholar]
- 7.Zaoui P, Barro C, Morel F. Differential expression and secretion of gelatinases and tissue inhibitor of metalloproteinase-1 during neutrophil adhesion. Biochim Biophys Acta. 1996;1290:101–112. doi: 10.1016/0304-4165(96)00008-6. [DOI] [PubMed] [Google Scholar]
- 8.Lizio M, Abugessaisa I, Noguchi S, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47:D752–D758. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol. 1991;97:679–685. doi: 10.1111/1523-1747.ep12483956. [DOI] [PubMed] [Google Scholar]
- 10.Petitfrère E, Kadri Z, Boudot C, et al. Involvement of the p38 mitogen-activated protein kinase pathway in tissue inhibitor of metalloproteinases-1-induced erythroid differentiation. FEBS Lett. 2000;485:117–121. doi: 10.1016/s0014-5793(00)02210-9. [DOI] [PubMed] [Google Scholar]
- 11.Lambert E, Dassé E, Haye B, Petitfrère E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Aaberg-Jessen C, Christensen K, Offenberg H, et al. Low expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in glioblastoma predicts longer patient survival. J Neurooncol. 2009;95:117–128. doi: 10.1007/s11060-009-9910-8. [DOI] [PubMed] [Google Scholar]
- 13.Kuvaja P, Talvensaari-Mattila A, Pääkkö P, Turpeenniemi-Hujanen T. The absence of immunoreactivity for tissue inhibitor of metalloproteinase-1 (TIMP-1), but not for TIMP-2, protein is associated with a favorable prognosis in aggressive breast carcinoma. Oncology. 2005;68:196–203. doi: 10.1159/000086774. [DOI] [PubMed] [Google Scholar]
- 14.Song G, Xu S, Zhang H, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35:148. doi: 10.1186/s13046-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Guo M, Whitsett JA, Xu Y. `LungGENS': a web-based tool for mapping single-cell gene expression in the developing lung. Thorax. 2015;70:1092–1094. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madtes DK, Elston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001;24:599–607. doi: 10.1165/ajrcmb.24.5.4192. [DOI] [PubMed] [Google Scholar]
- 17.Manoury B, Caulet-Maugendre S, Guénon I, Lagente V, Boichot E. TIMP-1 is a key factor of fibrogenic response to bleomycin in mouse lung. Int J Immunopathol Pharmacol. 2006;19:471–487. doi: 10.1177/039463200601900303. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz V, Ordóñez RM, Berumen J, et al. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1026–L1036. doi: 10.1152/ajplung.00183.2003. [DOI] [PubMed] [Google Scholar]
- 19.Dong J, Ma Q. TIMP1 promotes multi-walled carbon nanotube-induced lung fibrosis by stimulating fibroblast activation and proliferation. Nanotoxicology. 2017;11:41–51. doi: 10.1080/17435390.2016.1262919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb M, Bonniaud P, Galt T, et al. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of "fibrosis-prone" and "fibrosis-resistant" mouse strains. Am J Respir Cell Mol Biol. 2002;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 21.Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Todorova L, Gürcan E, Westergren-Thorsson G, Miller-Larsson A. Budesonide/formoterol effects on metalloproteolytic balance in TGFbeta-activated human lung fibroblasts. Respir Med. 2009;103:1755–1763. doi: 10.1016/j.rmed.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Chen C, Finger SN, et al. Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur Respir J. 2009;34:145–155. doi: 10.1183/09031936.00084808. [DOI] [PubMed] [Google Scholar]
- 24.Tong L, Smyth D, Kerr C, Catterall J, Richards CD. Mitogen-activated protein kinases Erk1/2 and p38 are required for maximal regulation of TIMP-1 by oncostatin M in murine fibroblasts. Cell Signal. 2004;16:1123–1132. doi: 10.1016/j.cellsig.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Ji H, Tang H, Lin H, et al. Rho/Rock cross-talks with transforming growth factor-beta/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep. 2014;2:787–792. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Hu H, Huynh ML, et al. Mechanisms of tissue inhibitor of metalloproteinase 1 augmentation by IL-13 on TGF-beta 1-stimulated primary human fibroblasts. J Allergy Clin Immunol. 2007;119:1388–1397. doi: 10.1016/j.jaci.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Trudeau JB, Schoonover KJ, et al. Interleukin-13 augments transforming growth factor-beta1-induced tissue inhibitor of metalloproteinase-1 expression in primary human airway fibroblasts. Am J Physiol Cell Physiol. 2005;288:C435–C442. doi: 10.1152/ajpcell.00035.2004. [DOI] [PubMed] [Google Scholar]
- 28.Guan S, Liu Q, Han F, et al. Ginsenoside Rg1 ameliorates cigarette smoke-induced airway fibrosis by suppressing the TGF-beta1/Smad pathway in vivo and in vitro. Biomed Res Int. 2017;2017 doi: 10.1155/2017/6510198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Luo Z, Zheng J, et al. IL-33 can promote the process of pulmonary fibrosis by inducing the imbalance between MMP-9 and TIMP-1. Inflammation. 2018;41:878–885. doi: 10.1007/s10753-018-0742-6. [DOI] [PubMed] [Google Scholar]
- 30.Recillas-Román S, Montaño M, Ruiz V, et al. Wood smoke extract promotes extracellular matrix remodeling in normal human lung fibroblasts. Int J Toxicol. 2021;40:506–516. doi: 10.1177/10915818211044809. [DOI] [PubMed] [Google Scholar]
- 31.O'Kane CM, Elkington PT, Jones MD, et al. STAT3, p38 MAPK, and NF-kappaB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2010;43:465–474. doi: 10.1165/rcmb.2009-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Baek MS, Yoon DS, et al. Vitamin D inhibits expression and activity of matrix metalloproteinase in human lung fibroblasts (HFL-1) cells. Tuberc Respir Dis. 2014;77:73–80. doi: 10.4046/trd.2014.77.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Q, Liu X, Al-Mugotir M, et al. Thrombin and TNF-alpha/IL-1beta synergistically induce fibroblast-mediated collagen gel degradation. Am J Respir Cell Mol Biol. 2006;35:714–721. doi: 10.1165/rcmb.2005-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan R, Zhang Y, Zheng M, Zang B, Jin M. Hydroxysafflor yellow a suppresses MRC-5 cell activation induced by TGF-beta1 by blocking TGF-beta1 binding to TbetaRII. Front Pharmacol. 2017;8:264. doi: 10.3389/fphar.2017.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Liu X, Kohyama T, et al. Cigarette smoke stimulates MMP-1 production by human lung fibroblasts through the ERK1/2 pathway. COPD. 2004;1:13–23. doi: 10.1081/COPD-120030164. [DOI] [PubMed] [Google Scholar]
- 36.Wieczfinska J, Pawliczak R. Thymic stromal lymphopoietin and apocynin alter the expression of airway remodeling factors in human rhinovirus-infected cells. Immunobiology. 2017;222:892–899. doi: 10.1016/j.imbio.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Russell RE, Culpitt SV, DeMatos C, et al. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;26:602–609. doi: 10.1165/ajrcmb.26.5.4685. [DOI] [PubMed] [Google Scholar]
- 38.Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162:1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- 39.Liang Y, Yang N, Pan G, Jin B, Wang S, Ji W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell Mol Biol Lett. 2018;23:52. doi: 10.1186/s11658-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jan JS, Yang CH, Wang MH, et al. Hirsutanol a attenuates lipopolysaccharide-mediated matrix metalloproteinase 9 expression and cytokines production and improves endotoxemia-induced acute sickness behavior and acute lung injury. Mar Drugs. 2019;17:360. doi: 10.3390/md17060360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhang D, Fucci QA, Dollery CM, Owen CA. Surface-bound matrix metalloproteinase-8 on macrophages: contributions to macrophage pericellular proteolysis and migration through tissue barriers. Physiol Rep. 2021;9:e14778. doi: 10.14814/phy2.14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan R, Mo Y, Chien S, et al. The role of hypoxia inducible factor-1alpha in the increased MMP-2 and MMP-9 production by human monocytes exposed to nickel nanoparticles. Nanotoxicology. 2011;5:568–582. doi: 10.3109/17435390.2010.537791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rand L, Green JA, Saraiva L, Friedland JS, Elkington PT. Matrix metalloproteinase-1 is regulated in tuberculosis by a p38 MAPK-dependent, p-aminosalicylic acid-sensitive signaling cascade. J Immunol. 2009;182:5865–5872. doi: 10.4049/jimmunol.0801935. [DOI] [PubMed] [Google Scholar]
- 44.Le Y, Cao W, Zhou L, et al. Infection of Mycobacterium tuberculosis promotes both M1/M2 polarization and MMP production in cigarette smoke-exposed macrophages. Front Immunol. 2020;11:1902. doi: 10.3389/fimmu.2020.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo CY, Huang HY, He JR, et al. Increased matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio in smokers with airway hyperresponsiveness and accelerated lung function decline. Int J Chron Obstruct Pulmon Dis. 2018;13:1135–1144. doi: 10.2147/COPD.S161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkington PT, Nuttall RK, Boyle JJ, et al. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–1604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Guo Y, Dong R, Dai R, Zhou M. Suppressor of cytokine signaling 1-modulated metalloproteinases and tissue inhibitor of metalloproteinase in pulmonary fibrosis. Mol Med Rep. 2015;12:3855–3861. doi: 10.3892/mmr.2015.3810. [DOI] [PubMed] [Google Scholar]
- 48.Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1444–L1452. doi: 10.1152/ajplung.2001.281.6.L1444. [DOI] [PubMed] [Google Scholar]
- 49.Slawinska-Brych A, Mizerska-Kowalska M, Król SK, Stepulak A, Zdzisinska B. Xanthohumol impairs the PMA-driven invasive behaviour of lung cancer cell line A549 and exerts anti-EMT action. Cells. 2021;10:1484. doi: 10.3390/cells10061484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen KH, Hung JH, Liao YC, Tsai ST, Wu MJ, Chen PS. Sinomenine inhibits migration and invasion of human lung cancer cell through downregulating expression of miR-21 and MMPs. Int J Mol Sci. 2020;21:3080. doi: 10.3390/ijms21093080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao PM, Maitre B, Delacourt C, Buhler JM, Harf A, Lafuma C. Divergent regulation of 92-kDa gelatinase and TIMP-1 by HBECs in response to IL-1beta and TNF-alpha. Am J Physiol. 1997;273:L866–L874. doi: 10.1152/ajplung.1997.273.4.L866. [DOI] [PubMed] [Google Scholar]
- 52.Elkington PT, Emerson JE, Lopez-Pascua LD, et al. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J Immunol. 2005;175:5333–5340. doi: 10.4049/jimmunol.175.8.5333. [DOI] [PubMed] [Google Scholar]
- 53.Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One. 2012;7:e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tacon CE, Wiehler S, Holden NS, Newton R, Proud D, Leigh R. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-{kappa}B. Am J Respir Cell Mol Biol. 2010;43:201–209. doi: 10.1165/rcmb.2009-0216OC. [DOI] [PubMed] [Google Scholar]
- 56.Brilha S, Chong DLW, Khawaja AA, et al. Integrin alpha2beta1 expression regulates matrix metalloproteinase-1-dependent bronchial epithelial repair in pulmonary tuberculosis. Front Immunol. 2018;9:1348. doi: 10.3389/fimmu.2018.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragaller M, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010;3:43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lew TW, Kwek TK, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 59.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2:1009. [PMC free article] [PubMed] [Google Scholar]
- 62.Welker C, Huang J, Gil IJN, Ramakrishna H. 2021 acute respiratory distress syndrome update, with coronavirus disease 2019 focus. J Cardiothorac Vasc Anesth. 2022;36:1188–1195. doi: 10.1053/j.jvca.2021.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166. doi: 10.1186/1741-7015-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva PL, Pelosi P, Rocco PRM. Personalized pharmacological therapy for ARDS: a light at the end of the tunnel. Expert Opin Investig Drugs. 2020;29:49–61. doi: 10.1080/13543784.2020.1699531. [DOI] [PubMed] [Google Scholar]
- 65.García-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5:283. doi: 10.21037/atm.2017.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner RL, Beltran L, Younkin EM, et al. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol. 2001;24:537–544. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]
- 67.Allen JR, Ge L, Huang Y, et al. TIMP-1 promotes the immune response in influenza-induced acute lung injury. Lung. 2018;196:737–743. doi: 10.1007/s00408-018-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kubota Y, Nishiwaki K, Ito M, Sugimoto A. The role of tissue inhibitors of metalloproteinases in organ development and regulation of ADAMTS family metalloproteinases in Caenorhabditis elegans. Genetics. 2019;212:523–535. doi: 10.1534/genetics.119.301795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hästbacka J, Linko R, Tervahartiala T, et al. Serum MMP-8 and TIMP-1 in critically ill patients with acute respiratory failure: TIMP-1 is associated with increased 90-day mortality. Anesth Analg. 2014;118:790–798. doi: 10.1213/ANE.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 70.Jones TK, Reilly JP, Anderson BJ, et al. Elevated plasma levels of matrix metalloproteinase-3 and tissue-inhibitor of matrix metalloproteinases-1 associate with organ dysfunction and mortality in sepsis. Shock. 2022;57:41–47. doi: 10.1097/SHK.0000000000001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almuntashiri S, Jones TW, Wang X, Sikora A, Zhang D. Plasma TIMP-1 as a sex-specific biomarker for acute lung injury. Biol Sex Differ. 2022;13:70. doi: 10.1186/s13293-022-00481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo Y, Ma L, Zhang F, Sun R, Li T. Neutrophil elastase ameliorates matrix metalloproteinase-9 to promote lipopolysaccharide-induced acute lung injury in mice 1. Acta Cir Bras. 2016;31:382–388. doi: 10.1590/S0102-865020160060000004. [DOI] [PubMed] [Google Scholar]
- 73.Chen G, Ge D, Zhu B, Shi H, Ma Q. Upregulation of matrix metalloproteinase 9 (MMP9)/tissue inhibitor of metalloproteinase 1 (TIMP1) and MMP2/TIMP2 ratios may be involved in lipopolysaccharide-induced acute lung injury. J Int Med Res. 2020;48:1–10. doi: 10.1177/0300060520919592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee MM, Yoon BJ, Osiewicz K, et al. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect Immun. 2005;73:661–665. doi: 10.1128/IAI.73.1.661-665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chernikov IV, Staroseletz YY, Tatarnikova IS, et al. siRNA-mediated Timp1 silencing inhibited the inflammatory phenotype during acute lung injury. Int J Mol Sci. 2023;24:1641. doi: 10.3390/ijms24021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain V, Vashisht R, Yilmaz G, Bhardwaj A. StatPearls; Treasure Island (FL): 2022. Pneumonia Pathology. [PubMed] [Google Scholar]
- 77.Giacalone VD, Margaroli C, Mall MA, Tirouvanziam R. Neutrophil adaptations upon recruitment to the lung: new concepts and implications for homeostasis and disease. Int J Mol Sci. 2020;21:851. doi: 10.3390/ijms21030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiang TY, Tsao SM, Yeh CB, Yang SF. Matrix metalloproteinases in pneumonia. Clin Chim Acta. 2014;433:272–277. doi: 10.1016/j.cca.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 79.Chiang TY, Yu YL, Lin CW, Tsao SM, Yang SF, Yeh CB. The circulating level of MMP-9 and its ratio to TIMP-1 as a predictor of severity in patients with community-acquired pneumonia. Clin Chim Acta. 2013;424:261–266. doi: 10.1016/j.cca.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 80.Herr C, Mang S, Mozafari B, et al. Distinct patterns of blood cytokines beyond a cytokine storm predict mortality in COVID-19. J Inflamm Res. 2021;14:4651–4667. doi: 10.2147/JIR.S320685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartog CM, Wermelt JA, Sommerfeld CO, Eichler W, Dalhoff K, Braun J. Pulmonary matrix metalloproteinase excess in hospital-acquired pneumonia. Am J Respir Crit Care Med. 2003;167:593–598. doi: 10.1164/rccm.200203-258OC. [DOI] [PubMed] [Google Scholar]
- 82.Bircan HA, Çakir M, Yilmazer Kapulu I, Sütcü R, Kaya S, Öztürk O. Elevated serum matrix metalloproteinase-2 and -9 and their correlations with severity of disease in patients with community-acquired pneumonia. Turk J Med Sci. 2015;45:593–599. doi: 10.3906/sag-1402-51. [DOI] [PubMed] [Google Scholar]
- 83.Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med. 2020;7:191. doi: 10.3389/fmed.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parameswaran K, Willems-Widyastuti A, Alagappan VK, Radford K, Kranenburg AR, Sharma HS. Role of extracellular matrix and its regulators in human airway smooth muscle biology. Cell Biochem Biophys. 2006;44:139–146. doi: 10.1385/CBB:44:1:139. [DOI] [PubMed] [Google Scholar]
- 86.Higashimoto Y, Yamagata Y, Iwata T, et al. Increased serum concentrations of tissue inhibitor of metalloproteinase-1 in COPD patients. Eur Respir J. 2005;25:885–890. doi: 10.1183/09031936.05.00092804. [DOI] [PubMed] [Google Scholar]
- 87.Park HS, Kim HA, Jung JW, et al. Metalloproteinase-9 is increased after toluene diisocyanate exposure in the induced sputum from patients with toluene diisocyanate-induced asthma. Clin Exp Allergy. 2003;33:113–118. doi: 10.1046/j.1365-2222.2003.01563.x. [DOI] [PubMed] [Google Scholar]
- 88.Cao TBT, Quoc QL, Yang EM, et al. Tissue inhibitor of metalloproteinase-1 enhances eosinophilic airway inflammation in severe asthma. Allergy Asthma Immunol Res. 2023;15:e25. doi: 10.4168/aair.2023.15.4.451. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belleguic C, Corbel M, Germain N, et al. Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy. 2002;32:217–223. doi: 10.1046/j.1365-2222.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 90.Kumar M, Bhadoria DP, Dutta K, et al. The alpha(1)AT and TIMP-1 gene polymorphism in the development of asthma. Comp Funct Genomics. 2012;2012 doi: 10.1155/2012/968267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ko FW, Diba C, Roth M, et al. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005;127:1919–1927. doi: 10.1378/chest.127.6.1919. [DOI] [PubMed] [Google Scholar]
- 92.Oshita Y, Koga T, Kamimura T, Matsuo K, Rikimaru T, Aizawa H. Increased circulating 92 kDa matrix metalloproteinase (MMP-9) activity in exacerbations of asthma. Thorax. 2003;58:757–760. doi: 10.1136/thorax.58.9.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casaro M, Souza VR, Oliveira FA, Ferreira CM. OVA-induced allergic airway inflammation mouse model. Methods Mol Biol. 2019;1916:297–301. doi: 10.1007/978-1-4939-8994-2_28. [DOI] [PubMed] [Google Scholar]
- 94.Lin SC, Chou HC, Chiang BL, Chen CM. CTGF upregulation correlates with MMP-9 level in airway remodeling in a murine model of asthma. Arch Med Sci. 2017;13:670–676. doi: 10.5114/aoms.2016.60371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sands MF, Ohtake PJ, Mahajan SD, et al. Tissue inhibitor of metalloproteinase-1 modulates allergic lung inflammation in murine asthma. Clin Immunol. 2009;130:186–198. doi: 10.1016/j.clim.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee KS, Jin SM, Lee H, Lee YC. Imbalance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in toluene diisocyanate-induced asthma. Clin Exp Allergy. 2004;34:276–284. doi: 10.1111/j.1365-2222.2004.01849.x. [DOI] [PubMed] [Google Scholar]
- 97.Cho JY, Miller M, McElwain K, et al. Remodeling associated expression of matrix metalloproteinase 9 but not tissue inhibitor of metalloproteinase 1 in airway epithelium: modulation by immunostimulatory DNA. J Allergy Clin Immunol. 2006;117:618–625. doi: 10.1016/j.jaci.2005.12.1324. [DOI] [PubMed] [Google Scholar]
- 98.Agarwal AK, Raja A, Brown BD. StatPearls; Treasure Island (FL): 2022. Chronic Obstructive Pulmonary Disease. [PubMed] [Google Scholar]
- 99.Karakioulaki M, Papakonstantinou E, Stolz D. Extracellular matrix remodelling in COPD. Eur Respir Rev. 2020;29 doi: 10.1183/16000617.0124-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arbaningsih SR, Syarani F, Ganie RA, Lelo A. The levels of vitamin D, metalloproteinase-9 and tissue inhibitor metalloproteinase-1 in COPD patients, healthy smokers and non-smokers of Indonesian citizens. Open Access Maced J Med Sci. 2019;7:2123–2126. doi: 10.3889/oamjms.2019.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Navratilova Z, Zatloukal J, Kriegova E, Kolek V, Petrek M. Simultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology. 2012;17:1006–1012. doi: 10.1111/j.1440-1843.2012.02197.x. [DOI] [PubMed] [Google Scholar]
- 102.Dimic-Janjic S, Hoda MA, Milenkovic B, et al. The usefulness of MMP-9, TIMP-1 and MMP-9/TIMP-1 ratio for diagnosis and assessment of COPD severity. Eur J Med Res. 2023;28:127. doi: 10.1186/s40001-023-01094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaker SB, von Wachenfeldt KA, Larsson S, et al. Identification of patients with chronic obstructive pulmonary disease (COPD) by measurement of plasma biomarkers. Clin Respir J. 2008;2:17–25. doi: 10.1111/j.1752-699X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 104.D'Armiento JM, Goldklang MP, Hardigan AA, et al. Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema. PLoS One. 2013;8:e56352. doi: 10.1371/journal.pone.0056352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Linder R, Rönmark E, Pourazar J, Behndig A, Blomberg A, Lindberg A. Serum metalloproteinase-9 is related to COPD severity and symptoms - cross-sectional data from a population based cohort-study. Respir Res. 2015;16:28. doi: 10.1186/s12931-015-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gilowska I, Kasper L, Bogacz K, et al. Impact of matrix metalloproteinase 9 on COPD development in polish patients: Genetic polymorphism, protein level, and their relationship with lung function. Biomed Res Int. 2018;2018 doi: 10.1155/2018/6417415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uysal P, Uzun H. Relationship between circulating Serpina3g, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 and -2 with chronic obstructive pulmonary disease severity. Biomolecules. 2019;9:62. doi: 10.3390/biom9020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Higashimoto Y, Iwata T, Okada M, Satoh H, Fukuda K, Tohda Y. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med. 2009;103:1231–1238. doi: 10.1016/j.rmed.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 109.Eltom S, Stevenson C, Birrell MA. Cigarette smoke exposure as a model of inflammation associated with COPD. Curr Protoc Pharmacol. 2013;5:Unit5.64. doi: 10.1002/0471141755.ph0564s60. Chapter. [DOI] [PubMed] [Google Scholar]
- 110.Wright JL, Tai H, Wang R, Wang X, Churg A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L125–L133. doi: 10.1152/ajplung.00539.2005. [DOI] [PubMed] [Google Scholar]
- 111.Xu J, Xu F, Wang R, Seagrave J, Lin Y, March TH. Cigarette smoke-induced hypercapnic emphysema in C3H mice is associated with increases of macrophage metalloelastase and substance P in the lungs. Exp Lung Res. 2007;33:197–215. doi: 10.1080/01902140701459514. [DOI] [PubMed] [Google Scholar]
- 112.Funada Y, Nishimura Y, Yokoyama M. Imbalance of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 is associated with pulmonary emphysema in Klotho mice. Kobe J Med Sci. 2004;50:59–67. [PubMed] [Google Scholar]
- 113.Gao W, Yuan C, Zhang J, et al. Klotho expression is reduced in COPD airway epithelial cells: effects on inflammation and oxidant injury. Clin Sci. 2015;129:1011–1023. doi: 10.1042/CS20150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Linder R, Rönmark E, Pourazar J, Behndig AF, Blomberg A, Lindberg A. Proteolytic biomarkers are related to prognosis in COPD- report from a population-based cohort. Respir Res. 2018;19:64. doi: 10.1186/s12931-018-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsay JJ, Hu Y, Goldberg JD, et al. Value of metalloproteinases in predicting COPD in heavy urban smokers. Respir Res. 2020;21:228. doi: 10.1186/s12931-020-01496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piesiak P, Brzecka A, Kosacka M, Passowicz-Muszyńska E, Dyła T, Jankowska R. [Concentrations of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in serum of patients with chronic obstructive pulmonary disease] Pol Merkur Lekarski. 2011;31:270–273. [PubMed] [Google Scholar]
- 117.Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatr Pulmonol. 2019;54:S84–S96. doi: 10.1002/ppul.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaggar A, Hector A, Bratcher PE, Mall MA, Griese M, Hartl D. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur Respir J. 2011;38:721–727. doi: 10.1183/09031936.00173210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rath T, Zwaschka L, Hage L, et al. Identification of neutrophil activation markers as novel surrogate markers of CF lung disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roderfeld M, Rath T, Schulz R, et al. Serum matrix metalloproteinases in adult CF patients: relation to pulmonary exacerbation. J Cyst Fibros. 2009;8:338–347. doi: 10.1016/j.jcf.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 121.Devereux G, Steele S, Jagelman T, et al. An observational study of matrix metalloproteinase (MMP)-9 in cystic fibrosis. J Cyst Fibros. 2014;13:557–563. doi: 10.1016/j.jcf.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 122.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 123.Ley B, Collard HR, King TE., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 124.Wei LQ, Li ZH, Kang J, Hou XM, Yu RJ. [Changes of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in the bronchoalveolar lavage fluid and the serum of patients with idiopathic pulmonary fibrosis] Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:399–402. [PubMed] [Google Scholar]
- 125.Yuan YD, Zhang Y, Sun CH. [Association of genetic polymorphisms of angiotensin converting enzyme and matrix metallo proteinase-1 with idiopathic pulmonary fibrosis] Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:38–45. [PubMed] [Google Scholar]
- 126.Zhang HT, Fang SC, Wang CY, et al. MMP-9 1562C>T gene polymorphism and efficacy of glucocorticoid therapy in idiopathic pulmonary fibrosis patients. Genet Test Mol Biomarkers. 2015;19:591–597. doi: 10.1089/gtmb.2015.0057. [DOI] [PubMed] [Google Scholar]
- 127.Xu L, Bian W, Gu XH, Shen C. Differing expression of cytokines and tumor markers in combined pulmonary fibrosis and emphysema compared to emphysema and pulmonary fibrosis. COPD. 2017;14:245–250. doi: 10.1080/15412555.2017.1278753. [DOI] [PubMed] [Google Scholar]
- 128.Tashiro J, Rubio GA, Limper AH, et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front Med. 2017;4:118. doi: 10.3389/fmed.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27–42. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 130.Manoury B, Nenan S, Leclerc O, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fattman CL, Gambelli F, Hoyle G, Pitt BR, Ortiz LA. Epithelial expression of TIMP-1 does not alter sensitivity to bleomycin-induced lung injury in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L572–L581. doi: 10.1152/ajplung.00291.2007. [DOI] [PubMed] [Google Scholar]
- 132.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chuliá-Peris L, Carreres-Rey C, Gabasa M, Alcaraz J, Carretero J, Pereda J. Matrix metalloproteinases and their inhibitors in pulmonary fibrosis: EMMPRIN/CD147 comes into play. Int J Mol Sci. 2022;23:6894. doi: 10.3390/ijms23136894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang H, Mao J, Gao L, Liu J, Wu T. Effect of antisense TIMP-1 cDNA on the expression of TIMP-1 and MMP-2 in lung tissue with pulmonary fibrosis induced by bleomycin. Mol Med Rep. 2013;7:149–153. doi: 10.3892/mmr.2012.1140. [DOI] [PubMed] [Google Scholar]
- 135.Manoury B, Nénan S, Guénon I, Lagente V, Boichot E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharmacol. 2007;7:900–911. doi: 10.1016/j.intimp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 136.Bonniaud P, Kolb M, Galt T, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 137.Kralisch S, Klein J, Lossner U, et al. Proinflammatory adipocytokines induce TIMP-1 expression in 3T3-L1 adipocytes. FEBS Lett. 2005;579:6417–6422. doi: 10.1016/j.febslet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 138.Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol. 2019;10:137. doi: 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zagoriti Z, El Mubarak MA, Farsalinos K, Topouzis S. Effects of exposure to tobacco cigarette, electronic cigarette and heated tobacco product on adipocyte survival and differentiation in vitro. Toxics. 2020;8:9. doi: 10.3390/toxics8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dias SP, Brouwer MC, van de Beek D. Sex and gender differences in bacterial infections. Infect Immun. 2022;90 doi: 10.1128/iai.00283-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liarte S, Chaves-Pozo E, Abellán E, Meseguer J, Mulero V, García-Ayala A. 17beta-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev Comp Immunol. 2011;35:19–27. doi: 10.1016/j.dci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166. doi: 10.1186/1465-9921-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woolley DE, Roberts DR, Evanson JM. Inhibition of human collagenase activity by a small molecular weight serum protein. Biochem Biophys Res Commun. 1975;66:747–754. doi: 10.1016/0006-291x(75)90573-2. [DOI] [PubMed] [Google Scholar]
- 145.Burkhardt J, Petit-Teixeira E, Teixeira VH, et al. Association of the X-chromosomal genes TIMP1 and IL9R with rheumatoid arthritis. J Rheumatol. 2009;36:2149–2157. doi: 10.3899/jrheum.090059. [DOI] [PubMed] [Google Scholar]
- 146.Anderson CL, Brown CJ. Polymorphic X-chromosome inactivation of the human TIMP1 gene. Am J Hum Genet. 1999;65:699–708. doi: 10.1086/302556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peng J, Gao K, Gao T, et al. Expression and regulation of tissue inhibitors of metalloproteinases (TIMP1 and TIMP3) in goat oviduct. Theriogenology. 2015;84:1636–1643. doi: 10.1016/j.theriogenology.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 148.Nasiri-Ansari N, Spilioti E, Kyrou I, et al. Estrogen receptor subtypes elicit a distinct gene expression profile of endothelial-derived factors implicated in atherosclerotic plaque vulnerability. Int J Mol Sci. 2022;23:10960. doi: 10.3390/ijms231810960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim KH, Burkhart K, Chen P, et al. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol. 2005;33:271–279. doi: 10.1165/rcmb.2005-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X, Zhang W, Chen X, Cai Y. Prevention of bleomycin-induced pulmonary inflammation and fibrosis in mice by bilobalide. Evid Based Complement Alternat Med. 2023;2023 doi: 10.1155/2023/1973163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cabrera S, Selman M, Lonzano-Bolaños A, et al. Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L593–L601. doi: 10.1152/ajplung.00320.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parsons CJ, Bradford BU, Pan CQ, et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106–1115. doi: 10.1002/hep.20425. [DOI] [PubMed] [Google Scholar]