Abstract
Clinical isolates of coagulase-negative staphylococci often elaborate a biofilm involved in adherence to medical devices and resistance to host defenses. The biofilm contains the capsular polysaccharide/adhesin (PS/A), which mediates cell adherence to biomaterials, and another antigen, termed polysaccharide intercellular adhesin (PIA), which is thought to mediate bacterial accumulation into cellular aggregates. PIA is a polymer of β-1,6-linked N-acetyl glucosamine residues with a molecular mass of <30,000 kDa. We found that recombinant Staphylococcus carnosus and Staphylococcus aureus carrying a plasmid with genes of the ica locus, which was reported to encode the biosynthetic proteins for production of PIA, were also able to synthesize PS/A. PS/A and a chemically and immunologically identical polysaccharide isolated from S. carnosus carrying the ica genes on plasmid pCN27 were found to be high-molecular-mass (>250,000 kDa), acid-stable polymers of β-1,6-linked glucosamine substituted on the amino group primarily with succinate, although some preparations also contained acetate. Moreover, all recombinant staphylococcal strains with the ica genes had the biologic properties previously attributed to PS/A. ica-positive strains readily formed an in vitro biofilm on plastic, adhered 3- to 10-fold more to catheters during a 30-min assay compared with control strains carrying only the cloning vector, adsorbed out antibodies to PS/A from immune serum, and elaborated a capsule visualized by immunoelectron microscopy with antisera to PS/A. These properties were also seen with PS/A-producing strains of Staphylococcus epidermidis, but not with transposon mutants lacking PS/A. An antiserum raised to PIA contained high-titer antibody to PS/A that was readily adsorbed out by PS/A-positive strains of S. epidermidis and recombinant strains of staphylococci carrying the ica genes. We conclude that the ica locus encodes production of PS/A and that the properties of S. epidermidis associated with initial bacterial adherence, biofilm formation, and intercellular adhesion can be correlated with elaboration of PS/A.
Coagulase-negative staphylococci (CoNS), including Staphylococcus epidermidis, are now recognized as important pathogens in preterm neonates, patients undergoing chemotherapy, and other patients with indwelling medical devices. Clinical isolates are often highly adherent to plastic surfaces because they elaborate an extracellular material which is referred to as biofilm or slime. The exact nature and chemical components of the biofilm have not been fully characterized. The capsular polysaccharide (PS) has been reported to be a component of the cell surface and biofilm layer, to mediate cell adherence to biomaterials, and to protect the bacterial cell from host defenses, such as opsonophagocytosis (8, 21, 35). Hence, it is referred to as capsular PS/adhesin (PS/A) (35). However, the exact chemical constituents of PS/A have not previously been determined. Other PSs are thought to be prominent components of slime (2, 4, 14, 23), and several investigators have described proteins that purportedly mediate bacterial adherence to biomaterials (15, 34). Mack et al. (23, 25) have chemically characterized one component of the biofilm to include two structurally related homoglycans composed of about 130 residues of β-1,6-linked glucosamine that are mostly (>80%) N acetylated. This material is referred to as the PS intercellular adhesin (PIA). Recently, Baldassarri et al. (2) have shown that the slime-associated antigen previously characterized by Christensen et al. (4) is chemically the same as PIA.
The major basis for differentiating PIA from PS/A comes from a study by Heilmann et al. (12), who cloned a fragment of DNA from S. epidermidis RP62A into plasmid pCA44. The result was plasmid pCN27, which has recently been reported to contain four open reading frames comprising the ica (intercellular adhesin) locus (7). After transformation of Staphylococcus carnosus TM300 with pCN27, the recombinant strain elaborated a material with the reported properties of PIA; it mediated intercellular clumping of bacteria but not the initial adherence of bacteria to polystyrene, as determined in a simple colorimetric assay for biofilm production (12). The recombinant S. carnosus(pCN27) strain adhered well to glass (12), which is also a property of PS/A (27, 35). Thus, the reported properties of S. carnosus(pCN27) conform to the model proposed by Mack et al. (25, 26), in which adherence of S. epidermidis to biomaterials is a two-step phenomenon involving initial adherence mediated by PS/A and/or one of several proteins (11, 15, 34, 36) and then accumulation of cells into the biofilm due to elaboration of PIA. However, these findings did not take into account the fact that PS/A can be present on CoNS strains that do not elaborate a biofilm detectable by the colorimetric assay but nonetheless adhere in high numbers to silastic catheters (27). Thus, biofilm production is not an adequate test for detecting elaboration of PS/A.
An additional limitation of the findings obtained with the recombinant S. carnosus(pCN27) strain is that the actual material elaborated by the recombinant strain giving rise to intercellular bacterial accumulation was not isolated and characterized. In a recent study using an in vitro assay for analysis of the enzymatic functions of the ica genes (7), the proteins encoded by two genes, icaA and icaD, were shown to be able to synthesize an N-acetylglucosamine oligomer up to 20 residues in length from UDP-N-acetylglucosamine precursors. A third gene in the ica locus, icaC, was needed to obtain N-acetylglucosamine polymers reactive with antisera raised to purified PIA (7). In spite of this clear finding from Gerke et al. (7) that the icaADC genes encode proteins that can synthesize a β-1,6-linked N-acetylglucosamine polymer in vitro, it still has not been shown that these genes encode the biosynthetic proteins for production of the PIA polymer in vivo. In addition, the use of UDP-N-acetylglucosamine in these studies as the biosynthetic precursor instead of UDP-glucosamine (which could not be undertaken due to lack of commercial availability of UDP-glucosamine) could exclude detection of alternatively substituted glucosamine polymers by the gene products of the ica locus. To characterize further the recombinant antigen made by the proteins encoded in the ica locus, we undertook an investigation of the biologic and immunochemical properties of S. carnosus(pCN27) and its biofilm antigens. In this paper, we demonstrate that the genes in the ica locus contained in pCN27 (12) encode production of proteins that can synthesize PS/A, which is chemically related to PIA but distinguished from PIA by molecular size, biophysical properties, and the presence of succinate groups on the majority of the amino groups of the glucosamine residues that make up the polymer.
MATERIALS AND METHODS
Bacterial strains.
The strains of S. epidermidis, S. carnosus, and Staphylococcus aureus used in this study and their associated plasmids are described in Table 1. pCN27 containing the ica locus was isolated from S. carnosus(pCN27) and electroporated into S. aureus RN4220 as described previously (1, 22). Curiously, we were unable to transform S. aureus RN4220 with the cloning vector pCA44; thus, we instead used pSK265, which also encodes chloramphenicol resistance, as a control for S. aureus(pCN27). Transformed cells were selected on Trypticase soy agar (TSA) supplemented with 5 μg of chloramphenicol/ml. We have determined that the transposon inserts in PS/A-negative strains M187-sn3 and M187-sn6 are not in the ica locus (unpublished observation), whereas the PIA-negative transposon mutant 1457-M11 appears to have a Tn917 insert in this locus (unpublished observation).
TABLE 1.
Bacterial strains used in this study
| Strain | Comment(s) | Reference or source |
|---|---|---|
| S. epidermidis M187 | Clinical isolate (peritonitis) | 27 |
| S. epidermidis M187-sn3 | PS/A-negative transposon mutant | 27 |
| S. epidermidis M187-sn6 | PS/A-negative transposon mutant | 27 |
| S. carnosus TM300 | Host strain | 19 |
| S. carnosus(pCN27) | S. carnosus transformed with pCN27 containing the ica locus | 12 |
| S. carnosus(pCA44) | S. carnosus transformed with pCA44 (control plasmid) | 12 |
| S. aureus RN4220 | S. aureus recA-deficient strain | 30 |
| S. aureus(pSK265) | S. aureus RN4220 transformed with pSK265 | This study |
| S. aureus(pCN27) | S. aureus RN4220 with pCN27 containing the ica locus | This study |
| S. epidermidis RP62A | ATCC 35984; prototype PS/A-positive strain | 5 |
| S. epidermidis 1457 | Prototype PIA-positive strain | 25 |
| S. epidermidis 1457-M11 | Isogenic biofilm and PIA-negative transposon mutant of S. epidermidis 1457 | 25 |
Antiserum.
An antiserum to purified PS/A was raised as previously described (35). An antiserum to PIA raised in rabbits was supplied by D. Mack, Krankenhaus Eppendorf, Hamburg, Germany.
Purification of PS/A.
Crude slime was prepared from cultures grown in a chemically defined medium (CDM) (13) containing RPMI 1640 AUTO-MOD, an RPMI 1640 preparation modified to allow sterilization by autoclaving (Sigma Chemical Co., St. Louis, Mo.) as a starting base. Phenol red was omitted because it was readily bound by purified PS/A (unpublished results). The CDM was supplemented with additional amino acids, vitamins, and nucleotides to give it a final composition similar to that described elsewhere (13). The medium was further supplemented with dextrose and sucrose; each was autoclaved separately and then added to a final concentration of 1%.
Cultures were inoculated with a single colony of S. epidermidis (27) from a TSA plate that had been incubated overnight at 37°C. The slime-positive phenotype of the inoculum was confirmed via subculture on Congo red agar (6). Batch cultures were grown with vigorous mixing at 37°C, with 2 liters of O2/min bubbled through a sparger and the pH maintained at 7.0 by the automatic addition of 5 M NaOH with a pH titrator. Cultures were grown until they ceased to need addition of NaOH to maintain the pH at 7.0 (i.e., for 48 to 72 h).
Slime was extracted from the bacterial cells directly into the culture supernatant with the use of divalent cations, low pH, and heat. MgCl2 was added to the culture to a final concentration of 100 mM, and the pH was adjusted to 5.0. The culture was heated at 65°C for 90 min with vigorous stirring. The cell bodies were sedimented at 9,000 × g for 15 min. The supernatant was concentrated to ∼1,000 ml via tangential-flow filtration (10,000-molecular-weight [MW] cutoff filter), and the following buffer exchanges and treatments were performed while the solution was still in the filtration device. The buffer was exchanged with distilled water (dH2O; pH 5.0) to remove excesses of Mg2+ ions, the pH was adjusted to 7.4 with 0.1 M Tris–0.15 M NaCl, and the solution was treated for 4 h at room temperature with 5 mg each of RNase and DNase and then for 2 h with 5 mg of trypsin. The buffer was changed to 10 mM EDTA (pH 8.6), and sodium deoxycholate (DOC) was added to a final concentration of 3%.
The cell bodies were reextracted by treatment with 5 mg each of RNase and DNase for 4 h at 37°C and then with 5 mg of trypsin for 2 h; the cells were sedimented, and the supernatant was added to the concentrated culture supernatant as it was being treated with trypsin. The cell bodies were resuspended in 10 mM EDTA–3% DOC (pH 8.6), stirred for 2 h, and sedimented again. The supernatant was added to the concentrated culture supernatant as it was being treated with 10 mM EDTA–3% DOC (pH 8.6). Finally, the pooled extracts were concentrated to 50 ml via stirred-cell filtration (30,000-MW-cutoff filter). Particulate material was sedimented by centrifugation at 2,000 × g for 30 min, and the supernatant was retained as the crude slime extract.
High-MW PS/A-enriched material was isolated from the crude slime extract by molecular sieve chromatography. A column (5.0 by 100 cm) was packed with Sepharose CL-4B (Pharmacia, Piscataway, N.J.) and washed with 10 mM EDTA containing 1% DOC (pH 8.6). Crude slime was applied in a volume of 10 to 20 ml, and the PS/A-containing fractions were identified by measurement of the absorption at 206 nm and immuno-dot blot analysis with polyclonal rabbit antiserum to PS/A. Material eluting in the void-volume fractions of the column was pooled and treated with proteinase K (0.1 mg/ml), which was proteolytically active in 1% DOC; the material was simultaneously concentrated to 100 ml with a stirred-cell filtration unit (30,000-MW-cutoff membrane). To destroy remnants of an alkali-labile cell wall antigen present in the preparations, the proteinase K-digested material was treated by the addition of NaOH to a final concentration of 500 mM, and the mixture was stirred at 22°C for 2 h and then neutralized with HCl. PS/A was recovered by methanol precipitation (50% [vol/vol]) and sedimented by centrifugation at 30,000 × g for 30 min. Residual DOC and impurities were removed by numerous washes of the precipitate with pure methanol. To remove residual teichoic acid, the methanol precipitate was suspended in 24% (vol/vol) hydrofluoric acid (HF) at 4°C for 48 h. The HF solution was diluted in water to a concentration of 12% HF and then neutralized with NaOH and dialyzed against running water. PS/A was mostly insoluble at this point. Any remaining soluble PS/A was precipitated by the addition of methanol to 50%, and the pellet was washed with pure methanol three to five times, suspended in deionized H2O, frozen, and lyophilized. Typical yields of PS/A ranged from 0.5 to 2 mg/liter of culture.
Purification of antigen from S. carnosus(pCN27).
The procedure described by Mack et al. (25) for the isolation of PIA antigen was used to purify antigens from S. carnosus(pCN27). In brief, S. carnosus(pCN27) was grown for 22 h at 37°C with shaking at 100 rpm/min in 900 ml of dialyzed Trypticase soy broth (TSB). The cells were collected by centrifugation and suspended in 20 ml of 0.1 M phosphate-buffered saline (PBS). The antigen was extracted by sonication of the cells for 10 min with 80 W of power. Cell debris was removed by centrifugation at 15,000 × g for 15 min at 4°C, and extracts were further clarified by centrifugation at 12,000 rpm for 60 min. The extracts were then filter sterilized and dialyzed against ammonium carbonate (0.4 M) concentrated to 50 ml with an Omegacell (10,000-MW-cutoff membrane; Filtron Technology Corp., Northborough, Mass.) and fractionated by gel filtration chromatography on a Sephacryl S-300 column. Material eluting in the fractions was detected by duplicate immuno-dot blot analysis with rabbit antiserum to PIA or PS/A. Immunologically reactive material was identified by immuno-dot blotting, pooled, dialyzed against water, and lyophilized.
Enzyme-linked immunosorbent assay (ELISA) and ELISA inhibition.
Immulon 1 plates (Dynatech Laboratories, Chantilly, Va.) were sensitized at 37°C for 12 h with 100 μl of either PS/A or recombinant antigen from S. carnosus(pCN27) (10 μg/ml) suspended in 0.04 M sodium phosphate buffer (pH 7.4). The plates were then washed three times with PBS, blocked with 5% skim milk for 2 h at 37°C, and again washed three times with PBS. For obtaining antibody-binding titers, sera were diluted twofold in 1% skim milk with 0.05% Tween and incubated at 37°C for 1 h, the plates were washed, and appropriate alkaline phosphatase-conjugated secondary antibodies were added. After incubation, washing, and color development with substrate reagents, the serum titer was determined as the reciprocal of the dilution giving an optical density at 405 nm (OD405) of 0.2, which was calculated by linear regression from the formula of the line plotting OD405 versus serum dilution (9).
Whole-cell absorptions of antiserum to PS/A or PIA that were to be used for ELISA inhibition were performed as follows. Bacteria were grown overnight in TSB or antibiotic-supplemented TSB. The cells were washed with PBS and suspended to an OD650 of 2.0. A 1-ml volume of each bacterial suspension was centrifuged (6,000 × g for 5 min), and a 500-μl volume of antiserum diluted 1:500 with 1% skim milk was added to each tube and thoroughly mixed. To prevent protein A on S. aureus strains from nonspecifically binding immunoglobulin G (IgG), these strains were first treated with trypsin (0.1 mg/ml for 30 min at 37°C) and then washed with PBS prior to their use as adsorbing antigens. The antiserum with adsorbing bacteria was incubated at 4°C for 45 min. Whole bacterial cells were removed by centrifugation (6,000 × g for 5 min), and a 100-μl volume of the adsorbed antiserum was added to wells of the ELISA plates coated with the antigens described above. The plates were incubated at 37°C for 1 h. After washing, anti-rabbit IgG conjugated with alkaline phosphatase (Sigma) was added to each well (100 μl of a 1:1,000 dilution in 1% skim milk). The plates were incubated at 37°C for 1 h, washed, and developed with p-nitrophenylphosphate. OD405 readings were taken with an ELISA plate reader (BioTek Instruments, Winoski, Ill.) after 60 min of incubation.
Immuno-dot blot.
Antibody binding to antigens was also evaluated by immuno-dot blotting. Five microliters of antigen (100 μg/ml in 0.1 M HCl) was placed onto nitrocellulose paper and left to dry. The paper was then treated for 30 min with 5% skimmed milk–PBS at room temperature, followed by three washing steps with PBS. Primary antisera (1:500 dilutions in 5% skimmed milk–PBS) were then added, and the blots were incubated for 30 to 45 min at room temperature and washed three times with PBS. The appropriate enzyme-conjugated antibody diluted 1:500 in skimmed milk-PBS was then added to the blot and incubated at room temperature for 30 to 45 min. A final wash with PBS was carried out before developing the blot with 5-bromo-4-chloro-3-indyolylphosphate–nitroblue tetrazolium (BCIP/NBT; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) as substrate. After developing, the blot was washed with PBS and allowed to dry at room temperature.
Quantitative biofilm assay.
The biofilm-forming capacities of the various strains were investigated by the method described by Heilmann et al. (12). Bacteria were cultivated overnight in 10 ml of TSB supplemented with 0.25% glucose. The culture was diluted 1:200 in TSB-glucose, and 200 μl of this cell suspension was used to inoculate sterile 96-well polystyrene microtiter plates (Costar Corp., Cambridge, Mass.). After cultivation for 24 h at 37°C, the plates were washed twice with sterile PBS and air dried, and the cells were adsorbed to the plates and stained with 0.025% safranin for 30 s. Absorbance at 490 nm was measured with an ELISA plate reader. Each assay was performed five times.
Biofilm formation on a glass surface was also evaluated in the manner described by Heilmann et al. (12).
Catheter adherence assay.
The catheter adherence assay was performed as described elsewhere (28, 35). In brief, staphylococcal strains were grown overnight on TSA plates, and the growth was suspended in TSB to an OD650 of 0.1. Catheters (PE10; Becton Dickinson and Co., Sparks, Md.) were cut to 15-mm lengths and placed into the cultures (five catheters per test strain), which were incubated at 37°C for 30 min. The catheters were removed and washed five times in sterile PBS. After washing, the catheters were placed into 1% proteose peptone (Difco, Ann Arbor, Mich.) and vortexed for 2 min. Confirmation that vortexing released the bacterial cells from the catheter was determined as described previously (28). Serial dilutions were then performed for bacterial enumeration, and means and standard errors were calculated for the adherence of each strain.
Complement-mediated phagocytic assay.
Staphylococcal strains were grown overnight on TSA with or without chloramphenicol (10 μg/ml), as appropriate; TSB cultures were then prepared, with a starting OD650 of 0.1, and grown to mid-log phase (OD650 of 0.4). Bacterial cells were washed with RPMI and diluted with 10% fetal calf serum in RPMI. A 100-μl volume (∼2 × 106 to 3 × 106 CFU) was then added to each assay tube. The complement source was adsorbed as follows to remove any specific antibody. A 1-ml volume of a culture of S. carnosus TM300 (OD650 of 2.0) was centrifuged at 15,000 × g for 5 min, the supernatant was removed, and 1 ml of complement (diluted 1:5 in 10% fetal calf serum in RPMI 1640) was added and incubated at 4°C for 45 min. The bacterial cells were removed by filter sterilization, and 100 μl of the diluted complement plus 100 μl (4 × 106 cells) of freshly isolated leukocytes from healthy volunteers was added to each assay tube. Tubes containing bacteria and only leukocytes were used as controls to ensure that leukocytes alone were not killing bacteria. The volume of each tube was then made up to 400 μl with 10% fetal calf serum in RPMI. The reaction mixture was incubated on a rotor rack for 90 min at 37°C. Serial dilutions were made for bacterial enumeration, and the mean percentage of killing in replicate tubes was calculated compared with that in tubes with bacteria and leukocytes alone.
Immunoelectron microscopy.
The bacterial test strains were grown overnight in TSB. A 1-ml volume of the cultures was centrifuged (15,000 × g for 5 min) and washed in sterile 0.1% PBS. The S. aureus strains were first treated with 1% human serum in PBS for 30 min at 37°C to block the protein A found on the cell surface. Electron microscopic grids (200-mesh, Formvar-carbon-coated copper grids; Electron Microscopy Sciences, Washington, Pa.) were placed on top of a 5-μl bacterial suspension on parafilm for 1 min. The grids were removed and placed on top of a drop of PBS–0.5% fish skin gelatin–0.1% Tween (blocking buffer) for 10 min. After blocking, the grids were placed on 5 μl of either normal or immune rabbit serum (diluted 1:10 in blocking buffer) for 20 to 30 min. Then, the grids were washed four times for 10 min with PBS. The secondary antibody was then applied for 20 to 30 min. For all but the S. aureus strains, the conjugate used was 15-nm colloidal gold-labeled protein A (Sigma) at a 1:10 dilution in the blocking buffer. For S. aureus strains, the secondary antibody used was gold-labeled anti-rabbit IgG (Sigma) at a 1:10 dilution in blocking buffer. Each grid was then washed four times for 10 min in dH2O. The grids were examined with a transmission electron microscope at magnifications of ×6 to 25,000.
Chemical analysis of antigens.
The method described by Smith and Gilkerson (33) was used for colorimetric determinations of amino sugars. Glucosamine (Sigma) was used to generate a standard curve. Additional chemical analysis was as described elsewhere (20), with gas-liquid chromatography–mass spectrometry (GLC-MS) and nuclear magnetic resonance (NMR) analysis of oligosaccharide and monosaccharide components released by hydrolysis in various concentrations of HCl (up to 6 N HCl) at 100°C for 2 to 14 h. Succinate was identified by GLC after the treatment of antigen in 5 N NaOH at 95°C for 30 min, acidification with concentrated sulfuric acid, and derivation to methyl esters in boron-trifluoride methanol.
Statistical analysis.
The t test was used for all two-sample comparisons and analysis of variance (ANOVA) for multisample comparisons was used as indicated in Results. P values were calculated with StatView software (Abacus Concepts, Berkeley, Calif.) on a Macintosh computer.
RESULTS
Characterization of PS/A antigen.
Previously, complete chemical characterization of PS/A was problematic because of the propensity of PS/A to bind to multiple bacterial and media constituents. Use of an RPMI-based CDM to prepare antigen appears to have minimized this problem, and use of both base (NaOH) and HF treatments to degrade contaminants significantly improved the purity of PS/A. A major fraction of PS/A purified for this study from either S. epidermidis RP62A or S. epidermidis M187 eluted in the high-molecular-mass, void-volume fractions of a Sepharose 4B column (>250,000 kDa) and gave a single precipitin band in immunodiffusion, as previously shown (35). In addition, sensitization of ELISA wells with dilutions of PS/A to as little as 0.02 μg/well resulted in the binding of significantly more of a 1:500 dilution of antibody to purified PS/A than of preimmune serum. Antiserum to purified PS/A bound to an extracellular capsule surrounding S. epidermidis strains (see below) and inhibited binding of biofilm-positive S. epidermidis to plastic (data not shown). It was of interest that PS/A purified by the method described above was insoluble at a pH higher than 4.0 and became progressively more soluble as the pH was decreased. Use of 3% DOC to solubilize PS/A during purification was found to be critical, but once the PS/A was precipitated from DOC solutions by methanol, it could not be resolubilized at a pH of >4.0, even in DOC buffers. PS/A was slightly soluble (∼500 μg/ml) in 50% propanol–50% butanol and in pyridine. The PS/A antigen was devoid of detectable phosphate (<0.01%) (18).
Chemical properties of PS/A and S. carnosus(pCN27)-produced recombinant antigen.
PIA has been defined by Mack et al. (23) as a linear polymer of β-1,6-linked, partially (∼80%) N-acetylated glucosamine residues, with an average chain length of 130 residues, corresponding to a molecular mass of ∼28,000 Da. Thus, PIA is considerably smaller than PS/A. In addition, purified PIA is soluble in aqueous buffers at neutral pH, whereas PS/A is not. To determine the nature of the antigen produced from proteins encoded by the ica genes in S. carnosus(pCN27), we isolated a polysaccharide from S. carnosus(pCN27) mostly using the method described by Mack et al. for purifying PIA (23), with omission of the final ion-exchange chromatography steps. These steps were omitted because the material that is immunologically reactive with antibodies to PIA eluted in the void volume of an S-300 column, whereas PIA, because of its small size, should elute much later. In addition, the immunologically reactive fractions obtained from S. carnosus(pCN27) were not reactive in the colorimetric assay of Smith and Gilkerson (33), which was used to follow purification of PIA (23). When this colorimetric method was also used to analyze PS/A, we could not detect amino sugars (<1%) in PS/A. Thus, PS/A and the recombinant antigen from S. carnosus(pCN27) are distinguished from PIA both by their large molecular sizes and by their lack of reactivity in the assay of Smith and Gilkerson (33) for detection of amino sugars.
When we further analyzed PS/A and the comparable antigen isolated from S. carnosus(pCN27) after hydrolysis in strong acid at high temperature for up to 14 h, using GLC-MS and NMR, we did find that glucosamine was the single sugar component in PS/A and the recombinant antigen isolated from S. carnosus(pCN27). In addition, a high quantity of succinate (ranging from 65 to 100% substitution of the glucosamine residues) was identified in both preparations. Acetate was also present in some, but not all, PS/A preparations, usually at levels <50% those of the succinate levels. Among different lots of PS/A produced from the same strain (S. epidermidis M187), there was variation in the acetate level from 0 to 33% substitution of the glucosamine residues. The succinate was released from the antigens by hydrolysis in 5 M NaOH for 30 min at 95°C, but not with 1 M NaOH under the same conditions. The lower amount of NaOH would readily release carboxyl-ester-linked succinate. Along with NMR results, this establishes the succinate as linked to the amino rather than hydroxyl groups of glucosamine. A minor component of PIA (termed polysaccharide II) was reported elsewhere (23) to be negatively charged due to carboxyl-ester-linked succinate, which was released by treatment under mild alkaline conditions (0.2 M NaOH, 37°C, 24 h). Finally, PS/A and the recombinant antigen isolated from S. carnosus(pCN27) lose their serologic reactivity after treatment with sodium periodate (0.2 M for 14 h), indicating a 1-6 linkage between the glucosamine residues, which has also been reported for PIA (23). The linkages between the glucosamines in PS/A were all determined to be in a β configuration. The detailed results of the GLC/MS and NMR studies are being prepared for publication. Thus, PS/A and the recombinant S. carnosus antigen are chemically identical to each other and related to PIA but differ from PIA principally on the basis of molecular size, solubility in aqueous solutions at neutral pH, and substitution of the majority of the amino groups on the glucosamine residues with succinate.
Specificity of antisera to PS/A and PIA.
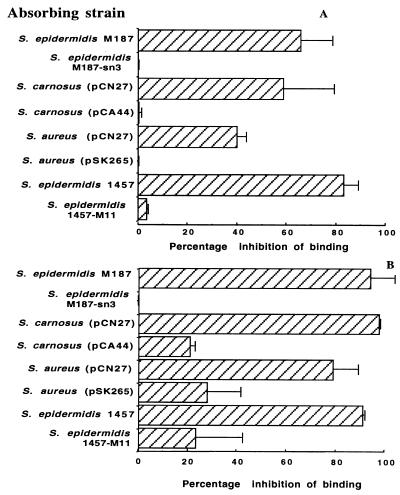
An ELISA was performed by coating a plate with PS/A containing no detectable acetate substituents and probing the immobilized antigen with polyclonal rabbit antisera to either PS/A or PIA. Antiserum to PS/A had a titer of 2,769, and antiserum to PIA had a titer of 2,742; normal sera had titers of ≤5.2. To investigate whether these antibodies were specific to PS/A and not a contaminant of the preparation, whole-cell absorptions of the rabbit antisera to PS/A and PIA were performed. Figure 1A shows the percentage of inhibition of binding of polyclonal antiserum to purified PS/A after adsorption with various strains of staphylococci. S. epidermidis M187, a biofilm and PS/A-positive clinical isolate, adsorbed significantly (P < 0.001 [t test]) more antibody from the PS/A antiserum than did an isogenic, unencapsulated transposon mutant, S. epidermidis M187-sn3, which makes no PS/A (27). In a similar manner, S. carnosus(pCN27) and S. aureus(pCN27) adsorbed out more antibody that bound to PS/A than did isogenic strains bearing control plasmids (P ≤ 0.005 [t tests]). Antibodies to PS/A were also removed by absorbing immune sera with S. epidermidis 1457, the prototype strain for isolating PIA (25), whereas its isogenic PIA-negative mutant, S. epidermidis 1457-M11, had little ability to remove antibodies to PS/A (P < 0.001 [t test]). A similar pattern of adsorption of antibody to PS/A was seen with antiserum raised to the PIA antigen (Fig. 1B).
FIG. 1.
ELISA inhibition with polyclonal antiserum to purified PS/A (A) and PIA (B) adsorbed with various staphylococcal strains. ELISA plates were coated with PS/A (2 μg/well). In all cases, significantly more antibody to PS/A was adsorbed by parental strains or strains carrying the ica locus than by the corresponding isogenic mutants or vector-control recombinant strains (P ≤ 0.005; unpaired t tests). Bars represent means, and error bars represent the standard errors of the means of five separate measurements.
Next, an ELISA plate coated with antigen isolated from S. carnosus(pCN27) (2 μg/well) was probed with antiserum to either PIA or PS/A. Antibodies in 1:500 dilutions of both sera bound strongly to the high-MW antigen isolated from S. carnosus(pCN27) (mean OD405s ± standard deviations, [SD], 1.07 ± 0.14 for anti-PS/A and 1.32 ± 0.370 for anti-PIA). These results indicate that antiserum raised to either PS/A or PIA binds to the antigen produced from the ica locus in recombinant S. carnosus(pCN27).
Adherence (biofilm) assay.
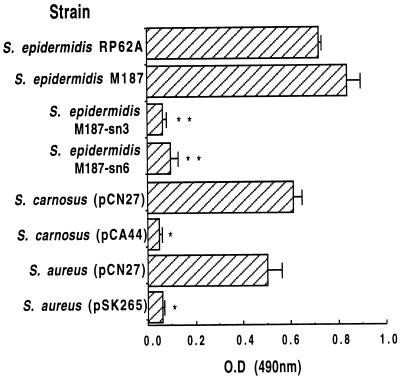
Mack et al. (25), Heilmann et al. (12), and others (37) proposed that PIA and PS/A have different biologic properties: PS/A mediates the initial early adherence of CoNS to polymers, and PIA mediates the formation of multilayered cell clusters. Heilmann et al. (10) claimed that these are phenotypically and genetically distinct properties of CoNS and reported that adherence to plastic (i.e., biofilm formation) was not a property of S. carnosus(pCN27). Using their method to measure cellular adherence to plastic, we confirmed that the previously defined PS/A-positive S. epidermidis strains M187 and RP62A are strongly adherent to a plastic surface (Fig. 2). Both S. carnosus(pCN27) and S. aureus(pCN27) exhibited a high degree of adherence and biofilm formation, comparable to that of S. epidermidis M187 and RP62A. As previously shown (27), PS/A-negative transposon mutants M187-sn6 and M187-sn3 showed negligible biofilm formation, as did S. carnosus(pCA44) and S. aureus(pSK265), which bear chloramphenicol resistance plasmids lacking the ica locus. The results shown in Fig. 2 were readily reproducible in four other instances (data not shown). When the adherence assay was conducted with glass tubes, the results were similar to those obtained by Heilmann et al. (12). Thus, using the method of Heilmann et al. (12), we demonstrated plastic adherence and biofilm formation by S. carnosus(pCN27), as well as by the S. aureus(pCN27) strain that we produced for this study.
FIG. 2.
Plastic adherence (biofilm assay). Staphylococcal strains were assessed for their abilities to adhere to the plastic, as visualized by safranin staining. Bars represent means, and error bars represent the standard errors of the means for five separate measurements. Significant differences in adherence were noted between all parental strains and isogenic mutants, as well as between S. carnosus and S. aureus strains with the ica locus in plasmid pCN27 and control strains with plasmids lacking the ica locus (∗, P ≤ 0.0001 [t tests for comparison with isogenic strain; ∗∗, P < 0.001 [ANOVA and Fisher PLSD for pairwise comparisons with strain S. epidermidis M187]).
Catheter adherence assay.
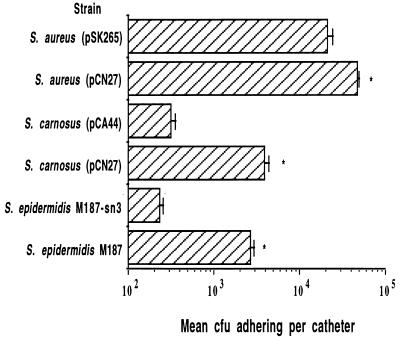
Further evidence that strains carrying the ica locus make PS/A was obtained in an assay measuring bacterial adherence to a catheter during a 30-min period (35), a defining property of PS/A (Fig. 3). S. carnosus(pCN27) and S. epidermidis M187 were more adherent to silastic catheters than S. epidermidis M187-sn3 and S. carnosus(pCA44) (P ≤ 0.0001 [t test]). There was a full log difference between the number of adherent PS/A-positive or ica-bearing bacterial cells and the number of adherent control (biofilm-negative) cells. In a similar fashion, S. aureus(pCN27) was three times more adherent to catheters compared with S. aureus(pSK265) (P < 0.0001 [t test]; Fig. 3). Therefore, the presence of the ica genes in pCN27 resulted in an increase in the rapid adherence of staphylococcal strains to plastic catheters.
FIG. 3.
Catheter adherence assay. After incubation for 30 min at 37°C, significant differences were noted between S. epidermidis parental strain M187 and isogenic mutant M187-sn3, S. carnosus(pCN27) and S. carnosus(pCA44), and S. aureus(pCN27) and S. aureus(pSK265) (∗, P ≤ 0.0001 [t tests]). Bars represent means, and error bars represent the standard errors of the means of five separate measurements.
Immunoelectron microscopy.
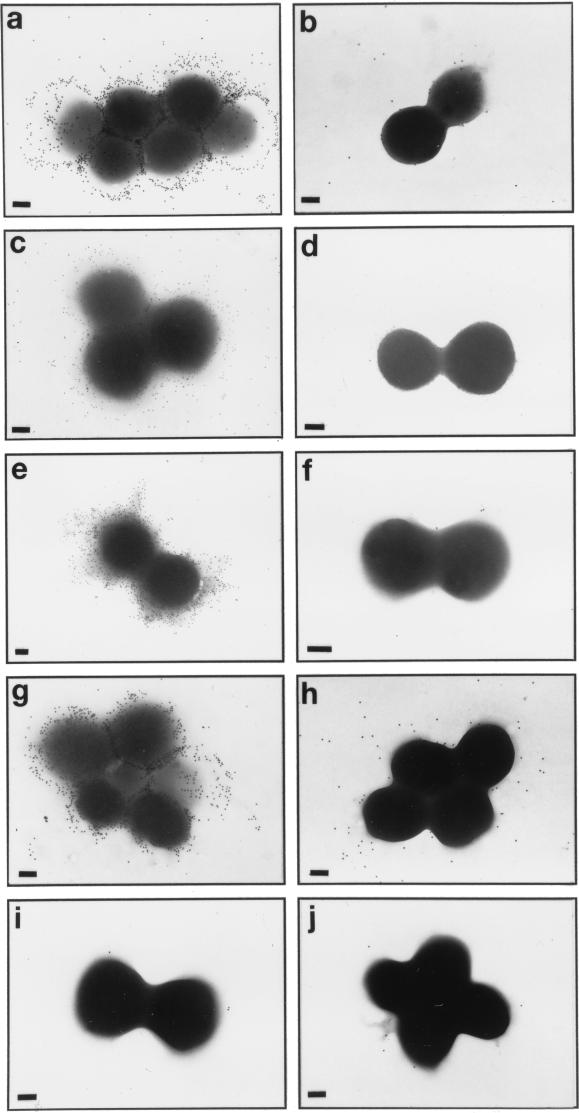
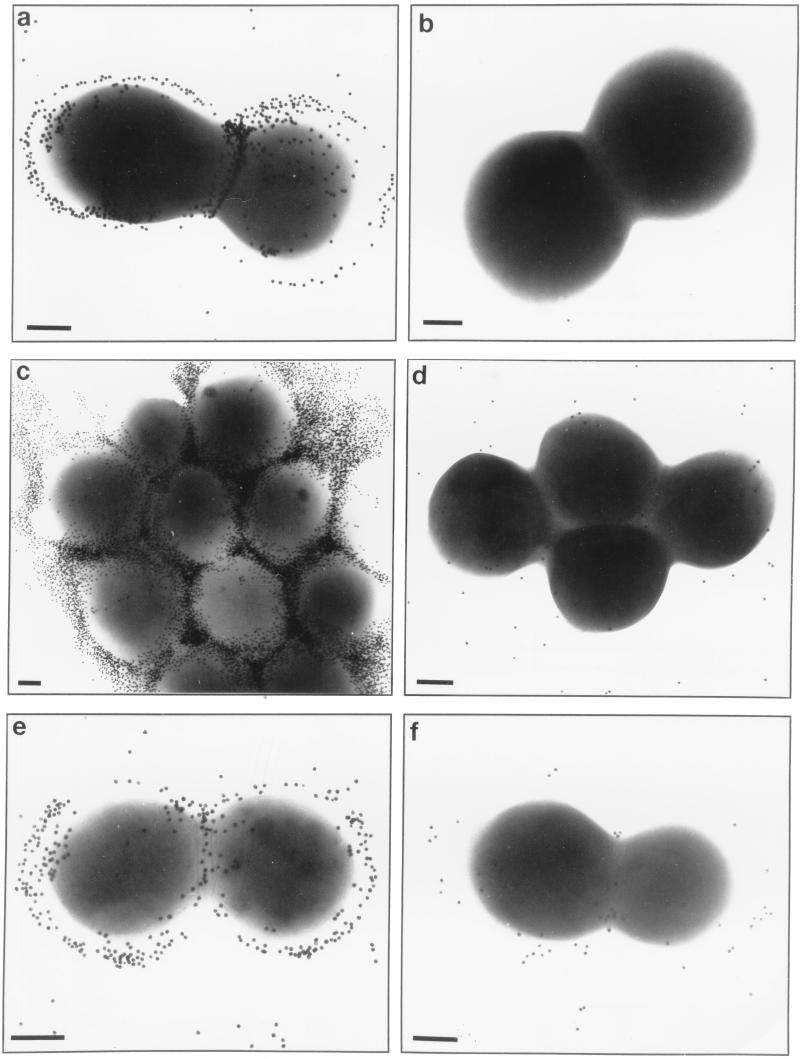
In addition to mediating rapid adherence to catheters and biofilm formation, PS/A represents the bacterial capsule for S. epidermidis. We investigated whether strains carrying the ica locus in pCN27 were encapsulated. Figure 4 shows transmission electron micrographs of the various strains probed first with polyclonal rabbit antiserum to PS/A and then with gold-labeled protein A, or, for S. aureus strains, gold-labeled anti-rabbit IgG. Clearly, antibodies to PS/A bound to an extracellular capsule in S. epidermidis M187, S. carnosus(pCN27), S. aureus(pCN27), and S. epidermidis 1457 (a PIA-producing strain). This binding was not seen with the PS/A-negative mutant S. epidermidis M187-sn3, with the strains carrying only the control plasmids, or with S. epidermidis 1457-M11, a transposon mutant made by Mack et al. (25) that reportedly does not produce PIA. Thus, plasmids with the ica locus caused heterologous strains of staphylococci to produce a capsule that could be visualized with an antiserum to PS/A. In addition, transposon mutagenesis that resulted in loss of PIA production by S. epidermidis 1457 also resulted in a loss of capsule expression. When an antiserum to PIA was used in comparable studies, an identical pattern of encapsulation (Fig. 5) confirmed the presence of antibodies to the S. epidermidis capsule in this antiserum.
FIG. 4.
Immunoelectron microscopy of staphylococcal strains probed first with antiserum to PS/A and then with gold-labeled protein A (CoNS strains) or gold-labeled anti-rabbit IgG (S. aureus strains). (a) S. epidermidis M187; (b) S. epidermidis M187-sn3; (c) S. carnosus(pCN27); (d) S. carnosus(pCA44); (e) S. aureus(pCN27); (f) S. aureus(pSK265); (g) S. epidermidis 1457; (h) S. epidermidis 1457-M11; (i and j) S. epidermidis M187 and S. carnosus(pCN27), respectively, probed with normal rabbit serum. Bars in each panel, 200 nm.
FIG. 5.
Immunoelectron microscopy of staphylococcal strains probed with antiserum to PIA and protein A-labeled gold. (a) S. epidermidis M187; (b) S. epidermidis M187-sn3; (c) S. carnosus(pCN27); (d) S. carnosus(pCA44); (e) S. epidermidis 1457; (f) S. epidermidis 1457-M11. Bars in each panel, 200 nm.
Opsonophagocytic killing of recombinant S. carnosus.
Another property of PS/A (and of bacterial capsules in general) is the ability to confer resistance to antibody-independent, complement-mediated opsonic killing (27, 31). We determined the susceptibility to complement-mediated killing of S. carnosus(pCN27) and S. carnosus(pCA44) and found that 73.5% ± 14.6% of the cells carrying the control plasmid were killed in 90 min by human peripheral-blood leukocytes and human complement adsorbed with S. carnosus, while S. carnosus(pCN27) resisted antibody-independent, complement-mediated opsonic killing (0.0% ± 17.5% killed; P < 0.001 [t test]). These results are comparable to those reported previously for isogenic PS/A-positive and PS/A-negative strains (31). Therefore, the antigen produced by the genes encoded by the ica locus promotes resistance to complement-mediated phagocytic killing, as does PS/A. We could not evaluate S. aureus in this assay, since the parent strain, RN4220, is not killed by complement and phagocytes in the absence of specific antibody (unpublished observation).
DISCUSSION
The coagulase-negative staphylococci are a major component of the normal microflora of the human skin and rarely cause infections in normal hosts (8). However, during the 1980s, CoNS strains became the fifth most common cause of nosocomial infections (3) and the most frequently isolated pathogen in nosocomial bacteremia (3). Of all species of CoNS isolated from these infections, S. epidermidis is the most common. Much of the pathogenic potential of this organism is related to elaboration of a biofilm involved in adherence to medical devices. Therefore, identification of the factors elaborated by S. epidermidis that promote adherence to polymeric surfaces is critical to an understanding of how this pathogen causes disease.
In this study, we isolated the PS antigen produced by recombinant S. carnosus(pCN27) carrying the cloned ica locus from S. epidermidis RP62A (12) and found that it encodes production of PS/A, which is related to but distinct from the antigen termed PIA. Although Gerke et al. (7) have recently shown that the ica locus encodes biosynthetic proteins that can polymerize UDP-N-acetylglucosamine in vitro into an oligomer chemically identical to PIA, our results indicate that in vivo an N-succinylated glucosamine polymer of larger molecular size with the biologic properties of PS/A also is made from the ica locus. The fact that both PS/A and PIA share a common β-1-6-linked-polyglucosamine backbone but differ in the primary substituent on the amino groups indicates that both PIA and PS/A can be made from the proteins encoded in the ica locus. Interestingly, we could not isolate a PIA-like antigen from S. carnosus(pCN27), but this could be due to any number of factors. In addition, Gerke et al. (7) showed that the product of the icaC gene was needed in order for the in vitro synthesized N-acetylglucosamine oligomer to react with antibody to PIA. These workers showed that the isolated icaA and icaB gene products could synthesize on oligomer up to 20 monosaccharides in length. When the gene product of the icaC locus was included in the reaction, a glucosamine polymer of higher molecular size was made as determined by its inability to migrate from the point of origin when spotted onto thin-layer chromatography gels. However, the exact function of the icaC gene product was not defined. Gerke et al. (7) speculated that the icaC gene product produced larger N-acetylglucosamine polymers better able to react with antibodies to PIA. However, if succinate or another appropriate substrate was present in the reaction mixture, a plausible alternative scenario is that the icaC gene product changes the amino group substituent from acetate to succinate. The resulting poorly soluble polymer would not migrate from the point of origin in thin-layer chromatographic gels and would have the correct substituent on the amino group of glucosamine to react with the antibodies raised to PIA, which also clearly bind to PS/A.
Antibodies to PS/A were present in high titers in antisera raised to PIA. The specificity of these antibodies to PS/A was shown by both direct binding to purified antigen and adsorption assays wherein native and recombinant staphylococcal strains carrying the ica genes and producing PS/A readily removed antibodies to PS/A from the PIA antiserum. Control strains and transposon mutants lacking detectable PS/A production failed to remove these antibodies. The specificity of these antibodies for PS/A was further shown by the ability of antisera to PIA to stabilize a bacterial capsule (visualized by electron microscopy) that was elaborated both by previously defined PS/A-positive strains (27, 35) and by recombinant staphylococcal strains carrying plasmids with the ica genes. A critical finding was that the isogenic mutants reported to differ in elaboration of PIA (25) differed in encapsulation; the PIA-deficient strain S. epidermidis 1457-M11 was also deficient in capsule production and elaboration of PS/A. It appears from these results that the ica locus of S. epidermidis encodes production of a PS antigen with all of the previously described immunologic properties of PS/A.
The chemical relatedness of PS/A and PIA and the chemical properties of PS/A suggest a basis for the finding that antisera to PIA contained antibodies that bound strongly to PS/A. Purified PS/A is poorly soluble at a pH of >4, likely explaining why large quantities of purified antigen were previously difficult to obtain. This poor solubility of PS/A and the chemical similarity of PS/A and PIA could account for the failure to detect PS/A in preparations of PIA when NMR analysis was used to characterize the PIA antigen (23). Intact PS/A, even when dissolved in deuterated chloride (DCl), gives very weak signals in the NMR, and we found it necessary to hydrolyze PS/A in strong acid to generate monosaccharides and oligosaccharides whose composition and structure could be analyzed by GC/MS and NMR. To purify PS/A, we found it necessary to treat the material with strong acid (24% HF) to dissociate it from charged contaminants. Strong binding of N-succinylated PS/A to other bacterial components suggests that PS/A could readily contaminate preparations of PIA used to immunize rabbits. We have found that PS/A is highly immunogenic in rabbits (29, 35), requiring only microgram quantities to elicit a brisk immune response (unpublished observation). Another possible basis for the presence of antibodies to PS/A in antisera raised to PIA is the shared polyglucosamine backbone, and the potential cross-reactivity of epitopes formed from acetylated glucosamines with epitopes formed from the succinylated glucosamines in PS/A. However, the reported molecular size of PIA (∼28 kDa) (23) indicates that it may be too small a PS antigen to elicit antibodies on its own (16). PS/A contamination may have also affected the recently reported results of Karamanos et al. (17), who indicated that a small (20-kDa), acidic, sulfated polysaccharide from S. epidermidis slime elicited antibodies that reacted strongly with many clinical isolates of biofilm-forming S. epidermidis.
The most critical finding in this report is that recombinant strains of staphylococci carrying the ica genes had all of the biologic properties previously ascribed to PS/A, strengthening our conclusion that this locus encodes production of PS/A. The activities of PS/A and PIA have been distinguished by an assay for in vitro production of biofilm, which was not detected in S. carnosus(pCN27) by the workers that produced this strain (12). However, as we have pointed out previously (29), a significant proportion of clinical CoNS isolates make PS/A and are highly adherent to catheters in vitro but do not make a biofilm detectable in vitro, indicating that elaboration of PS/A does not always lead to biofilm formation. We cannot explain why we were able to demonstrate biofilm formation using S. carnosus(pCN27), while Heilmann et al. were unable to do so (12). In addition, we have defined PS/A as the capsular PS antigen that mediates rapid adherence to catheters (35) and protects the organism from complement-mediated phagocytic killing (27, 31, 32); none of these properties was investigated by Heilmann et al. using S. carnosus(pCN27). Here, we found that recombinant S. carnosus(pCN27) bound 10-fold better to plastic catheters in a 30-min adherence assay compared with strains carrying control plasmids, and this rapid adherence to catheters was comparable to that of prototypical PS/A-producing strains (27, 35). S. carnosus(pCN27) also adhered significantly more to catheters than did its isogenic control strain. Critically important to our claim that the ica locus encodes proteins for synthesis of PS/A is the observation by electron microscopy of the encapsulation of recombinant S. carnosus and S. aureus carrying the ica genes on a plasmid. Since encapsulation is a defining property of PS/A, the ica locus must contain the genes needed to produce proteins required for the synthesis of the capsular PS/A antigen in a variety of staphylococci. S. carnosus(pCN27) also resisted complement-mediated phagocytosis, a key property of the PS/A antigen (27, 32). Taken together, these data show that mutants reportedly isogenic for PIA production (25) are actually isogenic for the PS/A capsule and that recombinant strains reported to produce PIA (10) produce PS/A. Thus, PS/A appears to be the biologically active fraction of S. epidermidis, mediating both initial adherence of bacteria to plastic and accumulation of cells into aggregates.
The difficulty of identifying the factors elaborated by pathogenic CoNS that mediate adherence to plastic and biofilm formation is evident from the plethora of papers describing different biofilm factors. Hussain et al. (14) reported that biofilm was composed mostly of teichoic acid, a finding consistent with the composition of crude slime reported earlier by Tojo et al. (35). However, these latter workers isolated a biologically active fraction from crude slime (PS/A) that has been well characterized as a virulence factor promoting both bacterial adherence to plastic in vitro (27, 35) and bacterial infection in animal models of endocarditis (31, 32). A third PS thought to be produced by S. epidermidis, known as slime-associated antigen (4), was characterized by Baldassarri et al. as a PS of small molecular size (2) that is similar if not identical to PIA. While this report, along with that of Mack et al. (23), indicates the probable presence of an N-acetylated polyglucosamine material of small molecular size in slime of some CoNS strains, none of the biologic properties attributed to this material can be confirmed to be due solely to PIA, because the reagents used in these studies encompassed strains and sera varying in their production of and specificities for PS/A.
Although much more remains to be learned about the properties of PS/A and the proteins encoded by genes in the ica locus that synthesize PS/A, the findings reported here clarify some important aspects of the biology of CoNS. PS/A is a high-molecular-weight, variably (65 to 100%) N-succinylated, β-1-6-linked polyglucosamine synthesized by proteins encoded in the ica locus of S. epidermidis. PS/A production is correlated with adherence of S. epidermidis to biomaterials, accumulation into cellular aggregates, encapsulation, resistance to phagocytosis, and virulence in animal models (31). Biofilm-producing, PS/A-positive S. epidermidis clearly make up the majority of significant clinical CoNS isolates (29). Moreover, it is likely that the studies of PIA production among clinical isolates of CoNS by Mack et al. (24) and Ziebuhr et al. (37) also measured PS/A production. Mack and colleagues used an antiserum raised to S. epidermidis 1457 to produce antibodies and adsorbed this antiserum with nonisogenic, nonadherent S. epidermidis strains to produce a reagent used to detect antigen elaboration among clinical isolates. As shown here, S. epidermidis 1457 is encapsulated and strongly binds antibodies to PS/A; thus, it is likely that the antibody reagent used to detect PIA production contained high titers of antibody to PS/A. Ziebuhr et al. (37) used the same antiserum to PIA that we used here to characterize antigen production by clinical isolates of S. epidermidis. Since this antiserum clearly has high titers of antibody to PS/A, these workers were also detecting PS/A production among strains of CoNS. In addition, Ziebuhr et al. (37) probed clinical isolates with the cloned ica locus and demonstrated the presence of these genes; again, they were detecting the potential for PS/A production among these strains. Overall, the results with the bacterial strains and reagents studied here indicate that PS/A is capable of mediating all of the significant biologic effects associated with biofilm-producing CoNS.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 23335.
We thank D. Mack, Krankenhaus Eppendorf, Hamburg, Germany, for providing S. epidermidis strains 1457 and 1457-M11 and antisera to PIA antigen; F. Gotz, University of Tuebingen, Tuebingen, Germany, for providing S. carnosus(pCN27), S. carnosus(pCA44), and S. carnosus TM300; and A. Onderdonk and C. Faye, Channing Laboratory, for help with succinate determinations.
REFERENCES
- 1.Augustin J, Gotz F. Transformation of Staphylococcus epidermidis and other Staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990;66:203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarri L, Donelli G, Gelosia A, Voglino M C, Simpson A W, Christensen G D. Purification and characterization of the staphylococcal slime-associated antigen and its occurrence among Staphylococcus epidermidis clinical isolates. Infect Immun. 1996;64:3410–3415. doi: 10.1128/iai.64.8.3410-3415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benerjee, S. S., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am. J. Med. 91(Suppl. 3B):86S–89S. [DOI] [PubMed]
- 4.Christensen G D, Barker L P, Mawhinney T P, Baddour L M, Simpson W A. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect Immun. 1990;58:2906–2911. doi: 10.1128/iai.58.9.2906-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deighton M, Borland R. Regulation of slime production in Staphylococcus epidermidis by iron limitation. Infect Immun. 1993;61:4473–4479. doi: 10.1128/iai.61.10.4473-4479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerke C, Kraft A, Subnuth S R, Schweitzer O, Gotz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 8.Goldmann D A, Pier G B. Pathogenesis of infections related to intravascular catheterization. Clin Microbiol Rev. 1993;6:176–192. doi: 10.1128/cmr.6.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatano K, Boisot S, DesJardins D, Wright D C, Brisker J, Pier G B. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect Immun. 1994;62:3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilmann C, Gerke C, Perdreau-Remington F, Gotz F. Characterization of tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 12.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M, Hastings J G M, White P J. A chemically defined medium for slime production by coagulase-negative Staphylococci. J Med Microbiol. 1991;34:143–147. doi: 10.1099/00222615-34-3-143. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M, Hastings J G M, White P J. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis. 1991;163:534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- 15.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat E A. Experimental Immunochemistry. In: Kabat E A, Mayer M M, editors. Experimental immunochemistry. 2nd ed. Springfield, Ill: Charles C. Thomas Publisher; 1961. pp. 241–267. [Google Scholar]
- 17.Karamanos N K, Syrokou A, Panagiotopoulou H S, Anastassiou E D, Dimitracopoulos G. The major 20-kDa polysaccharide of Staphylococcus epidermidis extracellular slime and its antibodies as powerful agents for detecting antibodies in blood serum and differentiating among slime-positive and -negative S. epidermidis and other staphylococcal species. Arch Biochem Biophys. 1997;342:389–395. doi: 10.1006/abbi.1997.0107. [DOI] [PubMed] [Google Scholar]
- 18.Keleti G, Lederer W H. Handbook of micromethods for the biological sciences. New York, N.Y: Van Nostrand Reinhold Co.; 1974. [Google Scholar]
- 19.Keller G, Schleifer K H, Gotz F. Construction and characterization of plasmid vectors for cloning in Staphylococcus aureus and Staphylococcus carnosus. Plasmid. 1983;10:270–278. doi: 10.1016/0147-619x(83)90041-0. [DOI] [PubMed] [Google Scholar]
- 20.Kogan G, Uhrin D, Brisson J R, Paoletti L C, Blodgett A E, Kasper D L, Jennings H J. Structural and immunochemical characterization of the type VIII group B streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 21.Kojima Y, Tojo M, Goldmann D A, Tosteson T D, Pier G B. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter related bacteremia due to coagulase-negative staphylococci. J Infect Dis. 1990;162:435–441. doi: 10.1093/infdis/162.2.435. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer G R, Iandolo J J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- 23.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 25.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic adherent Staphylococcus epidermidis—evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller E, Huebner J, Gutierrez N, Takeda S, Goldmann D A, Pier G B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993;61:551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller E, Takeda S, Goldmann D A, Pier G B. Blood proteins do not promote adherence of coagulase-negative staphylococci to biomaterials. Infect Immun. 1991;59:3323–3326. doi: 10.1128/iai.59.9.3323-3326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller E, Takeda S, Shiro H, Goldmann D, Pier G B. Occurrence of capsular polysaccharide adhesin among clinical isolates of coagulase-negative staphylococci. J Infect Dis. 1993;168:1211–1218. doi: 10.1093/infdis/168.5.1211. [DOI] [PubMed] [Google Scholar]
- 30.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 31.Shiro H, Meluleni G, Groll A, Muller E, Tosteson T D, Goldmann D A, Pier G B. The pathogenic role of Staphylococcus epidermidis capsular polysaccharide/adhesin in a low-inoculum rabbit model of prosthetic valve endocarditis. Circulation. 1995;92:2715–2722. doi: 10.1161/01.cir.92.9.2715. [DOI] [PubMed] [Google Scholar]
- 32.Shiro H, Muller E, Gutierrez N, Boisot S, Grout M, Tosteson T D, Goldmann D, Pier G B. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J Infect Dis. 1994;169:1042–1049. doi: 10.1093/infdis/169.5.1042. [DOI] [PubMed] [Google Scholar]
- 33.Smith R L, Gilkerson E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1979;98:478–480. doi: 10.1016/0003-2697(79)90170-2. [DOI] [PubMed] [Google Scholar]
- 34.Timmerman C P, Fleer A, Besnier J M, Degraaf L, Cremers F, Verhoef J. Characterization of a proteinaceous adhesin of Staphylococcus epidermidis which mediates attachment to polystyrene. Infect Immun. 1991;59:4187–4192. doi: 10.1128/iai.59.11.4187-4192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide/adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- 36.Veenstra G J, Cremers F F, van Dijk H, Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J Bacteriol. 1996;178:537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziebuhr W, Heilmann C, Gotz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]