Abstract
Mineral licks are key ecological components of the Amazon rainforest, providing critical dietary functions for herbivorous and frugivorous mammals and birds, which help maintain the structure and function of the forest itself through seed and nutrient dispersal. One of the most frequent visitors of interior forest mineral licks in the Amazon is the red brocket deer (Mazama americana), a large‐bodied ruminant frugivore and seed predator. While several hypotheses for the drivers of geophagy exist, including mineral supplementation, toxin adsorption, and habitat selection, robust data on geophagy for the red brocket deer for large numbers of mineral licks is nonexistent. We used soil data from 83 mineral licks in conjunction with camera trap data from 52 of those mineral licks and a mixed‐effects modeling approach to test the three proposed hypotheses of geophagy for the red brocket deer. We found that consumed soils at mineral licks had elevated concentrations of almost all major and minor biologically active minerals measured, including Ca, Na, Mg, K, Cu, Zn, and Mn. Model results suggest that all three hypotheses hold true to some extent for the red brocket deer, with the greatest support for the mineral supplementation hypothesis, in particular with respect to Mg, Ca, Na, Cu, and Zn. This study provides critical information on the feeding ecology of the red brocket deer in the wild, and the first robust analysis of geophagy of an Amazonian mammal involving a large sample size of interior forest mineral licks.
Keywords: Amazon, cervid, conservation, ecology, geophagy, mammal, mineral, ruminant, salt lick
Mineral licks are critical resources for herbivores across the world, including the Peruvian Amazon, but the services they provide visiting animals are largely unknown. We tested three hypotheses for geophagical behavior in the red brocket deer, one of the most common visitors to Amazonian mineral licks, at mineral licks in the Peruvian Amazon using camera traps, soil sampling, and a modeling framework. We found that all three hypotheses had at least some support, but the mineral supplementation hypothesis was the most heavily supported.
1. INTRODUCTION
The Amazon rainforest is one of the world's epicenters of biodiversity (Brown, 2014), and the conservation of that biodiversity has become a global priority (Schipper et al., 2008). Herbivorous and frugivorous mammals help maintain the structure and function of the Amazon rainforest by performing critical ecosystem services such as seed dispersal (Bodmer, 1991b; Brodie et al., 2009; Galetti et al., 2001) and nutrient transport (Doughty et al., 2016). These mammals also provide a source of food security for many rural populations that depend on subsistence hunting for animal protein (Alvard et al., 1995; Bizri et al., 2020; Griffiths, Bowler, et al., 2022; Mayor et al., 2021).
Soils in Amazonia are notoriously deficient in biologically active minerals, and many key herbivores and frugivores may be deficient in critical micro and macro nutrients needed for effective maintenance, reproduction, and growth. These species may compensate for mineral deficiencies in their diets by ingesting soil at natural mineral lick sites in the forest, a behavior known as geophagy (Abrahams & Parsons, 1996; Blake et al., 2011; Ferrari et al., 2008; Krishnamani & Mahaney, 2000). Alternative hypotheses for geophagy include toxin adsorption, where clays in ingested soils are assumed to bind toxic alkaloids from consumed plants and relieve gastrointestinal problems (Brightsmith et al., 2008; De Souza et al., 2002; Gilardi et al., 1999). A third plausible hypothesis which has received considerably less attention, and support, is the habitat selection hypothesis where animals may choose mineral licks based on their location, the suitability of the habitat they occur in, or the presence of predators in the habitat (Ayotte et al., 2008; Campbell et al., 2005; Tobler et al., 2009).
One of the most common visitors to interior forest Amazonian mineral licks is the red brocket deer (Mazama americana; Blake et al., 2011, 2013; Griffiths, Bowler, et al., 2020; Griffiths, Gilmore, et al., 2020; Tobler et al., 2009). The red brocket deer is a large‐bodied (12–65 kg) ruminant (Bodmer, 1989; Branan & Marchinton, 1985; Emmons & Feer, 1997; Jones et al., 2018; Robinson & Redford, 1986) that is found throughout tropical South America (Gallina‐Tessaro et al., 2019). The red brocket deer is primarily nocturnal, but often has a flexible activity pattern (Di Bitetti et al., 2008; Griffiths, Bowler, et al., 2020; Griffiths, Gilmore, et al., 2020), and is typically solitary (Reyna‐Hurtado & Chapman, 2019). It is most commonly found in terra firme forests, but can also inhabit swampy areas (Bodmer, 1991b; Tobler et al., 2009). The red brocket deer is primarily a frugivore (Danell et al., 2006; Lall et al., 2018; Prado, 2013) and seed predator (Gayot et al., 2004), though some small percentage of seeds may be dispersed (Bodmer, 1991b). The red brocket deer may consume browse seasonally as fruit becomes scarce (Emmons & Feer, 1997; Duarte et al., 2010). Reproduction of the red brocket deer is poorly understood, but thought to occur year‐round with about 0.76–0.82 young born per adult female per year (Gallina‐Tessaro et al., 2019; Mayor et al., 2011). The drivers of geophagy of the red brocket deer remain unknown, though its frugivorous diet suggests it may be deficient in some minerals, and its frequent visitation at mineral licks suggest it may be an important species for transporting minerals across the landscape (Doughty et al., 2016). Studies on geophagy of other cervids worldwide have attributed the behavior to a demand for mineral supplementation (Klaus & Schmidg, 1998; Lavelle et al., 2014; Weeks, 1978).
This study uses soil characteristic and camera trap data from a large number of mineral licks of Amazonian interior forest and a robust modeling approach to assess the drivers of geophagy for the red brocket deer. In particular, we test three different hypotheses posed for geophagy in the literature:
The demand for micro and macronutrients: the mineral supplementation hypothesis
The alleviation of gastrointestinal issues by alkaloid‐binding clays: the toxin adsorption hypothesis
The selection of mineral licks based on habitat: the habitat partitioning hypothesis
We estimated evidence for the validity of each of these hypotheses using the significance of specific soil‐ and landscape‐based covariates included in a generalized linear modeling framework.
2. MATERIALS AND METHODS
2.1. Study site
This study occurred in the Maijuna‐Kichwa Regional Conservation Area (MKRCA) and the titled lands of the Maijuna community of Sucusari, which adjoin the MKRCA to the south, in the northeastern Peruvian Amazon. The MKRCA is a 391,039‐ha protected area in the Department of Loreto, about 120 km northeast of the city of Iquitos, the capital of Loreto (Figure 1). The titled lands of the community of Sucusari encompass 4771 ha. The dominant habitat types in the region are primary, upland terra firme and seasonally flooded forests. The study area was completely encompassed by the watershed of the Sucusari River, a tributary of the Napo River, which has an area of about 508 km2. The region is characterized by a mean annual precipitation of 3100 mm and a mean annual temperature of 26°C (Marengo, 1998). There is a marked wet season that runs from November to May, with the dry season running from June to October (Espinoza Villar et al., 2009).
FIGURE 1.
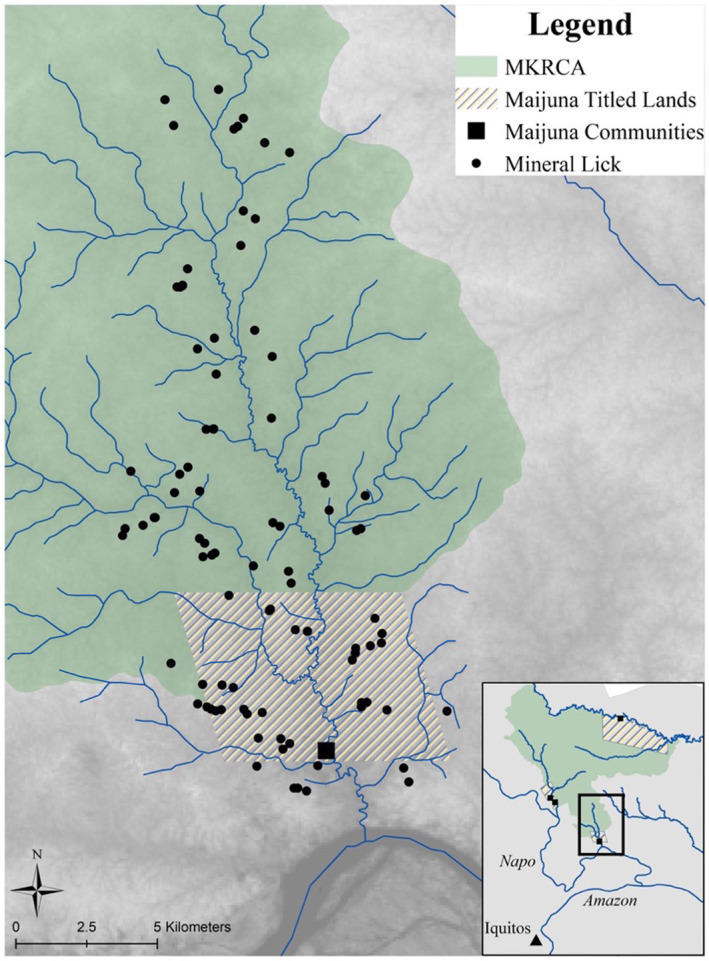
Map of the study site, the Maijuna‐Kichwa Regional Conservation Area (MKRCA) and the titled lands of the Maijuna community of Sucusari in the Peruvian Amazon. Map adopted from Griffiths, Jin, et al. (2022).
2.2. Camera trapping at mineral licks
These camera trapping methods were previously used to assess temporal visitation patterns of mammals (Griffiths, Bowler, et al., 2020; Stewart et al., 2022), natural history insights (Griffiths, Gilmore, et al., 2020) and dissimilarities among species assemblages (Griffiths et al., 2021) at mineral licks, along with the development of allometric relationships between mammal body mass and sodium deficiency (Duvall et al., 2023).
We set camera traps (Bushnell Aggressor, Boly Scout Guard) at 80 mineral licks in the Sucusari River basin from August, 2018 to June, 2019 in a series of four deployments that each lasted 60–80 days (Kays et al., 2020) across the dry and wet seasons (Griffiths, Bowler, et al., 2020). Up to 25 mineral licks were camera trapped in a single deployment before the cameras were rotated to new mineral licks. Mineral licks chosen for deployments were initially randomly selected; for the last deployment, mineral licks with less sampling effort due to camera malfunctions were prioritized. After the sampling period, we eliminated all mineral licks from the sample that did not achieve a total sampling effort of 55 camera nights, since mineral licks with fewer camera nights did not meet the sampling criteria for species richness established by Kays et al. (2020), leaving 52 mineral licks for analysis in this study.
At each mineral lick, we set camera traps facing the active faces of the lick, which were determined by looking for signs of recent animal activity (e.g. teeth and claw marks; Tobler et al., 2009). Camera traps were set 50 cm from the ground on burst photo mode, taking a set of three rapid‐fire images each time the camera was triggered (Tobler et al., 2008). Mineral licks with multiple active faces received more than one camera trap to achieve coverage of all active areas of the lick; the mean number of camera traps per lick was 1.2. The mineral licks in this study typically had saturated soil and often had standing water, regardless of season, with the lick area itself devoid of vegetation (Griffiths, Bowler, et al., 2020; Griffiths, Gilmore, et al., 2020).
We identified all red brocket deer in camera trap images, removed empty images and organized data for analyses using CameraBase v1.7 (Tobler, 2015). The number of individuals in the same photograph was also recorded. Sets of images were binned into independent visitation events, where multiple detections of red brocket deer within 1 h of each other were considered one visitation event at the mineral lick, following Tobler et al. (2008).
2.3. Soil sample collection and analysis
These soil collection and analysis methods were previously used to describe fundamental physical and chemical differences between soils from mineral licks and control soils (Griffiths, Jin, et al., 2022).
We revisited all mineral licks in the study in January 2022 to collect soil samples. At each mineral lick, a Maijuna expert and a trained research assistant collected soil from the feeding site of each lick, which was identified by looking for teeth and claw marks. Approximately 1 kg of soil was collected from feeding sites by scraping horizontally across exposed soil with a clean trowel (Mahaney & Krishnamani, 2003). Soil samples were placed in airtight, labeled plastic bags. In mineral licks where there were multiple active feeding sites with visibly distinct soil characteristics, one sample was taken from each feeding site, and these samples were analyzed separately. We then collected two samples of control soils by walking 5 m in a random direction, outside of the lick area devoid of vegetation. We scraped away organic debris from the topsoil and excavated 1 kg of subsoil (Mahaney & Krishnamani, 2003). We air dried all soil samples in the shade until they were no longer saturated, then they were rebagged and labeled for analysis. Soil samples were analyzed for their physical and chemical characteristics at SGS Peru in Lima, Peru (see Griffiths, Jin, et al., 2022).
2.4. Data analysis
We summarized red brocket deer visitation at mineral licks by calculating summary statistics of visit frequency (visits per 100 camera nights) at all mineral licks in the sample that had camera traps and red brocket deer visited at least once.
We used a generalized linear modeling approach to assess the drivers of geophagy of the red brocket deer. First, we binned soil samples from the same mineral lick by taking the mean of all relevant soil characteristics, leaving one “consumed” sample and one control sample at each mineral lick to account for pseudoreplication. We removed “consumed” samples (samples from feeding sites) which were never camera trapped since it is not clear whether red brocket deer would have visited those sites, leaving a total sample size of n = 129. We assigned a visit frequency to each consumed sample, which was the number of visits by red brocket deer per 100 camera nights at that mineral lick. All control soils were assigned a visit frequency of 0. If a mineral lick was camera trapped but never visited by red brocket deer (n = 6 mineral licks), a visit frequency of 0 was also assigned to those consumed samples. We used visit frequency as our response variable for modeling.
We constructed a global zero‐inflated model with two components: a binomial distribution to test the likelihood of a soil being consumed, and a Poisson distribution to test the predicted visit frequency of samples that were consumed. The covariates in the model were soil characteristics that were hypothesized to have some effect on red brocket deer physiology and landscape‐level mineral lick characteristics that may affect deer movement and mineral lick selection (Table 1; Tobler et al., 2009). Elevation, surface roughness, and slope were calculated using LANDSAT Collection 2 data. We also included interactions between exchangeable Ca and free P, and concentrations of Cu and Zn, Fe and Mn, and Cu and Fe since these are known to interact in the digestive systems of cervids, each impacting the absorption of the other (National Research Council (NRC), 2007). All covariates were tested for collinearity before including them in the model, with a correlation cutoff of r 2 > .7 for inclusion (Dormann et al., 2013). All continuous covariates were scaled before including them in the model.
TABLE 1.
Specific covariates tested in zero‐inflated models and the hypothesis for geophagy that the specific covariate relates to.
| Covariate tested | Hypothesis addressed |
|---|---|
| Exchangeable Na (meq/100 g) | 1. Mineral Supplementation |
| Exchangeable Mg (meq/100 g) | 1. Mineral Supplementation |
| Cu (ppm) | 1. Mineral Supplementation |
| Exchangeable K (meq/100 g) | 1. Mineral Supplementation |
| Exchangeable H (meq/100 g) | 1. Mineral Supplementation |
| Soluble B (ppm) | 1. Mineral Supplementation |
| Zn (ppm) | 1. Mineral Supplementation |
| Mn (ppm) | 1. Mineral Supplementation |
| Free P (ppm) | 1. Mineral Supplementation |
| Fe (ppm) | 1. Mineral Supplementation |
| Exchangeable Al (meq/100 g) | 1. Mineral Supplementation |
| Total N (%) | 1. Mineral Supplementation |
| Exchangeable Ca (meq/100 g) | 1. Mineral Supplementation |
| 2. Toxin Adsorption | |
| Clay content (%) | 2. Toxin Adsorption |
| CEC (meq/100 g) | 2. Toxin Adsorption |
| pH (1:1) | 2. Toxin Adsorption |
| Elevation (m) | 3. Habitat Partitioning |
| Slope (degrees) | 3. Habitat Partitioning |
| Surface roughness (m) | 3. Habitat Partitioning |
| Distance from water (km) | 3. Habitat Partitioning |
We used a backwards‐stepwise model selection process (Murtaugh, 2009), dropping one covariate at a time until dropping any more covariates resulted in a higher AIC, yielding the most parsimonious optimal model. Once the optimal model was selected, we tested covariates that were correlated and included in the optimal model (e.g., pH and exchangeable Ca) to see which lowered the AIC of the optimal model the most and should be included; however, in those cases, both covariates are discussed. Model fit was tested by visually examining residuals.
All analyses were conducted in R (version 4.2.1; R Core Team, 2022). Zero‐inflated models were constructed using the zeroinfl function in the pscl package (Jackman, 2020; Zeileis et al., 2008).
3. RESULTS
Red brocket deer visited 46 of the 52 mineral licks that had camera traps. The mean visit frequency at these sites was 37.17 (SD = 45.80) visits per 100 camera nights, with a range of 1.12–205.10 visits per 100 camera nights. On average, red brocket deer spent 9.57 min at mineral licks during a given visit, with a minimum mean visit duration of 2 min and a maximum mean visit duration of 41.02 min at a mineral lick.
The optimal model of drivers of geophagy of the red brocket deer included exchangeable Mg, Na, and Al, soluble B, and the interaction between Mg and P in the binomial half of the model. Many covariates were included in the Poisson half of the optimal model: soluble B, exchangeable Mg, K, Na, and Al, free P, total N, concentrations of Cu, Fe, Mn, and Zn, clay fraction, elevation, surface roughness, distance from the closest stream, and interactions between Mg and P, Fe and Mn, Cu and Fe, and Cu and Zn (Table 2). Soluble B, free P, exchangeable K and Al, concentration of Fe, and surface roughness all had a negative effect on predicted red brocket deer visitation while all other covariates had a positive effect. Red brocket deer visitation was predicted to increase dramatically at high concentrations of exchangeable Mg (Figure 2). Visitation was predicted to decrease to close to zero at exchangeable K concentrations greater than about 0.25 meq/100 g and soluble B concentrations greater than 1 ppm when all other covariates were held constant. Clay fraction, concentration of Cu, exchangeable Al, exchangeable Na, concentration of Mn, total N, and concentration of Zn all had a relatively moderate positive effect on predicted visitation (Figure 2). Overall, concentration of Fe, free P, and distance from the closest stream had a relatively small effect on predicted red brocket deer visitation (Figure 2). Red brocket deer were predicted to visit licks more frequently at higher elevations and lower surface roughness.
TABLE 2.
Optimal zero‐inflated model results testing hypotheses for the drivers of geophagy in the red brocket deer at Amazonian mineral licks.
| Covariate | Coeff. Est. | SE | z | p |
|---|---|---|---|---|
| Poisson | ||||
| Soluble B (ppm) | −0.597 | 0.042 | −14.276 | .000 |
| Exchangeable Mg (meq/100 g) | 0.314 | 0.071 | 4.447 | .000 |
| Free P (ppm) | −0.058 | 0.033 | −1.743 | .081 |
| Exchangeable K (meq/100 g) | −0.986 | 0.067 | −14.720 | .000 |
| Exchangeable Na (meq/100 g) | 0.143 | 0.032 | 4.533 | .000 |
| Total N (%) | 0.217 | 0.036 | 6.038 | .000 |
| Exchangeable Al (meq/100 g) | −0.089 | 0.053 | −1.667 | .096 |
| Cu (ppm) | 0.407 | 0.054 | 7.516 | .000 |
| Fe (ppm) | −0.147 | 0.060 | −2.468 | .014 |
| Mn (ppm) | 0.042 | 0.038 | 1.105 | .269 |
| Zn (ppm) | 0.483 | 0.051 | 9.450 | .000 |
| Clay fraction (%) | 0.350 | 0.062 | 5.622 | .000 |
| Elevation (m) | 0.227 | 0.044 | 5.110 | .000 |
| Surface roughness (m) | −0.720 | 0.056 | −12.943 | .000 |
| Distance from closest stream (km) | 0.209 | 0.046 | 4.583 | .000 |
| Exchangeable Mg x Free P | 0.214 | 0.032 | 6.672 | .000 |
| Cu × Fe | −0.081 | 0.042 | −1.923 | .055 |
| Fe × Mn | −0.190 | 0.042 | −4.552 | .000 |
| Cu × Zn | −0.368 | 0.061 | −6.083 | .000 |
| Binomial | ||||
| Soluble B (ppm) | −0.934 | 0.430 | −2.172 | 0.030 |
| Exchangeable Mg (meq/100 g) | −1.597 | 0.380 | −4.204 | 0.000 |
| Free P (ppm) | −0.332 | 0.278 | −1.196 | 0.232 |
| Exchangeable Na (meq/100 g) | −1.228 | 0.526 | −2.335 | 0.020 |
| Exchangeable Al (meq/100 g) | −1.513 | 0.365 | −4.150 | 0.000 |
| Exchangeable Mg × Free P | 0.600 | 0.319 | 1.882 | 0.060 |
Note: Statistically significant covariates shown in bold.
FIGURE 2.
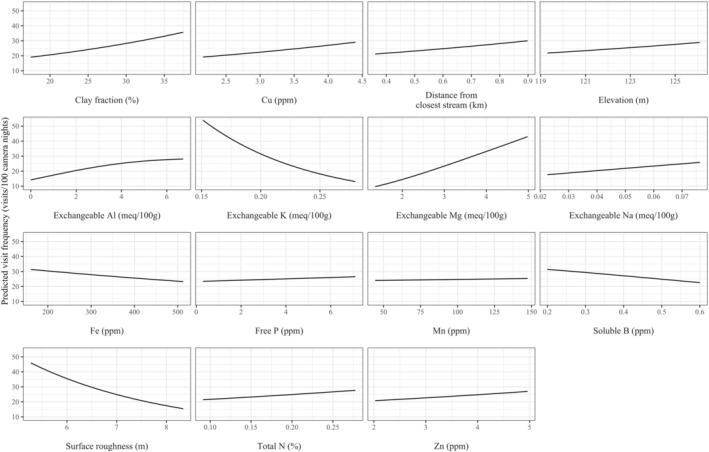
Optimal zero‐inflated model results showing the effects of mineral lick characteristics on the predicted visitation rates of the red brocket deer at Amazonian mineral licks. For display purposes, all covariates in the model that do not appear on individual graphs were held at the mean for consumed soils. Variables on x‐axes range across the inner quartile range of consumed soils.
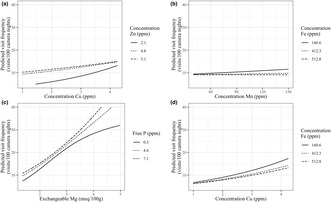
Four interactions were included in the optimal model: concentrations of Cu and Zn, Mn and Fe, Cu and Fe, and exchangeable Mg and free P. Predicted visitation was highest at high Cu and low Zn concentrations. However, at very low Cu concentrations, predicted visitation was slightly higher at sites with higher Zn concentrations (Figure 3a). The influence of Mn concentration on predicted visitation was highest at low Fe concentrations. At high Fe concentrations, Mn concentration had a negative effect on predicted visitation (Figure 3b). Exchangeable Mg and free P were included as an interaction since Ca and P are known to interact (NRC, 2007) and exchangeable Ca and Mg were highly correlated. The inclusion of Mg over Ca lowered the AIC of the optimal model significantly, but the interaction here also signifies the interaction between Ca and P. Predicted visitation increased most rapidly at high concentrations of both Mg and P, and the influence of exchangeable Mg on predicted visitation was lowest at low free P concentrations (Figure 3c). The influence of Cu concentration on predicted visitation was highest at low Fe concentrations, acting similarly to Mn (Figure 3d).
FIGURE 3.
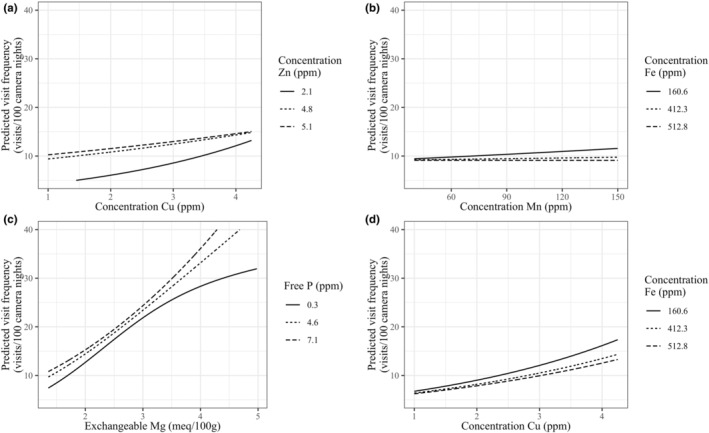
The interaction between (a) Cu and Zn, (b) Mn and Fe, (c) Mg and P, and (d) Cu and Fe concentrations on predicted red brocket deer visitation at Amazonian mineral licks. All other covariates in the model held at the mean for display purposes. Variables on x‐axes range across the inner quartile range of consumed soils.
Overall, two models fell within ∆AIC < 2 of the optimal model, one included Mn concentration (∆AIC = 0.128) and the other included both Mn concentration and exchangeable K (∆AIC = 1.294; Table S1).
4. DISCUSSION
This study is the first of its kind to use a large sample size of mineral licks in the same watershed (n = 52) camera trap records (n = 1733 independent visits) and soil samples (n = 218), in a robust modeling approach to test the three main hypotheses of geophagy for this cryptic cervid. Our results demonstrate the ecological significance of mineral licks for frugivorous species and provide substantial evidence for the mineral supplementation hypothesis.
The spatial variation in deer visitation, and the large variability in soil characteristics between mineral licks, emphasizes the importance of assessing a large sample size of mineral licks when drawing inferences about geophagy. In this study, we present results from a large sample size of mineral licks spread across the entirety of the Sucusari River watershed. Our soil samples likely encompass the full range of mineral lick characteristics available to red brocket deer in this watershed. The variation between licks has likely contributed to the incomplete understanding of the drivers of geophagy in the Amazon between studies with small sample sizes (Brightsmith et al., 2008; Brightsmith & Muñoz‐Najar, 2004; Emmons & Stark, 1979; Ghanem et al., 2013; Gilardi et al., 1999; Izawa, 1993; Molina et al., 2014). The limited sample sizes presented in other studies demonstrate the importance of increasing sampling to seek a larger sample size, which could be accomplished by working with local communities to identify these critical ecological sites.
In this study, we report high levels of collinearity between some soil characteristics, in particular Mg, Ca, and pH. Considering collinear variables together in the same set of analyses may cloud the interpretation of results (Dormann et al., 2013). In this case, we removed collinear covariates in our global model testing drivers of geophagy. Our optimal model showed that Mg may be the most important driver of geophagy. Since pH and Ca concentration were highly correlated with Mg, we can assume that these characteristics are also important though they were excluded due to collinearity. Testing these covariates together may have led to the mistaken assumption that the major cations Mg and Ca, along with pH were the only significant factors in driving geophagy.
Similarly, we account for any potential lack of independence in soil samples by taking the mean values within sample types for the models. In this case, because we took multiple soil samples from the same lick site (Mahaney & Krishnamani, 2003), the samples may not be independent, a condition which invalidates inferences from many approaches that have been used in other studies (e.g. basic descriptions of soils: De Souza et al., 2002; Izawa, 1993; Montenegro, 1998; correlations: Molina et al., 2014; Wilcox's rank sum test: Moe, 1993). We suggest that some of the variation in results from other studies is due to the lack of separation of collinear covariates and a lack of independence. We recommend that future studies use statistical methods that account for these complexities (e.g. PCA: Brightsmith et al., 2008; Razali et al., 2020; Sim et al., 2020).
The covariates that were predicted to cause the largest increase in visitation across their distributions were exchangeable Mg, exchangeable Na, Cu, and Zn. Mg is associated with bone growth, muscle development, and activation of enzymes in mammals (NRC, 2007), and high concentrations of Mg have been shown to increase absorption of Ca in horses (Hintz & Schryver, 1973). Mg, like Cu, has been associated with greater immune response in white‐tailed deer (Nichols et al., 2016). High concentrations of K have been shown to limit Mg absorption, a condition known as grass tetany (Ram et al., 1998). It's possible that if some fruits eaten by the red brocket deer are high in K, red brocket deer would need to supplement Mg in their diet or risk tetany (Kaspari, 2020). Accordingly, increased exchangeable K was associated with a decrease in predicted visitation. This model shows good evidence that Mg may be a major driver of mineral lick selection for the red brocket deer.
Cervids, including deer, elk, and moose, are known to have a relatively high need for Ca, Na, and P, particularly for bone and antler formation and growth and during lactation (Carciofi & Saad, 2001; Hellgren & Pitts, 1997; Landete‐Castillejos et al., 2007; Tajchman et al., 2018). While these animals may have similar physiological needs to red brocket deer, each animal's needs are determined by deficiencies in their diet, and the red brocket deer's frugivorous diet varies from the grazing/browsing diets of most other cervids. In the Amazon, a frugivorous diet is linked with lower micronutrient content than a grazing or browsing diet (Doughty et al., 2016; Ghanem et al., 2013). In general, supplementation of major cations can also improve rumen buffering (Beede & Collier, 1986). Cervids, in general, are particularly vulnerable to hypocalcaemia and hypophosphataemia while growing. While the red brocket deer has relatively small antlers compared to other cervid species, its highly frugivorous diet (Bodmer, 1989; Duarte et al., 2010) suggests that it may be deficient in some or all of the macro and micro minerals needed for growth. While both Ca and P are needed for growth, high concentrations of P can limit Ca absorption since P and Ca both compete for the same binding sites (NRC, 2007). Of particular importance, then, is the Ca:P ratio, and a Ca:P ratio of about 12:58–59 (or 0.2) has been considered optimal for cervids (Perkins, 1991). Interestingly, many of the consumed soils in this study were at or close to the optimal Ca:P ratio of 0.2, but many unconsumed and control soils were also near, or at, the optimal ratio. The interaction between Mg (and therefore Ca as well due to high correlation) and P was significant, indicating that the Ca:P ratio may be a determinant in mineral lick selection.
Na is a key mineral for herbivore growth, survival, and reproduction but is present in very low quantities in vegetation and fruit in the Amazon (Doughty et al., 2016). Many studies have described an association between Na content and mineral lick visitation (Brightsmith & Muñoz‐Najar, 2004; De Souza et al., 2002; Dudley et al., 2012; Lavelle et al., 2014; Stephenson et al., 2011), and Na deficiency has been a leading hypothesis for geophagy for decades. Our models describe a positive association between exchangeable Na and predicted visitation for the red brocket deer, adding to this body of literature. Kaspari (2020) describes how Na may also facilitate P uptake in ruminants which have P‐demanding gut microbiomes. Even compared to other native herbivorous and frugivorous species, including other ungulates, the red brocket deer's diet and body size suggest it may be the most deficient in Na (Duvall et al., 2023).
Our models predicted that mineral licks that had a Cu concentration of more than 10 ppm were almost guaranteed to be consumed by red brocket deer, and that proportion increased significantly when concentrations of Fe were relatively low. High levels of Fe have been shown to decrease absorption of Cu (NRC, 2007). Our models demonstrated this interaction between Cu and Fe, where the probability of soil consumption was the highest when soils were simultaneously rich in Cu and relatively poor in Fe. These results indicate that red brocket deer may select geophagical soils based on Cu content and the ratio of Cu:Fe and/or Cu:Zn to maximize Cu absorption. In cervids, Cu is most commonly associated with function of the immune system. In white‐tailed deer (Odocoileus virginianus), higher Cu intake was associated with less incidence of chronic wasting disease (Nichols et al., 2016) and a higher immune response to pathogens (Bartoskewitz et al., 2007). In red deer (Cervus elaphus), low levels of Cu were associated with lower carcass weight (Handeland et al., 2017). While other studies have measured Cu concentrations in lick soils (Emmons & Stark, 1979; Ghanem et al., 2013; Gilardi et al., 1999; Molina et al., 2014; Montenegro, 2004; Powell et al., 2009), our study is the first study to propose that Cu, and the relationship between Cu and Fe and Cu and Zn, may be an important driver of geophagy for red brocket deer.
Concentrations of exchangeable Al were significant in the optimal model, with relatively high concentrations of Al associated with a plateau in predicted visitation. In one study in North America, cervids were observed to avoid mineral licks with high levels of Al, potentially to avoid toxicity (Lavelle et al., 2014). Given that Al can be toxic to mammals, and is loosely negatively correlated with pH, we would expect high levels of Al to be negatively associated with consumption probability. The majority of consumed samples had an Al concentration of 0. The positive relationship and significance we show here is likely an artifact of the data, where some consumed samples had very high Al concentrations, skewing the results. Since some consumed soils also had very high levels of Na, P, Cu, and pH, the importance of these soil characteristics may also be overstated by models, but also match our hypotheses. It is also possible that elements co‐exist in the soil and animals are not able to find soils that contain only the minerals that they seek; the consumption of high Al content soils may be a consequence of seeking a mineral that co‐occurs with Al. We found that only Mg, Ca, and pH were highly correlated, but it is possible that this relationship between minerals occurs in specific mineral licks. However, the importance of Al in geophagous soils has previously been stated for primates (Ferrari et al., 2008), so it is possible that the high Al content is a legitimate driver of geophagy in other herbivores as well.
Overall, high intakes of many minerals, including Ca, P, Mn, and Cu can be toxic to cervids (Lavelle et al., 2014; McDowell, 2003). Similarly, high levels of several minerals can influence absorption of other minerals (NRC, 2007). In this study, we tested several of those interactions: Mg (and Ca) and P, Cu and Fe, Fe and Mn, and Cu and Zn. All of these interactions appeared in the optimal model and are known to interact in the digestive systems of ruminants (Tajchman et al., 2018) which indicates that simple comparisons between consumed and control soils are too simplistic to draw conclusions from, and more complex statistical methods are needed. The influence of potential toxicity could be tested with a large enough sample size by including polynomial terms in models of geophagy. Here, we faced a lack of convergence when polynomial terms were included, indicating that a greater sample size of brocket deer mineral licks are needed to test toxicity. However, it may be that brocket deer avoid ingesting toxic amounts of minerals simply by consuming a smaller quantity of soil.
Soil pH, correlated with Mg, also has a significant association with geophagy. This result is in line with other studies, which have posited that a main driver of geophagy is the detoxification of dietary alkaloids that were consumed (Freeland & Janzen, 1974; Gilardi et al., 1999; Krishnamani & Mahaney, 2000; Milton, 1998). However, this hypothesis is also linked to clay content of the soil, where a higher clay content has a higher adsorption capacity due to larger surface area. In our models, clay content in the soil was associated with increased visitation, as predicted under the toxin adsorption hypothesis. This result lends evidence to the conclusion that red brocket deer may receive dual benefits from soil ingestion. It should be noted that certain species of clays, such as kaolinite, can be critical for detoxification. While kaolinite is the dominant clay type in the Iquitos region (Irion, 1984; Kauffman et al., 1998), we did not measure the structure of clays present in this study. Other studies have determined that soils may alleviate gastrointestinal issues in other species and that clay content and pH are potential drivers of geophagy (Gilardi et al., 1999; Mahaney et al., 1996) or that species may derive dual benefits (Ayotte et al., 2006; Brightsmith et al., 2008; Diamond et al., 1999; Ferrari et al., 2008; Ghanem et al., 2013; Klaus et al., 1998; Kreulen, 1985; Krishnamani & Mahaney, 2000; Molina et al., 2014; Voigt et al., 2008).
There is some evidence that animals can detect Ca and Mg in soils (McCaughey & Tordoff, 2002; Tordoff, 2001), so it may be that cervids select soils with high Ca and Mg and derive other benefits, such as Cu supplementation, from that selection. The highly frugivorous diet of the red brocket deer (Bodmer, 1989; Danell et al., 2006; Gayot et al., 2004; Prado, 2013; Duarte et al., 2010) also supports that the main driver of geophagy is likely mineral supplementation, since plant‐based alkaloids are typically found in the mature leaves of plants (Julliot & Sabatier, 1993). The red brocket deer would only be consuming minor amounts of alkaloids.
The significance of landscape features in the model demonstrates that habitat selection may be an important factor in geophagy of the red brocket deer, a result that is not in line with results from Tobler et al. (2009), who reported a lack of habitat selection at mineral licks. Bodmer (1991a, 1991b) states that red brocket deer have a preference for inclined terrain in terra firme forests, and this may be the cause of the significance of landscape features here.
Several other studies, from the Amazon (Brightsmith et al., 2017; Montenegro, 2004; Setz et al., 1999), North America (Jones & Hanson, 1985), and other areas of the tropics (Matsubayashi et al., 2007; Mills & Milewski, 2007; Stephenson et al., 2011) have determined that mineral supplementation was likely the driver of geophagy at mineral licks, and there has been a particular emphasis on Na (Dudley et al., 2012; Holdø et al., 2002; Lavelle et al., 2014; Moe, 1993; Powell et al., 2009; Weeks, 1978). This study uses a robust statistical analysis on a large sample of interior forest Amazonian mineral licks, and it shows strong support for the mineral supplementation hypothesis in cervids, with some evidence that deer receive dual benefits.
CONCLUSIONS
Mineral licks are critical to the feeding ecology of Amazonian frugivores, in particular the red brocket deer. We suggest that red brocket deer receive dual benefits from mineral licks, satisfying both the mineral supplementation and toxin adsorption hypotheses, and that some selection of mineral licks based on habitat occurs. While we found evidence of all three hypotheses influencing red brocket deer visitation, our models suggest that the strongest driver of geophagy in the red brocket deer is mineral supplementation of Mg, Ca, Na, Cu, and Zn.
AUTHOR CONTRIBUTIONS
Brian M. Griffiths: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Lesa G. Griffiths: Funding acquisition (equal); project administration (equal); resources (lead); writing – review and editing (equal). Yan Jin: Conceptualization (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); resources (supporting); writing – review and editing (equal). Michael P. Gilmore: Conceptualization (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); supervision (equal); writing – review and editing (lead).
FUNDING INFORMATION
This work was supported by a Fulbright U.S. Student Grant awarded to Brian M. Griffiths.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant financial or nonfinancial interests to disclose.
Supporting information
Table S1.
ACKNOWLEDGEMENTS
We would like to acknowledge the Maijuna community of Sucusari for their eagerness to participate in ongoing studies at local mineral licks and their commitment to conservation. We would like to acknowledge the U.S. Fulbright Association and the University of Delaware for providing funding for this work. We would like to acknowledge OnePlanet, Inc., Amazon Explorama Lodges, and the Amazon Center for Environmental Education and Research (ACEER) for providing in‐kind support during the fieldwork component of this study. We would like to thank the contributions of the anonymous reviewers, whose comments we believe greatly improved previous versions of this manuscript.
Griffiths, B. M. , Griffiths, L. G. , Jin, Y. , & Gilmore, M. P. (2024). Drivers of geophagy by red brocket deer (Mazama americana) at Amazonian interior forest mineral licks. Ecology and Evolution, 14, e10968. 10.1002/ece3.10968
DATA AVAILABILITY STATEMENT
Analyses reported in this article can be reproduced using the data provided by Griffiths, Griffiths, et al. (2022).
REFERENCES
- Abrahams, P. W. , & Parsons, J. A. (1996). Geophagy in the tropics: A literature review. The Geographical Journal, 162, 63–72. 10.2307/3060216 [DOI] [Google Scholar]
- Alvard, M. , Alcorn, J. B. , Bodmer, R. E. , Hames, R. , Hill, K. , Hudson, J. , Lyman, R. L. , Puri, R. K. , Smith, E. A. , & Stearman, A. M. (1995). Intraspecific prey choice by Amazonian hunters. Current Anthropology, 36, 789–818. [Google Scholar]
- Ayotte, J. B. , Parker, K. L. , Arocena, J. M. , & Gillingham, M. P. (2006). Chemical composition of lick soils: Functions of soil ingestion by four ungulate species. Journal of Mammalogy, 87, 878–888. [Google Scholar]
- Ayotte, J. B. , Parker, K. L. , & Gillingham, M. P. (2008). Use of natural licks by four species of ungulates in northern British Columbia. Journal of Mammalogy, 89(4), 1041–1050. 10.1644/07-MAMM-A-345.1 [DOI] [Google Scholar]
- Bartoskewitz, M. L. , Hewitt, D. G. , Laurenz, J. C. , Pitts, J. S. , & Bryant, F. C. (2007). Effect of dietary copper and zinc concentrations on white‐tailed deer antler growth, body size, and immune system function. Small Ruminant Research, 73, 87–94. 10.1016/j.smallrumres.2006.11.005 [DOI] [Google Scholar]
- Beede, D. K. , & Collier, R. J. (1986). Potential nutritional strategies for intensively managed cattle during thermal stress. Journal of Animal Science, 62, 543–554. [Google Scholar]
- Bizri, H. R. E. , Morcatty, T. Q. , Valsecchi, J. , Mayor, P. , Ribeiro, J. E. S. , Vasconcelos Neto, C. F. A. , Oliveira, J. S. , Furtado, K. M. , Ferreira, U. C. , Miranda, C. F. S. , Silva, C. H. , Lopes, V. L. , Lopes, G. P. , Florindo, C. C. F. , Chagas, R. C. , Nijman, V. , & Fa, J. E. (2020). Urban wild meat consumption and trade in central Amazonia. Conservation Biology, 34, 438–448. 10.1111/cobi.13420 [DOI] [PubMed] [Google Scholar]
- Blake, J. G. , Mosquera, D. , Guerra, J. , Loiselle, B. A. , Romo, D. , & Swing, K. (2011). Mineral licks as diversity hotspots in lowland forest of eastern Ecuador. Diversity, 3, 217–234. 10.3390/d3020217 [DOI] [Google Scholar]
- Blake, J. G. , Mosquera, D. , & Salvador, J. (2013). Use of mineral licks by mammals and birds in hunted and non‐hunted areas of Yasuní National Park, Ecuador. Animal Conservation, 16(4), 430–437. [Google Scholar]
- Bodmer, R. E. (1989). Frugivory in Amazonian Artiodactyla: Evidence for the evolution of the ruminant stomach. Journal of Zoology, 219, 457–467. [Google Scholar]
- Bodmer, R. E. (1991a). Influence of digestive morphology on resource partitioning in Amazonian ungulates. Oecologia, 85, 361–365. [DOI] [PubMed] [Google Scholar]
- Bodmer, R. E. (1991b). Strategies of seed dispersal and seed predation in Amazonian ungulates. Biotropica, 23, 255–261. [Google Scholar]
- Branan, W. V. , & Marchinton, R. L. (1985). Biology of red brocket deer in Suriname with emphasis on management potential. Biology of Deer Production, 22, 41–44. [Google Scholar]
- Brightsmith, D. J. , Hobson, E. A. , & Martinez, G. (2017). Food availability and breeding season as predictors of geophagy in Amazonian parrots. Ibis, 160(1), 112–129. [Google Scholar]
- Brightsmith, D. J. , & Muñoz‐Najar, R. A. (2004). Avian geophagy and soil characteristics in southeastern Peru. Biotropica, 36, 534–543. [Google Scholar]
- Brightsmith, D. J. , Taylor, J. , & Phillips, T. D. (2008). The roles of soil characteristics and toxin adsorption in avian geophagy. Biotropica, 40, 766–774. [Google Scholar]
- Brodie, J. F. , Helmy, O. E. , Brockelman, W. Y. , & Maron, J. L. (2009). Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal‐dispersed tree. Ecological Applications, 19, 854–863. [DOI] [PubMed] [Google Scholar]
- Brown, J. H. (2014). Why are there so many species in the tropics? Journal of Biogeography, 41, 8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C. J. , Aureli, F. , Chapman, C. A. , Ramos‐Fernández, G. , Matthews, K. , Russo, S. E. , Suarez, S. , & Vick, L. (2005). Terrestrial Behavior of Ateles spp. International Journal of Primatology, 26(5), 1039–1051. 10.1007/s10764-005-6457-1 [DOI] [Google Scholar]
- Carciofi, A. C. , & Saad, C. E. d. P. (2001). Nutrition and nutritional problems in wild animals. In Fowler M. E. (Ed.), Biology, medicine, and surgery of South American wild animals (pp. 425–436). John Wiley & Sons, Ltd. [Google Scholar]
- Danell, K. , Bergström, R. , Duncan, P. , & Pastor, J. (2006). Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press. [Google Scholar]
- De Souza, L. L. , Ferrari, S. F. , Da Costa, M. L. , & Kern, D. C. (2002). Geophagy as a correlate of folivory in red‐handed howler monkeys (Alouatta belzebul) from eastern Brazilian Amazonia. Journal of Chemical Ecology, 28, 1613–1621. [DOI] [PubMed] [Google Scholar]
- Di Bitetti, M. S. , Paviolo, A. , Ferrari, C. A. , De Angelo, C. , & Di Blanco, Y. (2008). Differential responses to hunting in two sympatric species of brocket deer (Mazama americana and M. nana). Biotropica, 40, 636–645. 10.1111/j.1744-7429.2008.00413.x [DOI] [Google Scholar]
- Diamond, J. , Bishop, K. D. , & Gilardi, J. D. (1999). Geophagy in New Guinea birds. Ibis, 141, 181–193. 10.1111/j.1474-919X.1999.tb07540.x [DOI] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , Marquéz, J. R. G. , Gruber, B. , Lafourcade, B. , Leitão, P. J. , Münkemüller, T. , McClean, C. , Osborne, P. E. , Reineking, B. , Schröder, B. , Skidmore, A. K. , Zurell, D. , & Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 27–46. [Google Scholar]
- Doughty, C. E. , Wolf, A. , Baraloto, C. , & Malhi, Y. (2016). Interdependency of plants and animals in controlling the sodium balance of ecosystems and the impacts of global defaunation. Ecography, 39, 204–212. [Google Scholar]
- Duarte, J. M. B. , & González, S. (2010). Neotropical cervidology: Biology and medicine of Latin American deer. Funep/IUCN Jaboticabal. https://www.researchgate.net/profile/Jose‐Duarte‐7/publication/270050767_Neotropical_Cervidology_Biology_and_Medicine_of_Latin_American_Deer/links/549f1dc10cf267bdb8fdbb33/Neotropical‐Cervidology‐Biology‐and‐Medicine‐of‐Latin‐American‐Deer.pdf [Google Scholar]
- Dudley, R. , Kaspari, M. , & Yanoviak, S. P. (2012). Lust for salt in the western Amazon. Biotropica, 44, 6–9. 10.1111/j.1744-7429.2011.00818.x [DOI] [Google Scholar]
- Duvall, E. S. , Griffiths, B. M. , Clauss, M. , & Abraham, A. J. (2023). Allometry of sodium requirements and mineral lick use among herbivorous mammals. Oikos, 2023(9), e10058. 10.1111/oik.10058 [DOI] [Google Scholar]
- Emmons, L. , & Feer, F. (1997). Neotropical rainforest mammals: A field guide. University of Chicago Press. [Google Scholar]
- Emmons, L. H. , & Stark, N. M. (1979). Elemental composition of a natural mineral lick in Amazonia. Biotropica, 11, 311–313. [Google Scholar]
- Espinoza Villar, J. C. , Ronchail, J. , Guyot, J. L. , Cochonneau, G. , Naziano, F. , Lavado, W. , de Oliveira, E. , Pombosa, R. , & Vauchel, P. (2009). Spatio‐temporal rainfall variability in the Amazon basin countries (Brazil, Peru, Bolivia, Colombia, and Ecuador). International Journal of Climatology: A Journal of the Royal Meteorological Society, 29, 1574–1594. [Google Scholar]
- Ferrari, S. F. , Veiga, L. M. , & Urbani, B. (2008). Geophagy in New World monkeys (Platyrrhini): Ecological and geographic patterns. Folia Primatologica, 79, 402–415. [DOI] [PubMed] [Google Scholar]
- Freeland, W. J. , & Janzen, D. H. (1974). Strategies in herbivory by mammals: The role of plant secondary compounds. The American Naturalist, 108, 269–289. [Google Scholar]
- Galetti, M. , Keuroghlian, A. , Hanada, L. , & Morato, M. I. (2001). Frugivory and seed dispersal by the lowland tapir (Tapirus terrestris) in southeast Brazil. Biotropica, 33, 723–726. [Google Scholar]
- Gallina‐Tessaro, S. , Pérez‐Solano, L. A. , Reyna‐Hurtado, R. , & Escobedo‐Morales, L. A. (2019). Brocket deer. In Gallina‐Tessaro S. (Ed.), Ecology and conservation of tropical ungulates in Latin America (pp. 395–414). Springer. [Google Scholar]
- Gayot, M. , Henry, O. , Dubost, G. , & Sabatier, D. (2004). Comparative diet of the two forest cervids of the genus Mazama in French Guiana. Journal of Tropical Ecology, 20, 31–43. [Google Scholar]
- Ghanem, S. J. , Ruppert, H. , Kunz, T. H. , & Voigt, C. C. (2013). Frugivorous bats drink nutrient‐and clay‐enriched water in the Amazon rain forest: Support for a dual function of mineral‐lick visits. Journal of Tropical Ecology, 29, 1–10. [Google Scholar]
- Gilardi, J. D. , Duffey, S. S. , Munn, C. A. , & Tell, L. A. (1999). Biochemical functions of geophagy in parrots: Detoxification of dietary toxins and cytoprotective effects. Journal of Chemical Ecology, 25, 897–922. [Google Scholar]
- Griffiths, B. M. , Bowler, M. , Gilmore, M. P. , & Luther, D. (2020). Temporal patterns of visitation of birds and mammals at mineral licks in the Peruvian Amazon. Ecology and Evolution, 10, 14152–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, B. M. , Bowler, M. , Kolowski, J. , Stabach, J. , Benson, E. L. , & Gilmore, M. P. (2022). Revisiting optimal foraging theory (OFT) in a changing Amazon: Implications for conservation and management. Human Ecology, 50, 545–558. 10.1007/s10745-022-00320-w [DOI] [Google Scholar]
- Griffiths, B. M. , Cooper, W. J. , Bowler, M. , Gilmore, M. P. , & Luther, D. (2021). Dissimilarities in species assemblages among Amazonian mineral licks. Biotropica, 53, 1255–1260. [Google Scholar]
- Griffiths, B. M. , Gilmore, M. P. , & Bowler, M. (2020). Predation of a Brazilian porcupine (Coendou prehensilis) by an ocelot (Leopardus pardalis) at a mineral lick in the Peruvian Amazon. Food Webs, 24, e00148. 10.1016/j.fooweb.2020.e00148 [DOI] [Google Scholar]
- Griffiths, B. M. , Griffiths, L. , Jin, Y. , & Gilmore, M. P. (2022). Data from: Drivers of geophagy in red brocket deer (Mazama americana) at Amazonian interior forest mineral licks. Mendeley Data Repository. https://data.mendeley.com/datasets/s64mvk8v7k [DOI] [PMC free article] [PubMed]
- Griffiths, B. M. , Jin, Y. , Griffiths, L. , & Gilmore, M. P. (2022). Physical, landscape, and chemical properties of natural interior forest Amazonian mineral licks. Environmental Geochemistry and Health, 45, 3263–3276. [DOI] [PubMed] [Google Scholar]
- Handeland, K. , Viljugrein, H. , Lierhagen, S. , Opland, M. , Tarpai, A. , & Vikøren, T. (2017). Low copper levels associated with low carcass weight in wild Red Deer (Cervus elaphus) in Norway. Journal of Wildlife Diseases, 53, 176–180. [DOI] [PubMed] [Google Scholar]
- Hellgren, E. C. , & Pitts, W. J. (1997). Sodium economy in white‐tailed deer (Odocoileus virginianus). Physiological Zoology, 70, 547–555. [DOI] [PubMed] [Google Scholar]
- Hintz, H. F. , & Schryver, H. F. (1973). Magnesium, calcium and phosphorus metabolism in ponies fed varying levels of magnesium. Journal of Animal Science, 37, 927–930. [DOI] [PubMed] [Google Scholar]
- Holdø, R. M. , Dudley, J. P. , & McDowell, L. R. (2002). Geophagy in the African elephant in relation to availability of dietary sodium. Journal of Mammalogy, 83, 652–664. [Google Scholar]
- Irion, G. (1984). Clay minerals of Amazonian soils. In Robertson B. A., Hardy E. R., & Sioli H. (Eds.), The Amazon: Limnology and landscape ecology of a mighty Tropical River and its basin (pp. 537–579). Dr W. Junk Publishers. [Google Scholar]
- Izawa, K. (1993). Soil‐eating by Alouatta and Ateles . International Journal of Primatology, 14, 229–242. 10.1007/BF02192633 [DOI] [Google Scholar]
- Jackman, S. (2020). Pscl: Classes and methods for R developed in the political science computational laboratory. United States Studies Centre, University of Sydney, Sydney, New South Wales, Australia . R package version 1.5.5, https://github.com/atahk/pscl/
- Jones, K. R. , Lall, K. R. , & Garcia, G. W. (2018). Gross anatomy of the gastrointestinal tract of a red brocket deer (Mazama americana): A case study. Journal of Advanced Veterinary Research, 8, 26–31. [Google Scholar]
- Jones, R. L. , & Hanson, H. C. (1985). Mineral licks, geophagy, and biogeochemistry of North American ungulates. Iowa State Press. [Google Scholar]
- Julliot, C. , & Sabatier, D. (1993). Diet of the red howler monkey (Alouatta seniculus) in French Guiana. International Journal of Primatology, 14, 527–550. [Google Scholar]
- Kaspari, M. (2020). The seventh macronutrient: How sodium shortfall ramifies through populations, food webs and ecosystems. Ecology Letters, 23(7), 1153–1168. [DOI] [PubMed] [Google Scholar]
- Kauffman, S. , Paredes‐Arce, G. , & Marquina‐Pozo, R. (1998). Suelos de la zona de Iquitos. In Kalliola R. & Flores‐Paitán S. (Eds.), Geoecología y desarollo amazónico: Estudio integrado en la zona de Iquitos, Perú (pp. 139–229). University of Turku Press. [Google Scholar]
- Kays, R. , Arbogast, B. S. , Baker‐Whatton, M. , Beirne, C. , Boone, H. M. , Bowler, M. , Burneo, S. F. , Cove, M. V. , Ding, P. , & Espinosa, S. (2020). An empirical evaluation of camera trap study design: How many, how long and when? Methods in Ecology and Evolution, 11(6), 700–713. [Google Scholar]
- Klaus, G. , Klaus‐Hügi, C. , & Schmid, B. (1998). Geophagy by large mammals at natural licks in the rain forest of the Dzanga National Park, Central African Republic. Journal of Tropical Ecology, 14, 829–839. [Google Scholar]
- Klaus, G. , & Schmidg, B. (1998). Geophagy at natural licks and mammal ecology: A review. Mammalia, 62(4), 482–498. 10.1515/mamm.1998.62.4.482b [DOI] [Google Scholar]
- Kreulen, D. A. (1985). Lick use by large herbivores: A review of benefits and banes of soil consumption. Mammal Review, 15, 107–123. 10.1111/j.1365-2907.1985.tb00391.x [DOI] [Google Scholar]
- Krishnamani, R. , & Mahaney, W. C. (2000). Geophagy among primates: Adaptive significance and ecological consequences. Animal Behaviour, 59, 899–915. [DOI] [PubMed] [Google Scholar]
- Lall, K. R. , Jones, K. R. , & Garcia, G. W. (2018). Nutrition of six selected neo‐tropical mammals in Trinidad and Tobago with the potential for domestication. Veterinary Sciences, 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landete‐Castillejos, T. , Currey, J. D. , Estevez, J. A. , Gaspar‐López, E. , Garcia, A. , & Gallego, L. (2007). Influence of physiological effort of growth and chemical composition on antler bone mechanical properties. Bone, 41, 794–803. [DOI] [PubMed] [Google Scholar]
- Lavelle, M. J. , Phillips, G. E. , Fischer, J. W. , Burke, P. W. , Seward, N. W. , Stahl, R. S. , Nichols, T. A. , Wunder, B. A. , & VerCauteren, K. C. (2014). Mineral licks: Motivational factors for visitation and accompanying disease risk at communal use sites of elk and deer. Environmental Geochemistry and Health, 36, 1049–1061. 10.1007/s10653-014-9600-0 [DOI] [PubMed] [Google Scholar]
- Mahaney, W. C. , Hancock, R. G. V. , Aufreiter, S. , & Huffman, M. A. (1996). Geochemistry and clay mineralogy of termite mound soil and the role of geophagy in chimpanzees of the Mahale Mountains, Tanzania. Primates, 37(2), 121–134. [Google Scholar]
- Mahaney, W. C. , & Krishnamani, R. (2003). Understanding geophagy in animals: Standard procedures for sampling soils. Journal of Chemical Ecology, 29, 1503–1523. [DOI] [PubMed] [Google Scholar]
- Marengo, J. (1998). Climatología de la zona de Iquitos, Perú. In Kalliola R. & Flores‐Paitán S. (Eds.), Geoecologia y desarrollo Amazonico: estudio integrado en la zona de Iquitos (pp. 35–57). Peru University of Turku Press. [Google Scholar]
- Matsubayashi, H. , Lagan, P. , Majalap, N. , Tangah, J. , Sukor, J. R. A. , & Kitayama, K. (2007). Importance of natural licks for the mammals in Bornean inland tropical rain forests. Ecological Research, 22, 742–748. 10.1007/s11284-006-0313-4 [DOI] [Google Scholar]
- Mayor, P. , Bizri, H. R. E. , Morcatty, T. Q. , Moya, K. , Bendayán, N. , Solis, S. , Vasconcelos Neto, C. F. A. , Kirkland, M. , Arevalo, O. , Fang, T. G. , Pérez‐Peña, P. E. , & Bodmer, R. E. (2021). Wild meat trade over the last 45 years in the Peruvian Amazon. Conservation Biology, 36, e13801. 10.1111/cobi.13801 [DOI] [PubMed] [Google Scholar]
- Mayor, P. , Bodmer, R. E. , López‐Béjar, M. , & López‐Plana, C. (2011). Reproductive biology of the wild red brocket deer (Mazama americana) female in the Peruvian Amazon. Animal Reproduction Science, 128, 123–128. 10.1016/j.anireprosci.2011.09.009 [DOI] [PubMed] [Google Scholar]
- McCaughey, S. A. , & Tordoff, M. G. (2002). Magnesium appetite in the rat. Appetite, 38, 29–38. [DOI] [PubMed] [Google Scholar]
- McDowell, L. R. (2003). Minerals in animal and human nutrition. Elsevier. [Google Scholar]
- Mills, A. , & Milewski, A. (2007). Geophagy and nutrient supplementation in the Ngorongoro conservation area, Tanzania, with particular reference to selenium, cobalt and molybdenum. Journal of Zoology, 271, 110–118. [Google Scholar]
- Milton, K. (1998). Physiological ecology of howlers (Alouatta): Energetic and digestive considerations and comparison with the Colobinae. International Journal of Primatology, 19, 513–548. [Google Scholar]
- Moe, S. R. (1993). Mineral content and wildlife use of soil licks in southwestern Nepal. Canadian Journal of Zoology, 71, 933–936. [Google Scholar]
- Molina, E. , León, T. , & Armenteras, D. (2014). Characteristics of natural salt licks located in the Colombian Amazon foothills. Environmental Geochemistry and Health, 36, 117–129. 10.1007/s10653-013-9523-1 [DOI] [PubMed] [Google Scholar]
- Montenegro, O. L. (1998). The behavior of lowland tapir (Tapirus terrestris) at a natural mineral lick in the Peruvian Amazon . Master's Thesis, University of Florida.
- Montenegro, O. L. (2004). Natural licks as keystone resources for wildlife and people in Amazonia . Ph.D. University of Florida.
- Murtaugh, P. A. (2009). Performance of several variable‐selection methods applied to real ecological data. Ecology Letters, 12(10), 1061–1068. 10.1111/j.1461-0248.2009.01361.x [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) . (2007). Nutrient requirements of horses. National Academies Press. [Google Scholar]
- Nichols, T. A. , Spraker, T. R. , Gidlewski, T. , Cummings, B. , Hill, D. , Kong, Q. , Balachandran, A. , VerCauteren, K. C. , & Zabel, M. D. (2016). Dietary magnesium and copper affect survival time and neuroinflammation in chronic wasting disease. Prion, 10, 228–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, J. R. (1991). Supplemental feeding. Texas Parks & Wildlife Department, Fisheries & Wildlife Division. https://tpwd.texas.gov/publications/pwdpubs/media/pwd_bk_w7000_0033.pdf [Google Scholar]
- Powell, L. L. , Powell, T. U. , Powell, G. V. N. , & Brightsmith, D. J. (2009). Parrots take it with a grain of salt: Available sodium content may drive collpa (clay lick) selection in southeastern Peru. Biotropica, 41, 279–282. [Google Scholar]
- Prado, H. M. (2013). Feeding ecology of five Neotropical ungulates: A critical review. Oecologia Australis, 17, 459–473. [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Ram, L. , Schonewille, J. T. , Martens, H. , van't Klooster, A. T. , & Beynen, A. C. (1998). Magnesium absorption by wethers fed potassium bicarbonate in combination with different dietary magnesium concentrations. Journal of Dairy Science, 81, 2485–2492. [DOI] [PubMed] [Google Scholar]
- Razali, N. B. , Shafie, M. S. H. , Jobran, R. A. M. , Karim, N. H. A. , Khamis, S. , Mohd‐Taib, F. S. , Nor, S. M. , Ngadi, E. , Razali, S. H. A. , & Husin, S. M. (2020). Physical factors at salt licks influenced the frequency of wildlife visitation in the Malaysian tropical rainforest. Tropical Zoology, 33(3), 83‐96. [Google Scholar]
- Reyna‐Hurtado, R. , & Chapman, C. A. (Eds.) (2019). Movement ecology of Neotropical forest mammals: focus on social animals. Springer International Publishing. [Google Scholar]
- Robinson, J. G. , & Redford, K. H. (1986). Body size, diet, and population density of Neotropical forest mammals. The American Naturalist, 128, 665–680. [Google Scholar]
- Schipper, J. , Chanson, J. S. , Chiozza, F. , Cox, N. A. , Hoffmann, M. , Katariya, V. , Lamoreux, J. , Rodrigues, A. S. L. , Stuart, S. N. , Temple, H. J. , Baillie, J. , Boitani, L. , Lacher, T. E., Jr. , Mittermeier, R. A. , Smith, A. T. , Absolon, D. , Aguiar, J. M. , Amori, G. , Bakkour, N. , … Young, B. E. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science, 322, 225–230. [DOI] [PubMed] [Google Scholar]
- Setz, E. Z. F. , Enzweiler, J. , Solferini, V. N. , Amêndola, M. P. , & Berton, R. S. (1999). Geophagy in the golden‐faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. Journal of Zoology, 247, 91–103. [Google Scholar]
- Sim, S. F. , Azlan, J. M. , Abdul, N. A. H. M. , Lihan, S. , & Kan, P. L. (2020). Mineral characteristics of tropical salt licks in Sarawak, the northwest of Bornio Island. Journal of Sustainability Science and Management, 15, 53–62. [Google Scholar]
- Stephenson, J. D. , Mills, A. , Eksteen, J. J. , Milewski, A. V. , & Myburgh, J. G. (2011). Geochemistry of mineral licks at Loskop dam nature reserve, Mpumalanga, South Africa. Environmental Geochemistry and Health, 33, 49–53. [DOI] [PubMed] [Google Scholar]
- Stewart, H. D. , Tighe, E. , & Griffiths, B. M. (2022). Patterns of visitation of the Linnaeus's two‐toed sloth (Choloepus didactylus) at Amazonian mineral licks. European Journal of Wildlife Research, 68(2), 1–5.34876892 [Google Scholar]
- Tajchman, K. , Steiner‐Bogdaszewska, Z. , & Żółkiewski, P. (2018). Requirements and role of selected micro and macro elements in nutrition of cervids (Cervidae)‐review. Applied Ecology and Environmental Research, 16, 7669–7686. 10.15666/aeer/1606_76697686 [DOI] [Google Scholar]
- Tobler, M. W. (2015). Camera base version 1.7 . 1.7 ed.: San Diego Zoo Global.
- Tobler, M. W. , Carrillo‐Percastegui, S. E. , Pitman, R. L. , Mares, R. , & Powell, G. (2008). An evaluation of camera traps for inventorying large‐ and medium‐sized terrestrial rainforest mammals. Animal Conservation, 11(3), 169–178. 10.1111/j.1469-1795.2008.00169.x [DOI] [Google Scholar]
- Tobler, M. W. , Carrillo‐Percastegui, S. E. , & Powell, G. (2009). Habitat use, activity patterns and use of mineral licks by five species of ungulate in south‐eastern Peru. Journal of Tropical Ecology, 25, 261–270. [Google Scholar]
- Tordoff, M. G. (2001). Calcium: Taste, intake, and appetite. Physiological Reviews, 81, 1567–1597. [DOI] [PubMed] [Google Scholar]
- Voigt, C. C. , Capps, K. A. , Dechmann, D. K. , Michener, R. H. , & Kunz, T. H. (2008). Nutrition or detoxification: Why bats visit mineral licks of the Amazonian rainforest. PLoS One, 3, e2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks, H. P. (1978). Characteristics of mineral licks and behavior of visiting white‐tailed deer in southern Indiana. The American Midland Naturalist, 100, 384–395. 10.2307/2424838 [DOI] [Google Scholar]
- Zeileis, A. , Kleiber, C. , & Jackman, S. (2008). Regression models for count data in R. Journal of Statistical Software, 27, 1–25. http://www.jstatsoft.org/v27/i08/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
Analyses reported in this article can be reproduced using the data provided by Griffiths, Griffiths, et al. (2022).


