Abstract
Abstract
Objective parameters to quantify psoriatic inflammation are needed for interdisciplinary patient care, as well as preclinical experimental models. This study evaluates neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in psoriasis patients and five murine models of psoriasis-like skin disease based on topical imiquimod application and overexpression of IL-17A under different promotors. We performed a single-center prospective observational study in a German population, investigating psoriasis patients prior to, 4 weeks, and 16 weeks post begin of systemic anti-inflammatory therapy. Psoriasis area and severity index (PASI), blood count, and C-reactive protein (CRP) levels were attained at each timepoint. Additionally, five murine models of psoriasis-like skin disease involving five distinct experimental procedures differing in time of disease-onset and severity were investigated regarding PLR and NLR. Of 43 recruited psoriasis patients, 34 patients were followed up to 16 weeks. The cohort was 69.77% male, showing a median age of 32.0 years (range 19.0–67.0; IQR 26). The median PASI decreased from 16.35 (8.0–50.0; 10.20) to 1.6 (0–10.3; 2.56) after 16 weeks of systemic therapy. Spearman’s correlation showed statistically significant positive correlation for NLR with PASI (rs = 0.27, p = 0.006), however not for PLR. NLR, but not PLR, was significantly associated with PASI in a multiple linear regression analysis including age, sex, psoriasis arthritis, and smoking. In the murine models of psoriasis-like skin disease, both NLR and PLR were significantly increased in the acute-severe models compared to controls (p < 0.001, p = 0.005, and p = 0.02, respectively), demonstrating gradually less increased values from severe-acute to mild-late-onset psoriatic phenotype. NLR was significantly associated with PASI in psoriatic patients as well as psoriatic phenotype in different murine psoriasis models. Our data warrants investigation of NLR in psoriasis patients and preclinical psoriasis models as an objective biomarker of psoriatic skin inflammation.
Key messages
NLR, but not PLR, showed a statistically significant positive correlation with Psoriasis Area and Severity Index (PASI) in our human psoriasis cohort.
Both NLR and PLR were significantly increased in murine psoriasis models compared to matched controls, with gradually less increased values from severe-acute to mild-late-onset psoriatic phenotype.
NLR may represent an easily available, cheap, and objective parameter to monitor psoriatic inflammation in both clinical patient routine, as well as preclinical experimental murine models.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00109-023-02406-4.
Keywords: Psoriasis, Psoriasis-comorbidities, Interleukin-17A, Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio
Introduction
Psoriasis is a chronic inflammatory disorder mainly driven by a skewed IL-23/Th17 immune dysregulation [1]. On the skin, psoriasis commonly presents with erythrosquamous plaques, but systemic psoriatic inflammation seems to promote comorbidities such as psoriatic arthritis, cardiovascular disease (CVD), and metabolic changes in the form of elevated blood sugars, cholesterol, and triglycerides [1]. This necessitates interdisciplinary treatment of psoriasis patients, including specialists of dermatology as well as internal medicine, to enable prevention and treatment of associated comorbidities.
To quantify dermatological inflammation in psoriasis, the psoriasis area and severity index (PASI), in which the observer quantifies erythema, scaling, thickness, and expansion of the individual plaques, is widely used among dermatologists [2]. As this is based on subjective assessments, there is the risk of inter-observer-variability. Especially in the setting of clinical trials investigating response to novel therapies, this can be a severe confounder. Additionally, evaluating PASI can be challenging for clinicians lacking daily confrontation with psoriasis patients.
Tools to measure inflammation in psoriasis systemically are up to now not standardized. There is a need for simple, easily accessible, and objective tools to measure systemic psoriatic inflammation for the growing interdisciplinary team of physicians treating psoriasis patients (i.e. general practitioners, cardiologists). As cardiovascular comorbidity in fact forms the life-limiting aspect of psoriasis [3, 4], this holds especially true for interdisciplinary dermatological-cardiological patient care. Furthermore, both in human trials as well as translational research investigating underlying pathomechanisms in psoriasis in preclinical models, objective parameters for quantification of psoriatic inflammation are needed.
Recently, there have been increasing reports on the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as novel markers for systemic inflammation. Thought to reflect the balance between acute or chronic inflammation (via the absolute count of neutrophils or platelets) and adaptive immunity (via the lymphocyte count), they have been investigated as a potential prognostic marker in diseases ranging from infectious, oncologic, cardiovascular, to autoimmune diseases — including psoriasis [5–8]. Interestingly, an increased NLR was described to be associated with CVD outcome [9]. Whether NLR or PLR correlates with the severity of psoriasis, as well as longevity of psoriatic disease in patients, remains unclear. Additionally, to our knowledge, reports on NLR and PLR within murine models of psoriasis are lacking. We therefore prospectively investigated a single-center cohort of psoriasis patients undergoing systemic psoriasis-specific treatment. Furthermore, we examined multiple murine models of psoriasis-like skin disease (based on two underlying pathomechanisms — either imiquimod treatment or IL-17A overexpression) differing in time of disease-onset and severity regarding their PLR and NLR values: (i) the acute, severe psoriasis-like dermatitis model by applying the topical toll-like receptor 7/8 agonist imiquimod (IMQ) for 6 consecutive days [10]; (ii) a prolonged acute, severe psoriasis model by extending the IMQ application to 10 days [11]; (iii) the K14-IL-17Aind/+ mice with keratinocyte-specific IL-17A overexpression leading to an early-onset, chronic and severe psoriatic phenotype [12, 13]; and two models of delayed-onset, chronic psoriatic phenotype via IL-17A overexpression in CD11c+ cells leading to a moderate ((iv) homozygous CD11c-IL-17Aind/ind) to mild ((v) heterozygous CD11c-IL-17Aind/+ mice) psoriasis-like skin disease [14]. These are — as all mouse models of human disease — artificial [15] but nevertheless offer the opportunity to investigate potential translational value of NLR and PLR for disease severity assessment in preclinical studies.
Material and methods
Ethics
This study was conducted according to the Declaration of Helsinki. The study was approved by the ethics committee of Heidelberg (approval no. S-834/2020), and patients provided written and informed consent.
Human samples
Patients with confirmed psoriasis diagnosis by a dermatologist, eligible for systemic psoriatic therapy (PASI ≧ 10, upgrade criteria, e.g., scalp or nail involvement, or Dermatology Life Quality Index (DLQI) ≧ 10) and naïve to or post a 12-week washout of previous systemic psoriatic therapy, were included. Exclusion criteria were the presence of additional autoimmune, infectious, or malignant diseases; age < 18 years, pregnancy; and use of anti-platelet or anti-coagulant drugs. Overall, 43 subjects were enrolled in our study. Patient information and blood samples were taken prior to, 4 weeks. and 16 weeks post begin of systemic therapy, leading to three study visits overall within the in- and outpatient clinic in the Department for Dermatology, University of Heidelberg, Germany. The responsiveness to biological therapy was assessed by psoriasis area and severity index (PASI). Systemic therapy (ixekizumab, secukinumab, brodalumab, guselkumab, tildrakizumab, risankizumab, adalimumab, ustekinumab, dimethyl fumarate) was chosen by the treating physician based on intraindividual patient characteristics according to European and national guide-line recommendations and administered accordingly [16, 17].
Mice
Experiments were approved by the Animal Care and Use Committee from Rhineland-Palatine (Landesuntersuchungsamt Rheinland-Pfalz (LUA), Landau, approval no. G17-1–076) and Baden-Wuerttemberg (Regierungspräsidium Karlsruhe, Karlsruhe, approval no. G249/20). Guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes were followed. Mice of both sexes, as well as matched controls, were used. In short, the topical toll-like receptor 7/8 agonist imiquimod (IMQ) was applied to the shaved back and ears for 6 or 10 consecutive days before analysis of the peripheral blood [10]. The previously described transgenic psoriasis model K14-IL-17Aind/+ [12, 13], CD11c-IL-17Aind/ind, and CD11c-IL-17Aind/+ [14] were analyzed at the age of 7 to 26 weeks, depending on onset of phenotype [18].
Clinical biochemistry, blood parameters, and SAP-ELISA
Human blood count was retracted from electronic patient records. Regular CRP (mg/l) is censored below 2 mg/l. For murine studies, blood was measured in an automated hematometer (Element HT5 (Scil animal care company, Viernheim, Germany) and VetScan HM5 (Abaxis, Union City, CA, USA)). NLR and PLR were calculated by neutrophil and platelet count divided by the lymphocyte count, respectively. Serum amyloid P (SAP) was measured using the Mouse Pentraxin 2/SAP Quantikine® ELISA (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (version 9; GraphPad Software Inc., San Diego, CA, USA). Data are displayed as mean ± standard error of the mean (S.E.M). Data were analyzed for normal distribution (D’Agostino & Pearson test). As this test was significant, nonparametric Kruskal–Wallis test with Dunn’s multiple comparison or comparison of selected columns was used. For matched comparisons, Friedman test or mixed effects analysis with Geisser-Greenhouse correction was used, if single values were missing. We used nonparametric Spearman correlations or simple linear regression where appropriate. A multiple linear regression was performed for both NLR and PLR as dependent variables including age, sex, psoriasis arthritis, and smoking for the primary timepoint of inclusion. p values of < 0.05 were considered significant and marked by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Patient cohort characteristics
Of the 43 patients included in the study, 69.78% were male and showed a median age of 32.0 years (range 19.0–67.0; IQR 26) (see Table 1). Median disease duration at the time of study inclusion was 8.0 years (0.5–48; 17.75). Patients displayed a median body mass index (BMI) of 30.45 (18.37–53.46; 9.31), 25.58% suffered from additional psoriasis arthritis (PsA), 11.63% from arterial hypertension, 4.65% from diabetes mellitus type II, and 55.81% were smokers. Of the 43 patients, 34 patients were followed up prospectively over the time course of up to 16 weeks post systemic therapy start. The median PASI decreased from 16.35 (8.0–50.0; 10.20) to 4.2 (0.0–14.4; 3.38) to 1.6 (0–10.3; 2.58) after 4 and 16 weeks of systemic therapy, respectively. Targets of systemic therapy were IL-17A in 39.53%, IL-23 in 25.58%, TNF-α (adalimumab) in 9.30%, and anti-IL-12/IL-23 (ustekinumab) or dimethyl fumarate (DMF) in 2.33%.
Table 1.
Overview over patient characteristics (total cohort n = 43)
| n (%) or median (range; IQR) | |
|---|---|
| Male sex | 30 (69.77) |
| Age [years] | 32.0 (19.0–67.0; 26) |
| BMI | 30.45 (18.37–53.46; 9.31) |
| Disease duration [years] | 8.0 (0.5–48; 17.75) |
| Psoriasis arthritis | 11 (25.58) |
| Art. hypertension | 5 (11.63) |
| Diabetes mellitus type II | 2 (4.65) |
| Smoking | 24 (55.81) |
| PASI pre-therapy | 16.35 (8.0–50.0; 10.20) |
| PASI 4 weeks post therapy | 4.2 (0.0–14.4; 3.38) |
| PASI 16 weeks post therapy | 1.6 (0–10.3; 2.58) |
|
Anti-IL-17 therapy Ixekizumab Secukinumab Brodalumab |
17 (39.53) 14 (32.56) 2 (4.65) 1 (2.33) |
|
Anti-IL-23 therapy Guselkumab Tildrakizumab Risankizumab |
11 (25.58) 4 (9.30) 4 (9.30) 3 (6.98) |
|
Anti-TNF-α therapy Adalimumab |
4 (9.30) |
|
Anti-IL-12/23 therapy Ustekinumab |
1 (2.33) |
| Dimethyl fumarate therapy | 1 (2.33) |
BMI body mass index, PASI psoriasis area and severity index, IQR interquartile range
Analysis of systemic inflammation markers in psoriasis patients
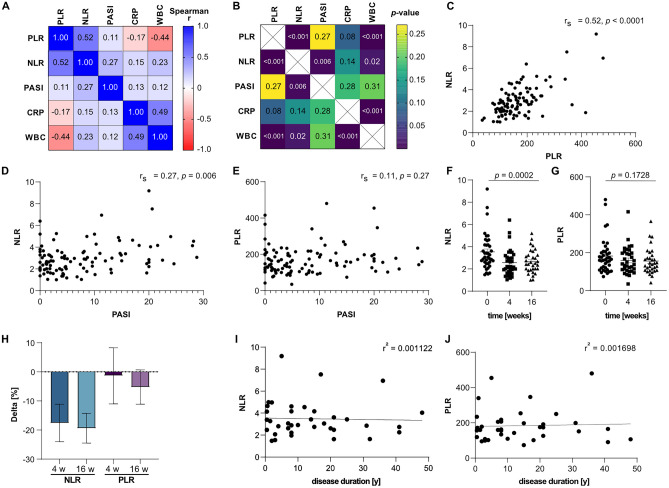
Spearman’s correlation showed statistically significant positive correlation for NLR and PLR (rs = 0.52, p < 0.0001; Fig. 1A, B, C), as well as for WBC and CRP (rs = 0.49, p < 0.001) in psoriasis patients. Values for PASI correlated with NLR (rs = 0.27, p = 0.006; Fig. 1D), however not with PLR (Fig. 1E), CRP, and WBC (Fig. 1A, B). Both NLR and PLR correlated with WBC, however in a positive (rs = 0.23) and negative way (rs = − 0.44), respectively. Multiple linear regression analyses including age, sex, psoriasis arthritis, and smoking for the pre-therapy timepoint showed statistically significant associations with PASI for NLR, but not for PLR (Suppl. Table 1). Analysis of absolute count of neutrophils, platelets, and lymphocytes showed no association with PASI in correlation analysis (Suppl. Figure 1A-C). Grouping of values for the therapeutic time points showed a significant decrease of NLR (Fig. 1F), but not for PLR (Fig. 1G) over time of systemic therapy in a repeated measure matched analysis on an intraindividual level. In addition, this was reflected by the negative intraindividual delta for NLR- and (to a lesser extent) for PLR-values over time of anti-psoriatic therapy (Fig. 1H). There was no association apparent for NLR (Fig. 1I) or PLR (Fig. 1J) with the duration of disease at the baseline timepoint of maximum PASI before starting systemic therapy.
Fig. 1.
NLR and PLR in human psoriasis cohort. A Correlation matrix of PLR, NLR, PASI, CRP, and WBC with respective Spearman’s rho (rs). B p values of respective correlations plotted in A. C Correlation of NLR and PLR, rs = 0.52, p < 0.0001. Correlation of NLR (rs = 0.27, p = 0.006) D and PLR (rs = 0.11, p = 0.27) (E) with PASI. Grouping of NLR (F) and PLR (G) by time points of study. H Delta of NLR and PLR in % compared to baseline for 4 weeks and 16 weeks of therapy, respectively. Simple regression of NLR (r2 = 0.001122) (I) and PLR (r2 = 0.001698) (J) over disease duration. Spearman’s correlation in A–E. Mixed effect analysis for matched pairs in F and G. Simple linear regression in I–J
Analysis of systemic inflammation markers in murine models of psoriasis-like skin disease
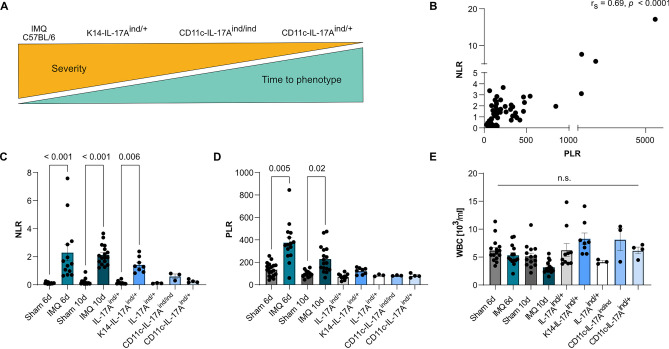
We analyzed five murine models of psoriasis-like dermatitis based on topical IMQ application and IL-17A overexpression under different promotors, described previously (Fig. 2A) [10, 11, 13, 14]. To corroborate systemic inflammation, serum amyloid P (SAP) was measured in the respective mouse models (Suppl. Figure 2A). Increased values were seen for all investigated models in comparison to matched control animals. In addition, levels of SAP gradually decreased with severity in the transgenic models based on IL-17A overexpression, as well as with chronicity for the topical imiquimod model. NLR and PLR correlated with each other significantly (Fig. 2B, rs = 0.69, p < 0.0001). Both NLR and PLR were significantly increased in the IMQ-induced psoriasis models compared to sham-treated controls, with higher values in the acute 6-day versus 10-day application model (Fig. 2C, D). Regarding the chronic models of psoriasis-like skin disease, elevated NLR and PLR values were observed in K14-IL-17Aind/+, with a decrease of the difference to control mice from K14-IL-17Aind/+, to CD11c-IL-17Aind/ind, and to CD11c-IL-17Aind/+ (Fig. 2C, D). No difference in WBC (Fig. 2E) was seen across models and conditions. Additionally, absolute values for neutrophils, platelets, and lymphocytes did not follow the same trend as observed for NLR and PLR (Suppl. Figure 2B-D).
Fig. 2.
NLR and PLR in different murine psoriasis models. A Schematic overview over investigated murine psoriasis models, described previously. B Correlation of NLR with PLR in murine psoriasis models (rs = 0.06862, p < 0.0001). C NLR, D PLR, and E WBC in different murine psoriatic dermatitis models. Spearman’s correlation in B, Kruskal–Wallis test corrected with Dunn’s test for multiple comparisons (C–E). n = 3–17 per group
Discussion
Increasing interdisciplinary treatment of psoriasis patients due to better understanding of comorbidities and wide-spread use of systemic psoriasis therapies necessitate objective parameters to monitor psoriatic inflammation. Systemic markers for psoriatic inflammation such as inflammatory cytokines (i.e., IL-17A, IL-6, TNF-α) are not part of clinical routine and time- and cost-consuming [19].
We therefore analyzed psoriasis patients before starting established systemic therapy (targeting IL-17A, IL-23, TNF-α, IL-12/23, and DMF) and for a follow-up up to 16 weeks in a single-center prospective study. In parallel, we investigated five murine models of psoriasis-like skin disease, based on topical IMQ application and overexpression of IL-17A under different promotors. While the examined mouse models do reflect hallmarks of psoriasis (each to different extents), as artificial systems with manipulated cytokines/immune cells, they do not recapitulate the human situation congruently. The discussion on how to choose the most valid mouse model and their translational value regarding human disease is continuously ongoing [15]. With the plethora of murine psoriasis models (see Gudjonsson et al. [20] and Gangwar et al. [21]), it is of highest importance to obtain reliable, objective proxies for psoriatic inflammation in preclinical models. Particularly, if collected murine and human data goes hand in hand, this opens avenues for valuable translational insights.
Interestingly, the well-established markers for systemic inflammation CRP and WBC showed no intraindividual correlation with PASI during a 4-month time course of systemic therapy in psoriasis patients (Fig. 1A, B). This is in contrast to the conclusion of a review on the topic of CRP and psoriasis, which inferred CRP to be interchangeable with PASI as a measure of disease severity in patients without psoriatic arthritis [22], and stresses the necessity for further inflammatory markers. However, as we investigated intraindividual changes of inflammatory markers, no comparison to healthy controls or patients with mild psoriasis (PASI < 5) can be drawn. The absent correlation of WBC with PASI seen in the patient cohort was corroborated across all mouse models (Fig. 2E). These findings in patients and murine psoriasis models suggest absolute WBC to be unfit to reflect psoriatic inflammation.
The role of neutrophils, partly driven by the neutrophil-recruiting cytokine IL-17A, is well established as a key driver in psoriatic disease [23]. Their function in the context of neutrophil extracellular traps and reactive oxygen species formation in the psoriatic plaque of the skin has been further investigated in systemic psoriatic inflammation, i.e., cardiovascular inflammation [11, 13, 18]. Additionally, the inflammatory function of platelets has recently been gaining attention in psoriatic research [24, 25], beyond mere reactive thrombocytosis [26]. However, as intraindividual cell counts may vary, the ratio of neutrophils/platelets to lymphocytes may provide an intra-individually normalized value enabling better inter-patient comparison and provide context to acute-vs.-chronic inflammation. A meta-analysis from 2019 by Paliogiannis et al. [6] comes to the conclusion that both NLR and PLR are increased in psoriasis compared to healthy controls. Reports on NLR and PLR as follow-up markers during systemic therapy for psoriasis vulgaris are to date mostly limited to TNF-α inhibitors (etanercept, infliximab, adalimumab [27–29]) or the IL-12/23 inhibitor ustekinumab [27–29], with little data on anti-IL-17A therapy (brodalumab [30], secukinumab, ixekizumab [29]) and IL-23 inhibitors [31]. Therefore, this study extends first data on these values, especially regarding IL-23 inhibitors (guselkumab, tildrakizumab, risankizumab). Interestingly, both values have also been investigated in the context of cardiovascular disease. Elevated NLR demonstrated a predictive value for hypertension incidence [32, 33]. Prognostically, a recent multicenter study found high NLR and PLR values to be independently associated with cardiac death in patients with acute decompensated heart failure [34]. Additionally, a multicenter study found increased baseline NLR to represent an independent predictive biomarker for incident major adverse cardiovascular events in five randomized controlled trials investigating cardiovascular anti-inflammatory drugs (canakinumab, rosuvastatin, bococizumab, or methotrexate) [35].
In patients, NLR, but not PLR, decreased in parallel with PASI under systemic psoriasis treatment in a statistically significant matter (Fig. 1D–H), corroborating previous reports showing increased NLR in psoriasis patients [27–30]. Interestingly, absolute count of neither peripheral lymphocytes, neutrophils, nor platelets correlated with PASI in our human cohort (Suppl. Figure 1B, C), highlighting the potential value of NLR. The findings within the patient cohort were confirmed in the analyzed murine psoriasis-like skin disease models, showing statistically significant elevated ratios in both NLR and PLR (Fig. 2C, D). As reflected in systemic SAP levels (Suppl. Figure 2A), a gradual decrease of NLR and PLR from the severe-acute to mild-late-onset models was apparent. The effect was more pronounced for NLR than PLR, mirroring and underlining the findings in the human cohort. While some studies demonstrated elevated PLR in psoriasis patients [6, 27, 36] and the evidence of a role of platelets in inflammatory diseases, specifically psoriasis, is increasing [24], our findings underscore the conclusion of other reports [37, 38], indicating PLR to not be a marker to well reflect severity of psoriatic inflammation. Further studies are needed to definitively clarify the role of PLR in psoriasis.
Of note, just recently, NLRs (and the cytokine IL-6) were found to be predictive biomarkers in psoriasis patients for treatment response under TNFalpha inhibitors [31]. Thus, this biomarker might serve to individualize and personalize regulation of biologic treatment in autoimmune disease. The next step will be to analyze these markers also in regard to associated cardiovascular disease in psoriasis patients in a long-term approach.
Additionally, to our knowledge, we are the first to investigate NLR and PLR in the context of psoriatic disease duration. With a large range of 0.5 to 50 years of history of psoriasis, we found no correlation for NLR or PLR with disease duration (Fig. 1H, I). In contrast, the murine data may indicate NLR and PLR to be higher in early-onset compared to late-onset disease. However, no clear differentiation between acuteness and disease duration can be drawn due to the experimental setup of the murine models [10, 11, 13, 14]. Taken together, this study suggests both NLR and PLR to not correlate with psoriatic disease duration.
Most likely, NLR and PLR are not disease specific, such as other inflammatory markers as CRP. However, it is noteworthy that only NLR correlated positively with psoriatic dermal inflammation quantified via PASI. Importantly, these easily attained hematological parameters showed correlation with psoriatic disease severity and disease onset in murine models, rendering these values applicable to study psoriatic phenotype and therapeutic responses in preclinical models. Recently, Dey et al. demonstrated a significant correlation of NLR with PASI under anti-TNF-α (adalimumab, etanercept), anti-IL-12/23 (ustekinumab), or anti-IL-17A (secukinumab, ixekizumab) therapy in 316 psoriasis patients [29]. This study underscores the relevance of NLR, as the median PASI was lower in this study than in our study cohort (baseline 6.0 (3.1–11.7) versus 16.35 (8.0–50.0), respectively), and the authors further reported an association of NLR with non-calcified coronary artery burden by coronary computed tomography angiography within psoriasis patients. The latter point might hint towards NLR not only reflecting dermal inflammation, but potentially acting as a marker for cardiovascular disease in psoriasis patients. This warrants further investigation into this easily attainable marker in local and systemic psoriatic inflammation.
Conclusion
In conclusion, we demonstrate NLR — but not PLR — to correlate with PASI intra-individually in psoriasis patients. This correlation was corroborated in five murine models of psoriasis-like skin disease based on topical imiquimod application and IL-17A overexpression under different promotors, in which the elevation of NLR — but not PLR — appeared to reflect severity and early-onset of psoriatic inflammation. Collectively, our findings highlight NLR as an easily accessible, cheap and convenient proxy for psoriatic inflammation in patients and preclinical psoriasis models.
Limitations
The reported data cannot address a causal understanding for the associations seen for NLR, PLR, and PASI. We have not investigated additional markers for organ-specific inflammation, i.e., cardiovascular inflammation. Additionally, our patient cohort was of small sample size (preventing comparisons of effects of individual therapeutic target classes and leading to underpowering multivariate analyses), predominantly male, and the biologic applied based on the physician’s choice. Therefore, results in the human cohort based on the German population must be seen as hypothesis-generating. Nevertheless, especially NLR might be a good clinical marker for classification of disease severity in psoriasis patients and related murine models of psoriasis-like skin disease. As murine models of human disease have to be considered cautiously, further corroboration of our data in additional murine psoriasis models independent of topical treatment or IL-17A overexpression is needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Kornelia Junge, Heike Böhli, Olivia Bochnig, Therezia Bokor-Billmann, and Katharina Kälber for their support in patient coordination. We thank Katharina Perius for excellent technical assistance and scientific input. We thank Björn Clausen for advice with the CD11c-IL17Aind/+ mice.
Abbreviations
- BMI
Body mass index
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- DLQI
Dermatology Life Quality Index
- DMF
Dimethyl fumarate
- IL-12
Interleukin-12
- IL-17A
Interleukin-17A
- IL-23
Interleukin-23
- IMQ
Imiquimod
- IQR
Interquartile range
- LYM
Lymphocytes
- NEU
Neutrophils
- NLR
Neutrophil-to-lymphocyte ratio
- PASI
Psoriasis area and severity index
- PLR
Platelet-to-lymphocyte ratio
- PLT
Platelets
- PsA
Psoriasis arthritis
- SAP
Serum Amyloid P
- SD
Standard deviation
- S.E.M.
Standard error of the mean
- T.N.F-
Tumor necrosis factor alpha
- WBC
White blood cells
Author contribution
All authors contributed to the study conception and design. Material preparation by Katharina S. Kommoss, Tabea Bieler, Julia Ringen, Silvia Mihalceanu, Johannes Wild, Ari Waisman, Alexander Enk, Knut Schäkel, Mathias Heikenwälder, and Susanne Karbach. Data collection by Katharina S. Kommoss, Tabea Bieler, Julia Ringen, Annika Lehmann, Anna Brand, Berenice Fischer, Daniela Kramer, and Johannes Wild. Data analysis by Katharina S. Kommoss, Tabea Bieler, Julia Ringen, Annika Lehmann, Anna Brand, Lukas Hobohm, Karsten Keller, and Susanne Karbach. The first draft of the manuscript was written by Katharina S. Kommoss and Susanne Karbach, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. KSK is funded by the Physician-Scientist Programme of Heidelberg University, Faculty of Medicine. TB received funding from the Federal Ministry of Education and Research (BMBF) and the Ministry of Science Baden-Württemberg within the framework of the Excellence Strategy of the Federal and State Governments of Germany. SK and JW are supported by the Boehringer Ingelheim Foundation “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications.” KSK, AE, MH, DK, AW, and SK are supported by TRR156/2–246807620 (“The Skin as Sensor and Effector Organ Orchestrating Local and Systemic Immunity,” DFG). DK and AW are supported by TRR355/1 (project number 490846870). DK receives support from the Peter-Hans Hofschneider foundation. SK is funded by the Deutsche Herzstiftung and by the DZHK Excellence Programme (“Interleukin-17A mediated inflammation in myocardial infarction in a mouse model of psoriasis-like skin disease”). M.H. was supported by SFBTR1335 project ID 360372040, SFB 1479 (Project ID: 441891347), the Rainer Hoenig Stiftung, Research Foundation Flanders (FWO) under grant 30826052 (EOS Convention MODEL-IDI), German-Israeli Cooperation in Cancer Research (DKFZ-MOST) and the Helmholtz-Gemeinschaft; M.H. was also supported by seed funding from HI-TRON and by Wilhelm Sander funding (2020.170.1).
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the ethical permits under which the data was generated, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was conducted according to the Declaration of Helsinki. The study was approved by the ethics committee of Heidelberg (approval no. S-834/2020), and patients provided written and informed consent. Animal experiments were approved by the Animal Care and Use Committee from Rhineland-Palatine (Landesuntersuchungsamt Rheinland-Pfalz (LUA), Koblenz, approval no. G17-1–076) and Baden-Wuerttemberg (Regierungspräsidium Karlsruhe, Karlsruhe, approval no. G249/20). Guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes were followed.
Consent to participate
Informed consent was obtained from all individual participants included in the study (see ethics approval).
Consent for publication
As no individual person’s data is published (details, images or videos), this does not apply to this study.
Conflict of interests
SK declares having received consultancy honoraria from Almiral and lecture honoraria from Janssen-Cilag GmbH. KS discloses that he was an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen-Cilag GmbH, LEO Pharma, Novartis, Pfizer and UCB. AE discloses speakers’ honoraria/travel grants for Janssen-Cilag GmbH, Abbvie and Eli Lilly. The authors declare that these conflicts are not associated with this work. The other authors have no conflicts of interest to declare.
Footnotes
Mathias Heikenwälder and Susanne Karbach joint senior authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rendon A, Schakel, K (2019) Psoriasis pathogenesis and treatment. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- 2.Fredriksson,T, Pettersson UJD (1978) Severe psoriasis–oral therapy with a new retinoid 157:238–244 [DOI] [PubMed]
- 3.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM (2010) Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol 163:586–592 [DOI] [PMC free article] [PubMed]
- 5.Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. doi: 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paliogiannis P, Satta R, Deligia G, Farina G, Bassu S, Mangoni AA, Carru C, Zinellu A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med. 2019;19:37–45. doi: 10.1007/s10238-018-0538-x. [DOI] [PubMed] [Google Scholar]
- 7.Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology. 2019;70:802–818. doi: 10.1177/0003319719845186. [DOI] [PubMed] [Google Scholar]
- 8.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angkananard T, Anothaisintawee T, Ingsathit A, McEvoy M, Silapat K, Attia J, Sritara P, Thakkinstian A. Mediation effect of neutrophil lymphocyte ratio on cardiometabolic risk factors and cardiovascular events. Sci Rep. 2019;9:2618. doi: 10.1038/s41598-019-39004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 11.Wild J, Ringen J, Bieler T, Knopp T, Lagrange J, Molitor M, Sies K, Kropp A, Keller K, Daiber A, Munzel T, Rauh M, Waisman A, Wenzel P, Titze J Karbach S (2022) Epicutaneous application of imiquimod to model psoriasis-like skin disease induces water-saving aestivation motifs and vascular inflammation. J Invest Dermatol 142:3117–3120.e2 [DOI] [PubMed]
- 12.Croxford AL, Karbach S, Kurschus FC, Wortge S, Nikolaev A, Yogev N, Klebow S, Schuler R, Reissig S, Piotrowski C, Brylla E, Bechmann I, Scheller J, Rose-John S, Thomas Wunderlich F, Munzel T, von Stebut E, Waisman A (2014) IL-6 regulates neutrophil microabscess formation in IL-17A-driven psoriasiform lesions. J Invest Dermatol 134:728–735 [DOI] [PubMed]
- 13.Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, Koukes L, Yogev N, Nikolaev A, Reissig S, Ullmann A, Knorr M, Waldner M, Neurath MF, Li H, Wu Z, Brochhausen C, Scheller J, Rose-John S, Piotrowski C, Bechmann I, Radsak M, Wild P, Daiber A, von Stebut E, Wenzel P, Waisman A, Munzel T. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. 2014;34:2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- 14.Wohn C, Brand A, van Ettinger K, Brouwers-Haspels I, Waisman A, Laman JD, Clausen BE. Gradual development of psoriatic skin lesions by constitutive low-level expression of IL-17A. Cell Immunol. 2016;308:57–65. doi: 10.1016/j.cellimm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol. 2010;6:704–714. doi: 10.1038/nrrheum.2010.157. [DOI] [PubMed] [Google Scholar]
- 16.Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, De Jong E, Garcia-Doval I, Gisondi P, Kaur-Knudsen D, Mahil S, Malkonen T, Maul JT, Mburu S, Mrowietz U, Reich K, Remenyik E, Ronholt KM, Sator PG, Schmitt-Egenolf M, Sikora M, Stromer K, Sundnes O, Trigos D, Van Der Kraaij G, Yawalkar N, Dressler C (2020) EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 34:2461–2498 [DOI] [PubMed]
- 17.Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, De Jong E, Garcia-Doval I, Gisondi P, Kaur-Knudsen D, Mahil S, Malkonen T, Maul JT, Mburu S, Mrowietz U, Reich K, Remenyik E, Ronholt KM, Sator PG, Schmitt-Egenolf M, Sikora M, Stromer K, Sundnes O, Trigos D, Van Der Kraaij G, Yawalkar N, Dressler C (2021) EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol 35:281–317 [DOI] [PubMed]
- 18.Schuler R, Brand A, Klebow S, Wild J, Veras FP, Ullmann E, Roohani S, Kolbinger F, Kossmann S, Wohn C, Daiber A, Munzel T, Wenzel P, Waisman A, Clausen BE, Karbach S. Antagonization of IL-17A attenuates skin inflammation and vascular dysfunction in mouse models of psoriasis. J Invest Dermatol. 2019;139:638–647. doi: 10.1016/j.jid.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 20.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 21.Gangwar RS, Gudjonsson JE, Ward NL. Mouse models of psoriasis: a comprehensive review. J Invest Dermatol. 2022;142:884–897. doi: 10.1016/j.jid.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beygi S, Lajevardi V, Abedini R. C-reactive protein in psoriasis: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:700–711. doi: 10.1111/jdv.12257. [DOI] [PubMed] [Google Scholar]
- 23.Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in psoriasis Front Immunol. 2019;10:2376. doi: 10.3389/fimmu.2019.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herster F, Karbach S, Chatterjee M, Weber ANR. Platelets: underestimated regulators of autoinflammation in psoriasis. J Invest Dermatol. 2021;141:1395–1403. doi: 10.1016/j.jid.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Herster F, Bittner Z, Codrea MC, Archer NK, Heister M, Loffler MW, Heumos S, Wegner J, Businger R, Schindler M, Stegner D, Schakel K, Grabbe S, Ghoreschi K, Miller LS, Weber ANR. Platelets aggregate with neutrophils and promote skin pathology in psoriasis. Front Immunol. 2019;10:1867. doi: 10.3389/fimmu.2019.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer AI. Thrombocytosis. N Engl J Med. 2004;350:1211–1219. doi: 10.1056/NEJMra035363. [DOI] [PubMed] [Google Scholar]
- 27.Asahina A, Kubo N, Umezawa Y, Honda H, Yanaba K, Nakagawa H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: response to therapy with biologics. J Dermatol. 2017;44:1112–1121. doi: 10.1111/1346-8138.13875. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann JHO, Knoop C, Schakel K, Enk AH, Hadaschik EN (2021) Evaluation of psoriasis area and severity index as a proxy for bio-markers of systemic disease under treatment with tumour necrosis factor-alpha and interleukin 12/23 antagonists in patients with psoriasis: a retrospective cohort study of 186 treatment cycles. Acta Derm Venereol 101:adv00462 [DOI] [PMC free article] [PubMed]
- 29.Dey AK, Teague HL, Adamstein NH, Rodante JA, Playford MP, Chen MY, Bluemke DA, Gelfand JM, Ridker PM, Mehta NN. Association of neutrophil-to-lymphocyte ratio with non-calcified coronary artery burden in psoriasis: findings from an observational cohort study. J Cardiovasc Comput Tomogr. 2021;15:372–379. doi: 10.1016/j.jcct.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Wiles C, Martinez LR, Han G. Neutrophil-to-lymphocyte ratio decreases after treatment of psoriasis with therapeutic antibodies. J Eur Acad Dermatol Venereol. 2017;31:e491–e492. doi: 10.1111/jdv.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen CSB, Kvist-Hansen A, Siewertsen M, Enevold C, Hansen PR, Kaur-Knudsen D, Zachariae C, Nielsen CH, Loft N, Skov L (2023) Blood cell biomarkers of inflammation and cytokine levels as predictors response to biologics in patients with psoriasis. Int J Mol Sci 24:6111 [DOI] [PMC free article] [PubMed]
- 32.Jhuang YH, Kao TW, Peng TC, Chen WL, Li YW, Chang PK, Wu LW. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens Res. 2019;42:1209–1214. doi: 10.1038/s41440-019-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, Wang C, Xia Y, Guo X, Li C, Bao X, Su Q, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. 2015;28:1339–1346. doi: 10.1093/ajh/hpv034. [DOI] [PubMed] [Google Scholar]
- 34.Tamaki S, Nagai Y, Shutta R, Masuda D, Yamashita S, Seo M, Yamada T, Nakagawa A, Yasumura Y, Nakagawa Y, Yano M, Hayashi T, Hikoso S, Nakatani D, Sotomi Y, Sakata Y, Investigators OC-HF. Combination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as a novel predictor of cardiac death in patients with acute decompensated heart failure with preserved left ventricular ejection fraction: a multicenter study. J Am Heart Assoc. 2023;12:e026326. doi: 10.1161/JAHA.122.026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, Tabas IA, Mehta NN, Ridker PM. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42:896–903. doi: 10.1093/eurheartj/ehaa1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann JHO, Enk AH. Evaluation of psoriasis area and severity index thresholds as proxies for systemic inflammation on an individual patient level. Dermatology. 2022;238:609–614. doi: 10.1159/000520163. [DOI] [PubMed] [Google Scholar]
- 37.Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM, Lee MG. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43:305–310. doi: 10.1111/1346-8138.13061. [DOI] [PubMed] [Google Scholar]
- 38.Yurtdas M, Yaylali YT, Kaya Y, Ozdemir M, Ozkan I, Aladag N. Neutrophil-to-lymphocyte ratio may predict subclinical atherosclerosis in patients with psoriasis. Echocardiography. 2014;31:1095–1104. doi: 10.1111/echo.12511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the ethical permits under which the data was generated, but are available from the corresponding author on reasonable request.