Abstract
Objectives
To evaluate the efficacy of eye exercises in preventing and controlling myopia.
Methods
We searched studies on eye exercises from nine Chinese and English databases from their inception to December 15, 2022. Using random-effect models and sensitivity/subgroup analyses, we estimated the effects of eye exercises compared to control on changes in three measures: visual acuity, refractive error (both quantified using standardized mean differences, SMDs), and protective/mitigating effects (assessed through risk ratios, RRs).
Results
Eleven studies were included in the meta-analysis, with 921 participants. Nine studies had some concerns of bias in at least two domains, and two studies had a high risk of bias in two domains. Seven studies used visual acuity to measure myopia; visual acuity declined after eye-exercise interventions (SMD = −0.67, 95% CI −1.28 to −0.07, Z = 2.17, P = 0.03) and the effect was not better than control (SMD = −0.50, 95% CI −1.16 to 0.16, Z = 1.49, P = 0.14). Two studies used refractive error to measure myopia; the effect of eye-exercise interventions did not differ from control (SMD = −1.74, 95% CI −6.27 to 2.79, Z = 0.75, P = 0.45). Seven studies reported on protective/mitigating effects; eye exercises exhibited a greater protective/mitigating effect than control (RR = 0.40, 95% CI 0.23–0.71, Z = 3.13, P < 0.01).
Conclusions
Overall, the results suggest that eye exercises have limited to no efficacy in preventing or controlling myopia progression. Until robust evidence supports their efficacy, available evidence suggests retiring the eye-exercise policy.
Subject terms: Epidemiology, Vision disorders
Abstract
方法: 在九个主要中文和英文数据库里搜索与眼保健操相关的对照实验研究, 日期为从数据库建立起至2022年12月15日, 并使用荟萃分析 (随机效应模型和敏感性分析) 估计眼保健操相对对照组在三个近视指标上的效果, 即视敏度, 屈光误差 (均使用标准化均数差, SMD), 以及保护/缓解效果 (风险比, RR) 。
结果: 共有11项研究纳入了这个荟萃分析, 涉及921名参与者——有九项研究在至少两个方面有偏倚问题, 而有两项研究在两个方面有高偏倚风险。其中七项研究使用视敏度来评估近视: 眼保健操干预后, 视力下降了 (SMD = −0.67, 95% CI −1.28 to −0.07, Z = 2.17, P = 0.03), 且其效果并不优于对照组 (SMD = −0.50, 95% CI −1.16 to 0.16, Z = 1.49, P = 0.14) 。两项研究使用屈光误差来评估近视: 眼保健操干预的效果与对照组无异 (SMD = −1.74, 95% CI −6.27 to 2.79, Z = 0.75, P = 0.45) 。七项研究报告了保护/缓解效果: 眼保健操的保护/缓解效果优于对照组 (RR = 0.40, 95% CI 0.23–0.71, Z = 3.13, P < 0.01) 。
结论: 荟萃分析表明, 眼保健操在预防或控制近视进展方面的疗效有限或无效。因此, 在有充分证据支持其疗效之前, 建议停止继续实施眼保健操政策。
Introduction
Myopia is a rapidly growing public health challenge, affecting more than 2 billion people currently—a number projected to grow to 5 billion by 2050, about half of the population worldwide [1]. In East and Southeast Asia, it has become an epidemic where more than 80% of young adults are myopic—a rapid rise from 20–30% in the mid-20th century [2, 3]. As it becomes more prevalent, it is also developing at a younger age [3]. Early onset of myopia is strongly associated with high myopia in adulthood [4]—more than 50% of those with myopia onset at 7 or 8 years of age develop high myopia (versus less than 5% of those with onset at 12 years or older) [5]. High myopia is a common cause of vision impairment and blindness, as it heightens the risk of cataracts, glaucoma, retinal detachment, and myopic macular degeneration [6]. In economic impact, myopic macular degeneration and uncorrected myopia—the leading cause of vision impairment—were estimated to be responsible for about US$250 billion in lost global productivity in 2015 [7]. Myopia thus presents an enormous challenge for health services, from screening and providing spectacles to managing eye diseases [8].
Because of its rapidly growing prevalence and its societal burden and personal costs (e.g., reduced quality of life), myopia control has become a top public health priority in countries such as China. A national survey of ~2.5 million Chinese students reported 52.7% of them to be myopic by the end of 2020: 80.5% in high school, 71.1% in middle school, 35.6% in primary school, and 14.3% in six-year-olds [9]. A large proportion of myopic schoolchildren have no refractive correction [10], which undermines their school learning and health—for example, according to a recent city-wide study the ratio was about 60% in a southern municipal city, Shantou [11]; the ratio was even higher in migrant children, estimated to be 85% [12, 13]. China has recently set up specific, numeric goals for preventing and controlling myopia—goals that are part of evaluation metrics for all provincial and local governments (eTable 1 in Supplementary Material). The target is to reduce the prevalence of myopia by at least 0.5% annually from 2018–2023 (for provinces with high prevalence, at least 1% annually), such that by 2030, the prevalence would be reduced to <70% in high school, <60% in middle school, <38% in primary school, and 3% in six-year-olds. To achieve these goals, a suite of implementation requirements has been made at the levels of family, school, medical institute, student, and government agency [14, 15]. Prominent among these are compulsory eye exercises [16] for schoolchildren, to be performed twice a day during school days—a policy dating to the 1960s, based on eye acupressure from traditional Chinese medicine (for an introduction of its history and rationale, see eTable 2 in Supplementary Material). This requirement was reaffirmed in response to the adverse impact of COVID-19 in a renewed concerted national plan issued in 2021.
This compulsory school policy has affected schoolchildren in mainland China for more than half a century. Yet, there has been no meta-analysis of controlled trials to evaluate the efficacy of eye exercises in myopia prevention or control. Long overdue, this important question acquires particular urgency in light of the recent nationwide policy goals and the negative impacts of the COVID-19 pandemic. Even though these goals are considered modest [17], progress has been stunted because of pandemic lockdown measures from 2020 to 2022 [18]. Home confinement has been associated with a substantial myopic shift, particularly in young children (aged 6–8 years) [19]; for example, from grade 2 to grade 3, myopia incidence almost doubled from late 2019 to late 2020 (20.8%, with lockdown) compared with the same period from 2018 to 2019 (13.3%, without lockdown) [20]. The cumulative effects over the past 3 years (2020–2022) may be even more pronounced, creating unprecedented challenges for controlling myopia. On the other hand, there is some evidence that the effects induced by lockdown may be transient [21]. In any case, adopting effective, evidence-based measures to combat myopia development is critical. A challenge in evaluating the causal effect of eye exercises, however, is that some studies on this topic are published in Chinese that are not indexed in English databases. Here, we aimed to evaluate the overall efficacy of eye exercises in preventing myopia and slowing its progression, by conducting a meta-analysis of studies that compared eye exercise interventions with controls that did not use eye exercises. We searched nine Chinese and English databases from their inception to December 15, 2022 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22].
Materials and methods
Search strategy and selection criteria
We assessed the effectiveness of eye exercises against myopia onset or progression (Fig. 1). We (WC and FX) searched nine databases, both Chinese (i.e., CNKI) and English (i.e., Web of Science, Google Scholar, EBSCO, PubMed, Cochrane Library, Science Direct, Scopus, and Embase), spanning from database inception to December 15, 2022. The search terms were “eye exercise” AND “myopia” in Chinese for the Chinese database and (“myopia” OR “short sightedness” OR “nearsightedness”) AND (“ocular gymnastics” OR “eye exercises” OR “eye exercise”) for the English databases (see eFig. 1 in Supplementary Material for a full list of the search terms used).
Fig. 1. PRISMA Flowchart for the Study Selection Process.
This flowchart illustrates the study selection process according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. It enumerates the records identified, screened, evaluated for eligibility, and ultimately included in the meta-analysis.
Four coders independently screened the literature: WC and YW from database inception to April 30, 2020; FX and XG from May 1, 2020 to December 15, 2022. The screening started with titles and abstracts first and then the full text, using the following selection criteria. Specifically, to be included, papers must meet all five criteria: (1) available as a journal publication or a dissertation; (2) using eye exercises for intervention; (3) employing a control group that did not use eye exercises; (4) reporting at least one myopia-related indicator (e.g., axial length, visual acuity, refractive error, or protective/mitigating effects); and (5) reporting data that enabled effect size extraction or estimation. Discrepancies between coders were resolved through discussion. All excluded articles during the full-text screening stage are listed in eTable 3 in Supplementary Material. RevMan (version 5.4) was used to screen and organize articles.
Data analysis
The analysis focused on outcome evaluation, risk of bias, and study heterogeneity. For each study, we (WC and FX) extracted the article information, sample size, intervention(s), and myopia indicator(s). Of the four myopia indicators, outcomes in visual acuity and refractive error were evaluated using standardized mean differences (SMDs, with 95% CI), by dividing the mean difference between two groups with the standard deviation. Protective/mitigating effects were assessed using the risk ratios (RRs, with 95% CI), by calculating the ratio of the risk of developing myopia (or progression) in the eye-exercise group to the risk in the control group. Axial length (the distance from the cornea to the retina) was not reported in any study.
Risk of bias was independently rated by two coders (FX and XG) using the Cochrane Risk of Bias Tool 2.0 (commonly recommended for randomized trials). Bias was rated across seven domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other biases. Discrepancies in ratings were resolved after discussion. Intercoder consistency (reliability) was evaluated using linearly weighted PABAK (prevalence-adjusted, bias-adjusted kappa), which accounted for two characteristics of the ratings: (1) some ratings (e.g., “some concerns”) were much more prevalent than others (e.g., “high risk”), and (2) degree of disagreement differed among the three ratings (e.g., “low risk” and “high risk” were more different than “low risk” and “some concerns”).
Study heterogeneity was quantified using the I2 statistic (range from 0% to 100%, with higher values representing larger heterogeneity). The degree of heterogeneity was defined based on conventional standards: not substantial (I2 < 50%) or substantial (I2 ≥ 50%). When the heterogeneity was substantial, outsized influences of individual studies on the overall results were probed using sensitivity analysis (subgroup analysis was not appropriate given the small number of included papers). Random-effects models were used to summarize effect sizes. Analyses were conducted by FX using RevMan (version 5.4) and R/RStudio (version 2022.07.1). Data and code are available online (https://osf.io/dr5jk/).
Results
Identification and characteristics of articles for analysis
The initial search yielded 1765 articles, of which 1754 were excluded: 423 were duplicates, 1223 did not have a control group, 16 did not have full-text, and 92 did not fulfill other inclusion criteria (as detailed in Fig. 1). The list of excluded articles during the full-text screening stage is provided in eTable 3 in Supplementary Material. In total, 11 studies (9 in Chinese and 2 in English) were included in the meta-analysis.
The 11 included studies [23–33] were between 1974 and 2021; three were dissertations [24, 25, 30]. All were controlled trials, including 2 non-randomized controlled trials and nine randomized controlled trials (Table 1). They assessed three types of outcomes: visual acuity, refractive error, and protective/mitigating effect. In total, the meta-analysis included 921 participants: 399 in eye-exercise groups and 522 in control groups. All studies were conducted in children except one (6–26 years old).
Table 1.
Basic Characteristics of the Included Studies.
| Eye exercise group | Control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Myopia target | Design | Participants (eyes), n | Age | Participants (eyes), n | Age | Activity | Eye exercise duration | Trial duration | Outcome variable | |
| Luo et al. [23] | Progression | RCT | 55 (110) | Mean (SD) 12 y (3) | 55 (110) | Mean (SD) 12 y (3) | 3D visual training combined with ciliary muscle exercise training | - | 3 mo | Visual acuity | |
| Wu [24] | Progression | RCT | 20 (40) | Mean (SD) 14 y (3) | 20 (40) | Mean (SD) 14 y (3) | point massage | 10 m | 30 d | Visual acuity, refractive error, protective/mitigating effectb | |
| 20 (40) | Mean (SD) 14 y (3) | Dazhui vibration | |||||||||
| 20 (40) | Mean (SD) 13 y (3) | Dazhui vibration plus point massage | |||||||||
| Sun [25] | Progression | RCT | 30 (60) | Mean (SD) 11 y (2) | 30 (60) | Mean (SD) 11 y (2) | Massage | 10 m | 3 mo | Protective/mitigating effectb | |
| Lv et al. [26] | Progression | RCT | 47 (90a) | Mean (SD) 12 y (4) | 55 (105a) | Mean (SD) 11 y (3) | Quadruple therapy (traditional Chinese medicine diet, auricular plaster therapy, sticking around the eye, and the fog [undercorrection]) | 10 m | 3 mo | Protective/mitigating effectb | |
| Zhang [27] | Incidence | NRCT | 28 (56) | Range, third-graders (8–10 y) | 24 (48) | Range, third-graders (8–10 y) | No intervention | 5 m | 1 d | Protective/mitigating effect | |
| Han and Mu [28] | Progression | RCT | 25 (50) |
Range, 7–15 y |
25 (50) |
Range, 7–15 y |
Badminton training | 10 m | 3 mo | Visual acuity, protective/mitigating effect | |
| He et al. [29] | Progression | RCT | 68 (136) |
Range, 7–15 y |
68 (136) |
Range, 7–15 y |
Yoga eye therapy | 15 m | 3 mo | Visual acuity, protective/mitigating effect | |
| Ma [30] | Incidence | NRCT | 62 (124) | Range, third-graders (8–10 y) | 61 (122) | Range, third-graders (8–10 y) | Eyesight gymnastics with physical exercise for health maintenance | 10 m | 4 mo | Visual acuityb | |
| Wang et al. [31] | Progression | RCT | 30 (54a) | Range, 6–16 y | 30 (54a) | Range, 7–16 y | Eye muscle massage | 10 m | 30 d | Visual acuityb | |
| 30 (55a) | Head and neck massage and scraping | ||||||||||
| 30 (53a) | Eye muscle massage with head and neck massage and scraping | ||||||||||
| Wang [32] | Progression | RCT | 24 (39a) | Mean (SD) 14 y (4) | 24 (42a) | Mean (SD) 13 y (3) | Auricular plaster therapy | 20 m | 12 wk | Refractive error, protective/mitigating effectb | |
| Hayashi and Du [33] | Incidence | RCT | 10 (20) | Mean (SD) 23 y (2) | 10 (20) | Mean (SD) 25 y (2) | Facial massage roller | 5 m | 1 d | Visual acuity | |
| 10 (20) | Mean (SD) 24 y (3) | Automated eye massager | |||||||||
| 10 (20) | Mean (SD) 23 y (2) | No intervention | |||||||||
| 10 (20) | Mean (SD) 25 y (2) | Facial massage roller | 2 mo | ||||||||
| 10 (20) | Mean (SD) 24 y (3) | Automated eye massager | |||||||||
| 10 (20) | Mean (SD) 23 y (2) | No intervention | |||||||||
Numbers marked with a hash sign (a) denote the number of diseased eyes (hence the total number of eyes was not double the number of participants). Studies marked with an asterisk (b) derived their results from the count of eyes rather than participants; among the six studies, only Wang [32] factored in the non-independence between fellow eyes during data analysis. Two of the studies, Hayashi and Du [33] and Wang [32], are in English; the rest are in Chinese. Dazhui refers to the acupuncture point “GV 14.”
RCT randomized controlled trial, NRCT non-randomized controlled trial.
Quality of included studies
Overall, out of the 11 studies, nine had some concerns of bias in at least two domains, and two studies had high risk of bias in two domains (eFig. 2 in Supplementary Material). Intercoder consistency in bias rating was high for the first five domains: random sequence generation (% of agreement = 100%, PABAK = 1, 95% CI 1–1, P < 0.001); allocation concealment (% of agreement = 95.5%, PABAK = 0.90, 95% CI 0.67–1, P < 0.001); blinding of participants and personnel (% of agreement = 77.3%, PABAK = 0.49, 95% CI 0.09–0.88, P = 0.02); blinding of outcome assessment (% of agreement = 90.9%, PABAK = 0.80, 95% CI 0.49–1, P < 0.01); and incomplete outcome data (% of agreement = 86.4%, PABAK = 0.69, 95% CI 0.20–1, P = 0.01). Consistency was low for the final two domains: selective reporting (% of agreement = 59.1%, PABAK = 0.08, 95% CI −0.38 to 0.54, P = 0.71) and other biases (% of agreement = 54.5%, PABAK = −0.02, 95% CI –0.25 to 0.21, P = 0.83). Thus, to better assess the effect of the eye-exercise intervention, sensitivity analysis was subsequently conducted with and without the two high-risk studies [26, 30].
The results from the risk of bias assessments were corroborated by the funnel plots (eFig. 3 in Supplementary Material), which are intended to evaluate the overall publication bias within each outcome measure: visual acuity (within-group and between-group), refractive error, and protective/mitigating effect. Owing to the small number of included studies and the somewhat subjective nature of funnel plots, the results can only be suggestive. Nevertheless, the patterns hint at the presence of publication bias: the distributions are asymmetrical, and the proportion of studies falling outside the bounds (within which 95% of studies are expected to remain in the absence of biases and heterogeneity) is high, ranging from 22% (for protective/mitigating effect) to 100% (for refractive error).
In addition, six [24–26, 30–32] out of the 11 studies derived their results from eye count rather than participant count, with five of them [24–26, 30, 31] failing to account for the non-independence of data between fellow eyes (e.g., shared genetics and environment) in their analysis (see Table 1). Thus, to mitigate the potential overestimation of effects due to the lack of statistical independence, subsequent sensitivity analyses were carried out to exclude studies that did not account for data non-independence.
Studies measuring visual acuity
Seven studies used visual acuity to measure myopia. Acuity was converted to a common decimal scale. The effect of the eye-exercise intervention was evaluated in one of two ways: (1) within-group changes following intervention (i.e., before and after the eye-exercise intervention); and (2) between-group differences following different interventions (i.e., eye-exercise intervention vs. control).
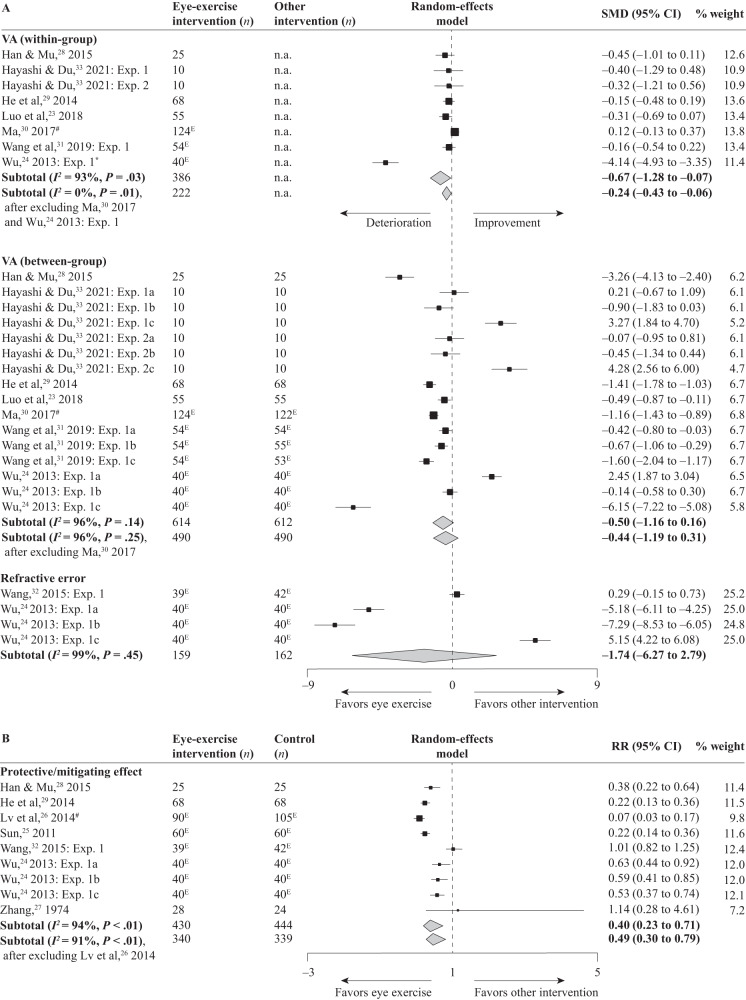
For within-group changes (Fig. 2A), data include 168 participants (218 eyes) from eight experiments. The duration of the controlled trials ranged from 1 day to 4 months. Across experiments, visual acuity declined after the eye-exercise intervention (SMD = −0.67, 95% CI −1.28 to −0.07, Z = 2.17, P = 0.03). The study heterogeneity was high (I2 = 93%), and one study [24] was found to greatly contribute to the high heterogeneity. After excluding this study, the I2 value declined to 8%, and the combined effect size became not significant (SMD = −0.12, 95% CI −0.28 to 0.04, Z = 1.51, P = 0.13). Another study [30] was found to be of high bias; after excluding it, the combined effect size was significant (SMD = −0.81, 95% CI −1.56 to −0.07, Z = 2.13, P = 0.03). The pattern was the same when both studies were excluded (SMD = −0.24, 95% CI −0.43 to −0.06, Z = 2.54, P = 0.01).
Fig. 2. Forest Plots for the Four Comparisons.
The first panel (A) presents the outcomes of comparing eye exercises with alternative interventions in terms of visual acuity (VA) and refractive error, while the second panel (B) displays the results of contrasting eye exercises with a control condition for protective/mitigating effects. SMD, standardized mean difference; n.a., not applicable; CI, confidence intervals; Exp, experiment; E, eyes (instead of participants); *, study that contributes to high heterogeneity; #, study of high-risk of bias. The dashed line represents a null effect. In the Hayashi and Du [33] study, experiments 1 and 2 refer to acute (short-term) and chronic (long-term) experiments, respectively; letters a, b and c refer respectively to comparisons with the no-intervention control, facial massage roller intervention, and automated eye massage intervention. In the Wang et al. [31] study, letters a, b and c refer respectively to comparisons with the eye muscle massage, head and neck massage and scraping, and combined intervention. In the Wu [24] study, letters a, b and c refer respectively to comparisons with the point massage, Dazhui vibration intervention, and combined intervention.
For between-group differences (Fig. 2A), data include 208 participants (406 eyes) in the eye-exercise groups and 208 participants (404 eyes) in the control groups from 16 different experiments. Before interventions, the two types of groups had comparable visual acuity in each of the experiments. After interventions, across experiments, visual acuity remained similar between the two types of groups (SMD = −0.50, 95% CI −1.16 to 0.16, Z = 1.49, P = 0.14), but was somewhat higher in the eye-exercise groups when the study with adult participants [33] was excluded (SMD = −1.21, 95% CI −1.98 to −0.43, Z = 3.04, P < 0.01). The heterogeneity was high as well (I2 = 96%), but no single study contributed to the heterogeneity. One study [30] was found to be of high bias; after excluding it, the combined effect size remained not significant (SMD = −0.44, 95% CI −1.19 to 0.31, Z = 1.14, P = 0.25).
Studies measuring refractive error
Two studies used refractive error to measure myopia (Fig. 2A). Data include 159 eyes in the eye-exercise groups and 162 eyes in the control groups from four experiments. Across experiments, the effects of interventions did not differ from each other (SMD = −1.74, 95% CI −6.27 to 2.79, Z = 0.75, P = 0.45). The study heterogeneity was high (I2 = 99%), but no single study could be identified to have contributed to it.
Studies measuring protective/mitigating effect
Seven studies reported protective/mitigating effects that evaluated relative risk (Fig. 2B). Data include 121 participants (309 eyes) in the eye-exercise groups and 117 participants (327 eyes) in the control groups from nine experiments. Across experiments, eye exercises had a higher protective/mitigating effect than control (RR = 0.40, 95% CI 0.23–0.71, Z = 3.13, P < 0.01). Again, the study heterogeneity was high (I2 = 94%), but no single study could be identified to have contributed to it. One study [26] was found to be of high bias; after excluding it, the combined effect size was reduced but remained significant (RR = 0.49, 95% CI 0.30–0.79, Z = 2.94, P < 0.01).
Sensitivity analyses: data non-independence
In studies measuring visual acuity, three of them [24, 30, 31] used eye counts for analyses but failed to account for data non-independence. After excluding them, the findings remained the same: visual acuity declined after the eye-exercise intervention (I2 = 0%, SMD = −0.27, 95% CI −0.48 to −0.05, Z = 2.46, P = 0.01); post interventions, visual acuity was similar between eye-exercise and other intervention groups (SMD = −0.05, 95% CI −0.97 to 0.87, Z = 0.10, P = 0.92).
In studies measuring protective/mitigating effects, three of them [24–26] also used eye counts for analyses without accounting for data non-independence. After excluding them, the findings were no longer statistically significant: the protective/mitigating effect was similar between eye-exercise and other intervention groups (I2 = 95%, RR = 0.53, 95% CI 0.18–1.59, Z = 1.13, P = 0.26).
Subgroup analyses: myopia incidence and myopia progression
Myopia incidence and myopia progression may have different risk factors and responses to interventions and treatments. Therefore, subgroup analyses were conducted to evaluate incidence and progression separately. Of the 11 studies, three examined myopia incidence [27, 30, 33] and the rest (eight studies) focused on myopia progression.
For myopia incidence, two studies measured visual acuity [30, 33] and one measured protective effect [27]. Visual acuity remained unchanged before and after the eye-exercise intervention (SMD = 0.06, 95% CI −0.18 to 0.29, Z = 0.47, P = 0.64), and there was no difference between eye-exercise groups and control groups (SMD = 0.56, 95% CI −0.56 to 1.68, Z = 0.98, P = 0.33). Similarly, there was no difference in the protective effect on myopia incidence between eye-exercise and control groups (RR = 1.14, 95% CI 0.28–4.61, Z = 0.19, P = 0.85).
Concerning myopia progression, visual acuity declined post eye-exercise intervention (SMD = –0.98, 95% CI –1.91 to –0.04, Z = 2.05, P = 0.04). As before, the study heterogeneity was high (I2 = 96%), with one study [24] greatly contributing to heterogeneity, but excluding the study did not change the effect on visual acuity (SMD = −0.23, 95% CI −0.43 to −0.03, Z = 2.31, P = 0.02). Visual acuity, while comparable before interventions, was higher in the eye-exercise groups than the control groups after interventions (SMD = −1.23, 95% CI −2.16 to −0.30, Z = 2.60, P = 0.01); however, the study heterogeneity was high (I2 = 97%) and could not be attributed to any single study. Finally, the mitigating effect was greater in the eye-exercise groups than the control (RR = 0.37, 95% CI 0.21–0.68, Z = 3.24, P < 0.01), but again, high heterogeneity was observed (I2 = 95%) and it was not attributable to any individual study. After excluding one study with high bias [26], the pattern remained unchanged (RR = 0.46, 95% CI 0.28–0.76, Z = 3.04, P < 0.01).
Discussion
The present meta-analysis of controlled trials shows that eye-exercise interventions were not effective in preventing myopia incidence, as evidenced by studies measuring visual acuity and protective effect. The findings on myopia progression were mixed: eye-exercise interventions were no better than the control in studies measuring refractive error, but they led to better outcomes in studies measuring visual acuity and mitigating effect.
To interpret the limited meta-analytic evidence for the efficacy of eye exercises in controlling myopia progression, it is important to note that the evidence from a meta-analysis is only as good as the quality of the studies it encompasses. In aggregate, the studies were highly heterogeneous and may be subject to publication bias, as indicated in eFig. 3 in Supplementary Material. Each individual study was also affected by at least five major weaknesses: a lack of axial length measurement (the primary physical change associated with myopia progression); small sample sizes (mostly with n < 100 per group); potential biases (summarized in eFig. 2 in Supplementary Material); failure to consider side effects (like infections such as conjunctivitis [pink eye] and styes from unwashed hands); and the absence of established effective interventions as control. In addition, six studies based their results on the count of eyes rather than participants, with five failing to factor in the non-independence between fellow eyes during data analysis (see Table 1). Indeed, after excluding the studies that failed to account for non-independent data, the protective/mitigating effect became not significant. Thus, these issues, from heterogeneity and publication bias to weaknesses in design and analysis, suggest that the findings from these studies must be interpreted cautiously.
Our meta-analysis evaluates controlled trials from database inception to December 15, 2022, providing comprehensive causal evidence on the efficacy of eye exercises in controlling myopia. It complements a recent meta-analysis that examined the association between eye exercises and myopia in school children in China, which focused on four databases (instead of nine here) using a shorter time frame (2006 to 2021) and included both interventional and observational studies [34]. Their included studies (12 total) showed that, after accounting for confounding factors like age and gender, there was no association between myopia and eye exercises. Although further subgroup analyses showed a moderate protective effect of eye exercises on myopia in studies with larger samples and those from the Chinese databases, the findings are only suggestive due to the post-hoc and observational nature of the subgroup analyses (not based on randomized comparisons). For instance, it is not clear why positive effects of eye exercises surfaced only in the Chinese databases and not in the English ones. Given that higher-quality studies from China typically were (and still are) prioritized for submission to English rather than Chinese journals (for instance, in our meta-analysis, the only study addressing the non-independence between fellow eyes was published in English; see Table 1), it stands to reason that more weight should be assigned to the negative findings in the English databases.
Our interpretation of the meta-analytic evidence dovetails with earlier indirect and observational findings. For instance, despite the compulsory nationwide practice of eye exercises in mainland China, the prevalence of myopia among young adults has steadily risen from 20–30% in the 1980s to 80–90% today—comparable to rates in other East Asian regions and Singapore where no eye-exercise policy has been in place [3]. Intriguingly, myopia is much more prevalent in China than in regions without such interventions (e.g., Australia); similarly, within China itself, urban students, known for better compliance with the policy, exhibit a much higher myopia rate than their rural counterparts [35]—~96.6% of urban students perform eye exercises regularly [36], as opposed to 15% of rural students [37]. Furthermore, other observational studies have largely found the effectiveness of eye exercises wanting [38]. For example, one study revealed a modest impact on alleviating near vision symptoms but none on myopia reduction [36], a finding contradicted by another study [37]. Likewise, another study noted a statistically significant, albeit likely clinically insignificant, effect on reducing accommodative lag [39], but a different study reported no association between eye exercises and the risk of myopia onset [40]. These observations are not consistent with the purported goal of eye exercises.
In contrast to eye exercises, other interventions to control myopia, such as spending time outdoors, have received strong support. In particular, robust evidence indicates that outdoor time can protect against developing myopia, both in interventions directed at schools [41] and at families [42, 43], even though the exact mechanisms of action and its effect on delaying myopia progression are not yet fully understood [44]. A distinct advantage of outdoor-time intervention is the added benefit of promoting an active lifestyle that helps to enhance mental and physical health more generally. Interventions targeting outdoor time agree with our current understanding of the etiology of myopia: changes in lifestyle over the past several decades, particularly decreased time spent outdoors, likely have played a major role [3]. Other risk factors, like increased near-work time and education pressure, may also contribute by reducing outdoor time [3]. Outdoor-time intervention can also be combined with non-lifestyle interventions, such as low-dose atropine and optical interventions, which have been shown to slow myopia progression [45].
Given the absence of substantial evidence supporting eye exercises and the compelling evidence favouring interventions like increased outdoor time, it is challenging to justify the continuation of the eye-exercise policy. One may argue that the lack of efficacy is due to students having failed to perform eye exercises correctly—owing to unfamiliarity with the right pressure, accurate acupoint location, or fundamental massage technique [40]. But this misses the larger issue: if, after over 50 years of implementation, most students still cannot execute it properly, is the fault with the students or with the intervention itself? Others may suggest that even if eye exercises do not help myopia, they might alleviate eye fatigue, or at the very least, do no harm anyway. However, this argument overlooks the missed opportunity to engage in health-promoting activities such as outdoor play or rest. Thus, to justify maintaining the status quote, robust evidence showing the effectiveness of eye exercises in controlling myopia is required. This evidence ideally should come from studies that measure axial length (i.e., physical rather than just functional indexes of myopia), use large sample sizes, minimize biases, examine side effects, and use outdoor time as a control intervention.
Our study has both strengths and limitations. Strengths include a comprehensive evaluation of studies published in both Chinese and English until December 15, 2022, and the use of controlled trials for more robust causal inference. Limitations include high heterogeneity among studies, possible publication bias, and weaknesses in individual studies such as small sample sizes. Additionally, a lack of sufficient studies precluded subgroup analysis for different age groups.
Conclusions
This comprehensive meta-analysis of controlled trials reveals that eye exercises are ineffective in preventing myopia onset, as indicated by visual acuity and protective effect measurements. Findings on myopia progression are inconsistent: eye exercises result in improved outcomes in studies assessing visual acuity and mitigating effect, but not in those examining refractive error. Multiple issues, including heterogeneity, publication bias, and weaknesses in design and analysis, are identified in existing research. In light of the limited evidence supporting their effectiveness, and the robust evidence favouring alternative interventions such as increased outdoor time, it is recommended that policymakers retire the eye-exercise policy. The time and resources saved may be better redirected toward evidence-based interventions—for the health of schoolchildren and for the success of myopia control policies.
Supplementary material is available at Eye’s website.
Summary
What is known about this topic
Myopia is a major public health challenge, with high prevalence in East Asia.
Eye exercises have been used as a myopia control policy in China for over 50 years, but their efficacy has not been established.
What this study adds
This systematic review and meta-analysis synthesizes evidence from 11 controlled trials on eye exercises for myopia control.
Overall, the results suggest eye exercises have limited to no efficacy in preventing or controlling myopia progression.
Given the lack of robust evidence supporting their effectiveness, the study recommends retiring the longstanding eye exercise policy in China.
Supplementary information
Acknowledgements
Yichen Wu and Xinran Ge contributed to the literature search, screening, and coding; and Xiani Jia contributed to the editing of a preliminary draft of methods and results.
Author contributions
ZL conceived and designed the study. WC and FX searched the literature and screened articles. FX and WC analyzed the data, constructed the table, and drew the figures under the supervision of ZL. ZL wrote the manuscript. FX and WC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. FX and WC contributed equally to this work.
Funding
The study was supported by the National Key R&D Program of China (STI2030-Major Projects+2021ZD0204200), National Natural Science Foundation of China (32071045), Guangdong Basic and Applied Basic Research Foundation (2019A1515110574), and Shenzhen Fundamental Research Program (JCYJ20210324134603010). The funding bodies had no role in the study design, data collection, analysis, and interpretation, report writing, or the decision to submit for publication.
Data availability
This study is registered on the Open Science Framework. Extracted data and code are available online (https://osf.io/dr5jk/); additional requests may be made to the corresponding author.
Competing interests
All authors approved the final version of the manuscript and were responsible for the decision to submit the manuscript. The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Feng Xiao, Weiye Cheng.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02739-x.
References
- 1.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Rose KA. Myopia: is the nature-nurture debate finally over? Clin Exp Optom. 2019;102:3–17. doi: 10.1111/cxo.12845. [DOI] [PubMed] [Google Scholar]
- 3.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–49. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Chua SY, Sabanayagam C, Cheung YB, Chia A, Valenzuela RK, Tan D, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36:388–94. doi: 10.1111/opo.12305. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Ding X, Guo X, Chen Y, Zhang J, He M. Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a Chinese cohort. JAMA Ophthalmol. 2020;138:1129–34. doi: 10.1001/jamaophthalmol.2020.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikuno Y. Overview of the complications of high myopia. Retina. 2017;37:2347–51. doi: 10.1097/IAE.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019;126:338–46. doi: 10.1016/j.ophtha.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, et al. The Lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9:e489–551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People’s Republic of China: The 2020 national survey on myopia among children and adolescents. Available at: http://www.gov.cn/xinwen/2021-07/13/content_5624709.htm. Accessed 21 Jan 2023.
- 10.He M, Huang W, Zheng Y, Huang L, Ellwein LB. Refractive error and visual impairment in school children in rural southern China. Ophthalmology. 2007;114:374–82. doi: 10.1016/j.ophtha.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Li Y, Qiu K, Zhang R, Lu X, Luo L et al. Prevalence of myopia and uncorrected myopia among 721 032 schoolchildren in a city-wide vision screening in southern China: the Shantou myopia study. Br J Ophthalmol. 2022. 10.1136/bjo-2021-320940. [DOI] [PubMed]
- 12.Ma X, Zhou Z, Yi H, Pang X, Shi Y, Chen Q, et al. Effect of providing free glasses on children’s educational outcomes in China: cluster randomized controlled trial. BMJ. 2014;349:g5740. doi: 10.1136/bmj.g5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XQ, Yi HM, Lu LN, Zhang LX, Ma XC, Jin L, et al. Population prevalence of need for spectacles and spectacle ownership among urban migrant children in eastern China. JAMA Ophthalmol. 2015;133:1399–406. doi: 10.1001/jamaophthalmol.2015.3513. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Chen Y, Tan Z, Xiong R, McGuinness MB, Muller A. Interventions recommended for myopia prevention and control among children and adolescents in China: a systematic review. Br J Ophthalmol. 2023;107:160–66. doi: 10.1136/bjophthalmol-2021-319306. [DOI] [PubMed] [Google Scholar]
- 15.Jan C, Li L, Keay L, Stafford RS, Congdon N, Morgan I. Prevention of myopia, China. Bull World Health Org. 2020;98:435–37. doi: 10.2471/BLT.19.240903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawstron JA, Burley CD, Elder MJ. A systematic review of the applicability and efficacy of eye exercises. J Pediatr Ophthalmol Strabismus. 2005;42:82–8. doi: 10.3928/01913913-20050301-02. [DOI] [PubMed] [Google Scholar]
- 17.Morgan IG, Jan CL. China turns to school reform to control the myopia epidemic: a narrative review. Asia Pac J Ophthalmol. 2022;11:27–35. doi: 10.1097/APO.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Xu L, Tan CS, Lanca C, Foo LL, Sabanayagam C, et al. Systematic review and meta-analysis on the impact of COVID-19 pandemic-related lifestyle on myopia. Asia Pac J Ophthalmol. 2022;11:470–80. doi: 10.1097/APO.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Li Y, Musch DC, Wei N, Qi X, Ding G, et al. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021;139:293–300. doi: 10.1001/jamaophthalmol.2020.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Zhao F, Ding X, Zhang S, Li Z, Guo Y, et al. Rates of myopia development in young Chinese schoolchildren during the outbreak of COVID-19. JAMA Ophthalmol. 2021;139:1115–21. doi: 10.1001/jamaophthalmol.2021.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Han Y, Musch DC, Li Y, Wei N, Qi X, et al. Evaluation and follow-up of myopia prevalence among school-aged children subsequent to the COVID-19 home confinement in Feicheng, China. JAMA Ophthalmol. 2023;141:333–40. doi: 10.1001/jamaophthalmol.2022.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Liu X, Chen Z, Huang X, Chen H. Application research of 3D visual training combined with ciliary muscle exercise training for juvenile myopia rectification. Nurs Pract Res. 2018;15:90–1. [Google Scholar]
- 24.Wu L. Clinical observation on treating juvenile myopia by Dazhui vibrating manipulation plus point massage. thesis, Fujian University of Traditional Chinese Medicine, 2013. Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201302&filename=1013186311.nh&uniplatform=NZKPT&v=xv-P_wO5R9tNyPd-g0H6KtdkdB4AAgfwuM6GsPvN4L3__bL3mip2XnUTo_8glVZv. Accessed 3 Feb 2023.
- 25.Sun J. The clinical research of applying tonifying spleen Qi Tuina method in treating spleen Qi deficiency type of pediatric myopia. thesis, Shandong University of Traditional Chinese Medicine, 2011. Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD2012&filename=1011204980.nh&uniplatform=NZKPT&v=BRxh8Ytmbk4bWBgfeDdAiIMxdl7ooRxfa538LkIz_g9sMyFkLerxGfVu3FSX8Ctp. Accessed 3 Feb 2023.
- 26.Lv Y, Dou S, Chen Y, Hong M, Ke M. Juvenile myopia treatment by four combined methods for 105 eyes. Chin Med Mod Distance Edu. 2014;12:10–11. [Google Scholar]
- 27.Zhang L. Observation of the influence of eye exercise on visual functions of pupils. Guangxi Med. 1974;04:37–8. [Google Scholar]
- 28.Han B, Mu T. Observation on the effect of badminton training on adolescent pseudomyopia. Chin J Conval Med. 2015;24:174–75. [Google Scholar]
- 29.He J, Hao Y, Peng Q, Chen J. Efficacy of yoga eye exercise on juvenile pseudomyopia. J Beihua Univ (Natural Sci Edit) 2014;15:85–7. [Google Scholar]
- 30.Ma Z. Research on the creation and compilation of gymnastic exercise for sports health care and prevention and treatment of myopia in teenagers thesis, Harbin Sport University, 2017. Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201801&filename=1017070627.nh&uniplatform=NZKPT&v=2rKcXhWX2PxuHzkadJ4HdPBphBb2RIcooEH8Zg9f0eMDoF7sSAWMRVoVhLm59jhO. Accessed 3 Feb 2023.
- 31.Wang Y, Bao X, Shi Y, Wang Z. Clinical study of muscle massage around eyes combined with scraping of head and neck in the treatment of adolescent pseudomyopia. J Heibei Trad Chin Med Pharmacol. 2019;34:35–8. [Google Scholar]
- 32.Wang Y-Q. Effect of eye exercises in combination with auricular plaster therapy on adolescent pseudomyopia patients. In Proceedings of the 3rd AASRI Conference on CIB, 2015. ISBN:978-1-60595-308-3.
- 33.Hayashi N, Du L. Acute and chronic periocular massage for ocular blood flow and vision: a randomized controlled trial. Int J Ther Massage Bodywork. 2021;14:5–13. [PMC free article] [PubMed] [Google Scholar]
- 34.Tang J, Pei Y, Wang J, Yan N, Luo Y, Zhou W, et al. The association between Chinese eye exercises and myopia in children and adolescents: a systematic review and meta-analysis. Frontiers in Public Health. 2023;11:950700. doi: 10.3389/fpubh.2023.950700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He MG, Zheng YF, Xiang F. Prevalence of myopia in urban and rural children in Mainland China. Optometry Vision Sci. 2009;86:40–4. doi: 10.1097/OPX.0b013e3181940719. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z, Vasudevan B, Jhanji V, Gao TY, Wang NL, Wang Q, et al. Eye exercises of acupoints: their impact on refractive error and visual symptoms in Chinese urban children. BMC Complement Altern Med. 2013;13:306. doi: 10.1186/1472-6882-13-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Z, Vasudevan B, Fang SJ, Jhanji V, Mao GY, Han W, et al. Eye exercises of acupoints: their impact on myopia and visual symptoms in Chinese rural children. BMC Complement Altern Med. 2016;16:349. doi: 10.1186/s12906-016-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z-P, Ouyang M-Z, Zhang R, Tang X, Zhong H-J. Association between Chinese eye exercises and onset of myopia: a meta-analysis. Int J Clin and Exp Med. 2019;12:4580–88. [Google Scholar]
- 39.Li SM, Kang MT, Peng XX, Li SY, Wang Y, Li L, et al. Efficacy of Chinese eye exercises on reducing accommodative lag in school-aged children: a randomized controlled trial. PLoS One. 2015;10:e0117552. doi: 10.1371/journal.pone.0117552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang MT, Li SM, Peng X, Li L, Ran A, Meng B, et al. Chinese eye exercises and myopia development in school age children: a nested case-control study. Sci Rep. 2016;6:28531. doi: 10.1038/srep28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–48. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 42.Li SM, Ran AR, Kang MT, Yang X, Ren MY, Wei SF, et al. Effect of text messaging parents of school-aged children on outdoor time to control myopia: a randomized clinical trial. JAMA Pediatr. 2022;176:1077–83. doi: 10.1001/jamapediatrics.2022.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Guo L, Zhang J, Zhao F, Hu Y, Guo Y, et al. Effect of school-based family health education via social media on children’s myopia and parents’ awareness: a randomized clinical trial. JAMA Ophthalmol. 2021;139:1165–72. doi: 10.1001/jamaophthalmol.2021.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104:593–99. doi: 10.1136/bjophthalmol-2019-314675. [DOI] [PubMed] [Google Scholar]
- 45.Wildsoet CF, Chia A, Cho P, Guggenheim JA, Polling JR, Read S, et al. IMI - Interventions Myopia Institute: interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019;60:M106–31. doi: 10.1167/iovs.18-25958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is registered on the Open Science Framework. Extracted data and code are available online (https://osf.io/dr5jk/); additional requests may be made to the corresponding author.