Abstract
Background/objectives
Studies have reported an association between herpes zoster ophthalmicus (HZO) and stroke. We sought to validate this association with rigorous controls for both medical comorbidities and social factors using a nationwide U.S. administrative medical claims database.
Subjects/methods
A two-step approach was taken: first a retrospective case-control study was performed, followed by a self-controlled case series (SCCS). For the case control study, cox proportional hazard regression with inverse proportional treatment weighting assessed the hazard for stroke. In the SCCS, incidence of stroke was compared prior to and after the diagnosis of HZO.
Results
For the case–control study, 25,720 cases and 75,924 controls met our eligibility criteria. 1712 (6.7%) and 4544 (6.0%) strokes occurred in the case and control groups respectively, conferring an 18% increased risk of stroke in the observed 1-year post-HZO period (HR = 1.18, 95% CI: 1.12–1.25, p < 0.001). SCCS analysis showed the risk for stroke was highest in the month immediately after HZO episode compared to any other time range (1–30 days after, relative risk 1.58, p < 0.001) and even higher when assessing time more distal time points prior to the HZO diagnosis (days 1–30 after HZO diagnosis had RR = 1.69 (95% CI: 1.38–2.07) and RR = 1.93 (95% CI: 1.55–2.39) compared with days −120 to −91 and −150 to −121 prior to index, respectively (p < 0.001).
Conclusions
After accounting for stroke risk factors, our analysis confirms the association between HZO and stroke, with highest risk in the immediate month after an episode.
Subject terms: Epidemiology, Risk factors
Introduction
Herpes zoster typically presents as a dermatomal painful vesicular rash, and represents a reactivation of latent varicella zoster virus. In herpes zoster ophthalmicus (HZO), the reactivation occurs in the distribution of the ophthalmic division of the trigeminal nerve. HZO occurs in ~10–20% of herpes zoster patients [1], and patients may have corneal epithelial defects, stromal infiltration, decreased corneal sensation, or ocular inflammation in nearly any part of the eye [2].
Recent evidence has documented an increased rate of stroke following any herpes zoster infection [3–7]. This link has been further extended to HZO specifically. The earliest studies to report a link between HZO and stroke were large population based studies from the Taiwan National Health Insurance Research Database [8, 9]. Incidence of stroke was 8.1% in HZO patients compared to 1.7% in an age matched control group [8]. Subsequent metanalyses consisting of twelve studies examining 7.9 million patients showed that both unspecified herpes zoster and herpes zoster ophthalmicus were associated with a 20–40% increased odds of cerebrovascular events [10].
Retrospective cohort studies have revealed that conditions that predispose patients to vascular disease, including lifestyle factors such as smoking and obesity, are significantly more common in subjects with herpes zoster [11]. This highlights that appropriate controls for confounding variables are needed in studies. The multitude of factors contributing to stroke, including non-medical factors like geographic location, income, and education, also substantiates the need for rigorous controls in studies evaluating stroke risk [12–14].
Although prior studies have offered various methods to control for demographic variables and medical co-morbidities, the goal of our study was to add to the current literature on stroke risk in HZO patients by additionally controlling for geographic area, income, health care usage, and education. To validate our findings a two-step approach was taken: first a rigorously controlled retrospective case-control study was performed, followed by a self-controlled case series.
Methods
Dataset
Data were abstracted from Optum’s de-identified Clinformatics® Data Mart Database. The database contains all outpatient medical claims (office visits, procedures, and medications given) as well as demographic data and some laboratory values for all patients enrolled in commercial and Medicare Advantage insurance plans, including patients from all 50 states. The subset of data available for this study included all patient visits from Jan 1st, 2002 to June 30th, 2021 within the database. Due to the de-identified nature of the database, this study was deemed exempt by the University of Pennsylvania IRB. All research adhered to the tenets of the Declaration of Helsinki.
Cohorts
All patients who were diagnosed with herpes zoster with ocular involvement were identified in the database. An index date was set for each patient equal to the date of earliest diagnosis. Patients were excluded if they did not have at least 2 years prior to the index date in the database, or if they were less than 55 years old. Patients were also excluded if they had a concurrent or previous diagnosis of herpes simplex outside of the HZO diagnosis. (See Supplementary Table 1 for all codes used within the study).
Controls were matched on age, gender, and race up to 3:1 and were required to have the same insurance plan eligibility start date (±4 months) as the matched patient. Controls were subject to the same exclusion criteria as noted above, and were also excluded if they had a previous diagnosis of HZO.
Covariates of interest
In addition to the matched variables, covariates of interest included history of hypertension, hypercholesterolemia, kidney disease, atrial fibrillation or flutter, heart arrhythmia, ischemic heart disease, coronary artery bypass graft, heart failure, myocardial infarction, peripheral vascular disease, atherosclerosis, diabetes mellitus, chronic liver disease, and pulmonary disease (Supplementary Table 1). Next, history of a herpes zoster vaccine was accounted for. Lastly, non-clinical variables included health care usage, smoking, education level, and geographic location.
Outcomes and statistical analysis
The primary outcome was defined as having a diagnosis of any new ischemic stroke, hemorrhagic stroke, or non-specific cerebrovascular accident after the HZO index date. Cox proportional-hazards models were constructed with inverse proportional treatment weighting (IPTW). Censoring occurred when patients left the insurance plan, reached 2 years after the index date or were diagnosed with a non-HZO form of herpes simplex. In addition, controls were censored for any diagnosis of HZO. Two sensitivity analyses were performed. The first varied the outcome definition by excluding patients diagnosed with transient ischemic attacks (TIAs) and the second censored patients after one year of observation instead of two.
Self-controlled case series (SCCS)
In this analysis, every HZO patient acts as their own control with time frames before and after the diagnosis date compared to each other. The main outcome was the occurrence of the first stroke for each patient (if one occurred at all) and the time frame that it occurred in.
All HZO patients were identified using the ICD codes in Supplementary Table 1. Again, the index date was set based on the earliest date of diagnosis. Patients were excluded if they did not have at least 2 years prior and 1 year after the index date in the database, if they were less than 55 years old, or if they had a diagnosis of herpes simplex within 6 months prior or 1 year following the index date. Lastly, since diagnosis codes are not time stamped within the day, anyone with a diagnosis of a stroke on the index date was excluded.
A stroke was defined as having a new ICD9 or ICD10 code for an ischemic stroke or hemorrhagic stroke. After all new occurrences of stroke were identified, incidence rate ratios were created from comparing the rate of strokes in specific 30-day time frames before and after the index date.
Results
Cohort study
After all inclusion and exclusion criteria was met, a total of 25,720 HZO patients were identified and matched to 75,924 controls (see Fig. 1 for numbers excluded at each criteria). The average age of patients was 71.8 in the control and 71.7 in the HZO group, and the majority of patients were female (60% in the control and 59.9% in the HZO group). After IPTW, all covariates showed good balance between cohorts with all standardized mean difference ≤0.049. (See Supplementary Table 2 for all baseline characteristics for both cohorts used in the Cox regression analysis). During the follow-up period, 1712 (6.7%) new diagnoses of stroke were made in the HZO cohort compared to 4544 (6%) in the control cohort. After IPTW, Cox regression analysis revealed an increased risk of stroke with a 1.18 hazard ratio (95% CI: 1.12 to 1.25, p < 0.001; Table 1) among patients with HZO versus matched controls. Figure 2 shows the Kaplan Meir curves for this analysis.
Fig. 1. Flowchart of patients included in cohort study.

A total of 25,720 HZO patients were identified and matched to 75,924 controls.
Table 1.
HZO patients are at increased risk of stroke compared to matched controls. Results from IPTW Cox regression model.
| Cohort | N | Person years | N of strokes (%) | KM estimated cumulative incidencea | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|
| Primary outcome | <0.001 | |||||
| Control | 75,924 | 114,055 | 4544 (6.0%) | 0.047 | REF | |
| HZO | 25,720 | 32,261 | 1712 (6.7%) | 0.055 | 1.18 (1.12, 1.25) | |
| Sensitivity outcome excluding TIA from the outcome definition | <0.001 | |||||
| Control | 75,924 | 115,401 | 3262 (4.3%) | 0.033 | REF | |
| HZO | 25,720 | 32,753 | 1219 (4.7%) | 0.040 | 1.17 (1.10, 1.26) | |
| Sensitivity outcome with censoring at 1 year instead of 2 | <0.001 | |||||
| Control | 75,924 | 65,231 | 3000 (4.0%) | 0.028 | REF | |
| HZO | 25,720 | 18,784 | 1194 (4.6%) | 0.036 | 1.22 (1.14, 1.31) |
aFor primary outcome and sensitivity outcome 1, the KM estimate is at 1 year. And for sensitivity outcome 2, the KM estimate is at 6 M.
Fig. 2. HZO patients have a high cummulative probability of stroke compared to matched controls.
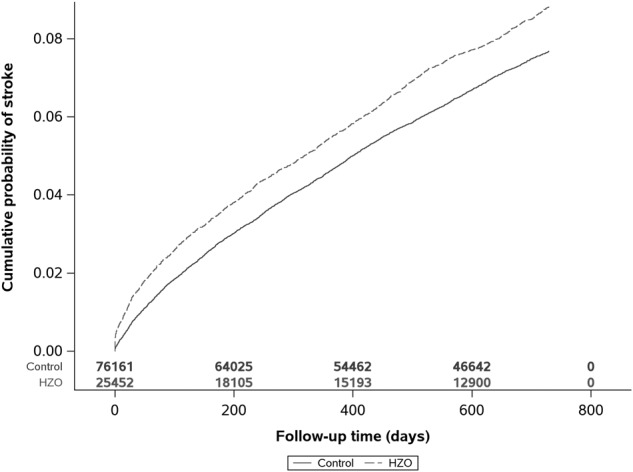
IPTW Kaplan Meier curve of cumulative probability of stroke relative to days after HZO episode.
The sensitivity analysis yielded nearly identical results with the first analysis which removed “TIA” diagnoses codes from the outcome, still showing an increased stroke risk in HZO patients (HR 1.17, 95%CI 1.10 to 1.26, p < 0.001). The second sensitivity analysis, which censored for 1 year of observation instead of 2, demonstrated a slightly stronger association between stroke and HZO (HR 1.22, 95% CI 1.14 to 1.31, p < 0.001) (Table 1).
Self-controlled case series
A total of 20,149 patients were included for the SCCS; demographics and comorbidities of included patients are shown in Table 2. The average age of patients included was 71.6 (SD 9.3), and a majority were female (60.2%).
Table 2.
Demographics of included patients in SCCS.
| Total (N = 20149) | |
|---|---|
| Age | |
| N | 20,149 |
| Mean (SD) | 71.6 (9.3) |
| Median | 72.0 |
| Q1, Q3 | 63.0, 79.0 |
| Range | (55.0–89.0) |
| Gender | |
| Male | 8012 (39.8%) |
| Female | 12,136 (60.2%) |
| Unknown | 1 (0.0%) |
| Race | |
| White | 15,633 (77.6%) |
| Black | 1389 (6.9%) |
| Hispanic | 1543 (7.7%) |
| Asian | 660 (3.3%) |
| Unknown | 924 (4.6%) |
| Education level | |
| Less than or equal to HS Diploma | 4465 (22.2%) |
| Less than Bachelor Degree | 11,249 (55.8%) |
| Bachelor Degree Plus | 3905 (19.4%) |
| Unknown | 530 (2.6%) |
| Household income | |
| <$40K | 4868 (24.2%) |
| $40K–$49K | 1563 (7.8%) |
| $50K–$59K | 1721 (8.5%) |
| $60K–$74K | 2229 (11.1%) |
| $75K–$99K | 2947 (14.6%) |
| $100K+ | 4591 (22.8%) |
| Unknown | 2230 (11.1%) |
| Geographic location | |
| Upper Midwest | 5008 (24.9%) |
| Southern Midwest | 2864 (14.2%) |
| Northeast | 2311 (11.5%) |
| Mountain | 2199 (10.9%) |
| Pacific | 3281 (16.3%) |
| South Atlantic | 4473 (22.2%) |
| Unknown | 13 (0.1%) |
| Index year | |
| 2002 | 130 (0.6%) |
| 2003 | 349 (1.7%) |
| 2004 | 461 (2.3%) |
| 2005 | 518 (2.6%) |
| 2006 | 541 (2.7%) |
| 2007 | 779 (3.9%) |
| 2008 | 1246 (6.2%) |
| 2009 | 1463 (7.3%) |
| 2010 | 1415 (7.0%) |
| 2011 | 1486 (7.4%) |
| 2012 | 1680 (8.3%) |
| 2013 | 1465 (7.3%) |
| 2014 | 1569 (7.8%) |
| 2015 | 1500 (7.4%) |
| 2016 | 888 (4.4%) |
| 2017 | 1088 (5.4%) |
| 2018 | 1212 (6.0%) |
| 2019 | 1356 (6.7%) |
| 2020 | 1003 (5.0%) |
| History of hypertension | |
| No | 4851 (24.1%) |
| Yes | 15,298 (75.9%) |
| History of hypercholesterolemia | |
| No | 4309 (21.4%) |
| Yes | 15,840 (78.6%) |
| Smoking | |
| No | 15,385 (76.4%) |
| Yes | 4764 (23.6%) |
| CKD | |
| No disease | 15,000 (74.4%) |
| CKD | 4504 (22.4%) |
| ESRD | 645 (3.2%) |
| Ischemic heart diseae | |
| No | 13,861 (68.8%) |
| Yes | 6288 (31.2%) |
| Heart failure | |
| No | 17,265 (85.7%) |
| Yes | 2884 (14.3%) |
| Atherosclerosis | |
| No | 17,147 (85.1%) |
| Yes | 3002 (14.9%) |
| DM | |
| No | 13,679 (67.9%) |
| Yes | 6470 (32.1%) |
| Chronic Liver disease | |
| No | 19,964 (99.1%) |
| Yes | 185 (0.9%) |
| Chronic Pulmonary Disease | |
| No | 12,987 (64.5%) |
| Yes | 7162 (35.5%) |
| Peripheral vascular disease | |
| No | 16,722 (83.0%) |
| Yes | 3427 (17.0%) |
| Atrial fibrillation/flutter | |
| No | 17,250 (85.6%) |
| Yes | 2899 (14.4%) |
| Congestive heart failure | |
| No | 16,893 (83.8%) |
| Yes | 3256 (16.2%) |
| Previous myocardial infarction | |
| No | 17,836 (88.5%) |
| Yes | 2313 (11.5%) |
| Arrhythmia | |
| No | 14,502 (72.0%) |
| Yes | 5647 (28.0%) |
| Cor Artery Bypass graft | |
| No | 19,883 (98.7%) |
| Yes | 266 (1.3%) |
| History of shingles vaccine | |
| No | 19,842 (98.5%) |
| Yes | 307 (1.5%) |
| Health Care Usage in the year prior to index date | |
| N | 20149 |
| Mean (SD) | 8.8 (7.2) |
| Median | 7.0 |
| Q1, Q3 | 4.0, 12.0 |
| Range | (0.0–76.0) |
Table 3 summarizes the relative risk of stroke after the diagnosis of HZO compared with time frames prior to HZO onset. Using the 90 to 60 day period prior to the diagnosis of HZO as a reference, the risk of stroke was significantly increased after the diagnosis of HZO with the highest risk of stroke occurring within the first 30 days of diagnosis (RR = 1.58, 95% CI 1.30–1.93, p < 0.001), but the elevated risk extended as far out as a year (RR = 1.33, 95% CI 1.07–1.65, p = 0.009).
Table 3.
Relative risk ratio of stroke between observation periods.
| Observation periods | N of stroke | Risk ratio (95% CI) | P value |
|---|---|---|---|
| −90 to −61 days prior to index | 130 | REF | |
| 1–30 days after index | 206 | 1.58 (1.30, 1.93) | <0.001 |
| 31–60 days after index | 172 | 1.32 (1.08, 1.62) | 0.007 |
| 61–90 days after index | 158 | 1.22 (0.99, 1.49) | 0.06 |
| 151–180 days after index | 165 | 1.27 (1.03, 1.56) | 0.02 |
| 331–360 days after index | 173 | 1.33 (1.07, 1.65) | 0.009 |
| −120 to −91 days prior to index | 122 | REF | <0.001 |
| 1–30 days after index | 206 | 1.69 (1.38, 2.07) | |
| −150 to −121 days prior to index | 107 | REF | <0.001 |
| 1–30 days after index | 206 | 1.93 (1.55, 2.39) |
The risk of stroke was even higher when assessing more distal time points prior to the HZO diagnosis. For example, relative risk ratio of stroke was 1.69 (95%CI 1.38–2.07, p < 0.001) 1 to 30 days after diagnosis of HZO compared to the period 120 to 91 days prior to diagnosis. Similarly, relative risk ratio of stroke was 1.93 (95% CI 1.55–2.39, p < 0.001) 1 to 30 days after diagnosis of HZO compared to 150 to 121 days prior to diagnosis.
Discussion
Stroke is a leading cause of death and disability in the United States [15]. Medical comorbidities, environmental influences, socioeconomic factors, and genetics all can contribute to the risk of stroke [12]. A variety of studies have recently alluded to the fact that herpes zoster opthalmicus may also increase risk of stroke [3–5], however given the numerous variables that can contribute to stroke risk an appropriately controlled study to assess for stroke risk is difficult to design. Our two-step approach using both a rigorously matched cohort study and complementary self-controlled case series further confirms the association between HZO and stroke.
Our cohort study is unique in the fact that cohorts were balanced not only by demographics and medical co-morbidities, but also geographic area, income, health care usage, and education. To our knowledge, prior studies within the United States have not accounted for these factors which are known to impact the incidence of stroke [13, 14]. Given vaccination has been associated with protection from stroke after zoster [16], we also controlled based on vaccination status. In complement to the cohort study, using the SCCS method provides a unique approach to avoid confounders since the analysis is within the same person. In addition, given our large sample size of the cohort study, we had an 80% power to detect a risk of difference of 1.08.
Recognition of the link between zoster and stroke is crucial, as it raises important questions regarding clinical management of these patients. Based on our SCCS data stroke risk is highest in the 30 days after diagnosis. This is in concordance with a prior Danish nationwide population-based cohort study which found that risk of stroke was highest in the first 2 weeks after zoster [17]. These results suggest that timely management is crucial, but begs the question as to what interventions work best to reduce morbidity and mortality from stroke in these patients.
Prior studies have advocated that systemic antivirals may reduce stroke risk. A self-controlled case-series based in the UK including over 6000 patients established stroke risk was lower in those who were treated with an antiviral, suggesting a protective effect [18]. This has been further supported by a more recent retrospective cohort study done in New Zealand which demonstrated that HZO patients treated with acyclovir have a 76.2% lower hazard of stroke [19]. In addition to prompt initiation of antivirals, the question arises of if patients should be referred for workup after diagnosis of HZO to ensure other stroke risk factors are also optimized. In our SCCS study, the majority of patients had underlying hypertension and hyperlipidemia (Table 2). It is unclear if prompt management of these factors could help mitigate stroke risk.
Despite recommendations, only 34.5% of the overall US population 60 years and older was vaccinated for herpes zoster in 2018 [20]. Herpes zoster vaccine not only decreases incidence of disease, but also reduces disease burden and disease complications like post-herpetic neuralgia [21]. Vaccination and stroke risk in herpes zoster has been a debated topic. Although some studies have demonstrated that vaccination reduces stroke risk [16], others have found no association [22]. Our study controlled for vaccination status (Supplementary Table 2), however given the study design we cannot directly speak to the association between vaccination and stroke risk within patients diagnosed with HZO. This would be a valuable future investigation, especially given prior studies have focused on nonspecific zoster and not HZO patients.
The mechanism behind the increased risk of stroke after HZO is likely multifactorial. Systemic inflammation has been associated with modulation of thrombotic pathways, which could potentially also lead to an ischemic event [23, 24]. Of note, the ophthalmic branch of the trigeminal ganglion sends branches to the middle and anterior cerebral artery and internal carotid artery. Further, significant changes have been identified on brain imaging and angiograms in patients with stroke occurring after herpes zoster [25]. It has been postulated that viral particles have the potential to travel along nerve fibers to blood vessels to initiate a pro-inflammatory and pro-thrombotic response, suggesting that direct inoculation of viral particles in the cerebral vasculature may lead to stroke [26]. Importantly, this hypothesis accounts for a vascular insult during active viral infection, which is consistent with our findings of stroke risk being most imminent in the time immediately after HZO diagnosis.
There are limitations to our study. First, given the deidentified nature of the database we are unable to verify diagnosis codes directly with clinical findings. To this point, we are unable to determine how the diagnosis of herpes zoster infection was made and if it was confirmed based on PCR testing. Although the appearance of HZO can often be sufficiently distinctive that a clinical diagnosis is accurate, HZO can often be mistaken for other conditions [27], and PCR testing can help provide confirmation. Herpes zoster and herpes simplex may also be difficult to differentiate clinically, which can make PCR testing helpful. Additionally, although we controlled for the presence of various risk factors, we are not able to account for the degree of control of underlying conditions. For example, an equivalent amount of patients in each group of our cohort study had a diagnosis of hypertension, however it is possible that one cohort had better controlled blood pressures than the other. Confirming our results with the SCCS, however, makes this less likely. Lastly, given an insurance-based data set was used, results may not be generalizable to other populations including those who are uninsured or covered by other insurance companies.
In conclusion, after carefully controlling for stroke risk factors, our study confirms the association between HZO and stroke with the highest risk being in the immediate month after diagnosis. Confirming this link between HZO and subsequent stroke has important implications for both treatment of the reactivated viral illness and prevention of stroke.
Summary
What was known before
Several studies have determined there is an associated with herpes zoster and stroke, but did not provide rigorous control for other social factors that may affect stroke including geographic location, income, and education.
What this study adds
We sought to validate the association between herpes zoster and stroke with rigorous controls for both medical comorbidities and social factors using a nationwide U.S. administrative medical claims database.
To validate our findings a two-step approach was taken: first a rigorously controlled retrospective case-control study was performed, followed by a self-controlled case series.
Supplementary information
Acknowledgements
Meeting presentation: Results to be presented at the ARVO annual meeting, New Orleans, LA, April 25th, 2023.
Author contributions
ASG, BVB, and SEO designed the study. YY responsible for data analysis and statistics. ASG, TP, and BVB wrote up the report. YY and SEO provided edits to the written report.
Funding
National Institutes of Health University of Pennsylvania Core Grant for Vision Research (2P30EYEY001583). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Funding from each of the above sources was received in the form of block research grants to the Scheie Eye Institute. None of the funding organizations had any role in the design or conduction of the study.
Data availability
The source data for this study were licensed by Optum, and hence we are not allowed to share the licensed data publicly.
Competing interests
ASG, TP, YY have no financial disclosures. BVB has no relevant financial disclosures and has consulted for EyePoint Pharmaceuticals. SEO is a consultant for Abbvie.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02708-4.
References
- 1.Liesegang T. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115:S3–12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Puri LR, Shrestha GB, Shah DN, Chaudhary M, Thakur A. Ocular manifestations in herpes zoster ophthalmicus. Nepal J Ophthalmol. 2011;3:165–71. doi: 10.3126/nepjoph.v3i2.5271. [DOI] [PubMed] [Google Scholar]
- 3.Sundström K, Weibull CE, Söderberg-Löfdal K, Bergström T, Sparén P, Arnheim-Dahlström L. Incidence of herpes zoster and associated events including stroke—a population-based cohort study. BMC Infect Dis. 2015;15:488. doi: 10.1186/s12879-015-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim M-C, Yun S-C, Lee H-B, Lee PH, Lee S-W, Choi S-H, et al. Herpes zoster increases the risk of stroke and myocardial infarction. J Am College Cardiol. 2017;70:295–6. doi: 10.1016/j.jacc.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Schink T, Behr S, Thöne K, Bricout H, Garbe E. Risk of stroke after herpes zoster—evidence from a german self-controlled case-series study. PLoS One. 2016;11:e0166554. doi: 10.1371/journal.pone.0166554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yawn BP, Wollan PC, Nagel MA, Gilden D. Risk of stroke and myocardial infarction after herpes zoster in older adults in a US community population. Mayo Clin Proc. 2016;91:33–44. doi: 10.1016/j.mayocp.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson BJ, Rausch DA, Irwin DE, Liang M, Yan S, Yawn BP. Analysis of vascular event risk after herpes zoster from 2007 to 2014 US insurance claims data. Mayo Clin Proc. 2019;94:763–75. doi: 10.1016/j.mayocp.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Lin H-C, Chien C-W, Ho J-D. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–7. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 9.Kang J-H, Ho J-D, Chen Y-H, Lin H-C. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–8. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 10.Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, Kiefe C, et al. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One. 2017;12:e0181565. doi: 10.1371/journal.pone.0181565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breuer J, Pacou M, Gauthier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;82:206–12. doi: 10.1212/WNL.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–95. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence: the NHANES I epidemiologic follow up study. Am J Epidemiol. 1996;144:665–73. doi: 10.1093/oxfordjournals.aje.a008979. [DOI] [PubMed] [Google Scholar]
- 14.Ahacic K, Trygged S, Kåreholt I. Income and education as predictors of stroke mortality after the survival of a first stroke. Stroke Res Treat. 2012;2012:983145. doi: 10.1155/2012/983145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Stroke Facts. Centers for Disease Control and Prevention n.d. https://www.cdc.gov/stroke/facts.htm.
- 16.Parameswaran GI, Wattengel BA, Chua HC, Swiderek J, Fuchs T, Carter MT, et al. Increased stroke risk following herpes zoster infection and protection with zoster vaccine. Clin Infect Dis. 2022:ciac549. 10.1093/cid/ciac549. [DOI] [PubMed]
- 17.Sreenivasan N, Basit S, Wohlfahrt J, Pasternak B, Munch TN, Nielsen LP, et al. The short- and long-term risk of stroke after herpes zoster—a nationwide population-based cohort study. PLoS ONE. 2013;8:e69156. doi: 10.1371/journal.pone.0069156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58:1497–503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer JJ, Liu K, Danesh-Meyer HV, Niederer RL. Prompt antiviral therapy is associated with lower risk of cerebrovascular accident following herpes zoster ophthalmicus. Am J Ophthalmol. 2022;242:215–20. doi: 10.1016/j.ajo.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Terlizzi E, Black L. Shingles vaccination among adults aged 60 and over: United States, 2018. NCHS Data Brief. No 370 National Center for Health Statistics 2020 1–8. [PubMed]
- 21.Young L. Zoster vaccine live for the prevention of shingles in the elderly patient. CIA. 2008;3:241–50. doi: 10.2147/CIA.S1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minassian C, Thomas SL, Smeeth L, Douglas I, Brauer R, Langan SM. Acute cardiovascular events after herpes zoster: a self-controlled case series analysis in vaccinated and unvaccinated older residents of the United States. PLoS Med. 2015;12:e1001919. doi: 10.1371/journal.pmed.1001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:8–9. doi: 10.1055/s-0037-1617146. [DOI] [PubMed] [Google Scholar]
- 24.Nagel MA, Mahalingam R, Cohrs RJ, Gilden D. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets. 2010;10:105–11. doi: 10.2174/187152610790963537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–60. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grose C. Heightened risk of ischemic stroke after recent herpes zoster ophthalmicus: GROSE. J Med Virol. 2018;90:1283–4. doi: 10.1002/jmv.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawyer AR, Williams G. Misdiagnosis of burns: herpes zoster ophthalmicus. J Burn Care Res. 2006;27:914–6. doi: 10.1097/01.BCR.0000245647.13372.5B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data for this study were licensed by Optum, and hence we are not allowed to share the licensed data publicly.


