Abstract
Onchocerca lupi is a zoonotic filarioid parasite of dogs and cats with widespread distribution. A specific non-invasive diagnostic assay for the detection of O. lupi infections remains unavailable. This study aimed to assess the accuracy, specificity, and sensitivity of an ELISA test designed using nine peptides from two O. lupi proteins. Sera (n = 54) collected from O. lupi infected dogs from endemic areas (Portugal and USA), alongside sera from dogs positive for Dirofilaria immitis, D. repens, Cercopithifilaria bainae, and Acanthocheilonema reconditum (n = 53) from a non-endemic area for O. lupi, as well as from helminth-free dogs (n = 60), were tested. The checkerboard titration method was applied for the optimization of peptide concentrations and conjugate anti-dog dilutions. Sensitivity, specificity, and optimal cut-off values were calculated using ROC curve analysis. All peptides reacted against sera of O. lupi, with no correlation between optic density (OD) values and microfilariae (mfs) loads. Sensitivity and specificity values ranging from 85.45 to 100%, and 88.89% to 100%, respectively, were recorded for all peptides examined, with 100% specificity and sensitivity observed for peptides 40_3, 40_5, 130_3, 120_3 and 40_1, 130_5, respectively. The maximum cut-off value was observed for peptides 40_5 (0.765) and 40_3 (0.708). Testing of sera from dogs positive for other filarioids resulted in lower OD values (up to 1.565) for peptides 40_3 and 40_5 when compared with O. lupi (up to 2.929). The availability of this assay will be of value in epidemiological studies of canine O. lupi infection in both endemic and non-endemic areas, and in assessing the risk for zoonotic transmission.
Subject terms: Infectious diseases, Proteomics
Introduction
Over the past decade, Onchocerca lupi (Spirurida, Onchocercidae) has attracted growing interest from the scientific community across continents1. From original taxonomic description in a Caucasian wolf2, this filarioid nematode has been widely reported as a causative agent of ocular infection in domestic dogs and cats, as well as in wild carnivores (wolves, coyotes), particularly in Europe and North America3–6. In animals, O. lupi microfilariae (mfs) are found in the cutaneous tissues7,8, whilst adult worms reside in the ocular connective tissues (i.e., eyelids, conjunctiva, and sclera) and, although infections are often asymptomatic, clinical signs ranging from acute or chronic ocular disease (i.e., periorbital swellings, photophobia or blindness) may be observed1,5,8.
Notably, important gaps in knowledge of the fundamental biology of this parasite still remain, in particular regarding its arthropod vector. DNA of O. lupi was detected in the blackfly species Simulium tribulatum9 and Simulium griseum5, as well as in other blood feeding arthropods, e.g., mosquitoes or biting midges (Culicoides spp.)10,11.
In the early 2010s, a case of human infection by O. lupi was diagnosed in Turkey12. This report was subsequently followed by other reports of human onchocerciasis due to O. lupi in both Europe12–16 and the USA17–21, thus highlighting the urgent need for specific diagnostic tools to better understand the epidemiology of this zoonotic nematode.
In animal hosts, diagnosis of O. lupi infection relies on the identification of adult parasites in ocular nodules in symptomatic cases7, or on ultrasound examination and computed tomography in asymptomatic animals22. Regardless of the imaging techniques, ultimately, diagnosis is achieved by morphological and molecular analyses of subcutaneous mfs in skin biopsies7,8,23. However, this diagnostic approach is invasive, time-consuming, and may lead to false-negative results, since mfs detection is highly dependent on their anatomical location, density, prepatent period and/or previous microfilaricidal treatments, as well as on operator skills8. The performance of serological enzyme-linked immunosorbent assay (ELISA) kits developed for the detection of antibodies against Onchocerca gibsoni (i.e., Og4C3)24 and Dirofilaria immitis (DiroCHEK®, SNAP® Heartworm and SNAP® 4Dx® Plus)25, have been evaluated for serodiagnosis of O. lupi infection or to assess any cross-reactivity by testing sera from dogs with confirmed onchocerciasis. Similarly, the sensitivity and specificity of a western blot assay against O. lupi paramyosin26 has been evaluated, as well as the immunogenic properties of six reactive peptides from O. lupi Paramyosin (Ol-PARA) and Major Antigen (Ol-MJA)27. However, the reactivity of these peptides against sera of dogs positive for O. lupi infection is yet to be demonstrated.
This study aimed to assess the accuracy, specificity, and sensitivity of an indirect ELISA targeting a total of nine peptides, including the six linear epitopes previously characterized from Ol-PARA and Ol-MJA and three additional peptides from Ol-MJA.
Results
Identification of novel peptides and indirect ELISA optimization
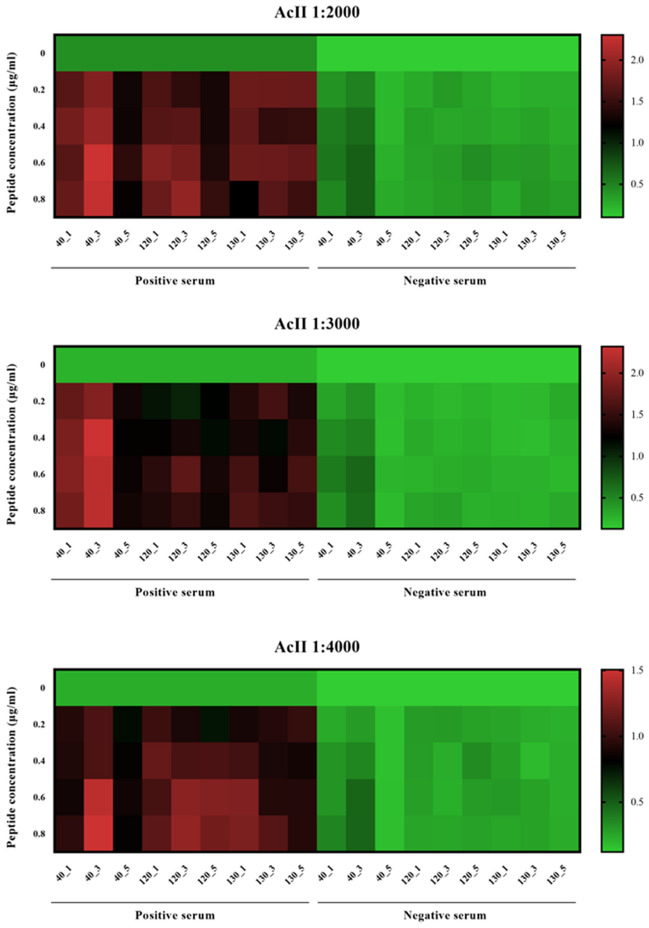
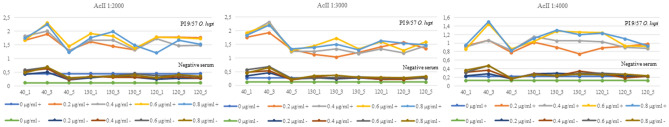
BLASTp analysis of the amino acid sequence of Ol-MJA displayed the highest identity (83.08%) with GenBank MCP9261943.1, a spindle-and centromere-associated protein from D. immitis. Sequence alignment revealed a three peptide-insertion in Ol-MJA (aa 8 to 11) (Fig. 1), hereafter referred to as 40_1: HSDALDKLRP; 40_3: RLKKDLIK; 40_5: VDGEGGSLSLS. All peptides were confirmed to be immunoreactive against the reference serum P1Ol 9/57 by indirect ELISA (Table 1). Checkerboard titration revealed an optimum peptide concentration of 0.2 μg/ml and an optimal dilution of anti-dog conjugate at 1:3000. The optimum P/N ratio was observed for all peptides examined, except for 130_1, 130_3 and 130_5 (Table 2). OD values up to 0.431 were observed as background binding for 0 μg/ml peptide concentration with horseradish peroxidase (HRP) anti-dog conjugate dilution at 1:2000 (Figs. 2, 3).
Figure 1.
Alignment of amino acid sequences of Major Antigen of Onchocerca lupi and of spindle-and centrosome-associated protein of Dirofilaria immitis. Peptides 40_1, 40_3 and 40_5 are indicated in bold.
Table 1.
Serum samples from Onchocerca lupi infected dogs identified according to adult, microfilaridermia detection (mfs loads) and identification and country of collection.
| ID samples | Country | Positivity | Mfs load | Proteins | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD Major antigen peptides | OD Paramyosin peptides | |||||||||||
| 130_1 | 130_3 | 130_5 | 40_1 | 40_3 | 40_5 | 120_1 | 120_3 | 120_5 | ||||
| OL-4 | USA | Clinical suspicion, serum only | – | 0.698 | 0.759 | 0.581 | 0.839 | 1.098 | 0.802 | 2.048 | 0.775 | 0.633 |
| OL-5 | USA | Clinical suspicion, serum only | – | 0.929 | 0.978 | 0.784 | 0.785 | 1.428 | 0.896 | 1.128 | 0.881 | 0.681 |
| OL-7 | USA | Clinical suspicion, serum only | – | 1.854 | 1.824 | 1.569 | 1.298 | 2.001 | 1.389 | 1.624 | 1.754 | 0.816 |
| OL-8 | USA | Adult: qPCR | – | 1.926 | 1.944 | 1.584 | 0.833 | 1.950 | 1.520 | 1.484 | 1.300 | 1.220 |
| OL-9 | USA | Clinical suspicion, serum only | – | 0.995 | 1.233 | 1.001 | 0.984 | 1.392 | 1.538 | 1.228 | 0.975 | 0.981 |
| OL-14 | USA | Adult: qPCR | – | 1.184 | 1.151 | 0.987 | 1.405 | 1.359 | 1.217 | 1.388 | 1.169 | 1.291 |
| OL-17 | USA | Clinical suspicion, serum only | – | 0.933 | 1.081 | 0.923 | 0.918 | 1.173 | 1.313 | 1.316 | 1.022 | 0.944 |
| OL-19 | USA | Adult: qPCR | – | 0.549 | 0.656 | 0.485 | 0.846 | 0.795 | 0.660 | 0.764 | 0.566 | 0.691 |
| OL-22 | USA | Clinical suspicion, serum only | – | 0.713 | 0.765 | 0.632 | 0.736 | 0.674 | 0.934 | 1.123 | 0.762 | 0.441 |
| OL-24 | USA | Adult: qPCR | – | 1.105 | 1.266 | 0.904 | 1.258 | 1.461 | 1.412 | 1.475 | 0.881 | 0.805 |
| OL-28 | USA | Adult: qPCR | – | 0.980 | 0.961 | 1.451 | 1.131 | 1.276 | 1.016 | 0.984 | 0.886 | 0.879 |
| OL-30 | USA | Conjunctival tissue: qPCR | – | 1.142 | 1.129 | 1.003 | 1.191 | 1.251 | 1.089 | 1.406 | 1.340 | 1.218 |
| OL-32 | USA | Conjunctival tissue: qPCR | – | 0.585 | 0.766 | 0.420 | 0.707 | 0.835 | 0.587 | 0.691 | 0.573 | 0.588 |
| OL-33 | USA | Conjunctival tissue: qPCR | – | 0.546 | 0.717 | 0.564 | 1.290 | 1.531 | 1.333 | 1.039 | 0.806 | 0.752 |
| OL-34 | USA | Conjunctival tissue: qPCR | – | 0.375 | 0.438 | 0.444 | 0.780 | 0.767 | 0.928 | 0.422 | 0.348 | 0.402 |
| OL-35 | USA | Adult: qPCR | – | 0.794 | 0.938 | 0.786 | 0.995 | 1.544 | 1.097 | 1.358 | 1.045 | 1.168 |
| OL-36 | USA | Clinical suspicion, serum only | – | 0.603 | 0.610 | 0.566 | 0.558 | 0.720 | 0.713 | 0.729 | 0.515 | 0.493 |
| NINA | Portugal | Skin: mfs, qPCR | – | 0.947 | 0.905 | 0.645 | 0.927 | 1.378 | 1.130 | 1.786 | 1.730 | 1.625 |
| P0 Ol 9/57 | Portugal | Skin: mfs, qPCR | 35 | 2.044 | 1.891 | 1.224 | 1.492 | 2.464 | 2.026 | 2.366 | 1.728 | 1.892 |
| P0 Ol 1/32 | Portugal | Skin: mfs, qPCR | 23 | 0.924 | 1.073 | 0.796 | 0.992 | 1.220 | 1.099 | 1.464 | 1.036 | 0.659 |
| P0 Ol 3/4 | Portugal | Skin: mfs, qPCR | 2 | 0.936 | 0.456 | 0.433 | 0.707 | 0.835 | 0.587 | 0.416 | 0.309 | 0.343 |
| P0 Ol 6/58 | Portugal | Skin: mfs, qPCR | 1 | 0.936 | 0.731 | 1.059 | 1.039 | 1.079 | 1.237 | 1.626 | 1.200 | 1.045 |
| P0 Ol 11/53 | Portugal | Skin: mfs, qPCR | 1 | 1.915 | 1.466 | 1.686 | 1.307 | 1.477 | 1.623 | 2.020 | 1.871 | 1.819 |
| P1 Ol 1/32 | Portugal | Skin: mfs, qPCR | 4 | 0.924 | 1.106 | 0.952 | 0.992 | 1.220 | 1.099 | 0.858 | 0.890 | 1.017 |
| P1 Ol 10/63 | Portugal | Skin: mfs, qPCR | 1 | 2.205 | 2.157 | 1.867 | 1.855 | 2.659 | 2.884 | 2.384 | 2.257 | 2.486 |
| P1 Ol 7/42 | Portugal | Skin: mfs, qPCR | 4 | 0.519 | 0.533 | 0.518 | 0.781 | 0.955 | 0.871 | 0.858 | 0.890 | 1.017 |
| P1 Ol 9/57 | Portugal | Skin: mfs, qPCR | 18 | 2.314 | 2.054 | 1.067 | 1.815 | 2.366 | 1.768 | 0.910 | 0.810 | 1.572 |
| P1 Ol 6/58 | Portugal | Skin: mfs, qPCR | 1 | 1.740 | 1.798 | 1.695 | 1.803 | 2.286 | 2.123 | 1.905 | 1.776 | 1.943 |
| P2 Ol 6/58 | Portugal | Skin: mfs, qPCR | 1 | 2.643 | 2.962 | 3.270 | 2.254 | 2.727 | 2.757 | 2.334 | 1.634 | 1.998 |
| P2 Ol 11/53 | Portugal | Skin: mfs, qPCR | 1 | 2.021 | 2.104 | 2.024 | 1.517 | 1.736 | 1.949 | 1.766 | 1.521 | 1.448 |
| P2 Ol 1/32 | Portugal | Skin: mfs, qPCR | 2 | 1.079 | 1.384 | 1.021 | 0.859 | 1.171 | 1.314 | 1.078 | 0.839 | 0.847 |
| P2 Ol 7/42 | Portugal | Skin: mfs, qPCR | 1 | 1.453 | 1.237 | 1.358 | 1.586 | 1.593 | 2.362 | 1.480 | 1.637 | 1.515 |
| P2 Ol 9/57 | Portugal | Skin: mfs, qPCR | 1 | 1.952 | 1.565 | 1.536 | 1.518 | 1.539 | 2.238 | 2.259 | 1.709 | 1.311 |
| P2 Ol 10/63 | Portugal | Skin: mfs, qPCR | 1 | 1.107 | 1.092 | 0.983 | 1.124 | 1.348 | 1.487 | 1.418 | 1.185 | 1.349 |
| P3 Ol 1/32 | Portugal | Skin: mfs, qPCR | 7 | 0.953 | 0.952 | 0.866 | 0.657 | 0.651 | 1.158 | 1.249 | 1.084 | 0.829 |
| P3 Ol 7/42 | Portugal | Skin: mfs, qPCR | 3 | 0.575 | 0.668 | 0.681 | 0.591 | 0.753 | 0.791 | 0.597 | 0.671 | 0.641 |
| P3 Ol 11/53 | Portugal | Skin: mfs, qPCR | 2 | 1.181 | 1.355 | 1.219 | 0.948 | 1.294 | 1.140 | 0.784 | 1.066 | 1.180 |
| P3 Ol 9/57 | Portugal | Skin: mfs, qPCR | 9 | 1.935 | 1.375 | 1.652 | 1.277 | 1.670 | 1.939 | 1.882 | 1.609 | 1.490 |
| P4 Ol 1/32 | Portugal | Skin: mfs, qPCR | 5 | 1.318 | 1.601 | 1.336 | 1.392 | 0.928 | 1.288 | 1.209 | 0.952 | 1.125 |
| P4 Ol 3/4 | Portugal | Skin: mfs, qPCR | 5 | 0.503 | 0.579 | 0.400 | 0.592 | 0.922 | 0.655 | 0.684 | 0.734 | 0.877 |
| P4 Ol 5/55 | Portugal | Skin: mfs, qPCR | 8 | 2.425 | 1.424 | 1.446 | 1.469 | 1.512 | 1.592 | 1.786 | 1.619 | 1.719 |
| P4 Ol 7/42 | Portugal | Skin: mfs, qPCR | 14 | 0.543 | 0.704 | 0.618 | 0.551 | 0.568 | 0.853 | 0.909 | 0.850 | 0.875 |
| P4 Ol 9/57 | Portugal | Skin: mfs, qPCR | 29 | 1.454 | 1.255 | 1.304 | 1.499 | 1.567 | 1.646 | 1.730 | 2.014 | 1.624 |
| P4 Ol 10/63 | Portugal | Skin: mfs, qPCR | 10 | 2.117 | 1.887 | 1.879 | 2.132 | 2.929 | 2.323 | 2.031 | 1.863 | 2.342 |
| Lindo | UKa | Adult: qPCR | – | 0.601 | 0.545 | 0.763 | 0.575 | 0.829 | 0.765 | 0.636 | 0.599 | 0.543 |
| RUCA 15 | Portugal | Skin: mfs, qPCR | – | 1.497 | 1.186 | 1.255 | 1.593 | 2.111 | 2.507 | 1.418 | 1.253 | 1.393 |
| Peluda 14 | Portugal | Skin: mfs, qPCR | – | 1.176 | 0.793 | 0.892 | 0.774 | 1.547 | 1.701 | 1.188 | 1.002 | 1.061 |
| OLD 14 | Portugal | Skin: mfs, qPCR | – | 1.231 | 1.010 | 1.247 | 0.946 | 1.544 | 1.185 | 1.193 | 1.352 | 1.382 |
| Labrador | Portugal | Skin: mfs, qPCR | – | 1.775 | 1.678 | 1.803 | 1.725 | 2.087 | 2.555 | 1.629 | 1.490 | 1.510 |
| CMD5 | Portugal | Skin: qPCR | nd | 0.935 | 1.049 | 0.855 | 0.482 | 0.559 | 0.497 | 0.372 | 0.474 | 0.405 |
| CM D11 | Portugal | Skin: qPCR | nd | 1.034 | 1.516 | 1.354 | 0.685 | 0.708 | 0.787 | 0.922 | 0.947 | 0.721 |
| CM D13 | Portugal | Skin: qPCR | nd | 1.151 | 0.952 | 1.394 | 1.216 | 0.895 | 2.556 | 1.865 | 1.765 | 2.072 |
| CM D18 | Portugal | Skin: qPCR | nd | 1.013 | 1.059 | 1.324 | 0.707 | 1.265 | 1.161 | 1.033 | 0.754 | 0.892 |
| CM D21 | Portugal | Skin: qPCR | nd | 1.156 | 1.030 | 1.726 | 1.882 | 2.530 | 2.042 | 1.689 | 1.278 | 2.502 |
The optical density (OD) value for each peptide examined is indicated.
qPCR: quantitative PCR.
aAnimal imported from Portugal.
Table 2.
OD450 ratio (P/N value) between Onchocerca lupi positive (P1Ol 9/57) and negative (helminth free dog) reference sera, according to anti-dog conjugate dilutions and peptide concentrations tested.
| Peptide concentration | Peptides | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40_1 | 40_3 | 40_5 | 130_1 | 130_3 | 130_5 | 120_1 | 120_3 | 120_5 | |
| Anti-dog conjugate dilution 1:2000 | |||||||||
| 0 μg/ml | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| 0.2 μg/ml | 4.0 | 3.8 | 6.1 | 5.7 | 3.9 | 4.3 | 7.5 | 6.5 | 6.5 |
| 0.4 μg/ml | 3.4 | 3.2 | 5.9 | 4.8 | 5.5 | 4.2 | 6.0 | 4.5 | 5.4 |
| 0.6 μg/ml | 3.0 | 3.4 | 5.7 | 5.7 | 5.1 | 3.1 | 4.8 | 4.6 | 5.4 |
| 0.8 μg/ml | 3.6 | 3.3 | 4.3 | 5.5 | 5.7 | 3.8 | 4.1 | 4.2 | 4.3 |
| Anti-dog conjugate dilution 1:3000 | |||||||||
| 0 μg/ml | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 |
| 0.2 μg/ml | 5.0 | 4.5 | 6.3 | 4.2 | 4.2 | 4.6 | 6.5 | 6.7 | 4.4 |
| 0.4 μg/ml | 3.9 | 4.3 | 6.3 | 4.1 | 5.1 | 4.1 | 6.0 | 5.6 | 5.3 |
| 0.6 μg/ml | 3.4 | 3.3 | 5.7 | 5.6 | 6.0 | 4.4 | 5.8 | 4.7 | 6.6 |
| 0.8 μg/ml | 4.0 | 3.4 | 6.1 | 4.1 | 4.1 | 4.6 | 5.8 | 5.8 | 4.7 |
| Anti-dog conjugate dilution 1:4000 | |||||||||
| 0 μg/ml | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| 0.2 μg/ml | 3.9 | 3.7 | 4.8 | 3.6 | 3.1 | 2.8 | 3.5 | 4.1 | 4.6 |
| 0.4 μg/ml | 3.0 | 3.0 | 5.1 | 4.1 | 4.6 | 3.1 | 3.7 | 4.9 | 3.9 |
| 0.6 μg/ml | 2.8 | 3.1 | 5.1 | 3.8 | 5.7 | 4.4 | 4.2 | 3.5 | 4.1 |
| 0.8 μg/ml | 2.6 | 3.2 | 4.8 | 4.4 | 5.2 | 4.5 | 4.9 | 4.2 | 4.1 |
Figure 2.
Heatmap from positive and negative serum samples for Onchocerca lupi tested against different concentrations of peptides and anti-dog conjugate dilutions at 1:2000, 1:3000 and 1:4000.
Figure 3.
Checkerboard titration of each peptide and anti-dog dilution. Raw data obtained from positive and negative reference sera are indicated by dots. + : Positive serum; −: Negative serum.
The highest P/N values were observed for peptides 40 (up to 6.3 for 40_5) and 120 (up to 6.7 for 120_3). In addition, peptide 40_3 resulted in the highest OD values (i.e., up to 2.318) when testing P1Ol 9/57 for all anti-dog conjugate dilutions and peptide concentrations examined (Fig. 3). The minimum background noise was observed using the blocking reagent (Roche), which was confirmed as optimum for the indirect ELISA.
Initial validation of peptides for diagnosis of Onchocerca lupi infection by indirect ELISA
When testing canine sera from dogs positive for O. lupi, the highest (3.270) and lowest (0.309) OD values were recorded for peptides 130_5 and 120_3, respectively (Table 1). No correlation was observed between OD values and skin mfs burden for any of the peptides (Table 1).
The optimal discrimination and the best predictive performance of the ELISA assay, determined by AUC values (ranging from 0.9603 for peptide 120_3 to 0.9959 for peptide 130_1), were confirmed by analysis of the ROC curves of positive sera for all peptides (Table 3). Sensitivity and specificity values ranging from 85.45% to 100%, and 88.89% to 100%, respectively, were recorded for all peptides examined. In particular, the highest specificity (100%) was observed for peptides 40_3, 40_5, 130_3 and 120_3, whilst peptides 40_1 and 130_5 returned the highest sensitivity (100%) (Table 3). The highest optimal cut-off value (0.765) was observed for peptide 40_5, followed by 40_3 (0.708); the lowest cut-off value was detected for peptides 40_1 (0.4) and 130_5 (0.42) (Table 3).
Table 3.
Receiver operating characteristic (ROC) data for serum samples of dogs with confirmed or clinically suspected Onchocerca lupi infection.
| Peptides | Optimal cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|
| Major antigen | ||||||
| 40_1 | 0.4 | 100 | 88.89 | 94.83 | 100 | 0.9838 |
| 40_3 | 0.708 | 92.59 | 100 | 100 | 87.1 | 0.9918 |
| 40_5 | 0.765 | 88.89 | 100 | 100 | 81.82 | 0.9842 |
| 130_1 | 0.503 | 96.3 | 96.3 | 96.43 | 100 | 0.9959 |
| 130_3 | 0.533 | 94.55 | 100 | 100 | 90 | 0.9933 |
| 130_5 | 0.42 | 100 | 88.89 | 94.83 | 100 | 0.9838 |
| Paramyosin | ||||||
| 120_1 | 0.684 | 89.09 | 96.3 | 98 | 81.25 | 0.9704 |
| 120_3 | 0.671 | 85.45 | 100 | 100 | 77.14 | 0.9603 |
| 120_5 | 0.633 | 87.27 | 96.3 | 97.96 | 78.79 | 0.9727 |
PPV: positive predictive value; NPV: negative predictive value; AUC: area under the curve.
Sensitivity and specificity of indirect ELISA testing using canine sera positive for other filarioid nematodes
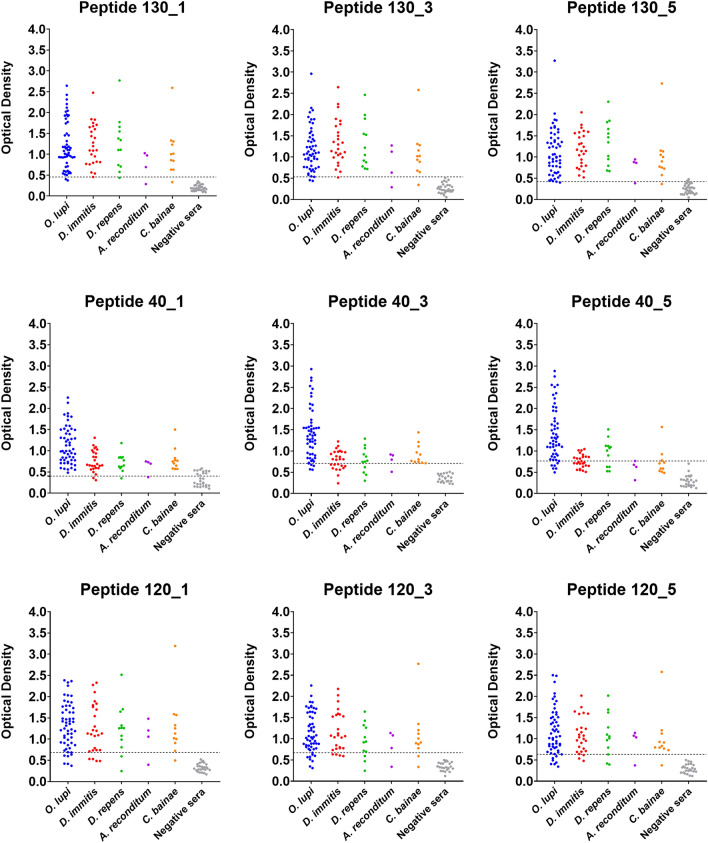
ROC analysis returned the lowest AUC values for peptide 40_1 (0.8833) followed by peptide 120_3 (0.8611) against sera of dogs positive for Acanthocheilonema reconditum. The highest AUC values (= 1) were observed for peptide 130_1 against D. immitis and D. repens, and 130_3 and 130_5 against D. repens (Table 4). Overall, lower specificity values (ranging from 75 to 96.43%) were recorded for peptides 40_1, 40_3 and 40_5 against sera of dogs positive for other filarioid nematodes. Conversely, a specificity of 100% was observed for peptides 130 and 120 (Table 4). Lower OD values were observed for peptides 40_3 and 40_5 against D. immitis, D. repens, A. reconditum and Cercopithifilaria bainae (OD up to 1.565), when compared to those observed against O. lupi (OD up to 2.929) (Fig. 4). Overall, suboptimal OD cut-off values (up to 0.613 for 40_3, against C. bainae) were observed for peptides 40 when compared with those against O. lupi (up to 0.765 for 40_5) (Tables 3 and 4).
Table 4.
ELISA parameters according to peptides of Major Antigen and Paramyosin proteins tested against serum samples of dogs infected by other common canine filarioid nematodes.
| Peptides | Filarioid nematodes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dirofilaria immitis | Dirofilaria repens | Cercopithifilaria bainae | Acanthocheilonema reconditum | |||||||||||||||||||||
| Cut-off | Se (%) | Sp (%) | PPV (%) | NPV (%) | AUC | Cut-off | Se (%) | Sp (%) | PPV (%) | NPV (%) | AUC | Cut-off | Se (%) | Sp (%) | PPV (%) | NPV (%) | AUC | Cut-off | Se (%) | Sp (%) | PPV (%) | NPV (%) | AUC | |
| Major antigen | ||||||||||||||||||||||||
| 40_1 | 0.557 | 84.62 | 85.71 | 61.2 | 85.71 | 0.8846 | 0.591 | 83.33 | 88.89 | 76.92 | 92.31 | 0.9182 | 0.569 | 100 | 80.65 | 62.5 | 100 | 0.9323 | 0.696 | 75 | 92.59 | 60 | 96.15 | 0.8333 |
| 40_3 | 0.559 | 92.31 | 78.57 | 80 | 91.67 | 0.8997 | 0.493 | 91.67 | 77.78 | 64.70 | 95.45 | 0.9198 | 0.613 | 100 | 96.43 | 90.9 | 100 | 0.9893 | 0.575 | 90.91 | 96.3 | 90.9 | 96.30 | 0.9798 |
| 40_5 | 0.507 | 100 | 75 | 78.79 | 100 | 0.9066 | 0.523 | 100 | 82.14 | 70.59 | 100 | 0.9435 | 0.484 | 100 | 77.78 | 64.7 | 100 | 0.8889 | 0.625 | 75 | 81.84 | 37.5 | 95.65 | 0.787 |
| 130_1 | 0.456 | 100 | 100 | 100 | 100 | 1 | 0.579 | 100 | 100 | 100 | 100 | 1 | 0.335 | 100 | 92.59 | 84.61 | 100 | 0.9933 | 0.286 | 100 | 88.89 | 57.14 | 100 | 0.9722 |
| 130_3 | 0.654 | 96.15 | 100 | 100 | 96.55 | 0.9973 | 0.718 | 100 | 100 | 100 | 100 | 1 | 0.645 | 90.91 | 100 | 100 | 100 | 0.9798 | 0.633 | 75 | 100 | 100 | 96.43 | 0.9074 |
| 130_5 | 0.52 | 100 | 94.44 | 92.86 | 100 | 0.9968 | 0.668 | 100 | 100 | 100 | 100 | 1 | 0.575 | 90.91 | 96.3 | 90.9 | 96.3 | 0.9798 | 0.382 | 100 | 85.19 | 100 | 100 | 0.963 |
| Paramyosin | ||||||||||||||||||||||||
| 120_1 | 0.711 | 84.62 | 96.43 | 95.65 | 87.1 | 0.9602 | 0.807 | 83.33 | 96.3 | 90.9 | 92.86 | 0.9167 | 0.723 | 90.91 | 96.15 | 90.9 | 96.15 | 0.972 | 1.056 | 75 | 100 | 100 | 100 | 0.9167 |
| 120_3 | 0.734 | 84.62 | 100 | 84.61 | 87.5 | 0.9815 | 0.697 | 75 | 100 | 100 | 90 | 0.8981 | 0.779 | 81.82 | 100 | 100 | 93.10 | 0.936 | 0.782 | 75 | 100 | 100 | 96.43 | 0.8611 |
| 120_5 | 0.535 | 96.15 | 89.29 | 89.28 | 96.15 | 0.9835 | 0.632 | 83.33 | 95.12 | 83.34 | 95.12 | 0.9451 | 0.733 | 90.91 | 100 | 100 | 96.43 | .09697 | 1.036 | 75 | 100 | 100 | 96.43 | 0.9167 |
Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; AUC: area under curve.
Figure 4.
Optical density (OD) obtained for all peptides examined according to each pathogen and negative control sera.
Discussion
In this study, we assessed the performance of an indirect ELISA based on primary antibody detection using specific peptides from two O. lupi proteins as antigens. We showed that all peptides are highly immunoreactive, also when tested against sera of dogs with low O. lupi microfilaridermia (i.e., OD = 2.643, mfs = 1). Our findings indicate that this non-invasive serological test may be applied to the detection of asymptomatic and/or amicrofilaremic infections, as well as of infections associated with aberrant sites of worm localisation28–30. Furthermore, the high positive predictive values (PPV) observed for some peptides (100% for 40_3, 40_5, 130_3 and 120_3) underscores the ability of this assay to discriminate between true vs. false positive results. Testing of dog sera with O. lupi infections revealed the high diagnostic accuracy of the indirect ELISA, as demonstrated by the high value of the AUC (> 0.9), as well as of specificity (100%) and sensitivity (from 85.45 to 94.55%) recorded for some of the peptides belonging to the Major Antigen (40_3, 40_5, 130_3) and Paramyosin (120_3) proteins. In addition, the robustness of this assay is also demonstrated by the high cut-off values recorded for peptide 40_3 and 40_5 (i.e., 0.708 and 0.765, respectively). However, the ELISA displayed a moderate cross-reactivity with lower specificity when peptides 40_3 and 40_5 were tested against canine sera from dogs infected by other filarioids (i.e., from: 75% for D. immitis to 96.43% for C. bainae), as well as lower overall cut-off threshold (up to 0.613 for C. bainae) and OD values (OD up to 1.565 for C. bainae) when compared with those for O. lupi (OD up to 2.929). The latter observation is of particular relevance, as it indicates that the ELISA with peptides 40_3 and 40_5 may support the diagnosis of canine O. lupi infection, also given the lower cut-off values for other filarioid nematodes (i.e., D. immitis, D. repens) that might be responsible for co-infections5,31–33 of dogs living in endemic areas, such as USA and Portugal34,35. Furthermore, these data may suggest that the ELISA may support screening of D. immitis-experimentally infected dogs36.
A limitation of this study is the unavailability of sera from dogs infected by other helminth species; nevertheless, the cross-reactivity between sera of dogs for which O. lupi infection is either suspected or confirmed and those of dogs infected with the most common filarioid species was assessed. In particular, moderate to high reactivity (sensitivity, 100%; specificity, 100%; OD ~ 2.5) was observed for some peptides against sera from animals positive for D. immitis and D. repens (i.e., peptides 130) and C. bainae and A. reconditum (i.e., peptides 120). These data contrast our previous finding obtained using microarray-based epitope mapping27 and highlight the limitations of this technology for high-throughput screening of sera37.
Overall, based on our data, peptides 40_3 and 40_5 yielded the best results for screening of canine O. lupi infection. Nevertheless, given that, thus far, no other ‘gold standard’ is available for diagnosis of canine onchocerciasis, we recommend that, until further validation can be carried out using additional independent assays, our ELISA test should be paired with microscopy-based and molecular detection tests, including conventional and real-time PCR7,8,38,39.
Moreover, although beyond the aim of our study, the reactivity of peptides 120 and 130 against infections by other filarioid species (C. bainae and A. reconditum and D. immitis and D. repens), deserves further investigation, as does the applicability of our assay to the diagnosis of feline infection by O. lupi. Indeed, cases of feline infections by this parasite are increasingly being reported (e.g., in Portugal, USA, and Romania), thus raising questions on the potential role of cats as reservoir of infection40–42. Furthermore, given that cases of O. lupi infections are being identified in animals from geographical areas where this parasite is considered non-endemic (e.g., UK) or of previously unknown endemicity (Israel)43,44, alongside cases of human infection5,12, the availability of a rapid, specific and sensitive tool for serodiagnosis of O. lupi infection is urgently needed, as it will assist the implementation of surveillance programmes aimed to investigate the geographic distribution and the epidemiology of this parasite. In turn, knowledge of O. lupi distribution will enhance current understanding of parasite epidemiology and fundamental biology, as well as risk of zoonotic transmission. Such efforts may also be aided by the determination of the O. lupi genome and/or transcriptome, and subsequent identification of additional epitopes for specific and accurate diagnosis of infection.
Materials and methods
Ethics statement
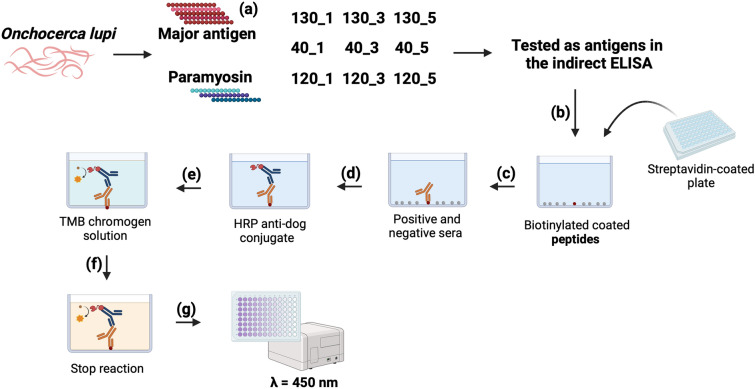
The study was conducted according to the Guidelines on Good Clinical Practices (The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines and Information Technology Unit, VICH Topic GL9; https://www.emea.eu.int/pdfs/vet/vich/059598en.pdf). The procedures were approved by the ethical commission at the University of Èvora (identification number: AE02Fila2013), complying with Portuguese legislation for the protection of animals (Decree-Law no. 113/2013), by Texas A&M University’s Approval of Animal Use Protocol (IACUC 2022-0261 CA) and by the Ethics Committee of the Department of Veterinary Medicine of the University of Bari, Italy (Prot. Uniba 12/20). The methods were carried out in accordance with the regulations of the university and with the recommendations in the ARRIVE guidelines. A flowchart outlining the procedures leading to the development of the indirect ELISA assay described in this study is available from Fig. 5.
Figure 5.
Schematic flowchart outlining the development of the indirect ELISA assay for serodiagnosis of Onchocerca lupi. Identification of new peptides from Major antigen protein (a). Coated peptides (antigen) onto wells of ELISA plate (b) interact with the first antibody from positive and negative canine serum samples (c). Adding the secondary antibody (conjugated antibody-HRP) (d). The reaction is developed by adding a substrate (e) which is cleaved by the conjugated enzyme and changes the reaction color after incubation (f). Results are read by ELISA plate reader (G). The figure was created with BioRender.com.
Identification and synthesis of Onchocerca lupi linear peptides
The amino acid (aa) sequence of Ol-MJA protein27 was compared with those of Onchocercidae (taxid: 6296) and Nematoda (taxid: 6231) species, available from the NR protein database, using BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins)45.
The newly identified peptides, alongside those previously described from Ol-MJA and from Ol-PARA (27; Ol-MJA: 130_1: LQNDQLQSEIQRLR; 130_3: IGRIEKLELERNEY; 130_5: QREAIESSLNALE; Ol-PARA: 120_1: LEEARRRLE; 120_3: SRLQSEVEVLIVDL; 120_5: MQVDEEHKMF) were synthesized as biotinylated synthetic peptides with a (Gly)4 linker (Purity ≥ 95%, N-Terminal modification, Biotin) and purchased from GenScript Biotech (Rijswijk, Netherlands). All peptides were tested as antigens in the indirect ELISA.
Assay standardization
The checkerboard titration method46 was used for optimization of the peptide concentration and conjugate anti-dog dilutions. Briefly, streptavidin-coated High-Capacity 96 well Plates (Thermo Fisher Scientific, Rockford, USA) were activated and rinsed three times with 200 μl PBS + 0.1% Tween-20 (PBS-T, washing buffer). Plates were incubated overnight at 4 °C with 100 μl of the selected biotinylated peptides diluted from 0.2 to 0.8 μg/ml in carbonate buffer, pH 9.6. The plates were subsequently rinsed three times with PBS-T under continuous shaking at 300 rpm at 35 °C for 30 min, thereby eliminating unbound peptides. Plates were blocked with 200 μl Blocking Reagent (Roche Diagnostics, Mannheim, Germany, GmBH) at 35 °C, 300 rpm for 30 min and washed three times with PBS-T 01%. Blocking with other reagents (i.e., PBS + 1% skim milk powder, buffer solution with gelatine) was also tested. Plates were dried by inversion on paper towel and 100 μl of positive and negative reference serum samples subjected to dilution in PBS-T at 1:40 and then incubated for 1 h at 35 °C and 300 rpm. Plates were washed four times with PBS-T and, once completely dried, incubated with 100 μl of HRP anti-dog conjugate, diluted at 1:2000, 1:3000 and 1:4000 (Invitrogen goat anti-canine IgG, Thermo Fisher Scientific, Waltham, USA), at 35 °C and 300 rpm for 1 h. After washing and drying, plates were incubated with 100 μl of TMB chromogen solution (Tetramethyl Benzidine, Sigma-Aldrich, St. Louis, Missouri, USA) for 10 min at room temperature. The colorimetric reaction was terminated with 50 μl stop solution (Invitrogen, Thermo Fisher Scientific, Vienna, Austria). The plate was then read using Absorbance 96 Plate reader Enzo (Byonoy, Hamburger, Germany) at a wavelength (λ) of 450 nm. The optimal conditions were selected based on the highest OD450 ratio between reference positive O. lupi (P1Ol 9/57) and negative serum samples (P/N value), testing all peptides with concentration ranging from 0 to 0.8 μg/ml. Background binding was assessed by testing peptides at concentrations ranging from 0 to 0.8 μg/ml with positive O. lupi and negative reference sera and with dilutions of HRP anti-dog conjugate at 1:2000, 1:3000 and 1:4000, respectively.
ELISA validation using field canine sera
Sera from dogs with either suspected or confirmed O. lupi infection (n = 54), available from previous studies conducted in endemic areas of Portugal (n = 3731) and the USA (n = 1725) were tested (Table 1), alongside sera (n = 53) from dogs living in a non-endemic area for O. lupi (Apulia and Sicily regions, Italy) that had previously tested positive for common filarioids of dogs (i.e., D. immitis, D. repens, C. bainae and A. reconditum) (Supplementary Table S132,38,47–49). Sera from young dogs (n = 60) that had tested molecularly or serologically negative for helminth infections were also included as negative controls.
Statistical analysis for determination of cut-off value and ELISA sensitivity and specificity
The diagnostic sensitivity (Se) and specificity (Sp) of the ELISA test, and the optimal cut-off, were calculated by plotting the receiver operating characteristic (ROC) curves (plots of sensitivity against [1 − specificity]). The area under the ROC curve (AUC) was estimated by non-parametric integration50 to measure diagnostic accuracy. ROC analyses were performed using Rstudio Version 1.6.0 with maximize metric method.
Supplementary Information
Acknowledgements
The authors are grateful to Caroline Sobotyk and Nancy McLean for their support in sample collection.
Author contributions
M.S.L.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing-original draft, writing-review and editing. V.N.L.F.: Formal analysis, writing-review and editing. C.M.: Resources, writing-review and editing. M.A.K.: Resources, writing-review and editing. G.G.V.: Resources, writing-review and editing. C.C.: Writing-review and editing. D.O.: Conceptualization, Funding acquisition, investigation, project administration, resources, supervision, visualization, writing-original draft, writing-review and editing.
Funding
This research was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT) and MUR (PRIN-PNRR 2022).
Data availability
All data analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-53759-w.
References
- 1.Rojas A, Morales-Calvo F, Salant H, Otranto D, Baneth G. Zoonotic ocular onchocercosis by Onchocerca lupi. Yale J. Biol. Med. 2021;94:331–341. [PMC free article] [PubMed] [Google Scholar]
- 2.Rodonaja TE. A new species of nematode, Onchocerca lupi n. sp, from Canis lupus cubanensis Soobshchenyia. Akad. Nauk. Gruzinskoy SSR. 1967;45:715–719. [Google Scholar]
- 3.Otranto D, et al. Clinical case presentation and a review of the literature of canine onchocercosis by Onchocerca lupi in the United States. Parasit. Vectors. 2015 doi: 10.1186/s13071-015-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verocai GG, et al. Onchocerca lupi nematodes in dogs exported from the United States into Canada. Emerg. Infect. Dis. 2016;22:1477–1479. doi: 10.3201/eid2208.151918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruntmeir J, Kelly M, Ramos RAN, Verocai GG. Cutaneous filarioid nematodes of dogs in the United States: Are they emerging, neglected, or underdiagnosed parasites? Front. Vet. Sci. 2023;10:1128611. doi: 10.3389/fvets.2023.1128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unterköfler MS, Huck A, Silbermayr K, Fuehrer HP. Autochthonous Onchocerca lupi infection of a domestic dog in Austria. Parasit. Vectors. 2023;16:46. doi: 10.1186/s13071-023-05681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otranto, D. etal. Zoonotic Onchocercalupi infection in dogs, Greece and Portugal, 2011–2012. Emerg.Infect.Dis. 19, 2000–2003 10.3201/eid1912.130264 (2013) [DOI] [PMC free article] [PubMed]
- 8.Otranto, D. etal. Cutaneous distribution and circadian rhythm of Onchocercalupi microfilariae in dogs. PLoSNeglTropDis.7, e2585 10.1371/journal.pntd.0002585 (2013) [DOI] [PMC free article] [PubMed]
- 9.Hassan HK, et al. Isolation of Onchocerca lupi in dogs and black flies, California, USA. Emerg. Infect. Dis. 2015;21:789–796. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manoj RRS, et al. Molecular detection of zoonotic filarioids in Culex spp. from Portugal. Med. Vet. Entomol. 2021;35:468–477. doi: 10.1111/mve.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roe, C. C. etal. Biting midges (Diptera: Ceratopogonidae) as putative vectors of zoonotic Onchocercalupi (Nematoda: Onchocercidae) in northern Arizona and New Mexico, southwestern United States. FrontVetSci.10, 1167070 10.3389/fvets.2023.1167070 (2023). [DOI] [PMC free article] [PubMed]
- 12.Otranto D, et al. Case report: First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am. J. Trop. Med. Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otranto D, et al. Human ocular filariasis: Further evidence on the zoonotic role of Onchocerca lupi. Parasit. Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilhan HD, et al. Onchocerca lupi infection in Turkey: A unique case of a rare human parasite. Acta Parasitol. 2013;58:384–388. doi: 10.2478/s11686-013-0152-8. [DOI] [PubMed] [Google Scholar]
- 15.Mowlavi G, et al. Human ocular onchocerciasis caused by Onchocerca lupi (Spirurida, Onchocercidae) in Iran. J. Helminthol. 2014;88:250–255. doi: 10.1017/S0022149X1300006016. [DOI] [PubMed] [Google Scholar]
- 16.Hasanreisoglu M, et al. Case report: A human case of Onchocerca lupi mimicking nodular scleritis. Am. J. Trop. Med. Hyg. 2021;105:1782–1785. doi: 10.4269/ajtmh.21-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhard, M.L. etal. Zoonotic Onchocercalupi infection in a 22-month-old child in Arizona: first report in the United States and a review of the literature. Am.J.Trop.Med.Hyg. 88, 601–605 10.4269/ajtmh.12-0733 (2013) [DOI] [PMC free article] [PubMed]
- 18.Berry, R. etal. Zoonotic Onchocercalupi presenting as a sub-cutaneous nodule in a 10-year-old girl: Report of the second case in the United States and a review of the literature. In AbstractBookofASDPAnnualMeeting.Chicago,USA.OralPresentations (2014).
- 19.Dudley RWR, et al. A cervical spine mass caused by Onchocerca lupi. Lancet. 2015;386:1372. doi: 10.1016/S0140-6736(14)62255-8. [DOI] [PubMed] [Google Scholar]
- 20.Cantey, P.T. etal. The emergence of zoonotic Onchocercalupi infection in the United States—a case-series. ClinInfectDis. 62, 778–783 10.1093/cid/civ983 (2016) [DOI] [PMC free article] [PubMed]
- 21.Bowers Wu, D., et al. Neuroinvasive Onchocerca lupi infection in a ten-year-old girl. Case Rep. Infect. Dis. 2022;2022:9773058. doi: 10.1155/2022/9773058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchini D, et al. Image diagnosis of zoonotic onchocercosis by Onchocerca lupi. Vet. Parasitol. 2014;203:91–95. doi: 10.1016/j.vetpar.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Roe CC, et al. Complete mitochondrial genome of Onchocerca lupi (Nematoda, Onchocercidae) Mitochond. DNA B Resour. 2021;6:2572–2574. doi: 10.1080/23802359.2021.1960211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannelli, A. etal. A preliminary investigation of serological tools for the detection of Onchocercalupi infection in dogs. ParasitolRes. 113, 1989–1991 10.1007/s00436-014-3844-6 (2014). [DOI] [PubMed]
- 25.de Oliveira, C. S., Savadelis, M. D., McLean, N. J. & Verocai, G. G. Assessing the potential cross-reactivity using a commercial heartworm ELISA kits of serum from dogs naturally infected with Onchocercalupi. VetParasitol. 280, 109070 10.1016/j.vetpar.2020.109070 (2020) [DOI] [PubMed]
- 26.Campbell B, et al. Paramyosin of canine Onchocerca lupi: Usefulness for the diagnosis of a neglected zoonotic disease. Parasit. Vectors. 2016;9:493. doi: 10.1186/s13071-016-1783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latrofa MS, et al. Major antigen and paramyosin proteins as candidate biomarkers for serodiagnosis of canine infection by zoonotic Onchocerca lupi. PLoS Negl. Trop. Dis. 2021;15:e0009027. doi: 10.1371/journal.pntd.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallitsch K, et al. Extensive aberrant migration of Onchocerca lupi in a dog. Top. Companion Anim. Med. 2022;49:100666. doi: 10.1016/j.tcam.2022.100666. [DOI] [PubMed] [Google Scholar]
- 29.Papaioannou N, et al. Obstructive, granulomatous tracheitis caused by Onchocerca sp. in a dog. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004;51:354–357. doi: 10.1111/j.1439-0442.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 30.Alho AM, et al. Aberrant laryngeal location of Onchocerca lupi in a dog. Parasitol. Int. 2016;65:218–220. doi: 10.1016/j.parint.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Colella V, et al. Evaluation of oxfendazole in the treatment of zoonotic Onchocerca lupi infection in dogs. PLoS Negl. Trop. Dis. 2018;12:e0006218. doi: 10.1371/journal.pntd.0006218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otranto D, et al. Efficacy of a spot-on formulation containing moxidectin 2.5%/imidacloprid 10% for the treatment of Cercopithifilaria spp. and Onchocerca lupi microfilariae in naturally infected dogs from Portugal. Parasit. Vectors. 2021;14:199. doi: 10.1186/s13071-021-04704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verocai GG, Sobotyk C, Lamison A, Borst MM, Edwards EE. Autochthonous, zoonotic Onchocerca lupi in a South Texas dog, United States. Parasit. Vectors. 2021;14:203. doi: 10.1186/s13071-021-04707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean NJ, Newkirk K, Adema CM. Canine ocular onchocerciasis: A retrospective review of the diagnosis, treatment, and outcome of 16 cases in New Mexico (2011–2015) Vet. Ophthalmol. 2017;20:349–356. doi: 10.1111/vop.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dantas-Torres F, Otranto D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020;282:109113. doi: 10.1016/j.vetpar.2020.109113. [DOI] [PubMed] [Google Scholar]
- 36.Chandrashekar R, et al. Experimental Dirofilaria immitis infection in dogs: Effects of doxycycline and advantage Multi® administration on immature adult parasites. Vet. Parasitol. 2014;206:93–98. doi: 10.1016/j.vetpar.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Lagatie, O., Van Dorst, B., Stuyver, L. J. Identification of three immunodominant motifs with atypical isotype profile scattered over the Onchocercavolvulus proteome. PLoSNeglTropDis. 11, e0005330 10.1371/journal.pntd.0005330 (2017). [DOI] [PMC free article] [PubMed]
- 38.Latrofa MS, et al. A real-time PCR tool for the surveillance of zoonotic Onchocerca lupi in dogs, cats and potential vectors. PLoS Negl. Trop. Dis. 2018;12:e0006402. doi: 10.1371/journal.pntd.0006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laidoudi Y, et al. Molecular approach for the diagnosis of blood and skin canine filarioids. Microorganisms. 2020;8:1671. doi: 10.3390/microorganisms8111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labelle, A. L., Daniels, J. B., Dix, M., Labelle, P. Onchocercalupi causing ocular disease in two cats. VetOphthalmol. 14, 105–110 10.1111/j.1463-5224.2011.00911.x (2011). [DOI] [PubMed]
- 41.Maia C, et al. Onchocerca lupi nematode in cat, Portugal. Emerg. Infect. Dis. 2015;21:2252–2254. doi: 10.3201/eid2112.150061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tudor P, et al. Feline ocular onchocercosis by Onchocerca lupi: Phylogenetic insights and implication for veterinary health. Acta Trop. 2023;237:106723. doi: 10.1016/j.actatropica.2022.106723. [DOI] [PubMed] [Google Scholar]
- 43.Rojas A, Salant H, Yasur-Landau D, Tsarfati H, Baneth G. First report of Onchocerca lupi from Israel and confirmation of two genotypes circulating among canine, feline and human hosts. Parasitology. 2020;147:1723–1727. doi: 10.1017/S0031182020001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGarry JW, et al. Onchocerca lupi in imported dogs in the UK: Implications for animal and public health. BMC Vet. Res. 2022;18:66. doi: 10.1186/s12917-022-03169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altschul SF, et al. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 46.Crowther JR. The ELISA Guidebook. 2. Humana Press; 2009. [DOI] [PubMed] [Google Scholar]
- 47.Brianti E, et al. New insights into the ecology and biology of Acanthocheilonema reconditum (Grassi, 1889) causing canine subcutaneous filariosis. Parasitology. 2012;139:530–536. doi: 10.1017/S0031182011002198. [DOI] [PubMed] [Google Scholar]
- 48.Panarese R, et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Int. J. Parasitol. 2020;50:555–559. doi: 10.1016/j.ijpara.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Otranto, D. etal. Morphological and molecular data on the dermal microfilariae of a species of Cercopithifilaria from a dog in Sicily. Vet.Parasitol. 182, 221–229 10.1016/j.vetpar.2011.05.043 (2011). [DOI] [PubMed]
- 50.Greiner M, Sohr D, Göbel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this published article.