Abstract
Purpose
To report the clinical outcomes of large diameter deep anterior lamellar keratoplasty (DALK) and converted two-piece microkeratome-assisted mushroom keratoplasty (MK) for herpetic corneal scars.
Methods
In this single-centre study, large diameter (9 mm) DALK was attempted in consecutive patients with herpetic corneal scars. In case of macroperforation or unsatisfactory clearance of the optical zone, the procedure was intraoperatively converted to two-piece microkeratome-assisted MK. Outcome measures were best spectacle-corrected visual acuity (BSCVA), refractive astigmatism, endothelial cell density (ECD), immunologic rejection, herpetic recurrence and graft failure rates in the two groups.
Results
DALK was successfully performed in 98 of 120 eyes, while the remaining 22 eyes required intraoperative conversion to MK. At 5 years, mean logMAR BSCVA was 0.10 ± 0.12 in the DALK group and 0.09 ± 0.15 in the MK group (P = 0.75). Refractive astigmatism at 5 years was 2.8 ± 1.4 D in the DALK group and 3.0 ± 1.7 D in the MK group (P = 0.67). ECD was higher in the DALK group than in the MK group at all time points (P < 0.001), with a mean annual cell loss of 10.9% after MK and 4.2% after DALK. The 5-year risk for immunologic rejection (DALK: 3%, MK: 5%, P = 0.38), herpetic recurrence (DALK: 6%, MK: 9%, P = 0.38), and graft failure (DALK: 4%, MK: 5%, P = 0.75) were comparable in both groups.
Conclusion
Large diameter (9 mm) DALK yields excellent visual and clinical outcomes in eyes with herpetic corneal scars. In case of intraoperative complications, DALK can be converted to two-piece microkeratome-assisted MK to maximize the refractive benefit of a large diameter graft while minimizing the risk of endothelial failure.
Subject terms: Corneal diseases, Eye abnormalities
Introduction
Herpetic keratitis remains among the leading causes of corneal infection [1]. Although most cases can be successfully managed with medical therapy, those resulting in visually significant stromal scarring often require corneal transplantation for visual rehabilitation [2, 3]. In the setting of previous inflammation and extensive vascularisation, deep anterior lamellar keratoplasty (DALK) would be the ideal surgical approach to decrease the risk of infectious recurrence, immune rejection and graft failure in high-risk herpetic scars which typically have a healthy endothelium. Specifically, DALK via pneumatic dissection would be preferred over manual lamellar dissection techniques as the former reliably results in a smooth graft–host interface compatible with optimal vision [4–6]. However, pneumatic dissection in eyes with herpetic corneal scars is generally considered technically challenging due to the alterations in the stromal consistency leading to high rates of conversion to penetrating keratoplasty (PK). Thus, big bubble DALK is not routinely performed for cases of corneal scarring [7].
Nonetheless, in cases with a significant macro-perforation or unsatisfactory clearance within the optical zone because of a full-thickness opacity, a strategy to manage conversion from DALK to MK has evolved to a two-piece microkeratome-assisted mushroom keratoplasty (MK) [8]. The two-piece MK graft consists of a 9.0-mm “mushroom hat” to maximize the refractive benefit of a large anterior lamellar surface, and a small posterior lamella, 6.0-mm in diameter (i.e. the mushroom “stem”), to minimize replacement of the healthy recipient endothelium and thereby reduce the risk of alloimmune rejection [9, 10]. This sequential approach allows to safely attempt DALK in eyes with herpetic corneal scars foreseeing the possibility of converting to a full-thickness graft in case of intraoperative complications.
The present study evaluates the long-term clinical outcomes of large diameter (9 mm) DALK and converted two-piece microkeratome-assisted MK for herpetic corneal scars.
Methods
This retrospective interventional case series conducted at a single tertiary care referral centre (Ospedali Privati Forlì “Villa Igea”, Forlì, Italy) evaluated the outcomes of consecutive eyes with herpetic corneal scars that underwent keratoplasty between January 2012 and January 2020. The study adhered to the tenets of the 2013 Declaration of Helsinki and was prospectively approved by the local Institutional Review Board (Comitato Etico Ospedali Privati Forlì in Forlì, CE-OPF 2022-0006, Italy). Written informed consent for the surgery and research was obtained from all participants. Eyes with persistent epithelial defects, neurotrophic keratitis, prior history of keratoplasty or other corneal surgery were not included in the study.
The main outcome measures were best spectacle-corrected visual acuity (BSCVA), refractive astigmatism, endothelial cell density (ECD) and postoperative complication rates. Preoperatively, all patients underwent complete ophthalmologic examination including slit-lamp examination, BSCVA, manifest refraction, applanation tonometry, fundoscopy, specular microscopy (EM-3000; Tomey Gmbh, Erlangen, Germany) and anterior segment optical coherence tomography (CASIA, Tomey, Erlangen, Germany). DALK surgery was indicated for loss of visual acuity due to herpetic corneal scars involving the optical zone in the presence of a healthy endothelium. ECD was assessed by either specular microscopy or in-vivo confocal microscopy, as necessary. The endothelium was deemed as clinically functional if the preoperative central ECD was at least 600 cells/mm2.
Antiviral prophylaxis treatment with oral acyclovir and ganciclovir 0.15% ophthalmic gel daily was initiated at least 6 months prior to the surgery and slowly tapered over 2 years (Table 1). All surgeries were performed after that the eye had been quiescent with no episodes of HSV reactivation for at least 6 months.
Table 1.
Herpetic keratitis prophylaxis protocol.
| Period | Oral acyclovir | Topical 0.15% ganciclovir | Topical dexamethasone |
|---|---|---|---|
| Month -6 to surgery | 800-mg 2x daily | 1x daily | – |
| 0 to Week 2 | 800-mg 5x daily | 4x daily | Every 2 h |
| Week 2 to Month 3 | 800-mg 5x daily | 3x daily | Every 3 h |
| Month 3 to Month 6 | 800-mg 3x daily | 3x daily | 4x daily |
| Month 6 to Month 12 | 800-mg 2x daily | 2x daily | 2x daily |
| Month 12 to Month 24 | 400-mg 2x daily | 1x daily | 1x daily |
| Month 24 onwards | – | 1x daily | 1x daily |
The first running suture was removed 3 months after surgery, whereas the second one was removed after 1 year. Follow-up visits were scheduled at month 1, 3, 6, 12 and yearly thereafter. All patients had the potential for at least 2-year follow-up.
BSCVA was assessed using the Snellen visual acuity chart at 4 meters and manifest refraction was evaluated using the cross-cylinder technique for cylinder refinement. A calibrated slit-lamp biomicroscope was used to assess graft status and presence of complications. Postoperative ECD was assessed by noncontact specular microscopy (EM-3000; Tomey Gmbh, Erlangen, Germany) using automatic focusing and digital capture of 15 images of the central cornea. Graft rejection was diagnosed as the presence of epithelial rejection line, subepithelial or stromal infiltrates, keratic precipitates or anterior chamber cell reaction with or without clinically apparent increase in stromal thickness or clarity. Graft failure was defined as the occurrence of any repeat keratoplasty for all and any indication; or in the absence of a re-graft, a cornea that was cloudy and did not clear; or a cornea that was initially clear after surgery and subsequently became irreversibly opaque.
Surgical technique
DALK was performed according to the authors’ previously described technique [11], and summarised briefly as follows: Anaesthesia and akinesia were obtained through peribulbar injection of 10.0-mL of 0.75% ropivacaine solution. Initial partial-thickness deep trephination was carried out using a vacuum trephine calibrated within 100-μm from the thinnest anterior segment optical coherence tomography (AS-OCT, CASIA, Tomey, Tokyo, Japan) pachymetry value at the 9.0-mm zone. In cases with highly irregular peripheral corneal thickness (>100-μm difference in corneal thickness at 9.0-mm zone), peripheral intrastromal hydration using 1.0-mL of normal saline for every clock hour of corneal thinning was performed to increase the stromal volume and allow deep trephination [12]. From the base of the deep trephination, a DALK probe was inserted and advanced centripetally by about 1.0-mm. The probe was replaced by a DALK cannula, which was further advanced by another 1.0-mm along the same track before performing pneumatic dissection. Regardless of bubble formation, a 9.0-mm en bloc anterior keratectomy from the base of the deep trephination.
When pneumatic dissection with a type 1 big-bubble succeeded, the bubble roof was incised using a 15-degree blade under viscoelastic protection and the deep stroma was excised up to the trephination edge. In cases of type 2 bubble formation, manual layer-by-layer dissection followed by limited removal of the type 2 bubble roof within the 4.0-mm optical zone was performed, thereby leaving a 4.0- to 5.0-mm peripheral stromal crown of manual dissected tissue. When a mixed bubble formed, only the type 1 bubble roof was opened, leaving the type 2 bubble untouched and simply allowing the air to spontaneously reabsorb after surgery. In the remaining cases, viscoelastic-assisted dissection or manual layer-by-layer dissection was performed. A 9-mm anterior lamellar graft was prepared by means of a 400-mm microkeratome head and sutured in place using interrupted nylon 10-0 sutures.
When microperforation occurred, the procedure was completed after the anterior chamber was filled with air, and DALK was completed according to the authors’ institutional technique. In cases in which there was either unsatisfactory clearance of the optical zone of a full-thickness opacity or a macro-perforation of the Descemet membrane, the procedure was converted to a 2-piece microkeratome-assisted mushroom-shaped PK, according to the authors’ previously described technique [9]. After a 9.0-mm en bloc anterior keratectomy from the base of the deep trephination, the central 6.0-mm optical zone was marked with a hand-held circular trephine and excised full-thickness using corneal scissors leaving a 1.5-mm peripheral rim of host stroma. Using a 250.0-μm microkeratome head, the donor cornea was split into anterior and posterior lamellae and punched to 9.0-mm and 6.0-mm, respectively. The 6.0-mm donor posterior lamella was positioned and fitted within the central hole of the recipient bed without sutures while the 9.0-mm donor anterior lamella was placed on top and sutured to the recipient bed using interrupted nylon 10-0 sutures.
Statistical analysis
All data had been initially collected prospectively and recorded in the institutional database (Microsoft Excel 2013, Microsoft Corp., Washington, USA). Ten-year follow-up data were analysed in this study. Statistical analysis was performed using SPSS (version 27.0, IBM Corp., New York, USA). Values were expressed as mean and standard deviation for continuous variables and individual counts and percentages for categorical values. BSCVA values were converted to logarithm of minimum angle of resolution (logMAR) units. Endothelial cell loss was calculated by subtracting postoperative ECD from baseline donor ECD and then dividing by baseline donor ECD and multiplying by 100. Analysis of repeated measures using linear mixed models were used to evaluate the difference in BSCVA, refractive astigmatism and ECD between DALK and MK. Adjustment with Bonferroni correction was applied to multiple pairwise comparisons. Intergroup comparisons of categorical data were tested using Fisher exact test or Pearson chi-square test, as appropriate. Cumulative probability of immunologic rejection and graft survival was determined by Kaplan-Meier analysis. The level of statistical significance was set at 0.05.
Results
In total, DALK was attempted in 120 eyes of 120 patients (66 males, mean age 48 ± 20 years) with herpetic corneal scars and a mean central corneal thickness of 545.6 ± 91.8 μm. Pneumatic dissection succeeded in 76 (63%) cases (with 68 cases with type 1, 5 with type 2 and 3 with mixed bubble formation). Visco-bubble dissection was successfully performed in 6 (5%) cases, while DALK was completed by manual dissection in 16 (13%) cases. Of 120 eyes, 22 (18.3%) required conversion to MK due to macroperforation (n = 19) or unsatisfactory clearance of the optical zone (n = 3). Mean length of follow-up was 4.5 ± 2.0 years for DALK and 4.5 ± 1.9 years for MK.
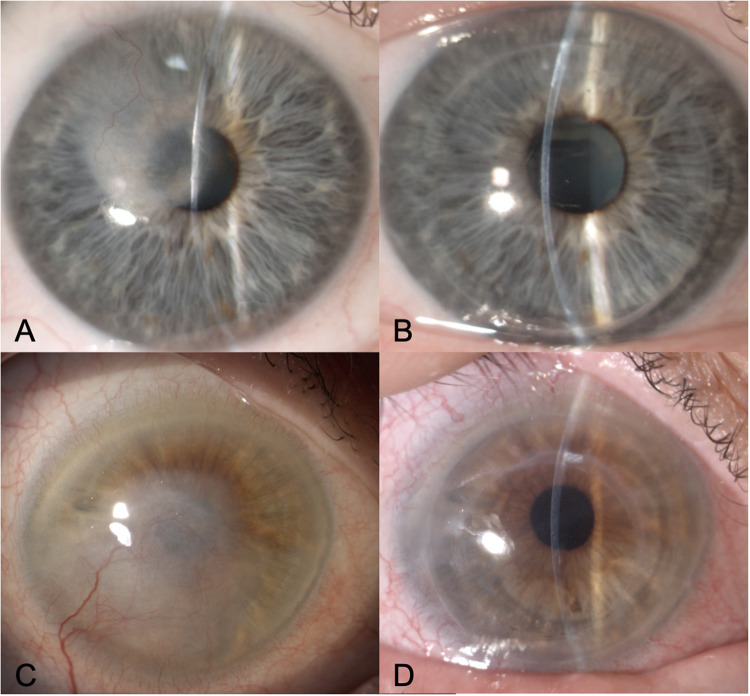
Figure 1 shows the preoperative and postoperative clinical pictures of two representative patients who underwent DALK and MK. Table 2 shows the visual outcomes in the two groups. No differences in BCVA between DALK and MK were observed at any time point. Excluding eyes with visual comorbidities, 5-year logMAR BSCVA was 0.10 ± 0.12 in the DALK group and 0.09 ± 0.15 in the MK group (P = 0.75).
Fig. 1. Clinical photos.
Preoperative and postoperative photos of two patients with vascularized herpetic corneal scarring who underwent deep anterior lamellar keratoplasty (A, B) and mushroom keratoplasty (C, D) respectively.
Table 2.
Clinical outcomes following DALK and MK for herpetic corneal scars.
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| Group | 1-year | 2-year | 3-year | 4-year | 5-year | |
| BSCVA (logMAR)* | DALK | 0.23 ± 0.17 (n = 92) | 0.17 ± 0.14 (n = 90) | 0.13 ± 0.13 (n = 71) | 0.11 ± 0.12 (n = 43) | 0.10 ± 0.12 (n = 33) |
| MK | 0.20 ± 0.14 (n = 20) | 0.20 ± 0.21 (n = 20) | 0.16 ± 0.17 (n = 19) | 0.11 ± 0.16 (n = 15) | 0.09 ± 0.15 (n = 11) | |
| P | 0.32 | 0.41 | 0.42 | 0.98 | 0.75 | |
| Refractive astigmatism (D)* | DALK | 3.9 ± 1.7 (n = 92) | 3.5 ± 1.6 (n = 90) | 3.3 ± 1.6 (n = 71) | 3.1 ± 3.3 (n = 43) | 2.8 ± 1.4 (n = 33) |
| MK | 3.9 ± 2.1 (n = 20) | 3.9 ± 2.1 (n = 20) | 3.5 ± 1.7 (n = 19) | 3.3 ± 1.4 (n = 15) | 3.0 ± 1.7 (n = 11) | |
| P | 0.99 | 0.33 | 0.61 | 0.62 | 0.67 | |
| ECD (cells/mm2) | DALK | 1939 ± 507 (n = 98) | 1899 ± 529 (n = 96) | 1867 ± 553 (n = 77) | 1851 ± 544 (n = 43) | 1826 ± 466 (n = 34) |
| MK | 1461 ± 412 (n = 22) | 1386 ± 446 (n = 21) | 1344 ± 412 (n = 20) | 1326 ± 445 (n = 15) | 1162 ± 174 (n = 11) | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
BSCVA Best spectacle-corrected visual acuity, ECD Endothelial cell density, D dioptre, DALK Deep anterior lamellar keratoplasty, logMAR logarithm of minimum angle of resolution, MK Mushroom keratoplasty.
*Excluding eyes with visually significant comorbidities in DALK (glaucoma (n = 2), age related macular degeneration (n = 3), epiretinal membrane (n = 2), cystoid macular oedema (n = 1)) and MK (age related macular degeneration (n = 1)).
Mean refractive astigmatism did not significantly differ between DALK and MK at any time point. One year postoperatively after complete suture removal, only 12% of DALK eyes and 14% of MK eyes had refractive astigmatism >4.5 D (P = 0.68). As shown in Table 2, ECD was significantly higher in the DALK group than in the MK group at all time points. Mean annual endothelial cell loss was 10.9% after MK, and 4.2% after DALK.
Table 3 summarises the postoperative complications in the two groups. The 5-year risk for immunologic rejection (DALK: 3%, MK: 5%, P = 0.38), herpetic recurrence (DALK: 6%, MK: 9%, P = 0.38), and graft failure (DALK: 4%, MK: 5%, P = 0.75) was comparable in both groups.
Table 3.
Complications following DALK and MK for herpetic corneal scars.
| DALK n = 98 | MK n = 22 | P | |
|---|---|---|---|
| Rebubbling | 5 (5%) | 1 (5%) | 0.91 |
| Elevated IOP | 2 (2%) | 0 (0%) | 0.50 |
| Cataract | 13 (13%) | 3 (14%) | 0.96 |
| Wound dehiscence | 0 (0%) | 0 (0%) | – |
| High astigmatism (RA > 4.5 D) | 12 (12%) | 3 (14%) | 0.86 |
| Immune rejection | 3 (3%) | 1 (5%) | 0.73 |
| Stromal rejection | 3 (3%) | 0 (0%) | 0.41 |
| Endothelial rejection | 0 (0%) | 1 (5%) | 0.03 |
| Herpetic recurrence | 6 (6%) | 2 (9%) | 0.61 |
| Graft failure | 4 (4%) | 1 (5%) | 0.92 |
IOP intraocular pressure, D dioptre, DALK deep anterior lamellar keratoplasty, MK mushroom keratoplasty, RA refractive astigmastism.
Discussion
Based on the results of the Collaborative Corneal Transplant Studies (CCTS), keratoplasty in eyes with herpetic keratitis has been traditionally classified at high risk for graft failure [13]. Both deep stromal vascularisation and viral latency confer increased rates of immunologic rejection and herpetic recurrence throughout the lifetime of the graft. Therefore, many corneal surgeons avoid performing corneal transplantation in such high-risk eyes.
In the last decade, however, innovations in surgical technique and instrumentation have significantly improved the outcomes of keratoplasty. Several groups have shown that DALK in eyes with HSV-related corneal scarring yields higher survival rates compared to standard PK [14–16]. However, the main concern in attempting DALK in these eyes is the difficulty in performing deep lamellar dissection due to poor intraoperative visibility and high risk of perforation [7]. It is important to note that in the previously published series, the techniques employed conventional graft diameter averaging 8.0 mm [14, 15]. In order to maximize the refractive advantage of DALK and achieve high-level visual acuity, larger diameter grafts would be desirable. However, should a conversion to PK be required, a 9 mm full-thickness graft would be significantly larger than the conventional 8–8.25 mm diameter, which is considered the optimal compromise to simultaneously minimize postoperative refractive error and the risk of immunologic rejection [9].
In recent years, however, the strategy to convert DALK to PK in case of unsuccessful or unsatisfactory lamellar dissection has evolved to a two-piece mushroom shaped graft [8, 17]. This technique has the advantages of maximizing the refractive benefit of the initially performed wide-diameter trephination while minimizing the risk of endothelial rejection conventionally associated with wide-diameter grafts. We have previously shown that 2-pieces MK converted from intended 9 mm DALK yields excellent visual and clinical results in eyes with keratoconus [9]. Thanks to the confidence that the outcomes of 2-piece MK are comparable to planned DALK, we have now adopted this sequential surgical approach for all eyes with stromal pathology and a healthy endothelium, including cases at high risk for conversion such as herpetic scars.
This study describes the long-term outcomes of 120 eyes with herpetic scars in which 9-mm DALK was attempted, 22 of which (18.3%) required intraoperative conversion to 2-piece MK for macroperforation or unsatisfactory clearance of the optical zone. Surprisingly, despite the presence of stromal scarring in all eyes, the conversion rate was in line with those previously reported for DALK performed in keratoconic eyes, which ranges from 14.9 to 35.3% [18–20]. This result stands in contrast with the recommendations from the Ophthalmic Technology Assessment report by the American Academy of Ophthalmology suggesting that manual dissection should be preferred in eyes with deep stromal scarring due to the low rates of big-bubble formation [7].
Best spectacle-corrected visual acuity did not differ significantly between successful DALK and converted MK at any time-point. This finding confirms that the optical quality of the microkeratome-dissected interface in MK is comparable with that obtained by means of pneumatic dissection in DALK. At 5 years, mean refractive astigmatism was 2.8 D after DALK and 3.0 D after MK, with no significant differences between the two groups. As expected given the larger diameter, these values compare favourably with those reported for 8-mm DALK performed for the same indication (5.6 D by Awan et al.) [15]. Minimizing post-surgical astigmatism is particularly important in patients with herpetic corneal scars who normally have a healthy fellow eye and may not be motivated to wear optical correction for a significant refractive error. In case of high astigmatism, our modified DALK technique with limited clearance of a 6-mm optical zone allows blunt relaxing incisions within the graft-host junction with minimal risk of perforation into the anterior chamber [21]. Similarly, since the stepped configuration of MK is self-sealing in case of limited dehiscence, it is thus possible to safely open the surgical wound at the steep meridian up to 2 clock hours to effectively reduce post-surgical astigmatism [8].
Herpetic reactivation can be immunologically triggered by local trauma during surgery, representing a major cause of corneal graft failure [22]. Antiviral prophylaxis has thereby become the standard for preventing HSV recurrence after keratoplasty and consequently reducing the rate of graft failure [23]. Nevertheless, there is still no consensus in terms of the optimal prophylactic regimen. The 5-year incidence of HSV recurrence after PK has been reported to be 22.1% in cases treated with acyclovir 200 mg 5 times daily for 3 months tapered to 400 mg 2 times daily for up to 18 months, and 15.8% in cases treated with acyclovir 800 mg 3–5 times daily tapered over 3 years. On the other hand, the presence of corneal neovascularisation in eyes with HSV-related stromal scarring confers a high-risk for immune rejection due to the violation of the corneal immune privilege [24]. Indeed, previous studies reported rejection rates up to 47.9% at 5 years following PK [16]. Although DALK eliminates the risk of endothelial rejection, stromal rejection is still possible. In a previous study by Awan et al., stromal rejection occurred in 44.4% of eyes which underwent DALK for herpetic corneal scarring [15]. By contrast, in this series the 5-year recurrence and rejection rates were only 4% and 3% after DALK and 5% and 6% after MK, respectively. This finding underscores the benefits of the initial high-dose and extended taper of antiviral and steroid prophylaxis to address the indefinite potential for both immunologic rejection and herpetic recurrence even years after corneal transplantation. Of note, we did not observe any case of persistent epithelial defect in this series. However, close monitoring for superficial punctate keratopathy is required in order to allow for prolonged topical antiviral prophylaxis.
As in other previous studies comparing DALK and PK [25], endothelial cell loss was higher following MK than following DALK. It should be noted however that in MK eyes endothelial cells were measured in the central 6-mm button. Since the button accounts for only 25% of the total endothelial population of the cornea, cell migration from the large peripheral reservoir across the posterior graft-host junction could explain why there were episodes of graft failure secondary to endothelial decay in the absence of rejection [9]. The low rate of immunologic rejection, herpetic recurrence and endothelial cell loss likely contributed to the excellent overall 5-year survival, which was 96% after DALK and 95% after MK, with no significant differences between the two procedures. These rates compare favourably with those reported after conventional PK by Wu et al. (78.8% at 5 years) [16] and Halberstadt et al. (40.9% at 5 years) [26], while are in line with those reported for primary MK (96% at 5 years) [17].
The present study has some limitations such as the retrospective design. Moreover, the relatively small sample size limits the power and generalizability of our analyses. Since the series reflects the results of two experienced surgeons at a tertiary referral centre, external validation in other clinical settings would be important. Finally, studies with longer follow-up are still required to assess whether the higher endothelial cell loss observed in MK would result in decreased long-term graft survival.
In conclusion, large-diameter big bubble DALK can be successfully performed in eyes with herpetic corneal scars. In case of intraoperative complications, 9-mm DALK can be converted to two-piece microkeratome-assisted MK to maximize the refractive benefit of a large diameter graft while minimizing the risk of endothelial failure.
Summary
What was known before
Herpetic corneal scarring often requires corneal transplantation for visual rehabilitation.
Penetrating keratoplasty provides suboptimal results including poor visual outcomes and limited graft survival due to immunologic rejection or herpetic recurrence.
What this study adds
Large diameter (9mm) DALK can be successfully performed in in eyes with herpetic corneal scars.
In case of intraoperative complications, conversion to two-piece microkeratome-assisted mushroom keratoplasty maximizes the refractive benefit of a large diameter graft while minimizing the risk of endothelial failure.
Supplementary information
Author contributions
Conceptualization, MP, ACY and MB; methodology, MP, ACY, RS, CB, GZ and MB; investigation, MP, ACY and MB; writing—original draft preparation, MP, ACY and RS; writing—review and editing, CB, GZ and MB.
Data availability
The dataset used and analysed during the current study is available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02744-0.
References
- 1.Kaye SB, Baker K, Bonshek R, Maseruka H, Grinfeld E, Tullo A, et al. Human herpesviruses in the cornea. Br J Ophthalmol. 2000;84:563–71. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobo LM, Coster DJ, Rice NS, Jones BR. Prognosis and management of corneal transplantation for herpetic keratitis. Arch Ophthalmol. 1980;98:1755–9. doi: 10.1001/archopht.1980.01020040607002. [DOI] [PubMed] [Google Scholar]
- 3.Ficker LA, Kirkness CM, Rice NS, Steele AD. The changing management and improved prognosis for corneal grafting in herpes simplex keratitis. Ophthalmology. 1980;96:1587–96. doi: 10.1016/S0161-6420(89)32668-6. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Parente G, Sincich A, Tassinari G. Influence of graft-host interface on the quality of vision after deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2011;30:497–502. doi: 10.1097/ICO.0b013e3181d25e4d. [DOI] [PubMed] [Google Scholar]
- 5.Scorcia V, Giannaccare G, Lucisano A, Soda M, Scalzo GC, Myerscough J, et al. Predictors of Bubble Formation and Type Obtained With Pneumatic Dissection During Deep Anterior Lamellar Keratoplasty in Keratoconus. Am J Ophthalmol. 2020;212:127–33. doi: 10.1016/j.ajo.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Busin M, Scorcia V, Leon P, Nahum Y. Outcomes of air injection within 2 mm inside a deep trephination for deep anterior lamellar keratoplasty in eyes with keratoconus. Am J Ophthalmol. 2016;164:6–13. doi: 10.1016/j.ajo.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118:209–18. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Myerscough J, Roberts H, Yu AC, Elkadim M, Bovone C, Busin M. Five-year outcomes of converted mushroom keratoplasty from intended deep anterior lamellar keratoplasty (DALK) mandate 9-mm diameter DALK as the optimal approach to keratoconus. Am J Ophthalmol. 2020;220:9–18. doi: 10.1016/j.ajo.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Busin M, Madi S, Scorcia V, Santorum P, Nahum Y. A two-piece microkeratome-assisted mushroom keratoplasty improves the outcomes and survival of grafts performed in eyes with diseased stroma and healthy endothelium (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2015;113:T1. [PMC free article] [PubMed] [Google Scholar]
- 10.Scorcia V, Busin M. Survival of mushroom keratoplasty performed in corneas with postinfectious vascularized scars. Am J Ophthalmol. 2012;153:44–50.e1. doi: 10.1016/j.ajo.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Busin M, Leon P, Nahum Y, Scorcia V. Large (9 mm) deep anterior lamellar keratoplasty with clearance of a 6-mm optical zone optimizes outcomes of keratoconus surgery. Ophthalmology. 2017;124:1072–108. doi: 10.1016/j.ophtha.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Bovone C, Myerscough J, Friehmann A, Elkadim M, Parmeggiani F, Busin M. Peripheral intrastromal hydration facilitates safe, deep trephination in corneas of irregular thickness. Cornea. 2020;39:207–9. doi: 10.1097/ICO.0000000000002067. [DOI] [PubMed] [Google Scholar]
- 13.The Collaborative Corneal Transplantation Studies Research Group. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–403. doi: 10.1001/archopht.1992.01080220054021. [DOI] [PubMed] [Google Scholar]
- 14.Sarnicola V, Toro P. Deep anterior lamellar keratoplasty in herpes simplex corneal opacities. Cornea. 2010;29:60–4. doi: 10.1097/ICO.0b013e3181a317d3. [DOI] [PubMed] [Google Scholar]
- 15.Awan MA, Roberts F, Hegarty B, Ramaesh K. The outcome of deep anterior lamellar keratoplasty in herpes simplex virus-related corneal scarring, complications and graft survival. Br J Ophthalmol. 2010;94:1300–3. doi: 10.1136/bjo.2009.169300. [DOI] [PubMed] [Google Scholar]
- 16.Wu SQ, Zhou P, Zhang B, Qiu WY, Yao YF. Long-term comparison of full-bed deep lamellar keratoplasty with penetrating keratoplasty in treating corneal leucoma caused by herpes simplex keratitis. Am J Ophthalmol. 2012;153:291. doi: 10.1016/j.ajo.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Yu AC, Friehmann A, Myerscough J, Socea S, Furiosi L, Giannaccare G, et al. Initial high-dose prophylaxis and extended taper for mushroom keratoplasty in vascularized herpetic scars. Am J Ophthalmol. 2020;217:212–23. doi: 10.1016/j.ajo.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Donoso R, Díaz C, Villavicencio P. Comparative study of keratoconus between Anwar’s deep anterior lamellar keratoplasty versus converted penetrating keratoplasty. Arch Soc Esp de Oftalmol. 2015;90:257–63. doi: 10.1016/j.oftal.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Gadhvi KA, Romano V, Fernández-Vega Cueto L, Aiello F, Day AC, Allan BD. Deep anterior lamellar keratoplasty for keratoconus: multisurgeon results. Am J Ophthalmol. 2019;201:54–62. doi: 10.1016/j.ajo.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Huang OS, Htoon HM, Chan AM, Tan D, Mehta JS. Incidence and outcomes of intraoperative descemet membrane perforations during deep anterior lamellar keratoplasty. Am J Ophthalmol. 2019;199:9–18. doi: 10.1016/j.ajo.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Elkadim M, Myerscough J, Bovone C, Busin M. A novel blunt dissection technique to treat modified deep anterior lamellar keratoplasty (DALK)-associated high astigmatism. Eye (Lond) 2020;34:1432–7. doi: 10.1038/s41433-019-0686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls SM, Shimeld C, Easty DL, Hill TJ. Recurrent herpes simplex after corneal transplantation in rats. Invest Ophthalmol Vis Sci. 1996;37:425–35. [PubMed] [Google Scholar]
- 23.Goldblum D, Bachmann C, Tappeiner C, Garweg J, Frueh BE. Comparison of oral antiviral therapy with valacyclovir or acyclovir after penetrating keratoplasty for herpetic keratitis. Br J Ophthalmol. 2008;92:1201–5. doi: 10.1136/bjo.2008.138065. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini M, Scorcia V, Giannaccare G, Lucisano A, Vaccaro S, Battaglia C, et al. Corneal neovascularisation following deep anterior lamellar keratoplasty for corneal ectasia: incidence, timing and risk factors. Br J Ophthalmol. 2022;106:1363–7. doi: 10.1136/bjophthalmol-2021-319339. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Deep anterior lamellar keratoplasty versus penetrating keratoplasty: a meta-analysis of randomized controlled trials. Cornea. 2016;35:169–74. doi: 10.1097/ICO.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 26.Halberstadt M, Machens M, Gahlenbek KA, Böhnke M, Garweg JG. The outcome of corneal grafting in patients with stromal keratitis of herpetic and non-herpetic origin. Br J Ophthalmol. 2002;86:646–52. doi: 10.1136/bjo.86.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and analysed during the current study is available from the corresponding author on reasonable request.