Abstract
The outer membrane protein OmpU of Vibrio cholerae O1 strain 86B3 was characterized with reference to colonization of the intestine by the organism. The purified OmpU exhibited a pI of 3.6. Upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis, it migrated to 38, 32, and 110 kDa when the sample was heated at 100°C for 2 min, 50°C for 15 min, and room temperature for 30 min, respectively. The purified OmpU was not hemagglutinative. Anti-OmpU serum did not agglutinate strain 86B3 or other V. cholerae organisms. OmpU adhered to the brush border of the rabbit small intestine; adhesion of the organisms to the intestine treated in advance with OmpU was not inhibited. Treating the organisms in advance with anti-OmpU Fab did not inhibit adhesion to the intestine. These results obtained in vitro suggest that OmpU is not involved in the adhesion of V. cholerae to the intestinal epithelium.
Vibrio cholerae O1 is a causative agent of cholera. Attachment of the organisms to the intestinal epithelium is the first step in the disease process, maximizing the effect of the cholera toxin produced by the adherent organisms. A number of colonization factors of V. cholerae O1 such as toxin-coregulated pili (14), mannose-sensitive hemagglutinin pili (8), fucose-sensitive hemagglutinin (7), and outer membrane proteins (OMPs) (9, 10, 12) have been proposed. Most of these colonization factors have been proposed as adherence factors but have not been fully characterized. Although toxin-coregulated pilus has been shown to be important for colonization of the intestine by classical V. cholerae O1, it has no adhesive properties (12, 13).
OmpU, one of the major OMPs of V. cholerae, is regulated by the toxR gene (4). Recently, Sperandio et al. reported that OmpU of V. cholerae was a potential adherence factor (12). They reported that anti-OmpU serum or F(ab′)2 fractions inhibited the adherence of V. cholerae to HeLa, HEp-2, Caco-2, and Henle 407 cells. On the other hand, Singh et al. examined the role of a 53-kDa protein of V. cholerae (10). They showed that the anti-53-kDa protein serum inhibited bacterial colonization in an animal model and concluded that a 53-kDa protein is involved in intestinal colonization. In this report, we describe further analysis of OmpU.
MATERIALS AND METHODS
Bacteria and culture conditions.
V. cholerae O1 86B3 (classical, Ogawa), 569B (classical, Inaba), A88 (El Tor, Ogawa), C128 (El Tor, Ogawa), and 82P7 (El Tor, Ogawa) were used for OmpU preparation. Bacteria were cultured in heart infusion broth (Eiken Co., Tokyo, Japan) at 37°C for 15 h with shaking.
Purification of OmpU.
OmpU was purified by the method of Chakrabarti et al. (2). Briefly, cells harvested by centrifugation (5,000 × g for 15 min) were suspended in 20 mM Tris-HCl buffer (pH 7.4) (Tris buffer) containing 5 mM phenylmethylsulfonyl fluoride and crushed in X-press. The crushed material was treated with DNase and RNase (each at 100 μg/ml), and the unbroken cells were removed by centrifugation (10,000 × g for 10 min). The supernatant was subjected to higher-speed centrifugation (105,000 × g for 1 h) to obtain the crude membrane fraction, and the sediment was treated with 1% (wt/vol) Sarkosyl to dissolve the inner membrane. It was again centrifuged at 105,000 × g for 1 h, and the pellet was washed several times in Tris buffer. The washed pellet was suspended in 4% Triton X-100–Tris buffer, incubated at 37°C for 30 min, and then centrifuged at 105,000 × g for 1 h to remove the remaining outer membrane debris. The supernatant was passed through a DEAE-cellulose column equilibrated with Tris buffer containing 0.1% Triton X-100. The OmpU was eluted from the column by 70 mM NaCl in Tris buffer containing 0.1% Triton X-100.
Isolation of rabbit BBM.
Rabbit brush border membranes (BBM) were isolated by the method of Weiser (16). Briefly, resected rabbit small intestine was rinsed with 0.154 M NaCl containing 1 mM dithiothreitol, placed in solution A (1.5 mM KCl, 9.6 mM NaCl, 27 mM sodium citrate, 8 mM KH2PO4, 5.6 mM NaHPO4 [pH 7.3]), and then incubated at 37°C for 15 min with gentle shaking. After the supernatant was removed, the tissue was transferred to phosphate-buffered saline (PBS) containing 1.5 mM EDTA and 0.5 mM dithiothreitol and incubated at 37°C for 15 min. The released cell clumps were washed with PBS by centrifugation three times at 900 × g for 5 min.
Adhesion of OmpU to rabbit BBM.
OmpU (100 μg/ml) was mixed with isolated BBM (ca. 106 cell clumps/ml) at 37°C for 30 min. The mixture was then washed with 0.1% Triton X-100–20 mM Tris-HCl (pH 7.4) five times. OmpU which had adhered to BBM was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (3) and Western blotting (15); it was also immunohistochemically detected by using a horseradish peroxidase-labeled antibody as previously reported (6).
Adhesion test.
Adhesion of 86B3 to the rabbit intestine was examined by the MASK method as reported elsewhere (5). Briefly, a piece of formalin-fixed intestine was incubated in a bacterial suspension in Krebs-Tris-Ringer’s buffer (128 mM NaCl, 5.1 mM KCl, 1.34 mM MgSO4 · 7H2O, 2.7 mM CaCl2, 10 mM Tris-HCl buffer [pH 7.4]) for 10 min at 30°C and then vigorously washed with the same buffer. The sample was prepared for scanning electron microscopy. Adherent organisms were counted in 30 randomly selected scanning electron microscope fields at a magnification of ×4,000. The adhesion indices were expressed as the mean counts per field.
Adhesion to cultured HEp-2 cells was examined as described by Sperandio et al. (12). Briefly, HEp-2 cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum. Bacteria (107 CFU/ml) were added to the cell monolayers for 3 h at 37°C in a 5% CO2 atmosphere. The cells were washed three times with PBS, fixed with methanol, stained with Giemsa stain, and observed by light microscopy. The adhesion index was expressed as the mean number of bacteria adhering to 30 HEp-2 cells selected at random.
Adhesion inhibition test.
For assays of inhibition, cells were treated by one of two methods before the adhesion test. In the first method, the organisms were treated with the Fab fraction of either anti-OmpU immunoglobulin G (IgG) or IgG from nonimmunized rabbits at 30°C for 30 min, and then the adhesion test was performed with the intact rabbit intestine or HEp-2 cells. In the second method, intestinal epithelium was treated with the purified OmpU suspension at 30°C for 30 min to block the receptor and then exposed to the intact bacteria.
Antibodies.
Anti-OmpU antibody was prepared by immunizing a rabbit with purified OmpU. The IgG fraction of the antisera was digested with papain, and the Fab fraction was collected by SP Sephadex C50 column chromatography.
Protein assay.
The protein content was assayed by the method of Smith et al. (11), with bovine serum albumin as the standard.
Amino acid sequence.
The N-terminal amino acid sequence of the purified OmpU was analyzed by automated Edman degradation on an Applied Biosystems 477A protein sequencer.
Agglutination assay.
Anti-OmpU serum was serially diluted twofold. The cells were suspended in 10 mM PBS at a concentration about 109 cells/ml. Equal volumes of antiserum and bacterial suspension were mixed on a glass depression slide, and agglutination was determined within 10 min.
RESULTS
Characterization of purified OmpU.
The molecular masses of OmpU estimated by SDS-PAGE were 38, 32, and 110 kDa when the sample was incubated at 100°C for 2 min, at 50°C for 15 min, and at room temperature (25°C) for 30 min, respectively. The isoelectric point was 3.6, and the N-terminal amino acid sequence was DGINQSGDKAGSTVYSAK. The OmpU did not agglutinate human, rabbit, or guinea pig erythrocytes.
Immunological examinations.
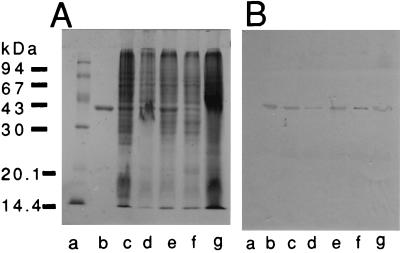
Anti-OmpU serum did not agglutinate the organisms. With Western blotting, the anti-OmpU serum and Fab fragments of anti-OmpU IgG reacted with the OmpU in the whole-cell lysate of V. cholerae as well as the purified form. OmpU was detected by immunoblotting not only from adhesive strains 86B3 and 82P7 but also from nonadhesive strains C128 and A88 and a weakly adhesive strain, 569B (Fig. 1).
FIG. 1.
SDS-PAGE profile of OmpU and V. cholerae whole-cell lysates (A) and Western blot analysis with anti-OmpU serum (B). Lanes: a, molecular mass markers; b, purified OmpU; c to g, OmpU from lysates of strains 86B3, 569B, A88, C128, and 82P7, respectively.
Adhesive properties.
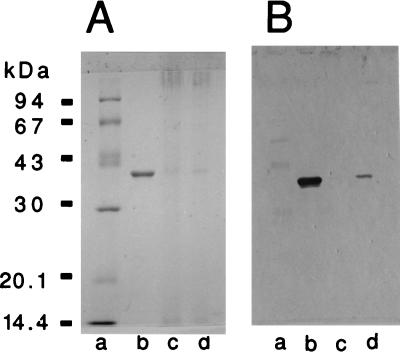
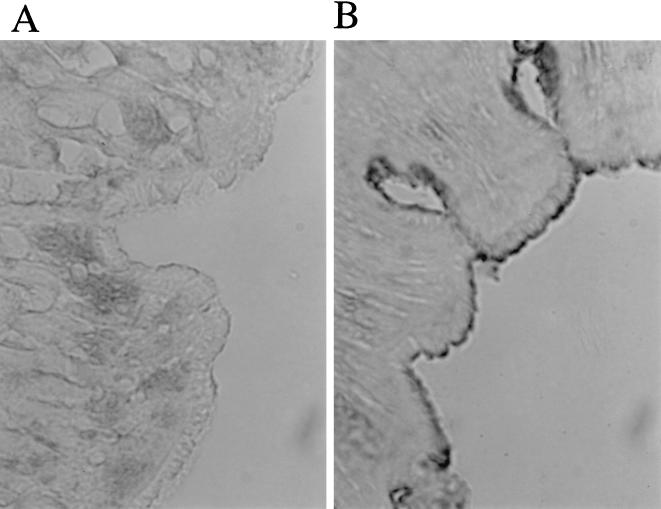
The purified OmpU adhered to isolated BBM, as detected by SDS-PAGE and Western blotting (Fig. 2). It also adhered to the epithelial surface of the intestinal tissue, as detected by the immunohistological technique. The intestine treated with OmpU developed an enzyme reaction on the villus surface (Fig. 3).
FIG. 2.
SDS-PAGE profile of rabbit BBM and OmpU (A) and Western blot analysis with anti-OmpU serum (B). Lanes: a, molecular mass markers; b, OmpU; c, BBM; d, BBM incubated with OmpU and then washed.
FIG. 3.
Adhesion of OmpU to the epithelium of intestine treated (B) and not treated (A) with purified OmpU. Adherent OmpU was detected by using the enzyme-labeled antibody technique, with a brown color developing along the villus surface.
Adhesion inhibition.
Strain 86B3 adhered well to HEp-2 cells and the intestinal epithelium. Neither treatment of HEp-2 cells and the intestine with purified OmpU (receptor block method) nor treatment of 86B3 with Fab fragments of the anti-OmpU antibody (adhesion block method) inhibited the adhesion. Strain 569B adhered very poorly (Tables 1 and 2).
TABLE 1.
Adhesion of V. cholerae 86B3 and 569B to HEp-2 cellsa
| Treatment | Adhesion (mean ± SD)
|
|
|---|---|---|
| 86B3 | 569B | |
| None | 14.3 ± 10.7 | 2.3 ± 2.8 |
| Nonimmunized serum | 13.0 ± 10.3 | 2.8 ± 2.8 |
| Anti-OmpU serum | 16.1 ± 10.4 | 2.9 ± 2.6 |
| Nonimmunized Fab | 11.2 ± 7.6 | 3.4 ± 4.1 |
| Anti-OmpU Fab | 15.6 ± 8.9 | 3.3 ± 3.6 |
Serum was diluted 20-fold; Fab concentration was 4 mg/ml. Results are means of three experiments.
TABLE 2.
Adhesion and inhibition of adhesion of V. cholerae 86B3 or 569B cells to the rabbit intestinal epithelium
| Pretreatment of intestine or organisms | Adhesion index (mean no. of adherent organisms/30 fields ± SD)
|
|
|---|---|---|
| 86B3 | 569B | |
| OmpU (μg/ml) | ||
| 0 | 212 ± 63 | 1 ± 2 |
| 12 | 288 ± 56 | NDa |
| 25 | 300 ± 83 | ND |
| 50 | 238 ± 84 | ND |
| 100 | 191 ± 62 | 3 ± 4 |
| Fab portion of anti-OmpU antibody (mg/ml) | ||
| 0 | 213 ± 70 | 1 ± 1 |
| 0.5 | 181 ± 71 | ND |
| 1 | 194 ± 91 | ND |
| 4 | 133 ± 50 | 1 ± 1 |
| Fab portion of preimmune sera of rabbits (4 mg/ml) | 126 ± 50 | 1 ± 1 |
ND, not done.
DISCUSSION
The results of this study indicate that in vitro, OmpU does not appear to be an adherence factor of V. cholerae. OmpU is immunogenic, and the antiserum reacted with OmpU as determined by Western blotting. However, the antiserum did not agglutinate V. cholerae live cells. Purified OmpU did not agglutinate erythrocytes but adhered to the intestinal epithelium. The discrepancies between these results and those of other investigators must be considered. Chakrabarti et al. reported that purified OmpU agglutinated rabbit and human erythrocytes but not sheep erythrocytes (2). However, in the present study, we could find no hemagglutination activity of OmpU. A possible reason for this discrepancy is the difference in strains used: Chakrabarti et al. obtained OmpU from strain 569B, whereas the OmpU used in the present study was obtained from strain 86B3. They reported that purified OmpU contained lipopolysaccharide (LPS). The presence of LPS may be the reason for hemagglutination, and the OmpU isolated from 86B3 may not contain LPS. The N-terminal amino acid sequence is slightly different in both strains. Sperandio et al. reported that anti-OmpU or its F(ab′)2 fraction completely inhibited adhesion of several V. cholerae strains to HEp-2 cells (12). However, in the present study, we did not observe such inhibition. We used the antiserum and Fab fraction of anti-OmpU IgG for the inhibition test. The Fab reacted with OmpU, as determined by Western blotting, and the antiserum did not agglutinate live 86B3 organisms. Therefore, the finding that adhesion was not inhibited is understandable. One reason for the discrepancy between the results of Sperandio et al. and ours appears to lie in the difference in V. cholerae strains used. In addition, Sperandio et al. used OmpU extracted from an SDS-polyacrylamide gel for raising antisera, whereas we used OmpU considered to be in the native state; these conditions may have contributed to the differences in findings.
Sengupta et al. isolated OMPs from V. cholerae and suggested that 40- to 43-kDa and 20-kDa OMPs play a role in the colonization of V. cholerae (9). The antisera against both OMPs agglutinated the live organisms, suggesting that these OMPs are exposed on the cell surface of V. cholerae. We could not find a relationship between OmpU and the 40- to 43-kDa OMPs because anti-OmpU serum did not agglutinate whole cells.
REFERENCES
- 1.Attridge S R, Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983;147:864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti S R, Chaudhuri K, Sen K, Das J. Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol. 1996;178:524–530. doi: 10.1128/jb.178.2.524-530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 4.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakasone N, Iwanaga M. Quantitative evaluation of colonizing ability of Vibrio cholerae O1. Microbiol Immunol. 1987;31:753–761. doi: 10.1111/j.1348-0421.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakasone N, Iwanaga M. Pili of a Vibrio parahaemolyticus strain as a possible colonization factor. Infect Immun. 1990;58:61–69. doi: 10.1128/iai.58.1.61-69.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakasone N, Iwanaga M. Cell-associated hemagglutinin of classical Vibrio cholerae O1 with reference to intestinal adhesion. FEMS Microbiol Lett. 1993;113:67–70. doi: 10.1111/j.1574-6968.1993.tb06489.x. [DOI] [PubMed] [Google Scholar]
- 8.Osek J, Jonson G, Svennerholm A-M, Holmgren J. Role of antibodies against biotype-specific Vibrio cholerae pili in protection against experimental classical and El Tor cholera. Infect Immun. 1994;62:2901–2907. doi: 10.1128/iai.62.7.2901-2907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengupta D K, Sengupta T K, Ghose A C. Major outer membrane proteins of Vibrio cholerae and their role in induction of protective immunity through inhibition of intestinal colonization. Infect Immun. 1992;60:4848–4855. doi: 10.1128/iai.60.11.4848-4855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S N, Srivastava R, Sinha V B, Srivastava B S. A 53 kDa protein of Vibrio cholerae classical strain O395 involved in intestinal colonization. Microb Pathog. 1994;17:69–78. doi: 10.1006/mpat.1994.1053. [DOI] [PubMed] [Google Scholar]
- 11.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 12.Sperandio V, Giron J A, Silveira W D, Kaper J B. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect Immun. 1995;63:4433–4438. doi: 10.1128/iai.63.11.4433-4438.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamamoto T, Nakashima K, Nakasone N, Honma Y, Higa N, Yamashiro T. Adhesive property of toxin-coregulated pilus of Vibrio cholerae O1. Microbiol Immunol. 1998;42:41–45. doi: 10.1111/j.1348-0421.1998.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiser M M. Intestinal epithelial cell surface membrane glycoprotein synthesis. J Biol Chem. 1973;248:2536–2541. [PubMed] [Google Scholar]