Abstract
Haemophilus influenzae requires heme for growth and can utilize both hemoglobin and hemoglobin-haptoglobin as heme sources. We previously identified a hemoglobin- and hemoglobin-haptoglobin-binding protein, HgpA, in H. influenzae HI689. Mutation of hgpA did not affect binding or utilization of either heme source. The hgpA mutant exhibited loss of a 120-kDa protein and increased expression of a 115-kDa protein. These data suggested that at least one other gene product is involved in binding of these heme sources by H. influenzae. A 3.2-kbp PCR product derived from HI689 was cloned. The nucleotide sequence indicated a separate, distinct gene with high homology to hgpA, which would encode a 115-kDa protein. Primers were designed for directional cloning of the structural gene in the correct reading frame. Sonicates of induced Escherichia coli harboring the cloned open reading frame bound both hemoglobin and hemoglobin-haptoglobin. An insertion/deletion mutant of H. influenzae at the newly identified locus, designated hgpB, was constructed. The 115-kDa protein was not detected in the mutant after affinity purification using biotinylated hemoglobin. An hgpA hgpB double-mutant strain exhibited a reduced ability to utilize hemoglobin-haptoglobin, although it was unaltered in the ability to utilize hemoglobin. Affinity isolation of hemoglobin-binding proteins from the double mutant resulted in isolation of an approximately 120-kDa protein. Internal peptide sequencing revealed this protein to be a third distinct protein, highly homologous to HgpA and HgpB. In summary a second hemoglobin- and hemoglobin-haptoglobin-binding protein of H. influenzae has been identified and characterized, and the presence of an additional protein of similar function has been revealed.
Haemophilus influenzae, a fastidious gram-negative bacteria, is the etiologic agent of human infections including otitis media, meningitis, epiglottitis, and pneumonia (42). H. influenzae lacks the ability to synthesize protoporphyrin IX, the immediate precursor of heme (10), and thus has an absolute growth requirement for a porphyrin source. Since the only known natural niche for H. influenzae is humans, the organism must adapt its mechanisms for acquiring heme accordingly. In vivo, heme is intracellular, in the form of hemoglobin or heme-containing enzymes, and thus unavailable to invading microorganisms (3, 23). Hemoglobin released by erythrocytes is avidly bound by the serum protein haptoglobin, and the hemoglobin-haptoglobin complex is rapidly cleared by hepatocytes (3, 30). Free heme, principally derived from the degradation of methemoglobin, is bound by either of the serum proteins hemopexin and albumin and cleared from the circulatory system by hepatocytes (3). Hemoglobin and the hemoglobin-haptoglobin, heme-hemopexin, and heme-albumin complexes can be utilized by H. influenzae as heme sources (39). The mechanism of acquisition of heme from these protein sources has not been elucidated.
We previously demonstrated that H. influenzae binds hemoglobin at the cell surface and that hemoglobin binding is suppressed by heme (12). Recently we cloned a gene encoding a heme-repressible hemoglobin-binding outer membrane protein from H. influenzae HI689 designated hgpA (20, 21). The hgpA nucleotide sequence revealed CCAA nucleotide repeating units immediately following the sequence encoding the leader peptide, and we proposed that these repeats may be involved in regulation of gene expression by a strand slippage mechanism (20). Insertional mutation of hgpA did not affect the ability of strain HI689 to bind hemoglobin or to utilize hemoglobin as a heme source (21). The hgpA mutant exhibited loss of a 120-kDa protein and apparently increased expression of a 115-kDa protein in affinity isolation procedures using biotinylated hemoglobin as the primary ligand (21). The recently sequenced H. influenzae Rd KW20 genome contains four loci with lengths of CCAA repeats and encoding proteins of high homology to HgpA, these gene products were designated as transferrin- or lactoferrin-binding proteins (11). Based on sequence homology, these proteins are likely to have functions similar to that of HgpA. Although genomic analysis is useful for identification of homologues, more rigorous investigations are necessary to definitively assign gene function (38).
The above data suggest that at least one gene product of H. influenzae other than HgpA binds hemoglobin and hemoglobin-haptoglobin, and homologues identified in the Rd KW20 genome sequencing project were candidates for this function. The goal of this investigation was to clone and characterize an additional gene mediating H. influenzae hemoglobin binding.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. H. influenzae type b strain HI689 and H. parainfluenzae strain 203 have been described previously (20, 28). Haemophilus strains were routinely maintained on brain heart infusion (BHI) agar (Difco, Detroit, Mich.) supplemented with 10 μg of both heme and β-NAD per ml (supplemented BHI [sBHI]). For experiments in heme-replete media, Haemophilus strains were grown at 37°C in sBHI broth. Heme-restricted growth of H. influenzae was performed in BHI supplemented with 10 μg of β-NAD per ml and 0.1 μg of heme per ml (heme-restricted BHI [hrBHI]) or in BHI supplemented with only 10 μg of β-NAD (heme-deplete BHI [hdBHI]). Escherichia coli strains were maintained on Luria-Bertani (LB) medium supplemented with antibiotics as indicated.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| H. influenzae | ||
| HI689 | Type b | 29a |
| HI689hgpAΔBglII | Type b, hgpAΔBglII Rbr | 20 |
| HI689hgpBΔBclI | Type b, hgpBΔBclI Tcr | This work |
| HI689hgpAΔBglII hgpBΔBclI | Type b, hgpAΔBglII hgpBΔBclI Rbr Tcr | This work |
| /hgpBΔBclI | ||
| Rd KW20 | Capsule-deficient type d derivative; strain used in sequencing project | 11 |
| H. parainfluenzae 203 | 20 | |
| E. coli | ||
| INVαF′ | Δ(lacZYA-argF) deoR+ F′ | Invitrogen |
| BL21(DE3)(pLysS) | F−ompT (DE3)(pLysS) Cmr | Novagen |
| GM2929 | F−dam-13::Tn9 (Cmr) | |
| Plasmids | ||
| pCRII | Plac, lacZα, Kanr, Ampr, Co1E1 origin, F1 origin, T7 and Sp6 promoters | Invitrogen |
| pGJB103 | Tcr | 41 |
| pGESYII | pACYC177 containing a 2.8-kbp Tcr cassette derived from pGJB103 | This work |
| pRSETA | Ampr, carrying T7 promoter metal-binding domain polylinker, and F1 origin | Invitrogen |
| pQM | pCRII, carrying a 3.2-kbp PCR product from H. influenzae which contains an internal stop codon | This work |
| pQMNEW | pCRII, carrying a 3.0-kbp PCR product without an internal stop codon | This work |
| pQMUC | pUC19, carrying a 3.2-kbp fragment from EcoRI-digested pQM | This work |
| pGEQMUC | pQMUC with internal deletion of 666- and 870-bp BclI fragments and insertion of a 2.8-kbp BamHI-digested Tcr marker | This work |
| pQMX | pRSETA, carrying 3.0-kbp PstI/HindIII fragment from pQMNEW | This work |
Rbr, ribostamycin resistance (15 μg/ml for H. influenzae); Tcr, tetracycline resistance (3 μg/ml for H. influenzae); Cmr, chloramphenicol resistance (50 μg/ml for E. coli); Kanr, kanamycin resistance (50 μg/ml for E. coli); Ampr, ampicillin resistance (50 μg/ml for E. coli); F−, F episome negative.
DNA isolation.
Bacterial genomic DNA was isolated by standard techniques as previously described (31). Plasmid DNA was isolated by the use of Qiagen plasmid kits (Qiagen, Chatsworth, Calif.) as directed by the manufacturer. DNA concentrations were assessed spectrophotometrically with a Shimadzu UV-1201S spectrophotometer with a DNA/Protein program pack (Shimadzu, Kyoto, Japan).
Cloning of hgpB by PCR.
A pair of primers, Phfj14 and Pstop (Table 2), were designed for use in PCR. Reactions were performed in a 50-μl mixture containing 2 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 10 pM each primer, and 2 U of Taq DNA polymerase (Gibco BRL, Gaithersburg, Md.), with 100 ng of H. influenzae HI689 chromosomal DNA as the template. PCR was carried out for 30 cycles, each cycle consisting of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and primer extension at 72°C for 3.5 min, with a final extension time of 10 min. A 3.2-kbp amplicon was directly ligated into the TA cloning vector pCRII (Invitrogen, San Diego, Calif.). The ligation was transformed into E. coli INVαF′ competent cells (Invitrogen) and recombinants were selected on LB agar containing 50 μg of carbenicillin per ml. A plasmid of the correct construct was identified and designated pQM.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| Phfj14 | 5′-CGGCTCATTATAGGAAACAAG-3′ |
| Pstop | 5′-CTAGAATTCAAACTGAACTGA-3′ |
| Pqm18 | 5′-CGTTTTACCTTTGCCGC-3′ |
| Pqm21 | 5′-CGTAGGATTGTCTGTGCC-3′ |
| Pqm50r | 5′-ATCCCAAAATGGAGTTTGGGAGAAATTTTCATA-3′ |
| Pqm50f | 5′-TATGAAAATTTCTCCCAAACTCCATTTTGGGAT-3′ |
| Ppsti | 5′-CTTGGGCTGCAGGCAAGTGTTGCTTATGCAG-3′ |
| Phindiii | 5′-GACTGTAAGCTTCTAGAATTCAAACTGAACTGA-3′ |
Automated sequencing (performed with an ABI model 373A apparatus by the Recombinant DNA/Protein Resource Facility, Oklahoma State University, Stillwater) of pQM indicated that the amplicon represented a distinct gene highly homologous to hgpA including a length of CCAA repeating units (20). The newly identified gene was designated hgpB. The length of CCAA repeating units in the cloned hgpB would result in the presence of a stop codon immediately following the CCAA repeats. In addition, a stop codon (TAA) was located at base pair position 1228, unrelated to the length of the CCAA repeats. To determine whether the stop codon at position 1228 was present in the genome of strain HI689, we used a second pair of primers, Pqm18 and Pqm21 (Table 2), to amplify the region, with strain HI689 chromosomal DNA as the template. Nucleotide sequence analysis of three independent amplicons indicated that the internal stop codon of the original clone, pQM, was an error introduced by Taq DNA polymerase. The error created during the PCR was corrected by site-directed mutagenesis using overlap extension PCR (1), utilizing primers Pqm50r and Pqm50f paired respectively with primers Ppsti and Phindiii (Table 2) for the primary PCR. The corrected sequence was confirmed by automated sequencing. The plasmid with the corrected sequence was designated pQMNEW. The primers at each end of the gene (designated Ppsti and Phindiii) additionally added PstI and HindIII sites to allow for directional cloning of the insert into the expression vector pRSETA.
Expression of HgpB in E. coli.
Plasmid pQMNEW was digested with PstI and HindIII. The fragment of appropriate size was gel purified by using a Geneclean II kit (Bio 101, Inc., Vista, Calif.) and ligated to gel-purified PstI- and HindIII-digested pRSETA. The ligation mixture was transformed into E. coli BL21(DE3)(pLysS), and recombinants were selected on LB agar containing 50 μg of carbenicillin per ml and 50 μg of chloramphenicol per ml. Plasmids were isolated from carbenicillin-resistant and chloramphenicol-resistant colonies and mapped by restriction enzyme digestion to identify clones containing the expected product. A positive clone was identified and designated pQMX.
Dot blot assay.
Binding of hemoglobin to both E. coli and Haemophilus strains was determined by a dot blot assay using biotinylated human hemoglobin as previously described (12, 20). Hemoglobin-haptoglobin binding assays were performed identically except that the primary ligand was biotinylated human hemoglobin-haptoglobin complex.
For dot blot assays using recombinant E. coli BL2(DE3)(pLysS), organisms were grown to mid-logarithmic phase in LB medium with appropriate antibiotic supplementation and induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. In some experiments, cells were lysed by sonication or by a freeze-thaw cycle prior to the dot blot assay.
For dot blot assays of Haemophilus strains, organisms were grown to mid-logarithmic phase in hrBHI.
Construction of a deletion/insertion mutant in hgpB.
The sequence of hgpB contains three BclI sites (Fig. 3). Since BclI results in GATC sticky ends, these were ideal sites for insertion of the BamHI fragment with the tetracycline resistance marker on pGESYII. Plasmid pGESYII was constructed in this laboratory to provide a readily excisable tetracycline resistance marker for use in H. influenzae. Briefly, plasmid pGJB103 (41) was digested with AatII and BanII, and the approximately 2.8-kbp fragment including the tetracycline resistance gene was gel purified. The overhanging ends of the AatII/BanII fragment were filled in with Klenow enzyme, and the fragment was ligated to BamHI linkers (Gibco-BRL). Following digestion with BamHI and gel purification, the fragment was ligated to BamHI-digested, gel-purified pACYC177 (4) to yield pGESYII. Thus, pGESYII contains an approximately 2.8-kbp tetracycline resistance marker (GESY) excisable by BamHI digestion.
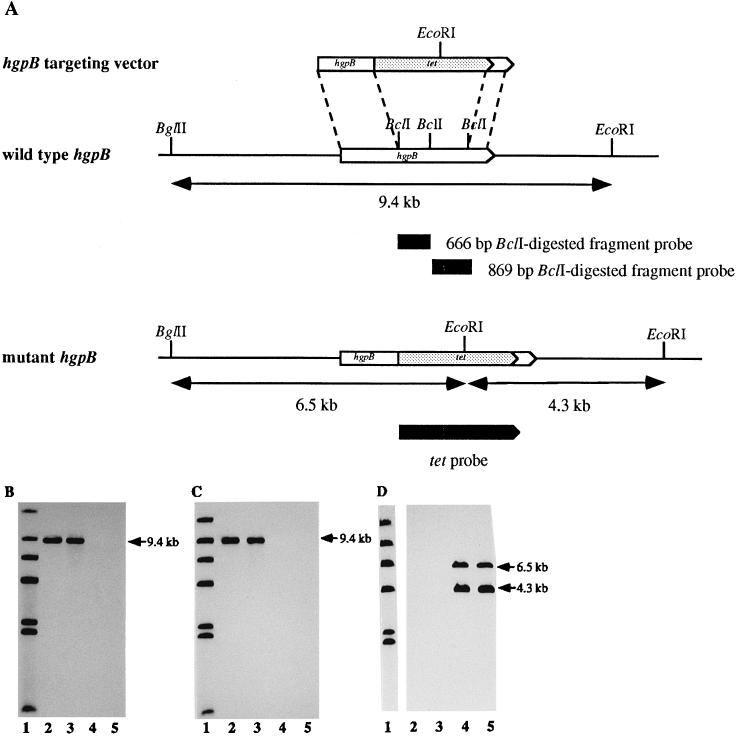
FIG. 3.
Production of an hgpB mutant in H. influenzae HI689. (A) hgpB locus and targeting construct. A 1,536-bp fragment from hgpB was deleted and replaced with the tetracycline resistance (tet) marker. The targeting construct was used to transform H. influenzae to tetracycline resistance. Southern analyses of H. influenzae HI689 and mutant derivatives were performed with the 666-bp BclI fragment from hgpB (B), the 870-bp BclI fragment from hgpB (C), and the tetracycline resistance cassette (D) as probes. Lanes: 1, labeled λ HindIII digest; 2, H. influenzae HI689 chromosomal DNA BglII/EcoRI digest; 3, H. influenzae HI689 hgpAΔBglII chromosomal DNA BglII/EcoRI digest; 4, H. influenzae HI689 hgpBΔBclI chromosomal DNA BglII/EcoRI digest; lanes 5, H. influenzae HI689 hgpAΔBglII/hgpBΔBclI chromosomal DNA BglII/EcoRI digest.
Construction of the mutant in hgpB was complicated by the presence of an additional BclI site in pCRII. Since there is no BclI site in pUC19, the insert of pQM was initially subcloned into pUC19. Mutation of hgpB was achieved as follows. The gel-purified 3.2-kbp EcoRI fragment of pQM was cloned into EcoRI-digested pUC19, generating plasmid pQMUC. Plasmid pQMUC was transformed into E. coli GM2929 to amplify nonmethylated pQMUC, since BclI is methylation sensitive. Purified pQMUC was completely digested with BclI and separated on a 0.8% (wt/vol) agarose gel. The 4.3-kbp fragment containing pUC19 flanked by portions of hgpB was gel purified and ligated to the 2.8-kbp GESY element. A plasmid of the correct construction was identified and designated pGEQMUC. Since strain Rd attains high-level competence, it was used as the initial recipient strain for homologous recombination of hgpB ΔBclI. H. influenzae Rd made competent by using the MII medium of Spenser and Herriott (34) was transformed with pGEQMUC. Recombinants were selected on sBHI agar containing 3 μg of tetracycline per ml. H. influenzae HI689 was transformed to tetracycline resistance with chromosomal DNA of one of the recombinant Rd clones. To reduce the chance of cotransformation with unrelated DNA, a second transformation was performed in which wild-type H. influenzae HI689 was transformed with chromosomal DNA of a tetracycline-resistant strain HI689 transformant. Appropriate chromosomal rearrangements were confirmed by Southern blot analysis.
Southern blot and DNA hybridization.
DNA was digested with restriction enzymes as directed by the manufacturers, separated on agarose gels (0.5 or 0.8% [wt/vol] agarose) in TBE buffer (0.045 M Tris-borate, 0.001 M EDTA), and transferred to Magnagraph nylon membranes (MSI, Westbrook, Mass.) by the method of Southern as described by Sambrook et al. (31).
The enhanced chemiluminescence (ECL) 3′-oligolabeling system (Amersham) was used as directed by the manufacturer to label the 3′ end of the oligonucleotides. The ECL random primer labeling kit (Amersham) was used as directed by the manufacturer to label DNA probes. Labeled oligonucleotides or DNA were used to probe Southern blots. Following stringency washing, hybridization was detected by using the ECL nucleic acid detection reagents (Amersham) as directed by the manufacturer. Blots were subsequently exposed to X-ray film (Fuji Photo Film Co., Tokyo, Japan).
Growth studies with H. influenzae.
H. influenzae strains were grown overnight in sBHI broth and then subcultured at a 0.1% inoculum into hdBHI broth. After incubation overnight to achieve heme depletion, the cultures were used to inoculate fresh hdBHI media (0.1% inoculum). Cultures were supplemented with human hemoglobin (10 μg/ml) or the human hemoglobin-haptoglobin complex (10-μg/ml hemoglobin equivalent). Hemoglobin-haptoglobin complex was made by mixing hemoglobin with haptoglobin in a ratio of 1 to 2 (wt/wt) at room temperature for 30 min. Growth was followed to stationary phase with a Klett colorimeter (Manostat, New York, N.Y.).
In some growth studies, bacteria were passaged through hemoglobin-haptoglobin prior to initiation of the growth study. Passage was achieved as follows. An overnight culture of bacteria in sBHI was subcultured at a 0.1% inoculum into 5 ml of hdBHI. Following growth overnight to induce heme restriction, the bacteria were harvested by centrifugation and resuspended in 2 ml of hdBHI supplemented with human hemoglobin-haptoglobin complex (10-μg/ml hemoglobin equivalent) and incubated overnight. This overnight culture was subsequently used to initiate growth studies as described above.
Affinity chromatography purification of H. influenzae hemoglobin-binding protein (Hgp).
Outer membrane proteins were isolated by selective solubilization with Triton X-100 as previously described (20, 40). Resuspended outer membranes were subjected to affinity chromatography using biotinylated human hemoglobin as the primary ligand as previously described (20).
Internal amino acid sequencing.
Purified hemoglobin-binding proteins were separated by sodium dodecyl sulfate by (SDS)-polyacrylamide gel electrophoresis on a NuPAGE 4 to 12% Bis-Tris gel (Novex, San Diego, Calif.) in 2-(N-morpholino)ethanesulfonic acid (MES)–SDS running buffer and stained with Coomassie blue. Stained gels were submitted to the Molecular Biology Resource Facility, University of Oklahoma Health Sciences Center, for in-gel digestion with trypsin and amino acid sequencing of the derived peptides as previously described (15, 19).
Nucleotide sequence accession number.
The GenBank accession number for the nucleotide sequence of hgpB is AF022910.
RESULTS
Cloning and sequencing of the DNA fragment homologous to hgpA.
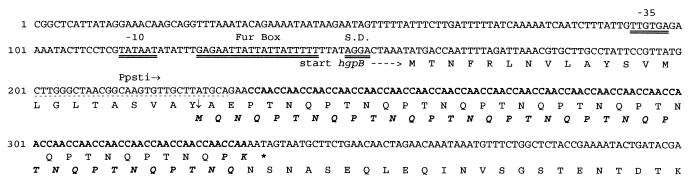
We previously cloned a gene, hgpA, encoding a hemoglobin-binding protein of H. influenzae. Primers were designed for use in PCR to amplify hgpA from H. influenzae. Amplification using strain HI689 chromosomal DNA as template resulted in an apparent single amplicon of approximately 3.2 kbp, which was cloned into pCRII. Partial mapping of one recombinant plasmid, pQM, revealed a restriction pattern different from that of pHFJ2, the original clone of hgpA (20). The insert of pQM was 3,162 bp, as determined by automated nucleotide sequencing. The nucleotide sequence data indicated that the insert of pQM represented a separate, distinct gene of high homology to hgpA (75% similarity and 61% identity), including a region of CCAA nucleotide repeats immediately following the putative leader peptide cleavage site (Fig. 1). Unlike the original clone of hgpA, the new clone had in-frame stop codons downstream of the multiple CCAA repeats. However, alteration of the reading frame across the CCAAs would account for a mature protein (Fig. 1), which we designated HgpB. We proposed that this alteration in reading frame may occur through a strand slippage mechanism resulting in addition or deletion of CCAA repeats (20). Confirmation that hgpB existed in strain HI689 as a gene distinct from hgpA was achieved by independent amplification and sequencing of an approximately 300-bp sequence internal to the putative hgpB. Cloned amplicons from several independent PCRs had the same sequence distinct from hgpA (data not shown). The predicted HgpB protein consists of 942 amino acids preceded by a 23-residue leader or signal peptide. The molecular mass of the mature protein was calculated to be 112,205 Da. Strong homology between the sequence 5′-TTGTGA-3′ at positions 93 to 98 and the consensus bacterial promoter −35 region (5′-TTGACA-3′) exists, and a perfect consensus bacterial promoter −10 sequence (5′-TATAAT-3′ at positions 114 to 119) follows 16 bp downstream (Fig. 1). A possible ribosome-binding site (33), 5′-AGGA-3′, is located at positions 149 to 152, 10 nucleotides upstream of the putative start site (Fig. 1). In addition, the sequence 5′-GAGAATTATTATTATTTTT-3′ at positions 126 to 144 (Fig. 1) shares 13 of 19 nucleotides with the 19-nucleotide symmetrical-dyad consensus ferric uptake regulator (Fur) protein-binding site and also exhibits high but not perfect symmetry. Because the assignment of promoter functions to these sequences is presently based only on sequence comparisons, verification of their function as elements of the promoter regulating transcription of the hemoglobin/hemoglobin-haptoglobin-binding protein genes requires further study.
FIG. 1.
Nucleotide sequence of the first 400 nucleotides of the 3.2-kbp PCR product cloned into pCRII and deduced amino acid sequences. The start site is indicated by the gene’s name (hgpB), and a short dashed arrow shows the direction of expression. The stop codon is marked with an asterisk. Two possible reading frames are shown. As originally cloned, hgpB is out of frame; however, removal of one CCAA results in a downstream mature protein (see text for explanation). The final CCAA unit and the amino acids in boldface italic type are not included in the predicted HgpB protein. The 25 CCAA repeating units are in boldface type. Double underlining indicates the putative −35 to −10 region, ribosomal binding (Shine-Dalgarno) site (S.D.), and putative Fur box. The vertical arrow is the putative signal sequence cleavage site. The dashed lines represents the position of the primer Ppsti, and the horizontal arrow indicates its direction.
HgpB is a homologue of the product of the predicted coding region HI0661 from the H. influenzae Rd KW20 genome, showing 95% similarity and 91% identity (11). An in-frame stop codon is found immediately downstream of the multiple CCAA repeats in hgpB as cloned and in the HI0661 locus in Rd KW20 (11). However, alteration in the number of CCAA repeats would in both cases lead to production of an approximately 115-kDa protein. To test the hypothesis that variable lengths of CCAA repeats may exist at the hgpB locus, clones of this region were sequenced. Using a (CCAA)6 oligonucleotide probe, we identified three hybridizing bands in an RsaI digest of strain HI689 chromosomal DNA. Using a probe derived from hgpB, we identified the specific RsaI fragment corresponding to hgpB (data not shown). A limited library of the appropriate RsaI fragment was constructed, and positive clones were identified with the (CCAA)6 oligonucleotide probe. Seven clones were sequenced and demonstrated variable lengths of CCAA repeats. Two clones possessed 32 repeats, one had 33, three had 34, and one had 37. The clone containing 33 CCAA repeats would encode a full-length protein as cloned.
Function of HgpB in E. coli.
On the basis of the sequence data, primers Ppsti and Phindiii (Table 2) were designed to amplify hgpB by PCR. Restriction enzyme sites were included in the primers to permit directional cloning in the expression vector pRSETA.
A hemoglobin-binding dot blot assay was used to investigate whether hgpB cloned in E. coli resulted in expression of a hemoglobin-binding phenotype. Lysed E. coli BL2(DE3)(pLysS) harboring pQMX bound biotinylated human hemoglobin following induction with IPTG (Fig. 2, wells 1 and 2). Compared to lysed organisms, whole cells of IPTG-induced E. coli BL2(DE3)(pLysS) harboring pQMX bound minimal hemoglobin (Fig. 2, well 3), because the recombinant protein is probably not well expressed at the E. coli cell surface. E. coli BL2(DE3)(pLysS) harboring pRSETA alone and induced with IPTG did not bind hemoglobin, whether the cells were lysed (Fig. 2, wells 4 and 5) or whole (Fig. 2, well 6). All tested strains were unable to bind hemoglobin without IPTG induction, whether whole or lysed (Fig. 2, wells 7 to 12). Additional dot blots showed that the cloned hgpB encoded hemoglobin-haptoglobin complex binding in E. coli (data not shown). These data demonstrated that pQMX contained a DNA fragment from H. influenzae HI689 which encodes hemoglobin and hemoglobin/haptoglobin binding in E. coli.
FIG. 2.
Dot blot analysis of human hemoglobin binding of E. coli with or without hgpB. Cells were either induced (wells 1 to 6) or not induced (wells 7 to 12) by IPTG. Wells 1 and 7, cells lysed by −70°C freezing of E. coli BL2(DE3)(pLysS pQMX); wells 2 and 8, sonicates of E. coli BL2(DE3)(pLysS pQMX); wells 3 and 9, whole cells of E. coli BL2(DE3)(pLysS pQMX); wells 4 and 10, cells lysed by −70°C freezing of E. coli BL2(DE3)(pLysS pRSETA); wells 5 and 11, sonicates of E. coli BL2(DE3)(pLysS pRSETA); wells 6 and 12, whole cells of E. coli BL2(DE3)(pLysS pRSETA).
Construction of H. influenzae hgpB and H. influenzae hgpA hgpB mutants.
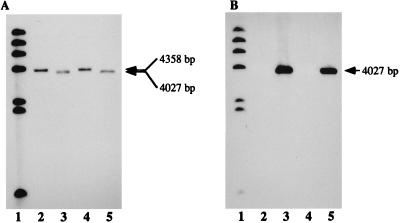
To investigate the potential phenotypic changes resulting from mutation, an antibiotic cassette was constructed to mutate hgpB. Cloned hgpB in plasmid pQMUC was interrupted with the tetracycline marker from pGESYII to generate pGEQMUC. The latter plasmid was used to transform H. influenzae Rd KW20 to tetracycline resistance. H. influenzae HI689 was transformed with the chromosomal DNA from one tetracycline-resistant Rd KW20 colony. One tetracycline-resistant HI689 colony was selected for further investigation. The isolate was carbenicillin sensitive, indicating that the entire plasmid had not been integrated. Southern hybridization, using the labeled 666- and 870-bp BclI fragments deleted from the hgpB gene and the tetracycline resistance marker as probes, confirmed that a single insertion of the tetracycline resistance marker had occurred in the correct site (Fig. 3). The labeled deletion regions hybridized to an approximately 9.4-kbp BglII/EcoRI fragment in the wild-type strain and the hgpA mutant and did not hybridize to the chromosomal DNA of the hgpB mutant. The labeled tetracycline resistance marker did not hybridize to wild-type chromosomal DNA but hybridized to approximately 6.5- and 4.3-kbp bands in BglII/EcoRI-digested mutant chromosomal DNA (Fig. 3). The mutant strain was designated HI689hgpBΔBclI. The chromosomal DNA of the HI689hgpBΔBclI mutant was transformed into the ribostamycin-resistant hgpA mutant, H. influenzae HI689hgpAΔBglII (21). Transformants were selected on sBHI with ribostamycin (15 μg/ml) and tetracycline (3 μg/ml). Southern hybridization confirmed that the double mutant in hgpA and hgpB had been correctly constructed (Fig. 3 and 4). Figure 4A shows a Southern blot probed with an internal fragment of hgpA; both the wild-type strain (lane 3) and the hgpB single mutant (lane 4) contain a hybridizing band at 4,358 bp. Since construction of the hgpA mutant resulted from deletion of an approximately 2.5-kbp BglII fragment and insertion of the approximately 2.2-kbp TSTE element, both the hgpA single mutant (lane 2) and the hgpA hgpB double mutant (lane 5) have a correspondingly smaller hybridizing band (4,027 bp). Figure 4B shows that the labeled TSTE element hybridized with a 4,027-bp fragment in only the hgpA single and hgpA hgpB double mutants.
FIG. 4.
Southern analyses of H. influenzae HI689 and mutant derivatives probed using a 2,557-bp BglII fragment of hgpA (A) and the ribostamycin resistance marker TSTE (B) as probes. Lanes: 1, labeled λ HindIII digest; 2, H. influenzae HI689 chromosomal DNA BglII/EcoRI digest; 3, H. influenzae HI689 hgpAΔBglII chromosomal DNA BglII/EcoRI digest; 4, H. influenzae HI689 hgpBΔBclI chromosomal DNA BglII/EcoRI digest; 5, H. influenzae HI689 hgpAΔBglII/hgpBΔBclI chromosomal DNA BglII/EcoRI digest.
Characterization of single and double mutants.
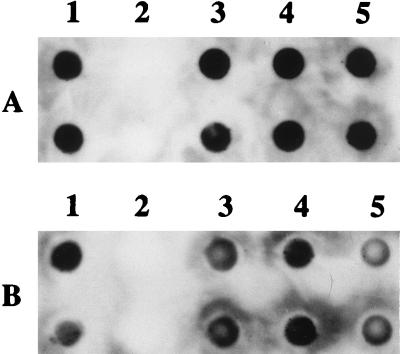
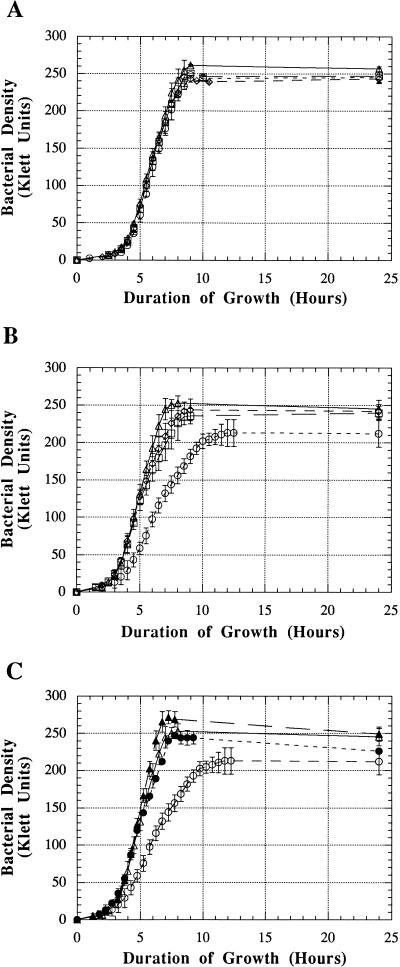
To determine the effect of mutation in hgpA and hgpB, either singly or together, dot blot binding assays, growth analyses, and hemoglobin affinity profiles were performed. Following growth in heme-restricted conditions, the single mutants HI689hgpAΔBglII and HI689hgpBΔBclI and the double mutant HI689hgpAΔBglII/hgpBΔBclI bound biotinylated hemoglobin as well as the wild-type strain HI689 (Fig. 5). Binding of the hemoglobin-haptoglobin complex was not significantly affected by single mutation, although binding by the double mutant may have been marginally less than that by the wild-type strain (Fig. 5). Growth characteristics of the mutants with either hemoglobin or the hemoglobin-haptoglobin complex as the sole heme source were also analyzed. Growth of the single and double mutants was unaltered in the presence of hemoglobin (Fig. 6A). When the sole heme source was the hemoglobin-haptoglobin complex, both of the single mutants were unaltered in growth characteristics. However, the double mutant grew at a significantly lower rate and to a significantly lower final density (Fig. 6B).
FIG. 5.
Dot blot analysis of human hemoglobin (A) and hemoglobin-haptoglobin complex (B) binding to H. influenzae HI689 (column 1), H. parainfluenzae 203 (column 2), H. influenzae HI689 hgpAΔBglII (column 3), H. influenzae HI689 hgpBΔBclI (column 4), and H. influenzae HI689 hgpAΔBglII/hgpBΔBclI (column 5). The rows in both panels represent 10-fold serial dilutions.
FIG. 6.
Growth of H. influenzae with hemoglobin or the hemoglobin-haptoglobin complex as the heme source. H. influenzae HI689 (Δ), H. influenzae HI689 following passage through hemoglobin-haptoglobin (▴), the single mutant HI689hgpAΔBglII (□), the single mutant HI689hgpBΔBclI (◊), the double mutant HI689hgpAΔBglII/hgpBΔBclI (○), and the double mutant HI689hgpAΔBglII/hgpBΔBclI following passage through hemoglobin-haptoglobin (•). (A) With hemoglobin (10 μg/ml) as the sole heme source; (B and C) with the hemoglobin-haptoglobin complex (10-μg/ml hemoglobin equivalent) as the sole heme source.
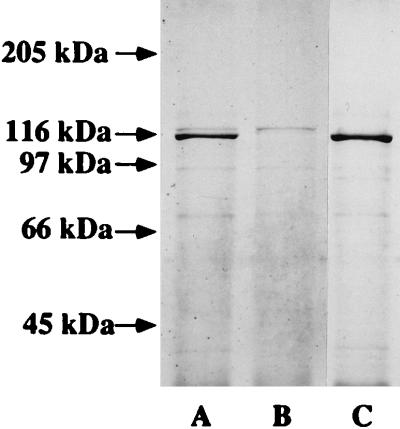
In addition to the binding assays and growth studies, bacteria grown in heme-restricted medium to mid-log phase were subjected to the affinity purification procedure using hemoglobin as the primary ligand. Affinity purification from the wild-type strain HI689 resulted in isolation of two bands of approximately 120 and 115 kDa (Fig. 7, lane A). The mutant strain HI689hgpBΔBclI did not yield a 115-kDa band, although a 120-kDa band was clearly visible (lane B). The hgpA single mutant, HI689hgpAΔBglII, did not yield the 120-kDa band but showed apparently increased expression of a 115-kDa protein (lane C).
FIG. 7.
SDS-polyacrylamide gel (7.5% acrylamide; Coomassie blue stained) of affinity-purified hemoglobin-binding proteins obtained from H. influenzae. Lanes: A, HI689; B, HI689hgpBΔBclI single mutant; C, HI689hgpAΔBglII single mutant. Numbers at left indicate sizes of molecular weight marker bands.
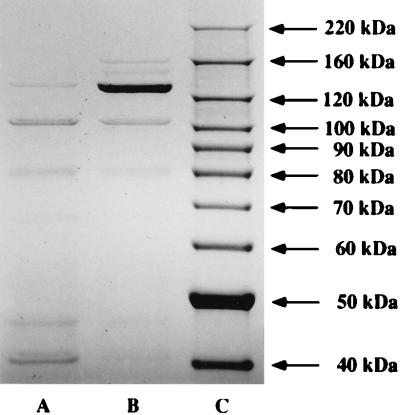
The double mutant HI689hgpAΔBglII/hgpBΔBclI exhibited a faint band at approximately 120 kDa (Fig. 7, lane A). We have shown that HI689 contains three loci with lengths of CCAA repeats (29). Since all of the CCAA-containing genes exhibit high homology, we hypothesized that this faint protein may represent the third CCAA-containing gene in this strain. Since the double mutant was apparently reduced in the ability to utilize the hemoglobin-haptoglobin complex, it is clear that these proteins are important for utilization of this heme source. We have previously proposed that the CCAA repeats may mediate phase variation through a strand slippage mechanism and that at any given time a mixed population of bacteria would exist, with some organisms containing an in-frame gene and others having an out-of-frame gene. We hypothesized that the third CCAA-containing gene in HI689 may also mediate utilization of hemoglobin-haptoglobin. To test this hypothesis, the double mutant was passaged through a medium where the sole heme source was hemoglobin-haptoglobin. Isolation of hemoglobin-binding proteins from the double mutant passaged through hemoglobin-haptoglobin resulted in isolation of significantly increased amounts of the approximately 120-kDa protein (Fig. 8, lane B). These data showing increased expression of an approximately 120-kDa protein from the double mutant passaged through hemoglobin-haptoglobin prompted us to perform growth studies to compare utilization of hemoglobin-haptoglobin by the double mutant either passaged or not passaged through hemoglobin-haptoglobin. The double mutant passaged through hemoglobin-haptoglobin grew as well as the wild-type strain and significantly better than the unpassaged mutant strain when the sole heme source was hemoglobin-haptoglobin (Fig. 6C). Growth studies were also performed with the wild-type strain passaged through hemoglobin-haptoglobin, the passaged wild-type strain consistently reached a slightly higher final density than the unpassaged wild-type strain (Fig. 6C).
FIG. 8.
SDS-polyacrylamide gel (NuPage 4 to 12% Bis-Tris gel; Coomassie blue stained) of affinity-purified hemoglobin-binding proteins obtained from H. influenzae. Lanes: A, HI689hgpAΔBglIIhgpBΔBclI double mutant; B, HI689hgpAΔBglIIhgpBΔBclI double mutant passaged through hrBHI supplemented with hemoglobin-haptoglobin (10-μg/ml hemoglobin equivalent) prior to growth for the hemoglobin-binding protein affinity isolation procedure; C, BenchMark molecular weight markers (Gibco BRL) (numbers at right indicate sizes).
Internal amino acid sequencing.
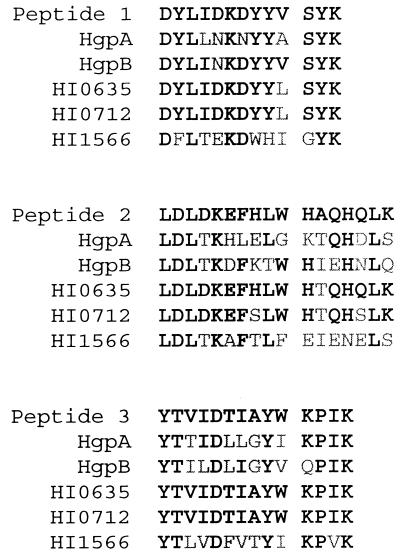
To confirm the identity of the affinity-purified protein from the double mutant, it was submitted for amino acid sequencing of internal peptides derived from trypsin digestion. Sequence was successfully obtained for three peptides (Fig. 9). Alignments of the three peptide sequences with the corresponding areas of HgpA, HgpB, and the loci HI0635, HI0712, and HI1566 indicated that the peptides were derived from a homolog of either the HI0635 or HI0712 product (Fig. 9). Peptide 3 was identical to the corresponding region in both HI0635 and HI0712, while peptide 1 varied from both only in the substitution of V for an L at position 10 of the peptide. Peptide 2 differed from HI0635 at only one position, while there were three differences between the peptide and HI0712. Although the protein is clearly not HgpA or HgpB, it is not possible from these data to determine whether the protein is a homologue of HI0635 or HI0712.
FIG. 9.
Sequence alignment between the amino acid sequences obtained from automated sequencing of internal peptides derived from the hemoglobin-binding protein isolated from the double mutant HI689hgpAΔBglIIhgpBΔBclI and the homologous peptides derived from the nucleic acid sequences of the H. influenzae HI689 genes hgpA and hgpB and the Rd KW20 ORFs HI0635, HI0712, and HI1566. Amino acid residues shown in boldface are identical; others are mismatched.
DISCUSSION
H. influenzae lacks the enzymes to convert δ-aminolevulinic acid to protoporphyrin IX, the immediate precursor of heme, and thus has an absolute growth requirement for a porphyrin source (10, 30). Heme, hemoglobin, the hemoglobin-haptoglobin complex, the heme-hemopexin and heme-albumin complexes, and protoporphyrin IX in the presence of an iron source can satisfy the heme requirement of H. influenzae in vitro (39). A heme-binding outer membrane protein (24), a heme-binding lipoprotein (14), and proteins binding the heme-hemopexin complex (5, 6, 13, 44) have been identified in H. influenzae and described.
We previously cloned a gene, hgpA, encoding a 120-kDa heme-repressible hemoglobin-binding outer membrane protein (HgpA) from H. influenzae HI689 (20). A major feature of this gene was a length of CCAA nucleotide repeats immediately following the sequence encoding the leader peptide (20). More recently, a second gene (hhuA) possessing a length of CCAA repeats and encoding a 115-kDa hemoglobin-haptoglobin utilization protein (HhuA) of H. influenzae nontypeable strain TN106 was reported (27). The gene hhuA is highly homologous to hgpA, showing 90% similarity and 84% identity, and it is likely that hhuA and hgpA represent the same gene in different strains (21). Four open reading frames (ORFs), designated HI0635, HI0661, HI0712, and HI1566, possessing CCAA nucleotide repeats have been identified in the genome of H. influenzae Rd KW20 (11, 17). The extents of homology between the four ORFs identified in Rd KW20 and hgpA are, respectively, for HI0635 66% similarity and 50% identity, for HI0661 74% similarity and 60% identity, for HI0712 68% similarity and 52% identity, and for HI1566 73% similarity and 55% identity. Insertional mutation of hgpA in strain HI689 did not affect the ability of the mutant to bind or utilize either hemoglobin or the hemoglobin-haptoglobin complex (unpublished data). In affinity isolation using biotinylated hemoglobin as the primary ligand, the hgpA mutant exhibited loss of a 120-kDa protein and increased expression of a 115-kDa protein (21).
In this report we identify a gene, hgpB, encoding a 115-kDa hemoglobin-binding protein (HgpB) of H. influenzae HI689 and possessing a length of CCAA nucleotide repeating units. The product of hgpB is highly homologous to the putative product of ORF HI0661 in the Rd KW20 chromosomal sequence (11), showing 95% similarity and 91% identity. Thus, hgpB represents a homologue of HI0661 in strain HI689. The original hgpB cloned amplicon contained 25 CCAA nucleotide repeats, giving rise to a protein with 8 QPTN tetrapeptide repeats, while the Rd KW20 HI0661 clone contained 20 CCAA repeats, encoding 6 QPTN tetrapeptide repeats. In both the hgpB clone and HI0661, the CCAA repeat region is followed by an in-frame stop codon. In both cases, alteration of the number of CCAA repeats would eliminate the stop codon, resulting in production of a nascent 115-kDa protein. Additional clones derived from a limited library and encompassing the CCAA-containing region of hgpB from strain HI689 were sequenced and demonstrated variation in the length of CCAA units from 31 to 37. These data indicate that alteration in the CCAA repeat unit length occurs, although additional experiments are required to determine whether the observed alteration occurred in H. influenzae or in the E. coli host strain. These data are consistent with a mixed population due to strand slippage (20).
Hemoglobin-binding proteins have been identified in various microorganisms, including Haemophilus ducreyi (8, 9, 36), H. influenzae (20), and Neisseria menigitidis (25, 37). These TonB-dependent outer membrane proteins share several conserved regions, including one near the amino terminus which likely interacts directly with the TonB protein (2, 32). HgpB exhibits significant homology with these other hemoglobin-binding proteins, particularly over regions considered indicative of TonB-dependent proteins (26). Utilization of heme from hemoglobin, the hemoglobin-haptoglobin complex, and the heme-hemopexin complex by H. influenzae is dependent on a functional tonB gene (18); a tonB homologue has been identified in the recently sequenced genome of H. influenzae Rd KW20 (11).
This report presents the first direct evidence that H. influenzae possesses more than one hemoglobin/hemoglobin-haptoglobin-binding protein. Since none of the hgpA, hgpB, or hgpA hgpB mutants were affected in the ability to either bind hemoglobin or utilize hemoglobin, it is likely that further proteins are involved in hemoglobin binding. The hgpA and hgpB single mutants were also unaltered in the ability to utilize hemoglobin-haptoglobin. The hgpA hgpB double mutant initially exhibited a reduced ability to utilize hemoglobin-haptoglobin; however, following passage of the mutant strain through hemoglobin-haptoglobin, growth in this heme source was equal to that of the wild-type strain. In affinity isolation using biotinylated hemoglobin, the hpgA single mutant exhibited loss of a 120-kDa protein and expression of a 115-kDa protein. The hgpB single mutant exhibited loss of a 115-kDa protein and presence of a 120-kDa protein. The hgpA hgpB double mutant expressed low levels of an approximately 120-kDa hemoglobin-binding protein, the isolation of which was dramatically increased following passage of the mutant strain through a medium with the hemoglobin-haptoglobin complex as the sole heme source. The presence of a band in the hgpA hgpB double mutant was not unexpected since we have shown that strain HI689 possesses a homologue of the strain Rd KW20 ORF designated HI0712 (unpublished observation). The predicted product of HI0712 in Rd KW20 is approximately 116 kDa, and upregulation of the homologous gene in HI689 may account for the presence of the approximately 120-kDa band in the double mutant. Amino acid sequencing of internal peptides derived from the protein isolated from the double mutant confirmed that this protein is not HgpA or HgpB but may be a homologue of HI0712. The 115-kDa protein expressed by the hgpA single mutant may be either hgpB or the HI0712 homologue, or possibly a mixture of both. The growth studies and affinity purification data obtained from the double mutant are consistent with expression of these proteins being mediated through strand slippage across the CCAA nucleotide repeats. We believe that passage of the double mutant through a medium where the sole heme source is hemoglobin-haptoglobin results in selection of a population where the gene encoding the remaining hemoglobin/hemoglobin-haptoglobin binding protein (the HI0712 homologue) is in frame and the protein is expressed. This occurs because the third protein is essential for growth of the double mutant in hemoglobin-haptoglobin; thus, only those organisms expressing the full-length protein will grow during passage through the medium where hemoglobin-haptoglobin is the sole heme source. Since the passaged population contains only organisms where the third gene is in frame, as many passaged as unpassaged organisms will yield greater amounts of the corresponding protein in the affinity purification procedure. Similarly, in the growth studies, selection of a population expressing the third protein results in restoration of growth to levels comparable to those seen with the wild-type strain. This is presumably because all organisms in the inoculum are capable of growth, whereas in the unpassaged mutant some portion of the cells lack any in-frame hemoglobin/hemoglobin-haptoglobin-binding protein genes and are thus incapable of growth in this medium. The wild-type strain similarly grew to a greater final density following passage through hemoglobin-haptoglobin compared to the unpassaged parent strain. We believe that passage of the wild-type strain has resulted in selection of a population where every organism expresses at least one of the three hemoglobin/hemoglobin-haptoglobin-binding proteins present in HI689, leading to the apparently enhanced ability of the passaged organisms to utilize hemoglobin-haptoglobin as a heme source.
The reason for the apparent level of redundancy in hemoglobin/hemoglobin-haptoglobin binding expressed by H. influenzae is unclear. It is possible the proteins interact with each other in the acquisition of heme from hemoglobin and/or hemoglobin/haptoglobin. Alternatively, the two or more hemoglobin-binding proteins present in H. influenzae may contribute to phase and antigenic variation similar to that seen with a number of other systems, including pilC of gonococcus (22), opacity proteins of gonococcus (35), and lipooligosaccharide of both Neisseria gonorrhoeae (7) and H. influenzae (16, 43).
In conclusion, we identified a second H. influenzae hemoglobin/hemoglobin-haptoglobin-binding protein, which we designated HgpB. A double mutant lacking expression of both identified hemoglobin/hemoglobin-haptoglobin-binding proteins, HgpA and HgpB, utilizes hemoglobin as a source of heme as well as the wild-type strain, although it is initially compromised in the ability to utilize the hemoglobin-haptoglobin complex. However when passaged through a medium where the sole heme source is hemoglobin-haptoglobin, the double mutant subsequently grows as well as the wild type in media containing hemoglobin-haptoglobin. An approximately 120-kDa hemoglobin-binding protein expressed by an hgpA hgpB double mutant was differentiated from HgpA and HgpB and may be a homologue of the product of the Rd KW20 locus HI0712. Further studies will characterize its role in hemoglobin/hemoglobin-haptoglobin utilization by H. influenzae HI689, by generation of a triple-mutant strain with mutations of hgpA, hgpB, and the HI0712 homologue.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI29611 from the National Institute of Allergy and Infectious Diseases to T.L.S. and by Health Research contract HN5-055 from the Oklahoma Center for the Advancement of Science to D.J.M. We acknowledge the support of the Children’s Medical Research Institute.
We thank Ken Jackson of the Molecular Biology Resource Facility, University of Oklahoma Health Sciences Center, for amino acid sequencing, and we thank Paul Whitby for helpful discussions.
REFERENCES
- 1.Aiyar A, Xiang Y, Leis J. Site-directed mutagenesis using overlap extension PCR. Methods Mol Biol. 1996;57:177–191. doi: 10.1385/0-89603-332-5:177. [DOI] [PubMed] [Google Scholar]
- 2.Bell P E, Nau C D, Brown J T, Konisky J, Kadner R J. Genetic suppression demonstrates interaction of TonB protein with outer membrane proteins in Escherichia coli. J Bacteriol. 1990;172:3826–3829. doi: 10.1128/jb.172.7.3826-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezkorovainy A. Iron proteins. In: Bullen J J, Griffiths E, editors. Iron and infection: molecular, physiological and clinical aspects. New York, N.Y: John Wiley & Sons; 1987. pp. 27–68. [Google Scholar]
- 4.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cope L D, Thomas S E, Latimer J L, Slaughter C A, Muller-Eberhard U, Hansen E J. The 100 kDa haem:haemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol Microbiol. 1994;13:863–873. doi: 10.1111/j.1365-2958.1994.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 6.Cope L D, Yogev R, Muller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme: hemopexin by Haemophilus influenzae type b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danaher R J, Levin J C, Arking D, Burch C L, Sandlin R, Stein D C. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J Bacteriol. 1995;177:7275–7279. doi: 10.1128/jb.177.24.7275-7279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkins C. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect Immun. 1995;63:1241–1245. doi: 10.1128/iai.63.4.1241-1245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins C, Chen C J, Thomas C E. Characterization of the hgpA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans N M, Smith D D, Wicken A J. Haemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae and Haemophilus parainfluenzae. J Med Microbiol. 1974;7:359–365. doi: 10.1099/00222615-7-3-359. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback R C, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Frangipane M E, Morton D J, Wooten J A, Pozsgay J M, Stull T L. Binding of human hemoglobin by Haemophilus influenzae. FEMS Microbiol Lett. 1994;118:243–248. doi: 10.1111/j.1574-6968.1994.tb06835.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanson M S, Pelzel S E, Latimer J, Muller-Eberhard U, Hansen E J. Identification of a genetic locus of Haemophilus influenzae type b necessary for the binding and utilization of heme bound to human hemopexin. Proc Natl Acad Sci USA. 1992;89:1973–1977. doi: 10.1073/pnas.89.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson M S, Slaughter C, Hansen E J. The hgpA gene of Haemophilus influenzae type b encodes a heme-binding lipoprotein conserved among heme-dependent Haemophilus species. Infect Immun. 1992;60:2257–2266. doi: 10.1128/iai.60.6.2257-2266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellman U, Wernstedt C, Gonez J, Heldin C-H. Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 16.High N J, Jennings M P, Moxon E R. Tandem repeats of the tetramer 5′-CAAT-3′ present in lic2A are required for phase variation but not lipopolysaccharide biosynthesis in Haemophilus influenzae. Mol Microbiol. 1996;20:165–174. doi: 10.1111/j.1365-2958.1996.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 17.Hood D W, Deadman M E, Jennings M P, Bisercic M, Fleischmann R D, Venter J C, Moxon E R. DNA repeats identify novel virulence genes in Haemophilus influenzae. Proc Natl Acad Sci USA. 1996;93:11121–11125. doi: 10.1073/pnas.93.20.11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeno P, Mini T, Moes S, Hintermann E, Horst M. Internal sequences from proteins digested in polyacrylamide gels. Anal Biochem. 1995;224:75–82. doi: 10.1006/abio.1995.1010. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Ren Z, Pozsgay J M, Elkins C, Whitby P W, Morton D J, Stull T L. Cloning of a DNA fragment encoding a heme-repressible hemoglobin-binding outer membrane protein from Haemophilus influenzae. Infect Immun. 1996;64:3134–3141. doi: 10.1128/iai.64.8.3134-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H, Ren Z, Whitby P W, Morton D J, Stull T L. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Genetic analysis of a hemoglobin binding protein (HgpA) of Haemophilus influenzae, abstr. B-223; p. 67. [Google Scholar]
- 22.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frame shift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:35–43. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee B C. Isolation of an outer membrane hemin-binding protein of Haemophilus influenzae type b. Infect Immun. 1992;60:810–816. doi: 10.1128/iai.60.3.810-816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis L A, Gray E, Wang Y P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 26.Lundrigan M D, Kadner R J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986;261:10797–10801. [PubMed] [Google Scholar]
- 27.Maciver I, Latimer J L, Liem H H, Muller-Eberhard U, Hrkal Z, Hansen E J. Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect Immun. 1996;64:3703–3712. doi: 10.1128/iai.64.9.3703-3712.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton D J, Musser J M, Stull T L. Expression of the Haemophilus influenzae transferrin receptor is repressible by hemin but not elemental iron alone. Infect Immun. 1993;61:4033–4037. doi: 10.1128/iai.61.10.4033-4037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton D J, Stull T L. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Haemophilus influenzae: species distribution of a family of genes containing CCAA nucleotide repeating units, abstr. B-224; p. 67. [Google Scholar]
- 29a.Musser J M, Barenkamp S J, Granoff D M, Selander S K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986;55:183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto B R, Verweij-van Vught A M, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook T, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Schoffler H, Braun V. Transport across the outer membrane of Escherichia coli via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989;217:378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- 33.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spenser H T, Herriott R M. Development of competence in Haemophilus influenzae. J Bacteriol. 1965;90:911–920. doi: 10.1128/jb.90.4.911-920.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern A, Brown M, Nickel P, Meyer T F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 36.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stojiljkovic I, Larson J, Hwa V, Anic S, So M. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss E J, Falkow S. Microbial pathogenesis: genomics and beyond. Science. 1997;276:707–712. doi: 10.1126/science.276.5313.707. [DOI] [PubMed] [Google Scholar]
- 39.Stull T L. Protein sources of heme for Haemophilus influenzae. Infect Immun. 1987;55:148–153. doi: 10.1128/iai.55.1.148-153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stull T L, Mack K, Haas J E, Smit J, Smith A L. A comparison of techniques for isolation of the outer membrane proteins of Haemophilus influenzae type b. Anal Biochem. 1985;150:471–480. doi: 10.1016/0003-2697(85)90537-8. [DOI] [PubMed] [Google Scholar]
- 41.Tomb J F, Barcak G J, Chandler M S, Redfield R J, Smith H O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989;171:3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turk D C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984;18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 43.Weiser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 44.Wong J C Y, Patel R, Kendall D, Whitby P W, Smith A, Holland J, Williams P. Affinity, conservation, and surface exposure of hemopexin-binding proteins in Haemophilus influenzae. Infect Immun. 1995;63:2327–2333. doi: 10.1128/iai.63.6.2327-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]