Abstract
Keloids, pathological scars resulting from skin trauma, have traditionally posed significant clinical management challenges due to their persistence and high recurrence rates. Our research elucidates the pivotal roles of lipids and their derivatives in keloid development, driven by underlying mechanisms of abnormal cell proliferation, apoptosis, and extracellular matrix deposition. Key findings suggest that abnormalities in arachidonic acid (AA) synthesis and non‐essential fatty acid synthesis are integral to keloid formation. Further, a complex interplay exists between lipid derivatives, notably butyric acid (BA), prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), and the regulation of hyperfibrosis. Additionally, combinations of docosahexaenoic acid (DHA) with BA and 15‐deoxy‐Δ12,14‐Prostaglandin J2 have exhibited pronounced cytotoxic effects. Among sphingolipids, ceramide (Cer) displayed limited pro‐apoptotic effects in keloid fibroblasts (KFBs), whereas sphingosine 1‐phosphate (S1P) was found to promote keloid hyperfibrosis, with its analogue, FTY720, demonstrating contrasting benefits. Both Vitamin D and hexadecylphosphorylcholine (HePC) showed potential antifibrotic and antiproliferative properties, suggesting their utility in keloid management. While keloids remain a prevalent concern in clinical practice, this study underscores the promising potential of targeting specific lipid molecules for the advancement of keloid therapeutic strategies.
Keywords: apoptosis, fibrosis, keloid, lipid metabolism, prostaglandins
Abbreviations
- cAMP/PKA
Cyclic Adenosine Monophosphate/Protein Kinase A
- FAs/FasL
Fas Cell Surface Death Receptor/FAs Ligand
- PPAR‐γ
Peroxisome Proliferator‐Activated Receptor Gamma
- PKC/IP3
Protein Kinase C/Inositol Triphosphate
- TGF‐β1/Smad
Transforming Growth Factor Beta 1/Sma‐ and Mad‐related protein
- MMP2/MMP9
Matrix Metalloproteinase‐2 and Matrix Metalloproteinase‐9
- P38‐MAPK
P38 Mitogen‐Activated Protein Kinase
- JNK/ERK
c‐Jun N‐terminal Kinase/Extracellular signal‐Regulated Kinase
1. INTRODUCTION
Keloids represent a pathological scarring that occurs following the healing of skin trauma, typically extending beyond the boundaries of the original wounds and persisting without natural regression. 1 Manifesting as raised, hard‐textured stripes or flaky lumps on the skin's surface, they pose a complex clinical management challenge due to notably high recurrence rates. 2 Most studies suggested that keloid formation is intricately tied to mechanisms of abnormal cell proliferation, apoptosis, and excessive extracellular matrix (ECM) deposition. 3 , 4 , 5 Numerous recent lipidomics studies underscored the crucial roles of lipids and their derivatives in keloid development, implicating ongoing inflammation, progressive fibrosis, and irregular cell proliferation and apoptosis in keloids. 6 , 7 We embarked on a meticulous and systematic investigation into the correlations between fatty acids and their derivatives, sphingomyelin derivatives, phospholipid analogues (such as HePC), and sterol derivatives (such as Vitamin D) and keloids. Our study aims to further illuminate the functions of lipids and their derivatives in keloid scarring and related mechanisms, providing a pivotal reference for devising new clinical interventions for keloids.
2. ASSOCIATION OF FATTY ACID‐BASED LIPID MOLECULES WITH KELOIDS
2.1. Abnormal metabolism of fatty acids in keloids
2.1.1. Disturbances in essential fatty acid (EFA) levels may promote keloid formation
Patients with keloids exhibit alterations in plasma levels of several fatty acids (FAs). 8 FAs, classified into saturated and unsaturated fatty acids based on structure, can also be categorised as essential and non‐essential fatty acids depending on the synthesis pathway. Furthermore, unsaturated fatty acids subdivide into monounsaturated and polyunsaturated fatty acids (PUFAs), with most of the EFAs belonging to the PUFA category. 9
Comparative analyses have revealed disruptions in plasma levels of several PUFAs in keloid patients relative to healthy individuals, suggesting their potential as biomarkers for keloids. 10 , 11 Notably, keloid skin demonstrates decreased levels of linoleic acid (LA), but elevated levels of arachidonic acid (AA) compared to normal skin. Regarding dietary intake, keloid patients consume higher than recommended levels of dietary LAs and AAs, specifically between 7 and 11 g/d. 8
It is hypothesised that AA metabolism plays a pivotal role in the fatty acid metabolism of keloid patients, with high levels of AA acting as a transport hub for fatty acid metabolism and mechanisms, including inflammation and fibrosis. 12 The free AA released by phospholipase A2 (PLA2) or diacylglycerol (DAG) lipase, activates protein kinase C (PKC), which in turn stimulates PLA2 to further release AA. This process is responsible for the overproduction of pro‐inflammatory prostaglandin E2 (PGE2). Moreover, LA can convert to AA, acting as a substrate for g‐linolenic acid (GLA) and duomo‐g‐linolenic acid (DGLA), and the anti‐inflammatory prostaglandin E1 (PGE1), to compensate for the over‐release of AA, leading to its further reduction. 4 , 13 Consequently, an excessive intake of AA stimulates the production of AA, and elevated levels of AA facilitate the production of pro‐inflammatory substances, contributing to keloid formation. 14
2.1.2. Altered synthesis of non‐essential fatty acids in keloid scars
Keloids, often considered to be a tumour‐like disease, showcase abnormal fibroblast proliferation, with keloid fibroblasts (KFBs) demonstrating one of their major metabolic features as increased lipid synthesis. 15 Notably, fatty acid de‐novo synthesis, critical in various human tissues, primarily employs exogenous lipids for generating new structural lipids. 16 Typically, endogenous fatty acid synthesis is suppressed, maintaining FASN expression at low levels. 17
In stark contrast, rapidly proliferating tumour cells exhibit a completely distinct process of de‐novo synthesis of FAs. 18 The aberrant synthesis of non‐essential FAs in keloids, particularly focusing on key enzymes involved in FA synthesis within the organism—namely sterol regulatory element‐binding protein‐1 (SREBP‐1) and FASN—has become a focal point of research. 19 SREBP‐1, a critical transcription factor, modulates the synthesis of steroids and lipids in the de‐novo synthesis pathway, and directly regulates the expression of pivotal enzymes. 20 FASN, instrumental in providing the energy necessary for the survival of proliferating cells and a key enzyme in the abnormal metabolism of endogenous fatty acids, has been observed to be overexpressed in various human epithelial cancers, including those of the prostate, ovaries, colon, lung, endometrium, and stomach. 21 Subsequent experiments indicated higher mRNA expression of SREBP1 and FASN in KFBs than in normal fibroblasts (NFBs), implying that the abnormal synthesis of endogenous FAs might contribute to keloid formation. 11
2.2. Lipid‐associated modulation in keloid scars
2.2.1. Butyric acids typifying fatty acids in the inhibition of keloid fibrogenesis
In comparison with physiologically normal skin, keloids discernibly exhibit divergent lipid profiles, with 27 disparate FAs and a total of 30 differentially expressed FAs identified. Specifically, eight short‐chain fatty acids (SCFAs) manifest alterations, with substantial diminutions in the expressions of butyric, isobutyric, malonic, valeric, and succinic acids. 11
SCFAs, produced through anaerobic bacterial fermentation of dietary fibre within the colon, especially butyrate and propionate, are known to exhibit histone deacetylase (HDAC) inhibitory activity. 22 Engaged in inflammation regulation, SCFAs curtail mast cell activation when in deficit, thereby limiting the production of inflammatory mediators. 23 The antifibrotic potential of butyrate and other HDAC inhibitors, as evidenced across various mesenchymal stem cells (MSCs), resides in their ability to mitigate cell proliferation, collagen synthesis, and α‐Smooth Muscle Actin (α‐SMA) expression through histone acetylation modulation. 24
An in‐vitro exploration involving the co‐culturing of butyric acid (BA) with keloid KFBs demonstrated that BA inhibited fibroblast proliferation and type III collagen expression while concurrently augmenting COX‐1 expression and facilitating PGE2 production. 25 This suggested that the scarcity of SCFAs in keloid scars modulates apoptosis, proliferation, and differentiation of KFBs, further exacerbating collagen deposition. Additionally, a potential therapeutic synergism for keloids was realised through the amalgamation of docosahexaenoic acid (DHA) and BA, which seemed to amplify inhibitory effects on α‐SMA, collagen types I and III, TGF‐β1, and type I TGF‐β1 receptors, concomitantly inhibiting cell proliferation, precipitating apoptosis, and dismantling stress fibers. 25 A secondary pathway, initiated by BA‐activated FAs in colonocytes, posits that a synergistic apoptotic effect is feasible through an intrinsic mitochondrial pathway. 26
The antifibrotic properties of DHA observed in human peritoneal fibroblasts encompass the inhibition of the expression of TGF‐β1, VEGF, and type I collagen. Given that DHA acts as a ligand for peroxisome proliferator‐activated receptor γ (PPARγ) and DHA‐derived lipid mediators robustly actuate PPARγ, extensive investigations corroborate that PPARγ expression or activation subdues the expression of type I collagen, α‐SMA, and TGF‐β across various fibroblast populations. 27 , 28
2.2.2. HePC: A phospholipid analogue suppressing KFBs' anomalous proliferation
Hexadecylphosphorylcholine (HePC), recognised as an alkyl phospholipid analogue, has garnered interest as an innovative antiproliferative agent, exerting its effects on the plasma membrane. 29 HePC, in vitro, substantively inhibits cellular growth across a spectrum of tumour cell lines, further manifesting antitumour activity in‐vivo. Investigations elucidated that HePC disrupts the proliferation of human epidermal keratinocytes and stifles phosphatidylcholine synthesis in‐vitro, establishing its efficacy in the treatment of non‐neoplastic, hyperproliferative skin disorders, inclusive of psoriasis. 30
Its potential applicability in keloid therapy has been explored. In‐vitro cultivation revealed that HePC impedes fibronectin synthesis and alters the constitution of the intracellular plasma membrane and membrane protein activity by obstructing phosphatidylcholine biosynthesis. This alteration culminates in marked antiproliferative characteristics by impeding cell cycle progression. 31 Nevertheless, the specific role of HePC in modulating keloid fibrosis and the keloid ECM demands additional scrutiny and is primed for subsequent investigative pursuit Table 1.
TABLE 1.
The regulatory roles of various molecules in the progression of keloids.
| Moleculars | Classification within lipids | Effects on keloids | Involved pathways | Expression in keloids |
|---|---|---|---|---|
| Arachidonic acid | Fatty acid | Enhance inflammation | cAMP/PKA | Upregulation |
| Butyric acid | Fatty acid | Inhibit proliferation; inhibit fibrosis; promote apoptosis | FAs/FAsL | Downregulation |
| DHA | Fatty acid | Synergistic with butyric acid; inhibit fibrosis | PPAR‐γ | Downregulation |
| HePC | Phospholipid analogue | Inhibit proliferation | PKC/IP3 | ‐ |
| PGE2 | Fatty acid derivative | Inhibit fibrosis; enhance inflammation | TGF‐β1/Smad; cAMP/PKA; MMP2/MMP9 | Downregulation |
| 15 deoxy‐PGJ 2 | Fatty acid derivative | Inhibit fibrosis; promote apoptosis; inhibt inflammation | PPAR‐γ; NF‐κB; P38‐MAPK | Downregulation |
| PGD2 | Fatty acid derivative | Inhibit fibrosis | cAMP/PKA | Downregulation |
| Ceramide | Sphingolipid derivatives | Promote apoptosis | FAs/FAsL | ‐ |
| S1P | Sphingolipid derivative | Enhance inflammation; promote fibrosis | JNK/ERK; MAPK | Upregulation |
| FTY720 | Sphingolipid analogue | Inhibit fibrosis; inhibt inflammation | JNK/ERK; MAPK | ‐ |
| Vitamin D | Steroid derivative | Inhibit fibrosis; inhibit proliferation | MMP9 | Downregulation |
3. RELEVANCE OF DERIVATIVES OF BOTH FAs AND SPHINGOLIPIDS TO KELOIDS
3.1. Inhibition of keloid progression through FA derivatives
3.1.1. Strategic curtailment of keloid formation facilitated by FA derivatives: A spotlight on prostaglandins
Keloids, pathologically hyperproliferative fibrotic lesions, betray a significant correlation with the multifaceted actions of eicosanoids, cytokines, and reactive oxygen species (ROS). AA, serving as a pivotal substrate for eicosanoids, including leukotrienes (LTs), prostaglandins (PGs), lipoxins (LXs), and thromboxanes (TXs), bespeaks an indelible signature in the complex pathophysiology of keloids. 32 Furthermore, notwithstanding its profound pro‐inflammatory modulation in PUFAs metabolism, emerging evidence posits a consequential role for ω‐3 FAs, particularly in attenuating anti‐apoptotic signaling and obfuscating pro‐inflammatory cues. Derivatives such as Docosapentaenoic acid (DPA) and DHA, stemming from alpha‐linolenic acid (ALA), have been implicated in the mitigation of inflammatory cytokine synthesis, inclusive of interleukin 6 (IL‐6) and tumour necrosis factor‐alpha (TNF‐α). 7
PGs, quintessential FA derivatives, are implicated in orchestrating a myriad of physiological and pathological cascades. 33 Their biogenesis, a subject of meticulous enzymatic control, commences with the conversion of membrane phospholipids to AA by phospholipases, followed by the transformation of AA to prostaglandin H2 (PGH2) via cyclooxygenase (COX) pathways. 34 PGH2 thus serves as a progenitor for biologically salient mediators such as thromboxane A2, PGE2, and prostacyclin. 35
COX‐2, an inducible isoform of COX, facilitates the synthesis of PGE2 and nitric oxide (NO) via nitric oxide synthase (NOS), particularly under the auspices of pro‐inflammatory mediators like TNF‐α, lipopolysaccharides, and interleukin‐1 beta (IL‐1β). 36 This orchestrated inflammatory milieu begets subsequent collateral damage to adjacent tissue cells at the wound locus, thereby decelerating the wound healing trajectory.
PGE2, omnipresent among PGs within the human physiology, and a noteworthy eicosanoid fibroblast product, mitigates fibroblast functionalities through autocrine secretion. 37 Anomalies in the synthesis or degradation of PGE2 have been correlative with a spectrum of pathological states, encompassing persistent inflammation and tumorigenesis. 38 , 39 Moreover, PGE2 has been attributed a bifurcated role in keloid development, concurrently advancing inflammatory progression and exhibiting anti‐fibrotic function, rendering it a nexus of contemporary research interest.
Prostaglandin D2 (PGD2), synthesised in both central nervous and peripheral tissues, safeguards pivotal roles in the modulation of inflammation and the preservation of homeostasis. 40 While mast cells are preeminent synthesisers of PGD2 in peripheral tissues, other leukocytes, such as dendritic cells (DC) and T‐helper 2 cells (Th2), also harbor the capability to synthesise PDG2. 41 , 42 PGD2, albeit relatively unstable with a plasma half‐life ~30 min, undergoes subsequent metabolism to yield lipid variants such as 15 deoxy‐PGJ 2, which hold particular relevance to keloids. 43 Additionally, PGD2 can undergo enzymatic metabolism, facilitated by aldo‐keto reductase 1C3 (AKR1C3), culminating in the formation of 9α,11β‐PGF2. Furthermore, 15 deoxy‐PGJ 2, engendered spontaneously through PGD2 dehydration, vies in production with other PGD2‐derived entities, including 9α,11β‐PGF2 11 Figure 1.
FIGURE 1.
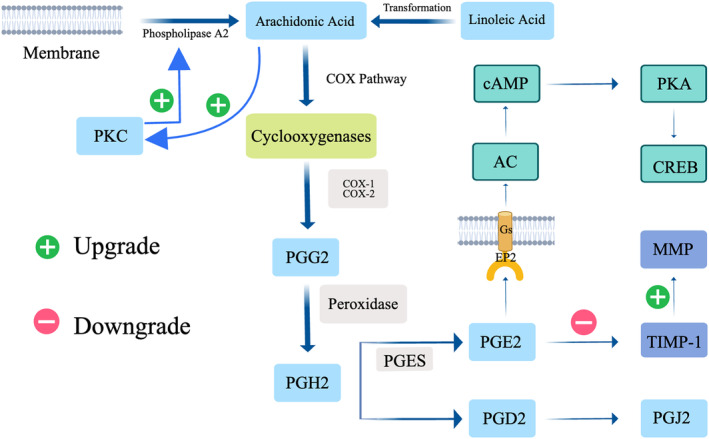
Illustrating the abnormal synthesis process of arachidonic acid in keloids, as well as the synthesis process of prostaglandins mediated by arachidonic acid. It also covers the pathways in which prostaglandin E2 is involved in regulating fibrosis in keloids. AC, Adenylyl Cyclase; cAMP, Cyclic Adenosine Monophosphate; CREB, cAMP Response Element‐Binding Protein; MMP, Matrix Metalloproteinase; PKC, Protein Kinase C; PGG2, Prostaglandin G2; PGH2, Prostaglandin H2; PGES, Prostaglandin E Synthase; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PKA, Protein Kinase A; PGJ2, Prostaglandin J2; TIMP‐1, Tissue Inhibitor of Metalloproteinases‐1.
3.1.2. In‐depth examination of prostaglandin‐mediated mechanisms in alleviating keloid fibrosis and anti‐apoptosis
PGE2, interfacing with its receptor EP2 within fibroblasts, instigates the cAMP/Protein Kinase A (PKA) signaling cascade, ensuing in the concomitant inhibition of cellular proliferation, migration, collagen synthesis, and myofibroblast differentiation. 44 , 45 Consequently, PGE2 heralds an anti‐fibrotic impact across an array of organ systems. 46 Additionally, PGE2 propagates tissue remodeling within skin tissues, while a diminution in dermal PGE2 levels has been associated with an amplification in collagen synthesis and scar ontogenesis. 47 Empirical investigations have illuminated a potential correlation between a diminished proficiency of KFBs to synthesise PGE2 and the overexpression of Transforming Growth Factor‐beta 1 (TGF‐β1), subsequently culminating in a downtrend in the endogenous expression of COX‐2 and PGE2 within KFBs. 48 This intimated that defects in endogenous PGE2, induced by TGF‐β1, may be pivotal in keloid fibrosis. The regulatory equilibrium of Matrix Metalloproteinase/Tissue Inhibitor of Metalloproteinase (MMP/TIMP) expression is also modulated by PGE2, escalating the expression of MMP‐2 and MMP‐9 whilst diminishing TIMP‐1 expression. 49 Moreover, PGE2, upon activation of EP2‐integrated with the cAMP signaling cascade‐augments cAMP levels, inhibiting collagen synthesis in dermal fibroblasts induced by TGF‐β1. The pro‐fibrotic effects of TGF‐β1 are contingent upon the Smad signaling pathway, whereas PGE2 obliterates TGF‐β1‐induced Smad 2/3 phosphorylation and Smad 2/3/4 complex formation, as well as nuclear translocation. Consequently, PGE2's anti‐fibrotic efficacy is ascribed to the inhibition of the TGF‐β1/Smad signaling pathway. 50 , 51
PGD2 orchestrates physiological functionalities via its distinct receptors, DP1 and DP2‐synonymously acknowledged as Chemoattractant Receptor‐Homologous Molecule expressed on Th2 Cells (CRTH2). 52 Activation of the DP1 receptor elicits an upsurge in cAMP levels and intracellular PKA activation, a phenomenon analogous to that of EP2. Albeit CRTH2's capacity to amalgamate with Gi proteins, inhibiting cAMP synthesis and enhancing intracellular Ca2+ concentration, it is imperative to note that CRTH2 is predominantly expressed within Th2 cells. 53 This revelation posits that receptor activation by PGD2 impedes the fibrotic process.
Fifteen deoxy‐PGJ 2, a ligand that actuates the peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ), inhibits the NF‐κB pathway and induces oxidative stress, participates in numerous biological processes. 54 15 deoxy‐PGJ 2 mediates its pro‐apoptotic and anti‐inflammatory impacts by actuating PPAR‐γ and obliterating NF‐κB. 55 The concentration of 15 deoxy‐PGJ 2 is instrumental in determining its inflammatory impact: it amplifies chemokine‐induced chemotaxis in eosinophils at suboptimal concentrations, whereas at maximal concentrations, it induces apoptosis and suppresses eosinophil survival. 56 Distinct from its PPAR‐γ‐dependent manner, various studies have discerned 15 deoxy‐PGJ 2 as possessing anti‐fibrotic properties. 57 , 58 Therapeutic application of 15 deoxy‐PGJ 2 to KFBs facilitated an enhancement in ROS formation, P38‐MAPK activation, and Superoxide Dismutase 1 (SOD1) transcription, thereby revealing cytotoxic impacts. Furthermore, aldo‐keto reductase 1C3 (AKR 1C 3) – the enzyme responsible for metabolising PGD2 – was observed to be overexpressed in keloids relative to normotrophic skin. This enzyme, which metabolises PGD2 to 9α,11β‐PGF 2, is thereby hypothesised as one of the defensive mechanisms of KFBs. 54 Consequently, inhibition of AKR 1C 3 could emerge as a potent local therapeutic strategy for keloids.
3.2. Sphingomyelin rheostat: Elucidating its paramountcy in keloid development
Sphingolipids, quintessential constituents of cellular membranes, pervade the plasma membranes of a plethora of mammalian cells, where their metabolites—including biologically salient signaling molecules such as ceramide (Cer), sphingosine (Sph), and sphingosine 1‐phosphate (S1P)—orchestrate pivotal signaling cascades implicating cell proliferation and apoptosis. The dynamic triad of Cer, Sph, and S1P has been metaphorically designated the ‘sphingomyelin rheostat’, attributable to the intrinsic link of their dynamic equilibrium to cellular vitality and apoptosis. 59
3.2.1. Interconversion within sphingolipid triangles and Sph's cytotoxicity to KFBs
Cer generation, a cornerstone of sphingolipid metabolism, is effectuated through two avenues: de‐novo synthesis within the endoplasmic reticulum and sphingolipids' hydrolysis via acid sphingomyelinase (ASM). 60 Post‐synthesis, Cer, in the milieu of ceramidase, undergoes deacylation, culminating in Sph production, which is subsequently shuttled to the Golgi apparatus via ceramide transporter proteins. 61 Thereupon, Sph, engaging a phosphate molecule in the presence of sphingosine kinase (SphK), begets S1P, which, through the action of sphingosine phosphate phosphatase, reverts to Sph in a reversible manner. Both Sph and Cer, imperative elements of the stress‐responsive regulatory system, embody inhibitory and pro‐apoptotic functionalities, whereas S1P is associated with pro‐growth and anti‐apoptotic activities. 62
3.2.2. Pro‐apoptotic potency of ceramide: Dampened efficacy in keloids
Cer propels apoptosis, acting as a secondary messenger in the FAs‐mediated apoptotic conduit, concurrently impeding fibroblast (FB) proliferation and enhancing their apoptosis. 63 Moreover, gamma interferon (IFN‐γ) throttles FB proliferation via the potentiation of Cer secretion. Nevertheless, in the keloid context, Cer's pro‐apoptotic potency is nullified, partially due to the augmented expression of insulin‐like growth factor‐1 (IGF‐I) in expeditiously proliferating KFBs, with keloids also evidencing overexpression of the insulin‐like growth factor‐1 receptor (IGF‐IR). 64 Furthermore, IGF‐I shields KFBs from FAs‐mediated apoptosis, and an additional impediment to keloid mitigation is the obstruction of the FAs‐mediated apoptotic pathway, wherein signal interruption is manifested upstream of Cer, precluding its receipt of the signal and subsequent pro‐apoptotic execution. 65 , 66
3.2.3. Divergent impacts of S1P and its analogue FTY720 on keloids
S1P, a crucial signaling transducer, wields substantial influence over cellular growth, differentiation, proliferation, and apoptosis. 67 S1P receptors (S1PRs), G protein‐coupled receptors targeted by S1P, encompass five subtypes: S1PR1–5, which modulate biological activities such as immune cell trafficking, angiogenic endeavors, and mitosis amid cellular proliferation. 68 In keloid tissue, S1PR1/S1PR2 mRNA and protein expressions were elevated by ~1.5‐fold and a substantive margin, respectively, compared to their normal skin tissue counterparts. By binding to S1PR, S1P oversees lymphatic cell transport and inflammatory cell recruitment to, and retention within inflamed tissues, thereby delineating a connection between S1P, collagen synthesis, and fibrosis during keloid development and progression. 69
Notably, FTY720, an immunosuppressant and S1P analogue, exerts anti‐inflammatory impacts and influences immune cell differentiation pathways, embodying a potentially potent actor in pathological scarring treatment. 70 Experimental data showed that FTY720 imparts distinct pleiotropic impacts on hyperproliferative scarring dermal fibroblasts (HSFs) and normal fibroblasts (NFBs), implicated diminished cellular viability, G0/G1 phase cell cycle arrest, apoptosis instigation, and inhibition of migration, contraction, and α‐SMA, collagen I and III expression, thereby underscore its prospective utility as an anti‐fibrotic agent. 71
3.2.4. The influence of vitamin D on fibrotic processes in keloids
Vitamin D (VD), a group of structurally analogous steroid derivatives, and its principal metabolite, 1,25‐dihydroxyvitamin D3 (1,25 D), play pivotal roles in processes such as calcium homeostasis, hormone secretion, and cell proliferation and differentiation. 72 Notably, in keloid skin, mRNA and protein expression of the functional vitamin D receptor (VDR)—a ligand‐dependent transcription factor within the steroidogenic nuclear receptor gene family and mediator of 1,25 D effects—is significantly reduced compared to normal skin. 73 1,25 D distinctly inhibits the proliferation of KFBs without triggering cytotoxicity. Ample research has pointed towards the involvement of VD in TGF‐β1‐related fibrosis in recognised VD target cells or organs. 74 , 75 In keloids, 1,25 D suppresses the TGF‐β1‐induced extracellular matrix production in KFBs, thereby reducing the expression of matrix proteins like type I collagen, Fibronectin (FN), and α‐SMA. Furthermore, aside from its fundamental antiproliferative and antifibrotic actions, 1,25 D uniquely induces the manifestation and secretion of hepatocyte growth factor in keloid fibroblasts and alleviates initial fibrosis by promoting MMP‐9 expression 76 , 77 Figure 2.
FIGURE 2.
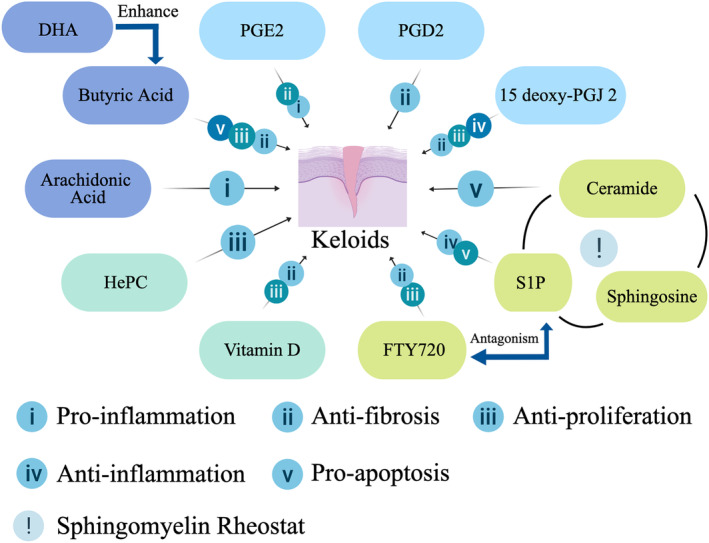
Depicting the regulatory roles of various molecules in the progression of keloids. Distinct theme colors represent different classifications. DHA, Docosahexaenoic acid; HePC, Hexadecylphosphocholine; S1P, Sphingosine‐1‐phosphate.
4. CONCLUSION
This article illuminates the intricate connections between lipids, lipid derivatives, and keloids, revealing a significant link with lipid metabolism in the progression of keloids. Abnormalities in AA synthesis and the synthesis of non‐essential FAs stand out as key characteristics of keloid scars. Delving into the impact of lipids on keloids, detrimental regulation primarily through BA, PGE2, and PGD2 is highlighted, particularly noting their role in inhibiting hyperfibrosis. Meanwhile, a combination of DHA with BA and 15‐deoxy‐Δ12,14‐Prostaglandin J2 exhibits noteworthy cytotoxicity. In the sphere of sphingolipid metabolism, Cer, Sph, and S1P emerge as pivotal. Specifically, Cer struggled to manifest its pro‐apoptotic effects in KFBs. Contrarily, S1P promoted keloid hyperfibrosis, while its analogue, FTY720, exhibited opposing effects. VD and HePC both offered antifibrotic and antiproliferative effects, thereby contributing to the negative regulation of keloid formation.
Despite being a common concern in plastic surgery and dermatology, keloids continue to be a challenge with no highly specific and effective treatment currently available; existing management typically navigates through surgical excision and radiotherapy. The explorations encapsulated in the current body of research provide a foundational understanding of the relationship between lipids, their derivatives, and keloids. Thus, targeting specific lipid molecules and derivatives opens a promising avenue for the development of novel interventions, aiming to enhance and refine therapeutic approaches to managing keloid scars.
FUNDING INFORMATION
This study was funded by 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University, Nos. ZYPY20001 and ZYPY20002.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Li C, Xie R, Zhang S, et al. Metabolism, fibrosis, and apoptosis: The effect of lipids and their derivatives on keloid formation. Int Wound J. 2024;21(2):e14733. doi: 10.1111/iwj.14733
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Berman B, Bieley HC. Keloids. J Am Acad Dermatol. 1995;33:117‐123. [DOI] [PubMed] [Google Scholar]
- 2. Tian F, Jiang Q, Chen J, Liu Z. Silicone gel sheeting for treating keloid scars. Cochrane Database Syst Rev. 2023;1:CD013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Li M, Qu C, et al. The polygenic map of keloid fibroblasts reveals fibrosis‐associated gene alterations in inflammation and immune responses. Front Immunol. 2021;12:810290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louw L. Keloids in rural black south Africans. Part 3: a lipid model for the prevention and treatment of keloid formations. Prostaglandins Leukot Essent Fatty Acids. 2000;63:255‐262. [DOI] [PubMed] [Google Scholar]
- 5. Wang Q, Wang P, Qin Z, et al. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. 2021;38:101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zang C, Liu Y, Chen H. The sphingolipids metabolism mechanism and associated molecular biomarker investigation in keloid. Comb Chem High Throughput Screen. 2023;26:2003‐2012. [DOI] [PubMed] [Google Scholar]
- 7. Yang J, Chen M, He L. To explore ideas from the altered metabolites: the metabolomics of pathological scar. J Craniofac Surg. 2022;33:1619‐1625. [DOI] [PubMed] [Google Scholar]
- 8. Louw L, Dannhauser A. Keloids in rural black south Africans. Part 2: dietary fatty acid intake and total phospholipid fatty acid profile in the blood of keloid patients. Prostaglandins Leukot Essent Fatty Acids. 2000;63:247‐253. [DOI] [PubMed] [Google Scholar]
- 9. de Carvalho CCCR, Caramujo MJ. The various roles of fatty acids. Mol Basel Switz. 2018;23:2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreira ACB, Hochman B, Furtado F, Bonatti S, Ferreira LM. Keloids: a new challenge for nutrition. Nutr Rev. 2010;68:409‐417. [DOI] [PubMed] [Google Scholar]
- 11. Yang J‐X, Li S‐Y, Chen M‐L, He L‐R. The role of altered fatty acid in pathological scars and their dermal fibroblasts. Chin J Traumatol Zhonghua Chuang Shang Za Zhi. 2022;25:218‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nomura T, Terashi H, Omori M, et al. Lipid analysis of normal dermis and hypertrophic scars. Wound Repair Regen. 2007;15:833‐837. [DOI] [PubMed] [Google Scholar]
- 13. Siess W, Siegel FL, Lapetina EG. Arachidonic acid stimulates the formation of 1,2‐diacylglycerol and phosphatidic acid in human platelets. Degree of phospholipase C activation correlates with protein phosphorylation, platelet shape change, serotonin release, and aggregation. J Biol Chem. 1983;258:11236‐11242. [PubMed] [Google Scholar]
- 14. Das UN. Interaction(s) between essential fatty acids, eicosanoids, cytokines, growth factors and free radicals: relevance to new therapeutic strategies in rheumatoid arthritis and other collagen vascular diseases. Prostaglandins Leukot Essent Fatty Acids. 1991;44:201‐210. [DOI] [PubMed] [Google Scholar]
- 15. Feng C, Shan M, Xia Y, et al. Single‐cell RNA sequencing reveals distinct immunology profiles in human keloid. Front Immunol. 2022;13:940645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: from cancer progression to treatment. Prog Lipid Res. 2020;80:101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pope ED, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mashima T, Seimiya H, Tsuruo T. De novo fatty‐acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763‐777. [DOI] [PubMed] [Google Scholar]
- 20. Cheng C, Ru P, Geng F, et al. Glucose‐mediated N‐glycosylation of SCAP is essential for SREBP‐1 activation and tumor growth. Cancer Cell. 2015;28:569‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones SF, Infante JR. Molecular pathways: fatty acid synthase. Clin Cancer Res. 2015;21:5434‐5438. [DOI] [PubMed] [Google Scholar]
- 22. Mirzaei R, Afaghi A, Babakhani S, et al. Role of microbiota‐derived short‐chain fatty acids in cancer development and prevention. Biomed Pharmacother Biomed Pharmacother. 2021;139:111619. [DOI] [PubMed] [Google Scholar]
- 23. Folkerts J, Redegeld F, Folkerts G, et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI‐mediated signaling. Allergy. 2020;75:1966‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maeshige N, Torii K, Tabuchi H, et al. Inhibitory effects of short‐chain fatty acids and ω‐3 polyunsaturated fatty acids on profibrotic factors in dermal fibroblasts. Eplasty. 2019;19:e4. [PMC free article] [PubMed] [Google Scholar]
- 25. Torii K, Maeshige N, Aoyama‐Ishikawa M, Miyoshi M, Terashi H, Usami M. Combination therapy with butyrate and docosahexaenoic acid for keloid fibrogenesis: an in vitro study. An Bras Dermatol. 2017;92:184‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan YY, Zhang J, Barhoumi R, et al. Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells. Am J Physiol. 1999;277:C310‐C319. [DOI] [PubMed] [Google Scholar]
- 27. Ishak WMW, Katas H, Yuen NP, Abdullah MA, Zulfakar MH. Topical application of omega‐3‐, omega‐6‐, and omega‐9‐rich oil emulsions for cutaneous wound healing in rats. Drug Deliv Transl Res. 2019;9:418‐433. [DOI] [PubMed] [Google Scholar]
- 28. Hogenkamp A, Ehlers A, Garssen J, Willemsen LEM. Allergy modulation by N‐3 long chain polyunsaturated fatty acids and fat soluble nutrients of the Mediterranean diet. Front Pharmacol. 2020;11:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lucas L, Hernández‐Alcoceba R, Penalva V, Lacal JC. Modulation of phospholipase D by hexadecylphosphorylcholine: a putative novel mechanism for its antitumoral activity. Oncogene. 2001;20:1110‐1117. [DOI] [PubMed] [Google Scholar]
- 30. Detmar M, Geilen CC, Wieder T, Orfanos CE, Reutter W. Phospholipid analogue hexadecylphosphocholine inhibits proliferation and phosphatidylcholine biosynthesis of human epidermal keratinocytes in vitro. J Invest Dermatol. 1994;102:490‐494. [DOI] [PubMed] [Google Scholar]
- 31. Blume‐Peytavi U, Geilen CC, Sommer C, Almond‐Roesler B, Orfanos CE. The phospholipid analogue hexadecylphosphocholine (HePC) inhibits proliferation of keloid fibroblasts in vitro and modulates their fibronectin and integrin synthesis. Arch Dermatol Res. 1997;289:164‐169. [DOI] [PubMed] [Google Scholar]
- 32. Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hinman JW. Prostaglandins. Annu Rev Biochem. 1972;41:161‐178. [DOI] [PubMed] [Google Scholar]
- 34. Peebles RS. Prostaglandins in asthma and allergic diseases. Pharmacol Ther. 2019;193:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep PR. 2008;60:3‐11. [PubMed] [Google Scholar]
- 36. Simon LS. Role and regulation of cyclooxygenase‐2 during inflammation. Am J Med. 1999;106:37S‐42S. [DOI] [PubMed] [Google Scholar]
- 37. Korn JH. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short‐term exposure to mononuclear cell products. J Clin Invest. 1983;71:1240‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Legler DF, Bruckner M, Uetz‐von Allmen E, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010;42:198‐201. [DOI] [PubMed] [Google Scholar]
- 40. Kohyama T, Liu XD, Wen FQ, Kim HJ, Takizawa H, Rennard SI. Prostaglandin D2 inhibits fibroblast migration. Eur Respir J. 2002;19:684‐689. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, Nagata K. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. J Immunol Baltim. 1950;2000(164):2277‐2280. [DOI] [PubMed] [Google Scholar]
- 42. Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti‐IgE. J Immunol Baltim. 1950;1982(129):1627‐1631. [PubMed] [Google Scholar]
- 43. Schuligoi R, Schmidt R, Geisslinger G, Kollroser M, Peskar BA, Heinemann A. PGD2 metabolism in plasma: kinetics and relationship with bioactivity on DP1 and CRTH2 receptors. Biochem Pharmacol. 2007;74:107‐117. [DOI] [PubMed] [Google Scholar]
- 44. Kolodsick JE, Peters‐Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. Prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537‐544. [DOI] [PubMed] [Google Scholar]
- 45. Bärnthaler T, Theiler A, Zabini D, et al. Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J Allergy Clin Immunol. 2020;145:818‐833.e11. [DOI] [PubMed] [Google Scholar]
- 46. Li K, Zhao J, Wang M, et al. The roles of various prostaglandins in fibrosis: a review. Biomolecules. 2021;11:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayashi T, Nishihira J, Koyama Y, Sasaki S, Yamamoto Y. Decreased prostaglandin E2 production by inflammatory cytokine and lower expression of EP2 receptor result in increased collagen synthesis in keloid fibroblasts. J Invest Dermatol. 2006;126:990‐997. [DOI] [PubMed] [Google Scholar]
- 48. Yeh F‐L, Shen H‐D, Lin M‐W, Chang C‐Y, Tai H‐Y, Huang M‐H. Keloid‐derived fibroblasts have a diminished capacity to produce prostaglandin E2. Burns. 2006;32:299‐304. [DOI] [PubMed] [Google Scholar]
- 49. Lee WJ, Ahn HM, Roh H, et al. Decorin‐expressing adenovirus decreases collagen synthesis and upregulates MMP expression in keloid fibroblasts and keloid spheroids. Exp Dermatol. 2015;24:591‐597. [DOI] [PubMed] [Google Scholar]
- 50. Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143‐153. [DOI] [PubMed] [Google Scholar]
- 51. Yang J, Li S, He L, Chen M. Adipose‐derived stem cells inhibit dermal fibroblast growth and induce apoptosis in keloids through the arachidonic acid‐derived cyclooxygenase‐2/prostaglandin E2 cascade by paracrine. Burns Dent Traumatol. 2021;9:tkab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Werder RB, Lynch JP, Simpson JC, et al. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN‐λ production. Sci Transl Med. 2018;10:eaao0052. [DOI] [PubMed] [Google Scholar]
- 53. Sawyer N, Cauchon E, Chateauneuf A, et al. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137:1163‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mantel A, Newsome A, Thekkudan T, Frazier R, Katdare M. The role of aldo‐keto reductase 1C3 (AKR1C3)‐mediated prostaglandin D2 (PGD2) metabolism in keloids. Exp Dermatol. 2016;25:38‐43. [DOI] [PubMed] [Google Scholar]
- 55. Scher JU, Pillinger MH. 15d‐PGJ2: the anti‐inflammatory prostaglandin? Clin Immunol Orlando Fla. 2005;114:100‐109. [DOI] [PubMed] [Google Scholar]
- 56. Huang C, Ogawa R. Roles of lipid metabolism in keloid development. Lipids Health Dis. 2013;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suk F‐M, Chen C‐H, Lin S‐Y, et al. 15‐deoxy‐Delta(12,14)‐prostaglandin J(2) inhibits fibrogenic response in human hepatoma cells. Toxicol Lett. 2009;187:22‐27. [DOI] [PubMed] [Google Scholar]
- 58. Han Z, Zhu T, Liu X, et al. 15‐deoxy‐Δ12,14 ‐prostaglandin J2 reduces recruitment of bone marrow‐derived monocyte/macrophages in chronic liver injury in mice. Hepatol Baltim. 2012;56:350‐360. [DOI] [PubMed] [Google Scholar]
- 59. McVey MJ, Weidenfeld S, Maishan M, et al. Platelet extracellular vesicles mediate transfusion‐related acute lung injury by imbalancing the sphingolipid rheostat. Blood. 2021;137:690‐701. [DOI] [PubMed] [Google Scholar]
- 60. Parveen F, Bender D, Law S‐H, Mishra VK, Chen C‐C, Ke L‐Y. Role of ceramidases in sphingolipid metabolism and human diseases. Cell. 2019;8:1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chaurasia B, Summers SA. Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol. 2021;83:303‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang S‐E, Kim K‐J, Ro K‐H, et al. Sphingosine may have cytotoxic effects via apoptosis on the growth of keloid fibroblasts. J Dermatol. 2004;31:1‐5. [DOI] [PubMed] [Google Scholar]
- 63. Andrieu‐Abadie N, Gouazé V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717‐728. [DOI] [PubMed] [Google Scholar]
- 64. Ishihara H, Yoshimoto H, Fujioka M, et al. Keloid fibroblasts resist ceramide‐induced apoptosis by overexpression of insulin‐like growth factor I receptor. J Invest Dermatol. 2000;115:1065‐1071. [DOI] [PubMed] [Google Scholar]
- 65. Katayama Y, Naitoh M, Kubota H, et al. Chondroitin sulfate promotes the proliferation of keloid fibroblasts through activation of the integrin and protein kinase B pathways. Int J Mol Sci. 2020;21:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xin Y, Min P, Xu H, Zhang Z, Zhang Y, Zhang Y. CD26 upregulates proliferation and invasion in keloid fibroblasts through an IGF‐1‐induced PI3K/AKT/mTOR pathway. Burns Dent Traumatol. 2020;8:tkaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Obinata H, Hla T. Sphingosine 1‐phosphate and inflammation. Int Immunol. 2019;31:617‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cartier A, Hla T. Sphingosine 1‐phosphate: lipid signaling in pathology and therapy. Science. 2019;366:eaar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jung SH, Song YK, Chung H, et al. Association between sphingosine‐1‐phosphate‐induced signal transduction via mitogen‐activated protein kinase pathways and keloid formation. Arch Dermatol Res. 2019;311:711‐719. [DOI] [PubMed] [Google Scholar]
- 70. Ryu J, Jhun J, Park M‐J, et al. FTY720 ameliorates GvHD by blocking T lymphocyte migration to target organs and by skin fibrosis inhibition. J Transl Med. 2020;18:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aoki M, Kondo A, Matsunaga N, et al. The immunosuppressant fingolimod (FTY720) for the treatment of mechanical force‐induced abnormal scars. J Immunol Res. 2020;2020:7057195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Urena‐Torres P, Souberbielle JC. Pharmacologic role of vitamin D natural products. Curr Vasc Pharmacol. 2014;12:278‐285. [DOI] [PubMed] [Google Scholar]
- 73. Hahn JM, Supp DM. Abnormal expression of the vitamin D receptor in keloid scars. Burns. 2017;43:1506‐1515. [DOI] [PubMed] [Google Scholar]
- 74. Hahn JM, Combs KA, Powell HM, Supp DM. A role for vitamin D and the vitamin D receptor in keloid disorder. Wound Repair Regen. 2023;31:563‐575. [DOI] [PubMed] [Google Scholar]
- 75. Suneetha PV, Sarin SK, Goyal A, Kumar GT, Shukla DK, Hissar S. Association between vitamin D receptor, CCR5, TNF‐alpha and TNF‐beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol. 2006;44:856‐863. [DOI] [PubMed] [Google Scholar]
- 76. Zhang GY, Cheng T, Luan Q, et al. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol. 2011;164:729‐737. [DOI] [PubMed] [Google Scholar]
- 77. Mamdouh M, Omar GA, Hafiz HSA, Ali SM. Role of vitamin D in treatment of keloid. J Cosmet Dermatol. 2022;21:331‐336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


