Key Points
Question
Do early adolescents with complex congenital heart disease (cCHD) have higher stress markers than healthy controls, and is there an association with executive functions and resilience?
Findings
In this case-control study of 100 early adolescents with cCHD and 104 controls, those with cCHD had higher stress markers in hair and lower executive function scores than those without cCHD. A significant interaction effect was observed between stress markers and executive function.
Meaning
This study’s results suggest evidence for clinically relevant alteration in physiological stress markers in adolescents with cCHD and an association with neurodevelopmental sequelae.
This case-control study compares physiological stress markers, executive function, and resilience among individuals aged 10 to 15 years with complex congenital heart disease vs healthy controls.
Abstract
Importance
Infants with complex congenital heart disease (cCHD) may experience prolonged and severe stress when undergoing open heart surgery. However, little is known about long-term stress and its role in neurodevelopmental impairments in this population.
Objective
To investigate potential differences between early adolescents aged 10 to 15 years with cCHD and healthy controls in physiological stress markers by hair analysis, executive function (EF) performance, and resilience.
Design, Setting, and Participants
This single-center, population-based case-control study was conducted at the University Children’s Hospital Zurich, Switzerland. Patients with different types of cCHD who underwent cardiopulmonary bypass surgery during the first year of life and who did not have a genetic disorder were included in a prospective cohort study between 2004 and 2012. A total of 178 patients were eligible for assessment at ages 10 to 15 years. A control group of healthy term-born individuals was cross-sectionally recruited. Data assessment was between 2019 and 2021. Statistical analysis was performed from January to April 2023.
Exposure
Patients with cCHD who underwent infant open heart surgery.
Main Outcomes and Measures
Physiological stress markers were quantified by summing cortisol and cortisone concentrations measured with liquid chromatography with tandem mass spectrometry in a 3-centimeter hair strand. EFs were assessed with a neuropsychological test battery to produce an age-adjusted EF summary score. Resilience was assessed with a standardized self-report questionnaire.
Results
The study included 100 patients with cCHD and 104 controls between 10 and 15 years of age (mean [SD] age, 13.3 [1.3] years); 110 (53.9%) were male and 94 (46.1%) were female. When adjusting for age, sex, and parental education, patients had significantly higher sums of hair cortisol and cortisone concentrations (β, 0.28 [95% CI, 0.12 to 0.43]; P < .001) and lower EF scores (β, −0.36 [95% CI, −0.49 to −0.23]; P < .001) than controls. There was no group difference in self-reported resilience (β, −0.04 [95% CI, −0.23 to 0.12]; P = .63). A significant interaction effect between stress markers and EFs was found, indicating a stronger negative association in patients than controls (β, −0.65 [95% CI, −1.15 to −0.15]; P = .01). The contrast effects were not significant in patients (β, −0.21 [95% CI, −0.43 to −0.00]; P = .06) and controls (β, 0.09 [95% CI, −0.11 to 0.30]; P = .38).
Conclusions and Relevance
This case-control study provides evidence for altered physiological stress levels in adolescents with cCHD and an association with poorer EF. These results suggest that future studies are needed to better understand the neurobiological mechanisms and timing of alterations in the stress system and its role in neurodevelopment.
Introduction
Infants with complex congenital heart disease (cCHD) often undergo multiple surgical procedures, intensive care unit (ICU) stays, and medical interventions. This likely causes intense and prolonged stress.1 Infant stress can disturb the hypothalamic-pituitary-adrenal (HPA) axis and the release of cortisol, which regulates normal stress reactions.2,3,4 Studies have investigated the association of early life stress and cortisol levels in infants with cCHD. Altered serum and salivary cortisol levels were associated with more complicated ICU stays and differed from reference values in children undergoing open heart surgery preoperatively and postoperatively.5,6 Another study found that cortisol levels differed between children aged 3 to 5 years who had undergone infant cardiac surgery and children with cCHD who did not require surgery.7 Whereas salivary and serum cortisol reflects acute stress, hair cortisol reflects a longer-term concentration,8 which constitutes a biomarker for chronic stress and persistent alterations in the HPA axis.9 This technique has proved successful in other studies investigating children and adolescents10 but has not been applied in studies involving patients with cCHD. Furthermore, the clinical relevance of altered stress markers in patients with cCHD remains unclear.
Children and adolescents with cCHD are at risk for executive function (EF) impairments.11,12 EFs are higher-order cognitive functions relevant for goal-directed behavior and to regulate cognition, behavior, emotions, and social functioning.13 However, the mechanisms underlying poor EFs in patients with cCHD remain unclear. An exploratory study in 15 children with tetralogy of Fallot found that the morphology of the inferior parietal gyrus was associated with preoperative cortisone levels and cognition. However, a direct link between cortisone/cortisol and cognition was not examined.14 Overall, little is known about the influence of stress on EFs in cCHD, but studies in healthy individuals indicate a link between low EF performance and high stress measured by cortisol levels.15,16,17 The underlying mechanisms remain unclear. However, cortisol can cross the blood-brain barrier and may be harmful for brain structure and function.18 Brain structures relevant to EFs (eg, prefrontal cortex, hippocampus) are particularly vulnerable to increased cortisol in humans and animal models.19,20 In contrast, EF difficulties may compromise psychological well-being due to increased academic and socioemotional difficulties, which in turn can increase stress.
While stress and high cortisol levels may be risk factors for lower EF and vice versa, better EF performance has been shown to correlate with better resilience in healthy children, adolescents,21 and young adults.22 “Resilience is a process that allows an individual to adapt to difficult life experiences requiring mental, emotional, and behavioral flexibility”23 and can be assessed with standardized questionnaires.24 To date, research investigating resilience in patients with cCHD is limited. However, a recent study showed adolescent patients with cCHD score lower than controls in a resilience questionnaire.25 Interindividual differences in resilience may mitigate both stress and EF impairments in the cCHD population, but research evidence is lacking.
We aimed to investigate if patients with cCHD had higher stress markers, quantified by hair cortisol and cortisone levels, lower EF performance, and lower resilience than healthy controls. Furthermore, we aimed to test the correlations between stress markers, EFs, and resilience.
Methods
Study Design and Population
This case-control study is part of a prospective cohort study (Teen Heart Study26) investigating neurodevelopmental outcomes and cerebral magnetic resonance imaging in early adolescents with cCHD and was conducted at the University Children’s Hospital Zurich, Switzerland, between April 2019 and September 2021.
This study included patients with cCHD who underwent cardiopulmonary bypass surgery (CPB) between 2004 and 2012 at the University Children’s Hospital Zurich. Patients were eligible if they underwent CPB surgery before 1 year of age, were not diagnosed with a genetic or dysmorphic syndrome, and were between 10 and 15 years of age at the time of assessment. The age range of 10 to 15 years has previously been defined as early adolescence.27 Of 178 eligible patients, 100 early adolescents with cCHD participated in the current study (participation rate: 56%). A control group of 104 healthy early adolescents between 10 and 15 years of age was recruited with the following exclusion criteria: preterm birth (<36 weeks of gestation), diagnosed with a neurological or substantial developmental disorder (eg, learning disorder or attention deficit hyperactivity disorder). Controls were recruited as friends of participating patients, through print and online advertisement. See the eFigure in Supplement 1 for the recruitment procedure. The study was approved by the ethics committee of the Canton of Zurich. Written informed consent was obtained prior to participation from the participants’ legal guardians and from participants if they were 14 years and older. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed.
Measures
Stress Markers
The analysis of the stress markers, cortisol and its metabolite cortisone, was conducted using 1 strand of hair collected from the posterior vertex region. A 3-centimeter hair segment was cut proximally to the scalp. At a mean growth rate of 1-centimeter per month, this represents the cumulative cortisol and cortisone concentration of the past 3 months. The sample was stored in aluminum foil at room temperature. Cortisol and cortisone were analyzed and quantified by liquid chromatography coupled with tandem mass spectrometry following a protocol by Voegel and colleagues.28 The sum of cortisol and cortisone was calculated for further statistical analysis as suggested by Voegel and colleagues.29
Executive Functions
EFs were investigated with an extensive standardized neuropsychological test battery.26,30,31,32,33,34 Details about the neuropsychological test measures of EFs are displayed in Table 1. A summary score for overall EF performance was built from these tests. See eText 1 and eTables 1, 2, and 3 in Supplement 1 for details.
Table 1. Neuropsychological Test Battery to Assess Executive Functions Performance.
| Executive function domains | Neuropsychological test | Test measurement |
|---|---|---|
| Working memory | Digit span forward and backward (WISC-IV) | No. of correct items |
| Letter-number sequencing (WISC-IV) | No. of correct items | |
| Corsi block tapping test (Corsi) | No. of correct items | |
| Inhibition | Subtest interference, color word interference task (D-KEFS) | Completion time |
| Go/NoGo (TAP) | No. of commission errors | |
| Cognitive flexibility | Subtest letter-number-switching, trail making task (D-KEFS) | Completion time |
| TAP flexibility (TAP) | Median reaction time | |
| Fluency | Subtests s-words and animals (RWT) | No. of correct items |
| Subtest filled-dots-only, design fluency test (D-KEFS) | No. of correct items | |
| Planning | Tower task (D-KEFS) | Total achievement score |
Abbreviations: D-KEFS, Delis-Kaplan Executive Function System; TAP, Test of Attentional Performance; RWT, Regensburger Verbal Fluency Test; WISC-IV, Wechsler Intelligence Scale for Children Fourth Edition, Corsi.
Resilience
Resilience was assessed with the Resilience Scale 13 (RS-13), which measures self-reported personality traits of acceptance of self and life and personal competences and has been validated in a German-speaking population. This questionnaire has good internal consistency and acceptable test-retest reliability, can be used in adolescents and adults, and has been applied in various patient samples.35,36 Participants rate the accuracy of 13 statements on a 7-point Likert scale. Higher scores indicate higher resilience. The sum score was calculated and used for statistical analysis.35
Participant Characteristics
Medical information was collected prospectively from patients’ medical records and included information on the neonatal period, the cCHD physiology, and the perioperative period. Sociodemographic variables were assessed in a parent-reported questionnaire: Parental education was calculated by the sum of maternal and paternal highest education, each on a 6-point scale (from 1 = no high school qualification to 6 = university degree).37 A 6-point Likert scale assessed whether their financial resources were sufficient to cover their living costs. Parental immigration background was assessed. Race and ethnicity were not assessed as part of this study as these variables were not included in the demographic questionnaire.
IQ was evaluated with a corrected short version of the Wechsler Intelligence Scale for Children 4th edition, including the subtests of matrices, similarities, letter number sequencing, and symbol search.38,39 The parent-reported life events scale assessed whether and/or which of 13 potentially stressful events (eg, parental separation, illness/death of a relative or familiar person) happened within the past 3 months.40 A sum score from 0 to 13 was calculated.
Statistical Analysis
For hypotheses testing, linear regression analysis was used to assess group differences (patients with cCHD vs controls) with respect to stress markers, EFs, and resilience, as well to investigate the association among these 3 outcomes. Post hoc, we investigated differences between patients with a univentricular and a biventricular cCHD regarding stress markers, EFs, and resilience. Furthermore, we tested an association between stress markers and clinical variables. At last, an interaction effect (group × cortisol) for the outcome EFs was tested and contrast effects per group were investigated post hoc. All post hoc analyses were false discovery rate–corrected. For consistency, all analyses were adjusted for sex, age, and parental education. Parental education was missing in 12 patients and 12 controls (12%) and was imputed by chained equation with 1 imputation and 5 iterations.41 All outcomes and factors included in the regression models were used for estimation of missing values. Standardized β and unstandardized B coefficients with 95% CIs were reported. Models were visually investigated for normal distribution of residuals.
To help interpret the EF summary score, each EF test contributing to the summary score was compared between patients and controls with a 2-sampled t test and between patients and available normative data with a 1-sampled t test. Results were considered statistically significant if 2-sided P < .05. All analyses were conducted using R version 4.2.0 (R Project for Statistical Computing) from January to April 2023.42
Results
Population Characteristics
The study included 100 patients with cCHD and 104 controls between 10 and 15 years of age; mean [SD] age was 13.3 [1.3] years; 110 (53.9%) were male and 94 (46.1%) were female; on average they were term-born with a mean (SD) gestational age of 39.2 (1.8) weeks. Demographic and clinical characteristics stratified by group are reported in Table 2. Statistical estimates of all EF tests that contributed to the EF summary score are displayed in eTable 4 in Supplement 1. Three cortisol-plus-cortisone samples were excluded due to suspected contamination. Patients who participated in this study did not significantly differ from patients who were eligible but rejected participation in clinical and demographic variables (eText 2 in Supplement 1).
Table 2. Demographic and Clinical Characteristics Stratified by Group.
| Population characteristics | cCHD (n = 100) | Control (n = 104) | P value |
|---|---|---|---|
| Innate | |||
| Sex, No. (%) | |||
| Female | 39 (39.0) | 55 (52.9) | .07a |
| Male | 61 (61.0) | 49 (47.1) | .07a |
| Age, mean (SD), y | 13.7 (1.2) | 13.0 (1.4) | <.001b |
| Parental education, median (IQR) | 8 (6-9) | 10 (8-12) | <.001b |
| Parental immigration background, No. (%) | 20 (20.0) | 13 (12.5) | .21a |
| Sufficient financial resources, median (IQR) | 6 (5-6) | 6 (5-6) | .003 |
| Life events in past 3 mo, median (IQR) | 0 (0-1) | 0 (0-1) | .17 |
| Gestational age, mean (SD), wk | 39.1 (2.0) | 39.2 (1.5) | .65b |
| IQ, mean (SD) | 99.2 (14.4) | 110.8 (9.2) | <.001b |
| Prenatal diagnosis, No. (%) | 23 (23.0) | NA | NA |
| Cyanotic cCHD, No. (%) | 73 (73.0) | NA | NA |
| Biventricular cCHD, No. (%) | 76 (76.0) | NA | NA |
| Preoperative (1st CPB surgery) | |||
| Preoperative saturation, mean (SD) | 86.4 (10.1) | NA | NA |
| Age at surgery, mean (SD), mo | 2.4 (2.8) | NA | NA |
| Intraoperative (1st CPB surgery) | |||
| Lowest temperature, mean (SD), °C | 29.0 (4.2) | NA | NA |
| Time on ECC, mean (SD), min | 169.5 (71.3) | NA | NA |
| Postoperative (1st CPB surgery) | |||
| Time in ICU, median (IQR), d | 8 (5-13) | NA | NA |
| Time in hospital, median (IQR), d | 26.5 (18.0-39.2) | NA | NA |
| No. CPB surgical procedures, median (IQR) | 1 (1-2) | NA | NA |
Abbreviations: cCHD, complex congenital heart disease; CPB, cardiopulmonary bypass; ECC, extracorporeal circulation; ICU, intensive care unit; NA, not applicable (these data do not exist because the controls did not undergo surgery and were not hospitalized).
Two-sampled χ2 test.
Two-sampled; two-sided Mann-Whitney U test. OR for χ2 tests; Cohen d for t tests; r for Mann-Whitney U test.
Group Comparisons
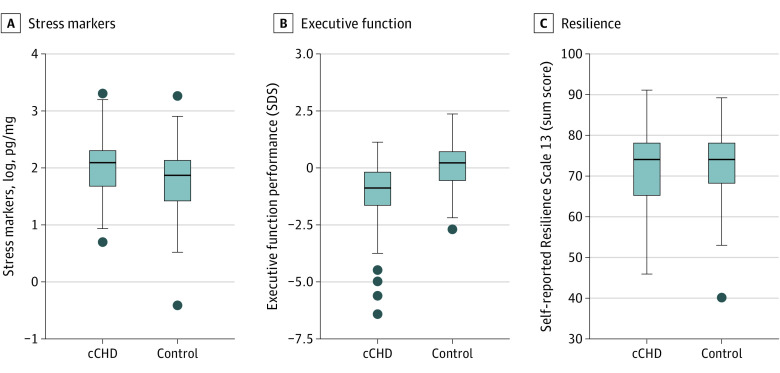
Data on stress markers was skewed and was thus transformed with the natural logarithm to reach normality of data distribution. Compared with controls, patients had significantly higher stress makers quantified by mean (SD) cortisol-plus-cortisone concentrations (cCHD: 9.1 [5.4] pg/mg; controls: 6.8 [3.8] pg/mg; cCHD log-transformed: 2.05 [0.56]; controls log-transformed: 1.76 [0.59]; β, 0.28 [95% CI, 0.12 to 0.43]; P < .001) and lower mean (SD) EFs (CHD: −1.2 [1.4]; controls: 0.01 [1.0]; β, −0.36 [95% CI, −0.49 to −0.23]; P < .001). There was no significant difference in mean (SD) resilience sum scores between patients and controls (CHD: 71.7 [9.4]); controls: 73.4 [8.5]; β, −0.04 [95% CI, −0.23 to 0.12]; P = .63) (Figure 1 and Table 3). Female early adolescents had higher levels of cortisol plus cortisone and higher scores for resilience than male early adolescents.
Figure 1. Group Comparisons for Stress Markers, Executive Functions, and Resilience.
The lower and upper borders of the box represent the first and third quartiles. The line within the box corresponds to the median. Outliers are represented by circles. cCHD indicates complex congenital heart disease; SDS, standard deviation score.
Table 3. Linear Regression Models Estimating Group Differencesa,b.
| Outcome | Factors | β (95% CI) | P value | R2 adjusted | P value model fit | |
|---|---|---|---|---|---|---|
| Standardized | Unstandardized | |||||
| Stress markers | Group | 0.28 (0.12 to 0.43) | 0.33 (0.14 to 0.52) | <.001 | 0.10 | <.001 |
| Sex | 0.25 (0.11 to 0.39) | 0.3 (0.12 to 0.47) | <.001 | |||
| Age | −0.03 (−0.18 to 0.11) | −0.01 (−0.08 to 0.05) | .66 | |||
| Parental education | −0.01 (−0.16 to 0.14) | −0.002 (−0.04 to 0.04) | .90 | |||
| Executive functions | Group | −0.36 (−0.49 to −0.23) | −0.93 (−1.30 to −0.57) | <.001 | 0.23 | <.001 |
| Sex | 0.01 (−0.14 to 0.11) | 0.03 (−0.36 to 0.30) | .84 | |||
| Age | 0.02 (−0.1 to 0.15) | 0.02 (−0.10 to 0.15) | .71 | |||
| Parental education | 0.24 (0.11 to 0.37) | 0.12 (0.05 to 0.20) | .001 | |||
| Resilience | Group | −0.04 (−0.23 to 0.12) | −0.72 (−3.67 to 2.23) | .63 | 0.09 | .07 |
| Sex | 0.02 (0.05 to 0.34) | 3.55 (0.85 to 6.24) | .01 | |||
| Age | −0.05 (−0.2 to 0.1) | −0.36 (−1.40 to 0.69) | .50 | |||
| Parental education | 0.05 (−0.11 to 0.21) | −0.18 (−0.40 to 0.76) | .54 | |||
Reference for group comparison: controls. Reference for sex comparison: male individuals.
Missing data: stress marker (n = 19 [9%]), executive function summary score (n = 6 [3%]), Resilience Scale 13 sum score (n = 30 [15%]).
There was no significant difference in stress markers between participants who experienced 1 or more life events the past 3 months compared with those who did not (t = –0.75; P = .46; ≥1 life events: 40 of 100 patients [40.0%], 31 of 104 controls [29.8%]). Post hoc analysis found no significant difference between patients with univentricular and biventricular cCHD in stress markers, EFs or resilience (eTable 5 in Supplement 1). Lastly, post hoc analysis showed no significant association between stress markers and any clinical variables (eTable 6 in Supplement 1).
Associations Between Stress Markers, EFs, and Resilience
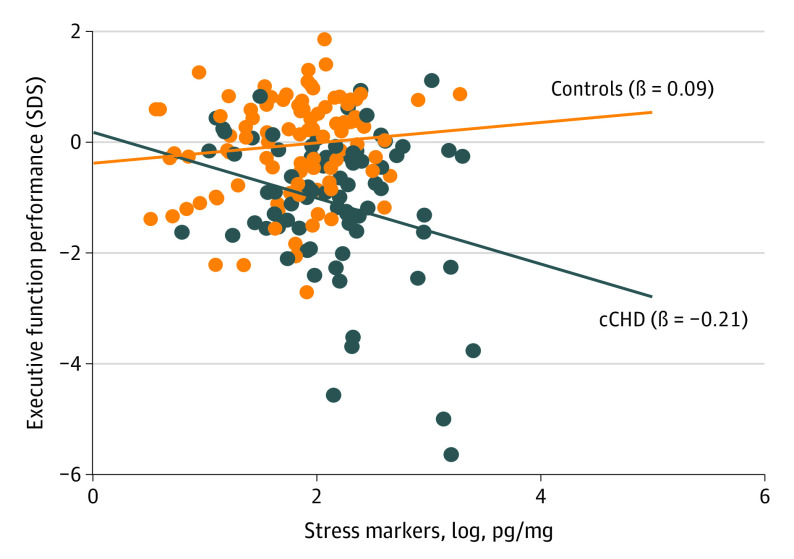
Stress markers were not significantly associated with EFs over the whole sample, but there was a significant interaction, indicating that the association between EFs and stress markers depended on group (β, −0.65 [95% CI, −1.15 to −0.15]; P = .01). Post hoc, there was a significant interaction, indicating that the association between EFs and stress markers depended on group. The association between higher stress markers and lower EFs was stronger in patients than in controls (ie, interaction), but these contrast effects were not significant (patients: β, −0.21 [95% CI, −0.43 to −0.00]; P = .06; controls: β, 0.09 [95% CI, −0.11 to 0.30]; P = .38) (Figure 2; eTable 7 in Supplement 1). Resilience was not significantly associated with stress markers or EFs (eTable 8 in Supplement 1).
Figure 2. Association of Executive Function and Stress Markers Stratified by Group.
Individual data points are represented by dots. Lines represent linear regressions for patients and controls. SDS indicates standard deviation score.
Discussion
To our knowledge, this is the first study that has investigated physiological stress markers and their associations with EF impairments in a large sample of early adolescents with cCHD. We found that early adolescents with cCHD (aged 10 to 15 years) have elevated long-term hair cortisol-plus-cortisone concentration. Furthermore, patients with cCHD had significantly lower EF performance than controls, but there was no difference in self-reported resilience. We identified a significant interaction effect, indicating that a negative association between stress markers and EFs is stronger in patients than in controls.
Altered Stress Markers in Early Adolescents With cCHD
Several studies have suggested that high early life stress may alter the function of the HPA axis and consequently alter stress markers such as cortisol and cortisone.43,44 Indeed, studies in infants and young children with cCHD have demonstrated that altered stress markers, measured by serum and salivary cortisol, are associated with complications during the ICU stay such as prolonged mechanical ventilation and inotrope medication and with the cardiopulmonary bypass surgery.5,6,7
In our cohort, we found no significant association between stress markers in hair at ages 10 to 15 years and length of ICU stay following open heart surgery in infancy and other clinical variables. However, length of ICU stay may not be a sufficiently sensitive marker for early life stress during hospitalization. We further found no difference in stress markers between patients with univentricular and biventricular cCHD, although patients with univentricular cCHD may be expected to experience more early life stress than those with biventricular cCHD, because univentricular patients typically undergo repeated open heart surgery during infancy, with prolonged hospital and ICU stays entailing higher risk of complications. Stressful events experienced during early life by patients with cCHD can be manifold and include physical pain (eg, pin picks due to needle injections), intubation, noise on the ICU, and emotional stress (eg, due to separation from parents). Future studies should examine such events separately and investigate associations with stress markers.
Although we did not find differences between subgroups of patients with cCHD, we found evidence for increased stress markers in the whole cCHD group. Early life stress may be one explanation for increased cortisol-plus-cortisone concentration during adolescence. Other explanations for higher stress markers in early adolescents with cCHD could be continued high stress associated with higher rate of emotional problems,45 and neurodevelopmental impairments at the time of measurement.46,47 Longitudinal studies are needed to assess perceived stress and biological stress markers starting in early life.
Stress Markers and Executive Function Performance
Previous meta-analyses have suggested that both early life and acute stress are associated with lower EF performance.48,49 Studies have reported mixed findings on an association between cortisol levels, EF, and overall cognitive performance in healthy individuals.50,51,52,53,54
Nits and colleagues55 proposed the neonatal stress embedding (NSE) model in the preterm population, which provides a comprehensive description of the biological embedding of stress and how this may affect long-term neurodevelopmental outcomes. The NSE model suggests that the effect of early life stress on neurodevelopment may be mediated by changes in the immune system, the autonomic nervous system, the HPA axis, and gene expressions, and that this effect may be moderated by the prenatal environment and parent–infant interactions. (Refer to Nits et al55 for details.)
In the current study, we observed higher levels of stress markers and lower EF performance in patients than controls and a significant group interaction for the association between stress markers and EF. Post hoc analysis showed that the association between higher stress markers and lower EF was higher in patients than in controls. These effects were small and did not survive multiple comparison correction. Thus, future studies should replicate these findings in a well-powered sample. However, these findings show evidence that alterations of the physiological stress system in patients with cCHD may drive neurodevelopmental impairments, particularly poorer EF performance. However, this study assessed stress markers and EFs cross-sectionally. Future longitudinal studies are needed to investigate whether early alterations of the physiological stress system in patients with cCHD predict poorer long-term neurodevelopmental outcome. Indeed, cortisol-plus-cortisone concentrations may serve as early biomarkers for neurodevelopmental outcome, and rigorous research is needed to explore this hypothesis.
Future studies should also investigate the mediating effects of brain alterations on the association of stress and EF impairments in patients with cCHD. The limbic structures, such as the hippocampus, amygdala, and prefrontal cortex, are especially vulnerable to stress and increased glucocorticoid release.56 Studies in adolescents with cCHD have shown that alterations in these brain regions are associated with impaired working memory, a core domain of EF.57,58 HPA dysfunction and associated increased cortisol levels may mediate these associations.
Of note, we computed an age-adjusted EF summary score based on an extensive neuropsychological test battery. The simple, explicit and replicable standardization procedure which was applied in this study did work well for our purpose, and could also be easily used in other studies. We used the data of the controls for age-adjusted standardization but not normative data because of substantial qualitative differences in the available norms for these tests. While this approach may be limited by the differences in parental education of patients and controls, our summary score is not biased by different norms for different tests (eg, regional origin and language of norm samples) and we performed adequate age-adjustment. This is particularly important considering the steep developmental trajectory of EF performance which parallels the maturation of brain network at this age.59 At last, using a summary score helps reduce multiple testing and type 1 error in studies. Considering that in patients with CHD, all EF domains seem to be equally affected,12 the use of a summary score seems valid.
Resilience
The current study showed similar self-reported resilience in early adolescents with cCHD and in healthy peers and no significant association with stress markers and EF. These results contradict published findings. Köble and colleagues have shown that adolescents with cCHD score significantly lower than controls in a self-reported resilience questionnaire.25 This study used the 11-item version (RS-11) of the same questionnaire that was used in our study (RS-13). Another study found higher resilience in adolescents with cCHD relative to controls assessed with the Connor-Davison Resilience Scale, which assesses similar personality traits as the RS-13.60 Considering these different results of 3 studies that used similar methodology (ie, age range, well-powered samples and validated self-reported resilience questionnaires), we assume that variability in resilience among patients with cCHD is very high. Future studies are needed to further elaborate resilience in cCHD and should particularly investigate predictors that can explain the large variability in outcome.
A study in healthy young adults showed that higher scores in a resilience questionnaire were associated with lower hair cortisol.61 Some studies also suggest that traits associated with resilience such as positive reappraisal and coping are directly dependent on EFs, indicating an interconnection between resilience and EF.21,22,62 Different interpretations and measurements of resilience render comparison across studies difficult. In this study, we used a standardized questionnaire, which assessed the personality traits of acceptance of self and life and personal competences.35 However, King and colleagues suggested that resilience should be measured not only with self-report questionnaires but also with observational behavioral measures such as measuring how children cope with stressful tasks to better understand this complex construct.63,64 This is relevant as challenges in EF, which are common in cCHD, may hinder introspection. Introspection is crucial for completing self-reported questionnaire. Consequently, studies that incorporate diverse information sources to assess resilience are likely more reliable in the context of EF difficulties.
Clinical Implications
Our results indicate high stress markers and an association with EF impairments in adolescents with cCHD. Interventions to reduce early life stress during hospitalization may reduce long-term alterations in the HPA axis and contribute to better neurodevelopmental outcomes in these patients. These interventions include reduced parent–infant separation, kangaroo care,65,66 promoted breastfeeding,67 music therapy,68 and/or trauma-informed care in the ICU.69,70 Promoting parental and child mental health throughout development may also reduce child stress and be an important target for interventions.71 Further studies with longitudinal designs and well-powered sample sizes should investigate these associations and may consider testing the usability of hair cortisol plus cortisone as a predictive biomarker for EFs in patients with cCHD.
Limitations
The limitations of our study need to be considered. This study is cross-sectional, and thus, no conclusions can be drawn about causality. Although hair cortisol-plus-cortisone levels are well-established markers for HPA axis activity and long-term stress,8,9 some confounders such as frequency of hair washing and hair color can influence the cortisol-plus-cortisone concentration.72 We excluded hair samples with dyed hair.
We analyzed physiological stress markers with cortisol-plus-cortisone levels, but we did not assess participants’ perceived psychological stress. Cortisol levels have been shown not to correlate well with perceived stress measured with standardized psychological questionnaires.9,73 Importantly, we found that differences in stress markers between patients and controls were not associated with recently experienced life events. Nevertheless, we acknowledge that the questionnaire used to assess life events may not capture the full range of stressful experiences. Thus, we cannot exclude that stress markers may be influenced by recently experienced stress.
Additionally, measuring resilience with the RS-13 may not have fully captured participants’ resilience, which has been described as a multifactorial and dynamic construct.74 More research is needed to identify more comprehensive ways of measuring this concept.
Additionally, while patients were recruited prospectively, controls were recruited cross-sectionally. Different recruitment approaches may limit the group comparison. Also, this was a single-center study with a relatively homogenous sample of controls who performed well on cognitive tests (mean IQ = 110.8), had high average parental education and more financial resources than patients. This may have led to less variability and therefore less pronounced effects in the association between stress markers and EFs within the control sample. Also, differences in childhood affluence, advantage, and opportunity may exist between the 2 groups and this limits the generalizability of our results. Nevertheless, all analyses were controlled for parental education and group differences remained significant.
Conclusions
This case-control study is the first, to our knowledge, to provide evidence that early adolescents with cCHD, who underwent infant open heart surgery, showed higher stress markers and lower EF performance than controls. These findings align with previous studies in infants and young children with cCHD showing altered stress markers in this population and underline the chronic burden of cCHD. The group interaction we found shows an association between higher stress markers and lower EF performance in the cCHD group, indicating that chronically high stress markers may play a role in the development of EF impairments in early adolescents with cCHD. Longitudinal studies are needed to better understand the neurobiological mechanisms and timing of alterations in the stress system and its role in neurodevelopmental outcomes in patients with cCHD.
eFigure. Recruitment Flow Chart
eText 1. EF Summary Score
eTable 1. Statistical Estimates for Each EF Test
eTable 2. Weights for EF Domains
eTable 3. Descriptive Statistical Estimates for EF Domains and Global Score in Healthy Controls
eText 2. Comparison of Participants vs Non-Participants
eTable 4. Comparison of Patient Data to Healthy Controls and Norms for Each EF Test
eTable 5. Group Difference Between Patients With Uni- or Biventricular CHD
eTable 6. Association Between Stress Markers and Clinical Variables
eTable 7. Associations Between Stress Markers and EFs
eTable 8. Association Between Resilience, Stress Markers and EFs
Data Sharing Statement
References
- 1.Field T. Preterm newborn pain research review. Infant Behav Dev. 2017;49:141-150. doi: 10.1016/j.infbeh.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Juruena MF, Bourne M, Young AH, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction by early life stress. Neurosci Lett. 2021;759:136037. doi: 10.1016/j.neulet.2021.136037 [DOI] [PubMed] [Google Scholar]
- 3.Kalmakis KA, Meyer JS, Chiodo L, Leung K. Adverse childhood experiences and chronic hypothalamic-pituitary-adrenal activity. Stress. 2015;18(4):446-450. doi: 10.3109/10253890.2015.1023791 [DOI] [PubMed] [Google Scholar]
- 4.van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87. doi: 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plumpton KR, Anderson BJ, Beca J. Thyroid hormone and cortisol concentrations after congenital heart surgery in infants younger than 3 months of age. Intensive Care Med. 2010;36(2):321-328. doi: 10.1007/s00134-009-1648-4 [DOI] [PubMed] [Google Scholar]
- 6.Taşar S, Dikmen N, Bulut İ, et al. Potential role of salivary cortisol levels to reflect stress response in children undergoing congenital heart surgery. Cardiol Young. Published online April 1, 2022. doi: 10.1017/S1047951122001081 [DOI] [PubMed] [Google Scholar]
- 7.McGauran M, Jordan B, Beijers R, et al. Long-term alteration of the hypothalamic-pituitary-adrenal axis in children undergoing cardiac surgery in the first 6 months of life. Stress. 2017;20(5):505-512. doi: 10.1080/10253890.2017.1349748 [DOI] [PubMed] [Google Scholar]
- 8.Bates R, Salsberry P, Ford J. Measuring stress in young children using hair cortisol: the state of the science. Biol Res Nurs. 2017;19(5):499-510. doi: 10.1177/1099800417711583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261-274. doi: 10.1016/j.psyneuen.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 10.Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, Stein DJ. Determinants of hair cortisol concentration in children: a systematic review. Psychoneuroendocrinology. 2018;87:204-214. doi: 10.1016/j.psyneuen.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 11.Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol. 2016;43(1):173-185. doi: 10.1016/j.clp.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 12.Feldmann M, Bataillard C, Ehrler M, et al. Cognitive and executive function in congenital heart disease: a meta-analysis. Pediatrics. 2021;148(4):e2021050875. doi: 10.1542/peds.2021-050875 [DOI] [PubMed] [Google Scholar]
- 13.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135-168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma SY, Liu YT, Cun YS, et al. Preoperative serum cortisone levels are associated with cognition in preschool-aged children with tetralogy of fallot after corrective surgery: new evidence from human populations and mice. World J Pediatr. Published online September 22, 2023. doi: 10.1007/s12519-023-00754-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner SL, Cepeda I, Krieger D, et al. [Formula: see text]Higher cortisol is associated with poorer executive functioning in preschool children: the role of parenting stress, parent coping and quality of daycare. Child Neuropsychol. 2016;22(7):853-869. doi: 10.1080/09297049.2015.1080232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePasquale CE, Tyrell FA, Kalstabakken AW, et al. Lifetime stressors, hair cortisol, and executive function: Age-related associations in childhood. Dev Psychobiol. 2021;63(5):1043-1052. doi: 10.1002/dev.22076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol B Psychol Sci Soc Sci. 2011;66 Suppl 1(Suppl 1):i71-i81. doi: 10.1093/geronb/gbq094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153(9):4111-4119. doi: 10.1210/en.2012-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merz EC, Desai PM, Maskus EA, et al. Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biol Psychiatry. 2019;86(12):921-929. doi: 10.1016/j.biopsych.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410-422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martel MM, Nigg JT, Wong MM, et al. Childhood and adolescent resiliency, regulation, and executive functioning in relation to adolescent problems and competence in a high-risk sample. Dev Psychopathol. 2007;19(2):541-563. doi: 10.1017/S0954579407070265 [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Zhang X, Wang J, et al. The associations of executive functions with resilience in early adulthood: a prospective longitudinal study. J Affect Disord. 2021;282:1048-1054. doi: 10.1016/j.jad.2021.01.031 [DOI] [PubMed] [Google Scholar]
- 23.American Psychological Association . Resilience. Accessed January 11, 2024. https://www.apa.org/topics/resilience
- 24.Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. 2011;9:8. doi: 10.1186/1477-7525-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köble K, Willinger L, Brudy L, Oberhoffer-Fritz R, Ewert P, Müller J. Resilience in children with congenital heart disease: a comparative study with health counterparts. Arch Dis Child. 2023;108(11):935-939. doi: 10.1136/archdischild-2023-325605 [DOI] [PubMed] [Google Scholar]
- 26.Ehrler M, Naef N, Tuura RO, Latal B. Executive function and brain development in adolescents with severe congenital heart disease (Teen Heart Study): protocol of a prospective cohort study. BMJ Open. 2019;9(10):e032363. doi: 10.1136/bmjopen-2019-032363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223-228. doi: 10.1016/S2352-4642(18)30022-1 [DOI] [PubMed] [Google Scholar]
- 28.Voegel CD, Baumgartner MR, Kraemer T, Wüst S, Binz TM. Simultaneous quantification of steroid hormones and endocannabinoids (ECs) in human hair using an automated supported liquid extraction (SLE) and LC-MS/MS - insights into EC baseline values and correlation to steroid concentrations. Talanta. 2021;222:121499. doi: 10.1016/j.talanta.2020.121499 [DOI] [PubMed] [Google Scholar]
- 29.Voegel CD, Kroll SL, Schmid MW, et al. Alterations of stress-related glucocorticoids and endocannabinoids in hair of chronic cocaine users. Int J Neuropsychopharmacol. 2022;25(3):226-237. doi: 10.1093/ijnp/pyab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petermann F, Petermann U. Kindheit und Entwicklung. Published online June 18, 2008. doi: 10.1026/0942-5403.17.2.71 [DOI]
- 31.Corsi P. Human memory and the medial temporal region of the brain. 1972. Accessed January 11, 2024. https://escholarship.mcgill.ca/concern/theses/05741s554
- 32.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. 2012. Accessed January 11, 2024. doi: 10.1037/t15082-000 [DOI]
- 33.Zimmermann PFB. Testbatterie zur Aufmerksamkeitsprüfung (TAP 2.3). Psytest; 2012. [Google Scholar]
- 34.Aschenbrenner S, Tucha O, Lange K. Regensburger Wortflüssigkeitstest. Hogrefe Göttingen; 2000. [Google Scholar]
- 35.Leppert K, Koch B, Brähler E, Strauß B. Die Resilienzskala (RS)–Überprüfung der Langform RS-25 und einer Kurzform RS-13. Klin Diagn Eval. 2008;1:226-243. [Google Scholar]
- 36.Zolkoski B, Bullock LM. Resilience in children and youth: a review. Child Youth Serv Rev. 2012;23(12):2295-2303. doi: 10.1016/j.childyouth.2012.08.009 [DOI] [Google Scholar]
- 37.Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G. Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol. 1989;31(4):440-456. doi: 10.1111/j.1469-8749.1989.tb04022.x [DOI] [PubMed] [Google Scholar]
- 38.Waldmann HC. Kurzformen des HAWIK-IV: statistische Bewertung in verschiedenen Anwendungsszenarien. Diagnostica. 2008;54(4):202-210. doi: 10.1026/0012-1924.54.4.202 [DOI] [Google Scholar]
- 39.Ehrler M, Latal B, Polentarutti S, von Rhein M, Held L, Wehrle FM. Pitfalls of using IQ short forms in neurodevelopmental disorders: a study in patients with congenital heart disease. Pediatr Res. 2020;87(5):917-923. doi: 10.1038/s41390-019-0667-2 [DOI] [PubMed] [Google Scholar]
- 40.Landolt MA, Vollrath M. Life event scale. University Children’s Hospital Zurich; 1998. [Google Scholar]
- 41.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 42.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2022. [Google Scholar]
- 43.Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614-626. doi: 10.3109/10253890.2011.577848 [DOI] [PubMed] [Google Scholar]
- 44.Lopez M, Ruiz MO, Rovnaghi CR, et al. The social ecology of childhood and early life adversity. Pediatr Res. 2021;89(2):353-367. doi: 10.1038/s41390-020-01264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abda A, Bolduc ME, Tsimicalis A, Rennick J, Vatcher D, Brossard-Racine M. Psychosocial outcomes of children and adolescents with severe congenital heart defect: a systematic review and meta-analysis. J Pediatr Psychol. 2019;44(4):463-477. doi: 10.1093/jpepsy/jsy085 [DOI] [PubMed] [Google Scholar]
- 46.Jackson JL, Gerardo GM, Daniels CJ, Vannatta K. Perceptions of disease-related stress: a key to better understanding patient-reported outcomes among survivors of congenital heart disease. J Cardiovasc Nurs. 2017;32(6):587-593. doi: 10.1097/JCN.0000000000000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz JH, Wang J, Berl MM, Armour AC, Cheng YI, Donofrio MT. Executive function and psychosocial quality of life in school age children with congenital heart disease. J Pediatr. 2018;202:63-69. doi: 10.1016/j.jpeds.2018.07.018 [DOI] [PubMed] [Google Scholar]
- 48.Goodman JB, Freeman EE, Chalmers KA. The relationship between early life stress and working memory in adulthood: a systematic review and meta-analysis. Memory. 2019;27(6):868-880. doi: 10.1080/09658211.2018.1561897 [DOI] [PubMed] [Google Scholar]
- 49.Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci Biobehav Rev. 2016;68:651-668. doi: 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Heuvel LL, Suliman S, Bröcker E, et al. The association between hair cortisol levels, inflammation and cognitive functioning in females. Psychoneuroendocrinology. 2022;136:105619. doi: 10.1016/j.psyneuen.2021.105619 [DOI] [PubMed] [Google Scholar]
- 51.Feeney JC, O’Halloran AM, Kenny RA. The Association Between Hair Cortisol, Hair Cortisone, and Cognitive Function in a Population-Based Cohort of Older Adults: Results From The Irish Longitudinal Study on Ageing. J Gerontol A Biol Sci Med Sci. 2020;75(2):257-265. doi: 10.1093/gerona/gly258 [DOI] [PubMed] [Google Scholar]
- 52.McLennan SN, Ihle A, Steudte-Schmiedgen S, Kirschbaum C, Kliegel M. Hair cortisol and cognitive performance in working age adults. Psychoneuroendocrinology. 2016;67:100-103. doi: 10.1016/j.psyneuen.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 53.Pluck G, Córdova MA, Bock C, Chalen I, Trueba AF. Socio-economic status, executive functions, and theory of mind ability in adolescents: relationships with language ability and cortisol. Br J Dev Psychol. 2021;39(1):19-38. doi: 10.1111/bjdp.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulopulos MM, Hidalgo V, Almela M, Puig-Perez S, Villada C, Salvador A. Hair cortisol and cognitive performance in healthy older people. Psychoneuroendocrinology. 2014;44:100-111. doi: 10.1016/j.psyneuen.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 55.Nist MD, Harrison TM, Steward DK. The biological embedding of neonatal stress exposure: a conceptual model describing the mechanisms of stress-induced neurodevelopmental impairment in preterm infants. Res Nurs Health. 2019;42(1):61-71. doi: 10.1002/nur.21923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3-23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latal B, Patel P, Liamlahi R, Knirsch W, O’Gorman Tuura R, von Rhein M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr Res. 2016;80(4):531-537. doi: 10.1038/pr.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrler M, Schlosser L, Brugger P, et al. Altered white matter microstructure is related to cognition in adults with congenital heart disease. Brain Commun. 2020;3(1):fcaa224. doi: 10.1093/braincomms/fcaa224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goddings AL, Roalf D, Lebel C, Tamnes CK. Development of white matter microstructure and executive functions during childhood and adolescence: a review of diffusion MRI studies. Dev Cogn Neurosci. 2021;51:101008. doi: 10.1016/j.dcn.2021.101008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glenn T, Cousino MK, Wernovsky G, Schuchardt EL. Resilient hearts: measuring resiliency in young people with congenital heart disease. J Am Heart Assoc. 2023;12(21):e029847. doi: 10.1161/JAHA.123.029847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-León MÁ, Pérez-Mármol JM, Gonzalez-Pérez R, García-Ríos MDC, Peralta-Ramírez MI. Relationship between resilience and stress: perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol Behav. 2019;202:87-93. doi: 10.1016/j.physbeh.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 62.Jackson JL, Gerardo GM, Monti JD, Schofield KA, Vannatta K. Executive function and internalizing symptoms in adolescents and young adults with congenital heart disease: the role of coping. J Pediatr Psychol. 2018;43(8):906-915. doi: 10.1093/jpepsy/jsx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King L, Jolicoeur-Martineau A, Laplante DP, Szekely E, Levitan R, Wazana A. Measuring resilience in children: a review of recent literature and recommendations for future research. Curr Opin Psychiatry. 2021;34(1):10-21. doi: 10.1097/YCO.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 64.Davydov DM, Stewart R, Ritchie K, Chaudieu I. Resilience and mental health. Clin Psychol Rev. 2010;30(5):479-495. doi: 10.1016/j.cpr.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 65.Whitelaw A, Sleath K. Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet. 1985;1(8439):1206-1208. doi: 10.1016/S0140-6736(85)92877-6 [DOI] [PubMed] [Google Scholar]
- 66.Harrison TM. Improving neurodevelopment in infants with complex congenital heart disease. Birth Defects Res. 2019;111(15):1128-1140. doi: 10.1002/bdr2.1517 [DOI] [PubMed] [Google Scholar]
- 67.Cong X, Wu J, Vittner D, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev. 2017;108:9-16. doi: 10.1016/j.earlhumdev.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue W, Han X, Luo J, Zeng Z, Yang M. Effect of music therapy on preterm infants in neonatal intensive care unit: systematic review and meta-analysis of randomized controlled trials. J Adv Nurs. 2021;77(2):635-652. doi: 10.1111/jan.14630 [DOI] [PubMed] [Google Scholar]
- 69.Sanders MR, Hall SL. Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J Perinatol. 2018;38(1):3-10. doi: 10.1038/jp.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber A, Harrison TM. Reducing toxic stress in the neonatal intensive care unit to improve infant outcomes. Nurs Outlook. 2019;67(2):169-189. doi: 10.1016/j.outlook.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCusker CG, Doherty NN, Molloy B, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. 2010;36(1):110-117. doi: 10.1111/j.1365-2214.2009.01026.x [DOI] [PubMed] [Google Scholar]
- 72.Dettenborn L, Tietze A, Kirschbaum C, Stalder T. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. 2012;15(6):578-588. doi: 10.3109/10253890.2012.654479 [DOI] [PubMed] [Google Scholar]
- 73.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 74.Ungar M, Ghazinour M, Richter J. Annual research review: what is resilience within the social ecology of human development? J Child Psychol Psychiatry. 2013;54(4):348-366. doi: 10.1111/jcpp.12025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Recruitment Flow Chart
eText 1. EF Summary Score
eTable 1. Statistical Estimates for Each EF Test
eTable 2. Weights for EF Domains
eTable 3. Descriptive Statistical Estimates for EF Domains and Global Score in Healthy Controls
eText 2. Comparison of Participants vs Non-Participants
eTable 4. Comparison of Patient Data to Healthy Controls and Norms for Each EF Test
eTable 5. Group Difference Between Patients With Uni- or Biventricular CHD
eTable 6. Association Between Stress Markers and Clinical Variables
eTable 7. Associations Between Stress Markers and EFs
eTable 8. Association Between Resilience, Stress Markers and EFs
Data Sharing Statement