Using a common cardiac thermal performance assay, we identified temperature ranges that define the thermal optima and upper thermal limits of the larval life stage of the imperiled longfin smelt larvae. We demonstrated the utility of a cardiac assay for defining appropriate thermal conditions for a species of conservation concern.
Keywords: Arrhenius breakpoint, cardiac performance, climate change, heart rate, larvae, San Francisco Estuary, thermal performance
Abstract
Upper thermal limits in many fish species are limited, in part, by the heart’s ability to meet increased oxygen demand during high temperatures. Cardiac plasticity induced by developmental temperatures can therefore influence thermal tolerance. Here, we determined how incubation temperatures during the embryonic stage influence cardiac performance across temperatures during the sensitive larval stage of the imperiled longfin smelt. We transposed a cardiac assay for larger fish to newly hatched larvae that were incubated at 9°C, 12°C or 15°C. We measured heart rate over increases in temperature to identify the Arrhenius breakpoint temperature (TAB), a proxy for thermal optimum and two upper thermal limit metrics: temperature when heart rate is maximized (Tpeak) and when cardiac arrhythmia occurs (TArr). Higher incubation temperatures increased TAB, Tpeak and TArr, but high individual variation in all three metrics resulted in great overlap of individuals at TAB, Tpeak and TArr across temperatures. We found that the temperatures at which 10% of individuals reached Tpeak or TArr and temperatures at which number of individuals at TAB relative to Tpeak (ΔN(TAB,Tpeak)) was maximal, correlated more closely with upper thermal limits and thermal optima inferred from previous studies, compared to the mean values of the three cardiac metrics of the present study. Higher incubation temperatures increased the 10% Tpeak and TArr thresholds but maximum ΔN(TAB,Tpeak) largely remained the same, suggesting that incubation temperatures modulate upper thermal limits but not Topt for a group of larvae. Overall, by measuring cardiac performance across temperatures, we defined upper thermal limits (10% thresholds; Tpeak, 14.4–17.5°C; TArr, 16.9–20.2°C) and optima (ΔN(TAB,Tpeak), 12.4–14.4°C) that can guide conservation strategies for longfin smelt and demonstrated the potential of this cardiac assay for informing conservation plans for the early life stages of fish.
Introduction
Water temperature influences most biochemical and physiological processes of ectotherms, such as fishes, and is among the most important drivers of fish population distribution and survival (Brett, 1971; Reynolds and Casterlin, 1979). The individual physiological performances across temperatures and thermal tolerance limits of fishes have been linked to broader ecological processes, such as species distributions (Eliason et al., 2011; Guo et al., 2021) and population productivity (Moyano et al., 2020), and have been applied to aquaculture programs to optimize culture methods (Brauner and Richards, 2020). As water temperatures continue to warm due to climate change, evaluating thermal tolerances and the influence of water temperature on fish physiological performance has become increasingly essential for the development of conservation strategies for imperiled fish species (Cooke et al., 2021).
Temperature begins shaping the physiology of fishes as early as embryogenesis. A substantial body of research has demonstrated that, generally, higher incubation temperatures shift the thermal optimum for physiological performance and upper thermal tolerance limits higher at the juvenile and adult life stages (reviewed in Jonsson and Jonsson, 2014, 2019). For example, higher embryonic incubation temperatures improve swimming performance of adult zebrafish (Danio rerio) in warmer water through alterations in muscle fiber composition and the muscle transcriptome (Scott and Johnston, 2012). Similarly, higher incubation temperatures increased upper cardiac thermal tolerance of juvenile Atlantic salmon (Salmo salar) through alterations in myocardial morphology and the ventricular proteome (Muir et al., 2022a, 2022b). There is comparatively less known about how incubation temperature affects the thermal physiology of the life stage immediately post-hatching, larvae. Some evidence suggests a similar pattern to juveniles and adults whereby higher incubation temperatures alter thermal tolerance by shifting their upper thermal limits higher (Dash et al., 2021).
Fish larvae are considered to be among the most thermally sensitive life stages for fish (Killen et al., 2007; Pörtner and Farrell, 2008; Pörtner and Peck, 2010). This life stage is characterized by extremely high mortality both in the wild and in captivity, which impacts the recruitment of a species in the wild (Hjort, 1914; Victor, 1986; Houde, 1994) and can impede the development of aquaculture programs (Holt et al., 2007; DiMaggio et al., 2013, 2017; Symonds et al., 2014; Degidio et al., 2017; Goetz et al., 2021; Groover et al., 2021). Therefore, targeted efforts to improve the survival of the larval stage could have a higher impact on a fish species' productivity. Temperature drives many of the factors that influence larval survival. For example, temperature affects growth rates, swimming ability, yolk utilization efficiencies, digestion and the energetic demands of development (Gunnarsson et al., 2011; Mueller et al., 2015; Yanagitsuru et al., 2021; Lo et al., 2022; McInturf et al., 2022; Zillig et al., 2022). Indeed, temperature correlates strongly with the recruitment of some fish species (Ghil and Vautard, 1991; Galloway et al., 1998; Sponaugle et al., 2006) and explains a substantial amount of the observed variation in larval mortality rates for wild populations (Pepin, 1991). Evaluating the physiological performance of larval fish across temperatures, and how thermal history during the embryonic stage influences this, is thus a critical step towards developing plans to protect wild populations and improve methods for conservation aquaculture. For example, optimizing temperature for larviculture is essential for effective aquaculture programs (Shields, 2001), and the relationship between fish thermal tolerance and natural water temperature is a critical consideration for successful population supplementation efforts (Karppinen et al., 2014; Yanagitsuru et al., 2022).
Differential physiological performance across temperatures has been postulated to be driven by changes in the capacity to supply oxygen to meet the organism’s oxygen demand (Pörtner, 2001; Pörtner et al., 2017). This capacity can be measured as aerobic scope, the difference between maximum and standard metabolic rates, and describes the scope for aerobically dependent activities such as feeding, growth and reproduction (Farrell et al., 2008; Auer et al., 2015a, 2015b). The temperature-dependent nature of aerobic scope can be described by the Fry aerobic scope curve (Fry and Hart, 1948), whereby aerobic scope is greatest at the organism's optimal temperature (Topt) and decreases at temperatures above or below Topt, until a critical temperature is reached where aerobic scope is zero (Tcrit), and only short-term survival is possible (Farrell, 2009). While aerobic scope is commonly measured for the juvenile and adult life stages of many fish species (Farrell, 2016), it may not be amenable to early life stages; comparatively fewer studies have measured aerobic scope in larvae due to their highly active states that prevent accurate measurements of standard metabolic rates and their poor response to the forced swimming protocols commonly used to measure maximum metabolic rates (Peck and Moyano, 2016).
While oxygen consumption from the external environment is used to calculate aerobic scope, oxygen delivery to tissues depends on cardiac output. As a result, aspects of cardiac output such as heart rate (fH) correlate well with aerobic scope across temperatures (Steinhausen et al., 2008; Eliason et al., 2011). Moreover, because fH is a relatively straightforward aspect of cardiac output to measure compared to stroke volume, and is the primary determinant of delivering oxygen to tissue during acute temperature increases (Farrell, 1991, 2009; Gamperl et al., 2011), measuring fH in association with acute temperature increases provides a simple and rapid (a few days compared to a few weeks to measure aerobic scope) method for evaluating thermal performance in individual fishes (Casselman et al., 2012), which is particularly important during the early life stages where ontogenetic changes in physiology can occur rapidly (Downie et al., 2023). This cardiac assay relies on a well-characterized cardiac response to temperature to identify multiple indices of thermal performance. As temperature rises, maximum heart rate (fHmax) increases exponentially until an Arrhenius breakpoint temperature (TAB) is reached, and as temperature increases beyond TAB, fHmax continues to rise until a temperature is reached that elicits maximum fHmax (Tpeak). Thereafter, further increases in temperature lead to cardiac arrhythmia, indicating the onset of cardiac failure, the temperature of which is termed TArr. These three indices, TAB, Tpeak and TArr provide the basis through which thermal performance is evaluated with this cardiac assay. TAB has been correlated with Topt for fishes when acclimation temperatures were relatively cool (Casselman et al., 2012; Anttila et al., 2013; Ferreira et al., 2014). While the exact mechanism behind this correlation has not been empirically determined, TAB represents the temperature where fHmax, and therefore cardiac output, is maximized while heart rate increases proportionally with the increasing metabolic demands that acute temperature increases pose; this is to say, oxygen delivery to tissues is maximized at TAB. Tpeak and TArr, on the other hand, indicate the temperatures where cardiac output can no longer increase to match the higher metabolic demands incurred by higher temperature and the onset of cardiac failure, respectively. Given that Tcrit is reliant on the failure of metabolic and cardiac performance, it is likely that Tpeak and TArr provide the basis for Tcrit. Indeed, both metrics have been measured in other species to be just below Tcrit and have therefore been used as measures of upper thermal tolerance (Casselman et al., 2012; Anttila et al., 2013). While more work is necessary to evaluate whether this cardiac assay method can be applied across species and acclimation temperatures, the rapid pace at which the assay can be conducted and its ability to measure multiple metrics of thermal performance within individual fish has garnered its attention, and it has become an increasingly common way to assess thermal performance in fishes (Casselman et al., 2012; Chen et al., 2013, 2015; Verhille et al., 2013; Sidhu et al., 2014; Drost et al., 2016; Anttila et al., 2017; Moyano et al., 2020; Muir et al., 2022a). Furthermore, this cardiac assay has a clear endpoint (i.e. cardiac arrhythmia) compared to the classical metric of Tcrit, critical thermal maximum, for which the loss of equilibrium endpoint can be difficult to ascertain in slow-moving larvae that often have undeveloped swim bladders and sometimes lay at the bottom of containers (Moyano et al., 2017). In summary, by measuring fHmax over acute temperature increases, TAB can be used as a proxy for Topt, and Tcrit can be approximated, albeit with an underestimation, with Tpeak and TArr.
Longfin smelt (Spirinchus thaleichthys) is an imperiled forage fish species that is distributed along the Pacific coast of North America from California to Alaska. While some landlocked populations exist in Washington, United States and British Columbia, Canada, most populations are anadromous with adults migrating from coastal marine habitats between October and April to streams and estuaries to spawn (Moyle, 2002; Garwood, 2017; Lewis et al., 2020). The timing of outmigration for longfin smelt is unclear but is thought to occur in the summer months (beginning June) as water temperatures rise (Rosenfield and Baxter, 2007). The San Francisco Estuary (SFE) population of longfin smelt is at the southernmost range of the species and is likely a genetic source population for several Northern coastal populations of longfin smelt (Garwood, 2017; Saglam et al., 2021). They were once one of the most abundant fish species in the SFE but are now reduced to approximately 1% of their historic (pre-1980s) abundance, have been officially proposed for listing as a federally endangered species, and are at risk of extirpation (Sommer et al., 2007; Hobbs et al., 2017; USFWS, 2023). Despite more than 50 years of population monitoring surveys, there is limited knowledge about the biology and physiology of the species and, in turn, a lack of understanding regarding how the species responds to variations in environmental conditions such as temperature (Hobbs et al., 2017). As temperatures continue to rise in the SFE due to climate change, and the particularly large spatial extent of temperature increases across the SFE during the longfin smelt spawning season, it is imperative to evaluate the upper thermal tolerance of the early life stages of longfin smelt and their capacity to acclimate to higher temperatures (Bashevkin et al., 2022).
To begin evaluating the upper thermal tolerance of longfin smelt larvae and how developmental temperatures could influence it, we took advantage of a well-documented cardiac response to temperature to measure TAB as a proxy for Topt, and Tpeak and TArr as measures of upper thermal limits. Nearly all previous studies using the cardiac assay described above have been conducted on post-larval life stages of fishes, so we therefore transposed the methods developed for larger fish to newly hatched larvae. We expected TAB, Tpeak and TArr to increase with incubation temperature. We also predicted that longfin smelt yolk-sac larvae would have a TAB between 8°C and 12°C as larval longfin smelt are most abundant at these temperatures in the SFE (Grimaldo et al., 2017) and because a recent laboratory study has shown improved growth performance at 9°C and 12°C (Yanagitsuru et al., 2021). Finally, we predicted that Tpeak and TArr would be around 15°C to 16°C as larval longfin smelt abundance declines in the SFE after 16°C and because larvae exhibited reduced growth performance at 15°C (Grimaldo et al., 2017; Yanagitsuru et al., 2021).
Materials and Methods
Broodstock and embryo collection
Longfin smelt broodstock were collected between November and December 2019 by the US Fish and Wildlife Services Chipps Island trawl with a midwater trawl, the UC Davis Fish Conservation and Culture Laboratory (FCCL) using a lampara net in the Sacramento San Joaquin Delta, and the UC Davis Otolith Geochemistry and Fish Ecology Laboratory using an otter trawl in the Alviso Marsh in South San Francisco Bay. Broodstock collections were approved under California Department of Fish and Wildlife MOU ID: Hobbs_LFS_2021 and Specific Use Permit IDs: S-191990002-19, 199-001, and D-0021521915-6. Collected fish were transported to the FCCL where they were held at 12°C in 10 parts per-thousand (ppt) water and fed live adult artemia. As soon as a female became gravid, a single female and male pair were strip-spawned into a plastic bowl where eggs and milt were mixed in freshwater from the Delta (0.2 ppt) at 12°C. A subset of 300 fertilized embryos from each clutch to be used for experiments were then transported to the UC Davis Fish Conservation Physiology Laboratory in conical tubes within coolers, lightly chilled with ice packs (approximately 1.5 hours transport time). Water temperatures within conical tubes were between 10.9°C and 12.1°C upon arrival. Subsets of 300 embryos from five single pair crosses, for a total of 1500 embryos, were incubated for experiments between December 2019 and January 2020.
Incubation temperatures
Embryos from each cross were distributed amongst three experimental incubation temperatures that spanned the range of temperatures that the early life stages of longfin smelt may experience in the SFE: 9°C, 12°C or 15°C. Embryos from each clutch were divided into three groups of 100 (one for each incubation temperature) within 270 ml plastic bowls that were filled with freshwater sourced from a well (0.4 ppt, pH 8.4–8.6) and floated in water baths at 12°C. Temperatures in each water bath were adjusted at a rate of approximately 0.5°C∙h−1 until experimental temperatures were reached. Water temperatures within bowls (9°C: 8.8 ± 0.3°C; 12°C: 12.0 ± 0.3°C; 15°C: 14.8 ± 0.4°C; mean ± SD) were monitored daily with an Omega HH82A digital thermocouple thermometer (Omega Engineering Inc., Norwalk, CT, USA), and water quality in each bowl was maintained with ~ 75% water changes daily. To prevent the spread of diseases, embryos afflicted by fungus, and dead embryos indicated by a milky yellow coloration similar to that observed in dead delta smelt embryos (Tsai et al., 2021), were removed daily. We did not record hatching rates but expect that there was slightly lower hatching in the 15°C incubation group (Yanagitsuru et al., 2021). Once hatched, individual larvae were gently pipetted into plastic bowls floating in water baths at their incubation temperature. Because more individuals hatched than could fit under the experimental setup (see Section 2.3), a subset of hatched individuals were randomly selected to be experimented upon the following day (one day post hatch).
Heart rate measurements
We developed an experimental setup that allowed us to transpose the cardiac assay initially developed by Casselman et al. (2012) for larger fish, to larvae. The cardiac assay experimental setup consisted of a petri dish fitted with a glass plate for placing larval fishes. Petri dishes were additionally perforated on the bottom and fitted with a 335-μm nylon mesh within a water bath. The perforations and nylon mesh allowed gentle water exchange from the water bath to hasten temperature equalization within the dish and water bath without disturbing the larvae. Water baths were temperature-controlled via recirculation with a Delta Star air-cooled heat pump (Aqualogic Inc., San Diego, CA, USA). The experimental setup was placed under a Leica S8APO stereomicroscope (Leica Microsystems, Chicago, IL, USA) mounted with a Canon EOS Rebel T6 SLR camera (Canon, Tokyo, Japan) to record videos of transparent larvae, which had hearts visible (Supplementary Fig. 1; Supplementary Video 1).
Prior to the start of an experiment, water bath temperature was set to 9°C. To ensure that larvae would not move during recordings, MS-222 was mixed into bath water to a concentration of 0.08 g∙L−1, which preliminary trials found to be the lowest concentration necessary to prevent spontaneous body movements for the duration of experiments (~5 hours). This MS-222 concentration is well below that which has been tested and found to have no effect on heart rate in the larvae of several other fish species (Moyano et al., 2020). Decrease in well water pH after addition of MS-222 at this dose was minimal (≤0.01 pH unit) likely due to the hardness of well water so pH adjustment with addition of NaHCO3 was not necessary (Allen and Harman, 1970). Once larvae were anaesthetized, up to 30 larvae were positioned within the petri dish under the microscope and left to acclimate for 1 hour. Water bath temperatures were then increased in a stepwise fashion from 9°C to 25°C at 2-°C increments between 9°C and 19°C (9, 11, 13, 15, 17, 19°C) and 1°C increments between 19°C and 25°C (19°C, 20°C, 21°C, 22°C, 23°C, 24°C, 25°C) at a rate of approximately 0.6°C∙min−1 between temperature steps. At each temperature step, larvae were exposed to the new temperature for 5 min and then filmed at 30 frames s−1 for 2.5 minutes under a bright light before increasing the temperature again (i.e. larvae were exposed to the new temperature step for a total of 7.5 min). Following the final recording at 25°C, larvae were euthanized with MS-222 overdose (0.5 g∙L−1 buffered to pH 8.0). A total of 121, 152 and 134 individuals were video recorded at each of the twelve temperature steps for the 9°C, 12°C and 15°C groups, respectively, for a total of 407 larvae. Therefore, a total of 4884 (i.e. 407 fish * 12 temperatures) videos of larval hearts were recorded. Heart rates were not measured in videos where hearts had reached arrhythmia.
DanioScope (Noldus, Wageningen, Netherlands), an activity analysis software that measures the frequency of activity spikes (i.e. inter-beat intervals) to calculate fH within selected areas, was used to measure fH for most individuals (Supplementary Fig. 2). The first 30 s of video were excluded from analyses due to camera instability and fH was measured in the 2 min between 0:30 and 2:30 at each temperature. Poor focus in some videos on the hearts of some larvae prevented DanioScope from recognizing activity spikes. For the larvae in these videos, fH was measured with human visual observation (method described below). Of the 4884 larval heart videos recorded (one video per temperature per larva), 238 hearts (4.9% of heart rates) were measured with human visual observation. Because this software has not previously been used with longfin smelt, we first validated this software for the species by comparing the fH measured with Danioscope and with human visual observation with 10 larvae. To measure fH visually, the video segment from 0:30 to 2:30 for the 10 larvae was split into seven 15-s clips using Bandicut video editing software (Bandicam Company, Irvine, CA, USA). A single observer counted the number of heart beats in each clip and divided it by 0.25 min to calculate beats per minute. fH for an individual was determined as the average of the fH calculated in the seven clips. Automatic recordings of fH with DanioScope strongly correlated with those obtained visually (t8 = 98.0, P < 0.001, R2 = 0.999), validating the accuracy of this software for longfin smelt larvae (Supplementary Fig. 3).
Using the fH measured for each individual across stepwise increases in temperature, we measured TAB, Tpeak and TArr. TAB was determined in R 3.6.3 (R Core Team, 2013) by plotting the log10 of fH over the inverse of temperature (1000 K−1) (Fig. 1) and using the ‘segmented’ package (Muggeo, 2017), which uses piece-wise linear regression to determine breakpoints where linear relationships change. Tpeak was determined by identifying the temperature at which the highest fH was measured for an individual. TArr was determined as the temperature at which cardiac arrhythmia was first observed, which could be identified from Danioscope activity plots (Supplementary Fig. 2B) when activity spikes were discontinuous (Supplementary Fig. 2C; Supplementary Video 2). When activity spikes were discontinuous, videos were verified visually for arrhythmia. We also measured the initial fH (fH0), fH at Tpeak (peak heart rate, fHpeak), and the rate of increase in fH with temperature prior to TAB (dfH/dT) by calculating the slope of the linear relationship between fH and temperature prior to TAB, for each individual. Finally, we calculated the difference between fHpeak and fH0 (∆fHpeak-H0) as an indicator of cardiac scope.
Figure 1.
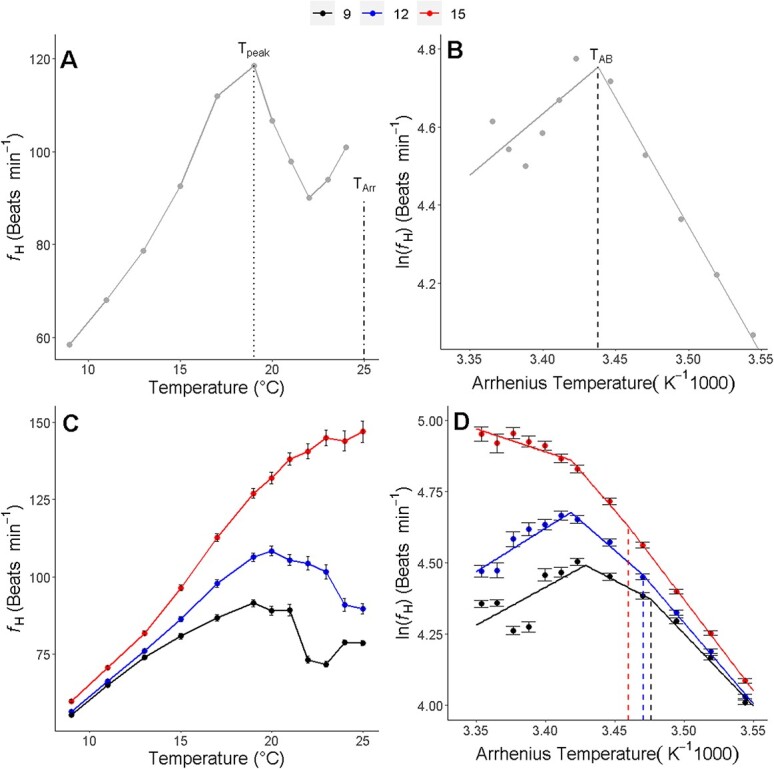
The effect of increasing temperature on heart rate (fH) of longfin smelt larvae. Representative plot of heart rate with temperature (A) and Arrhenius plot of fH with increasing temperatures (B) for an individual larva. Average heart rate with temperature for all individuals (C) and Arrhenius plots of fH with increasing temperatures (D) for different incubation temperature groups. Regression lines for representative Arrhenius plots are divided into two segments, representing the method by which Arrhenius breakpoint temperatures (TAB) were determined for each individual. Unlike individual Arrhenius plots, grouped Arrhenius plots were best fit as 3 segments due to variation in post-TAB changes in fH across individuals. Dotted line indicates temperature where heart rate peaks (Tpeak), dash-dotted line indicates temperature where cardiac arrhythmia was first observed (TArr), and dashed line indicates the TAB. Data presented as mean ± SEM.
Almost all previous studies on fish using this cardiac assay have been conducted on later life stages where it is recommended to pharmacologically induce fHmax with intraperitoneal injections of atropine sulphate and isoproterenol (e.g. Casselman et al., 2012). For very small larval fish (<7 mm length, < 50 μg mass; Yanagitsuru et al., 2021), it is exceedingly difficult to perform such injections and cardioactive drugs were thus not used in this study (Drost et al., 2016). However, pharmacological agents have relatively minor effects on the cardiac metrics measured in fish (Casselman et al., 2012) and atropine sulphate dissolved in water had either no effect or decreased fH depending on concentration in larval Arctic cod (Boreogadus saida) (Drost et al., 2016). Furthermore, bright lights, as used during our experiments, are known to induce hyperactivity in the larval stage of a similar fish species; delta smelt (Mundy et al., 2020). We thus report fH with the expectation that fHmax had been reached.
Statistical analyses
All statistical analyses were performed using R 3.6.3 (R Core Team, 2013). The validation of DanioScope measurements was analysed using a linear regression with fH measured with DanioScope as the response variable and fH measured visually as the fixed effect. fH0, fHpeak, ∆fHpeak-H0, dfH/dT, TAB, Tpeak and TArr were analysed separately as response variables using linear mixed effects models with incubation temperature as a fixed effect and clutch ID as a random effect. The significance of random effect was determined with a likelihood ratio test. Tukey’s multiple comparison post-hoc test was used to analyse differences between each pair of incubation temperature within clutches using the ‘lsmeans’ package (Lenth, 2016). Effect sizes as measured by marginal and conditional R2 values were calculated with the ‘r.squaredGLMM’ function from the ‘MuMIn’ package (Nakagawa and Schielzeth, 2013; Johnson, 2014). We generated a kernel density plot for each incubation temperature group to visualize the proportion of individuals at TAB across temperatures (Fig. 4) using ‘geom_density’ (Wickham et al., 2016). We then extracted the values from this density plot to estimate the proportion of individuals at TAB across temperatures. We additionally fit a logistic model on the proportion of individuals that reached Tpeak and TArr with incubation temperature, stepwise temperature, and their interactions as fixed effects and clutch ID as a random effect. We estimated the temperatures at which 10%, 50% and 95% of individuals would have their heart rates peak or become arrhythmic with the ‘dose.p’ function in R (Venables and Ripley, 2002). Finally, we fit the difference between the number of individuals at TAB and Tpeak (∆N(TAB,Tpeak), and TAB and TArr (∆N(TAB,TArr) to generalized additive models (GAMs) with temperature as a predictor variable using the ‘mgcv’ package (Wood, 2017). The GAMs were fit with a Gaussian distribution and thin plate regression spline smoothing functions. The k value was set to 4 and 9, for Tpeak and TArr, respectively. Statistical significance was accepted at P < 0.05. All values are reported as mean ± SEM unless otherwise specified.
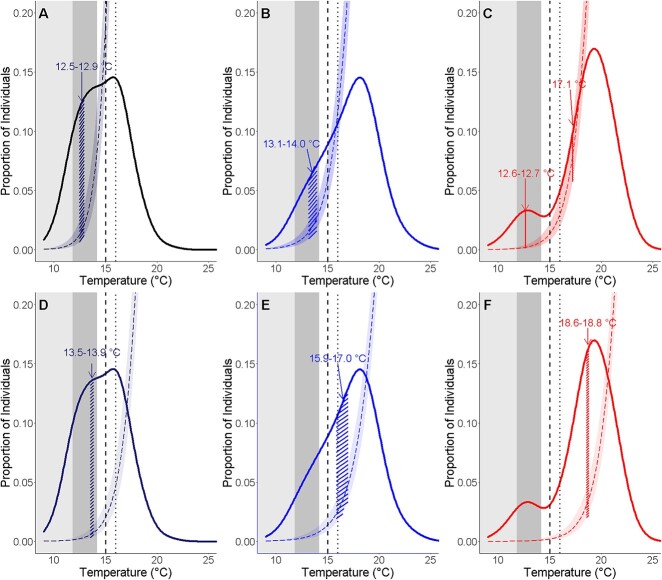
Figure 4.
Overlays of proportion of individuals at Arrhenius breakpoint temperatures (TAB) and temperatures where heart rate is maximized (Tpeak) and experience arrhythmia (TArr) reveal a temperature range where the proportion of individuals at TAB relative to Tpeak or TArr is maximized. Overlay of the proportion of individuals at TAB and that have reached Tpeak (A-C) and TArr (D-F) for 9°C (A,D), 12°C (B,E), and 15°C (C,F) incubation temperature groups. Smooths of proportion of individuals at TAB are kernel density estimates of proportion of individuals at TAB. Dashed and dotted curves are logistic curves of the proportion of individuals at Tpeak and TArr. Logistic curves are the same as in Fig. 3. Hashed areas under curve indicate the temperature ranges where TAB-Tpeak and TAB-TArr are maximized. Dark gray shaded area indicates temperature range that longfin smelt larvae are cultured at the Fish Conservation and Culture Laboratory (Supplementary Table 3), and light gray shaded areas indicate temperatures where larvae are most abundant in the San Francisco Estuary. Dotted lines indicate the temperature beyond which longfin smelt larvae are no longer observed in the field and dashed lines indicate the temperature where reduced larval growth rates have been measured.
Results
Heart rate
Incubation temperature had significant effects on fH0, fHpeak, ∆fHpeak-H0 and dfH/dT (Table 1). Averaged across clutches, fH0, fHpeak and ∆fHpeak-H0 increased with incubation temperatures but dfH/dT was only higher in the 15°C group, and there was no difference between the 9°C and 12°C groups. The random effect clutch ID had high variance (Supplementary Table 1), and a large difference between conditional and marginal R2 values for fH0, fHpeak and scope (Table 1). This was reflected in the relationships between fH0, fHpeak and ∆fHpeak-H0, which were more variable across clutches for each incubation temperature (Table 2).
Table 1.
Parameter estimates for fixed effects, marginal (R2m) and conditional R2 (R2c) values of linear mixed models for cardiac function metrics. See Supplementary Table 1 for parameter estimates of random effects. Bolded P values indicate statistical significance (P < 0.05).
| Metric | Parameter | Estimate | SE | Df | t value | P value | R2m | R2c |
|---|---|---|---|---|---|---|---|---|
| Initial heart rate (fH0) | Intercept | 48.87 | 1.66 | 13.66 | 29.51 | <0.001 | ||
| Incubation | 0.64 | 0.09 | 402.77 | 7.39 | <0.001 | 0.085 | 0.383 | |
| Peak heart rate (fHpeak) | Intercept | 5.12 | 7.56 | 12.70 | 0.68 | 0.510 | ||
| Incubation | 8.68 | 0.39 | 400.72 | 22.53 | <0.001 | 0.459 | 0.647 | |
| Difference between initial and peak heart rate (∆fHpeak-H0) | Intercept | −43.63 | 6.39 | 16.53 | −6.83 | <0.001 | ||
| Incubation | 8.03 | 0.36 | 400.98 | 22.44 | <0.001 | 0.482 | 0.626 | |
| Rate of increase in heart rate prior to Arrhenius breakpoint temperature (dfH/dT) | Intercept | −4.77 | 0.40 | 261.47 | −11.94 | <0.001 | ||
| Incubation | −0.11 | 0.03 | 402.92 | −3.62 | <0.001 | 0.032 | 0.050 | |
| Arrhenius breakpoint temperature (TAB) | Intercept | 9.13 | 0.73 | 70.84 | 12.56 | <0.001 | ||
| Incubation | 0.63 | 0.05 | 399.94 | 11.98 | <0.001 | 0.252 | 0.317 | |
| Temperature where heart rate reaches a maximum (Tpeak) | Intercept | 12.20 | 0.56 | 127.45 | 21.95 | <0.001 | ||
| Incubation | 0.57 | 0.04 | 404.08 | 13.64 | <0.001 | 0.310 | 0.346 | |
| Temperature where arrhythmia first occurs (TArr) | Intercept | 15.16 | 0.50 | 55.24 | 30.60 | <0.001 | ||
| Incubation | 0.51 | 0.04 | 353.45 | 14.48 | <0.001 | 0.355 | 0.427 |
Table 2.
Metrics of cardiac function: initial heart rate (fH0), peak heart rate (fHpeak), difference between initial and peak heart rate (∆fHpeak-H0), rate of increase in heart rate with temperature prior to Arrhenius breakpoint temperature (dfH/dT), Arrhenius breakpoint temperature (TAB), temperature where heart rate reaches a maximum (Tpeak), and temperature where arrhythmia first occurs (TArr) at different incubation temperatures for each clutch. P values for pairwise comparisons between incubation temperatures are listed for each cardiac function metric. Bolded P values indicate statistically significant (P < 0.05) differences among clutches and incubation temperature groups.
| Temp (°C) | Clutch 1 | Clutch 2 | Clutch 3 | Clutch 4 | Clutch 5 | Average | |
|---|---|---|---|---|---|---|---|
| f H0 (beats min−1) | 9 | 57.4 ± 0.4 | 58.2 ± 1.3 | 52.9 ± 0.4 | 48.9 ± 0.7 | 55.9 ± 0.6 | 55.5 ± 0.4 |
| 12 | 58.3 ± 0.4 | 61.2 ± 0.6 | 55.9 ± 0.6 | 54.6 ± 1.4 | 52.3 ± 1.0 | 56.9 ± 0.4 | |
| 15 | 60.0 ± 0.7 | 63.1 ± 1.2 | 59.8 ± 0.4 | 52.5 ± 0.6 | 54.2 ± 1.4 | 59.8 ± 0.5 | |
| f Hpeak (beats min−1) | 9 | 102.4 ± 2.0 | 97.0 ± 3.0 | 75.6 ± 1.1 | 68.8 ± 2.3 | 85.6 ± 1.4 | 87.9 ± 1.4 |
| 12 | 115.9 ± 2.1 | 127.1 ± 3.1 | 98.5 ± 3.1 | 96.8 ± 6.0 | 98.4 ± 3.4 | 109.2 ± 1.6 | |
| 15 | 132.5 ± 3.7 | 159.5 ± 6.0 | 138.7 ± 2.5 | 101.7 ± 2.2 | 128.6 ± 11.5 | 140.9 ± 2.5 | |
| ∆fHpeak-H0 (beats min−1) | 9 | 44.9 ± 1.8 | 38.9 ± 2.7 | 22.7 ± 0.8 | 19.2 ± 2.2 | 29.7 ± 1.3 | 32.4 ± 1.1 |
| 12 | 57.6 ± 2.0 | 65.9 ± 2.9 | 42.6 ± 2.8 | 42.3 ± 6.0 | 46.0 ± 3.4 | 52.2 ± 1.5 | |
| 15 | 72.5 ± 4.0 | 96.3 ± 5.3 | 79.1 ± 2.5 | 49.2 ± 2.0 | 74.3 ± 11.5 | 81.1 ± 2.2 | |
| dfH/dT (beats min−1∙°C−1) | 9 | 5.8 ± 0.2 | 6.7 ± 0.5 | 5.4 ± 0.1 | 4.8 ± 0.2 | 6.1 ± 0.2 | 5.9 ± 0.1 |
| 12 | 6.3 ± 0.1 | 6.2 ± 0.1 | 6.3 ± 0.3 | 5.7 ± 0.8 | 5.7 ± 0.4 | 6.0 ± 0.1 | |
| 15 | 5.9 ± 0.3 | 6.8 ± 0.3 | 6.6 ± 0.3 | 6.5 ± 0.1 | 7.3 ± 0.9 | 6.6 ± 0.1 | |
| TAB (°C) | 9 | 16.5 ± 0.3 | 14.7 ± 0.3 | 13.8 ± 0.3 | 14.1 ± 0.9 | 14.2 ± 0.5 | 14.7 ± 0.2 |
| 12 | 17.7 ± 0.4 | 17.9 ± 0.3 | 15.3 ± 0.4 | 16.8 ± 0.6 | 16.9 ± 0.5 | 17.0 ± 0.2 | |
| 15 | 18.8 ± 0.5 | 19.4 ± 0.3 | 17.9 ± 0.5 | 16.5 ± 0.7 | 18.0 ± 0.8 | 18.4 ± 0.2 | |
| Tpeak (°C) | 9 | 18.7 ± 0.3 | 17.6 ± 0.3 | 16.7 ± 0.2 | 16.6 ± 0.7 | 16.7 ± 0.2 | 17.3 ± 0.2 |
| 12 | 19.1 ± 0.4 | 20.4 ± 0.3 | 18.3 ± 0.3 | 18.9 ± 0.5 | 19.9 ± 0.5 | 19.5 ± 0.2 | |
| 15 | 20.3 ± 0.4 | 21.4 ± 0.3 | 20.6 ± 0.3 | 19.4 ± 0.4 | 20.2 ± 0.8 | 20.7 ± 0.2 | |
| TArr (°C) | 9 | 21.0 ± 0.2 | 20.1 ± 0.2 | 19.4 ± 0.2 | 18.6 ± 0.5 | 19.5 ± 0.2 | 19.8 ± 0.1 |
| 12 | 22.1 ± 0.3 | 22.3 ± 0.2 | 20.4 ± 0.2 | 20.8 ± 0.4 | 21.3 ± 0.3 | 21.4 ± 0.1 | |
| 15 | 22.4 ± 0.4 | 23.3 ± 0.2 | 22.9 ± 0.3 | 21.8 ± 0.4 | 22.3 ± 0.5 | 22.8 ± 0.2 |
Cardiac indices of thermal performance
Incubation temperature had a significant effect on TAB, Tpeak and TArr (Table 1). All three metrics increased with incubation temperatures (Fig. 2). The random effect clutch ID had low variances and small differences between conditional and marginal R2 values for all three metrics (Table 2). This was reflected in the small interclutch variation in the response to incubation temperature for all three metrics but the same trend whereby higher incubation temperatures resulted in higher TAB, Tpeak and TArr across clutches (Table 2). It should be noted that there were three individuals where TAB could not be calculated because a breakpoint was not present prior to the individual becoming arrhythmic; these individuals were not included in the TAB dataset. Additionally, not all individuals experienced arrhythmia within the range of temperatures tested (described below) and thus our measure of TArr is likely to be slightly underestimated.
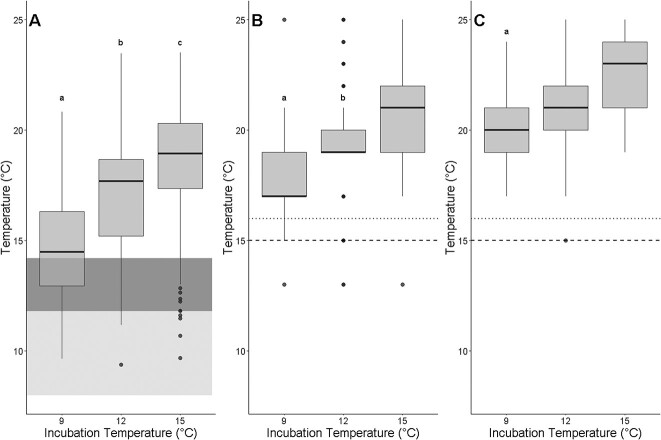
Figure 2.
Median cardiac metrics do not correlate with inferred optimal temperatures or upper thermal limits. Arrhenius breakpoint temperature (TAB) (A), temperature where heart rate reaches a maximum (Tpeak) (B), and temperature where arrhythmia first occurs (TArr) (C) of one day post-hatch longfin smelt larvae incubated at and held for one day at different temperatures. Boxplots represent median, first and third quartiles of cardiac performance metrics. Shaded dark gray area indicates the temperature range in which longfin smelt are cultured at the Fish Conservation and Culture Laboratory (Supplementary Table 3) and light gray shaded area indicates the temperatures where longfin smelt larvae are most abundant in the San Francisco Estuary. Dotted lines indicate the temperature beyond which longfin smelt larvae are no longer observed in the field and dashed lines indicate the temperature where reduced larval growth rates have been measured. Different letters indicate statistical significance between incubation temperatures within each cardiac performance metric.
There was a significant effect of temperature and incubation temperature, but not their interaction on the proportion of individuals that reached Tpeak or TArr (Table 3). Notably, there was extremely high individual variation in cardiac indices regardless of incubation temperature. For 9°C, 12°C and 15°C incubation temperatures, TAB ranged between 9.7°C to 20.8°C, 9.4°C to 23.5°C and 9.7°C to 23.5°C, and TArr ranged between 17°C to 24°C, 15°C to 25°C and 19–25°C for 9°C, 12°C and 15°C incubation groups, respectively. Tpeak ranged between 13°C to 25°C for all incubation temperatures. Logistic curves showed that the temperature at which 10, 50 and 95% of the proportion of individuals that had reached Tpeak or TArr increased with incubation temperature (Fig. 3). This was reflected in the significantly higher percentage of arrhythmic individuals by 25°C for the 9°C incubation temperature compared to the 12°C and 15°C incubation temperatures (F2,413 = 4.93, P = 0.009; 9–12°C: P = 0.007, 9–15°C: P = 0.036, 12–15°C: P = 0.954) where the proportion of arrhythmic individuals by 25°C was 95.8 ± 1.8, 82.1 ± 3.0 and 84.3 ± 3.2% for 9°C, 12°C and 15°C incubation temperatures, respectively.
Table 3.
Parameter estimates, odds ratios, marginal (R2m) and conditional R2 (R2c) values of generalized mixed models for the proportion of individuals at Tpeak and TArr across temperatures for different incubation temperatures as depicted in Fig. 3. Odds ratios were calculated as the exponential of parameter estimates. R2 values were calculated using the delta method. See Supplementary Table 2 for parameter estimates of random effects. Bolded P values indicates statistical significance (P < 0.05).
| Metric | Parameter | Estimate±SE | z-value | P value | Odds Ratio | R2m | R2c |
|---|---|---|---|---|---|---|---|
| Temperature where heart rate reaches a maximum (Tpeak) | Intercept | −10.17 ± 2.32 | −4.39 | <0.001 | |||
| Temperature | 0.92 ± 0.12 | 7.40 | <0.001 | 2.52 | 0.836 | 0.843 | |
| Incubation | −0.50 ± 0.19 | −2.59 | 0.010 | 0.61 | |||
| Temperature x Incubation | −0.003 ± 0.01 | −0.35 | 0.725 | 1.00 | |||
| Temperature where arrhythmia first occurs (TArr) | Intercept | −10.31 ± 2.50 | −4.12 | <0.001 | |||
| Temperature | 0.75 ± 0.12 | 6.40 | <0.001 | 2.12 | 0.783 | 0.793 | |
| Incubation | −0.52 ± 0.21 | −2.47 | 0.014 | 0.59 | |||
| Temperature x Incubation | 0.002 ± 0.01 | 0.23 | 0.821 | 1.00 |
Figure 3.
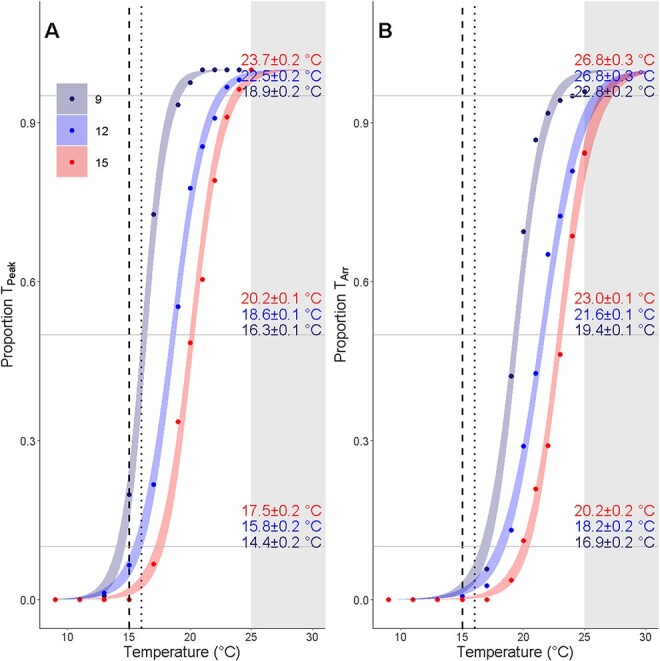
The 10% threshold for Tpeak and TArr correlate more closely with indicators of upper thermal limits measured by previous studies. Proportion of individuals reaching temperatures where heart rate reaches a maximum (Tpeak) (A) and where arrhythmia first occurs (TArr) (B) as test temperatures increased. Embryos were incubated at 9°C, 12°C, and 15°C. Gray shaded areas indicate temperatures beyond those tested experimentally and are thus predictions of the models. Horizontal lines indicate 10, 50 and 95% thresholds and temperatures listed correspond to the temperature at which these thresholds were met for each incubation temperature. Dotted lines indicate the temperature beyond which longfin smelt larvae are no longer observed in the field and dashed lines indicate the temperature where reduced larval growth rates have been measured.
The proportions of individuals that were at TAB across temperatures were distributed in a bell-shaped curve for 9°C and 12°C, and a bimodal distribution for 15°C (Fig. 4). The temperatures where the estimated proportion of individuals at TAB were within 90% of the maximal proportion of individuals at TAB ranged between 13.1°C to 16.6°C, 17.0°Cto 19.0°C, and 18.4°C to 20.3°C, for 9°C, 12°C and 15°C incubation temperatures, respectively. The TAB curve for the 15°C incubation temperature also had a second smaller peak at 12.8°C. Because there were some individuals reaching Tpeak or TArr simultaneous to other individuals at TAB, overlaying logistic curves for Tpeak and TArr with the TAB curves revealed a range of temperatures where there are more individuals at TAB relative to Tpeak or TArr (Fig. 4). Calculating ∆N(TAB,Tpeak) found that the temperatures where the proportion of individuals at TAB was maximized relative to Tpeak ranged between 12.5°C to 12.9°C and 13.4°C to 14.4°C for 9°C and 12°C incubation temperatures, respectively (Fig. 4A). For the 15°C incubation temperature, there were two ranges where this difference was maximized: 12.6°C to 12.7°C and 17.2°C. Maximum values of ∆N(TAB,Tpeak) trended towards lower values with higher incubation temperatures; maximum ∆N(TAB,Tpeak) for 9°C, 12°C and 15°C were 0.11, 0.05 and 0.03, respectively (Fig. 4A–C). ∆N(TAB-TArr) was maximized between 13.6°C to 13.9°C, 16.2°C to 16.9°C and 18.6°C to 18.8°C, for 9°C, 12°C and 15°C incubation temperatures, respectively (Fig. 4D–F). Similarly, maximum ∆N(TAB,TArr) trended towards lower values with higher incubation temperatures; maximum ∆N(TAB,TArr) for 9°C, 12°C and 15°C were 0.12, 0.06 and 0.03, respectively.
GAM plots showed a negative response for ∆N(TAB,Tpeak), indicating that there were more individuals at their upper thermal limit compared relative to those at TAB, starting between 16.5–17.7°C, 18.2–19.2°C, and 18.7–20.1°C, for 9°C, 12°C and 15°C incubation temperatures, respectively (Fig. 5A). For ∆N(TAB,TArr), GAM plots showed a negative response starting between 18.4°C to 19.1°C, 19.6°C to 20.4°C and 20.5°C to 21.7°C, for 9°C, 12°C and 15°C incubation temperatures, respectively (Fig. 5B). Breakpoints for GAM plots indicated the temperature when ∆N(TAB,Tpeak) and ∆N(TAB,TArr) began to decrease, that is, when the number of individuals at their upper thermal limit began to increase relative to those at TAB. For ∆N(TAB,Tpeak), there were breakpoints at 14.1°C, 16.2°C and 16.9°C, for 9°C, 12°C and 15°C, respectively. For ∆N(TAB,TArr), there were breakpoints at 16.6°C, 17.8°C and 19.5°C, for 9°C, 12°C and 15°C, respectively.
Figure 5.
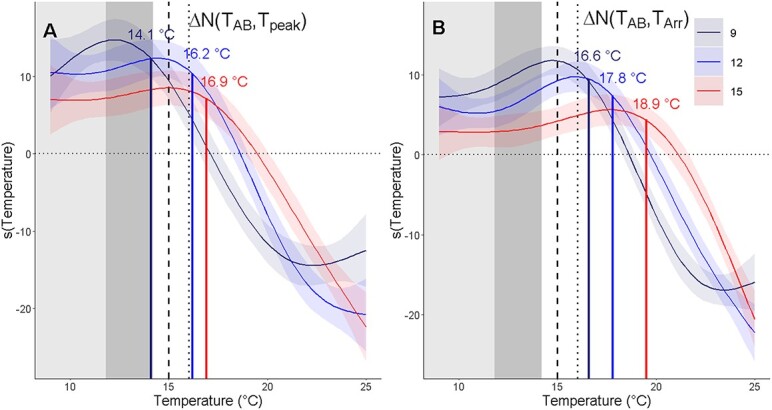
Smoothed fits (s(Temperature)) from GAMs of the relationship between number of individuals at TAB minus those that have reached Tpeak (∆N(TAB,Tpeak)) (A) and relationship between number of individuals at TAB minus those that have reached TArr (∆N(TAB,TArr)) (B) across temperatures for each incubation temperature. Vertical colored lines and associated temperatures indicate the temperature at which ∆N(TAB,Tpeak) and ∆N(TAB,TArr) begin decreasing with temperature. Plots are fitted smooths and 95% confidence intervals from GAMs. The y-axis units are centered on zero. The estimated degrees of freedom of the smooths for each incubation temperature are: ∆N(TAB,Tpeak): 9°C: 4.46, 12°C: 4.49, 15°C: 3.45, ∆N(TAB,TArr): 9°C: 5.61, 12°C: 5.18, 15°C: 4.49. Dark gray shaded area indicates temperature range that longfin smelt larvae are cultured at the Fish Conservation and Culture Laboratory (Supplementary Table 3), and light gray shaded areas indicate temperatures where larvae are most abundant in the San Francisco Estuary. Dotted lines indicate the temperature beyond which longfin smelt larvae are no longer observed in the field and dashed lines indicate the temperature where reduced larval growth rates have been measured.
Discussion
By transposing a cardiac thermal performance assay typically used for larger fish to newly hatched larvae, and using an activity analysis software, we rapidly assessed cardiac thermal performance of the larval life stage of the imperiled longfin smelt when incubated at different temperatures. We found that initial (fH0) and peak heart rates (fHpeak), and consequently the difference between peak and initial heart rates (∆fHpeak-H0) varied across clutches. However, the Arrhenius breakpoint temperature (TAB), temperature when heart rate peaks (Tpeak), and temperature when arrhythmia is first observed (TArr) remained relatively similar across clutches. This suggests that while the inherited properties governing cardiac function may have differed, the thermal tolerance range of longfin smelt are similar across clutches. We additionally found that higher incubation temperatures increased TAB, Tpeak and TArr but that the large and overlapping ranges of these three metrics measured within an incubation temperature rendered the mean and median values of these performance metrics incomplete for informing conservation strategies. We therefore suggest that analysing the proportions of larvae at TAB, Tpeak and TArr could provide a more appropriate method for identifying Topt and upper thermal limits.
Use of the cardiac assay
A notable result from the present study was the extremely high individual variation in TAB, Tpeak and TArr regardless of incubation temperatures; across all incubation temperatures, TAB ranged between 9.4°C and 23.5°C, Tpeak between 13°C and 25°C and TArr between 15°C and 25°C. This is consistent with a previous study that found highly variable growth and yolk resorption rates across temperatures in longfin smelt yolk-sac larvae (Yanagitsuru et al., 2021). While some of this variation across individuals can be attributed to parentage, there was low variance attributed to clutch ID for the cardiac performance metrics. This suggests that there is inherently high individual variation in thermal performance within a clutch of SFE longfin smelt. From a life history and evolutionary standpoint, this individual variation in newly hatched larvae is perhaps unsurprising. This life stage has not undergone the expected large mortality event typically observed during the transition from endogenous to exogenous feeding in larvae (Sifa and Mathias, 1987). Therefore, one of the first bottlenecks, where selective pressures can reduce phenotypic variation during recruitment, did not occur (Hjort, 1914). Were this study to be performed at a later life stage, it is possible that this individual variation would be greatly reduced as a result of the inevitable mass-mortality event during the larval life stage. Furthermore, this individual variation among larvae could be adaptive in the dynamic SFE environment where temperature, among other physicochemical properties, can vary greatly on tidal, seasonal and annual timescales (Knowles, 2002), as high phenotypic variation can increase the capacity of a population to persist under a variable environment (Price et al., 2003; Forsman and Wennersten, 2016).
Regardless of the underlying principles resulting in individual variation in longfin smelt larvae thermal tolerance, the extreme variation in cardiac indices complicates the interpretation of the thermal performance metrics. The large range of cardiac indices results in many individuals deviating greatly from the mean values of each of these metrics, and therefore, the mean values would misrepresent a large proportion of the study population. Related to this, individual variation across all three indices resulted in great overlap of the proxy for Topt as measured by TAB, and the proxies for upper thermal limits as measured by Tpeak and TArr, which results in a large mix of individuals that are at appropriate thermal conditions and those that are experiencing thermal stress. For example, in the 9°C incubation temperature group, rearing at mean TAB (14.7°C) would result in 14.0% of individuals at TAB, 14.0% having reached Tpeak and 1.5% having reached TArr.
To interpret cardiac indices for a species and/or life stage with high individual variation, we propose that upper thermal limits are best defined as the temperature where 10% of individuals exhibit Tpeak or TArr. The 10% thresholds for Tpeak and TArr correlate with sublethal and lethal thermal limits for longfin smelt larvae, respectively. For example, in the 9°C incubation temperature group, the 10% threshold for Tpeak (14.4°C) lies just below the temperature where growth rates are decreased in longfin smelt (15°C) (Yanagitsuru et al., 2021), and the 10% threshold for TArr (16.9°C) falls within a degree of the temperature where longfin smelt larvae are no longer observed in the field (16°C) (Grimaldo et al., 2017). This makes sense since the 10% threshold for the logistic curves of Tpeak and TArr indicate the temperature at which any further warming would lead to a rapid increase in the number of individuals that would reach these indices of upper thermal limit. For management, we suggest under the precautionary principle that the 10% threshold for Tpeak would ensure that most larvae remain below their upper thermal limit and thus provides a more suitable metric for use in conservation compared to TArr. For example, if the TArr 10% threshold for the 9°C incubation temperature was applied as a management target, but individuals do indeed begin experiencing thermal stress by Tpeak, then up to 67% of individuals could experience sublethal thermal stress at this temperature. Furthermore, the use of thermal thresholds based on lower incubation temperatures could also ensure most larvae do not experience thermally stressful conditions. For example, if the Tpeak 10% threshold for the 12°C incubation temperature was applied as a management target, but most wild fish actually incubate at 9°C, then up to 36.5% of wild individuals would hatch into thermally stressful conditions, likely resulting in mortality and recruitment failure.
We also propose that due to the overlapping range of these metrics, Topt can be better approximated as the temperature range where the number or proportion of individuals at TAB is maximized relative to Tpeak (i.e. maximum ∆N(TAB,Tpeak)) for captive-raised individuals. The temperature range where the difference in the proportion or number of individuals at TAB and Tpeak is maximized across all incubation temperatures correlates with the temperature range where longfin smelt larvae were successfully cultured in captivity (11.9–14.2°C; Supplementary Table 3). In contrast, the temperature range where ∆N(TAB,Tpeak) is maximized (12.5–14.0°C depending on incubation temperature) was slightly higher than the Topt inferred from field studies (8–12°C). This discrepancy could be explained by differences in fundamental and realized niches, whereby factors other than temperature such as other physicochemical variables (Dege and Brown, 2003; Grimaldo et al., 2020; Lewis et al., 2020) and food availability (Winder and Jassby, 2011; Lee et al., 2016; Barros et al., 2022; Burris et al., 2022; Fiksen and Reglero, 2022) drive the thermal habitats that organisms occupy in nature (Allen-Ankins and Stoffels, 2017).
Cardiac thermal performance of larval longfin smelt
Higher incubation temperatures increased mean fH0, fHpeak, ∆fHpeak-H0, TAB, Tpeak and TArr. These data suggest that higher incubation temperatures increased the upper bound of cardiac scope, shifted Topt to a higher temperature, and increased upper thermal limits, respectively. Increases to upper thermal limits with higher incubation temperatures is also reflected in the increase of the 10% threshold for Tpeak and TArr as well as the breakpoints where our GAMs for ∆N(TAB,Tpeak) begin decreasing. Within the temperatures tested, these shifts in cardiac indices suggest that higher incubation temperatures could provide an appreciable tolerance benefit for longfin smelt larvae in a warming world. However, incubation temperature had little influence on the temperature range of maximum ΔN(TAB,Tpeak). Taken together, this suggests that the upper thermal limit for a group of longfin smelt larvae is relatively plastic compared to Topt. Higher incubation temperatures may increase the resistance of larval longfin smelt to the direct effects of high temperatures but the relatively cooler temperature range of maximum ΔN(TAB,Tpeak) would provide the ideal thermal habitat for longfin smelt larvae regardless of their embryonic thermal history. Under climate change, this could mean that the predicted warmer temperatures during embryonic development could provide longfin smelt larvae with higher resistance to the projected increases to temperatures in April and May (Bashevkin et al., 2022), which begin exceeding the 10% Tpeak thresholds (Brown et al., 2013, 2016). However, continually increasing temperatures during the larval period predicted by climate models (Bashevkin et al., 2022) will result in increasingly larger proportions of larvae being shifted outside of their optimal thermal range. Since maximum ΔN(TAB,Tpeak) appears to overestimate field-based optimal thermal habitats, the proportion of longfin smelt larvae falling outside of their ideal temperature range in the SFE are likely to be even higher than would be expected from our results.
The constant incubation temperatures used in this study provide some baseline information on the thermal performance of longfin smelt larvae, but variable thermal regimes more closely reflect what organisms experience in nature. It is well established that variable thermal regimes produce complex phenotypic effects that would not be expected from constant thermal means (Massey and Hutchings, 2021). For example, daily sinusoidal fluctuations in incubation temperature yielded adults with higher critical thermal maxima but smaller size compared to constant mean temperatures in zebrafish (Schaefer and Ryan, 2006). The exact location where longfin smelt spawn is still under investigation (Gross et al., 2022) but areas within the range of potential spawning habitats in the SFE (i.e. San Pablo Bay, Suisun Marsh, Suisun Strait, Carquinez Strait) experience a lightly variable thermal regime during the spawning season, and it is likely that embryos experience daily temperature fluctuations in the SFE. The temperature regime within the spawning season is seasonally variable. Between November and February, temperatures are generally lower with median temperatures typically between 9°C and 15°C, and more stable with temperature fluctuations between ±0.5°C and ±1.5°C daily. In October, March, and April, temperatures increase to median temperatures typically between 12°C and 19°C, with more variable fluctuations typically between ±0.5°C and ±3.5°C (USGS Water Data for USA, https://waterdata.usgs.gov/nwis?). The results of our study most closely represent embryos that are spawned in the cooler and more stable winter months but future studies examining fluctuating incubation temperature regimes would provide a more complete understanding of the thermal habitat requirements of larvae hatched from embryos spawned during the early and late spawning season.
Management implications
While rising temperature is only one of numerous issues in the SFE, it is important to identify thermal limits for imperiled species living in habitats facing rising temperatures, such as the SFE. We demonstrated that a common cardiac assay has the potential to rapidly identify Topt and upper thermal limits of the larval life stage of a fish species. Our results suggest that it is not possible to provide a single Topt for all, let alone most, individuals from a group of longfin smelt larvae. However, we show that there is a temperature range that maximizes the proportion of individuals at Topt relative to those at their upper thermal limit, which correlates with temperatures at which larvae have been successfully cultured, but may overestimate optimal thermal habitats in the field. We also defined temperature thresholds that would minimize the number of larvae experiencing thermal stress to inform water management policies in the SFE. Our findings suggest that this cardiac assay is a promising tool for quickly identifying Topt for use in culture and for defining thermal thresholds that can be used for field-based management. In particular, this cardiac assay could improve current conservation aquaculture practices and inform supplementation of wild populations.
Conservation aquaculture provides a sustainable supply of research specimens necessary for physiological studies and provides a captive refuge population that can be released for supplementation of wild populations. However, a successful conservation aquaculture program requires the development of culturing methods that can often take years, and successful supplementation efforts requires knowledge of thermal tolerances to identify when and where to release fish (Yanagitsuru et al., 2022). Given the highly limited quantity of research specimens to work with and the time-sensitive nature of protecting imperiled species, it is essential for conservation aquaculture to rapidly identify proper rearing techniques with few individuals to support conservation research. Identifying proper larviculture methods is often the first bottleneck for the successful development of aquaculture for a new species. Traditional grow-out and survival experiments definitively determine the efficacy of culturing protocols, but these studies take time and use large quantities of larvae due to the high densities that larval fish in culture often require. Indeed, for longfin smelt it took over a decade to identify the proper abiotic conditions for culturing larvae (CDWR, 2020). Furthermore, given that population supplementation has already been initiated in endangered delta smelt in the SFE (Baerwald et al., 2023), and considering the population declines and likely impending endangered listing for longfin smelt, it is conceivable that supplementation will also be implemented in longfin smelt. It is therefore critical to quickly develop the culturing tools and obtain the necessary environmental tolerance data for informing future supplementation efforts. While temperature is just one condition, the use of this cardiac assay has the potential to quickly identify the appropriate range of temperatures for captive culture of the larvae of a new species (i.e. maximum ΔN(TAB,Tpeak)) with relatively few individuals, and define thermal tolerance thresholds for management efforts, such as supplementation.
Overall, our study demonstrates the utility of this cardiac assay for the early life stages of fishes and our results fill a critical gap in our knowledge of the thermal physiology of longfin smelt. Because mortality during the larval stage influences the success of recruitment in wild fish populations and the success of aquaculture programs, the thermal tolerance metrics that we establish provide a valuable step toward improving longfin smelt conservation in the SFE. The temperatures we defined for captive larviculture through ΔN(TAB,Tpeak) and upper thermal limits defined as 10% thresholds can be used to ensure that conservation actions encapsulate appropriate thermal conditions to develop effective rearing protocols for a conservation aquaculture program and to support effective supplementation efforts for this sensitive species. However, it is important to assess thermal tolerance across ontogeny for longfin smelt to inform conservation actions because some species may exhibit ontogenetic variation in thermal tolerance (Komoroske et al., 2014). Furthermore, future studies with this assay across more species and thermal regimes, and comparisons of the relationship between temperatures that maximize ΔN(TAB,Tpeak) and 10% thresholds of Tpeak and TArr with other thermal performance metrics (e.g. swimming, feeding and survival), are necessary to validate its general utility for fish larvae.
Supplementary Material
Acknowledgements
We thank the technical staff at the Otolith Geochemistry and Fish Ecology Laboratory at UC Davis, US Fish and Wildlife Service, FCCL, and California Department of Water Resources for broodstock collections. We also thank Galen Tigan, Luke Ellison, and FCCL staff for caring for broodstock and fertilizing the embryos used in this study. Further, we thank Thomas Hahn, Andrew Rypel, and Anne Todgham for comments on the manuscript. Finally, we thank two anonymous reviewers whose comments substantially improved the manuscript.
Contributor Information
Yuzo R Yanagitsuru, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
Florian Mauduit, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA; Department of Anatomy, Physiology, and Cell Biology, School of Veterinary Medicine, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
Alexis J Lundquist, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
Levi S Lewis, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
James A Hobbs, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA; California Department of Fish and Wildlife, Bay-Delta IEP, 2109 Arch Airport Rd,Stockton, CA, San Joaquin County, 95206, USA.
Tien-Chieh Hung, Fish Conservation and Culture Laboratory, Department of Biological and Agricultural Engineering, University of California, Davis, 17501 Byron Hwy, Byron, CA, Contra Costa County, 94514, USA.
Richard E Connon, Department of Anatomy, Physiology, and Cell Biology, School of Veterinary Medicine, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
Nann A Fangue, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Avenue, Davis, CA, Yolo County, 95616, USA.
Author Contributions
Y.R.Y., F.M., R.E.C. and N.A.F. conceptualized and designed experiments. Y.R.Y., F.M. and A.J.L. performed experiments and analysed data. J.A.H. and L.S.L. oversaw collection of broodstock. T.-C.H. oversaw rearing of adult longfin smelt and coordinated provisioning of embryos. Y.R.Y. drafted the original manuscript with detailed edits from F.M., J.A.H., L.S.L., T.-C.H., R.E.C., and N.A.F. Y.R.Y., J.A.H., L.S.L., T.-C.H., R.E.C. and N.A.F. acquired funding.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by a SeaGrant/Delta Science Fellowship (grant R/SF-93 to Y.R.Y.), the UC Davis Agricultural Experiment Station (grant 2098-H to N.A.F.), US Fish and Wildlife Service (grants F18AC00057, F18AC00060 & F20AC11492 to N.A.F. and R.E.C.), the California Department of Fish and Wildlife (Proposition 1, grant P1806019 to R.E.C., N.A.F., J.A.H., T.-C.H., and L.L.), and the California Department of Water Resources (Agreement 4600011161 to T.-C.H. and 4600014181 to R.E.C., N.A.F., T.-C.H. and L.L.)
Data Availability
Data supporting this paper are available in the Dryad data depository: https://doi.org/10.25338/B8H06D.
References
- Allen JL, Harman PD (1970) Control of pH in MS-222 anesthetic solutions. The Progressive Fish-Culturist 32: 100–100. 10.1577/1548-8640(1970)32[100:COPIMA]2.0.CO;2. [DOI] [Google Scholar]
- Allen-Ankins S, Stoffels RJ (2017) Contrasting fundamental and realized niches: two fishes with similar thermal performance curves occupy different thermal habitats. Freshw Sci 36: 635–652. 10.1086/693134. [DOI] [Google Scholar]
- Anttila K, Casselman MT, Schulte PM, Farrell AP (2013) Optimum temperature in juvenile salmonids: connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol Biochem Zool 86: 245–256. 10.1086/669265. [DOI] [PubMed] [Google Scholar]
- Anttila K, Mauduit F, Le Floch S, Claireaux G, Nikinmaa M (2017) Influence of crude oil exposure on cardiac function and thermal tolerance of juvenile rainbow trout and European sea bass. Environ Sci Pollut Res 24: 19624–19634. 10.1007/s11356-017-9609-x. [DOI] [PubMed] [Google Scholar]
- Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB (2015a) The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Funct Ecol 29: 479–486. 10.1111/1365-2435.12396. [DOI] [Google Scholar]
- Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB (2015b) Flexibility in metabolic rate confers a growth advantage under changing food availability. J Anim Ecol 84: 1405–1411. 10.1111/1365-2656.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald MR, Kwan N, Pien C, Auringer G, Carson EW, Cocherell DE, Ellison L, Fangue NA, Finger AJ, Gille DAet al. (2023) Captive-Reared Delta smelt (Hypomesus transpacificus) exhibit high survival in natural conditions using in situ enclosures. PloS One 18: e0286027. 10.1371/journal.pone.0286027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros A, Hobbs JA, Willmes M, Parker CM, Bisson M, Fangue NA, Rypel AL, Lewis LS (2022) Spatial heterogeneity in prey availability, feeding success, and dietary selectivity for the threatened longfin smelt. Estuaries and Coasts 45: 1766–1779. 10.1007/s12237-021-01024-y. [DOI] [Google Scholar]
- Bashevkin SM, Mahardja B, Brown LR (2022) Warming in the upper San Francisco estuary: patterns of water temperature change from five decades of data. Limnol Oceanogr 67: 1065–1080. 10.1002/lno.12057. [DOI] [Google Scholar]
- Brauner CJ, Richards JG (2020) Physiological performance in aquaculture: Using physiology to help define optimal conditions for growth and environmental tolerance. In Benfey TJ, Farrell AP, Brauner CJ eds, Fish Physiol, Vol. 38. Academic Press, Cambridge, Massachusetts, United States, pp. 83–121 [Google Scholar]
- Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerkd). Am Zool 11: 99–113. 10.1093/icb/11.1.99. [DOI] [Google Scholar]
- Brown LR, Bennett WA, Wagner RW, Morgan-King T, Knowles N, Feyrer F, Schoellhamer DH, Stacey MT, Dettinger M (2013) Implications for future survival of delta smelt from four climate change scenarios for the Sacramento–san Joaquin Delta, California. Estuar Coasts 36: 754–774. 10.1007/s12237-013-9585-4. [DOI] [Google Scholar]
- Brown LR, Komoroske LM, Wagner RW, Morgan-King T, May JT, Connon RE, Fangue NA (2016) Coupled downscaled climate models and ecophysiological metrics forecast habitat compression for an endangered estuarine fish. PloS One 11: e0146724. 10.1371/journal.pone.0146724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris ZPL, Baxter RD, Burdi CE (2022) Larval and juvenile Longfin smelt diets as a function of fish size and prey density in the San Francisco estuary. Calif Fish Wildlife J 108: e11. 10.51492/cfwj.108.11. [DOI] [Google Scholar]
- California Department of Water Resources (CDWR) . Longfin Smelt Science Plan 2020–2030. Academic Press, Cambridge, Massachusetts, United States, 2020 [Google Scholar]
- Casselman MT, Anttila K, Farrell AP (2012) Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J Fish Biol 80: 358–377. 10.1111/j.1095-8649.2011.03182.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Anttila K, Wu J, Whitney CK, Hinch SG, Farrell AP (2013) Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Can J Zool 91: 265–274. 10.1139/cjz-2012-0300. [DOI] [Google Scholar]
- Chen Z, Snow M, Lawrence CS, Church AR, Narum SR, Devlin RH, Farrell AP (2015) Selection for upper thermal tolerance in rainbow trout (Oncorhynchus mykiss Walbaum). J Exp Biol 218: 803–812. 10.1242/jeb.113993. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Fangue NA, Bergman JN, Madliger CL, Cech JJ Jr, Eliason EJ, Brauner CJ, Farrell AP (2021) Introduction: Physiology and the conservation and management of wild fish populations in the Anthropocene – A Systems Approach. In Cooke SJ, Fangue NA, Farrell AP, Brauner CJ, Eliason EJ eds, Fish Physiol. 39A. Academic Press, Academic Press, pp. 1–32 [Google Scholar]
- Dash P, Tandel RS, Pandey N, Sawant PB, Sarma D, Rawat KD, Chadha NK (2021) Effects of rearing temperature on egg incubation, growth, standard metabolic rate, and thermal tolerance of chocolate mahseer, Neolissochilus hexagonolepis. J Therm Biol 98: 102942. 10.1016/j.jtherbio.2021.102942. [DOI] [PubMed] [Google Scholar]
- Dege M, Brown LR (2003) Effect of outflow on spring and summertime distribution and abundance of larval and juvenile fishes in the upper San Francisco Estuary. In Feyrer F, Brown LR, Brown RL, Orsi JJ eds, Early Life History of Fishes in the San Francisco Estuary and Watershed American Fisheries Society Symposium, Bethesda, MD, pp. 49–66
- Degidio JML, Yanong RP, Watson CA, Ohs CL, Cassiano EJ, Barden K (2017) Spawning, embryology, and larval development of the milletseed butterflyfish Chaetodon miliaris in the laboratory. N Am J Aquac 79: 205–215. 10.1080/15222055.2017.1302025. [DOI] [Google Scholar]
- DiMaggio MA, Broach JS, Ohs CL, Grabe SW (2013) Captive volitional spawning and larval rearing of pigfish. N Am J Aquac 75: 109–113. 10.1080/15222055.2012.736447. [DOI] [Google Scholar]
- DiMaggio MA, Cassiano EJ, Barden KP, Ramee SW, Ohs CL, Watson CA (2017) First record of captive larval culture and metamorphosis of the Pacific blue Tang,Paracanthurus hepatus. J World Aquaculture Soc 48: 393–401. 10.1111/jwas.12426. [DOI] [Google Scholar]
- Downie AT, Lefevre S, Illing B, Harris J, Jarrold MD, McCormick MI, Nilsson GE, Rummer JL (2023) Rapid physiological and transcriptomic changes associated with oxygen delivery in larval anemonefish suggest a role in adaptation to life on hypoxic coral reefs. PLoS Biol 21: e3002102. 10.1371/journal.pbio.3002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost HE, Fisher J, Randall F, Kent D, Carmack EC, Farrell AP (2016) Upper thermal limits of the hearts of Arctic cod Boreogadus saida: adults compared with larvae. J Fish Biol 88: 718–726. 10.1111/jfb.12807. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. 10.1126/science.1199158. [DOI] [PubMed] [Google Scholar]
- Farrell AP (1991) From hagfish to tuna: a perspective on cardiac function in fish. Physiol Zool 64: 1137–1164. 10.1086/physzool.64.5.30156237. [DOI] [Google Scholar]
- Farrell AP (2009) Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J Exp Biol 212: 3771–3780. 10.1242/jeb.023671. [DOI] [PubMed] [Google Scholar]
- Farrell AP (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88: 322–343. 10.1111/jfb.12789. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT (2008) Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81: 697–709. 10.1086/592057. [DOI] [PubMed] [Google Scholar]
- Ferreira EO, Anttila K, Farrell AP (2014) Thermal optima and tolerance in the eurythermic goldfish (Carassius auratus): relationships between whole-animal aerobic capacity and maximum heart rate. Physiol Biochem Zool 87: 599–611. 10.1086/677317. [DOI] [PubMed] [Google Scholar]
- Fiksen Ø, Reglero P (2022) Atlantic bluefin tuna spawn early to avoid metabolic meltdown in larvae. Ecology 103: e03568. 10.1002/ecy.3568. [DOI] [PubMed] [Google Scholar]
- Forsman A, Wennersten L (2016) Inter-individual variation promotes ecological success of populations and species: evidence from experimental and comparative studies. Ecography 39: 630–648. 10.1111/ecog.01357. [DOI] [Google Scholar]
- Fry F, Hart JS (1948) The relation of temperature to oxygen consumption in the goldfish. Biol Bull 94: 66–77. 10.2307/1538211. [DOI] [PubMed] [Google Scholar]
- Galloway TF, Kjørsvik E, Kryvi H (1998) Effect of temperature on viability and axial muscle development in embryos and yolk sac larvae of the Northeast Arctic cod (Gadus morhua). Mar Biol 132: 559–567. 10.1007/s002270050421. [DOI] [Google Scholar]
- Gamperl AK, Swafford BL, Rodnick KJ (2011) Elevated temperature, per se, does not limit the ability of rainbow trout to increase stroke volume. J Therm Biol 36: 7–14. 10.1016/j.jtherbio.2010.08.007. [DOI] [Google Scholar]
- Garwood RS (2017) Historic and contemporary distribution of Longfin smelt (Spirinchus thaleichthys) along the California coast. Calif Fish Game 103: 96–117. [Google Scholar]
- Ghil M, Vautard R (1991) Interdecadal oscillations and the warming trend in global temperature time series. Nature 350: 324–327. 10.1038/350324a0. [DOI] [Google Scholar]
- Goetz FW, Anulacion BF, Arkoosh MR, Cook MA, Dickhoff WW, Dietrich JP, Fairgrieve WT, Hayman ES, Hicks MBR, Jensen Cet al. (2021) Status of sablefish, Anoplopoma fimbria, aquaculture. J World Aquac Soc 52: 607–646. 10.1111/jwas.12769. [DOI] [Google Scholar]
- Grimaldo L, Burns J, Miller RE, Kalmbach A, Smith A, Hassrick J, Brennan C (2020) Forage fish larvae distribution and habitat use during contrasting years of low and high freshwater flow in the San Francisco estuary. SFEWS 18: 6. 10.15447/sfews.2020v18iss3art5. [DOI] [Google Scholar]
- Grimaldo L, Feyrer F, Burns J, Maniscalco D (2017) Sampling uncharted waters: examining rearing habitat of larval Longfin smelt (Spirinchus thaleichthys) in the upper San Francisco estuary. Estuaries and Coasts 40: 1771–1784. 10.1007/s12237-017-0255-9. [DOI] [Google Scholar]
- Groover EM, Alo MM, Ramee SW, Lipscomb TN, Degidio JML, DiMaggio MA (2021) Development of early larviculture protocols for the melanurus wrasse Halichoeres melanurus. Aquaculture 530: 735682. 10.1016/j.aquaculture.2020.735682. [DOI] [Google Scholar]
- Gross E, Kimmerer W, Korman J, Lewis L, Burdick S, Grimaldo L (2022) Hatching distribution, abundance, and losses to freshwater diversions of longfin smelt inferred using hydrodynamic and particle-tracking models. Mar Ecol Prog Ser 700: 179–196. 10.3354/meps14168. [DOI] [Google Scholar]
- Gunnarsson S, Imsland AK, Árnason J, Gústavsson A, Arnarson I, Jónsson JK, Foss A, Stefansson S, Thorarensen H (2011) Effect of rearing temperatures on the growth and maturation of Arctic charr (Salvelinus alpinus) during juvenile and on-growing periods. Aquacult Res 42: 221–229. 10.1111/j.1365-2109.2010.02615.x. [DOI] [Google Scholar]
- Guo C, Ito SI, Yoneda M, Kitano H, Kaneko H, Enomoto M, Aono T, Nakamura M, Kitagawa T, Wegner NCet al. (2021) Fish specialize their metabolic performance to maximize bioenergetic efficiency in their local environment: conspecific comparison between two stocks of Pacific chub mackerel (Scomber japonicus). Front Mar Sci 8: 613965. 10.3389/fmars.2021.613965. [DOI] [Google Scholar]
- Hjort J (1914) Fluctuations in the Great Fisheries of Northern Europe Viewed in the Light of Biological Research, Vol. 20. ICES Conseil Permanent International pour l'Exploration de la Mer, Copenhagen, Denmark, pp. 1–228 [Google Scholar]
- Hobbs J, Moyle PB, Fangue N, Connon RE (2017) Is extinction inevitable for Delta smelt and Longfin smelt? An opinion and recommendations for recovery. San Fran Estuar Watershed Sci 15: 2. 10.15447/sfews.2017v15iss2art2. [DOI] [Google Scholar]
- Holt GJ, Faulk CK, Schwarz MH (2007) A review of the larviculture of cobia Rachycentron canadum, a warm water marine fish. Aquaculture 268: 181–187. 10.1016/j.aquaculture.2007.04.039. [DOI] [Google Scholar]
- Houde ED (1994) Differences between marine and freshwater fish larvae: implications for recruitment. ICES J Mar Sci 51: 91–97. 10.1006/jmsc.1994.1008. [DOI] [Google Scholar]
- Johnson PC (2014) Extension of Nakagawa & Schielzeth's R2GLMM to random slopes models. Methods Ecol Evol 5: 944–946. 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson B, Jonsson N (2014) Early environment influences later performance in fishes. J Fish Biol 85: 151–188. 10.1111/jfb.12432. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Jonsson N (2019) Phenotypic plasticity and epigenetics of fish: embryo temperature affects later-developing lift-history traits. Aquat Biol 28: 21–32. 10.3354/ab00707. [DOI] [Google Scholar]
- Karppinen P, Jounela P, Huusko R, Erkinaro J (2014) Effects of release timing on migration behaviour and survival of hatchery-reared Atlantic salmon smolts in a regulated river. Ecol Freshw Fish 23: 438–452. 10.1111/eff.12097. [DOI] [Google Scholar]
- Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc R Soc B 274: 431–438. 10.1098/rspb.2006.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N (2002) Natural and management influences on freshwater inflows and salinity in the San Francisco estuary at monthly to interannual scales. Water Resour Res 38: 25–21. 10.1029/2001WR000360. [DOI] [Google Scholar]
- Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue NA (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol 2: cou008. 10.1093/conphys/cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hung SS, Fangue NA, Haller L, Verhille CE, Zhao J, Todgham AE (2016) Effects of feed restriction on the upper temperature tolerance and heat shock response in juvenile green and white sturgeon. Comp Biochem Physiol A Mol Integr Physiol 198: 87–95. 10.1016/j.cbpa.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69: 1–33. 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- Lewis LS, Willmes M, Barros A, Crain PK, Hobbs JA (2020) Newly discovered spawning and recruitment of threatened Longfin smelt in restored and underexplored tidal wetlands. Ecology 101: e02868. 10.1002/ecy.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo VK, Martin BT, Danner EM, Cocherell DE, Cech JJ Jr, Fangue NA (2022) The effect of temperature on specific dynamic action of juvenile fall-run Chinook salmon, Oncorhynchus tshawytscha. Conserv Physiol 10: coac067. 10.1093/conphys/coac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey MD, Hutchings JA (2021) Thermal variability during ectotherm egg incubation: a synthesis and framework. J Exp Zool A: Ecol Integr Physiol 335: 59–71. 10.1002/jez.2400. [DOI] [PubMed] [Google Scholar]
- McInturf AG, Zillig KW, Cook K, Fukumoto J, Jones A, Patterson E, Cocherell DE, Michel CJ, Caillaud D, Fangue NA (2022) In hot water? Assessing the link between fundamental thermal physiology and predation of juvenile Chinook salmon. Ecosphere 13: e4264. 10.1002/ecs2.4264. [DOI] [Google Scholar]
- Moyano M, Candebat C, Ruhbaum Y, Álvarez-Fernández S, Claireaux G, Zambonino-Infante J-L, Peck MA. (2017). Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PloS One 12: e0179928. 10.1371/journal.pone.0179928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano M, Illing B, Polte P, Kotterba P, Zablotski Y, Gröhsler T, Hüdepohl P, Cooke SJ, Peck MA (2020) Linking individual physiological indicators to the productivity of fish populations: a case study of Atlantic herring. Ecol Indic 113: 106146. 10.1016/j.ecolind.2020.106146. [DOI] [Google Scholar]
- Moyle PB (2002) Inland Fishes of California. Univ of California Press, Berkeley, California [Google Scholar]
- Mueller CA, Eme J, Manzon RG, Somers CM, Boreham DR, Wilson JY (2015) Embryonic critical windows: changes in incubation temperature alter survival, hatchling phenotype, and cost of development in lake whitefish (Coregonus clupeaformis). J Comp Physiol B 185: 315–331. 10.1007/s00360-015-0886-8. [DOI] [PubMed] [Google Scholar]
- Muggeo VM (2017) Interval estimation for the breakpoint in segmented regression: a smoothed score-based approach. Aust N Z J Stat 59: 311–322. 10.1111/anzs.12200. https://cran.r-project.org/doc/Rnews/. [DOI] [Google Scholar]
- Muir CA, Bork BS, Neff BD, Damjanovski S (2022a) Proteomic analysis of temperature-dependent developmental plasticity within the ventricle of juvenile Atlantic salmon (Salmo salar). Current Res Physiol 5: 344–354. 10.1016/j.crphys.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir CA, Garner SR, Damjanovski S, Neff BD (2022b) Temperature-dependent plasticity mediates heart morphology and thermal performance of cardiac function in juvenile Atlantic salmon (Salmo salar). J Exp Biol 225: jeb.244305. 10.1242/jeb.244305. [DOI] [PubMed] [Google Scholar]
- Mundy PC, Carte MF, Brander SM, Hung TC, Fangue N, Connon RE (2020) Bifenthrin exposure causes hyperactivity in early larval stages of an endangered fish species at concentrations that occur during their hatching season. Aquat Toxicol 228: 105611. 10.1016/j.aquatox.2020.105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4: 133–142. 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- Peck MA, Moyano M (2016) Measuring respiration rates in marine fish larvae: challenges and advances. J Fish Biol 88: 173–205. 10.1111/jfb.12810. [DOI] [PubMed] [Google Scholar]
- Pepin P (1991) Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can J Fish Aquat Sci 48: 503–518. 10.1139/f91-065. [DOI] [Google Scholar]
- Pörtner H (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88: 137–146. 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Bock C, Mark FC (2017) Oxygen-and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol 220: 2685–2696. 10.1242/jeb.134585. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. 10.1126/science.1163156. 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- Price TD, Qvarnström A, Irwin DE (2003) The role of phenotypic plasticity in driving genetic evolution. Proc Biol Sci 270: 1433–1440. 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ [Google Scholar]
- Reynolds WW, Casterlin ME (1979) Behavioral thermoregulation and the “final preferendum” paradigm. Am Zool 19: 211–224. 10.1093/icb/19.1.211. [DOI] [Google Scholar]
- Rosenfield JA, Baxter RD (2007) Population dynamics and distribution patterns of longfin smelt in the San Francisco estuary. Trans Am Fish Soc 136: 1577–1592. 10.1577/T06-148.1. [DOI] [Google Scholar]
- Saglam IK, Hobbs JA, Baxter R, Lewis LS, Benjamin A, Finger AJ (2021) Genome-wide analysis reveals regional patterns of drift, structure, and gene flow in longfin smelt (Spirinchus thaleichthys) in the northeastern Pacific. Can J Fish Aquat Sci 78: 1793–1804. 10.1139/cjfas-2021-0005. [DOI] [Google Scholar]
- Schaefer J, Ryan A (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J Fish Biol 69: 722–734. 10.1111/j.1095-8649.2006.01145.x. [DOI] [Google Scholar]
- Scott GR, Johnston IA (2012) Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc Nat Acad Sci 109: 14247–14252. 10.1073/pnas.1205012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RJ (2001) Larviculture of marine finfish in Europe. Aquaculture 200: 55–88. 10.1016/S0044-8486(01)00694-9. [DOI] [Google Scholar]
- Sidhu R, Anttila K, Farrell AP (2014) Upper thermal tolerance of closely related Danio species. J Fish Biol 84: 982–995. 10.1111/jfb.12339. [DOI] [PubMed] [Google Scholar]
- Sifa L, Mathias JA (1987) The critical period of high mortality of larvae fish—a discussion based on current research. Chinese J Oceanol Limnol 5: 80–96. 10.1007/BF02848526. [DOI] [Google Scholar]
- Sommer T, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer F, Gingras M, Herbold Bet al. (2007) The collapse of pelagic fishes in the upper San Francisco estuary: El colapso de los peces pelagicos en la cabecera del Estuario San Francisco. Fisheries 32: 270–277. 10.1577/1548-8446(2007)32[270:TCOPFI]2.0.CO;2. [DOI] [Google Scholar]
- Sponaugle S, Grorud-Colvert K, Pinkard D (2006) Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida keys. Mar Ecol Prog Ser 308: 1–15. 10.3354/meps308001. [DOI] [Google Scholar]
- Steinhausen MF, Sandblom E, Eliason EJ, Verhille C, Farrell AP (2008) The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J Exp Biol 211: 3915–3926. 10.1242/jeb.019281. [DOI] [PubMed] [Google Scholar]
- Symonds JE, Walker SP, Pether S, Gublin Y, McQueen D, King A, Irvine GW, Setiawan AN, Forsythe JA, Bruce M (2014) Developing yellowtail kingfish (Seriola lalandi) and hāpuku (Polyprion oxygeneios) for New Zealand aquaculture. N.Z. J Mar Fresh 48: 371–384. 10.1080/00288330.2014.930050. [DOI] [Google Scholar]
- Tsai YJJ, Chase SN, Hung TC (2021) Validating the use of sodium hypochlorite for egg detachment and photograph-based egg counting in Delta smelt. Aquacult Res 52: 5936–5940. 10.1111/are.15472. [DOI] [Google Scholar]
- United States Fish and Wildlife Service (USFWS) (2023) Endangered and Threatened Wildlife and Plants; Endangered Species Status for the San Francisco Bay-Delta Distinct Population Segment of the Longfin Smelt. United States Fish and Wildlife Service, Washington DC, United States [Google Scholar]
- Venables, WN, Ripley, BD (2002). Modern Applied Statistics with S, Fourth edition. Springer, New York. ISBN 0-387-95457-0, http://www.stats.ox.ac.uk/pub/MASS4/, 10.1007/978-0-387-21706-2 [DOI] [Google Scholar]
- Verhille C, Anttila K, Farrell AP (2013) A heart to heart on temperature: impaired temperature tolerance of triploid rainbow trout (Oncorhynchus mykiss) due to early onset of cardiac arrhythmia. Comp Biochem Physiol A Mol Integr Physiol 164: 653–657. 10.1016/j.cbpa.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Victor BC (1986) Larval settlement and juvenile mortality in a recruitment-limited coral reef fish population. Ecol Monogr 56: 145–160. 10.2307/1942506. [DOI] [Google Scholar]
- Wickham H, Chang W, Wickham MH (2016) Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2: 1–189. [Google Scholar]
- Winder M, Jassby AD (2011) Shifts in zooplankton community structure: implications for food web processes in the upper San Francisco estuary. Estuar Coast 34: 675–690. 10.1007/s12237-010-9342-x. [DOI] [Google Scholar]
- Wood S (2017) Generalized Additive Models: An Introduction with R, Ed2nd. Chapman and Hall/CRC, Boca Raton, Florida, United States [Google Scholar]
- Yanagitsuru YR, Davis BE, Baerwald MR, Sommer TR, Fangue NA (2022) Using physiology to recover imperiled smelt species. In Fangue NA, Cooke SJ, Farrell AP, Brauner CJ, Eliason EJ eds, Fish Physiol. 39B. Academic Press, Cambridge, Massachusetts, United States, pp. 1–38 [Google Scholar]
- Yanagitsuru YR, Main MA, Lewis LS, Hobbs JA, Hung TC, Connon RE, Fangue NA (2021) Effects of temperature on hatching and growth performance of embryos and yolk-sac larvae of a threatened estuarine fish: Longfin smelt (Spirinchus thaleichthys). Annual review of fish diseases 537: 736502. 10.1016/j.aquaculture.2021.736502. [DOI] [Google Scholar]
- Zillig KW, Lusardi RA, Cocherell DE, Fangue NA (2023) Interpopulation variation in thermal physiology among seasonal runs of Chinook salmon. Fisheries Research Board of Canada Journal of the Fisheries Research Board of Canada 80: 1–13. 10.1139/cjfas-2022-0133. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this paper are available in the Dryad data depository: https://doi.org/10.25338/B8H06D.