Abstract
Background
In this study, we investigated the effect of preservation of the pulmonary branches of the vagus nerve during systematic dissection of mediastinal lymph nodes, when performing radical resection of lung cancer, on the postoperative complication rate.
Methods
The clinical data for 80 patients who underwent three-dimensional thoracoscopic radical resection of lung cancer in the Department of Thoracic Surgery at Huizhou Municipal Central Hospital between 2020 and 2022 were analyzed. The patients were divided into two groups according to whether the pulmonary branches of the vagus nerve were retained during intraoperative carinal lymph node dissection. The operation time, time until first postoperative defecation, duration for which a chest tube was needed, total chest drainage volume, average pain intensity during the first 5 postoperative days, incidence of postoperative pneumonia, and postoperative length of stay were compared between the two groups.
Results
There was no statistically significant difference in histological staging or in time until first postoperative defecation between the two groups (p > 0.05). However, there were significant differences in operation time, the duration for which a chest tube was needed, total chest drainage volume, average pain intensity during the first 5 postoperative days, white blood cell count and procalcitonin level on postoperative days 1 and 5, and postoperative length of stay between the two groups (p < 0.05).
Conclusion
Preserving the pulmonary branches of the vagus nerve during carinal lymph node dissection when performing three-dimensional thoracoscopic radical resection of lung cancer can reduce the risk of postoperative complications.
Keywords: Three-dimensional thoracoscopy, Pulmonary branches of vagus nerve, Radical resection of lung cancer, Postoperative rehabilitation
Background
The vagus nerve is the tenth cranial nerve and contains mixed afferent (sensory), efferent (motor), and parasympathetic nerve fibers that innervate several major organs, including the liver, lungs, spleen, kidneys, and gut. The vagus nerve also plays an important role in regulation of heart rate [1–3]. The pulmonary branches of the vagus nerve is the parasympathetic nerve of the lung and is distributed in the bronchial smooth muscle, glands, and vascular wall. Inferior to the azygos arch, the main branches of the vagus nerve pass posteromedially to the main right bronchus and pulmonary hilum; they then branch off, forming the posterior pulmonary plexus, which contains 77% (62–100%) of the total nerve supply to the right lung [4–6]. Stimulation of the corresponding receptors leads to contraction of bronchial smooth muscle, glandular secretions, vascular congestion, and mucosal swelling, promoting timely expectoration of sputum and preventing postoperative pulmonary infection [7, 8]. Perioperative pulmonary complications have several causes, including damage to the vagus nerve during thoracic surgery, severe pain caused by injury to the intercostal nerve, impaired postoperative lung function, and fear of coughing. Several studies in China and other countries have confirmed that preserving the vagus nerve during radical resection of upper gastrointestinal cancer can effectively reduce postoperative abdominal distention, constipation, vomiting, and other complications [4, 9, 10]. However, few studies have focused on complications after radical resection of lung cancer with preservation of the vagus nerve. In this study, we investigated the effect of preservation of the pulmonary branches of the vagus nerve during systematic dissection of the mediastinal lymph nodes on the postoperative complication rate after radical resection of lung cancer.
Methods
Clinical data and grouping
The clinical data for 80 patients who underwent three-dimensional (3D) thoracoscopic radical resection of lung cancer in the Department of Thoracic Surgery at Huizhou Municipal Central Hospital between 2020 and 2022 were analyzed. The patients were divided into two groups: those in whom the pulmonary branches of the vagus nerve was preserved during intraoperative carinal lymph node dissection (group A) and those in whom the pulmonary branches was not preserved (group B).
The inclusion criteria were as follows: age 18–65 years; primary lung cancer requiring radical surgery; and 3D thoracoscopic radical resection of lung cancer performed. The following exclusion criteria were applied: benign pulmonary disease not requiring lymph node dissection; advanced lung cancer requiring only wedge resection; serious underlying disease of the heart, liver, kidney, or other organ affecting postoperative recovery and prolonging the hospital stay; preoperative pulmonary infection or long-term abdominal discomfort, such as abdominal distension or diarrhea; an Eastern Cooperative Oncology Group score of 3 or 4; requirement for conversion to open surgery ; and injury or incision of the pulmonary branches of the vagus nerve during surgery.
Surgical approach
Radical surgery for lung cancer was performed using a 3D thoracoscope (Karl Storz SE & Co. KG, Tuttlingen, Germany). Double-lumen endotracheal intubation anesthesia was performed in both groups. Minimally invasive surgery was performed by double-hole thoracoscopy with the patient in the lateral decubitus position. The main surgical access incision was at the level of the third or fourth intercostal space in the midaxillary line and was approximately 3–4 cm in length. The exploratory incision was at the level of the sixth or seventh intercostal space in the axillary line and was approximately 1 cm in length. The thoracoscope and other instruments were inserted, and the pulmonary ligament and mediastinal pleura of the hilum were divided. The pulmonary artery, vein, bronchus, and fissure were completely dissected and transected with a stapler (Endo GIA; Medtronic, Minneapolis, MN, USA), and the hilar mediastinal lymph nodes were systematically dissected. The pulmonary branches of the vagus nerve was either preserved (group A) or not preserved (group B). The exposed left and right pulmonary brancheses of the vagus nerve during mediastinal lymph node dissection are shown in Figs. 1 and 2, respectively. No sealing device was used in either group. Two indwelling #16 chest tubes were placed for postoperative drainage. The chest tubes were removed when chest radiographs showed that the lung had completely reexpansion, no air leak was present, the drainage fluid was a light red or light yellow clear liquid, and the drainage volume was < 200 mL/day.
Fig. 1.
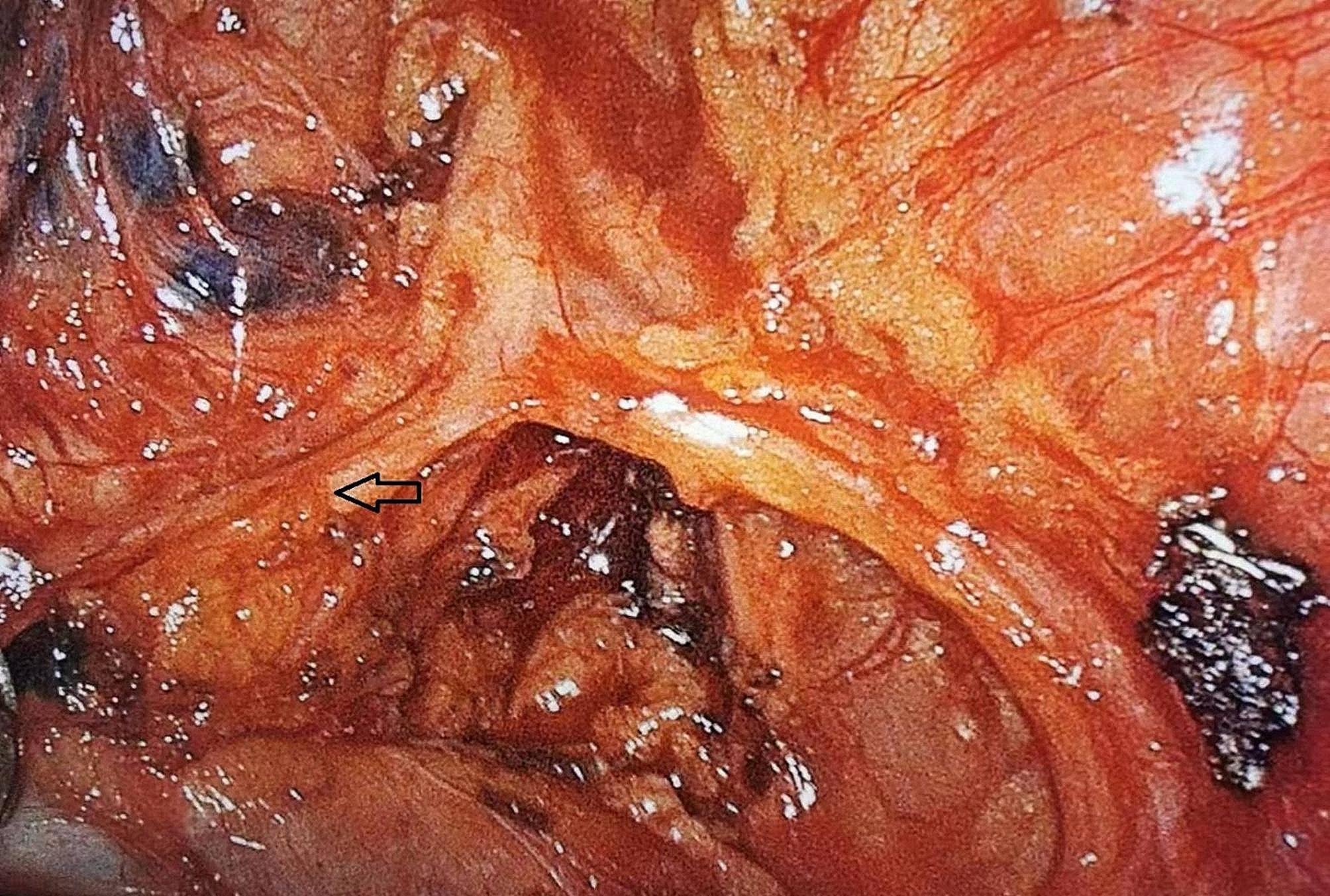
Exposure of the left pulmonary branches of the vagus nerve (arrow) during mediastinal lymph node dissection
Fig. 2.
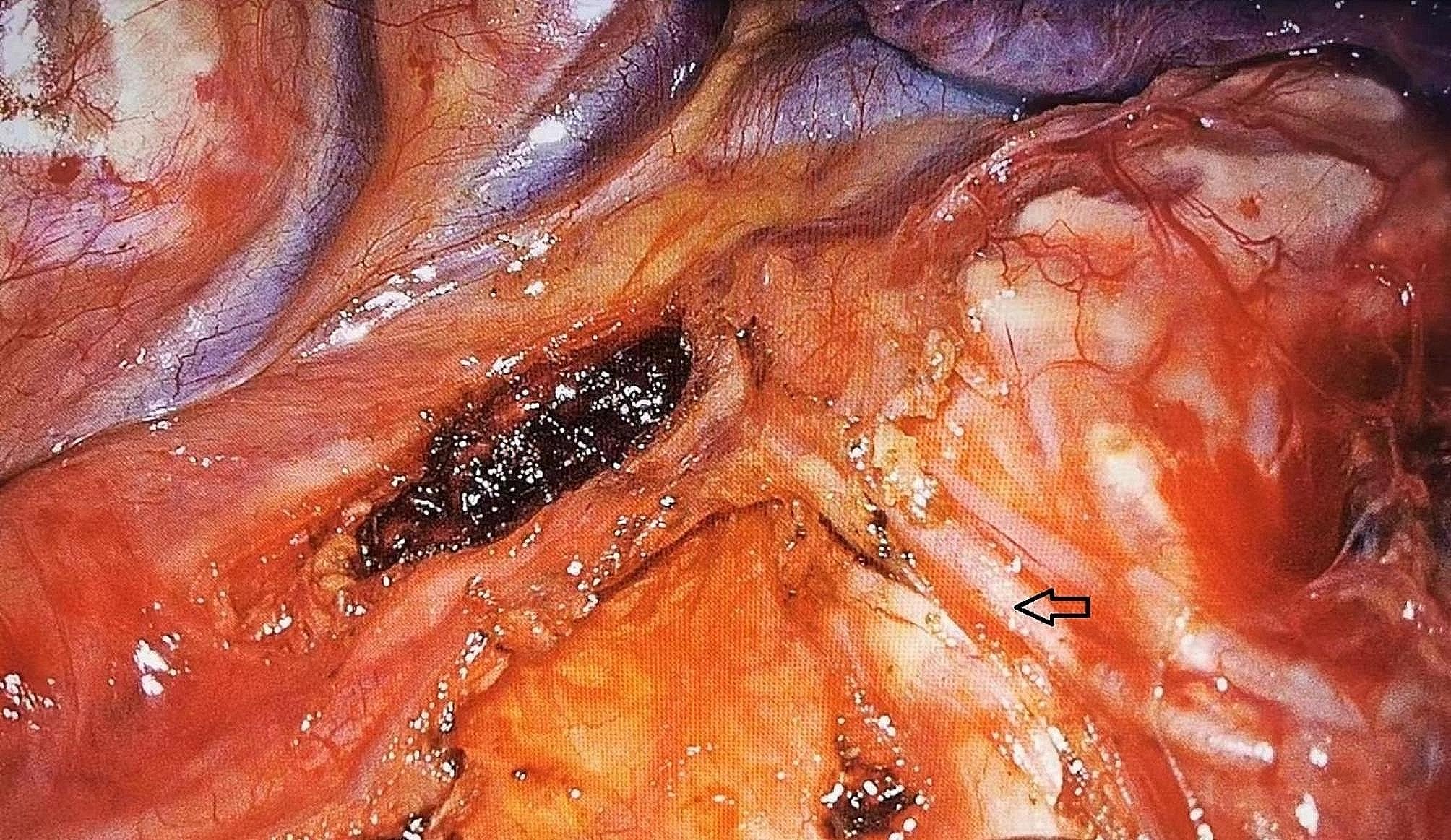
Exposure of the right pulmonary branches of the vagus nerve (arrow) during mediastinal lymph node dissection
Clinical data
The following data were compared between the two groups: operation time, histological stage, time until first postoperative defecation, duration for which a chest tube was needed, total chest drainage volume, average pain intensity during the first 5 postoperative days, incidence of postoperative pneumonia, and postoperative length of stay. The severity of postoperative pain was scored using an 11-point visual analog scale (0, no pain; 10, severe pain). Postoperative pneumonia was diagnosed by radiographic evidence of pulmonary exudate and an increased white blood cell (WBC) count and procalcitonin level in peripheral blood. The WBC count and procalcitonin level were recorded on postoperative days 1 and 5.
Statistical analysis
Quantitative data are expressed as the mean ± standard deviation and were compared between the two groups using the t-test if normally distributed and the rank sum test if not normally distributed. Qualitative data are expressed as the number and percentage and were compared between the two groups using the chi-squared test. All statistical analyses were performed using IBM SPSS Statistics Version 22 (IBM Corp., Armonk, NY, USA). A p-value of < 0.05 was considered statistically significant.
Results
The patients’ demographic and clinical data are shown in Table 1. There was no statistically significant difference in age, sex, weight, preoperative WBC count, histological stage, or time to first postoperative defecation between the two groups (p > 0.05). However, there were significant between-group differences in operation time, duration for which a chest tube was needed, total chest drainage volume, average pain intensity during the first 5 days, WBC count and procalcitonin level on postoperative days 1 and 5, and postoperative length of stay (p < 0.05).
Table 1.
Demographic and clinical data for the two study groups
| All patients n = 80 |
Group A n = 40 |
Group B n = 40 |
p value | |
|---|---|---|---|---|
| Mean age,years | 59.58 ± 10.112 | 59.05 ± 11.204 | 60.1 ± 9.001 | 0.645 |
| Sex (male, %) | 36(45%) | 17(42.5%) | 19(47.5%) | 0.653 |
| Mean weights(kg) | 59.12 ± 8.991 | 59.75 ± 7.801 | 58.49 ± 10.103 | 0.534 |
| Preoperative WBC(10^9/L) | 6.71 ± 2.29 | 6.49 ± 1.96 | 6.94 ± 2.59 | 0.386 |
| Operation time (mean hours ± SD) | 3.47 ± 0.782 | 3.76 ± 0.745 | 3.19 ± 0.715 | 0.001 |
| Histological Stage(n,%) | 0.599 | |||
| I A | 32(40%) | 17(42.5%) | 15(37.5%) | |
| I B | 32(40%) | 16(40%) | 16(40%) | |
| II B | 6(7.5%) | 2(5%) | 4(10%) | |
| III A | 10(12.5%) | 5(12.5%) | 5(12.5%) | |
| Time to first postoperative defecation (mean day ± SD) | 2.89 ± 2.365 | 2.88 ± 1.305 | 2.9 ± 3.103 | 0.237 |
| Chest tube duration time(mean day ± SD) | 4.7 ± 3.188 | 3.28 ± 1.502 | 6.13 ± 3.763 | 0.001 |
| Total chest drainage volume(ml ± SD) | 979.88 ± 678.675 | 653 ± 289 | 1306 ± 794 | 0.001 |
| Average pain intensity during the first 5 days (mean intensity ± SD) | 2.06 ± 0.789 | 1.76 ± 0.682 | 2.36 ± 0.78 | 0.001 |
| 1st day’s WBC(10^9/L) | 16.75 ± 4.146 | 15.59 ± 3.949 | 17.91 ± 4.058 | 0.01 |
| 1st day’s PCT(ng/ml) | 1.324 ± 3.065 | 0.502 ± 0.53 | 2.146 ± 4.167 | 0.001 |
| 5th day’s WBC(10^9/L) | 11.33 ± 3.408 | 9.93 ± 3.176 | 12.74 ± 3.067 | 0.001 |
| 5th day’s PCT(ng/ml) | 0.500 ± 0.670 | 0.263 ± 0.230 | 0.737 ± 0.861 | 0.001 |
|
Postoperative length of stay (mean day ± SD) |
11.19 ± 4.198 | 11.7 ± 3.376 | 10.68 ± 4.875 | 0.016 |
PCT, procalcitonin; SD, standard deviation; WBC, white blood cell
Discussion
In this study, we found a significantly longer mean operation time in group A than in group B despite no significant between-group difference in age, sex, weight, preoperative WBC count, or histological stage. This finding suggests that preserving the pulmonary branches of the vagus nerve during intraoperative dissection of the carinal lymph nodes increases the difficulty of surgery.
The vagus nerve releases acetylcholine (ACh) and promotes contraction of gastrointestinal smooth muscle, resulting in defecation symptoms. Therefore, preserving the vagus nerve during radical resection of a malignant upper gastrointestinal tumor can effectively reduce the likelihood of postoperative abdominal distention and constipation [9]. However, in the present study, we found no significant difference in the time to first postoperative defecation between the two study groups. We attribute this finding to the fact that the patients’ digestive tract symptoms were controlled mainly by the abdominal branch of the vagus nerve; therefore, intraoperative resection of the pulmonary branches of the vagus nerve did not have a significant impact.
There is some evidence indicating that vagotomy can lower the threshold of the nociceptive reflex associated with mechanical retraction, indicating that release of ACh by the vagus nerve can protect against postoperative pain [11]. In our present study, the average pain intensity score was lower in group A than in group B during the first 5 days after surgery, which is consistent with the findings of the previous report [11].
The WBC count and procalcitonin level were lower in group A than in group B on postoperative days 1 and 5, indicating that the vagus nerve plays an important role in the regulation of systemic and local inflammatory responses and can reduce the risk of inflammation [12]. It has been hypothesized that vagal receptors are activated by local proinflammatory factors that transmit signals to the central nervous system through afferent nerves and release ACh through efferent nerves, which are known to produce this neurotransmitter [13]. ACh inhibits the ability of immune cells to produce proinflammatory factors by activating α7 nicotinic ACh receptors (α7nAChR) on these cells [14]. When the vagal circuits are intact, activation of α7nAChR promotes phosphorylation of AKT1 in splenic α7nAChR-expressing CD11b + granulocytes and confines these cells to the spleen. Previous research found that disruption of vagal circuits reduced phosphorylation of AKT1 in splenic α7nAChR + CD11b + granulocytes and facilitated egress of these cells from the spleen to the lung [15]. Moreover, without functional vagal circuits or α7nAChR agonist stimulation, α7nAChR + CD11b + cells accumulated in lungs challenged by lipopolysaccharides or Escherichia coli, failed to clear bacteria, and promoted an inflammatory response. ACh also acts on the trachea and bronchial glands, which can result in contraction of bronchial smooth muscle, promote secretion of mucus and timely expectoration, and prevent postoperative accumulation of sputum, thereby reducing the risk of pulmonary infection [7, 8].
The duration for which a chest tube was needed was shorter and the total chest drainage volume was smaller in group A than in group B. We attribute this to the reduction of local inflammation of the lungs and pleura by preservation of the vagus nerve. This decrease in inflammation could reduce the permeability of the pleural capillaries and reduce infiltration of cells, proteins, and fluids from capillaries into the pleural cavity, thereby shortening the length of time for which a postoperative chest tube is needed and reducing the risk of infection in the pleural cavity.
Finally, the postoperative length of stay was shorter in group B than in group A. We consider that the time of discharge from hospital would have been affected by subjective factors, such as the patient’s willingness to be discharged.
Conclusion
Preserving the pulmonary branches of the vagus nerve during dissection of the carinal lymph nodes when performing 3D thoracoscopic radical resection of lung cancer can reduce the likelihood of postoperative complications. Our study is not without limitations. Retrospective analyses introduce potential bias, so we cannot establish causality. Second, only 80 patients were able to be included in the study, which also limits the power and generalizability of our conclusions.
Acknowledgements
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Abbreviations
- 3D
Three-dimensional
- WBC
White blood cell
- PCT
Procalcitonin
- ACh
Acetylcholine
Author contributions
Wencong Huang conceived the idea underpinning this research and drafted the manuscript. Jiantian Yang and Huiwen Chen collected the data and performed the statistical analysis. Wei Wei performed the operations and supervised the study. Peijian Li performed the operations. All authors read and approved the final manuscript.
Funding
The study was supported by the Huizhou Municipal Central Hospital Key Support Research Project Foundation.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Huizhou Municipal Central Hospital. All patients and their family members provided written informed consent before surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tracey KJ. The inflammatory reflex[J] Nature. 2002;420(6917):853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Song NLJ, Bogdan M, Juan G, Rafael P, Yu J. The vagus nerve and pulmonary disease. Fudan Univ J Med Sci. 2012;39:117–22. [Google Scholar]
- 3.DeMeester SR. New options for the therapy of Barrett’s high-grade dysplasia and intramucosal adenocarcinoma: endoscopic mucosal resection and ablation versus vagal-sparing esophagectomy[J] Ann Thorac Surg. 2008;85(2):747–S750. doi: 10.1016/j.athoracsur.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 4.Weijs TJ, Ruurda JP, Luyer MD, et al. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy[J] J Anat. 2015;227(4):431–9. doi: 10.1111/joa.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Li J, Liu G, et al. Nerves of the mediastinum[J] Thorac Surg Clin. 2011;21(2):239–49. doi: 10.1016/j.thorsurg.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Netter F. Netter’s Atlas of Human Anatomy 6/e - Professional Edition[J]. 2014.
- 7.Akiyama H, Tsurumaru M, Kawamura T, et al. Esophageal stripping with preservation of the vagus nerve[J] Int Surg. 1982;67(2):125–8. [PubMed] [Google Scholar]
- 8.Mazzone SB, Canning BJ. Autonomic neural control of the airways[J] Handb Clin Neurol. 2013;117:215–28. doi: 10.1016/B978-0-444-53491-0.00018-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Zhang Y, Du Z, et al. Vagal efferent fiber stimulation ameliorates pulmonary microvascular endothelial cell injury by downregulating inflammatory responses[J] Inflammation. 2013;36(6):1567–75. doi: 10.1007/s10753-013-9701-4. [DOI] [PubMed] [Google Scholar]
- 10.Weijs TJ, Ruurda JP, Luyer MD, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy[J] Surg Endosc. 2016;30(9):3816–22. doi: 10.1007/s00464-015-4683-y. [DOI] [PubMed] [Google Scholar]
- 11.Yu H. Li Gang,Tong Dongyi.Effect of bilateral cervical vagotomy on microglialactivation in spinal cordina rat model of persistent postoperative pain. J J Chin Physician January 2015 17 no.1:55–7.
- 12.Lubbers T, de Haan JJ, Luyer MD, et al. Cholecystokinin/Cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats[J] Ann Surg. 2010;252(2):376–82. doi: 10.1097/SLA.0b013e3181dae411. [DOI] [PubMed] [Google Scholar]
- 13.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia[J] Proc Natl Acad Sci U S A. 2008;105(31):11008–13. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey KJ. Reflex control of immunity[J] Nat Rev Immunol. 2009;9(6):418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Yang X, Su EM, et al. Signals of vagal circuits engaging with AKT1 in alpha7 nAChR(+)CD11b(+) cells lessen E. Coli and LPS-induced acute inflammatory injury[J] Cell Discov. 2017;3:17009. doi: 10.1038/celldisc.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.


