Abstract
Background
The inherited X-linked disorder, Fabry disease, is caused by deficient lysosomal enzyme α-galactosidase A, with progressive accumulation of globotriaosylceramide in multiple organs including the upper and lower airways.
Objectives
To assess pulmonary function at the time of the first pulmonary function test (PFT) performed among the National Danish Fabry cohort and define the prevalence of affected lung function variables.
Materials and Method
A cross-sectional retrospective cohort study of 86 adult patients enrolled in one or both international patient registry databases for Fabry disease, Fabry Registry or FollowME with at least one PFT. The Mainz Severity Score Index (MSSI) was calculated to determine the disease severity. Lung function variables were examined by multivariate regression adjusted for important variables for developing airway illness.
Results
Seventeen patients (20%) showed obstructive airflow limitation and 7 (8%) a restrictive lung deficiency. Smoking status (p = .016) and MSSI (p < .001) were associated with increasing obstructive airway limitation.
Conclusion
The prevalence of affected lung function among the National Danish Fabry cohort was 28%. Patients with classic gene variants frequently developed a decrease in lung function regardless of their smoking status, with significant relationship with disease severity.
Keywords: Fabry disease, pulmonary involvement, respiratory impairment, pulmonary function test
Introduction
Fabry disease is an inherited X-linked recessive lysosomal storage disorder, secondary to pathogenic variants in the GLA gene, responsible for encoding the lysosomal α-galactosidase A enzyme. The classic variants comprise the severe phenotypes in males and some females due to complete or severe loss of function of α-galactosidase A. Some females with classical variants and both males and females with late-onset variants demonstrate milder phenotypes, whereas some females are asymptomatic. 1 The deficiency of the enzyme activity results in a progressive accumulation of globotriaosylceramide (Gb3) in the lysosomes of multiple organs, leading to decreased perfusion, development of fibrosis and consequently organ damage. 2 A similar mechanism is suggested to concern also the smooth muscle of upper and lower airways leading to bronchial/bronchiolar obstruction and obstructive sleep apnoea.3–5
Although many patients seem to complain about shortness of breath, 1 the evaluation of pulmonary function in Fabry disease has generally been neglected, probably because symptoms of dyspnoea in Fabry patients have often been ascribed to cardiac or kidney involvement or to general fatigue with low physical performance. Despite lack of clear recommendations, the clinical impression has changed over time, and currently there is increased attention to include pulmonary causes as possible explanation of shortness of breath in Fabry patients. However, in a study by Svensson et al. 1 where multiple literature studies on pulmonary involvement in Fabry disease were included the pulmonary function tests (PFT) showed various results, and consistent findings could not be documented to determine if there is an obstructive or a restrictive airflow limitation in Fabry patients due to the lack of a larger cohort. Few, if ever, have investigated lung diseases among Fabry patients in an unselected large cohort or Nationwide registry.
The current treatments of Fabry disease focus on replacing the absent or deficient α-galactosidase A by enzyme replacement therapy (ERT) (available since 2001) or chaperone (migalastat) (available since 2016), which alleviate and prevent deposits of Gb3 in tissues.
The primary aim of the study was to assess pulmonary function at the time of the first PFT performed among the National Danish Fabry cohort and define the prevalence of affected lung function variables. The secondary aim was to classify the type of lung disease and to study the influence of smoking status and the disease severity.
Materials and method
Study design and population
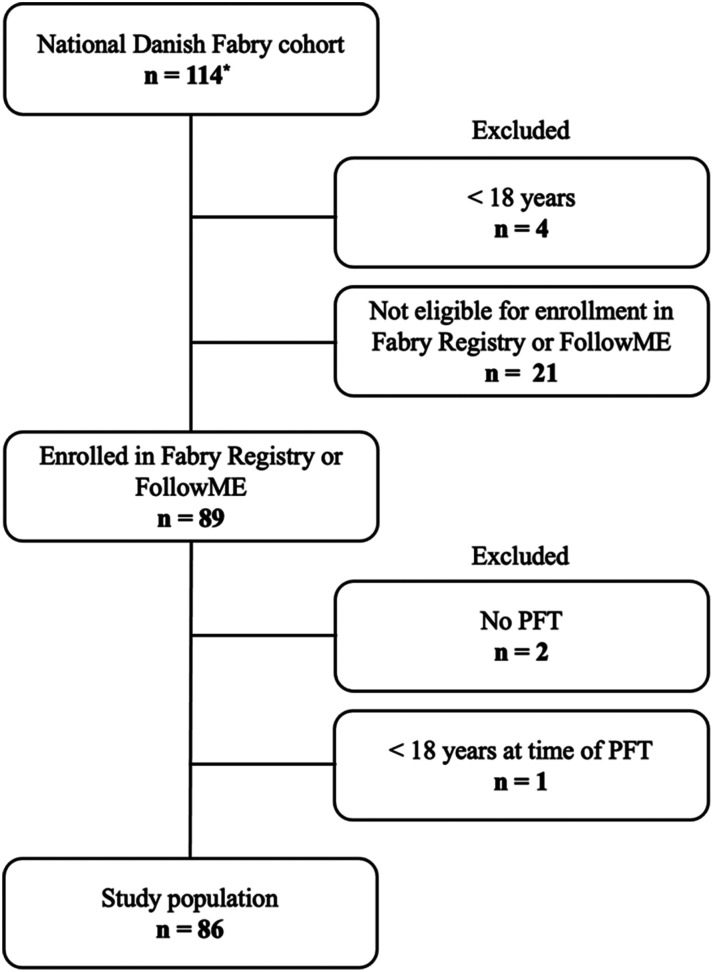
The study was an investigator initiated retrospective observational study of prospectively collected data from the National Danish Fabry cohort followed at Department of Endocrinology and Metabolism, Copenhagen University Hospital, Rigshospitalet in collaboration with other specialist departments in the Danish Fabry Team. All enrolled Danish Fabry probands and their affected family members were genetically verified. Data was included from the introduction of the first PFT as routine in 2007 (except 2 now deceased patient with PFTs in 2002 and 2003) until datalock June 2021, including data from deceased Fabry patients. Part of the data from the Fabry cohort has been published previously. 6 Patients aged 18 years and older, with at least one PFT and enrolled in one of two international patient registry databases for Fabry disease, Fabry Registry® (Genzyme-Sanofi) and FollowME® (Amicus), were included (Figure 1). Fifty percent of the patients were on ERT and 50% were not on ERT (by indication or own choice) nor migalastat (not licenced) at the time of the first PFT. The study was approved by the Danish Health and Medicine Authority (3-3013-667/1/), the Regional Health Research Ethics Committee (H-3-2014-FSP8) and the Danish Data Protection Agency 2014–641-0055 and 2013-41-1949.
Figure 1.
Patient flowchart of the National Danish Fabry cohort * includes 16 dead patients; PFT, pulmonary function test.
Pulmonary function test
All PFT were performed using the same equipment (Jaeger®), half of which (n = 45) were conducted at the Department of Respiratory Medicine, Bispebjerg Hospital, Copenhagen, Denmark, and in the periods 2007–2012 and 2020–2021 the other half (n = 41) PFTs at the Department of Clinical Physiology, Nuclear Medicine and PET, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark. All tests were completed according to American Thoracic Society’s and European Respiratory Society’s standards, 7 and performed by trained staff to ensure consistency of the procedure. A standardised calibration was performed daily at both sites. The procedure was completed in sitting position, with a nose clip, and the patient was asked to take a maximal inspiration followed by a forceful expel of air for as long as possible. Forced expiratory volume in the first-second (FEV1) and forced vital capacity (FVC) were determined. FEV1/FVC ratio was calculated, identifying obstructive or restrictive ventilatory defects. FEV1/FVC <70% indicated an obstructive defect, whereas FEV1/FVC >90% indicated a restrictive defect. 8 FEV1 and FVC values were calculated as percentages of predicted normal values, based on age, gender and height values used at both sites. 7
Smoking status
Smoking status for Fabry patients enrolled in the Fabry Registry was extracted from the registry and categorized as follows: Current smoker: currently smoking; former smoker: smoked at least 100 cigarettes over lifetime; or never smoker: not smoked at least 100 cigarettes over lifetime. The smoking status for Fabry patients enrolled in FollowME were viewed in their medical file and categorized as in Fabry Registry.
Mainz Severity Score Index
The Mainz Severity Score Index (MSSI) is a clinical scoring system to determine the severity of the signs and symptoms of Fabry disease and to monitor disease course (Supplementary Table 1). 9 It is composed of four sections that assess the general, neurological, cardiovascular and renal signs and symptoms of Fabry disease. The maximum MSSI is 76 points (maximum score of 18, 20, 20, 18 points for the general, neurological, cardiovascular and renal section, respectively). The severity of Fabry disease can be classified, based on the MSSI, as mild (score <20), moderate (score 20-40) and severe (score >40). 9 For this study, the MSSI was adjusted to exclude “characteristic facial appearance” in the general section, because clinical facial photos of dead patients were not possible to obtain. The maximum score in the general section was therefore 17 and the MSSI was maximum 75 points. Furthermore, orthostatic hypotension was rated as “mild vertigo,” cardiomyopathy >15 mm was rated as “severe cardiomyopathy (>15 mm)” and the rating “no” for the New York Heart Association (NYHA) classification was excluded. Cardiac involvement was rated positive if patients had at least one fulfilled cardiovascular variable in the MSSI.
Measurement of α-galactosidase A enzyme activity
Measurement of α-galactosidase A activity was determined in mixed leukocytes using a fluorimetric assay with 4-methylumbelliferyl-α-D-galactopyranoside as substrate.10,11 The fluorescence intensity was measured in a Fluostar Optima plate reader (BMG Labtech) at excitation and emission wavelengths of 360 nm and 450 nm, respectively.
Measurement of plasma Gb3, plasma lyso-Gb3 and urine-Gb3
Plasma-Gb3, plasma lyso-Gb3 and urine-Gb3 serve as disease severity biomarkers as aid in the diagnosis and subclassification of Fabry disease as well as treatment monitoring markers during treatment with ERT or Migalastat. Gb3 measurements were performed at Sahlgrenska University Hospital, Sweden up to 2006 by densitometric evaluation after high performance thin layer chromatography for plasma Gb3 and orcinol detection in the case of urine-Gb3 12 and afterwards in Genzyme’s laboratories, using a rapid liquid chromatography with tandem mass spectrometry method for quantification of total plasma lyso-Gb3 and urine-Gb3. 13 Gb3 units from Sahlgrenska were given in mg/L (reference: 1.6 – 3.3), while more recent measurements from Genzyme and LabCorp were given in μmol/L (reference: <7.0) and μg/mL (reference: 1.39 – 4.04), respectively. Urine-Gb3 was given in μg/mmol creatinine (reference: <81) at Genzyme, μg/mmol creatinine (reference: <16) at LabCorp and μmol/mol creatinine (reference: <10) at Sahlgrenska. The calibration of methods has changed over time, but no attempts were made to correct values for unification. Therefore, each individual measurement was compared to the respective method and laboratory derived normal reference range. Plasma lyso-Gb3 was only measured in Genzyme’s and LabCorp’s laboratory.
Statistical analysis
IBM SPSS Statistics 2017 version 25.0.0.0 was used for statistical analyses. Data are compared by conducting one-way Analysis of Variance (ANOVA). Changes in pulmonary function are examined with multivariate regression adjusted for variables of importance for developing airway illness: smoking status, cardiac involvement, respiratory medication, respiratory symptoms, MSSI and ERT. p-values less than or equal to 0.05 were considered statically significant.
Results
The patient characteristics of the 86 Fabry patients (32 males and 54 females) at the time of lung screening are listed in Table 1. Seventeen patients (20%, nine males) showed obstructive airflow limitation with an FEV1/FVC ratio ≤70% and seven patients (8%, six females) showed restrictive airflow limitation with an FEV1/FVC ratio ≥90%. The mean (SD) MSSI for the 86 Fabry patients was 23.4 (9.9). Two patients with known asthma were receiving anti-asthma treatment at the time of the PFT. Forty-three patients (50%) received ERT at the time of their first PFT (10 agalsidase-alfa and 33 agalsidase-beta), and 43 (50%) were not treated with ERT nor migalastat. The median (range) time from the initiation of ERT to the first PFT was 5.4 [0.6–16.3] years.
Table 1.
Characteristics of the 86 Fabry patients at time of first pulmonary function test between 2007 and 2021.
| Variable | n = 86 |
|---|---|
| Demographic | |
| Female | 54 (63) |
| Age (years) | 39.5 ± 15.6 |
| Height (cm) | 171.3 ± 9.0 |
| Weight (kg) | 71.7 ± 16.7 |
| BMI (kg/m2) | 24.4 ± 5.5 |
| Pulmonary function measurement | |
| FEV1 (L) | 3.0 ± 1.0 |
| FEV1 (% predicted) | 88.1 ± 14.8 |
| FVC (L) | 4.0 ± 1.1 |
| FVC (% predicted) | 98.6 ± 13.0 |
| FEV1/FVC ratio | 0.76 ± 0.01 |
| Smoking | |
| Current smoker | 27 (31) |
| Former smoker | 22 (26) |
| Never smoker | 37 (43) |
| Respiratory symptom | |
| Breathlessness | 7 (8) |
| Cough | 14 (16) |
| Wheezing | 2 (2) |
| Sputum | 3 (3) |
| Feeling pressure in the chest | 5 (6) |
| Nocturnal symptoms | 2 (2) |
| Exercise-induced dyspnoea | 31 (36) |
| MSSI | |
| Total score | 23.4 ± 9.9 |
| Mild score <20 | 35 (41) |
| Moderate score 20–40 | 48 (56) |
| Severe score >40 | 3 (3) |
| Cardiac involvement | 75 (87) |
| Lung treatment | |
| SABA | 10 (12) |
| ICS | 8 (9) |
| ICH-LABA | 11 (13) |
| Other type of lung medication | 2 (2) |
| Fabry treatment | |
| ERT | 43 (50) |
| Migalastat | 0 |
| Time from ERT initiation to first PFT (years) | 5.4 [0.6–16.3] |
Mean ± SD, n (%) or median [range]. n, number of patients; BMI: body mass index; FEV1: one-second forced expiratory volume in the first-second; FVC: forced vital capacity; FEV1/FVC ratio: forced expiratory volume in the first second divided by forced vital capacity; MSSI: Mainz Severity Score Index; SABA: short-acting β2-agonist; ICS: inhaled corticosteroids; ICS-LABA: inhaled corticosteroids combined with long-acting β2-agonist; ERT: enzyme replacement therapy; PFT: pulmonary function test.
The GLA gene pathogenic variants and α-galactosidase A enzyme activities are listed in Supplementary Table 2. Seventy-nine (92%) of the patients had GLA gene pathogenic variants classified as classic based on the International Fabry Disease Genotype-Phenotype Database (dbFGP) (https://dbfgp.org/dbFgp/fabry/), accessed November 16, 2021), 2 (2%) had GLA gene pathogenic variants classified as late-onset and 5 (6%) had previously unreported disease-causing variants in the GLA gene. All males and 35 (65%) females had an α-galactosidase A activity below normal (normal range: 20–65 nmol/h/mg protein) with a median [range] of 1.7 [0.3–11] nmol/h/mg protein and 11 [1.6–19] nmol/h/mg protein, respectively. Sixteen females had normal α-galactosidase A activity, and three females did not have available α-galactosidase A activity measurements.
Gb3 in plasma and urine was available in 73 (29 males and 49 females) and 42 (14 males and 28 females) patients, respectively. Lyso-Gb3 in plasma was available in 24 patients (11 males and 13 females). Nevertheless, males and females had plasma Gb3 concentrations, respectively, lower than the normal upper limit (high vs normal plasma Gb3: 8 (28%) versus 21 (72%); 4 (8%) versus 45 (92%)), while plasma lyso-Gb3 was, respectively, higher than the normal upper limit in males compared to females (high vs normal plasma lyso-Gb3: 9 (82%) versus 2 (18%); 2 (15%) versus 11 (85%)). Urine-Gb3 was higher than the normal upper limit in females (high vs normal urine-Gb3: 7 (50%) versus 7 (50%); 16 (57%) versus 12 (43%)).
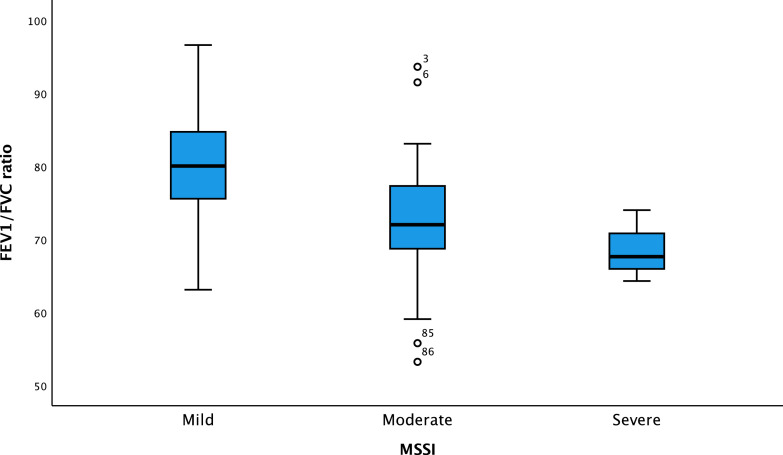
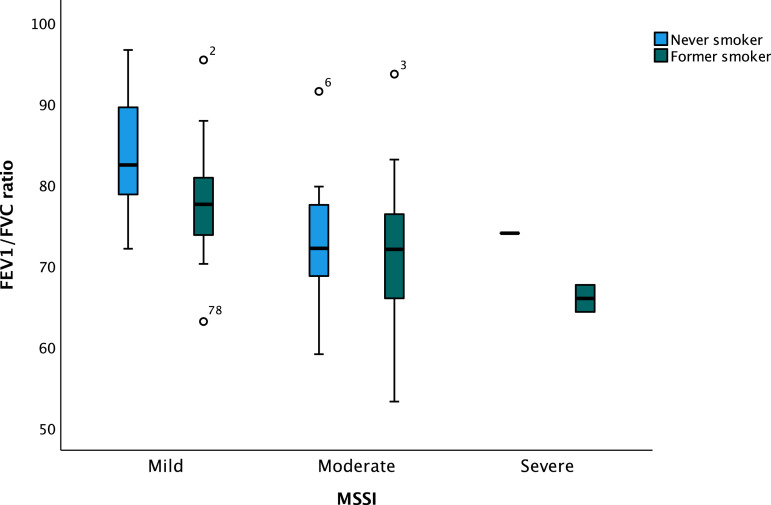
One-way ANOVA revealed that FEV1/FVC ratio was dependent on smoking status, being never (78.4 ± 8.6), former (73.5 ± 9.2) and current (74.0 ± 8.6), with a significant difference in smoking status and FEV1/FVC ratio being low value (F(1,84) = [6.002], p = .016). The analysis also revealed that FEV1/FVC ratio was dependent on MSSI being mild (80.7 ± 7.5), moderate (72.1 ± 8.3) and severe (68.6 ± 4.9), respectively, with a significant difference (F(2,83) = [13.439], p < .001) (Figure 2), as FEV1/FVC ratio was lower in MSSI being severe and higher in MSSI being mild. The relationship between FEV1/FVC ratio and MSSI in former and never smokers revealed that never (p < .001, Figure 3) and former (p = .031, Figure 3) smokers were significantly associated with MSSI and FEV1/FVC ratio was low in both groups, whereas this was not found among smokers.
Figure 2.
Box plot of the association between MSSI mild, moderate, and severe and FEV1/FVC ratio in the National Danish Fabry cohort. FEV1/FVC ratio: forced expiratory volume in the first-second divided by forced vital capacity; MSSI: Mainz Severity Score Index. ANOVA analysis p < .001.
Figure 3.
Box plot of the association between MSSI mild, moderate, and severe and FEV1/FVC ratio in never and former smoking patients in the National Danish Fabry cohort. FEV1/FVC ratio: forced expiratory volume in the first-second divided by forced vital capacity; MSSI: Mainz Severity Score Index. ANOVA analysis p < .001 (never smokers) and ANOVA analysis p = .031 (former smokers).
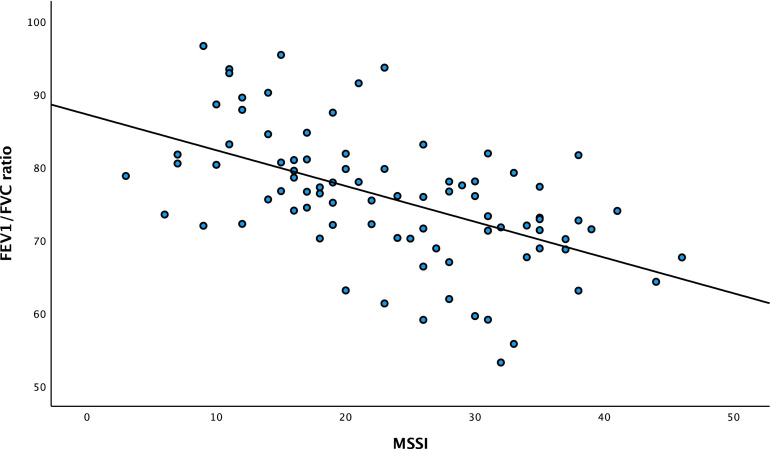
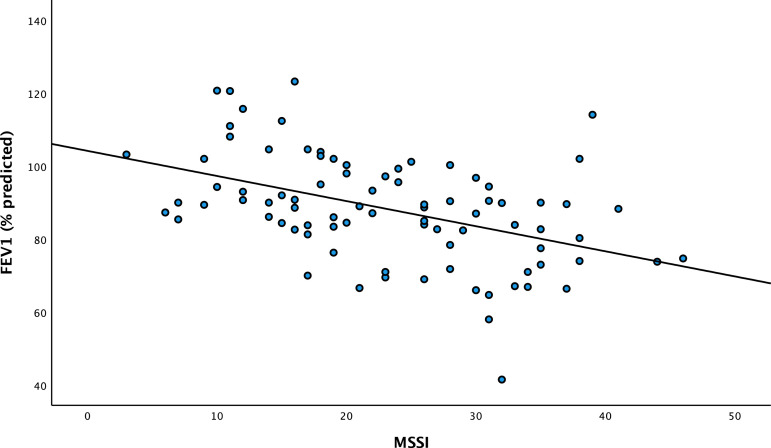
In the multivariate analysis of the relation between FEV1/FVC ratio and the variables of importance for developing airway illness, only high MSSI had a significantly and independently inverse association with impaired FEV1/FVC ratio (p < .001) (Figure 4) with a correlation coefficient of −0.29, and FEV1 (% predicted) (Figure 5) with a correlation coefficient of −0.21.
Figure 4.
Scatter plot of the association between MSSI and FEV1/FVC ratio in the National Danish Fabry. FEV1/FVC ratio: forced expiratory volume in the first-second divided by forced vital capacity; MSSI: Mainz Severity Score Index. Correlation coefficient of −0.29.
Figure 5.
Scatterplot of the association between MSSI on FEV1 (% predicted) in the National Danish Fabry cohort. FEV1: forced expiratory volume in the first-second; MSSI: Mainz Severity Score Index. Correlation coefficient of −0.21.
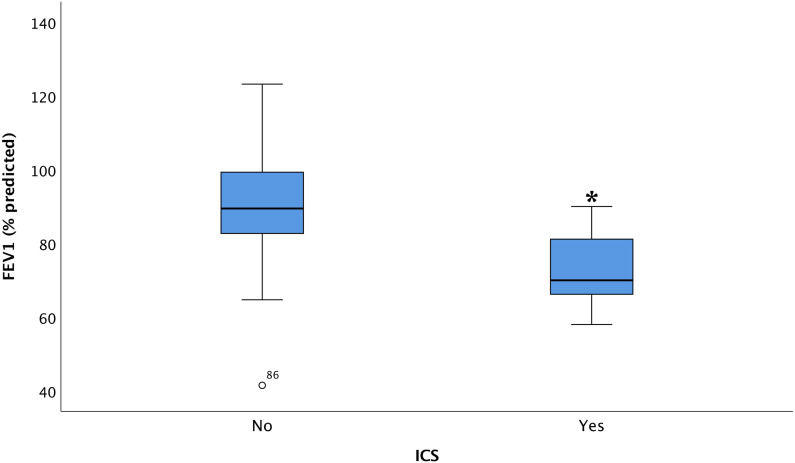
Examining airflow limitation, using FEV1 (% predicted) as the dependent variable and variables of importance for developing airway limitations revealed, that current use of inhaled corticosteroids (p < .001, Figure 6), other types of lung medications (p < .001), and moderate MSSI (p < .001), respectively, had a significant association with FEV1 (% predicted). Furthermore, chest tightness tended towards a negative association with FEV1 (% predicted) (p = .057).
Figure 6.
Box plot of the association between ICS and FEV1 (% predicted) in Fabry patients with or without use of ICS. FEV1: forced expiratory volume in the first second; ICS: inhalation corticosteroid. p < .001.
Fabry patients showed nocturnal symptoms (p = .001) and tendency to breathlessness (p = .067). Both nocturnal symptoms (p = .003) and breathlessness (p = .044) were due to cardiac illness, and not reduced pulmonary function.
Discussion
In this unselected nationwide study of the largest cohort in the Nordic area of patients suffering from Fabry disease we showed a significant manifestation of obstructive airflow limitation among Fabry patients, not entirely explained by tobacco consumption, but importantly with relationship to severity of the Fabry disease thus without relationship with respiratory symptoms.
Severity of disease measured by MSSI was correlated to a significant decline of both FEV1 (% predicted) and FEV1/FVC ratio. Cigarette smoking strengthened this association, whereas no association between respiratory symptoms and lung function was found among Fabry patients. These findings contributed to the notion that Fabry patients frequently develop an obstructive airflow limitation, not only in smokers, but also in never smokers, possibly due to Fabry disease mechanism. Furthermore, Fabry patients with obstructive airflow limitation were also found to have the classic Fabry phenotypes, with high concentrations of plasma lyso-Gb3 in males, urine-Gb3 in females and low α-galactosidase A activity (much lower in males compared to females). Hypothetically, this finding is most likely due to increased tissue destruction possibly at the alveolar level or bronchial mucosa or smooth muscles accumulation of Gb3, although other mechanisms may play a role. We would expect development of fibrosis due to its development in other organs by Gb3 accumulation, and we did find, that patients with obstructive defects had high levels of Gb3. We demonstrated, that pulmonary involvement seemed more prominent in patients with classic rather than late-onset gene variants as α-galactosidase A activity in late-onset phenotypes is very likely sufficient to clear the bronchial smooth-muscle cells from Gb3 accumulation. 4 Only 2% patients had late-onset phenotypes in our study and none of them had pulmonary involvement, versus patients with classic gene variants, where pulmonary involvement was seen in 28%.
Furthermore, the study showed that higher MSSI, were associated with a lower lung function, revealing that the increased disease burden and progression of Fabry disease can be explained by development of airway obstruction as well. It was not possible to verify if increasing MSSI leads to airway obstruction or if airway obstruction leads to increasing MSSI. The adjustments made in the MSSI questionnaire were minor, and not sufficient to change the MSSI substantially and the relation with FEV1/FVC ratio and FEV1 (% predicted) remained significant. Additionally, the study showed that there was no difference between patients receiving ERT and not receiving ERT as both groups developed airway obstruction.
Age, smoking status, and gender have previously been described as significant predictors for FEV1 (% predicted) decline and these factors were also among the main influencers in development of airflow limitation in Fabry patients. 14 Smokers developing airflow limitation, could be a general finding across different patient diagnoses, but an association between MSSI is specific for Fabry and different from the general findings. Also, a study by Rosenberg et al. 15 revealed that the obstructive impairment was much worse in Fabry patients who smoked, implying that even mild cigarette smoking was hazardous to Fabry patients. On the other hand, in our study, former smokers had a more pronounced decline in the FEV1/FVC ratio compared to never smoker, but Fabry patients who were never smokers also had signs of airflow limitation and were therefore also likely to develop obstructive airway limitation. Age was not found to be associated with either FEV1 (% predicted) nor FEV1/FVC ratio, even though it could be expected as age is a risk factor for bronchial obstruction, and FEV1 generally declines approximately 1%–2% per year after the age of 25 in healthy people. 14 In the current study, no gender differences were found possibly due to too small numbers and power, however the number of females was substantially higher than in most studies, since all Fabry genotype positive family members found by cascade screening were offered inclusion in the study. 16
Sixteen cohort studies from 1972 to 2018 focused on the pulmonary involvement in Fabry disease, and a summary of the studies is listed in chronical order in Supplementary Table 3.3–5,15,17–28 Furthermore, 12 longitudinal case reports have been published,29–40 using similar methods for pulmonary function examinations as those used in the cohort studies. The longitudinal case report outcomes were that restrictive and obstructive patterns were a general finding with reduced diffusion capacity and mild-moderate chronic airway limitation. However, none of these studies related to smoking nor to MSSI status.
There was no relationship between level of lung function and respiratory symptoms among patients suffering of chronic obstructive pulmonary disease, 41 leading to a large group of underdiagnosed airflow limitations, which is in agreement with findings in Fabry patients examined in this study. These findings are important for the clinicians which might be attempted to measure FEV1 in patients with respiratory symptoms, whereas a screening approach might be suggested based on our findings.
There are limitations to our study, first, the retrospective study design is a possible source of bias, although the main University hospital is the single Nationwide centre for Fabry. Second, plasma lyso-Gb3 concentrations usually decrease rapidly upon commencement of ERT, which 50% of the patients received, suggesting that the plasma lyso-Gb3 concentration in the patients would be higher if they were measured pre-ERT. Similarly, FEV1 (%predicted) would be lower in patients receiving inhaled corticosteroids and other type of lung medication if they were measured pre-medication. Third, the number of late-onset phenotypes was low, and the findings should therefore be confirmed in a larger cohort with more late-onset patients. Also, the low number of asthma patients might indicate that patients with Fabry disease suffer of non-Type-2 illness, although this study is too small to demonstrate any associations. Furthermore, this study has been initiated before lower limit of normal was introduced, which will account for some of the development of airway obstruction (REF: Howraman Meteran), on the other hand the level of FEV1 also was found to be reduced, which seems to suggest that the level of obstruction is due to pathologic process, rather than a decrease due to age. Also, 28% of the patients exhibited pulmonary involvement by PFT, and it is possible that specific pathogenic variants may be more frequently associated with pulmonary defect and that these pathogenic variants were not present in our cohort. Other limitations included the cross-sectional nature of the study, why causality could not be documented. In general, subgrouping was not possible due to too low numbers in each subgroup. Finally, our measurements do not allow assessment of diffusion capacity to investigate if the reduced lung capacity might be due to a diffusion defect.
Conclusion
The prevalence of affected lung function variables among the National Danish Fabry cohort, with 86 patients included in this study, was 28% and only Fabry patients with classic phenotypes developed a pulmonary defect. This is the first study to show that patients with Fabry disease frequently develop an obstructive lung function defect regardless of their smoking status being current, former, or never smoker, and it is worse in patients with high MSSI. Furthermore, the MSSI was associated with a reduction in both FEV1/FVC ratio and FEV1 (% predicted). Further studies with more late-onset patients, are needed to confirm a broader disease spectrum of these findings. However, this study suggests that care-takers of patients with Fabry disease, should be aware of the development of respiratory illness as part of the disease.
Supplemental Material
Supplemental Material for Respiratory impairments in patients suffering from Fabry disease – A cross-sectional study by Huma Ahmed, Vibeke Backer, Grigoris Effraimidis, Åse Krogh Rasmussen, Caroline Michaela Kistorp and Ulla Feldt-Rasmussen in Chronic Respiratory Disease
Acknowledgements
Ira Hagen Pedersen (specialist nurse) and Casper Kok (laboratory technician) are thanked for excellent assistance. Members from the Danish Fabry Team are thanked for continuously supplying data. Ulla Feldt-Rasmussen’s research salary was sponsored by the Kirsten and Freddy Johansen’s Fund. This investigator-initiated study was sponsored by Sanofi Genzyme.
Author contributions: H.A.: Methodology, Formal analysis, Writing – Original Draft, Visualization. V.B.: Conceptualization, Methodology, Formal analysis, Writing – Review and Editing, Visualization, Supervision. G.E.: Methodology, Formal analysis, Writing – Review and Editing, Visualization. Å.K.R.: Writing – Review and Editing, Visualization. C.M.K.: Writing – Review and Editing, Visualization. U.F.-R.: Conceptualization, Methodology, Investigation, Resources, Writing – Review and Editing, Visualization, Supervision, Funding acquisition.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Sanofi Genzyme and Kirsten and Freddy Johansen’s Fund.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Ulla Feldt-Rasmussen https://orcid.org/0000-0002-5903-3355
Data availability statement
The original anonymised data can be provided upon reasonable request to the corresponding author.
References
- 1.Svensson CK, Feldt-Rasmussen U, Backer V. Fabry disease, respiratory symptoms, and airway limitation – a systematic review. Eur Clin Respir J 2015; 2(1): 26721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardes TP, Foresto RD, Kirsztajn GM. Fabry disease: genetics, pathology, and treatment. Rev Assoc Med Bras 2020; 66(Suppl 1): 10–16. [DOI] [PubMed] [Google Scholar]
- 3.Duning T, Deppe M, Brand E, et al. Brainstem involvement as a cause of central sleep apnea: pattern of microstructural cerebral damage in patients with cerebral microangiopathy. PLoS One 2013; 8(4): e60304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzen D, Haile SR, Kasper DC, et al. Pulmonary involvement in Fabry disease: effect of plasma globotriaosylsphingosine and time to initiation of enzyme replacement therapy. BMJ Open Respir Res 2018; 5(1): e000277–e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzen D, Gerard N, Bratton DJ, et al. Prevalence and risk factors of sleep disordered breathing in fabry disease a prospective cohort study. Med (United States) 2015; 94(52): e2413–e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazdanfard PDW, Effraimidis G, Madsen CV, et al. Hearing loss in fabry disease: a 16 year follow-up study of the Danish nationwide cohort. Mol Genet Metab Reports 2022; 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–338. [DOI] [PubMed] [Google Scholar]
- 8.Hirai T. Pulmonary function tests. Med Radiol 2021; 80: 11–20. [Google Scholar]
- 9.Whybra C, Kampmann C, Krummenauer F, et al. The Mainz Severity Score Index: a new instrument for quantifying the Anderson - fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 2004; 65(4): 299–307. [DOI] [PubMed] [Google Scholar]
- 10.Hasholt L, Ballegaard M, Bundgaard H, et al. The D313Y variant in the GLA gene–no evidence of a pathogenic role in Fabry disease. Scand J Clin Lab Invest 2017; 77(8): 617–621. [DOI] [PubMed] [Google Scholar]
- 11.Feldt-Rasmussen U, Dobrovolny R, Nazarenko I, et al. Diagnostic dilemma: a young woman with Fabry disease symptoms, no family history, and a “sequencing cryptic” α-galactosidase a large deletion. Mol Genet Metab 2011; 104(3): 314–318. [DOI] [PubMed] [Google Scholar]
- 12.Apelland T, Gude E, Strøm EH, et al. Familial globotriaosylceramide-associated cardiomyopathy mimicking Fabry disease. Heart 2014; 100(22): 1793–1798. [DOI] [PubMed] [Google Scholar]
- 13.Al-Dirbashi OY, Jacob M, Al-Hassnan Z, et al. Diagnosis of methylmalonic acidemia from dried blood spots by HPLC and intramolecular-excimer fluorescence derivatization. Clin Chem 2005; 51(1): 235–237. [DOI] [PubMed] [Google Scholar]
- 14.Kerstjens HAM, Rijcken B, Schouten JP, et al. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997; 52(9): 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg DM, Ferrans VJ, Fulmer JD, et al. Chronic airflow obstruction in fabry’s disease. Am J Med 1980; 68: 898–905. [DOI] [PubMed] [Google Scholar]
- 16.Effraimidis G, Rasmussen ÅK, Dunoe M, et al. Systematic cascade screening in the Danish Fabry Disease Centre: 20 years of a national single-centre experience. PLoS One 2022; 17: e0277767–e0277818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartimmo EE, Jr, Guisan M, Moser KM, et al. Pulmonary Involvement in Fabry’s Disease: A Reappraisal Follow-Up of a San Diego Kindred and Review of the Literature. The American Journal of Medicine. 1972; 53: 755–764. [DOI] [PubMed] [Google Scholar]
- 18.Brown LK, Miller A, Bhuptani A, et al. Pulmonary involvement in Fabry disease. Am J Respir Crit Care Med 1997; 155(3): 1004–1010. [DOI] [PubMed] [Google Scholar]
- 19.Bierer G, Kamangar N, Balfe D, et al. Cardiopulmonary exercise testing in fabry disease. Respiration 2005; 72(5): 504–511. [DOI] [PubMed] [Google Scholar]
- 20.Bierer G, Balfe D, Wilcox WR, et al. Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease. J Inherit Metab Dis 2006; 29(4): 572–579. [DOI] [PubMed] [Google Scholar]
- 21.Aubert JD, Barbey F. Chapter 27 Pulmonary involvement in Fabry disease. Oxford Pharma Genesis. 2006; 1–7. [PubMed] [Google Scholar]
- 22.Magage S, Lubanda JC, Susa Z, et al. Natural history of the respiratory involvement in Anderson-Fabry disease. J Inherit Metab Dis 2007; 30(5): 790–799. [DOI] [PubMed] [Google Scholar]
- 23.Wang RY, Lelis A, Mirocha J, et al. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med 2007; 9(1): 34–45. [DOI] [PubMed] [Google Scholar]
- 24.Sorbello M, Veroux M, Cutuli M, et al. Anaesthesiologic protocol for kidney transplantation in two patients with Fabry Disease: a case series. Cases J 2008; 1(1): 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havndrup O, Christiansen M, Stoevring B, et al. Fabry disease mimicking hypertrophic cardiomyopathy: genetic screening needed for establishing the diagnosis in women. Eur J Heart Fail 2010; 12(6): 535–540. [DOI] [PubMed] [Google Scholar]
- 26.Koskenvuo JW, Kantola IM, Nuutila P, et al. Cardiopulmonary involvement in Fabry’s disease. Acta Cardiol 2010; 65(2): 185–192. [DOI] [PubMed] [Google Scholar]
- 27.Odler B, Cseh Á, Constantin T, et al. Long time enzyme replacement therapy stabilizes obstructive lung disease and alters peripheral immune cell subsets in Fabry patients. Clin Respir J 2017; 11(6): 942–950. [DOI] [PubMed] [Google Scholar]
- 28.Franzen DP, Nowak A, Haile SR, et al. Long-term follow-up of pulmonary function in Fabry disease: a bi-center observational study. PLoS One 2017; 12(7): e0180437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang RY, Abe JT, Cohen AH, et al. Enzyme replacement therapy stabilizes obstructive pulmonary Fabry disease associated with respiratory globotriaosylceramide storage. J Inherit Metab Dis 2008; 31(Suppl. 2): S369–S374. [DOI] [PubMed] [Google Scholar]
- 30.Kelly MM, Leigh R, Mckenzie R, et al. Induced sputum examination: diagnosis of pulmonary involvement in Fabry’s disease. Thorax 2000; 55: 720–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soterakis J, Sossi AJ, Chawla SK, et al. (eds) Pulmonary Involvement in Fabry’s Disease: Letters to the editor clinocopathologic oversight. The American Journal of Medicine. 1978; 64: 911–912. [DOI] [PubMed] [Google Scholar]
- 32.Kim W, Pyeritz RE, Bernhardt BA, et al. Pulmonary manifestations of Fabry disease and positive response to enzyme replacement therapy. Am J Med Genet 2007; 143(4): 377–381. [DOI] [PubMed] [Google Scholar]
- 33.John C, Parkinson E, Sunshine CA. Angiokeratoma corporis diffusum univerdale (Fabry) presenting as suspected myocardial infarction and pulmonary infarcts. American Journal of Medicine. 1961; 31: 951–958. [DOI] [PubMed] [Google Scholar]
- 34.Shirai T, Ohtake T, Kimura M, et al. Atypical Fabry’s disease presenting with cholesterol crystal embolization. Intern Med 2000; 39(8): 646–649. [DOI] [PubMed] [Google Scholar]
- 35.Bodamer OA, Ratschmann R, Paschke E, et al. Recurrent acroparaesthesia during febrile infections. Lancet 2004; 363(9422): 1698. [DOI] [PubMed] [Google Scholar]
- 36.Takao M, Mori T, Orikasa H, et al. Postmortem diagnosis of Fabry disease with acromegaly and a unique vasculopathy. Virchows Arch 2007; 451(3): 721–727. [DOI] [PubMed] [Google Scholar]
- 37.Elleder M, Bradová V, Smíd F, et al. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry’s disease - report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol 1990; 417(5): 449–455. [DOI] [PubMed] [Google Scholar]
- 38.Möhrenschlager M, Pontz BF, Lanzl I, et al. Fabry disease: case report with emphasis on enzyme replacement therapy and possible future therapeutic options. JDDG - J Ger Soc Dermatology 2007; 5(7): 594–597. [DOI] [PubMed] [Google Scholar]
- 39.Gaggl M, Kain R, Jaksch P, et al. A single lung transplant in a patient with fabry disease: causality or far-fetched? A case report. Case Rep Transplant 2013; 2013: 905743–905746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith P, Heath D, Rodgers B, et al. Pulmonary vasculature in Fabry’s disease. Histopathology 1991; 19(6): 567–569. [DOI] [PubMed] [Google Scholar]
- 41.Tantucci C, Modina D. Lung function decline in COPD. Int J COPD 2012; 7: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Respiratory impairments in patients suffering from Fabry disease – A cross-sectional study by Huma Ahmed, Vibeke Backer, Grigoris Effraimidis, Åse Krogh Rasmussen, Caroline Michaela Kistorp and Ulla Feldt-Rasmussen in Chronic Respiratory Disease
Data Availability Statement
The original anonymised data can be provided upon reasonable request to the corresponding author.