Abstract
Reduced diffusion capacity (DLCO) after COVID 19 pneumonia was reported in hospitalised patients after discharge. Here, we studied the restoration of DLCO over a 24 months period in COVID-19 pneumonia survivors (n = 317), who were categorised into “moderate” cases (no oxygen supply; no need for hospitalisation), “severe” cases (respiratory frequency > 30/min and/or peripheral oxygen SpO2 < 93%), and “critical” cases (respiratory failure and admission into the intensive care unit). COVID-19 pneumonia survivors with a decreased DLCO (<80%) at 3 months (n = 133) were invited for 6- and 24-months follow-up. At 3 months, impairment of DLCO was more severe in critical case (p < .01). Over time, the subgroups showed a similar level of improvement; and, there was no difference in recovery over time between the subgroups. At 24 months, the DLCO did not differ between the subgroups, with a mean DLCO of 73% for all patients. At 24 months, 65% of patients still had a DLCO < 80%, and in 40% of patients DLCO was <70% of predicted. Regardless the initial disease severity, all COVID-19 survivors showed improvement in DLCO during follow-up; however, DLCO had not normalised in the majority of patients with a DLCO <80% 3 months after hospital discharge.
Keywords: COVID-19 pneumonia, pulmonary function, diffusion capacity
Previously, we reported on 6-months follow-up of lung function in both non-hospitalized and hospitalized COVID-19 survivors. 1 We demonstrated a reduced diffusion capacity (DLCO), i.e. <80% of the predicted value, in the majority of patients (Table 1). Compared to vital capacity, DLCO was shown to be a more useful parameter to monitor residual pulmonary impairment. 1 A Belgian study, in hospitalised patients only, showed a reduced DLCO after 12 months in 25% of patients. 2 Zhang et al. were the first to report on 2 years follow up in an Asian population. They showed recovery in lung function the 1 year post COVID, followed by a subsequent decline in all patients, i.e. irrespective the severity of disease at presentation. This decline remained unexplained and was not paralleled by a decrease in 6-min walk distance or quality of life (QoL). 3 Since the group of patients recovering from COVID-19 is still expanding, 4 insight into the long-term recovery in various (ethnic) subgroups is still warranted; and, can lead to better and more efficient streamlined post-acute care. Therefore, we present our follow-up results on DLCO up to 24 months in first-wave COVID-19 pneumonia survivors. We studied whether the extent of recovery at 24 months differed in patients presenting with clinically moderate, severe or critical disease. 1
Table 1.
Baseline characteristics at 3 months.
| Total | Moderate disease | Severe disease | Critical (ICU) | p-value | |
|---|---|---|---|---|---|
| Patients (n) | 133 | 22 | 82 | 29 | |
| Age (years) | 58 ± 16 | 50 ± 16 | 59 ± 15 * | 59 ± 15 | .026 |
| Female (%) | 64 (48%) | 11 (50%) | 46 (56%) | 7 (24%)# | .012 |
| Underlying disease | 73 (55%) | 11 (50%) | 44 (54%) | 18 (62%) | .648 |
| Asthma/OSAS | 26 (20%) | 8 (36%) | 13 (16%) | 5 (17%) | .754 |
| Heartdisease/hypertension | 51 (38%) | 6 (27%) | 33 (40%) | 12 (41%) | .216 |
| Diabetes | 28 (21%) | 2 (9%) | 15 (18%) | 11 (38%)** | .021 |
| Solid tumour/malignancy | 7 (5%) | 2 (9%) | 2 (2%) | 3 (10%) | .625 |
| DLCO | 65.6 ± 9.7 | 69.1 ± 8.0 | 66.6 ± 9.7 | 60.0 ± 9.1**# | <.01 |
| DLCO 70–80% | 54 (41%) | 12 (55%) | 38 (46%) | 4 (14%) | |
| DLCO 60–70% | 45 (34%) | 8 (36%) | 26 (32%) | 11 (38%) | |
| DLCO < 60% | 34 (26%) | 2 (9%) | 18 (22%) | 14 (48%) | |
| FVC | 92.3 ± 15.3 | 96.5 ± 15.7 | 93.9 ± 15.0 | 84.5 ± 13.6*## | .006 |
| FEV1 | 94.4 ± 15.4 | 97.5 ± 16 | 94.9 ± 15.5 | 90.9 ± 14.5 | .293 |
| FEV1/FVC | 81.5 ± 7.6 | 80.9 ± 6.2 | 80.4 ± 8.1 | 85.1 ± 6.2## | .016 |
| Smoking status | .671 | ||||
| Current smoker | 10 (8%) | 1 (5%) | 6 (7%) | 3 (11%) | |
| Non-smoker | 80 (61%) | 15 (71% | 47 (57%) | 18 (64%) | |
| Former smoker | 41 (31%) | 5 (24%) | 29 (36%) | 7 (25%) | |
| BMI (kg/m 2 ) | 28.98 ± 6.06 | 29.5 ± 7.3 | 28.2 ± 5.7 | 30.7 ± 6.0 | .154 |
| Ethnicity | .447 | ||||
| Caucasian | 84 (64%) | 15 (68%) | 52 (64%) | 17 (59%) | |
| African American | 11 (8%) | 1 (4.5%) | 9 (11%) | 1 (3%) | |
| Southeast Asian | 2 (2%) | 1 (4.5) | 1 (%) | 0 (0%) | |
| Other/mixed | 35 (26%) | 5 (23%) | 19 (24%) | 11 (38%) |
*: p < .05 versus moderate disease.
**: p < .01 versus moderate disease.
***: p < .001 versus moderate disease.
#: p < .01 versus severe disease.
##: p < .05 versus severe disease.
This study is part of a prospective observational follow-up study in COVID-19 pneumonia survivors (n = 317) in OLVG, a large non-academic inner-city hospital in Amsterdam. Patients were discharged from the hospital between March 1th to June 1th 2020, i.e. the first wave of the COVID-19 pandemic in the Netherlands. Patients were not treated with corticosteroids in the acute phase; and, they received no specific medication during follow-up. All patients were diagnosed with COVID-19 pneumonia as previously described. 1 At 3 months, we studied spirometry and QoL (SF-36). All patients with a DLCO< 80% at 3 months after hospital discharge (n = 133) were invited for follow-up at 6- and 24 months; for this written informed consent was obtained. As we wanted to study the effect of COVID-19 on “healthy” lungs, patients with pre-existing lung disease were excluded. The study was approved by the advisory committee for scientific research of OLVG and by the medical ethics committee (MEC). According to WHO clinical management of COVID-19 guidelines, 5 we categorised patients into three groups, i.e., non-hospitalized “moderate” pneumonia cases, “severe” and “critical” cases, as described before. 1 Pulmonary function test were performed according to the ATS and ERS guidelines.6,7 Outcomes were expressed as percentage of the predicted values. Data were analysed in SPSS version 27.0. Between-group comparisons for baseline variables were tested by one-way ANOVA or Chi-square test. For the repeated measurements of DLCO, linear mixed-effects models with random intercepts were used to analyse development over time. We fitted different independent variables to compare: (1) a main effect for time (categorical), (2) main effects for time and group, and (3) main effects en interaction effect for time and group. The best model fit based on Akaike’s Information Criterion is presented with p-values for the Wald test. Results were considered statistically significant at p < .05.
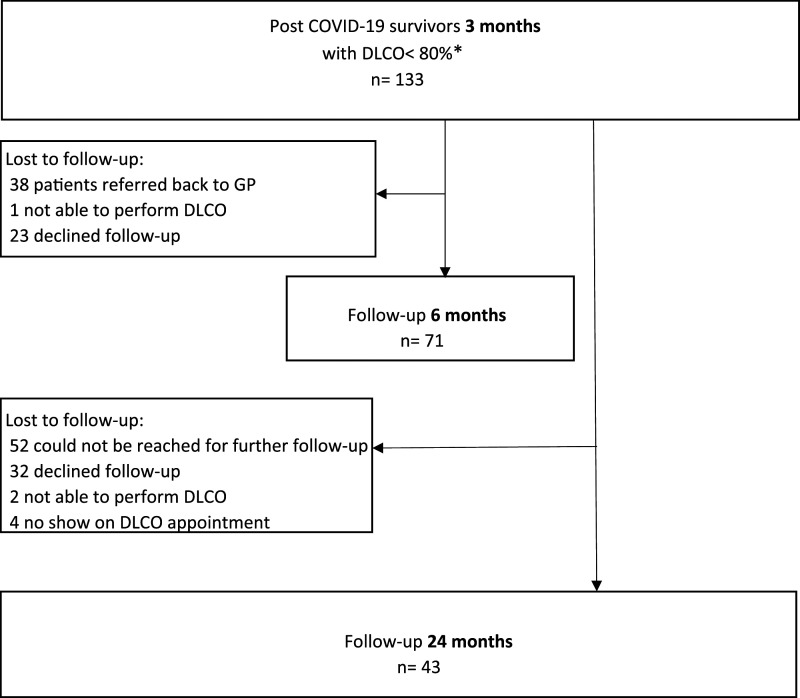
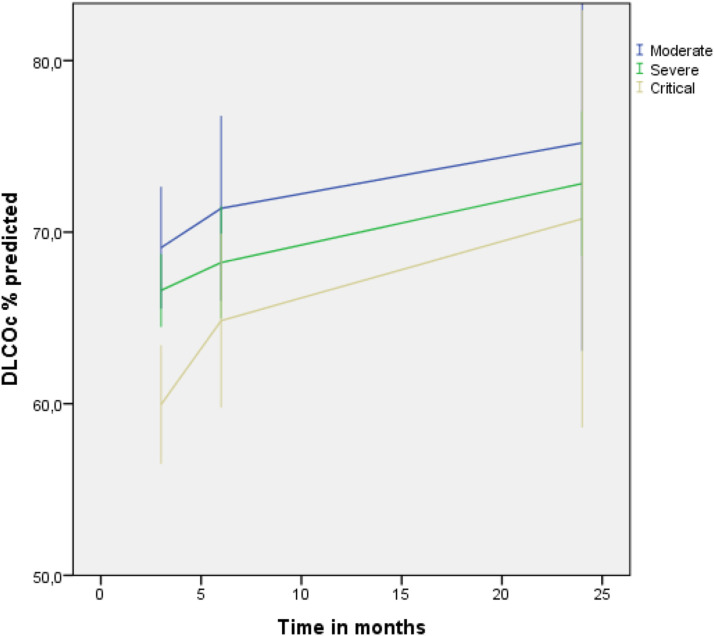
Three months after hospital discharge, we demonstrated a DLCO<80% in 133 patients. Baseline characteristics are shown in Table 1; males and diabetes being overrepresented in the critical group. At 3 months after hospital discharge, DLCO was significantly more impaired in the critical patients (p < .01). FVC was significantly lower in critical patients, but still within normal limits in the most patients (66%). From these 133 patients, 62 (47%) were lost to follow-up at 6 months (Figure 1). At 24 months, 43 patients performed a lung function test. No differences were detected in 3-months baseline characteristics between studied patients and those who were lost to follow-up. DLCO and its development over time, related to the severity of disease at presentation are presented in Figure 2.
Figure 1.
Flow chart of COVID-19 patients.
Figure 2.
Longitudinal trajectories of diffusing capacity for carbon monoxide (DLCO) at 3-, 6- and 24 months by disease severity. The figure shows mean DLCO of each time point. Error bars indicates 95% CI.
In contrast to Zhang et al, 3 over time, the three subgroups showed a similar level of improvement. And, at 24 months, the DLCO did not differ between the subgroups, with a mean DLCO of 73% for all patients. However, at 24 months, a DLCO<80% was still observed in 65% of the 43 patients studied, and in 17 patients (40%) DLCO was below 70%. In line with Zhang et al, in a subgroup of patients (n = 16), DLCO at 24 months did not correlated with SF-36 studied QoL. In conclusion, in this 2-years follow-up study in COVID-19 pneumonia survivors, we demonstrated a gradual improvement in DLCO over time; and, this improvement did not differ in the subgroups of pneumonia severity at presentation. However, in 65% of the patients studied at 24 months, we still observed a decreased DLCO. Although our statistical analyses accounted for missing data, our observations are based upon a relative small sample size. Therefore, future research is warranted to study restoration DLCO in larger cohort studies.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Marlise P de Roos https://orcid.org/0000-0001-9146-6700
References
- 1.de Roos MP, Siegerink S, Dijkstra N, et al. Pulmonary function and quality of Life in a prospective cohort of (non-) hospitalized COVID-19 pneumonia survivors up to six months. Chron Respir Dis 2022; 19: 14799731221114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorent N, Vande Weygaerde Y, Claeys E, et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res 2022; 8: 00004–2022. DOI: 10.1183/23120541.00004-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Li X, Huang L, et al. Lung-function trajectories in COVID-19 survivors after discharge: a two-year longitudinal cohort study. EClinicalMedicine 2022; 54: 101668. Available at: https://covid19.who.int [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int
- 5.Clinical management of severe acute respiratory infection (SARI) when COVID-19 356 disease is suspected Interim guidance. Geneva, Switzerland: World Health Organisation (WHO), 2020. [Google Scholar]
- 6.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med 2019; 200(8): e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017;49(1). 1600016. [DOI] [PubMed] [Google Scholar]