Abstract
Porphyromonas gingivalis can induce its uptake by host epithelial cells; however, the nature and role of the P. gingivalis molecules involved in this invasion process have yet to be determined. In this study, modulation of secreted P. gingivalis proteins following association with gingival epithelial cells was investigated. Western immunoblot analysis showed that contact with epithelial cells or epithelial cell growth media induces P. gingivalis 33277 to secrete several proteins with molecular masses between 35 and 95 kDa. Secretion of the Arg-gingipain and Lys-gingipain proteases was repressed under these conditions. The contact-induced secreted protein profile was altered in Arg-gingipain-deficient and Lys-gingipain-deficient mutants, indicating a possible role for these proteases in the secretion pathway. The P. gingivalis contact-dependent protein secretion pathway differs to some extent from type III protein secretion pathways in enteric pathogens, as a gene homologous to the invA family genes was not detected in P. gingivalis. The secreted proteins of P. gingivalis may play a role in the interactions of the organism with host cells.
Periodontal diseases comprise a group of pathological conditions of the periodontal tissues that surround and support the teeth. Although a variety of bacterial species are associated with the initiation and progression of periodontitis (50), accumulated evidence suggests that Porphyromonas gingivalis, a gram-negative anaerobe, is an important pathogen in severe manifestations of the disease (15, 49, 50). P. gingivalis possesses a wide range of virulence factors with potential relevance to the disease process including extracellular proteases, which can perturb host immune response and tissue integrity, toxic metabolites and cellular constituents, and adherence factors that promote colonization (20, 22, 29, 42, 52, 55, 56). Furthermore, P. gingivalis can efficiently invade and replicate within primary cultures of gingival epithelial cells (23, 24) and multilayered pocket epithelium (46). P. gingivalis can also invade transformed epithelial cells (10, 33). Fimbriae and proteases have been found to be involved in the invasion process (23, 33, 55); however, the molecular basis of P. gingivalis invasion is not well characterized.
Intracellular invasion is considered an important virulence factor and can be found in a variety of genera associated with both acute and chronic infections (3, 8, 13, 27, 28, 30, 34). Recently, a unique protein secretion pathway, closely related to bacterial invasion processes, was identified in a variety of gram-negative plant and animal pathogens (2, 4, 11, 45, 54). This secretion pathway has been designated type III and is also known as contact-dependent secretion because of a requirement for an inducing extracellular signal, usually resulting from the interaction with host cells or from environmental cues associated with the epithelial cell environment. It has been demonstrated that the target proteins of this pathway can be translocated directly into the host cell cytoplasm and can subvert intracellular signaling pathways (2, 4, 5, 11, 16–18, 26, 53, 58). In this study, we investigated the presence of a contact-dependent protein secretion pathway in P. gingivalis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
P. gingivalis 33277 and derivatives were grown anaerobically at 37°C in Trypticase soy broth supplemented per liter with 1 g of yeast extract, 5 mg of hemin, and 1 mg of menadione. When necessary, gentamicin and erythromycin were added to the medium at final concentrations of 200 and 10 μg/ml, respectively. Solid medium was prepared by supplementing with 5% sheep blood and 1.5% agar. Escherichia coli strains and Salmonella typhimurium were grown in Luria-Bertani broth containing the necessary antibiotics (100 μg of ampicillin per ml, 50 μg of kanamycin per ml, or 200 μg of trimethoprim per ml).
Plasmids used in this study are listed in Table 1.
TABLE 1.
Plasmids constructed and used in this study
| Plasmid(s) | Descriptiona | Source or reference |
|---|---|---|
| pJRD215 | Wide-host range cosmid vector, Kmr Smr Mob+ | 7 |
| pJRD/ET | 3.8-kb EcoRI fragment of R751::*Ω4 (47) containing tetX and ermF genes inserted into pJRD215, Kmr Smr Tcr (Emr Rep− in P. gingivalis) | This study |
| R751 | IncP plasmid used to mobilize suicide plasmids from E. coli to P. gingivalis 33277, Tpr Tra+ | 48 |
| pCR/KGP and pCR/RGP | 1.5-kb PCR-amplified fragments corresponding to the catalytic domain-coding regions of kgp and rgp, respectively, inserted into pCR II (Invitrogen), Kmr | This study |
| pJRD/ET/KGP and pJRD/ET/RGP | 1.5-kb XbaI-BamHI fragments containing the catalytic domains of kgp and rgp, respectively, cloned into pJRD/ET | This study |
Abbreviations for plasmids: Emr, erythromycin resistance; Kmr, kanamycin resistance; Mob+, can be mobilized; Rep−, incapable of replication; Smr, streptomycin resistance; Tpr, trimethoprim resistance; Tra+, capable of self-transfer.
Epithelial cell culture.
Primary cultures of gingival epithelial cells (GEC) were prepared from gingival explants and cultured in keratinocyte basal medium (KBM) (Clonetics) as described previously (23).
Preparation of secreted proteins.
Proteins secreted by P. gingivalis were collected under four conditions: from culture supernatant, from P. gingivalis cells washed and resuspended in phosphate-buffered saline (PBS) or in KBM, or from P. gingivalis cells reacted with 107 GEC treated with 2 μM cytochalasin D. In the latter three conditions incubation was for 3 h at 37°C. Viable counting showed that P. gingivalis cells did not increase in number under these conditions. Control experiments demonstrated that cytochalasin D did not affect P. gingivalis protein secretion. Prior to recovery of secreted proteins, the protease inhibitors TLCK (N-α-p-tosyl-l-lysine chloromethyl ketone), TPCK (N-tosyl-l-phenylalanine chloromethyl ketone), and phenylmethylsulfonyl fluoride were added to a final concentration of 0.2 mM. Bacterial cells were then removed from the recovered medium by centrifugation (9,000 × g for 10 min) and filtration (0.22-μm-pore-size filter). Proteins in the cell-free medium were precipitated by addition of 10% trichloroacetic acid (TCA) and incubation on ice for 1 h. The proteins were collected by centrifugation at 4°C (100,000 × g for 30 min), washed with ice-cold acetone, and resuspended in sample loading buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The secreted protein sample was analyzed by SDS-PAGE (21) and Western immunoblot assay (44).
Invasion assay.
P. gingivalis invasion of GEC was quantitated by the antibiotic protection assay described previously (23).
Molecular biology procedures.
DNA manipulation procedures and transformation of E. coli strains were performed according to the standard methods described by Ausubel et al. (1). PCR was performed using a thermocycler [Techne (Cambridge) Ltd.] with Taq DNA polymerase (GibcoBRL) according to the protocol described by the manufacturer of the enzyme.
N-terminal amino acid sequences.
The N-terminal amino acid sequences of the major secreted proteins of P. gingivalis were determined from samples bound to a polyvinylidene difluoride protein sequencing membrane (Bio-Rad) (25) by automated protein sequencing at the Department of Biochemistry, University of Washington, Seattle.
Oligonucleotides.
Two degenerate synthetic oligonucleotide primers for PCR amplification of the invA family gene fragment were designed based on the amino acid sequences of the conserved regions of the InvA family proteins (2, 12). The conserved regions chosen for this study corresponded to the regions between amino acids 50 and 59 (VLLL[F]TTTLL[F]R) and between amino acids 220 and 229 (V[I]A[S]QIPALLIS[A]) of S. typhimurium InvA. The primers synthesized were INV1 (5′-GTNYTNYTNYTNCANCANYTNYTNMGNYTN-3′) and INV2 (5′-NSDDATNARNARNGKNGGKATYTGNSDNAY-3′), where D = A, T, or G; K = T or G; M = A or C; N = A, C, T, or G; R = A or G; S = C or G; and Y = C or T.
The following oligonucleotides were synthesized to amplify the catalytic domain-coding regions of two major cysteine protease genes (kgp and rgpA) of P. gingivalis 33277 based on the DNA sequences published previously (35, 36); KGP1, 5′-GATGTTTATACAGATCATGG-3′; KGP2, 5′-ACGTACATCGTTTGCAGGTT-3′; RGP1, 5′-TCAACACCGGTAGAGGAAAA-3′; and RGP2, 5′-GCGAAGAAGTTCGGGGGCAT-3′.
Construction of P. gingivalis 33277 mutants.
Mutants were constructed by insertional mutagenesis using a suicide plasmid pJRD/ET (Table 1). The catalytic domain coding regions of kgp and rgpA were amplified from the chromosomal DNA of P. gingivalis 33277 by PCR and subcloned into the T-A cloning vector pCR II (Invitrogen). The plasmids, pJRD/ET/KGP and pJRD/ET/RGP, were constructed by subcloning the 1.5-kb fragment of kgp or rgpA, respectively, into the suicide plasmid pJRD/ET and were introduced into P. gingivalis 33277 by conjugation as described previously (37). Erythromycin-resistant transconjugants were randomly selected. The chromosomal DNA of each transconjugant was isolated and used for Southern hybridization to confirm the insertion of the plasmid in the rgpA or kgp genes. Insertion of the plasmid into the catalytic domain will occur upstream of the hemagglutinin domain-coding sequences. Lack of the expression and secretion of the gene products was confirmed by analyzing culture supernatant or whole cells of each mutant by SDS-PAGE or by Western blotting with monospecific Arg-gingipain (RGP) or Lys-gingipain (KGP) antibodies (43). Furthermore, mutants were tested for loss of enzyme activity with the specific substrates BApNA (for RGP) and Z-Lys-pNA (for KGP).
RESULTS
Major secreted proteins of P. gingivalis in normal culture conditions.
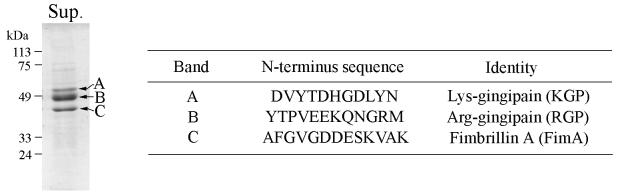
Prior to the study of contact-dependent protein secretion in P. gingivalis, the secreted proteins of the bacteria cultivated under normal culture conditions were analyzed. Secreted proteins were collected from cell-free culture supernatant by TCA precipitation and separated by SDS-PAGE. As shown in Fig. 1, three major secreted proteins were detected, with molecular masses of approximately 55, 48, and 43 kDa. These three proteins were also detected by immunoblotting with P. gingivalis antibodies (not shown). N-terminal sequencing identified the proteins as the mature forms of two major extracellular cysteine proteases of P. gingivalis, KGP and RGP, and the structural component of the major fimbriae, fimbrillin A (FimA), respectively.
FIG. 1.
Proteins of P. gingivalis 33277 secreted into the culture supernatant. Proteins were collected from the culture media by TCA precipitation, and 5 μg was separated by SDS-PAGE and stained with Coomassie brilliant blue R-250. The SDS-PAGE profile is shown on the left. The N-terminal amino acid sequence and the identity of the main protein bands (A, B, and C) are shown on the right. Sup., protein sample of the culture supernatant.
Secreted protein profile of P. gingivalis 33277 following contact with epithelial cells.
To investigate the presence of the contact-dependent protein secretion pathway, P. gingivalis 33277 was incubated with GECs, and the secreted proteins were analyzed by Western immunoblot assay using the antiserum raised against P. gingivalis whole cells (22). P. gingivalis secreted proteins with molecular masses ranging from 35 to 95 kDa following contact with GECs (Fig. 2, lanes 3 and 4). These proteins were not derived from extracellular vesicles as a high-speed centrifugation (at 100,000 × g for 30 min), prior to TCA precipitation, did not affect the secreted protein profile. The identity of the 43-kDa protein was confirmed as FimA with antiserum raised against the purified P. gingivalis fimbriae (not shown). Thus, fimbrillin is secreted both with and without contact with GECs. Of the other two major secreted proteins in normal culture conditions, neither KGP (55 kDa) nor RGP (48 kDa) was detected by antibodies monospecific to the catalytic domains of the enzymes (43) (not shown). This suggests that secretion of KGP and RGP may be inhibited under conditions that induce contact-dependent secretion.
FIG. 2.
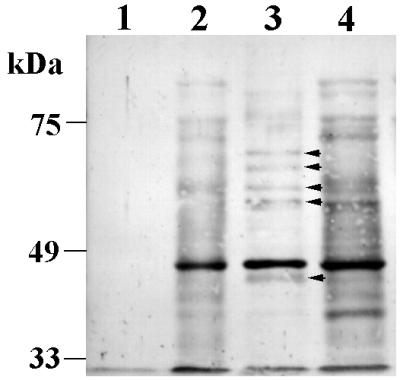
Contact-dependent protein secretion of P. gingivalis 33277. Western immunoblot with antiserum raised against P. gingivalis whole cells. Protein samples were collected by TCA precipitation from the cell-free supernatant after incubating 5 × 109 P. gingivalis cells in PBS (lane 1), in KBM (lane 2), with GEC in PBS (lane 3), or with GEC in KBM (lane 4). Proteins secreted by contact with GEC but not by contact with KBM are indicated by arrows.
Protein secretion by P. gingivalis in various environments.
KBM was also able to stimulate P. gingivalis to secrete proteins (Fig. 2, lane 2) comparable to those secreted by contact with GECs in KBM (lane 4). PBS did not induce protein secretion in P. gingivalis (Fig. 2, lane 1). The component(s) of KBM activating the protein secretion remains to be determined. The P. gingivalis antibodies did not reacted with a TCA-precipitated protein sample of KBM (not shown).
To distinguish proteins secreted specifically by contact with GECs and not KBM, P. gingivalis was incubated with washed GECs in PBS (Fig. 2, lane 3). Compared with the protein profiles in KBM, GECs were solely responsible for the secretion of at least five different proteins (arrows marked in lane 3). Thus, GEC and KBM may provide slightly different induction signals that result in differing secreted protein profiles.
Insertional mutagenesis of kgp and rgpA genes of P. gingivalis.
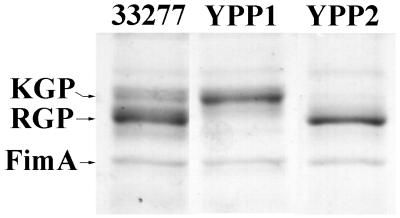
As shown above, contact with host cells seemed to inhibit secretion of KGP and RGP, the major secreted proteins of P. gingivalis 33277, under normal culture conditions. To assess the involvement of these proteins in contact-dependent secretion, we performed insertional mutagenesis to create RGP-deficient or KGP-deficient mutants (YPP1 and YPP2, respectively) of P. gingivalis 33277. Disruption of the genes by insertion of the suicide plasmids was confirmed by Southern hybridization (not shown). In addition, SDS-PAGE analysis of the secreted protein samples of each mutant confirmed the deficiency of the gene products (Fig. 3).
FIG. 3.
Secreted protein profiles of P. gingivalis strains 33277, YPP1, and YPP2. Proteins were collected from the culture supernatant by TCA precipitation, and 5 μg was separated by SDS-PAGE and stained with Coomassie brilliant blue R-250.
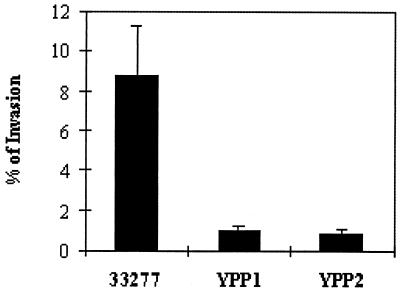
The invasive capacities of the respective P. gingivalis strains are shown in Fig. 4. Both mutants showed an almost 10-fold reduction in invasion compared to the level obtained for the parent strain, confirming that the proteins play important roles in P. gingivalis invasion.
FIG. 4.
Invasion of P. gingivalis strains 33277, YPP1, and YPP2. Invasion was calculated as the number of bacteria recovered intracellularly and expressed as a percentage of the total bacteria reacted with GEC. Error bars represent standard errors (n = 3).
Contact-dependent secreted protein profiles of the insertional mutants.
Figure 5 shows the secreted protein profiles of the protease-deficient mutants. Both mutants continued to secrete proteins when induced by KBM; however, differences in the protein profiles were observed. RGP deficiency (YPP1) had a negative affect on FimA secretion and on secretion of the 95-kDa protein (Fig. 5, lane 2). The KGP-deficient mutant, YPP2 (Fig. 5, lane 3), appeared to secrete an increased amount of protein compared to that of the other strains. YPP2 also secreted a novel protein of 75 kDa. Identical numbers of bacteria were utilized in these studies and the effects were consistent over several repetitions.
FIG. 5.
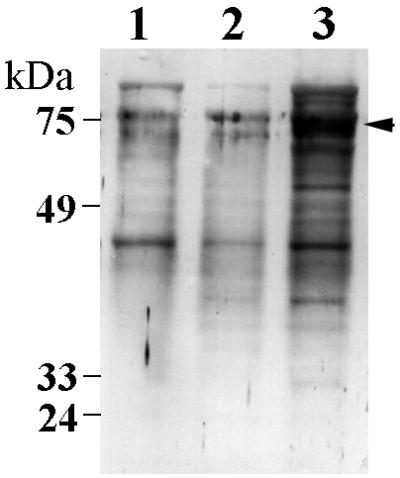
Contact-dependent protein secretion of P. gingivalis strains 33277 (lane 1), YPP1 (lane 2), and YPP2 (lane 3). Proteins were collected by TCA precipitation from cell-free supernatants after incubating 5 × 109 cells of each strain in KBM and were analyzed by Western immunoblotting using antiserum raised against P. gingivalis whole cells. The 75-kDa protein secreted only by YPP2 is indicated by the arrow.
The invA family gene in P. gingivalis.
The results described above suggested that P. gingivalis may have a type III, contact-dependent, protein secretion pathway. Hence, the presence of genes encoding functional components of this secretion pathway was examined by degenerate PCR and by Southern hybridization. Chromosomal DNA of P. gingivalis 33277 was amplified with degenerate primers designed from the amino acid sequences of the conserved regions of the InvA family proteins which are involved in type III secretion.
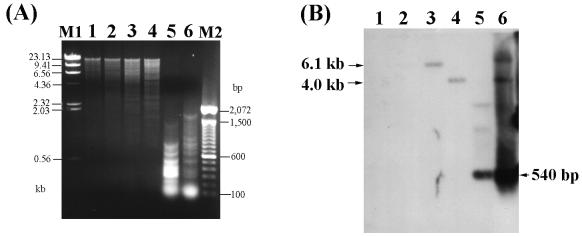
As shown in Fig. 6, the degenerate primers were able to amplify a 540-bp DNA fragment from the chromosomal DNA of S. typhimurium CS019 (lane 1) or from a recombinant plasmid containing the invA gene (obtained from Samuel I. Miller, University of Washington) (lane 2). The size of the fragment was identical to that expected from the DNA sequence of the gene. However, no amplified DNA fragment was detected from the chromosomal DNA of P. gingivalis 33277 (Fig. 6, lane 3). Variations in Mg2+ concentrations and annealing temperatures during PCR did not affect this result.
FIG. 6.
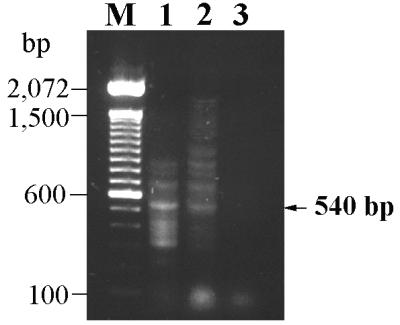
PCR with the degenerate primers INV1 and INV2. Templates used were the plasmid containing invA (lane 1), chromosomal DNA of S. typhimurium CS019 (lane 2), and chromosomal DNA of P. gingivalis 33277 (lane 3). Lane M is a 100-bp DNA ladder (GibcoBRL).
Southern hybridization was performed with the recombinant plasmid containing the invA gene as a probe. The probe hybridized with the 6.1-kb HindIII and 4.0-kb PstI fragments of S. typhimurium DNA (Fig. 7, lanes 3 and 4). The 540-bp PCR-amplified DNA fragments reacted with the probe (lanes 5 and 6), confirming that the degenerate primers can be used to amplified the invA gene. No hybridized band was detected with the P. gingivalis DNA, even under the low-stringency conditions employed.
FIG. 7.
Southern hybridization to examine the presence of the invA family gene in P. gingivalis 33277. Results obtained by agarose gel electrophoresis (A) and Southern blotting (B) are shown. Lanes: 1, HindIII-digested P. gingivalis DNA; 2, PstI-digested P. gingivalis DNA; 3, HindIII-digested S. typhimurium DNA; 4, PstI-digested S. typhimurium DNA; 5, PCR products of S. typhimurium invA (Fig. 3, lane 1); 6, PCR products of S. typhimurium DNA (Fig. 3, lane 2). M1 and M2 are a λ-HindIII DNA marker and a 100-bp DNA ladder, respectively.
These studies suggest that the invA family gene may not present in P. gingivalis and that P. gingivalis may have a functionally conserved but structurally distinct contact-dependent protein secretion pathway.
DISCUSSION
This study has identified a contact-dependent protein secretion pathway of an important pathogen in adult periodontitis, P. gingivalis. General features of such contact (or type III) secretion pathways are (i) the absence of a typical amino-terminal signal sequence in the secreted proteins and the translocation of the target proteins through two membranes without cleavage of their amino termini, (ii) the requirement for the products of a large gene cluster to assist protein secretion, and (iii) the requirement for an inducing extracellular signal, usually resulting from the interaction with host cells or factors specific to the host cell environment (4, 6, 45). The secreted protein profile of P. gingivalis 33277 following contact with cultured epithelial cells was analyzed by Western immunoblotting. The bacteria secreted several proteins which appeared as more than five different protein bands with molecular masses between 35 and 95 kDa. The protein profiles were quite different from that of the cell-free culture supernatant sample in which the major secreted proteins were the mature forms of two cysteine proteases (KGP and RGP) (36, 41) and the major structural subunit protein of the fimbriae (FimA) (57). This indicates that contact with host cells induces P. gingivalis to secrete specific target proteins and suggests the presence of a type III secretion pathway in the microorganism. FimA was also secreted following epithelial cell contact. However, sequence analysis of the fimA gene has demonstrated an amino-terminal signal sequence (9), indicating that the type II, sec-dependent, general secretion pathway is responsible for FimA secretion. Initial attempts to investigate the identities of the contact-secreted proteins by amino acid sequencing were unsuccessful due to amino-terminal blockage. Removal of P. gingivalis vesicles from the cell-free supernatants showed no effect on the proteins secreted by contact with host cells or under normal culture conditions. This indicates that the proteins detected in the study were not vesicle associated but secreted directly to the outside environment.
Unlike PBS or P. gingivalis growth medium (Trypticase soy broth), KBM stimulated the protein secretion by P. gingivalis. The protein profile was similar to that obtained by contact with GEC, although some different proteins were observed. The secretion-stimulating components of KBM are unknown to date. There have been several reports demonstrating that type III secretion pathways can be stimulated by the extracellular environment. Serum was found to activate the type III secretion pathway of S. typhimurium (58), and the release of Ipa molecules from Shigella can be activated by fetal bovine serum, Congo red, and components of the extracellular matrix such as fibronectin, laminin, or collagen type IV (26, 38, 53). In Yersinia spp., Yops can be secreted by a type III secretion pathway by depleting Ca2+ from the medium (6). Thus, the contact-dependent secretion pathway of P. gingivalis shares with the invasive enteric pathogens the feature of inducibility by environmental conditions.
The structural components of the type III secretion pathways of various animal and plant pathogens are highly conserved at the protein sequence level (4). On this basis, we investigated the presence of type III pathway components in P. gingivalis by PCR using degenerate primers designed from the consensus sequences of the InvA family proteins. invA was first identified as one of the genes in the genetic locus that allows Salmonella spp. to enter cultured epithelial cells. Homologous proteins are present in various pathogens, including Shigella and Yersinia spp. These proteins can even demonstrate functional conservation as evidenced by the ability of the Shigella mxiA gene to complement a noninvasive invA Salmonella mutant (4, 14). The InvA family functions as one of the accessory proteins in the type III secretion pathway, possibly by forming a channel in the inner membrane through which the exported polypeptides are translocated (4). However, no evidence indicating the presence of this gene in P. gingivalis was obtained in this study. Thus, either the contact-dependent secretion pathway in P. gingivalis does not utilize an InvA-like protein or the equivalent protein in P. gingivalis has diverged considerably from that in other organisms.
FimA, RGP, and KGP, the proteins secreted under normal culture conditions, all possess amino-terminal signal peptides (35, 36, 39, 40), indicating secretion by the type II (sec-dependent) secretion pathway. FimA continued to be secreted following contact with epithelial cells; however, secretion of RGP and KGP was repressed. This indicated that expression of these proteases, which are not found in other organisms possessing type III secretion pathways, may be antagonistic to contact-dependent secretion. Disruption of the kgp gene enhanced the total level of contact-dependent secretion and resulted in the secretion of a novel 75-kDa protein. This supports the concept that KGP, a lysine-specific cysteine protease, may degrade either the targets or the effector molecules of the secretion pathway. In contrast, disruption of the rgpA gene did not elevate levels of secreted proteins. Indeed, secretion of the 95-kDa protein and of FimA was reduced in the RGP mutant. Here, it should be noted that two different rgp genes, rgpA and rgpB, have been identified in P. gingivalis 33277 (31). The two genes share extensive homology in the protease domains but not in their carboxyl-terminal domains. In this study, Southern blot analysis showed that the insertional mutation disrupted rgpA in all potential RGP-deficient mutants selected. Disruption of this gene resulted in loss of the 48-kDa mature form of RGP in the secreted protein profile. This is consistent with the results reported by Nakayama et al. (31) and suggests the possible involvement of the carboxyl-terminal domain in the protein secretion. Two different research groups recently reported the involvement of RGP in the fimbriation of P. gingivalis. Nakayama et al. (32) showed that a rgpA-rgpB double mutant possessed very few fimbriae on the cell surface. Tokuda et al. (51) demonstrated that a rgpA mutant of P. gingivalis 381 did not express detectable levels of the FimA protein, as determined by both Western and Northern blotting, or visible fimbriae, following electron microscopy of the cells. Our results are consistent with these observations. The involvement of RGP in the contact-dependent secretion of the 95-kDa protein remains to be determined; however, it is possible that this protein represents one of the many complexes of this protease with hemagglutinin domains (41, 42).
The precise role of the contact-dependent secreted proteins of P. gingivalis in the invasive process remains to be determined. Certainly, despite differences in protein secretion, both mutant strains YPP1 and YPP2 displayed a log reduction in epithelial cell invasion. However, loss of protease function may affect the initial association between P. gingivalis and host cells (19, 20, 23, 42, 51, 56) that does not require contact-secreted proteins. A tentative model could thus be postulated whereby proteases are required for efficient adherence of P. gingivalis, either through effects of fimbrial or hemagglutinin expression (20, 32, 56) or by exposing “cryptitopes” within epithelial cell receptors (19, 42). Following contact with the epithelial cells, protease secretion is downregulated, thus (in the case of KGP) elevating levels of contact-dependent secreted proteins that may be required to transduce a signal to the epithelial cells that subsequently stimulates bacterial uptake.
Proteins secreted by the type III protein secretion pathway play major roles in the invasive process of enteric pathogens. Studies of this pathway and the target molecules have increased the understanding of host-pathogen interactions and of pathogenic mechanisms. Although much is known about a wide variety of P. gingivalis virulence factors, including proteases, fimbriae, and hemagglutinins, the protein secretion pathways of P. gingivalis have not been investigated. We report here the presence of a contact-dependent protein secretion pathway for P. gingivalis. This pathway appears to differ from those of other pathogenic bacteria, possibly as an adaptation to the highly proteolytic nature of P. gingivalis. Further studies will involve the identification of the target proteins and the characterization of the genes or gene cluster(s) related to the secretion pathway.
ACKNOWLEDGMENTS
We thank Sam Miller for providing S. typhimurium strains and plasmids and James Travis and Jan Potempa for gifts of the Kgp and Rgp antibodies. We also thank Carol Belton for assistance with the cell culture and Shiwei Cai for technical assistance.
The support of the National Institute of Dental Research (DE11111) is gratefully acknowledged.
REFERENCES
- 1.Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 2.Bogdanove A J, Wei Z-M, Zhao L, Beer S V. Erwinia amylovora secretes hairpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns J L, Jonas M, Chi E Y, Clark D K, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun. 1996;64:4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collazo C M, Galán J E. The invasion-associated type-III protein secretion system in Salmonella—a review. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 5.Collazo C M, Galán J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 7.Davison J, Heusterpreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pRJD215, a wide-host-range cosmid vector with multiple cloning sites. Gnee. 1987;351:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 8.de Vries F P, van der Ende A, van Putten J P M, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson D P, Kubiniec M A, Yoshimura F, Genco R J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988;170:1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galán J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner J A, Cover T L. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginocchio C C, Galán J E. Functional conservation among members of the Salmonella typhimurium InvA family of proteins. Infect Immun. 1995;63:729–732. doi: 10.1128/iai.63.2.729-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontani M, Ono H, Shibata H, Okamura Y, Tanaka T, Fujiwara T, Kimura S, Hamada S. Cystein protease of Porphyromonas gingivalis 381 enhances binding of fimbriae to cultured human fibroblasts and matrix proteins. Infect Immun. 1996;64:756–762. doi: 10.1128/iai.64.3.756-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuramitsu H, Tokuda M, Yoneda M, Duncan M, Cho M-I. Multiple colonization defects in a cysteine protease mutant of Porphyromonas gingivalis. J Peridontal Res. 1997;32:140–142. doi: 10.1111/j.1600-0765.1997.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lamont R J, Bevan C A, Gil S, Persson R E, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276. doi: 10.1111/j.1399-302x.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamont R J, Hersey S G, Rosan B. Characterization of the adherence of Porphyromonas gingivalis to oral streptococci. Oral Microbiol Immunol. 1992;7:193–197. doi: 10.1111/j.1399-302x.1992.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- 26.Ménard R, Sansonetti P, Pasot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer D H, Lippmann J E, Fives-Taylor P M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer D H, Sreenivasan P K, Fives-Taylor P M. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyata Y, Takeda H, Kitano S, Hanazawa S. Porphyromonas gingivalis lipopolysaccharide-stimulated bone resorption via CD14 is inhibited by broad-spectrum antibiotics. Infect Immun. 1997;65:3513–3519. doi: 10.1128/iai.65.9.3513-3519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosleh I M, Boxberger H-J, Sessler M J, Meyer T F. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama K, Yoshimura F, Kadowaki T, Yamamoto K. Involvement of arginine-specific cysteine proteinase (arg-gingipain) in fimbriation of Porphyromonas gingivalis. J Bacteriol. 1996;178:2818–2824. doi: 10.1128/jb.178.10.2818-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Njoroge T, Genco R J, Sojar H T, Genco C A. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine-specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto K, Misumi Y, Kadowaki T, Yoneda M, Yamamoto K, Ikehara Y. Structural characterization of argingipain, a novel arginine-specific cysteine proteinase as a major periodontal pathogenic factor from Porphyromonas gingivalis. Arch Biochem Biophys. 1995;316:917–925. doi: 10.1006/abbi.1995.1123. [DOI] [PubMed] [Google Scholar]
- 37.Park Y, McBride B C. Characterization of the tpr gene product and isolation of a specific protease-deficient mutant of Porphyromonas gingivalis W83. Infect Immun. 1993;61:4139–4146. doi: 10.1128/iai.61.10.4139-4146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsot C, Ménard R, Gounon P, Sansonetti P J. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol Microbiol. 1995;16:291–300. doi: 10.1111/j.1365-2958.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 39.Pavloff N, Pemberton P A, Potempa J, Chen W-C A, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 40.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 41.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 42.Pike R N, Potempa J, McGraw W, Coetzer T H T, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renart J, Sandoval I V. Western blots. Methods Enzymol. 1984;104:455–460. doi: 10.1016/s0076-6879(84)04114-8. [DOI] [PubMed] [Google Scholar]
- 45.Salmond G P C, Reeves P J. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 46.Sandros J, Papapanou P N, Nannmark U, Dahlén G. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 47.Shoemaker N B, Getty C, Gardner J F, Salyers A A. Tn4351 transposes in Bacteroides spp. and mediates integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoemaker N B, Guthrie E P, Salyers A A, Gardner J F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985;162:626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 50.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 51.Tokuda M, Duncan M, Cho M-I, Kuramitsu H K. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Travis J, Pike R, Imamura T, Potempa J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodontal Res. 1997;32:120–125. doi: 10.1111/j.1600-0765.1997.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 53.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoneda M, Kuramitsu H K. Genetic evidence for the relationship of Porphyromonas gingivalis cysteine protease and hemagglutinin activity. Oral Microbiol Immunol. 1996;11:129–134. doi: 10.1111/j.1399-302x.1996.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura F, Takahashi K, Nodasaka Y, Suzuki T. Purification and characterization of a novel type fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984;160:949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zierler M K, Galán J E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]