Abstract
Organismal functional strategies form a continuum from slow- to fast-growing organisms, in response to common drivers such as resource availability and disturbance. However, whether there is synchronisation of these strategies at the entire community level is unclear. Here, we combine trait data for >2800 above- and belowground taxa from 14 trophic guilds spanning a disturbance and resource availability gradient in German grasslands. The results indicate that most guilds consistently respond to these drivers through both direct and trophically mediated effects, resulting in a ‘slow-fast’ axis at the level of the entire community. Using 15 indicators of carbon and nutrient fluxes, biomass production and decomposition, we also show that fast trait communities are associated with faster rates of ecosystem functioning. These findings demonstrate that ‘slow’ and ‘fast’ strategies can be manifested at the level of whole communities, opening new avenues of ecosystem-level functional classification.
Subject terms: Community ecology, Ecosystem ecology
Although co-occurring species may differ widely in their response traits, coordinated functional trait shifts may emerge at the community level in response to environmental factors. Here, the authors use data from 150 grassland sites to identify a coordinated slow-fast strategy response to land-use intensification across above- and belowground taxa.
Introduction
Understanding how functional strategies respond to environmental drivers is one of the longest-standing and most fundamental questions in ecology (e.g., r/K strategist theory1, CSR (competitive/stress-resistant/ruderals) strategies2). Due to evolutionary trade-offs, species allocate resources differently to their capacity to grow, reproduce, and survive, and for several taxa, it is well established that this leads to sets of co-varying traits that represent ecological strategies3–5. At the community level, a range of positive and negative biotic interactions6 and abiotic factors constrain which individuals, bearing specific traits, persist in a community7. In any given environment this is likely to lead to the dominance of the trait set best adapted to local conditions, leading to trait similarity between co-occurring species and resulting in community-level trait covariation.
Functional strategies, and their trait proxies, have been particularly well studied and characterised in vascular plants, both at the species2,8–10 and community levels11, but have also been described for groups as diverse as fishes, arthropods12, and more recently, microorganisms13. While the hypotheses underlying such patterns are often underdeveloped compared to those for plants, similar drivers of strategy orientation have been identified, namely resource availability and disturbance; and these are consistently seen to act concurrently to shape both individual species and community-level strategies traits in terms of growth, reproduction, and survival across the tree of life14,15. Body size in particular is a fundamental trait of functional strategies that shows consistent responses to these drivers, with undisturbed environments filtering for larger (‘slow’) organisms and disturbed environments for smaller ('fast') organisms across groups14,16, a finding that is consistent with general theory17.
At the level of guilds and communities, winning strategies can be seen as manifesting as an emergent property, and represented in community-level trait measures, typically the community abundance-weighted trait mean11,18 (CWM). While there can be significant trait variation within a community, a CWM captures the average functional strategy of the community; and changes in CWM across space and time reflect both turnover in species with different trait values, and variation in their relative abundance, in response to changes to the species pool and environmental conditions. The combined response of multiple trait CWMs thus represents a change in the overall functional strategy at a community level. This means that slow-fast strategy responses – encompassing a range of traits – may emerge at the community level from a concurrent change in individual CWM traits related to ‘slow’ and ‘fast’ strategies. In particular, communities of resource-acquisitive, fast-growing organisms with numerous offspring, a fast pace of life and good dispersal abilities tend to be found in resource-rich and disturbed habitats, while resource-conservative, slow-growing organisms with longer lifespan and fewer offspring tend to be favoured in undisturbed or resource-poor habitats14,15,19–21.
Consistent with these common responses at both species and community levels, associations between traits have been reported between interacting guilds, including plants and soil microorganisms22–24, plants and arthropods25, and plants and frugivores26. These shared responses between guilds to similar environmental drivers, along with the existence of widespread strategies within guilds, suggest the possibility of synchronised responses across trophic groups and therefore the potential existence of trait syndromes at the level of entire communities. The possibility of such community-level coupling27 has been discussed previously, in particular in terms of linkages between plants, herbivores and the soil food web28. However, while there is theoretical and empirical support for a community-level slow-fast trait response, conflicting observations challenge this hypothesis. First, while the slow-fast spectrum is well defined in plants, additional axes of variation in functional and life-history strategies (e.g., reproductive strategies, and their defining traits such as the timing of reproductive onset and reproductive allocation) have been identified in both plants and other organisms29,30, and these may respond to different drivers and dominate the distribution of certain organismal groups, decoupling them from the response of others3,31. Furthermore, guilds might vary in their strength of response to the drivers of the slow-fast functional axis32 and could respond over different spatiotemporal scales33 leading to weak overall coupling.
If community-level slow-fast responses to disturbance and resources are present, then they may be driven by shared direct responses to the environment such as rapid reproduction and effective dispersal of both plants and their consumers in highly disturbed environments. In contrast, synchronous responses could also be mediated by bottom-up34 trophic interactions: at higher resource availability, we expect higher productivity and resource concentrations, and faster rates of resource transfer between trophic levels, encouraging organisms with faster growth and reproduction both above and belowground28,35. Thus, the slow-fast response of the entire community to resource and disturbance drivers might emerge from both direct effects (mainly through shared responses to disturbance) and indirect effects (e.g., a trophic cascade driven by the resource availability component).
Finally, many studies have shown that community-level trait measures of individual guilds explain variation in individual ecosystem functions, which is to be expected given the link between functional traits and rates of metabolic and tissue turnover processes23,36,37. Meanwhile, several recent studies have demonstrated that overall ecosystem functioning can be described in terms of just a few fundamental functional axes38–40. Given this, we predict that the entire community ‘slow-fast’ trait axis corresponds to an ecosystem functioning ‘slow-fast’ axis, with ‘fast’ functioning defined as fast process rates (e.g., high productivity, rapid decomposition and nutrient turnover).
While theory often considers the effects of resource availability and disturbance as orthogonal (e.g., CSR theory2), these drivers are often confounded in real ecosystems. Agricultural systems in particular, tend to be found on a continuum between low intensity (low disturbance, no nutrient input) and high intensity (high disturbance, such as mowing, and high nutrient input). We can thus expect to find a continuum between ‘slow and steady’ to ‘grow fast, die young’ strategies along land-use intensity gradients – and indeed land-use intensification tends to select for faster strategies of plants41, arthropods25,42, and microorganisms43.
Despite the evidence base presented above, until now, a lack of coordinated multitrophic abundance, functional trait, and ecosystem function data has hindered the investigation of synchronised responses at the level of entire communities, and their link to ecosystem functioning. However, a recent explosion in trait data availability44–47, combined with large-scale research platforms that survey multiple organismal groups and ecosystem functions simultaneously48–50, now allows this long-standing question to be addressed. Here, we use data from the large-scale and long-term Biodiversity Exploratories49 to test the hypotheses that there is a common, whole community-level slow-fast response to disturbance and resource availability in the form of land-use intensity, and that this community-level strategy drives a slow-fast ecosystem functioning response. The Biodiversity Exploratories is, to our knowledge, the most comprehensive data source for multiple guilds, with abundance data available from the same sites and at all trophic levels, both above- and belowground, including bacteria, fungi, protists, as well as plants, invertebrates and vertebrates33. These are collected in 150 grassland plots in three regions of Germany. We focus on the land-use intensity gradient of the Exploratories, which is a combined gradient of both resource availability (fertilisation, with high fertilisation being usually applied to the most inherently productive sites) and disturbance (mowing, which is strongly correlated with fertilisation; and grazing)51. Because existing theory on slow-fast strategies was lacking for some guilds, we first conducted expert workshops to identify traits expected to represent the slow-fast continuum for each guild (Tables S1 and S2). Based on pre-defined hypotheses from existing theories, observational studies, and expert knowledge, we selected several traits for each guild to represent the slow-fast continuum, and generated expectations of how these respond to resource availability and disturbance (Fig. 1, see Table S2 for full detail). Focusing on these pre-selected traits, we then tested three hypotheses: (H1) there is a synchronous functional response across trophic levels, shifting from ‘slow and steady’ to ‘grow fast, die young’ strategies with increasing land-use intensity. (H2) Land-use intensity drives the slow-fast response of the entire community through both direct and indirect (through a trophic cascade) pathways. (H3) The entire community ‘slow-fast’ trait axis corresponds to an ecosystem functioning ‘slow-fast’ axis, with ‘fast’ functioning defined as fast process rates (e.g., high productivity, rapid nutrient turnover). We show that the slow-fast response is coordinated among most guilds and drives an ecosystem functioning slow-fast axis, thus forming a whole ecosystem slow-fast axis.
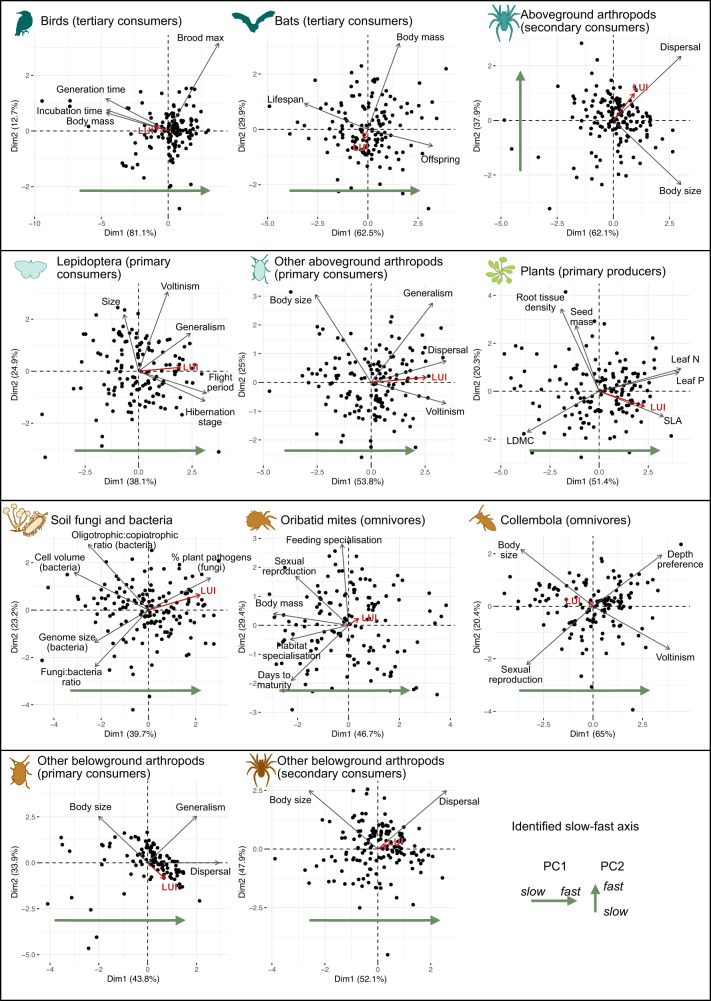
Fig. 1. Expected trait variations in ‘slow’ (low resource, low disturbance) and ‘fast’ (high resource, high disturbance) communities.
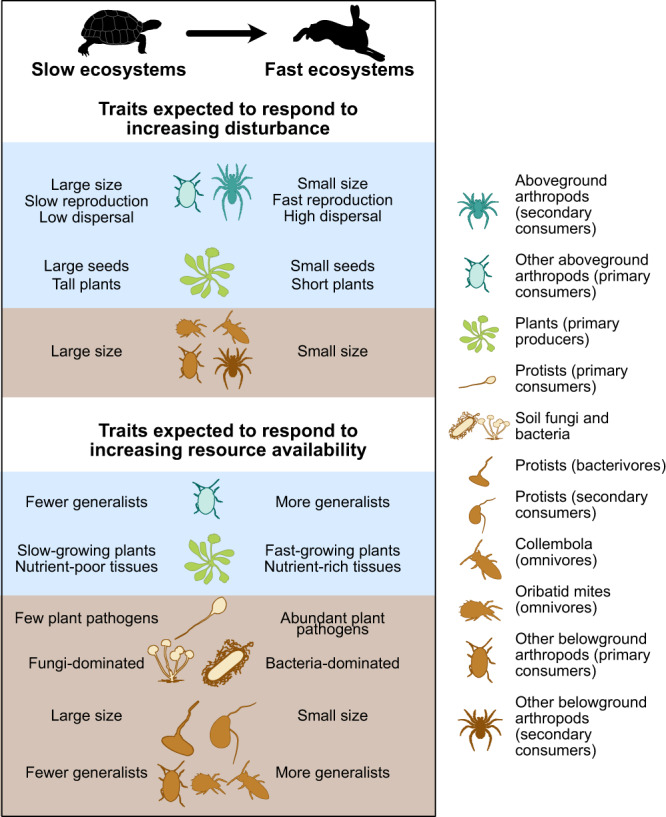
The tortoise and the hare icons represent Aesop’s fable “The Tortoise and the Hare”, which is the origin of the phrase “slow and steady wins the race”, here a potentially winning ecological strategy under conditions of low resource availability and low disturbance. The resource and disturbance gradients are, in this study, respectively represented by a fertilisation and a grazing/mowing gradient. Only a subset of hypotheses is shown8,15,19,21,22,24,57,61,64,147,268, see Table S2 for full details and references. Belowground guilds are shown in brown, aboveground guilds in blue. Icons were acquired and adapted from Phylopic.org (artists: M. Dahirel, B. Lang, M. Crook, J. A. Venter, H. H. T. Prins, D. A. Balfour, R. Slotow, T. M. Keesey, A. A. Farke, Y. Wong, G. Monger).
Results
We first identified ‘slow-fast’ traits at the community level within individual trophic guilds. To do this, we classified individual species into trophic guilds of broadly comparable trophic status (e.g., aboveground primary consumers or belowground omnivores). Different taxonomic groups with the same trophic status were aggregated into a single trophic guild if all trait data was consistent, otherwise they were kept separate. For instance, we had comparable trait data for many aboveground arthropods (size, feeding generalism, dispersal ability compiled from ref. 52, but data for Lepidoptera was available for different traits (hibernation stage, voltinism, etc) and compiled from a range of other sources53–56. Therefore, this group was treated separately from other primary consumer arthropods. This led to the definition of 14 homogeneous trophic guilds (hereafter ‘guilds’), listed in Table 1. For each guild, abundance-weighted, community-level mean (CWM) trait values were calculated and corrected for environmental covariates (see Methods). Some expected trait responses of different guilds to resource availability and disturbance are shown in Fig. 1 (see Table S2 for full details).
Table 1.
Trophic guilds taxonomic composition and assigned trophic position
| Guild | Taxa included | Trophic position |
|---|---|---|
| Birds (tertiary consumers) | Insectivorous and predatory birds | 3 |
| Bats (tertiary consumers) | Bats | 3 |
| Herb- and litter-dwelling arthropods (secondary consumers) | Omnivorous Hemiptera, carnivorous Coleoptera, Araneae, Orthoptera and Hemiptera | 2 |
| Lepidoptera (primary consumers) | Butterflies and day-flying moths | 1 |
| Other herb- and litter-dwelling arthropods (primary consumers) | Herbivorous Orthoptera, pollinators, herbivorous and detritivorous Coleoptera, and herbivorous Hemiptera | 1 |
| Vascular plants (primary producers) | All vascular plants | 0 |
| Microbial communities (decomposers) | Bacteria and fungi, including saprotrophs and parasites | 1 |
| Protists (primary consumers) | Plant parasites | 1 |
| Protists (bacterivores) | Bacterivorous protists | 2 |
| Protists (secondary consumers) | Predatory and omnivorous protists | 2 |
| Collembola (omnivores) | Collembola | 2 |
| Oribatid mites (omnivores) | Oribatid mites | 2 |
| Other belowground arthropods (primary consumers) | Herbivorous, detritivorous and fungivorous Coleoptera and Hemiptera | 2 |
| Belowground arthropods (secondary consumers) | Omnivorous or carnivorous Hemiptera, Coleoptera and Araneae | 3 |
Identification of guild-level slow-fast axes
We assumed that for each guild, the main axis of covariation between the selected slow-fast traits (Fig. 2) should represent the guild’s overall slow-fast response (‘axis’). If the selected traits were not strongly associated (weak covariation) in a principal coordinates analysis (PCA), we concluded that there was no observable slow-fast axis in the considered guild. Conversely, if most selected traits within a guild covaried, then their main axis of covariation was retained as the guild’s slow-fast axis. This covariation axis was defined through a principal component analysis with all traits within each guild, and retained if it explained more than twice of the shared variance as expected if traits were independent; and if the axis was correlated (r > 0.4) with at least 60% of the traits (see specific criteria in Methods).
Fig. 2. Identification of slow-fast axes for each guild.
PCA were run on the CWM of traits hypothesised to be related to the slow-fast strategies in each guild to identify a common slow-fast axis (green arrows). This was the first PC axis for all guilds, except aboveground secondary consumer arthropods. The evidence supported the hypothesised slow-fast axis for most guilds, and partially supported it for three guilds. For Lepidoptera, there was no response from body size, contrary to expectations. Body size was measured as wing length, an indicator of overall body size (which is expected to be a “slow” trait; smaller size helps to survive disturbance) but also of dispersal ability (a “fast” trait, as larger wings promote recolonisation after disturbance). Both effects might cancel each other leading to no response of size. For secondary consumer, aboveground arthropods, dispersal and body size are slightly confounded because larger body size increases dispersal abilities. However, these two traits are opposed on the second axis of the PCA, which we use as our slow-fast index. The last guild for which we found only partial support for the slow-fast axis was bats. High body mass is usually considered a ‘slow’ trait, and is expected to be positively correlated to lifespan and negatively to the number of offspring (trade-off between survival and reproduction). However, hibernation saves resources and leads, in hibernating bats (most of the species observed in our study), to a correlation between number of offspring and body mass228, leading to the results observed here. LUI: land-use intensity. Icons were acquired and adapted from Phylopic.org (artists: M. Dahirel, B. Lang, M. Crook, J. A. Venter, H. H. T. Prins, D. A. Balfour, R. Slotow, T. M. Keesey, A. A. Farke, Y. Wong, G. Monger).
The existence of the hypothesised guild-level slow-fast axis was supported by the data for most guilds, and it explained on average 52% (± sd 13) of the total variation in the 4.2 (± 1.8) CWM traits per guild that were included in the analyses. For three protist guilds (primary consumers, bacterivores, secondary consumers), only one trait was available, cell size, and we therefore assumed the existence of a ‘slow-fast’ axis defined by large to small cell size in subsequent analyses1,57. Of the remaining 11 trophic guilds, we found strong support for a community-level ‘slow-fast’ axis in nine guilds: plants (primary producers), aboveground arthropods (primary consumers), aboveground arthropods (secondary consumers), birds (tertiary consumers), belowground bacteria and fungi (decomposers), belowground arthropods (primary consumers), Collembola (omnivores), Oribatid mites (omnivores), and other belowground arthropods (secondary consumers). For instance, aboveground arthropods (primary consumers) displayed a clear trade-off between ‘slow’ communities dominated by large body size and slow reproduction, and ‘fast’ communities dominated by generalist-feeding species with smaller body size, higher dispersal abilities and multiple generations per year (PC1: 53.8% variance, Fig. 2). Similarly, Collembola (omnivores) displayed a trade-off between ‘slow’ communities, dominated by soil-surface-dwelling species with a large body size and sexual reproduction, and ‘fast’ communities, characterised by more organisms living deeper in the soil and capable of parthenogenesis, which leads to faster generation times (PC1: 65% variance). There was partial support for the existence of a ‘slow-fast’ axis in the remaining guilds (Fig. 2).
Slow-fast trait continuum across trophic levels
Next, we explored the degree of correspondence between the community-level ‘slow-fast’ trait axes of individual guilds. The data supported our overarching hypothesis H1: most of the guild-level ‘slow-fast’ axes were closely correlated with each other and with the land-use intensity gradient. A Principal Components Analysis (PCA) conducted on the slow-fast axes of all guilds with sufficient data availability (all except belowground arthropods (primary consumers), see Methods) revealed that 25% of the combined variation in all the guilds’ slow-fast axes was explained by the first PC axis of variation. This axis was strongly correlated with land-use intensity (Pearson correlation r = 0.74, p < 10−20, Fig. 3a). We also observed a significant and positive correlation between land-use intensity and the slow-fast axis score of nine (out of 14) guilds (p ranging from 1.10−14 to 0.04 and |r | > 0.2), with this correlation being particularly strong at low trophic levels. In contrast to our expectations, the highest trophic levels tended to have ‘slower’ traits at high land-use intensity (birds, r = −0.2, p = 1.5 10−2). We also tested for the existence of the whole community slow-fast axis by running a PCA on all considered traits from all guilds simultaneously (rather than aggregated into guild-level slow-fast axes, themselves extracted from PCAs). This analysis also supported the existence of a whole community slow-fast axis, with ‘slow’ and ‘fast’ traits clearly separated across sites (Fig. 3b).
Fig. 3. The slow-fast trait axis of individual guilds is strongly related to land-use intensity.
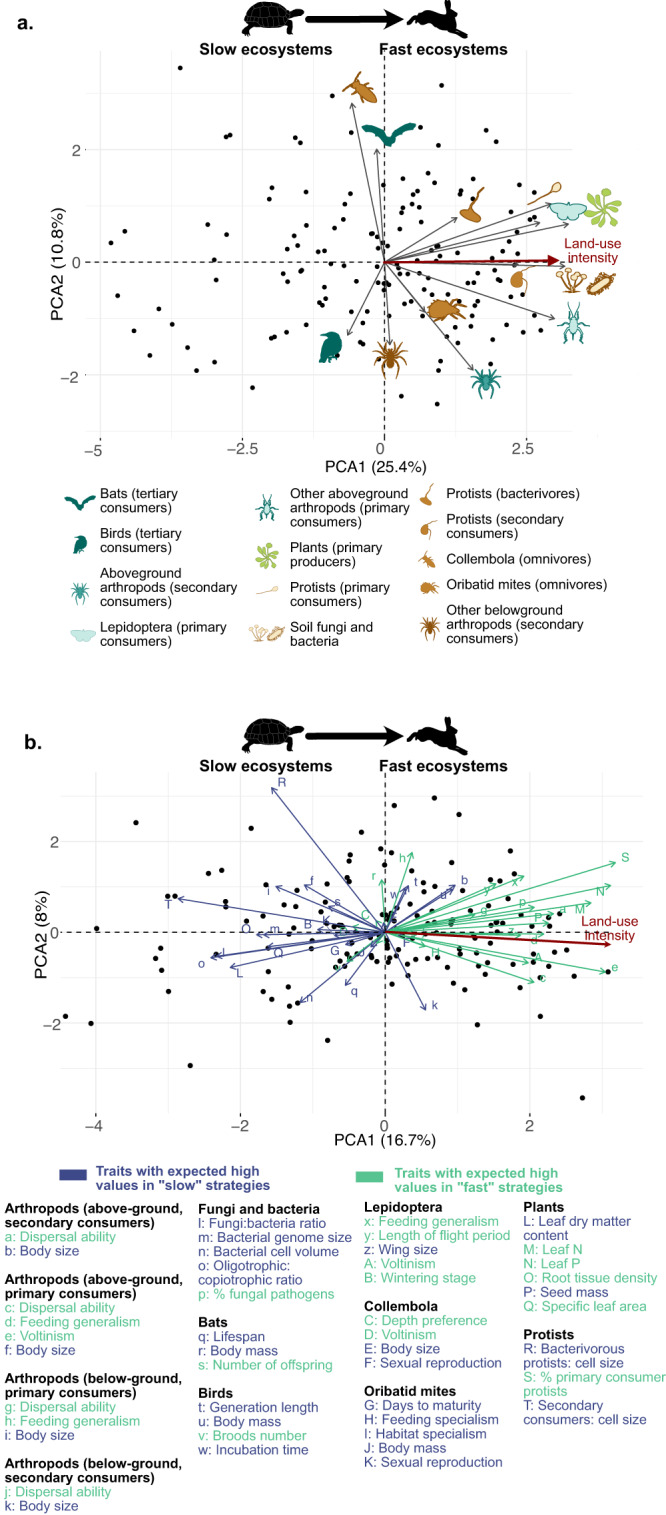
a Whole community slow-fast axis, based on guild-level slow-fast axes. The variables included in the PCA are the slow-fast axes of each guild, which were estimated as the PC axis that best represents the hypothesised slow-fast axis (PC1 in 90% of cases) in a PCA of the selected CWM traits expected to be related to slow-fast strategies for each guild individually. These guild-level PCA can be found in Fig. 2. Land-use intensity (which is projected on the PC axes, shown in dark red) is strongly associated with Axis 1. Belowground guilds are shown in brown, aboveground guilds in blue, and plants in green. b Whole community slow-fast axis, based on all traits. In contrast to the results shown in a, the PCA was conducted on all traits, at the CWM level. Each trait was weighted as 1/n, with n with n the number of traits available for the guild, so that all guilds are weighted equally. Traits expected to be ‘slow' are coded in blue, ‘fast' in green. Sample size: 150 (sample sizes for each individual guild are shown in Fig. 4, missing values were imputed to run the PCA, see Methods). Icons were acquired and adapted from Phylopic.org (artists: M. Dahirel, B. Lang, M. Crook, J. A. Venter, H. H. T. Prins, D. A. Balfour, R. Slotow, T. M. Keesey, A. A. Farke, Y. Wong, G. Monger).
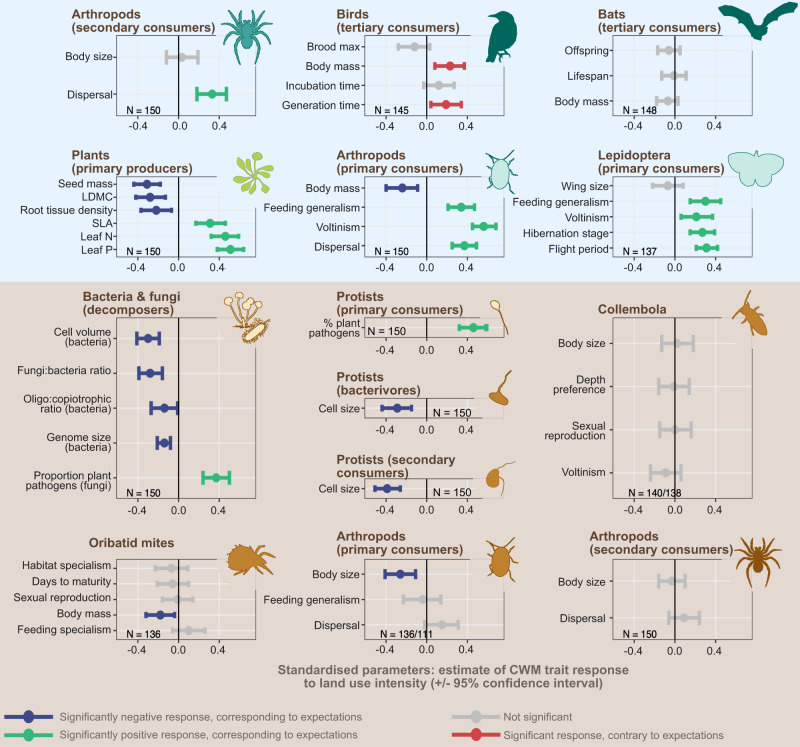
The observed covariation in slow-fast traits across guilds demonstrates that land-use intensity drives differentiation between entire communities, with ‘slow’ and ‘fast’ communities characterised by distinct trait syndromes (Fig. 3, Table S2). This conclusion was supported by the analysis of individual traits’ responses to land-use intensity, which shows that the CWMs of 54% of individual traits responded to land-use intensification in the hypothesised direction, and only two (4%) responding in an opposing direction (Fig. 4). ‘Slow’ communities are found at low resource availability and infrequent disturbance, and are characterised by slow-growing resource-conservative plants (correlation of leaf dry matter content with land-use intensity: r = −0.28, p = 7 10-4; root tissue density: r = −0.22, p = 7 10-3). Their soil microbial communities are dominated by fungi rather than bacteria (fungal:bacteria ratio: r = −0.28, p = 3 10-5), consistent with previous observations that low nutrient availability selects for fungal-dominated communities and slow-growing plants21,22. Bacterial communities in ‘slow’ communities also contain a higher proportion of small-celled organisms with large genomes (cell volume: r = −0.30, p < 10-8; genome size: r = −0.14, p = 2 10-4), an adaptation to growth in nutrient-poor (oligotrophic) conditions58 (oligotrophic:copiotrophic ratio: r = −0.14, p = 0.02). In contrast to the overall trend, birds had slower strategies in high intensity than low-intensity grasslands (slower generation length, r = 0.19, p = 0.02 and larger body size, r = 0.23, p = 0.005).
Fig. 4. Observed responses of individual traits at the guild-level (CWM) to land-use intensity, shown as estimated parameter +/− 95% confidence intervals.
The responses shown were extracted for each trait individually as the slope ± confidence interval of a linear model with the CWM trait as a response to land-use intensity after correction for other environmental covariates (see Figure S1). P-values (two-sided t-tests) were adjusted for multiple testing. Land use intensity explained between 0 (non-significant traits) and 40% (Aboveground primary consumer arthropods: voltinism) of the total variation in individual trait CWM (Table S2). Belowground guilds are shown over a brown background, aboveground guilds over blue. A standardised response below 0 (blue lines) indicate a negative response to land-use intensity, in accordance with hypotheses (expected higher trait values in ‘slow’ communities). Responses above 0 (green line) indicate a positive response to land-use intensity, in accordance to hypotheses (expected higher trait values in ‘fast’ communities). Red lines indicate traits with opposite responses to that hypothesised. 95% intervals crossing the 0 line indicate no significant response (grey lines). The number of replicates (sites with available data) are shown for each guild. Collembola: 138 for voltinism, 140 for the other traits. Arthropods belowground (primary consumers): 111 for feeding generalism, 136 for other traits. Icons were acquired and adapted from Phylopic.org (artists: M. Dahirel, B. Lang, M. Crook, J. A. Venter, H. H. T. Prins, D. A. Balfour, R. Slotow, T. M. Keesey, A. A. Farke, Y. Wong, G. Monger).
At the ‘fast’ end of the spectrum, high resource availability is associated with fast-growing plants with high nutrient content (leaf N content: r = 0.46, p < 10-8, leaf P content: r = 0.51, p < 10-8), which is often related to lower levels of physical and chemical defence59. This strategy was associated with a higher dominance of pathogenic fungi60 (proportion of pathogenic soil fungi, r = 0.37, p < 10-8) and plant pathogenic soil protists61 (r = 0.46, p < 10-8). In addition, high land-use intensity selected for smaller body size in four guilds (bacterivore protists: r = −0.29, p = 3 10-4; secondary consumer protists: r = −0.39, p < 10-8, aboveground arthropods (primary consumer) size: r = −0.24, p = 4 10-3; belowground arthropods (primary consumers): r = −0.24, p = 2 10-3) and faster reproduction19 in two guilds (voltinism of Lepidoptera (primary consumers): r = 0.21, p = 0.01; and other aboveground arthropods (primary consumers): r = 0.56, p < 10-8). This response is likely driven by disturbance, which causes higher mortality in large-bodied arthropods62,63, and favours early reproductive maturity and fast reproduction as organisms can reproduce before disturbance, and population numbers can recover quickly afterwards. In addition, dispersal ability was greater in the fast communities of high land-use intensity (dispersal ability of aboveground arthropod (secondary consumers): r = 0.33, p = 3 10-5, aboveground arthropods (primary consumers): r = 0.37, p < 10-8). This higher dispersal capacity enables faster recolonisation after disturbance15,62.
The slow-fast trait responses of some consumer guilds was potentially driven not directly by resources and disturbance, but indirectly via losses in plant diversity associated with increased fertilisation64,65. This commonly leads to the loss of associated specialist plant species that are typically found in slow ecosystems33,66,67. As such specialist plant species often have higher degrees of physical and chemical defences, they tend to be associated to specialist herbivores68. In our study, this effect was manifested by an increased dominance of generalist species with land-use intensity, both among Lepidoptera and other aboveground arthropods (primary consumers) (feeding generalism of Lepidoptera: r = 0.30, p = 3 10-4; and of aboveground arthropods (primary consumers): r = 0.34, p < 10-8).
Changes in community-level traits along environmental gradients can be due to changes in species identity (taxonomic turnover) or variation in species relative abundance. In our case, we found that both factors were responsible for the observed slow-fast responses to land-use intensity within the different guilds (average turnover across guilds: 0.95 ± 0.03; average nestedness: 0.03 ± 0.03, Table S3).
Land-use intensity effect on community-level slow-fast response
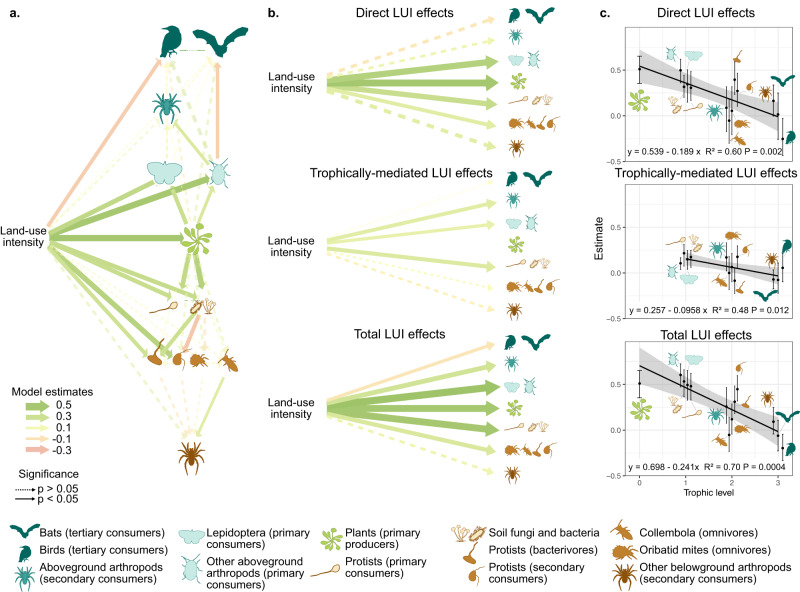
The observed entire community-level slow-fast continuum could be driven by a common, but independent, response of individual guilds to land-use intensity, or mediated by cascading bottom-up trophic interactions between guilds, and we hypothesised that both pathways were important (Hypothesis 2). To test this, we used Structural Equation Modelling (SEM) to assess the effect of these pathways on the ‘slow-fast’ axes of the 13 guilds. We found that both pathways were important, supporting Hypothesis 2: slow-fast strategies axes are shaped both directly via shared responses to land-use intensity and indirectly via trophic interactions. Land-use intensity showed significant direct effects on the ‘slow-fast’ axis of eight guilds out of 13 (average estimate: 0.20 ± 0.06), and indirect, i.e., trophically-mediated, effects on six guilds (0.07 ± 0.02). The pathways combined to make significant total effects of land-use intensity (0.27 ± 0.07) on nine out of the 13 guilds (Fig. 5a).
Fig. 5. Direct and trophically-mediated effects of land-use intensity on the slow-fast axis of individual trophic guilds.
a Full SEMs including all guilds. Two independent models were fitted for below- and aboveground guilds; plants were included in both. b Average direct, trophically-mediated (indirect) and total land-use intensity (LUI) effects on each trophic level (averaged across guilds within each trophic level from the SEM estimates shown in a. c Decreasing direct, indirect and total land-use intensity effects with trophic level. Each dot represents the estimated effect (mean ± standard error) of land-use intensity on the slow-fast axis of each individual guild in the full SEM. Sampling size per guild: 150 for all guilds except Oribatid mites (136), Collembola (138), Lepidoptera (137), birds (145) and bats (148). In a and b colours indicate the path estimate value from negative (orange) to positive (green); p-values are extracted from two-sided z-tests; in c two-sided t-tests; they were not corrected for multiple testing. Precise p-values can be found in Tables S6 and S7. Belowground, primary consumer arthropods were excluded (see Methods section). Icons were acquired and adapted from Phylopic.org (artists: M. Dahirel, B. Lang, M. Crook, J. A. Venter, H. H. T. Prins, D. A. Balfour, R. Slotow, T. M. Keesey, A. A. Farke, Y. Wong, G. Monger).
Next, we obtained a general assessment of how land-use intensity affected the slow-fast axis of each trophic level, by averaging the direct, indirect and total effects for all guilds at each trophic level (producers vs. primary vs. secondary vs. tertiary consumers). This showed that the ‘slow-fast’ axes of lower trophic levels responded more strongly than higher trophic levels to land-use intensity, via both the direct and indirect paths. While there was a significant total effect of land-use intensity on all trophic levels, except belowground secondary consumers (Fig. 5b), the direct (p = 2 10-3), indirect (p = 0.01) and total (p = 4 10-4) effects all significantly decreased in intensity with trophic levels, indicating more synchronous trait responses at lower trophic levels (Fig. 5c).
Slow-fast ecosystem functioning response
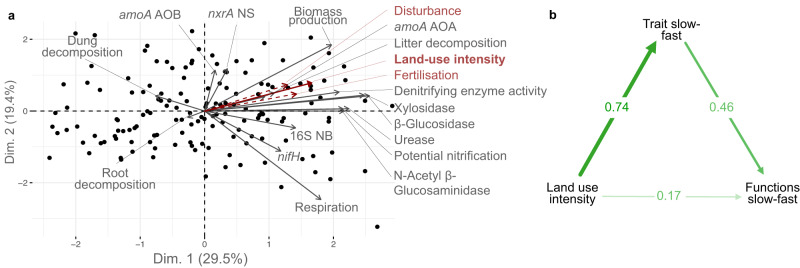
To test our third hypothesis, that the community-level slow-fast continuum was related to whole ecosystem function, we first conducted a PCA based on 15 ecosystem functions related to carbon fluxes, nitrogen fluxes, biomass production and decomposition. This showed that ecosystem functions covary, from ‘slow’ sites characterised by slow decomposition, biomass production and low enzyme activities to ‘fast’ sites with faster nutrient cycling (first axis of the PCA: 29% variance, Fig. 6a). This whole ecosystem functioning slow-fast axis was better explained by the entire community ‘slow-fast’ traits axis (r = 0.4, R2 = 0.34, p < 10-8) than by other hypothesised drivers of ecosystem functioning (single guild community traits measures (plants and microbes), land-use intensity or taxonomic diversity, R2 from 0.11 to 0.26, Table S4). The exception to this was fungal-bacterial ratio, which was correlated with the whole slow-fast axis (r = −0.84, P < 10-8) and better explained the functions slow-fast axis (R2 = 0.47, Figs. S5 and S6). This was likely due to the prevalence of soil-related measures in our selected set of functions. Additionally, some individual functions were more strongly associated with specific guilds or traits (e.g., fast plant community traits were positively associated with biomass production (Fig. S5)). Results were similar when using the all traits slow-fast axis (PC1 in Fig. 3b) as the main community slow-fast axis (Fig. S4).
Fig. 6. The ecosystem functions slow-fast axis is linked more strongly to the trait slow-fast axis than to land-use intensity.
a PCA of the 15 selected ecosystem functions. Functions within each bundle (carbon fluxes, nitrogen fluxes, biomass production and decomposition) are weighted inversely to the number of functions in the bundle. The first axis shows a slow-fast axis from slow decomposition, biomass production and low enzyme activities to faster nutrient cycling. Land-use intensity was projected onto the PCA, and is strongly associated with axis 1. b Direct and indirect links between land-use intensity, the entire community traits slow-fast axis (identified in Fig. 3a: PC1 of the PCA) and the ecosystem function slow-fast axis. DEA denitrification enzyme activity; urease, β glucosidase, N-Acetyl β glucosaminidase, xylosidase: activity of the respective enzyme; amoA_AOA: abundance of ammonia oxidation gene of archaea; amoA_AOB: abundance of ammonia oxidation gene of bacteria; nifH: abundance of nitrogen fixation gene in soil bacteria; nxrA NS: abundance of nitrite oxidation gene of Nitrobacter bacteria; 16S NB: abundance of nitrite-oxidizing bacteria; dung decomposition; plant aboveground biomass production; litter decomposition; root decomposition. Sample size: 150 sites (sample sizes for each individual guild are shown in Fig. 4, missing values were imputed to run the PCA, see Methods section).
A mediation analysis found that the effect of land-use intensity on the ecosystem functioning ‘slow-fast’ axis was both direct and mediated by the functional traits ‘slow-fast’ axis, as both direct and indirect paths were significant (Fig. 6b). Together these results support Hypothesis 3 and suggest that the whole community fast-slow trait continuum is linked to a slow-fast axis of whole ecosystem functioning.
Sensitivity analyses
While there were strong hypothetical reasons to expect body size to be a key trait driving fast-slow variation at the community level, it can respond to a range of drivers and drive life-history variation in the absence of other trait responses; as a result, the effect of body mass is often removed in analyses that seek to identify life-history trade-offs69,70. To assess the robustness of our results to this trait, we conducted additional analyses in which we excluded all body size data (resulting in the exclusion of bacterivorous and predatory protists as other trait data were not available for these groups). Even in the absence of body size were still able to identify a strong guild-level slow-fast axis for most guilds, except Oribatid mites. This resulted in somewhat weaker, but still consistent results regarding the synchrony of slow-fast axes across guilds and the effect of the whole community slow-fast axis on ecosystem functioning (Tables S8–S10, Figs. S7–S9).
Other sensitivity analyses included checking the impact of using raw CWM data (uncorrected for environmental covariates) and unweighted (instead of using abundance-weighted) trait values. These are discussed in the supplementary analyses (Tables S11–S18; Figs. S10–S20); they show overall weaker, but consistent results with those presented here.
Discussion
Our results provide strong evidence for the existence of synchronous, whole ecosystem-level responses to environmental drivers, and more specifically the existence of a slow-fast axis of variation at the level of entire multitrophic communities. We show that there are similar functional responses to resource availability and disturbance across taxa and trophic levels, from large organisms with slow reproduction and slow dispersal at low land-use intensity to small, fast-paced organisms in more intensively managed sites. The findings of this axis extend earlier studies which investigated the coordinated responses of a few trophic guilds24–26,71 and demonstrate a previously unrecognised emergent property of multitrophic community assembly. We also demonstrate that entire community-level synchrony is driven by both the shared responses of individual guilds to land management and trophically-mediated cascades. Synchrony was stronger at the lower trophic levels which make up the vast majority (>99%) of community biomass72, with weaker effects for the less abundant organisms of higher trophic levels (e.g., birds). This is likely due to higher trophic level organisms, e.g., birds and bats, being less dependent on local management conditions and instead responding to larger-scale drivers, such as landscape composition33. Belowground organisms also tended to show weaker responses than aboveground ones, likely due lower trait data availability weakening our capacity to detect a response and lower disturbance of the soil environment than the aboveground environment by the intensification studied here - mowing and grazing33. Finally, we provide evidence that this whole community strategy variation mediates the effect of land-use intensity on overall ecosystem functioning. More specifically, communities dominated by faster, non-conservative strategies (e.g., faster reproduction, shorter lifespan) have faster metabolic rates per unit biomass, a higher digestibility and/or tissue resource concentrations and rapid turnover of tissues. All these factors lead to a faster transfer of resources from organic to inorganic pools and between trophic levels28,73,74. In terms of ecosystem functioning, this equates to greater productivity and gas fluxes, higher soil enzyme activities, and faster decomposition and rates of organic matter mineralisation. This extends previous findings that demonstrate the linkages between traits of individual guilds and ecosystem functions23,36,37.
The theory describing slow and fast strategies, and the identity of their defining traits, is very well developed in some organisms such as plants8,30,75. In most other taxa, however, such theories are only emerging (e.g. microorganisms13, arthropods76). Here, we built upon both in-depth expert discussions and observational studies to define and test hypotheses on the responses of individual taxa to resource availability and disturbance. While this allowed us to generate insights into possible slow-fast strategies for multiple functional groups our need to do this also highlights the need for further theory development on the functional strategies of many taxa. Such theoretical advances would provide key building blocks towards the improved understanding of the response of slow-fast functional strategies to environmental drivers at the level of individual organisms, guilds and entire communities. Such theory could also support the integration of other axes of functional variation, which have been described for individual taxa at species3,31, and community level77.
Our results highlight that the average functional strategy of guilds, as represented by the CWM of multiple traits, can be meaningfully related to land-use intensity, linkages with other guilds, and measures of ecosystem functioning. By focusing on CWM trait means, we did not consider the role of strategy variation within a community, which can be considerable78. This functional diversity can represent multiple winning strategies within a community and/or niche differentiation from the optimal strategy, and thus the avoidance of competitive exclusion. Functional and taxonomic diversity both within and across taxa has been shown to play an important role in driving multitrophic interactions79 as well as ecosystem functioning80 and plant diversity is commonly related to higher levels of ecosystem functioning81–83. Previous studies have explored the relative response of dominant strategies, versus their variability, in response to both environmental factors84 and as drivers of ecosystem functioning85. Expanding this approach from single groups to whole communities could provide new insights into the behaviour of functional diversity as a multitrophic property that may drive ecosystem structure and functioning at a ‘whole systems’ level.
It is worth noting that the whole ecosystem ‘slow-fast’ gradient observed here was manifested across a relatively short environmental gradient - all sites were temperate agricultural grasslands. It remains to be seen whether the results hold across time, as suggested by dynamic linkages between plant and microbial traits in time22, and for other systems, in particular a diversity of longer, natural and more orthogonal gradients of disturbance and resource availability. We hypothesise that across stronger environmental gradients of climate and soils, e.g., between biomes, and when incorporating a more comprehensive array of slow-fast traits, ecosystem-level trait synchrony will be even more marked. Correspondence between global gradients in climate and soils and plant community-level trait measures86 support this idea, especially given that the traits of other trophic levels are often strongly associated with plant communities22–24. The close relationship between our ecosystem slow-fast axis and fungal-bacterial ratio also suggests that this axis is compatible with earlier literature pointing to a slow-fast functioning gradient from fungal-to bacteria-dominated communities21,28,87 and that fungal-bacterial ratio could act as an indicator variable for the whole ecosystem slow-fast continuum. Such synchrony may also correspond to recently described global trends in the covariance of multiple ecosystem functions39,88. It could also be related to other widely reported patterns of ecological change, such as the global “community downsizing” of animal communities that occurs in response to both climate change and anthropogenic disturbance89,90 and its impact on ecosystem functioning91, though further theoretical integration would also be required. When combined with these results, our findings highlight the potential for a new generation of whole ecosystem-level studies that go beyond the description of single trophic guild-level strategies to characterise communities, or single functions to characterise ecosystem responses. Instead, these would work at the level of universal ecosystem-level functional types and axes38,39. This may form the basis of a new typology for classifying ecosystems, and provide a generalisable, predictive and simple means of describing their responses to land-use and environmental change.
Methods
Study area
The study was conducted as part of the long-term Biodiversity Exploratories project (www.biodiversity-exploratories.de). Data was collected in 150 grassland plots in three regions of Germany: the Schwäbische Alb plateau and UNESCO Biosphere Reserve in south-western Germany; the Hainich National Park and surrounding areas in central Germany (both are hilly regions with calcareous bedrock) and the UNESCO Biosphere Reserve Schorfheide-Chorin in the post-glacial lowlands of north-eastern Germany. The three regions differ in climate, geology and topography, but each is characterised by a gradient of grassland land-use intensity that is typical for large parts of temperate Europe49. In each region, 50 plots (50 m × 50 m) were chosen in secondary wet, mesic and dry grasslands by stratified random sampling from a total of 500 candidate plots on which initial vegetation, soil and land-use surveys were conducted. This ensured that plots covered the whole range of land-use intensities and management types, while minimising confounding factors such as spatial position or soil type.
Land-use intensity
Land-use intensity was assessed annually via questionnaires sent to land managers in which they reported the level of fertilisation (kg N ha−1 yr−1), the number of mowing events per year (from one to three cuts, starting in May and continuing up to September/October in the most intensive plots), and the number and type of livestock and their duration of grazing (number of livestock units ha−1 yr−1)49,51. Mowing and grazing intensities determine the frequency, and intensity, at which aboveground biomass is removed; and thus represent the intensity of disturbance in the plot. Fertilisation provides additional nutrients, and is typically mostly applied in naturally productive plots: it thus represents a resource availability gradient. In our study system, mowing and fertilisation intensities are positively correlated (r = 0.7), while grazing and mowing intensities are negatively correlated (r = −0.6). Thus, independent effects of each land-use component, and the respective effect of resource availability and disturbance, cannot be reliably estimated. We therefore used a compound index of land-use intensity, characterising a combined resource availability and disturbance gradient. The land-use intensity index (LUI) was calculated as the square-root-transformed sum of standardised measures of global mowing, fertilisation and grazing intensities across the three regions for each year92. We calculated the mean LUI for each plot over the years 2008–2018 because this reflects the average LUI around the years when most of the data was collected. At low LUI (0.5–0.7), grasslands are typically neither fertilised or mown, but grazed by one cow (>2 years old) per hectare for 30 days (or one sheep per hectare for the whole year). At an intermediate LUI (around 1.5), grasslands are usually fertilised with less than 30 kg N ha−1y−1, and are either mown twice a year or grazed by one cow per hectare for most of the year (300 days). At a high LUI (3), grasslands are typically intensively fertilised (60–120 kg N ha−1y−1), are mown 2–3 times a year or grazed by three cows per hectare for most of the year (300 days), or are managed by a combination of grazing and mowing. The study area did not cover very high intensity grasslands (e.g., cut more than three times per year and ploughed annually).
In some figures, LUI was added as a supplementary variable to e.g., PCAs, along with a ‘fertilisation’ variable and a ‘disturbance’ index. The fertilisation variable is the standardised fertilisation value. The disturbance index is calculated as the square-root of the sum of mowing and grazing intensities, both standardised by dividing by their overall average.
Acquisition of environmental covariates
To measure pH and soil texture, composite samples were taken in 2011 in all 150 plots, by mixing 14 mineral topsoil samples (0–10 cm, using a split tube manual soil corer with 5 cm diameter). Ten g of sieved and air-dried soil were mixed with 25 ml 0.01 M CaCl2 solution and shook for 2 h. Afterwards the pH of the soil suspension was measured using a glass electrode. The pH of each sample was measured twice. We determined soil texture by separating soil particles into sand (2–0.063 mm), silt (0.063–0.002 mm) and clay (<0.002 mm) by sieving and sedimentation (DIN-ISO 11277). Open datasets from Biodiversity Exploratories: refs. 93–95.
The topographic wetness index (TWI) combines measures of upslope contributing area (determining the amount of water received from upslope areas) and slope (determining the loss of water from the site to downslope areas) and has been shown in previous analyses to be a better predictor than local humidity measures33. It is defined as ln(a/tanB), where a is the specific catchment area (cumulative upslope area which drains through a Digital Elevation Model (DEM, http://www.bkg.bund.de) cell, divided by per unit contour length) and tanB is the slope gradient in radians calculated over a local region surrounding the cell of interest. TWI was calculated from raster DEM data with a cell size of 25 m for all plots, using GIS tools (flow direction and flow accumulation tools of the hydrology toolset and raster calculator). The TWI measure used was the average value for a 4 × 4 window centred on the plot, i.e., 16 DEM cells corresponding to an area of 100 m × 100 m. Dataset from the Biodiversity Exploratories: ref. 96.
Finally, we measured mean annual temperature as the temperature 2 m aboveground level in each plot, aggregated at the year level and averaged between 2008 and 2015. Open dataset from Biodiversity Exploratories: ref. 97.
Sampling protocols by trophic guild
In each plot, we measured the relative abundance of multiple guilds using standard methodology. Most data was extracted from an aggregated diversity dataset from the Biodiversity Exploratories98,99. Original datasets are listed below.
Vascular plants
We sampled vascular plants in an area of 4 m × 4 m in all 150 plots, and estimated the percentage cover of each occurring species every year from 2008 to 2019. Open dataset from Biodiversity Exploratories: ref. 100.
Soil fungi and bacteria
Composite soil samples were taken in 2011, 2014 and 2017 in all plots, by mixing 14 mineral topsoil samples (0–10 cm, using a split tube manual soil corer with 5 cm diameter).
For bacteria the analysis only included data from 2011 (148 plots) and 2014 (150 plots). 10 g of the homogenised soil was put immediately on liquid nitrogen and stored until RNA extraction. RNA was extracted using a custom protocol (Lueders protocol). Total RNA was isolated from soils and reverse transcribed into cDNA. Amplicons of the V3 region of the 16S rRNA gene were sequenced on an Illumina Hiseq platform using universal bacterial primers.
For fungi, total microbial DNA was isolated from the bulk soil sample using a MoBioPowerSoil DNA Isolation Kit. A PCR approach was used to amplify fungal ITS-rDNA by using the primer pair ITS4/fITS7, containing the Illumina adapter sequences. PCR products were then purified, cleaned and sequenced using Illumina MiSeq. Data was available for 150 plots in 2011, 2014 and 2017.
Open datasets from Biodiversity Exploratories: refs. 101–105. Other dataset from the Exploratories: ref. 106.
Soil protists
Soil DNA was extracted in 2011 and in 2017, from 400 mg of soil (not sieved), 3- to 6-times, using the DNeasy PowerSoil Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s protocol. Data for one plot in 2011 was missing, data for all 150 plots was available in 2017. Specific primers for Cercozoa and Endomyxa were used to amplify, by two semi-nested PCRs, the V4 region of the 18 S rRNA gene. Libraries were prepared using TruSeqDNA PCR-Free (Illumina, San Diego, CA, US). Sequencing was performed with a MiSeq v3 Reagent kit of 300 cycles (on a MiSeq Desktop Sequencer, Illumina). The bioinformatics pipeline was conducted with Mothur v. 39.5. After assembling and quality filtering, sequences were clustered at 97% similarity, and rare clusters (<0.01% of the reads) were removed. OTUs were identified in the PR2 database using BLASTn with an e-value of 1e50 and keeping only the best hit. Chimeric OTUs were identified using UCHIME and removed61.
Open datasets from Biodiversity Exploratories: refs. 107,108.
Collembola and Oribatid mites
In 2019, we sampled four soil cores of 4.5 cm × 10 cm per plot. Collembola and Oribatid mites were then extracted following a Kempson extraction and identified. Collembola data was available for 140 plots. For Oribatid mites, it was available in 149 plots but some plots had to be excluded due to low trait data coverage (see below), resulting in 136 used plots in total. Datasets from the Biodiversity Exploratories: refs. 109,110.
Lepidoptera
Lepidoptera were recorded using sweep netting along transects of 300 m during 30 min, done three times per plot in 2008. Data was available for 137 plots. Open dataset from the Exploratories: ref. 19.
Other arthropods
All arthropods of the herb layer were sampled twice per year between 2008 and 2017 in June and August to represent different phenological windows within the peak season of adult arthropod activity. Total number of plots sampled each year varied from 143 to 150, all plots were sampled at least eight years (average: 9.8 years). Based on monthly samplings at the beginning of the study, we identified these two months to represent the best trade-off between reducing sampling effort and covering most species. Arthropods were sampled by sweep netting along a 150-m long transect comprising three of the virtual borders of a site by conducting 60 double sweeps per site. Sweep netting was only conducted on days without rain, low wind speed and after morning dew had dried. All samples were sorted to order level in the laboratory. For taxonomic groups that occurred in larger numbers and for which expert taxonomists were available, adult specimens were identified at species level: Araneae, Coleoptera, Hemiptera (Heteroptera and Auchenorrhyncha), Orthoptera. Species that spend a significant part of their lifecycle in the soil or litter were classified as belowground species. Open data from the Exploratories: ref. 111.
Birds
Birds were recorded using audio-visual point counts during breeding times (March-June), at the centre of each respective grassland plot (50 m × 50 m). This was done every year between 2008 and 2012 and again in 2018. Records were available for 72–128 plots per year, with four years of records available per plot on average. Open datasets from the Biodiversity Exploratories: refs. 112–117.
Bats
Acoustic recordings were conducted along the edges of each plot, using a combination of point-stop and transect monitoring in 2009 and 2010. Point-stops were located at each corner of the plot. Transects were walked slowly and in a direct line between the point stops. Survey time at point-stops and during transect walks were 3 min each. This resulted in a total survey time of 24 min along a 200 m transect per plot. Recordings were made using a Pettersson D 1000× ultrasound detector (Pettersson Electronic AG, Uppsala, Sweden). Echolocation calls were identified to species level or to Sonotype using the software Avisoft SAS Lab Pro, Version 5.0.24 and onward (Raimund Specht, Avisoft Bioacoustics, Berlin Germany). Records were available for 148 plots in total, including 134 in 2009 and 130 in 2010. Open datasets from Biodiversity Exploratories: refs. 118,119.
Aggregation to functional guilds
These taxa were compiled into functional guilds in which trophic status was broadly comparable (e.g., aboveground primary consumers or belowground omnivores). Within each functional guild, organisms of different taxa were aggregated if trait data was comparable, and if not, the functional guild was classified at the taxonomic level (Table 1). Aboveground guilds included vascular plants (primary producers); Lepidoptera (primary consumers – incl. butterflies and day-flying moths); other aboveground primary consumers arthropods (incl. primary consumers of Orthoptera, Coleoptera, and Hemiptera); aboveground secondary consumers arthropods (including omnivorous Hemiptera, carnivorous Coleoptera, Araneae, Orthoptera and Hemiptera); bats (tertiary consumers); and birds (tertiary consumers, i.e., excluding herbivorous and granivorous birds). Belowground guilds included: bacterial and fungal communities (symbionts, decomposers and parasites); plant parasite protists (primary consumers); bacterivorous protists; secondary consumers protists (incl. predators and omnivores); Collembola (omnivores); Oribatid mites (omnivores); other herbivorous, detritivorous and fungivorous belowground arthropods (primary consumers, incl. Coleoptera and Hemiptera); and predatory and omnivorous, soil-dwelling arthropods (incl. Araneae, Coleoptera, and Hemiptera).
Functional trait data acquisition and treatment
Although it is general in overall concept, and has been discussed in a wide range of contexts, the spectrum of slow-fast strategies and their associated trade-offs has a well developed, trait-specific theory for only a few specific taxa, such as plants. Such theories are also emerging in the literature for other taxa13,120 but in many cases are not fully developed yet. Selecting appropriate traits thus required us to combine hypotheses from existing group-specific theories8, more general ecological theories (e.g., r/K1,4), empirical observations, and expert knowledge. We conducted multiple expert consultation discussions within subteams of the authors to identify traits potentially related to a resource availability and disturbance, and thus potentially falling on a slow-fast axis, i.e., size, dispersal abilities, feeding niche, etc. Detailed hypotheses guiding the choice of traits for each guild can be found in Table S2.
Trait data was obtained from multiple sources. Abundance data and trait databases were matched using the GBIF taxonomy (packages taxize, traitdataform121) when necessary. Guild-specific details on trait data acquisition and treatment are described below.
Plants
Aboveground plant traits were compiled from the TRY database5,23,36,45,122–220. TRY data was first cleaned to remove duplicates, non-mature, non-healthy plants and plants which were grown in non-standard exposition (e.g. shade) as well as non-standard trait measurements. The trait data was then subsetted by data from central Europe to avoid geographic bias. Finally, resulting trait values were averaged by contributing sources, with outlier sources being excluded; and were then averaged for each species221. Belowground trait data was obtained from pot experiments122. Where appropriate, data from synonyms was used. When individuals were identified at the genus level, average trait values for all other species from the considered genus found during the survey was used. Community-weighted means were calculated by excluding tree saplings which are not part of the stable grassland communities and do not have the (adult) traits usually reported in databases.
Birds
Bird trait data for body length and incubation time was obtained from the literature and own datasets47,222–226. Data on functional groups and trophic levels was extracted from ref. 225 and the Avonet dataset47. Identification of ground-nesting species followed ref. 223. Maximum longevity and generation length were extracted from ref. 222. While trait data was also available for herbivorous species, the abundance data was not sufficient to include this group as 46 plots did not have any primary consumers (primarily herbivorous, frugivorous, and granivorous species). Thus, only secondary consumers (including carnivores, invertivores, and omnivores whose diet includes vertebrates or insects) were included in the study.
Bats
Bat traits data was obtained from ref. 227 (morphological traits) and ref. 228 (life-history traits). Both datasets were then restricted to species occurring in Germany based on ref. 229.
Abundance data was based on acoustic recording, which did not always allow for species-level identification: some individuals were classified as genera (Myotis sp., Plecotus sp.) or similarly acoustic groups (Nyctaloids). In that case, traits were attributed based on the average values of species from the same genus present in Germany, or on all species in the Nyctaloid group (Nyctalus octule, Vespertilio murinus, Eptesicus serotinus, Nyctalus leisleri, Eptesicus nilssonii), respectively. The interpolation was usually relatively conservative (trait mean > 2×trait sd).
Arthropods: Araneae, Coleoptera, Hemiptera and Orthoptera
Body size (in mm), feeding guild, stratum use and dispersal ability of Araneae, Coleoptera, Hemiptera and Orthoptera were assembled from various literature sources and validated in correspondence with taxonomic experts for the respective groups. These were augmented by published trait data on feeding generalism from ref. 52 and voltinism from multiple sources3,230–232. Species were classified as secondary consumers if one of their life stages was mostly carnivorous, and as primary consumers otherwise (i.e., all life stages primarily herbivorous, fungivorous or detritivorous). Species were classified as belowground if at least one of their life stages was primarily soil- or ground-dwelling, and aboveground otherwise. Voltinism was coded as a numeric variable (0.5: semivoltine, 1: univoltine; 1.5: uni- or bivoltine; 2: bivoltine; 3: multivoltine). Dispersal was coded as a numerical variable (in five gradations (from low to high: 0, 0.25, 0.5, 0.75, 1) based on flight ability and wing dimorphism for insects and ballooning for spiders). Body size was log-transformed to avoid non-normal distribution. When sampled individuals were only identified at genus level in the abundance dataset, they were attributed the average traits of the other species in the genus only when the trait data was homogeneous (i.e., dominant category if it is representative of 90% of the species in the genus among the sample species, and if the mean is > 2×standard deviation for quantitative variables); and was considered as a missing value otherwise. Open dataset from the Biodiversity Exploratories: ref. 233.
Arthropods: Lepidoptera
Traits for Lepidoptera were compiled from multiple databases. We used as primary sources ref. 55 for moth traits and ref. 19 for butterfly traits. In addition, we completed this data with traits from ref. 54, and the European and Maghreb Butterfly Trait database56. When datasets disagreed, we primarily kept the data from ref. 55 which was specifically curated for German moths. Voltinism was coded as a numerical variable: 0.5 for semivoltine species, 1 for strictly univoltine species, 1.25 for univoltine species with partial generation, 1.5 for uni- or multivoltine species, and 2 for multivoltine species. Feeding generalism was coded as a numerical variable: 1 for monophagous species, 2 for oligophagous species (within one plant genus), 3 for oligophagous species (within one plant family), and 4 for polyphagous species. Body size was provided as different metrics for the different databases: estimated dry mass, forewing minimum and maximum length in ref. 54, forewing average length in ref. 56, and body mass and wing length in ref. 55. The most complete was the forewing maximum54; we used data imputation (function mice, using default parameters) to impute the missing values in this variable, using all other size-related variables as predictors. The distribution of the data was similar before and after imputation, indicating reliable imputation. Body size was then log-transformed. Overwintering stage was coded as a numeric variable, from 1 (overwintering as egg), 2 (larvae), 3 (pupa) and 4 (adult). When datasets disagreed, we retained the latest stage of development as overwintering stage. Flight period was measured as the maximum number of flight months for adults. Again, ref. 54 was the most complete database. We used this data when available, and imputed missing values (function mice, default parameters) based on additional data from ref. 56. Flight period was then log-transformed. We completed the resulting data for three additional species (Lythria purpuraria, Zygaena carniolica, Aphantopus hyperantus) based on https://lepiforum.org/https://lepiforum.org/ and http://www.pyrgus.de/. Open datasets from Biodiversity Exploratories: refs. 53,234,235.
Arthropods: Collembola and Oribatid mites
Data on adult body mass and habitat specificity was collected on specimens collected during sampling (see above). Habitat specificity was coded numerically from 1 (non-specific habitat, most generalist), 2 (soil-dwelling), 3 (surface-dwelling) and 4 (litter-dwelling, which are the most specific species). Feeding specialisation was coded numerically from 1 (omnivorous, most generalists), 2 (herbifungivorous), 3 (herbivorous) and 4 (fungivorous, most specialist). (V. Wolters and A Zaytsev, pers. comm). Time to maturity (in days) and reproduction type (sexual or parthogenetic) were provided by experts and completed from the literature and coded as binary variables. Seven plots had low trait data coverage (<50%); they were given NA values for all CWM values and not used in the analysis. Dataset from the Biodiversity Exploratories236.
Bacteria and fungi
We used a published dataset46 to characterise bacterial communities.
For bacteria, trait data was used at genus level when available. If no genus-level data was available, it was extrapolated to the order level if the values of all genera within the order were consistent (mean > standard deviation for quantitative traits, and >60% of all genera sharing one trait value for qualitative traits). If the data was not consistent within the order, it was kept as missing values. Then, cell volume was estimated as (cell radius)^23.14×cell length and log-transformed before further analysis. Other traits such as doubling time were considered but had very low coverage (e.g., for doubling time median of OTUs with available data over all plots <20%) and were excluded from the analysis. Community-weighted means were calculated using OTUs numbers as an approximation for relative abundances. We completed these traits with community levels characteristics. For bacteria, we quantified the relative abundance (approximated as OTU number) of groups commonly classified as oligotrophs (Acidobacteria, Verrucomicrobia, Planctomycetes) to copiotrophs (Actinobacteria, Betaproteobacteria, Gammaproteobacteria, Proteobacteria, Bacteroidetes).
For fungi, we calculated the relative abundance of pathotroph fungi among all fungi. Finally, we also measured the PLFA-based fungal-bacteria ratio from 2011 and 2014 (150 plots each year); data that were first published by ref 22. This was done by sampling two g of field moist soil (from the same composite samples as described above) for lipid extraction and fractionation following the alkaline methylation method described in ref. 237. Samples were measured by gas chromatography (AutoSystem XL. PerkinElmer Inc., Massachusetts, USA) using a flame ionization detector, an HP-5 capillary column and helium as the carrier gas. Fatty acid nomenclature used was described by ref. 238. Total bacterial PLFAs were calculated as the sum of Gram-positive (a15:0, i15:0, i16:0 and i17:0) and Gram-negative (cy17:0 and cy19:0)239 plus the FAME 16:1ω?7 which is widespread in bacteria in general. Fungal biomass was represented by the PLFA 18:2ω?6.,9239. Open datasets from the Biodiversity Exploratories: refs. 104,105.
Protists
Genus-level protist trait data was obtained from ref. 240 and completed for size class data by K. Dumack. Size classes were coded numerically: 1 (<10 microns), 2 (11-30 microns), 3 (31–50 microns), 4 (>51 microns). Trophic levels were classified as plant parasites, bacterivores, or secondary consumers (non-plant parasites, eukaryvores, omnivores).
Primary consumers (plant parasites) had only one trait combination (size class 1) so we characterised these communities only by the relative abundance of plant parasites among all protists.
Measurement of ecosystem functions
We selected 15 ecosystem function indicators from five bundles of related functions: decomposition (dung, litter and root decomposition), nitrogen cycling in soils (potential nitrification, activity of urease and denitrification enzyme, gene abundances of ammonia oxidising bacteria and archaea (amoA AOA and amoA AOB), abundances of nitrogen fixation (nifH) and nitrite reductase genes (nxrA)), functions related to the carbon cycle in soils (activity of ß-glucosidase, N-acetyl- ß-glucosaminidase, xylosidase), aboveground biomass production and total soil respiration. Most ecosystem functions data was extracted from a synthesis dataset of the Biodiversity Exploratories: ref. 241.
Soil enzymes
Soil samples were taken from each plot in the beginning of May 2011 and 2014 as a mixed sample from 14 soil cores of the top horizon (0–10 cm). Denitrification enzyme activity (DEA) was measured in 2011 only according to refs. 242,243. Urease activity was determined in 2011 only by incubating 1 g of fresh soil with 1.5 ml of 0.08 M substrate (urea) solution at 37 °C for 2 h244. Released ammonium was extracted with 12 ml of a 1 M potassium chloride/0.01 M hydrochloric solution and determined by a modified Berthelot reaction. The abundance of ammonia oxidation gene of archaea, bacteria and nitrogen fixation gene in soil bacteria different functional genes (nifH, amoA) is quantified via real-time qPCR analysis in both 2011 and 2014 and averaged across years. The abundance of ammonia nitrite-oxidizing Nitrobacter bacteria and archaea was estimated in 2014 using respective amoA gene, while NS-like and NB-like NOBs were targeted by primer sets for 16S rRNA genes for NS and nxrA primers genes specific for NB245. The abundance of nitrite-oxidizing Nitrospira was estimated using 16S rRNA gene primer specific for Nitrospira245. For potential nitrification, ammonium and nitrate concentrations were measured after CaCl2 extraction, potential nitrification was determined according to ref. 246 and averaged across years. Activities of the soil enzymes soil enzyme ß-glucosidase, N-acetyl-ß-glucosaminidase, and ß-xylosidase were determined according to ref. 247 as described in detail in ref. 248, using fluorescent 4-methylumbelliferone substrates (4-MUF; Sigma-Aldrich, St. Louis, USA) and a buffered solution (pH 6.1).
Open datasets from Biodiversity Exploratories: refs. 249–252.
Decomposition rates
To measure dung decomposition rates, five dung samples were placed on each site in 2014. We used dung with a fresh weight of approx. 220.7 ( ± 19.9) g of cow, 34.4 ( ± 3.8) g of horse, 50.5 ( ± 3.6) g of sheep, 32.6 ( ± 1.6) g of deer, 14.5 ( ± 1.4) g of fox and 47.6 ( ± 2.4) g of wild boar. All dung samples were placed on cellulose paper. After 48 h dung samples of removal experiments were collected, transferred into small paper bags, labelled (date, site-ID, dung type) and stored in a freezer at −20 °C. Removal samples were transferred into drying ovens and kept there at 60 °C for at least five days. Afterwards the dry weight for each dung sample was weighed (Mettler Toledo “EL 2001” ( ± 0.01 g), Columbus, Ohio) and noted for further calculations. Open dataset from Biodiversity Exploratories: ref. 253.
We measured the decomposition of fine roots (<2 mm) within the upper 10 cm of the mineral soil in 2012 with standardised herbaceous roots. Root material was left to decompose in litterbags with mesh size of 100 µm during six months (until October 2012), and mass loss was determined. Open dataset from Biodiversity Exploratories: ref. 254.
To measure litter decomposition, around 1.5 g of dry plant material collected in each plot in spring 2012 were put back in their original location in January 2013. The aboveground vegetation was removed, and bags were fixed to the ground with nails or wood sticks. There were ten replicate bags in each plot, five of them were collected two months later, and the five last ones two more months later. The dry biomass remaining in each bag was measured to calculate daily decomposition rates. Open dataset from Biodiversity Exploratories: ref. 255.
Soil respiration
Soil respiration was measured from late June–July in 2018 and 2019, using the soda-lime adsorption absorption method with an open and static chamber to determine soil CO2 efflux. Soda-lime, i.e., mainly Ca(OH)2 and NaOH, was used as the adsorption absorption material. We installed four chambers, forming a square of 10 m side length (around the weather station), and one bottom-sealed trap served as control. The mass of soda-lime for each measurement was ~12 g/d and the exposure time was three days. Aboveground vegetation was carefully clipped and removed from the installation area and PVC rings was were plugged down to 1 cm soil depth. Soda-lime and lid straps were installed five to six days after vegetation clipping. Just before installation, we re-wetted the soda-lime to compensate for the initial moisture content of about 18%, since CO2 needs to be hydrated before reacting with soda-lime. The CO2 flux is calculated from the dry soda-lime mass differences considering i) the exposure time, ii) the area of the chamber, iii) the coefficient of 1.69 to account for the water lost during the reaction and iv) after correcting with controls (bottom-sealed) traps. The soil efflux is calculated by the equation256,257: Rs = [(WGsample-WGblank)/CA] × [24/T] × [12/44] × 1.69 where Rs is the soil respiration in [gCO2-C m-2 d-1], WG is the weight gain [g], CA is the chamber basal area in [m-2], T is the implementation time in [h], 12/44 is the ratio of carbon atomic mass over carbon dioxide molecular mass and 1.69 compensates for the H2O formed during CO2 sorption and lost during drying. Open dataset from Biodiversity Exploratories: ref. 258.
Plant biomass production
On two subplots of 1.5 m × 2 m size (varying position every year between 2009 and 2017), aboveground biomass was harvested between mid-May and mid-June by clipping the vegetation at a height of 5 cm. Samples were dried at 80 °C for 48 h and weighted. An arithmetic mean of biomass per m2 for each plot plot was calculated. Data was then averaged across years 2009–2017. Open datasets from Biodiversity Exploratories: refs. 259–267.
Data analysis
All the data analyses were conducted using the R software v. 4.0.3268.
Initially, in a confirmatory analysis, species-level fast-slow axes were identified from PCA that included all species within most guilds (Fig. 2).
As we hypothesised changes in community level properties across environmental gradients (rather than changes in individual species), we focused on changes in average trait values across gradients. We thus did not consider the diversity of functional strategies in a community, or the potential for differing responses of individual species, in this analysis. Average trait values can represent both changes in species identity (taxonomic turnover) and variation in their relative abundance along the gradient. Thus, all further analyses were conducted at the level of community abundance-weighted trait mean18 (CWM). CWM traits captures the average functional strategy of the community both in terms of response to environmental conditions (response traits) and how it reciprocally affects the functioning or other biotic components of the system (effect traits269). In practice, the same traits can influence both response and effect and thus can correlate strongly with both environmental drivers and ecosystem functioning270. CWM were calculated using relative abundance data depending on the considered guild (e.g., cover for plants, number of individuals for arthropods, number of Amplicon Sequence Variants (ASV) (fungi) or Operational Taxonomic Units (OTU) (bacteria, protists), use of the habitat and activity for birds and bats). As a result of the Biodiversity Exploratories design, data from different guilds was sometimes collected in an uncoordinated way (e.g., every year, every three years, or on fewer consecutive years). To allow comparison across guilds, when data was available for more than one year, we first calculated CWM values independently for each year, then aggregated the CWM across years. Data collected in different years, but from the same plot, were considered comparable because both land-use intensity and multivariate CWM for all guilds that were sampled more than once differed more across plots than across time. This was shown by conducting variance partitioning analyses (using the varpart function, R package vegan) with either the land-use intensity or the trait CWM value for each Plot Year combination as the response matrix, and the plot and year (as factors) as explanatory matrices. The variance explained by the plot term was on average >15 higher by that explained by the year term (Table S5). Biologically this is because high intensity fields tend to be managed at high intensity year after year, and vice-versa.
Each guild was represented by two to six traits (4.2 on average), except protists for which only one was available. Taxa with missing trait data were excluded from the corresponding CWM calculation (except in a few specific cases where data was imputed, see Methods section on functional traits); the resulting coverage (% of abundance, in terms of cover or individuals with available trait data) was always above 80% except for bacterial and Oribatid mites traits (Table S2). The CWM approach was favoured over joint species distribution modelling of trait-environment relationships271, as it was the trait values of entire guilds and communities, not species, that was the appropriate unit of replication in our study. It was therefore essential to summarise variables at the whole guild, community, and ecosystem levels prior to analysis.
To gain a more reliable estimate of how CWM trait data was related to the hypothesised drivers, it was necessary to correct for environmental covariates before analysis. As such correction has been shown to produce biased parameter estimates in the presence of correlation between the environmental covariates272, we identified highly correlated variables from our list of potential covariates (mean annual temperatures and precipitation, topographic wetness index, soil clay and sand content, pH, depth and region). We excluded soil depth and sand content which were both highly anticorrelated to soil clay content ( | r | > 0.72); and precipitation which was highly anticorrelated with temperature (r = −0.77). We thus retained mean annual temperature, topographic wetness index, soil clay content and pH as well as the region as covariates. To investigate the importance of these factors relative to land-use intensity, our focus variable, we fitted linear models with each trait CWM as a response and all covariates and land-use intensity as explanatory variables. This showed that on average the region explained as much variance as land-use intensity (around 8%), followed by the topographic wetness index (2%), but that there was high variability across guilds. For instance, bats and Collembola were primarily driven by the region, secondary consumer protists by soil pH, and plants or aboveground primary consumer arthropods by land-use intensity (Fig. S1). After that, we fitted linear models with each trait CWM as a response, and the covariates, excluding land-use intensity, as explanatory variables. The residuals of these linear models were used for all further analyses. Results of sensitivity analyses with uncorrected values can be found in Tables S15–S18 and Figs. S15–S20.
In cases where abundance data was not available for all 150 plots, guild-level analyses were run on all available plots (never <110 plots). To test for the response of each individual CWM trait to land-use intensity, we calculated the slope of the regression of the CWM trait against land-use intensity (excluding missing values) between each trait and land-use intensity (p-values corrected for false detection rates using the p.adjust function, n = 47, shown in Fig. 4). For each guild, we then sought to identify a slow-fast axis based on pre-established hypotheses based on either environmental filtering through resource availability and disturbance, or indirect, trophically-mediated mechanisms. Indeed, because traits represent consistent functional strategies, ‘slow’ and ‘fast’ traits are expected to covary. This guild-level aggregation from individual traits CWM to guild-level slow-fast axes was necessary to make the guilds comparable in the subsequent analyses. Also, this approach was chosen because the main objective of the study was not to establish the existence of such guild-level slow-fast axes, but rather examine their association at the whole community level. We ran a Principal Component Analysis (PCA) on all CWM traits of each considered guild separately. For these PCA, we used the R package factoextra, retained three principal components axes (or two if only two traits were available) and identified the PCA axis that best represented a slow-fast continuum. This was axis 1 for all guilds except the aboveground predatory arthropods (Fig. 2). Notably, the fast-slow axis was also always that which most strongly correlated with LUI, except for belowground primary consumer arthropods and birds where axes 1 and 3 (respectively) had a similarly strong correlation (Fig. S2). Guild-level PCA axes were considered to represent a slow-fast continuum (and called hereafter ‘guild slow-fast axes’) if they fulfilled all following conditions: (i) the considered axis explained at least twice the variance expected at random (i.e., 2/(number of traits), with 1/(number of traits) the average variance per axis expected if traits are independent); and ii) the considered axis was significantly correlated with all traits (p-value < 0.05, corrected for false detection rates, n = 34), with at least 60% correlations ≥ 0.4. The ‘slow-fast’ axis was considered only partially represented if it (i) explained at least twice the variance expected at random; (ii) was correlated (r > 0.25) with at least 60% of the traits, or (iii) had correlations in the unexpected direction with one trait.
For analyses across guilds, we excluded one guild (belowground primary consumer arthropods) as more than 20% of plot data were missing due to absence of this functional guild in the samples associated to some plots.
To test whether the slow-fast responses were synchronous across guilds, we ran a PCA on the previously identified slow-fast axes of all guilds, and projected the land-use intensity index (LUI) as a supplementary variable on this PCA. This approach of combining multiple PCAs into a “main” PCA follows the same logic as Multiple Factor Analysis (MFA273,274) and allowed us to simultaneously analyse the response of guilds with different traits and individual responses. Missing values for the slow-fast axis at the plot level (shown at the individual trait level in Table S2) were given the average of the considered guild across all plots to allow comparison across guilds (imputed values: 1.8% of all values, 0–12% range within guilds). This was done to allow comparison across guilds, and because PCA cannot be used with incomplete data. Additionally, we also tested for the existence of the whole community slow-fast axis by running a PCA on all considered traits (rather than guild-level slow-fast axes, themselves extracted from PCAs, Fig. 3b).
In follow-up analyses, we used Structural Equation Models to assess whether the ecosystem-level slow-fast continuum was driven by a common, but independent, response of individual guilds to land-use intensity, or mediated by trophic interactions between them leading to a cascade of trait matching. Because plants can affect the traits of other organisms not only through direct consumption but also by e.g., structuring the habitat275, we also included paths between all guilds and plants. We also allowed for some correlation paths between guilds when it made biological sense and significantly improved model fit (e.g., between birds and bats, which might be jointly affected by landscape-scale variables). Models were fitted using the lavaan R package276, separately for below and aboveground guilds; plants were included in both. The hypothesised model structures are shown in Fig. S3 and model statistics in Tables S6 and S7.
The SEM were fitted using the maximum likelihood estimator using all available data (i.e., excluding NAs/imputed data points but using all existing data for each estimate). Estimates were bootstrapped 200 times. To assess how path strength varied across trophic levels, we extracted the coefficients and corresponding confidence intervals for all direct, indirect and total effects of land-use intensity on each trophic level. We then fitted linear models with estimated direct, indirect and total effects of LUI as the response variables and trophic level as the explanatory variable. Effect estimates were weighted by the inverse of the standard error to account for variable uncertainties across guilds. To evaluate the overall ecosystem response to LUI, we averaged the direct, indirect and total effects at each trophic level, independently above- and belowground. This was done by defining custom parameters within the SEM as the average of all corresponding parameters (direct/indirect/total for all guilds within each trophic level). This allowed the same bootstrap procedure to also estimate average effects and their confidence intervals.
Finally, we investigated the possible linkage between the entire community slow-fast axis and a potential ecosystem functioning slow-fast axis. We selected 15 ecosystem function indicators from five bundles of related functions (see above). All functions were corrected for environmental variables (as described above for traits) before analysis. All selected functions were hypothesised to represent ‘fast’ ecosystem functioning (e.g., fast nutrient cycling) at high values.
We first investigated the correlation between individual traits and functions; or guild slow-fast axes and function bundles (Figs. S5 and S6). We then conducted a PCA on all functions, weighted so that each functional bundle would have the same final weight (e.g., functions related to N fluxes were weighted 1/8, decomposition functions were weighted 1/3, etc.). This was done to prevent bundles with more functions (e.g. N fluxes) from having a disproportionate impact upon the PCA. The first axis (29% of total variance) represented a continuum from slow to fast nutrient cycling with high potential nitrification, and high activities of most enzymes. The plot-level PC scores of this ecosystem functions axis were then regressed against the ‘slow-fast’ axes of individual guilds (microbes and plants, expected to be the main driver of soil functioning), the overall ecosystem functional trait slow-fast axis, land-use intensity and multidiversity, a measure of the overall species diversity of all considered groups (Table S4). This was calculated as the average richness of all taxonomic groups, scaled by the total species richness of each group32, and also corrected for environmental variables, as described above. Finally, to test the direct and indirect effect of land-use intensity on the slow-fast ecosystem functioning axis, we fitted a SEM (using the lavaan package) testing the indirect effect via the entire community slow-fast axis (Fig. 6b).
Additionally, we tested for the direct and indirect effects of land-use intensity on the ecosystem slow-fast axis, but on the community slow-fast axis that was derived from the ‘all traits’ PCA (rather than guild-level slow-fast axes extracted from individual PCAs, Fig. S4).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The work was partly funded by the DFG Priority Program 1374 ‘Infrastructure-Biodiversity- Exploratories’, and by Senckenberg Biodiversity and Climate Research Center. The soil analyses (EK, SM) was funded by the DFG (KA 1590/8-5, MA 4436/1-5). DB, ASZ, KB, KJ and VW have received funding in the context of Resilience DFG project 413730012. ASZ was supported by the RSF 23-14-00201 grant. Fieldwork permits were issued by the state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg. The authors thank Konstans Wells, Kirsten Reichel-Jung, Sonja Gockel, Kerstin Wiesner, Katrin Lorenzen, Andreas Hemp, Martin Gorke maintained the plot and project infrastructure; Simone Pfeiffer, Maren Gleisberg, Christiane Fischer, Jule Mangels and Victoria Grießmeier for administrative support, and Jens Nieschulze, Michael Owonibi and Andreas Ostrowski for database management. Eduard Linsenmair, Dominik Hessenmöller, Daniel Prati, Ernst-Detlef Schulze, Wolfgang W. Weisser and Elisabeth Kalko helped establish the Biodiversity Exploratories project. The administration of the Hainich National Park, the UNESCO Biosphere Reserves Swabian Alb and Schorfheide-Chorin and all land owners provided logistical support. Tom Lachaise, Jörg Overmann and Johannes Sikorski contributed data. Icons were acquired and adapted (colour, width-height ratio) from Phylopic.org. Lepidoptera, fungi, secondary consumer arthropods, bats, protists, Oribatid mites: public domain. Birds: Maxime Dahirel (CC BY 3.0 Deed). Collembola: Birgit Lang (CC BY 3.0 Deed). Bacteria: Matt Crook (CC BY-SA 3.0 Deed). Hare: Jan A. Venter, Herbert H. T. Prins, David A. Balfour & Rob Slotow (vectorised by T. Michael Keesey) (CC BY 3.0 Deed). Tortoise: Andrew A. Farke, shell lines added by Yan Wong (CC BY 3.0 Deed). Primary consumer arthropods: Gareth Monger (CC BY 3.0 Deed). Link to the corresponding licences: CC BY 3.0 Deed: https://creativecommons.org/licenses/by/3.0/. CC BY-SA 3.0 Deed: https://creativecommons.org/licenses/by-sa/3.0/.
Author contributions
M.N., F.D.S. and P.M. designed the study. M.N. and P.M. conducted the analyses with inputs from G.L.P. and A.L.B. M.N., P.M., G.L.P., D.B., F.T.d.V., J.B., A.M.F.D., S.G., K.G., A.M., R.A.S., N.K.S., J.A.T., A.S.Z. participated to workshops and expert discussions to define guild-specific trait lists and hypotheses. M.N. and P.M. wrote the manuscript with significant inputs from G.L.P., A.L.B., D.B., J.B., S.G., J.A.T., M.M.G., E.K., K.B., K. Jung, J. K., C.P., M.C.R., M. Schlöter, S. Schulz, M. Staab, M.v.K. and V.W. G.L.P., D.B., J.B., A.M.F.D., K.G., R.S., N.S., J.T., A.Z., M.G., K. John, K. Jung, E.K., J.K., C.P., M. Schloter, S. Schulz, M. Staab, V.W., A.A., S.B., R.S.B., R.B., M.B., F.B., K.D., H.Y.G., N.H., J.H., K.J., V.H.K, T.K., S.M., J.M., S.C.R., N.V.S., I.S., M.S., S. Seibold, S.A.S., E.S., M.T., M.v.K., T.W., M.F. and P.M. contributed data. All authors commented on the manuscript. Author order was determined as follow: main authors, workshop and discussion participants (alphabetical), other authors with significant input (alphabetical), other data contributors (alphabetical), senior author.
Peer review
Peer review information
Nature Communications thanks Bertrand Fournier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Funding
Open access funding provided by University of Bergen.
Data availability
This work is based on data collected within several projects of the Biodiversity Exploratories program (DFG Priority Program 1374). Most datasets from the Biodiversity Exploratories are publicly available in the Biodiversity Exploratories Information System (Bexis) (10.17616/R32P9Q). The CWM data generated in this study have been deposited under accession code 31689 https://www.bexis.uni-jena.de/ddm/data/Showdata/31689. The raw trait and abundance, and ecosystem functions datasets are listed below, most of which are publicly available. To give data owners and collectors time to perform their analysis the Biodiversity Exploratories’ data and publication policy includes by default an embargo period of three years from the end of data collection/data assembly. Access to the remaining datasets can thus be obtained by contacting the Biodiversity Exploratories office or data owners. At the end of the embargo period these datasets will be made publicly available via the same data repository. Full list of used datasets (both from the Biodiversity Exploratories and external, previously published datasets)3,19,45,47,53–56,93–97,101–119,221–235,240,249–254,268–109,110,277.
Code availability
Full code to replicate the analyses is stored on GitHub at https://github.com/mneyret/trait_synchronies under the 10.5281/zenodo.10286643.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Margot Neyret, Email: margot.neyret@univ-grenoble-alpes.fr.
Peter Manning, Email: peter.manning@uib.no.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-45113-5.
References
- 1.MacArthur, R. H. & Wilson, E. O. The Theory of Island Biogeography (Princeton University Press, 1967).
- 2.Grime, J. P. Plant Strategies and Vegetation Processes (John Wiley & Sons Ltd, 1979).
- 3.Bakewell AT, Davis KE, Freckleton RP, Isaac NJB, Mayhew PJ. Comparing life histories across taxonomic groups in multiple dimensions: how mammal-like are insects? Am. Nat. 2020;195:70–81. doi: 10.1086/706195. [DOI] [PubMed] [Google Scholar]
- 4.Stearns SC. Life-history tactics: a review of the ideas. Q. Rev. Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- 5.Adler PB, et al. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. USA. 2014;111:740–745. doi: 10.1073/pnas.1315179111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayfield MM, Levine JM. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010;13:1085–1093. doi: 10.1111/j.1461-0248.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- 7.Diaz S, Cabido M, Casanoves F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998;9:113–122. doi: 10.2307/3237229. [DOI] [Google Scholar]
- 8.Díaz S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- 9.Weigelt A, et al. An integrated framework of plant form and function: the belowground perspective. New Phytol. 2021;232:42–59. doi: 10.1111/nph.17590. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann J, et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020;6:eaba3756. doi: 10.1126/sciadv.aba3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruelheide H, et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018;2:1906–1917. doi: 10.1038/s41559-018-0699-8. [DOI] [PubMed] [Google Scholar]
- 12.Grime, J. P. & Pierce, S. The Evolutionary Strategies that Shape Ecosystems (John Wiley & Sons, 2012).
- 13.Westoby M, et al. Trait dimensions in bacteria and archaea compared to vascular plants. Ecol. Lett. 2021;24:1487–1504. doi: 10.1111/ele.13742. [DOI] [PubMed] [Google Scholar]
- 14.Pedley SM, Dolman PM. Multi-taxa trait and functional responses to physical disturbance. J. Anim. Ecol. 2014;83:1542–1552. doi: 10.1111/1365-2656.12249. [DOI] [PubMed] [Google Scholar]
- 15.Simons NK, Weisser WW, Gossner MM. Multi-taxa approach shows consistent shifts in arthropod functional traits along grassland land-use intensity gradient. Ecology. 2016;96:15–0616.1. [PubMed] [Google Scholar]
- 16.Suraci JP, et al. Disturbance type and species life history predict mammal responses to humans. Glob. Change Biol. 2021;27:3718–3731. doi: 10.1111/gcb.15650. [DOI] [PubMed] [Google Scholar]
- 17.Lytle DA. Disturbance regimes and life‐history evolution. Am. Nat. 2001;157:525–536. doi: 10.1086/319930. [DOI] [PubMed] [Google Scholar]
- 18.Garnier E, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. doi: 10.1890/03-0799. [DOI] [Google Scholar]
- 19.Börschig, C., Klein, A.-M., von Wehrden, H. & Krauss, J. Traits of butterfly communities change from specialist to generalist characteristics with increasing land-use intensity. Basic Appl. Ecol. 547–554. 10.1016/j.baae.2013.09.002 (2013).
- 20.Daou L, Garnier É, Shipley B. Quantifying the relationship linking the community weighted means of plant traits and soil fertility. Ecology. 2021;102:e03454. doi: 10.1002/ecy.3454. [DOI] [PubMed] [Google Scholar]
- 21.de Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006;38:2092–2103. doi: 10.1016/j.soilbio.2006.01.008. [DOI] [Google Scholar]
- 22.Boeddinghaus RS, et al. Plant functional trait shifts explain concurrent changes in the structure and function of grassland soil microbial communities. J. Ecol. 2019;107:2197–2210. doi: 10.1111/1365-2745.13182. [DOI] [Google Scholar]
- 23.Buzzard V, et al. Continental scale structuring of forest and soil diversity via functional traits. Nat. Ecol. Evol. 2019;3:1298–1308. doi: 10.1038/s41559-019-0954-7. [DOI] [PubMed] [Google Scholar]
- 24.de Vries FT, et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 2012;15:1230–1239. doi: 10.1111/j.1461-0248.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 25.Le Provost G, et al. Trait‐matching and mass effect determine the functional response of herbivore communities to land‐use intensification. Funct. Ecol. 2017;31:1600–1611. doi: 10.1111/1365-2435.12849. [DOI] [Google Scholar]
- 26.McFadden IR, et al. Global plant-frugivore trait matching is shaped by climate and biogeographic history. Ecol. Lett. 2022;25:686–696. doi: 10.1111/ele.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochoa-Hueso R, et al. Ecosystem coupling: a unifying framework to understand the functioning and recovery of ecosystems. One Earth. 2021;4:951–966. doi: 10.1016/j.oneear.2021.06.011. [DOI] [Google Scholar]
- 28.Wardle DA, et al. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Caro RC, et al. Anthropogenic impacts on threatened species erode functional diversity in chelonians and crocodilians. Nat. Commun. 2023;14:1542. doi: 10.1038/s41467-023-37089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salguero-Gómez R, et al. Fast–slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl. Acad. Sci. USA. 2016;113:230–235. doi: 10.1073/pnas.1506215112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capdevila P, et al. Longevity, body dimension and reproductive mode drive differences in aquatic versus terrestrial life-history strategies. Funct. Ecol. 2020;34:1613–1625. doi: 10.1111/1365-2435.13604. [DOI] [Google Scholar]
- 32.Allan E, et al. Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl. Acad. Sci. USA. 2014;111:308–313. doi: 10.1073/pnas.1312213111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Provost G, et al. Contrasting responses of above- and belowground diversity to multiple components of land-use intensity. Nat. Commun. 2021;12:3918. doi: 10.1038/s41467-021-23931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. Am. Nat. 1960;94:421–425. doi: 10.1086/282146. [DOI] [Google Scholar]
- 35.Audusseau H, Kolb G, Janz N. Plant fertilization interacts with life history: variation in stoichiometry and performance in nettle-feeding butterflies. PLoS ONE. 2015;10:e0124616. doi: 10.1371/journal.pone.0124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadotte MW. Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 2017;20:989–996. doi: 10.1111/ele.12796. [DOI] [PubMed] [Google Scholar]
- 37.Evans R, et al. Defining the functional traits that drive bacterial decomposer community productivity. ISME J. 2017;11:1680–1687. doi: 10.1038/ismej.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning P, et al. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018;2:427–436. doi: 10.1038/s41559-017-0461-7. [DOI] [PubMed] [Google Scholar]
- 39.Migliavacca M, et al. The three major axes of terrestrial ecosystem function. Nature. 2021;598:468–472. doi: 10.1038/s41586-021-03939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eskelinen A, et al. Resource‐enhancing global changes drive a whole‐ecosystem shift to faster cycling but decrease diversity. Ecology. 2020;101:e03178. doi: 10.1002/ecy.3178. [DOI] [PubMed] [Google Scholar]
- 41.Allan E, et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015;18:834–843. doi: 10.1111/ele.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kőrösi Á, Dolek M, Nunner A, Lang A, Theves F. Pace of life and mobility as key factors to survive in farmland – Relationships between functional traits of diurnal Lepidoptera and landscape structure. Agric. Ecosyst. Environ. 2022;334:107978. doi: 10.1016/j.agee.2022.107978. [DOI] [Google Scholar]
- 43.Leff JW, et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA. 2015;112:10967–10972. doi: 10.1073/pnas.1508382112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher RV, et al. Open Science principles for accelerating trait-based science across the Tree of Life. Nat. Ecol. Evol. 2020;4:294–303. doi: 10.1038/s41559-020-1109-6. [DOI] [PubMed] [Google Scholar]
- 45.Kattge J, et al. TRY – a global database of plant traits. Glob. Change Biol. 2011;17:2905–2935. doi: 10.1111/j.1365-2486.2011.02451.x. [DOI] [Google Scholar]
- 46.Madin JS, et al. A synthesis of bacterial and archaeal phenotypic trait data. Sci. Data. 2020;7:170. doi: 10.1038/s41597-020-0497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobias JA, et al. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 2022;25:581–597. doi: 10.1111/ele.13898. [DOI] [PubMed] [Google Scholar]
- 48.Bruelheide H, et al. Designing forest biodiversity experiments: general considerations illustrated by a new large experiment in subtropical China. Methods Ecol. Evol. 2014;5:74–89. doi: 10.1111/2041-210X.12126. [DOI] [Google Scholar]
- 49.Fischer M, et al. Implementing large-scale and long-term functional biodiversity research: the biodiversity exploratories. Basic Appl. Ecol. 2010;11:473–485. doi: 10.1016/j.baae.2010.07.009. [DOI] [Google Scholar]
- 50.Li D, et al. Standardized NEON organismal data for biodiversity research. Ecosphere. 2022;13:e4141. doi: 10.1002/ecs2.4141. [DOI] [Google Scholar]
- 51.Blüthgen N, et al. A quantitative index of land-use intensity in grasslands: integrating mowing, grazing and fertilization. Basic Appl. Ecol. 2012;13:207–220. doi: 10.1016/j.baae.2012.04.001. [DOI] [Google Scholar]
- 52.Gossner MM, et al. A summary of eight traits of Coleoptera, Hemiptera, Orthoptera and Araneae, occurring in grasslands in Germany. Sci. Data. 2015;2:150013. doi: 10.1038/sdata.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Börshig, C. & Krauss, J. Diversity and abundance of day active butterflies and day-active moths along a land use intensity gradient (2008). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/12526 (2011).
- 54.Cook, P. M. et al. Traits data for the butterflies and macro-moths of Great Britain and Ireland. NERC EDS Environ. Inf. Data Cent. 10.5285/5b5a13b6-2304-47e3-9c9d-35237d1232c6 (2021). [DOI] [PubMed]
- 55.Mangels J, Fiedler K, Schneider FD, Blüthgen N. Diversity and trait composition of moths respond to land-use intensification in grasslands: generalists replace specialists. Biodivers. Conserv. 2017;26:3385–3405. doi: 10.1007/s10531-017-1411-z. [DOI] [Google Scholar]
- 56.Middleton-Welling J, et al. A new comprehensive trait database of European and Maghreb butterflies, Papilionoidea. Sci. Data. 2020;7:351. doi: 10.1038/s41597-020-00697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavalier-Smith T. r- and K-tactics in the evolution of protist developmental systems: Cell and genome size, phenotype diversifying selection, and cell cycle patterns. Biosystems. 1980;12:43–59. doi: 10.1016/0303-2647(80)90037-4. [DOI] [PubMed] [Google Scholar]
- 58.Piton G, et al. Using proxies of microbial community-weighted means traits to explain the cascading effect of management intensity, soil and plant traits on ecosystem resilience in mountain grasslands. J. Ecol. 2020;108:876–893. doi: 10.1111/1365-2745.13327. [DOI] [Google Scholar]
- 59.Endara M-J, Coley PD. The resource availability hypothesis revisited: a meta-analysis. Funct. Ecol. 2011;25:389–398. doi: 10.1111/j.1365-2435.2010.01803.x. [DOI] [Google Scholar]
- 60.Liu X, Zhang L, Huang M, Zhou S. Plant diversity promotes soil fungal pathogen richness under fertilization in an alpine meadow. J. Plant Ecol. 2021;14:323–336. doi: 10.1093/jpe/rtaa099. [DOI] [Google Scholar]
- 61.Fiore-Donno, A. M., Richter-Heitmann, M. & Bonkowski, T. Functional traits of two important protistan lineages (Cercozoa and Endomyxa) reveal contrasting distributions of plant parasites and phagotrophs in temperate grassland and forest soils. 14, (2020).
- 62.Birkhofer K, et al. Land‐use type and intensity differentially filter traits in above‐ and below‐ground arthropod communities. J. Anim. Ecol. 2017;86:511–520. doi: 10.1111/1365-2656.12641. [DOI] [PubMed] [Google Scholar]
- 63.Hanson HI, Palmu E, Birkhofer K, Smith HG, Hedlund K. Agricultural land use determines the trait composition of ground beetle communities. PLoS ONE. 2016;11:e0146329. doi: 10.1371/journal.pone.0146329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chisté, M. N. et al. Losers, winners, and opportunists: how grassland land‐use intensity affects orthopteran communities. Ecosphere7, e01545 (2016).
- 65.Gámez-Virués S, et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 2015;6:8568. doi: 10.1038/ncomms9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boch, S., Biurrun, I. & Rodwell, J. Grasslands of Western Europe. in Encyclopedia of the World’s Biomes (eds. Goldstein, M. I. & DellaSala, D. A.) 678–688 (Elsevier, 2020). 10.1016/B978-0-12-409548-9.12095-0.
- 67.Socher SA, et al. Direct and productivity-mediated indirect effects of fertilization, mowing and grazing on grassland species richness. J. Ecol. 2012;100:1391–1399. doi: 10.1111/j.1365-2745.2012.02020.x. [DOI] [Google Scholar]
- 68.Ali JG, Agrawal AA. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012;17:293–302. doi: 10.1016/j.tplants.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Bielby J, et al. The fast‐slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 2007;169:748–757. doi: 10.1086/516847. [DOI] [PubMed] [Google Scholar]
- 70.Stearns SC. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos. 1983;41:173–187. doi: 10.2307/3544261. [DOI] [Google Scholar]
- 71.Garibaldi LA, et al. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 2015;52:1436–1444. doi: 10.1111/1365-2664.12530. [DOI] [Google Scholar]
- 72.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chapin, F. S., Matson, P. A. & Vitousek, P. M. Principles of Terrestrial Ecosystem Ecology. (Springer, 2011). 10.1007/978-1-4419-9504-9.
- 74.Yvon-Durocher G, Allen AP. Linking community size structure and ecosystem functioning using metabolic theory. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:2998–3007. doi: 10.1098/rstb.2012.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reich PB. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol. 2014;102:275–301. doi: 10.1111/1365-2745.12211. [DOI] [Google Scholar]
- 76.Gibb H, et al. Ecological strategies of (pl)ants: Towards a world-wide worker economic spectrum for ants. Funct. Ecol. 2023;37:13–25. doi: 10.1111/1365-2435.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeMalach N, Ron R, Kadmon R. Mechanisms of seed mass variation along resource gradients. Ecol. Lett. 2019;22:181–189. doi: 10.1111/ele.13179. [DOI] [PubMed] [Google Scholar]
- 78.Lamanna C, et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA. 2014;111:13745–13750. doi: 10.1073/pnas.1317722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scherber C, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- 80.Walde M, et al. Both diversity and functional composition affect productivity and water use efficiency in experimental temperate grasslands. J. Ecol. 2021;109:3877–3891. doi: 10.1111/1365-2745.13765. [DOI] [Google Scholar]
- 81.Allan E, et al. More diverse plant communities have higher functioning over time due to turnover in complementary dominant species. Proc. Natl. Acad. Sci. USA. 2011;108:17034–17039. doi: 10.1073/pnas.1104015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 83.Craven D, Polley HW, Wilsey B. Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2018;2:1579. doi: 10.1038/s41559-018-0647-7. [DOI] [PubMed] [Google Scholar]
- 84.Ricotta C, Moretti M. CWM and Rao’s quadratic diversity: a unified framework for functional ecology. Oecologia. 2011;167:181–188. doi: 10.1007/s00442-011-1965-5. [DOI] [PubMed] [Google Scholar]
- 85.Zhu J, Jiang L, Zhang Y. Relationships between functional diversity and aboveground biomass production in the Northern Tibetan alpine grasslands. Sci. Rep. 2016;6:34105. doi: 10.1038/srep34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joswig, J. S. et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 10.1038/s41559-021-01616-8 (2021). [DOI] [PMC free article] [PubMed]
- 87.Bardgett RD, et al. Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils. 1996;22:261–264. doi: 10.1007/BF00382522. [DOI] [Google Scholar]
- 88.Gounand I, Little CJ, Harvey E, Altermatt F. Global quantitative synthesis of ecosystem functioning across climatic zones and ecosystem types. Glob. Ecol. Biogeogr. 2020;29:1139–1176. doi: 10.1111/geb.13093. [DOI] [Google Scholar]
- 89.Lindo Z. Warming favours small-bodied organisms through enhanced reproduction and compositional shifts in belowground systems. Soil Biol. Biochem. 2015;91:271–278. doi: 10.1016/j.soilbio.2015.09.003. [DOI] [Google Scholar]
- 90.Martins IS, et al. Widespread shifts in body size within populations and assemblages. Science. 2023;381:1067–1071. doi: 10.1126/science.adg6006. [DOI] [PubMed] [Google Scholar]
- 91.Donoso I, et al. Downsizing of animal communities triggers stronger functional than structural decay in seed-dispersal networks. Nat. Commun. 2020;11:1582. doi: 10.1038/s41467-020-15438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ostrowski, A., Lorenzen, K., Petzold, E. & Schindler, S. Land use intensity index (LUI) calculation tool of the Biodiversity Exploratories project for grassland survey data from three different regions in Germany since 2006, BEXIS 2 module. 10.5281/zenodo.3865579 (2020).
- 93.Schöning, I., Solly, E., Klötzing, T., Schrumpf, M. & Trumbore, S. Mineral soil pH values of all experimental plots (EP) of the Biodiversity Exploratories project from 2011, Soil (core project). v8. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/14447 (2015).
- 94.Schöning, I. et al. MinSoil 2014 - Soil pH. v3. Biodiversity Exploratories Information System. Dataset.https://www.bexis.uni-jena.de/ddm/data/Showdata/19067 (2015).
- 95.Schöning, I., Heublein, J., Klötzing, T., Schrumpf, M. & Trumbore, S. MinSoil 2011 - Soil Texture. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/14686 (2013).
- 96.Le Provost, G. et al. Aggregated environmental and land-use covariates of the 150 grassland EPs used in ‘Contrasting responses of above- and belowground diversity to multiple components of land-use intensity’. v5. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/31018 (2021). [DOI] [PMC free article] [PubMed]
- 97.Hänsel, F., Forteva, S., Wöllauer, S. & Nauss, T. Öffentlich verfügbare Klimadaten der Exploratorien / Open Climate Data of the Exploratories Project. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/tcd/PublicClimateData/Index (2019).
- 98.Penone, C. et al. Assembled species information from grassland EPs (2008–2020) for multidiversity synthesis - November 2020. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27706 (2021).
- 99.Penone, C. et al. Assembled RAW diversity from grassland EPs (2008–2020) for multidiversity synthesis - November 2020. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27707 (2021).
- 100.Bolliger, R., Prati, D. & Fischer, M. Vegetation Records for Grassland EPs, 2008–2020. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27386 (2021).
- 101.Buscot, F., Goldmann, K. & Wubet, T. Abundant soil fungi on all 150 grassland EPs (from Soil Sampling Campain 2011; Illumina MiSeq) - ASV abundances. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/26470 (2021).
- 102.Buscot, F., Goldmann, K. & Wubet, T. Abundant soil fungi on all 150 grassland EPs (from Soil Sampling Campain 2014; Illumina MiSeq) - ASV abundances. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/26471 (2020).
- 103.Overmann, J. & Sikorski, J. 16S rRNA gene (V3 region) RNA-based analysis of microbial soil communities at sequence variant level in 148 grassland EP plots, using QIIME2-based bioinformatics, 2011. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/24866 (2019).
- 104.Kandeler, E., Marhan, S., Berner, D. & Boeddinghaus, R. S. Microbial soil properties of all grassland EPs, soil sampling campaign (SSC) 2011, SCALEMIC. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/20250 (2017).
- 105.Kandeler, E., Marhan, S., Berner, D. & Boeddinghaus, R. S. Microbial soil properties of all grassland EPs, soil sampling campaign (SSC) 2014, SCALEMIC. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/20251 (2017).
- 106.Sikorski, J., Overmann, J. & Marzini, C. 16S rRNA gene (V3 region) RNA-based analysis of microbial soil communities at sequence variant level in 150 grassland EP plots, using QIIME2-based bioinformatics, 2014. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/25066 (2019).
- 107.Fiore-Donno, A. M. & Bonkowski, M. Cercozoa and Endomyxa (Rhizaria, protists), Illumina Sequences, all EPs, grassland and forest, 2011. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/24426 (2019).
- 108.Fiore-Donno, A. M. & Bonkowski, M. Cercozoa and Endomyxa (Rhizaria, protists), Illumina Sequences, all EPs, grassland and forest, 2017. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/24466 (2019).
- 109.Baulechner, D., Wolters, V., John, K. & Zaytsev, A. Acari abundance (Oribatida to species level) on all 150 grassland EPs from 2019. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27406.
- 110.Baulechner, D., Wolters, V., Zaytsev, A. & John, K. Collembolan species abundance on all 150 grassland EPs from 2019. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27007 (2020).
- 111.Weisser, W. et al. Sweep net samples from grasslands since 2008: Araneae, Coleoptera, Hemiptera, Orthoptera. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21969 (2019).
- 112.Tschapka, M., Renner, S. & Jung, K. Bird survey data 2008, all 300 EPs. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21446 (2017).
- 113.Tschapka, M., Renner, S. & Jung, K. Bird survey data 2009, all 300 EPs. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21447 (2017).
- 114.Tschapka, M., Renner, S. & Jung, K. Bird survey data 2010, all 300 EPs. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21448 (2017).
- 115.Tschapka, M., Renner, S. & Jung, K. Bird survey data 2011, all 300 EPs. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21449 (2017).
- 116.Goldmann, K., Buscot, F. & Wubet, T. Abundant fungi on all EPs (from Soil Sampling Campain 2011) - merged grassland and forest data: taxonomic look-up table. Version 3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/24306 (2018).
- 117.Teuscher, M. & Fischer, M. Bird survey and trait data on all grassland and forest EPs 2018. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/31521 (2021).
- 118.Jung, K. & Tschapka, M. Bat activity in all Exploratories, summer 2009, using acoustic monitoring. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/19849 (2016).
- 119.Jung, K. & Tschapka, M. Bat activity in all Exploratories, summer 2010, using acoustic monitoring. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/19850 (2016).
- 120.Westoby M, et al. Cell size, genome size, and maximum growth rate are near‐independent dimensions of ecological variation across bacteria and archaea. Ecol. Evol. 2021;11:3956–3976. doi: 10.1002/ece3.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneider, F. EcologicalTraitData/traitdataform: Conforming v0.10 of the Ecological Trait-data Standard (ETS). 10.5281/ZENODO.1489527 (2020).
- 122.Lachaise T, Bergmann J, Rillig MC, van Kleunen M. Below- and aboveground traits explain local abundance, and regional, continental and global occurrence frequencies of grassland plants. Oikos. 2021;130:110–120. doi: 10.1111/oik.07874. [DOI] [Google Scholar]
- 123.Burrascano S, et al. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 2015;16:244–253. doi: 10.1556/168.2015.16.2.12. [DOI] [Google Scholar]
- 124.Kleyer M, et al. The LEDA traitbase: a database of life-history traits of the Northwest European flora. J. Ecol. 2008;96:1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x. [DOI] [Google Scholar]
- 125.Atkin OK, et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015;206:614–636. doi: 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- 126.Lhotsky B, Csecserits A, Kovács B, Botta-Dukát Z. New plant trait records of the Hungarian flora. Acta Bot. Hung. 2016;58:397–400. doi: 10.1556/ABot.58.2016.3-4.8. [DOI] [Google Scholar]
- 127.Takkis, K. Changes in Plant Species Richness and Population Performance in Response to Habitat Loss and Fragmentation. PhD thesis from University of Tartu (2014).
- 128.Siefert A, Fridley JD, Ritchie ME. Community functional responses to soil and climate at multiple spatial scales: when does intraspecific variation matter? PLoS ONE. 2014;9:e111189. doi: 10.1371/journal.pone.0111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Louault F, Pillar VD, Aufrère J, Garnier E, Soussana J-F. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Veg. Sci. 2005;16:151–160. doi: 10.1111/j.1654-1103.2005.tb02350.x. [DOI] [Google Scholar]
- 130.Li Y, Shipley B. Community divergence and convergence along experimental gradients of stress and disturbance. Ecology. 2018;99:775–781. doi: 10.1002/ecy.2162. [DOI] [PubMed] [Google Scholar]
- 131.Sandel, B., Corbin, J. D. & Krupa, M. Using plant functional traits to guide restoration: a case study in California coastal grassland. Ecosphere2, art23 (2011).
- 132.Campetella G, et al. Patterns of plant trait–environment relationships along a forest succession chronosequence. Agric. Ecosyst. Environ. 2011;145:38–48. doi: 10.1016/j.agee.2011.06.025. [DOI] [Google Scholar]
- 133.Onoda Y, et al. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017;214:1447–1463. doi: 10.1111/nph.14496. [DOI] [PubMed] [Google Scholar]
- 134.Ciccarelli D. Mediterranean coastal dune vegetation: are disturbance and stress the key selective forces that drive the psammophilous succession? Estuar. Coast. Shelf Sci. 2015;165:247–253. doi: 10.1016/j.ecss.2015.05.023. [DOI] [Google Scholar]
- 135.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013;27:1254–1261. doi: 10.1111/1365-2435.12116. [DOI] [Google Scholar]
- 136.Peco B, de Pablos I, Traba J, Levassor C. The effect of grazing abandonment on species composition and functional traits: the case of dehesa grasslands. Basic Appl. Ecol. 2005;6:175–183. doi: 10.1016/j.baae.2005.01.002. [DOI] [Google Scholar]
- 137.Giarrizzo E, et al. Re-visiting historical semi-natural grasslands in the Apennines to assess patterns of changes in species composition and functional traits. Appl. Veg. Sci. 2017;20:247–258. doi: 10.1111/avsc.12288. [DOI] [Google Scholar]
- 138.Freschet GT, Cornelissen JHC, Van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 2010;98:362–373. doi: 10.1111/j.1365-2745.2009.01615.x. [DOI] [Google Scholar]
- 139.La Pierre KJ, Smith MD. Functional trait expression of grassland species shift with short- and long-term nutrient additions. Plant Ecol. 2015;216:307–318. doi: 10.1007/s11258-014-0438-4. [DOI] [Google Scholar]
- 140.Fonseca CR, Overton JMcC, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 2000;88:964–977. doi: 10.1046/j.1365-2745.2000.00506.x. [DOI] [Google Scholar]
- 141.Dwyer JM, Hobbs RJ, Mayfield MM. Specific leaf area responses to environmental gradients through space and time. Ecology. 2014;95:399–410. doi: 10.1890/13-0412.1. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez-Akre, E., McShea, W., Bourg, N. & Anderson-Teixera, K. Leaf traits data (SLA) for 56 woody species at the Smithsonian Conservation Biology Institute-ForestGEO Forest Dynamic Plot. [Data set]. Version 1.0. (2015).
- 143.Spasojevic MJ, Turner BL, Myers JA. When does intraspecific trait variation contribute to functional beta‐diversity? J. Ecol. 2016;104:487–496. doi: 10.1111/1365-2745.12518. [DOI] [Google Scholar]
- 144.Bond-Lamberty B, Wang C, Gower ST, Norman J. Leaf area dynamics of a boreal black spruce fire chronosequence. Tree Physiol. 2002;22:993–1001. doi: 10.1093/treephys/22.14.993. [DOI] [PubMed] [Google Scholar]
- 145.Liebergesell M, et al. Functional resilience against climate-driven extinctions – comparing the functional diversity of European and North American tree floras. PLoS ONE. 2016;11:e0148607. doi: 10.1371/journal.pone.0148607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paine CET, et al. Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. J. Ecol. 2015;103:978–989. doi: 10.1111/1365-2745.12401. [DOI] [Google Scholar]
- 147.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 148.Maire V, et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015;24:706–717. doi: 10.1111/geb.12296. [DOI] [Google Scholar]
- 149.Maire, V. et al. Data from: Global effects of soil and climate on leaf photosynthetic traits and rates. 1125213 bytes. 10.5061/DRYAD.J42M7 (2016).
- 150.Falster DS, et al. BAAD: a biomass and allometry database for woody plants: ecological archives E096-128. Ecology. 2015;96:1445–1445. doi: 10.1890/14-1889.1. [DOI] [Google Scholar]
- 151.Herz K, et al. Drivers of intraspecific trait variation of grass and forb species in German meadows and pastures. J. Veg. Sci. 2017;28:705–716. doi: 10.1111/jvs.12534. [DOI] [Google Scholar]
- 152.Herz K, et al. Predicting individual plant performance in grasslands. Ecol. Evol. 2017;7:8958–8965. doi: 10.1002/ece3.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prentice IC, et al. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol. 2011;190:169–180. doi: 10.1111/j.1469-8137.2010.03579.x. [DOI] [PubMed] [Google Scholar]
- 154.Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 2009;15:976–991. doi: 10.1111/j.1365-2486.2008.01744.x. [DOI] [Google Scholar]
- 155.Walker, A. P. A global data set of leaf photosynthetic rates, leaf N and P, and specific leaf area. 1.103161 MB. 10.3334/ORNLDAAC/1224 (2014).
- 156.Smith SW, Woodin SJ, Pakeman RJ, Johnson D, Wal R. Root traits predict decomposition across a landscape‐scale grazing experiment. New Phytol. 2014;203:851–862. doi: 10.1111/nph.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guy AL, Mischkolz JM, Lamb EG. Limited effects of simulated acidic deposition on seedling survivorship and root morphology of endemic plant taxa of the Athabasca Sand Dunes in well-watered greenhouse trials. Botany. 2013;91:176–181. doi: 10.1139/cjb-2012-0162. [DOI] [Google Scholar]
- 158.Vergutz, L., Manzoni, S., Porporato, A., Novais, R. F. & Jackson, R. B. A global database of carbon and nutrient concentrations of green and senesced leaves. 0.22148 MB. 10.3334/ORNLDAAC/1106 (2012).
- 159.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 160.Lukeš P, Stenberg P, Rautiainen M, Mõttus M, Vanhatalo KM. Optical properties of leaves and needles for boreal tree species in Europe. Remote Sens. Lett. 2013;4:667–676. doi: 10.1080/2150704X.2013.782112. [DOI] [Google Scholar]
- 161.Meir P, et al. Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ. 2002;25:343–357. doi: 10.1046/j.0016-8025.2001.00811.x. [DOI] [Google Scholar]
- 162.Tribouillois H, et al. A functional characterisation of a wide range of cover crop species: growth and nitrogen acquisition rates, leaf traits and ecological strategies. PLoS ONE. 2015;10:e0122156. doi: 10.1371/journal.pone.0122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spasojevic MJ, Suding KN. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. J. Ecol. 2012;100:652–661. doi: 10.1111/j.1365-2745.2011.01945.x. [DOI] [Google Scholar]
- 164.Price CA, Enquist BJ. Scaling mass and morphology in leaves: an extension of the WBE model. Ecology. 2007;88:1132–1141. doi: 10.1890/06-1158. [DOI] [PubMed] [Google Scholar]
- 165.Medlyn BE, et al. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ. 1999;22:1475–1495. doi: 10.1046/j.1365-3040.1999.00523.x. [DOI] [Google Scholar]
- 166.Baruch Z, Goldstein G. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia. 1999;121:183–192. doi: 10.1007/s004420050920. [DOI] [PubMed] [Google Scholar]
- 167.Wilson KB, Baldocchi DD, Hanson PJ. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 2000;20:565–578. doi: 10.1093/treephys/20.9.565. [DOI] [PubMed] [Google Scholar]
- 168.Martin AR, et al. Inter- and intraspecific variation in leaf economic traits in wheat and maize. AoB Plants. 2018;10:ply006. doi: 10.1093/aobpla/ply006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pahl AT, Kollmann J, Mayer A, Haider S. No evidence for local adaptation in an invasive alien plant: field and greenhouse experiments tracing a colonization sequence. Ann. Bot. 2013;112:1921–1930. doi: 10.1093/aob/mct246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cornelissen JHC, et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Veg. Sci. 2003;14:311–322. doi: 10.1111/j.1654-1103.2003.tb02157.x. [DOI] [Google Scholar]
- 171.Laughlin DC, Leppert JJ, Moore MM, Sieg CH. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2010;24:493–501. doi: 10.1111/j.1365-2435.2009.01672.x. [DOI] [Google Scholar]
- 172.Dalke IV, Novakovskiy AB, Maslova SP, Dubrovskiy YA. Morphological and functional traits of herbaceous plants with different functional types in the European Northeast. Plant Ecol. 2018;219:1295–1305. doi: 10.1007/s11258-018-0879-2. [DOI] [Google Scholar]
- 173.Gos P, et al. Relative contribution of soil, management and traits to co-variations of multiple ecosystem properties in grasslands. Oecologia. 2016;180:1001–1013. doi: 10.1007/s00442-016-3551-3. [DOI] [PubMed] [Google Scholar]
- 174.Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a mediterranean old-field succession. Funct. Ecol. 2006;20:21–30. doi: 10.1111/j.1365-2435.2006.01080.x. [DOI] [Google Scholar]
- 175.Thuiller, W. Traits of European Alpine Flora - Wilfried Thuiller - OriginAlps Project- Centre National de la Recherche Scientifique.
- 176.Blonder B, et al. Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants. 2015;7:plv049. doi: 10.1093/aobpla/plv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cornelissen, J. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. 10.2307/2261479 (1996).
- 178.Sheremetev, S. N. Herbs on the soil moisture gradient (water relations and the structural-functional organization) (in Russian). 271 (KMK, Moscow, 2005).
- 179.Wang, H. et al. The China Plant Trait Database. 10.1594/PANGAEA.871819 (2017).
- 180.Dahlin KM, Asner GP, Field CB. Environmental and community controls on plant canopy chemistry in a Mediterranean-type ecosystem. Proc. Natl. Acad. Sci. USA. 2013;110:6895–6900. doi: 10.1073/pnas.1215513110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boucher FC, Thuiller W, Arnoldi C, Albert CH, Lavergne S. Unravelling the architecture of functional variability in wild populations of Polygonum viviparum L. Funct. Ecol. 2013;27:382–391. doi: 10.1111/1365-2435.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Laughlin DC, Fulé PZ, Huffman DW, Crouse J, Laliberté E. Climatic constraints on trait-based forest assembly. J. Ecol. 2011;99:1489–1499. doi: 10.1111/j.1365-2745.2011.01885.x. [DOI] [Google Scholar]
- 183.Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 184.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 185.Quested HM, et al. Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology. 2003;84:3209–3221. doi: 10.1890/02-0426. [DOI] [Google Scholar]
- 186.Craine JM, Towne EG, Ocheltree TW, Nippert JB. Community traitscape of foliar nitrogen isotopes reveals N availability patterns in a tallgrass prairie. Plant Soil. 2012;356:395–403. doi: 10.1007/s11104-012-1141-7. [DOI] [Google Scholar]
- 187.Han W, et al. Floral, climatic and soil pH controls on leaf ash content in China’s terrestrial plants: Ecological controls on leaf ash. Glob. Ecol. Biogeogr. 2012;21:376–382. doi: 10.1111/j.1466-8238.2011.00677.x. [DOI] [Google Scholar]
- 188.Cornelissen JHC, et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct. Ecol. 2004;18:779–786. doi: 10.1111/j.0269-8463.2004.00900.x. [DOI] [Google Scholar]
- 189.Scherer-Lorenzen M, Schulze E-D, Don A, Schumacher J, Weller E. Exploring the functional significance of forest diversity: a new long-term experiment with temperate tree species (BIOTREE) Perspect. Plant Ecol. Evol. Syst. 2007;9:53–70. doi: 10.1016/j.ppees.2007.08.002. [DOI] [Google Scholar]
- 190.Adriaenssens, S. Dry Deposition and Canopy Exchange for Temperate Tree Species Under High Nitrogen Deposition. (Ghent Univ., 2012).
- 191.Chen Y, Han W, Tang L, Tang Z, Fang J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography. 2013;36:178–184. doi: 10.1111/j.1600-0587.2011.06833.x. [DOI] [Google Scholar]
- 192.Yguel B, et al. Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecol. Lett. 2011;14:1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 193.Smith NG, Dukes JS. LCE: leaf carbon exchange data set for tropical, temperate, and boreal species of North and Central America. Ecology. 2017;98:2978–2978. doi: 10.1002/ecy.1992. [DOI] [PubMed] [Google Scholar]
- 194.Rolo V, López-Díaz ML, Moreno G. Shrubs affect soil nutrients availability with contrasting consequences for pasture understory and tree overstory production and nutrient status in Mediterranean grazed open woodlands. Nutr. Cycl. Agroecosyst. 2012;93:89–102. doi: 10.1007/s10705-012-9502-4. [DOI] [Google Scholar]
- 195.Preston KA, Cornwell WK, Denoyer JL. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytol. 2006;170:807–818. doi: 10.1111/j.1469-8137.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 196.Schmitt M, et al. The evolution of aluminum accumulation in ferns and lycophytes. Am. J. Bot. 2017;104:573–583. doi: 10.3732/ajb.1600381. [DOI] [PubMed] [Google Scholar]
- 197.Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia. 2001;129:611–619. doi: 10.1007/s004420100752. [DOI] [PubMed] [Google Scholar]
- 198.Iversen CM, et al. A global fine‐root ecology database to address below‐ground challenges in plant ecology. New Phytol. 2017;215:15–26. doi: 10.1111/nph.14486. [DOI] [PubMed] [Google Scholar]
- 199.Akhmetzhanova AA, et al. A rediscovered treasure: mycorrhizal intensity database for 3000 vascular plant species across the former Soviet Union. Ecology. 2012;93:689–690. doi: 10.1890/11-1749.1. [DOI] [Google Scholar]
- 200.Werner GDA, et al. Symbiont switching and alternative resource acquisition strategies drive mutualism breakdown. Proc. Natl. Acad. Sci. USA. 2018;115:5229–5234. doi: 10.1073/pnas.1721629115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koele N, Dickie IA, Oleksyn J, Richardson SJ, Reich PB. No globally consistent effect of ectomycorrhizal status on foliar traits. New Phytol. 2012;196:845–852. doi: 10.1111/j.1469-8137.2012.04297.x. [DOI] [PubMed] [Google Scholar]
- 202.Diaz S, et al. The plant traits that drive ecosystems: evidence from three continents. J. Veg. Sci. 2004;15:295–304. doi: 10.1111/j.1654-1103.2004.tb02266.x. [DOI] [Google Scholar]
- 203.Bragazza L. Conservation priority of Italian Alpine habitats: a floristic approach based on potential distribution of vascular plant species. Biodivers. Conserv. 2009;18:2823–2835. doi: 10.1007/s10531-009-9609-3. [DOI] [Google Scholar]
- 204.Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26:163–182. doi: 10.1046/j.1365-3040.2003.00991.x. [DOI] [Google Scholar]
- 205.Paula S, et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:1420–1420. doi: 10.1890/08-1309.1. [DOI] [Google Scholar]
- 206.Schweingruber, F. H. & Landolt, W. The Xylem Database. Swiss Federal Research Institute WSL Updated. (2005).
- 207.Wirth, C. & Lichstein, J. W. The Imprint of Species Turnover on Old-Growth Forest Carbon Balances - Insights From a Trait-Based Model of Forest Dynamics. in Old-Growth Forests: Function, Fate and Value (eds. Wirth, C., Gleixner, G. & Heimann, M.) 81–113 (Springer, 2009). 10.1007/978-3-540-92706-8_5.
- 208.Green, W. USDA PLANTS Compilation, version 1. http://bricol.net/downloads/data/PLANTSdatabase/ (2002).
- 209.NRCS: The PLANTS Database. http://plants.usda.gov (2009).
- 210.Lin Y-S, et al. Optimal stomatal behaviour around the world. Nat. Clim. Change. 2015;5:459–464. doi: 10.1038/nclimate2550. [DOI] [Google Scholar]
- 211.Cavender-Bares J, Keen A, Miles B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:S109–122. doi: 10.1890/0012-9658(2006)87[109:PSOFPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 212.Cornelissen JHC, Diez PC, Hunt R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996;84:755–765. doi: 10.2307/2261337. [DOI] [Google Scholar]
- 213.Moles AT, Falster DS, Leishman MR, Westoby M. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. J. Ecol. 2004;92:384–396. doi: 10.1111/j.0022-0477.2004.00880.x. [DOI] [Google Scholar]
- 214.Fagúndez J, Izco J. Seed morphology of two distinct european species of Erica L. (Ericaceae) Acta Bot. Malacit. 2008;33:47–55. doi: 10.24310/abm.v33i0.6988. [DOI] [Google Scholar]
- 215.Kühn I, Durka W, Klotz S. BiolFlor — a new plant-trait database as a tool for plant invasion ecology. Divers. Distrib. 2004;10:363–365. doi: 10.1111/j.1366-9516.2004.00106.x. [DOI] [Google Scholar]
- 216.Vile, D. Significations fonctionnelle et écologique des traits des espèces végétales exemple dans une succession post-culturale méditerranéenne et généralisations. PhD thesis, Univ. Montpellier (2005).
- 217.Moretti M, Legg C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography. 2009;32:299–309. doi: 10.1111/j.1600-0587.2008.05524.x. [DOI] [Google Scholar]
- 218.Chave J, et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 219.Zanne, A. E. et al. Global wood density database. http://hdl.handle.net/10255/dryad.10235 (2009).
- 220.Ordoñez JC, et al. Plant strategies in relation to resource supply in mesic to wet environments: does theory mirror nature? Am. Nat. 2010;175:225–239. doi: 10.1086/649582. [DOI] [PubMed] [Google Scholar]
- 221.Neyret, M., Lachaise, T., van Kleunen, M., Bergmann, J. & Manning, P. Community-weighted mean for plant below-ground traits - 300 forest and grassland EPs - 2008-2018. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/27608 (2021).
- 222.Bird JP, et al. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 2020;34:1252–1261. doi: 10.1111/cobi.13486. [DOI] [PubMed] [Google Scholar]
- 223.McMahon BJ, Doyle S, Gray A, Kelly SBA, Redpath SM. European bird declines: do we need to rethink approaches to the management of abundant generalist predators? J. Appl. Ecol. 2020;57:1885–1890. doi: 10.1111/1365-2664.13695. [DOI] [Google Scholar]
- 224.Renner SC, Hoesel Wvan. Ecological and functional traits in 99 bird species over a large-scale gradient in Germany. Data. 2017;2:12. doi: 10.3390/data2020012. [DOI] [Google Scholar]
- 225.Pigot AL, et al. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat. Ecol. Evol. 2020;4:230–239. doi: 10.1038/s41559-019-1070-4. [DOI] [PubMed] [Google Scholar]
- 226.Penone, C., Renner, S., Teuscher, M. & Fischer, M. Bird traits for all species in the Exploratories EPs. https://www.bexis.uni-jena.de/ddm/data/Showdata/31368 (2022).
- 227.Conenna I, et al. Global patterns of functional trait variation along aridity gradients in bats. Glob. Ecol. Biogeogr. 2021;30:1014–1029. doi: 10.1111/geb.13278. [DOI] [Google Scholar]
- 228.Wilkinson GS, South JM. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 229.Schall, O. & Petermann, R. National Report on Bat Conservation in the Federal Republic of Germany 2010-2013. https://www.eurobats.org/sites/default/files/documents/pdf/National_Reports/Inf.MoP7_.20-National%20Implementation%20Report%20of%20Germany.pdf (2014).
- 230.Neff F, et al. Long-term restoration success of insect herbivore communities in semi-natural grasslands: a functional approach. Ecol. Appl. 2020;30:e02133. doi: 10.1002/eap.2133. [DOI] [PubMed] [Google Scholar]
- 231.Nickel, H. & Remane, R. Check list of the planthoppers and leafhoppers of Germany, with notes on food plants, diet width, life cycles, geographic range and conservation status (Hemiptera, Fulgoromorpha and Cicadomorpha). Beitr. Zur Zikadenkunde5, 27–64 (2002).
- 232.Saulich A, Musolin D. Seasonal Development of Plant Bugs (Heteroptera, Miridae): Subfamily Mirinae, Tribe Stenodemini. Entomol. Rev. 2021;101:147–161. doi: 10.1134/S0013873821020019. [DOI] [Google Scholar]
- 233.Staab, M., Simons, N. K., Gossner, M. M., Weisser, W. W. & Blüthgen, N. Body size and life-history traits of arthropod species. v7. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/31122 (2022).
- 234.Blüthgen, N., Mangels, J. & Schneider, F. Morphological traits of moths (Standardized). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/23926 (2018).
- 235.Mangels, J. & Blüthgen, N. Life history traits of moths. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21228 (2017).
- 236.Baulechner D. et al. Collembola traits from all grassland plots (2023). v0. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/31481 (2023).
- 237.Frostegård Å, Tunlid A, Bååth E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods. 1991;14:151–163. doi: 10.1016/0167-7012(91)90018-L. [DOI] [Google Scholar]
- 238.Frostegård, Å., Tunlid, A. & Bååth, E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 10.1128/aem.59.11.3605-3617.1993 (1993). [DOI] [PMC free article] [PubMed]
- 239.Ruess L, Chamberlain PM. The fat that matters: soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 2010;42:1898–1910. doi: 10.1016/j.soilbio.2010.07.020. [DOI] [Google Scholar]
- 240.Dumack K, Fiore-Donno AM, Bass D, Bonkowski M. Making sense of environmental sequencing data: ecologically important functional traits of the protistan groups Cercozoa and Endomyxa (Rhizaria) Mol. Ecol. Resour. 2020;20:398–403. doi: 10.1111/1755-0998.13112. [DOI] [PubMed] [Google Scholar]
- 241.Schenk, N. et al.Assembled ecosystem measures from grassland EPs. Biodiversity Exploratories Information Systemhttps://www.bexis.uni-jena.de/ddm/data/Showdata/27087 (2020).
- 242.Smith MS, Tiedje JM. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 1979;11:261–267. doi: 10.1016/0038-0717(79)90071-3. [DOI] [Google Scholar]
- 243.Keil D, et al. Effects of warming and drought on potential N2O emissions and denitrifying bacteria abundance in grasslands with different land-use. FEMS Microbiol. Ecol. 2015;91:fiv066. doi: 10.1093/femsec/fiv066. [DOI] [PubMed] [Google Scholar]
- 244.Schinner, F., Öhlinger, R., Kandeler, E. & Margesin, R. Methods in Soil Biology (Springer, 1996).
- 245.Ollivier J, et al. Effects of repeated application of sulfadiazine-contaminated pig manure on the abundance and diversity of ammonia and nitrite oxidizers in the root-rhizosphere complex of pasture plants under field conditions. Front. Microbiol. 2013;4:22. doi: 10.3389/fmicb.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hoffmann H, Schloter M, Wilke B-M. Microscale-scale measurement of potential nitrification rates of soil aggregates. Biol. Fertil. Soils. 2007;44:411–413. doi: 10.1007/s00374-007-0227-5. [DOI] [Google Scholar]
- 247.Marx M-C, Wood M, Jarvis SC. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001;33:1633–1640. doi: 10.1016/S0038-0717(01)00079-7. [DOI] [Google Scholar]
- 248.Berner D, et al. Land-use intensity modifies spatial distribution and function of soil microorganisms in grasslands. Pedobiologia. 2011;54:341–351. doi: 10.1016/j.pedobi.2011.08.001. [DOI] [Google Scholar]
- 249.Kandeler, E., Berner, D., Marhan, S. & Boeddinghaus, R. S. Soil enzyme activities of all grassland EPs, soil sampling campaign (SSC) 2011, SCALEMIC. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/20246 (2017).
- 250.Stempfhuber, B. & Schloter, M. Abundance of nitrogen fixing microbes and ammonia oxidizers in grassland. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/13986 (2017).
- 251.Stempfhuber, B. & Schloter, M. Nitrogen pools and potential nitrification taken at soil sampling campaign 2014. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/19847 (2016).
- 252.Kovacevic, V., Schulz, S. & Schloter, M. Nitrifiers abundances at grassland EPs. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21547 (2019).
- 253.Frank, K. & Blüthgen, N. Decomposition (dung removal in g on 300 EPs, season 2014/2015), Taxon: dung beetles (Invertebrates, Scarabaeoidea) - Dungwebs. v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21206 (2017).
- 254.Solly, E. & Schöning, I. MinSoil 2011 root decomposition. v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/16666 (2013).
- 255.Fischer, M. & Grassein, F. Litter decomposition (2013, all grassland EPs). v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/18926 (2015).
- 256.Grogan P. Co2 Flux measurement using soda lime: correction for water formed during co2 adsorption. Ecology. 1998;79:1467–1468. doi: 10.1890/0012-9658(1998)079[1467:CFMUSL]2.0.CO;2. [DOI] [Google Scholar]
- 257.Keith H, Wong SC. Measurement of soil CO2 efflux using soda lime absorption: both quantitative and reliable. Soil Biol. Biochem. 2006;38:1121–1131. doi: 10.1016/j.soilbio.2005.09.012. [DOI] [Google Scholar]
- 258.Apostolakis, A., Schöning, I., Schrumpf, M., Klötzing, T. & Trumbore, S. MinSoil 2018-19: soil respiration in forests and grasslands. v4. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/26908 (2020).
- 259.Boch, S., Mueller, J., Socher, S., Prati, D. & Fischer, M. Measurement of biomass (2009, all grassland EPs). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/16209 (2017).
- 260.Fischer, M., Schäfer, D., Boch, S. & Prati, D. Vegetation records for 150 grassland EPs in 2016, header data without species identities (incl. biomass). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/21187 (2017).
- 261.Prati, D., Fischer, M., Minker, J. & Schmitt, B. Measurement of biomass (2013, all grassland EPs). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/16786 (2013).
- 262.Schäfer, D., Prati, D. & Fischer, M. Vegetation records for 150 grassland EPs in 2017, header data without species identities (incl. biomass). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/23486 (2018).
- 263.Schäfer, D., Fischer, M., Klaus, V. & Busch, V. Vegetation records for 150 grassland EPs in 2014, header data without species identities (incl. biomass). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/19807 (2016).
- 264.Schäfer, D., Fischer, M., Klaus, V. & Busch, V. Vegetation records for 150 grassland EPs in 2015, header data without species identities (incl. biomass). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/19809 (2016).
- 265.Schmitt, B., Prati, D., Fischer, M. & Minker, J. Measurement of biomass (2011, all grassland EPs). v3. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/14346 (2012).
- 266.Schmitt, B., Prati, D., Fischer, M. & Minker, J. Measurement of biomass (2012, all grassland EPs). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/14986 (2012).
- 267.Schmitt, B., Prati, D. & Fischer, M. Measurement of biomass (2010, all grassland EPs). v2. Biodiversity Exploratories Information System. Dataset. https://www.bexis.uni-jena.de/ddm/data/Showdata/12706 (2011).
- 268.R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria 2023).
- 269.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 270.Suding KN, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 2008;14:1125–1140. doi: 10.1111/j.1365-2486.2008.01557.x. [DOI] [Google Scholar]
- 271.Pollock LJ, et al. Understanding co‐occurrence by modelling species simultaneously with a Joint Species Distribution Model. Methods Ecol. Evol. 2014;5:397–406. doi: 10.1111/2041-210X.12180. [DOI] [Google Scholar]
- 272.Freckleton RP. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 2002;71:542–545. doi: 10.1046/j.1365-2656.2002.00618.x. [DOI] [Google Scholar]
- 273.Bécue-Bertaut M, Pagès J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput. Stat. Data Anal. 2008;52:3255–3268. doi: 10.1016/j.csda.2007.09.023. [DOI] [Google Scholar]
- 274.Escofier B, Pagès J. Multiple factor analysis (AFMULT package) Comput. Stat. Data Anal. 1994;18:121–140. doi: 10.1016/0167-9473(94)90135-X. [DOI] [Google Scholar]
- 275.Kéfi S, et al. More than a meal... integrating non-feeding interactions into food webs. Ecol. Lett. 2012;15:291–300. doi: 10.1111/j.1461-0248.2011.01732.x. [DOI] [PubMed] [Google Scholar]
- 276.Rosseel Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012;48:1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 277.Wolfgang Wagner. Europäische Schmetterlinge und ihre Ökologie. http://www.pyrgus.de/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This work is based on data collected within several projects of the Biodiversity Exploratories program (DFG Priority Program 1374). Most datasets from the Biodiversity Exploratories are publicly available in the Biodiversity Exploratories Information System (Bexis) (10.17616/R32P9Q). The CWM data generated in this study have been deposited under accession code 31689 https://www.bexis.uni-jena.de/ddm/data/Showdata/31689. The raw trait and abundance, and ecosystem functions datasets are listed below, most of which are publicly available. To give data owners and collectors time to perform their analysis the Biodiversity Exploratories’ data and publication policy includes by default an embargo period of three years from the end of data collection/data assembly. Access to the remaining datasets can thus be obtained by contacting the Biodiversity Exploratories office or data owners. At the end of the embargo period these datasets will be made publicly available via the same data repository. Full list of used datasets (both from the Biodiversity Exploratories and external, previously published datasets)3,19,45,47,53–56,93–97,101–119,221–235,240,249–254,268–109,110,277.
Full code to replicate the analyses is stored on GitHub at https://github.com/mneyret/trait_synchronies under the 10.5281/zenodo.10286643.