Abstract
Steinernema hermaphroditum is the only identified entomopathogenic nematode that is consistently hermaphroditic and thus offers a great opportunity to use genetic approaches to probe symbiosis. Evolutionarily, ecologically, and morphologically distinct from laboratory nematodes commonly used in the laboratory, with both forward and reverse genetics tools available, this species also provides an opportunity to explore other areas of biology, especially using comparative studies. Here, we describe an improved solid medium-based culturing method for S. hermaphroditum that we found particularly helpful for phenotypic analysis and genetic manipulation. We document the rapid increase in the size of the worm; and show that the uniform growth of the worm on this medium provides a good basis for developmental studies. Finally, we measure the brood size of individual animals, which, although far larger, has a very similar trajectory to that of the hermaphroditic Caenorhabditis elegans, suggesting common reproductive restraints.
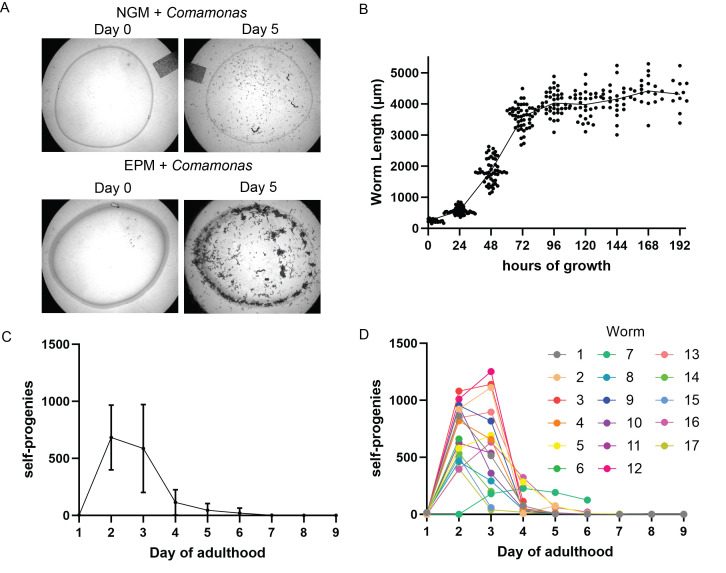
Figure 1. An improved solid medium culturing method for Steinernema hermaphroditum.
(A) Enriched Peptone Medium (EPM) agar plates seeded with Comamonas aquatica can sustain a much larger population of S. hermaphroditum than NGM agar. Starting with three young larvae, the resulting population exhausted a lawn of C. aquatica bacteria grown on NGM by day 5 (the mark left by the lawn edge can still be seen), while the C. aquatica lawn grown on EPM agar was still present and was supporting F 1 animals that had grown much larger in size. Pictures are representative of six replicates. (B) S. hermaphroditum grew rapidly but uniformly on C. aquatica /EPM plates. Starting from a near-synchronous population, with an average length of 241 ± 48 µm (n = 42), the worms developed rapidly, increasing in length to 571 ± 119 µm (n = 52) at 24 hours, 1844 ± 384 µm (n = 52) at 48 hours, 3592 ± 373 µm (n = 51) at 72 hours, 4028 ± 371 µm (n = 39) at 96 hours, 3982 ± 423 µm (n = 31) at 120 hours, 4152 ± 472 µm (n = 25) at 144 hours, 4416 ± 437 µm (n = 19) at 168 hours, and 4312 ± 512 µm (n = 11) at 192 hours. (C) S. hermaphroditum hermaphrodites produced 1363 ± 623 self-progeny (n = 17) with 3 ± 6 (n = 17) on day 1, 683 ± 284 (n = 17) on day 2, 587 ± 385 (n = 16) on day 3, 115 ± 111 (n = 13) on day 4, 45 ± 59 (n = 10) on day 5, 21 ± 43 (n = 8) on day 6, 2 ± 2 (n = 5) on day 7, 0 (n = 4) on day 8, 0 ± 1 (n = 4) on day 9. Values are mean ± SD (17 worms were analyzed; for each time point, the data from all the surviving worms were used). (D) Brood size analysis of individual animals. Each color point represents the number of self-progeny produced by an individual animal during the day of adulthood indicated on the X axis, with identically colored lines connecting the colored dots. Data from the same animals are presented as summaries in in C and as individuals in D. All worms were incubated at 25°C.
Description
The nematode Steinernema hermaphroditum (Griffin et al. 2001; Stock et al. 2004; Bhat et al. 2019), is the only entomopathogenic nematode characterized that is consistently hermaphroditic (Cao et al. 2022), a reproductive mode that has been critical for the successful use of most of the common laboratory cultured nematodes in molecular genetics research (Felix 2006; Sommer 2006; Gupta et al. 2007; Corsi et al. 2015). A genetically tractable entomopathogenic nematode is a highly attractive animal model for symbiosis research, as they are not only parasitic to insect hosts but also themselves the host in a mutualistically symbiotic relationship with their pathogenic bacteria that help them kill their insect prey. In addition to its relationships with its insect host and its bacterial endosymbiont, the biology of S. hermaphroditum offers other opportunities, particularly for developmental studies. Specifically, as a clade IV nematode, it is evolutionarily distant from most of the small free-living nematodes commonly used as genetic models (such as the clade V Caenorhabditis elegans and Pristionchus pacificus ), and it is morphologically distinctively different from these nematodes. Our laboratory has shown that S. hermaphroditum is genetically tractable, with tools developed for both forward and reverse genetic analyses (Cao et al. 2022; Cao 2023; Schwartz et al. 2023), and efforts have been made to adapt or establish experimental methods with S. hermaphroditum as the animal model (Garg et al. 2022; Alani et al. 2023; Huynh et al. 2023).
Previously, we showed that S. hermaphroditum could be cultured on bacterial lawns grown on Nematode Growth Medium (NGM) agar in Petri plates (Cao et al. 2022), using as the bacteria either its native symbiont Xenorhabdus griffiniae HGB2511 (Cao et al. 2022) or Comamonas aquatica DA1877 (Avery and Shtonda 2003; Watson et al. 2014), on which the worm grows faster. Culturing S. hermaphroditum on C. aquatica offers several advantages. First, unlike Xenorhabdus , C. aquatica is not pigmented, offering an unobstructed view of the transparent S. hermaphroditum large and small, resembling the experience of examining C. elegans growing on Escherichia coli OP50 and is particularly helpful for developmental and phenotypic analysis. Second, C. aquatica may provide a less challenging environment for S. hermaphroditum than does X. griffiniae ; for example, worms recover better on C. aquatica after microinjection (Cao 2023; Schwartz et al. 2023). Third, Xenorhabdus bacteria are known to exist in two forms (Akhurst 1980) and are light sensitive (Xu and Hurlbert 1990) ; both characteristics may cause unintended variations in experiments if not well controlled.
However, we found that NGM is a poor medium for C. aquatica , and the poor growth led to inefficient food for effective maintenance of the worm, exacerbated by the body size and the brood size of the animal (see below). In addition, we observed that worms cultured on C. aquatica /NGM frequently wandered off the bacterial lawn and left the plate entirely, likely also a result of poor bacterial growth conditions. We thus experimented with other media, resulting in conditions that significantly abrogated this problem (see below).
We adopted the Enriched Peptone Plates with nystatin recipe from Evans (2006) with agar concentration modified to 1.8% (weight/volume) (See Methods for the complete protocol), which we abbreviate as EPM (Enriched Peptone Medium). EPM supports a much higher level of C. aquatica growth than NGM, but the bacterial lawn remained highly transparent and was evenly grown. C. aquatica /EPM overcomes the limitations of C. aquatica /NGM, as it supports a far larger number of animals per plate, and the vast majority of the animals stay on the lawn. As in the example shown in Fig. 1A, in which 3 larvae were placed on either a 10 cm NGM plate or a 10 cm EPM plate seeded with C. aquatica , the EPM plates allowed the F 1 animals to develop to a larger size in comparison to the NGM plates, on which the food was more quickly exhausted, making EPM plates more useful for genetic analysis. If cultured on DA1877 on EPM at 25°C, the size of S. hermaphroditum hermaphrodites increased rapidly, reaching an average length of about 4 mm, with some individuals exceeding 5 mm ( Fig. 1B ). Animal growth over time showed little variation ( Fig. 1B ), providing a good basis for developmental studies. The lack of bacterial pigmentation and ample food supply facilitated quantification of the self-fertilizing brood size of S. hermaphroditum . On average, an unmated hermaphrodite has 1,363 ± 623 progeny (n = 17) ( Fig. 1C ), with some animals having a brood size exceeding 2,000 ( Fig. 1D ). Compared to the rather uniform size increase, larger variations were observed between individual animals. Nonetheless, it is clear that peak productivity and the vast majority of reproduction occurs on the second and third day of adulthood and drops precipitately afterwards. This resembles the time course of selfing reproduction seen in the clade V hermaphroditic nematode C. elegans (Maupas 1900; Hirsh et al. 1976; Gems and Riddle 1996; Hughes et al. 2007), which may indicate a shared reproductive constraint. As with most hermaphroditic nematodes described (Maupas 1900; Felix and Sternberg 1996; Sommer et al. 1996; Pires-daSilva 2007; Shinya et al. 2014), S. hermaphroditum hermaphrodites have a somatic body similar morphologically to the females of related species, suggesting they have a female origin. The brood size of C. elegans by self-fertilizing was shown very early to be restricted by sperm (Maupas 1900) , which are only produced during late larval development, after which the germline shifts from spermatogenesis to oogenesis (Hirsh et al. 1976; Ward and Carrel 1979). The sperm followed by oocyte pattern was also followed by the hermaphrodite of other androdioecious (hermaphrodite with, often rare, males) species (Rudel et al. 2005; Shinya et al. 2014), but alternative patterns are possible, as has been shown in the hermaphrodite of trioecious Auanema species, of which sperm are produced continuously (McCaig et al. 2017). The similar trend in progeny production we observed in S. hermaphroditum led us to reason that hermaphroditism in this species also involves the production of sperm in the gonad before shifting to the production of oocytes, and a major restriction on the brood size was the number of sperm available. We also observed large numbers of unfertilized oocytes laid by post-reproductive hermaphrodites, as has previously been seen in post‑reproductive C. elegans (Maupas 1900; Hirsh et al. 1976; Ward and Carrel 1979), further suggesting that the hermaphroditic reproduction of this clade IV nematode is similar to that of the clade V nematode C. elegans , despite their phylogenetic distance and difference in brood size.
In summary, we have developed improved culturing methods for S. hermaphroditum that we believe could facilitate the adaptation of the animal as a model, particularly for laboratories already familiar with techniques for working with C. elegans. We analyzed the growth and reproduction of S. hermaphroditum using this culture method, both to provide a baseline for the method and because using previously existing methods we had found some analyses to be more difficult than they should be. In doing so, we showed that the brood size of unmated S. hermaphroditum hermaphrodites is very large (reaching more than 2,000 in some worms), is still quite variable (with some variation likely resulting from the early death of some worms from eggs hatching internally; “bagging”) and is likely limited by the number of sperm the hermaphrodite produces. Most importantly, we hope we have shown that S. hermaphroditum is a very different nematode but can be easily adopted by any lab with experience working with C. elegans .
Methods
Solid medium-based culturing method for S. hermaphroditum
Our recipe for Enriched Peptone Medium (EPM) agar was adapted from Evans (2006) with a minor modification (decreased agar concentration) and briefly described in Schwartz et al. (2023), in which the Enriched Peptone Medium EPM was utilized for culturing worms for microinjections and recovery afterwards. Briefly, for 1 liter:
(1) Weigh 1.2 g of sodium chloride (NaCl), 20 g of peptone (Bacto Peptone, Gibco), 18 g of agar (Bacto Agar, BD Diagnostics), and add deionized water to 1 liter.
(2) Autoclave. Let cool down to 55°C with stirring.
(3) Add solutions sterilized by autoclaving or filtration: 1 ml cholesterol (5 mg/ml in ethanol), 1 ml 1 M magnesium sulfate (MgSO 4 ), 25 ml 1 M KPO 4 (pH 6.0), and 10 ml of Nystatin suspension (10,000 unit/mL, N1638, Sigma)
For maintenance of S. hermaphroditum , 10 cm Petri plates are recommended.
Nematode Growth Medium (NGM) was prepared similarly to what was described by Brenner (1974). Comamonas aquatica DA1877 (Avery and Shtonda 2003; Watson et al. 2014) were cultured overnight in Luria-Bertani (LB) medium in an Erlenmeyer flask at 200 rpm, 37°C, and seeded on EPM or NGM agar in Petri plates the next day. Except when otherwise noted, all S. hermaphroditum were cultured at 25°C on Petri plates containing EPM agar with a lawn of C. aquatica DA1877.
Obtaining a synchronized population of S. hermaphroditum
To obtain a near-synchronous population of young S. hermaphroditum , we modified the synchronization method based on Stiernagle (2006) and what we described in Cao et al. (2022). Briefly, gravid adults were washed off from the plates using M9 buffer (For 1 liter: 3 g KH 2 PO 4 , 6 g Na 2 HPO 4 , 5 g NaCl, and with 1 ml 1 M MgSO 4 added after autoclaving) and collected into a 15 ml centrifuge tube. The worm-suspension was then centrifuged to remove the supernatant and water was added to the worm pellet to a total volume of 3.5 ml. 0.5 ml of 5 M NaOH and 1 ml of household bleach (8% available chlorine) were then added to the solution. The solution was mixed by gently shaking and allowed to react for about 5 minutes; most of the tissues besides the embryos should be dissolved by this point; longer reactions substantially decreased the survivability of the embryos. After this, the embryos were collected by centrifugation and washed with 10 ml of M9 buffer three times. After the washes, the embryos were seeded on NGM agar plates without bacteria and allowed to hatch overnight, and the larvae were collected the next day for experimentation.
Comparison of S. hermaphroditum culture on C. aquatica /NGM and C. aquatica /EPM
Growth comparisons between the media, as shown in Fig. 1A, were done by placing three semi -synchronized larvae on each of six 10 cm Petri plates containing C. aquatica /NGM and six 10 cm Petri plates containing C. aquatica /EPM ( Fig. 1A, left ). The plates were then assayed every 24 hours, and images were taken at the start of the experiment and on the fifth day after the start of the experiment, at which point the C. aquatica lawns on all six NGM plates were exhausted but were still present on all six EPM plates ( Fig. 1A, left ). Similar results were also observed in another independent trial.
Growth rate analysis
To measure the growth of S. hermaphroditum hermaphrodites on C. aquatica /EPM plates, five semi-synchronized larvae each were transferred onto C. aquatica /EPM plates, and images were acquired every 24 hours using WormLab (MBF Bioscience, Williston, VT) equipment and software. The camera was a Nikon AF Micro 60/2.8D with zoom magnification. Animals were moved to new plates upon the hatching of progeny, and plates that included males were excluded from further analysis. Worm length was measured by processing the image with ImageJ (Fiji) (Schindelin et al. 2012) by converting the image to 8-bit, thresholding to obtain the shape of the worm, and by using the “skeletonize” and “analyze skeleton” feature (Arganda‐Carreras et al. 2010). Most worms, but not every worm, were measured at each time point.
Brood size analysis
J4 worms were placed individually on C. aquatica /EPM plates and maintained at 25°C. Every 24 hours, mothers were examined and transferred to fresh plates to remove them from the eggs they had laid. One day after the mother had been removed the progeny they had deposited were counted, removing each animal as it was counted using a Pasteur pipette connected to a vacuum aspirator to avoid double counting; plates were then checked again on the next day for overlooked progeny.
Acknowledgments
Acknowledgments
We thank members of the Sternberg Lab for their support and valuable suggestions. We would especially like to thank Wilber Palma for his help in medium preparation, Alakananda Das for her suggestions on worm length measurement analysis, Hillel Schwartz for valuable suggestions, and both he and Erich Schwarz (Cornell University) for critical reading of the manuscript. The C. aquatica strain DA1877 was obtained from the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Funding Statement
<p>This research was supported by National Science Foundation (NSF) Enabling Discovery through GEnomics (EDGE) grant 2128267 to PWS.</p>
References
- AKHURST R. J. Morphological and Functional Dimorphism in Xenorhabdus spp., Bacteria Symbiotically Associated with the Insect Pathogenic Nematodes Neoaplectana and Heterorhabditis. Microbiology. 1980 Dec 1;121(2):303–309. doi: 10.1099/00221287-121-2-303. [DOI] [Google Scholar]
- Alani OS, Cao M, Goodrich-Blair H, Heppert JK. Conjugation and transposon mutagenesis of Xenorhabdus griffiniae HGB2511, the bacterial symbiont of the nematode Steinernema hermaphroditum (India). MicroPubl Biol. 2023 Apr 25;2023 doi: 10.17912/micropub.biology.000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arganda-Carreras I, Fernández-González R, Muñoz-Barrutia A, Ortiz-De-Solorzano C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc Res Tech. 2010 Oct 1;73(11):1019–1029. doi: 10.1002/jemt.20829. [DOI] [PubMed] [Google Scholar]
- Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003 Jul 1;206(Pt 14):2441–2457. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AH, Chaubey AK, Shokoohi E, William Mashela P. Study of Steinernema hermaphroditum (Nematoda, Rhabditida), from the West Uttar Pradesh, India. Acta Parasitol. 2019 May 10;64(4):720–737. doi: 10.2478/s11686-019-00061-9. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May 1;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Mengyi. CRISPR-Cas9 genome editing in Steinernema entomopathogenic nematodes . 2023 Nov 25; doi: 10.1101/2023.11.24.568619. [DOI]
- Cao M, Schwartz HT, Tan CH, Sternberg PW. The entomopathogenic nematode Steinernema hermaphroditum is a self-fertilizing hermaphrodite and a genetically tractable system for the study of parasitic and mutualistic symbiosis. Genetics. 2022 Jan 4;220(1) doi: 10.1093/genetics/iyab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics. 2015 Jun 1;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans Thomas. Transformation and microinjection. WormBook. 2006 doi: 10.1895/wormbook.1.108.1. [DOI] [Google Scholar]
- Félix MA. Oscheius tipulae. WormBook. 2006 Aug 16;:1–8. doi: 10.1895/wormbook.1.119.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix MA, Sternberg PW. Symmetry breakage in the development of one-armed gonads in nematodes. Development. 1996 Jul 1;122(7):2129–2142. doi: 10.1242/dev.122.7.2129. [DOI] [PubMed] [Google Scholar]
- Garg P, Tan CH, Sternberg PW. DiI staining of sensory neurons in the entomopathogenic nematode Steinernema hermaphroditum. MicroPubl Biol. 2022 Feb 24;2022 doi: 10.17912/micropub.biology.000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996 Feb 22;379(6567):723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- Griffin CT, O'Callaghan KM, Dix I. A self-fertile species of Steinernema from Indonesia: further evidence of convergent evolution amongst entomopathogenic nematodes? Parasitology. 2001 Feb 1;122(Pt 2):181–186. doi: 10.1017/s003118200100717x. [DOI] [PubMed] [Google Scholar]
- Gupta BP, Johnsen R, Chen N. Genomics and biology of the nematode Caenorhabditis briggsae. WormBook. 2007 May 3;:1–16. doi: 10.1895/wormbook.1.136.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976 Mar 1;49(1):200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2006 Dec 28;3(2):e25–e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T, O'Halloran D, Hawdon J, Eleftherianos I. The nematode parasite Steinernema hermaphroditum is pathogenic to Drosophila melanogaster larvae without activating their immune response. MicroPubl Biol. 2023 Sep 25;2023 doi: 10.17912/micropub.biology.000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupas E. Modes et formes de reproduction des nematodes. 1900 Jan 8.
- McCaig CM, Lin X, Farrell M, Rehain-Bell K, Shakes DC. Germ cell cysts and simultaneous sperm and oocyte production in a hermaphroditic nematode. Dev Biol. 2017 Aug 24;430(2):362–373. doi: 10.1016/j.ydbio.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Pires-daSilva A. Evolution of the control of sexual identity in nematodes. Semin Cell Dev Biol. 2007 Jan 19;18(3):362–370. doi: 10.1016/j.semcdb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Rudel D, Riebesell M, Sommer RJ. Gonadogenesis in Pristionchus pacificus and organ evolution: development, adult morphology and cell-cell interactions in the hermaphrodite gonad. Dev Biol. 2005 Jan 1;277(1):200–221. doi: 10.1016/j.ydbio.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012 Jun 28;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz HT, Tan CH, Peraza J, Raymundo KLT, Sternberg PW. Molecular identification of a peroxidase gene controlling body size in the entomopathogenic nematode Steinernema hermaphroditum. Genetics. 2023 Dec 11; doi: 10.1093/genetics/iyad209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya R, Hasegawa K, Chen A, Kanzaki N, Sternberg PW. Evidence of hermaphroditism and sex ratio distortion in the fungal feeding nematode Bursaphelenchus okinawaensis. G3 (Bethesda) 2014 Aug 12;4(10):1907–1917. doi: 10.1534/g3.114.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer RJ. Pristionchus pacificus. WormBook. 2006 Aug 14;:1–8. doi: 10.1895/wormbook.1.102.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer R, Carta LK, Kim SY, Sternberg PW. Morphological, genetic and molecular description of Pristionchus pacificus sp. n.(Nematoda: Neodiplogasteridae). Fundamental and applied Nematology. 1996 Jan 1;19:511-22.
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006 Feb 11;:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock S. Patricia, Griffin Christine T., Chaerani Rani. Morphological and molecular characterisation of Steinernema hermaphroditum n. sp. (Nematoda: Steinernematidae), an entomopathogenic nematode from Indonesia, and its phylogenetic relationships with other members of the genus. Nematology. 2004;6(3):401–412. doi: 10.1163/1568541042360555. [DOI] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979 Dec 1;73(2):304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, Walhout AJ. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014 Feb 13;156(4):759–770. doi: 10.1016/j.cell.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hurlbert RE. Toxicity of Irradiated Media for Xenorhabdus spp. Appl Environ Microbiol. 1990 Mar 1;56(3):815–818. doi: 10.1128/aem.56.3.815-818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]