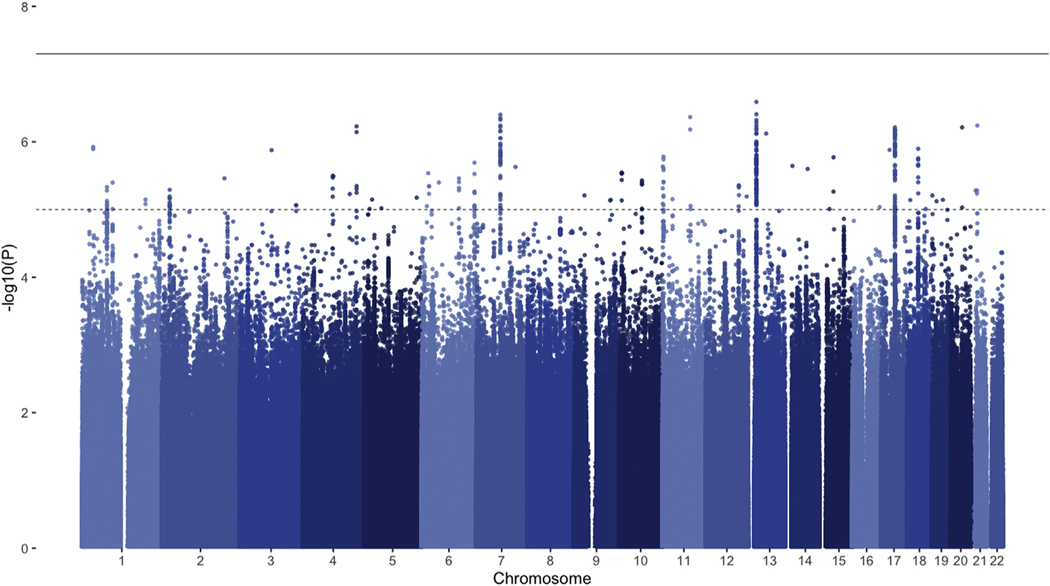
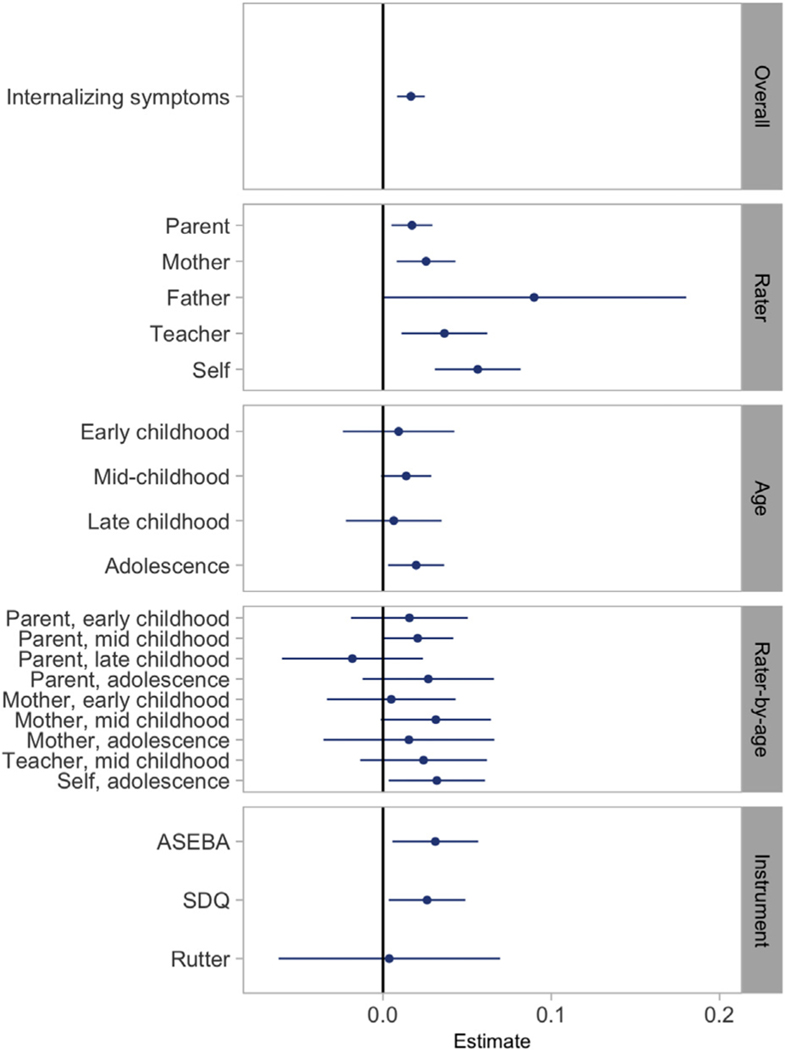
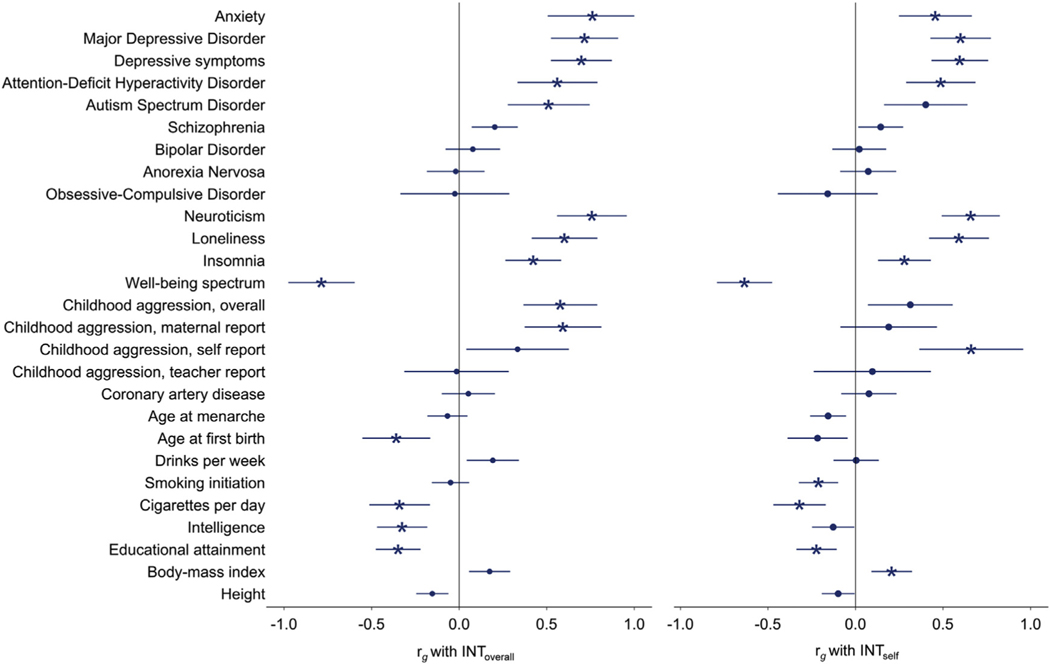
Eshim S Jami
Eshim S Jami, MSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Anke R Hammerschlag
Anke R Hammerschlag, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Hill F Ip
Hill F Ip, MSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Andrea G Allegrini
Andrea G Allegrini, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Beben Benyamin
Beben Benyamin, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Richard Border
Richard Border, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Elizabeth W Diemer
Elizabeth W Diemer, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Chang Jiang
Chang Jiang, MS
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ville Karhunen
Ville Karhunen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Yi Lu
Yi Lu, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Qing Lu
Qing Lu, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Travis T Mallard
Travis T Mallard, MA
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Pashupati P Mishra
Pashupati P Mishra, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ilja M Nolte
Ilja M Nolte, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Teemu Palviainen
Teemu Palviainen, MSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Roseann E Peterson
Roseann E Peterson, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Hannah M Sallis
Hannah M Sallis, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Andrey A Shabalin
Andrey A Shabalin, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ashley E Tate
Ashley E Tate, MSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Elisabeth Thiering
Elisabeth Thiering, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Natàlia Vilor-Tejedor
Natàlia Vilor-Tejedor, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Carol Wang
Carol Wang, BSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ang Zhou
Ang Zhou, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Daniel E Adkins
Daniel E Adkins, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Silvia Alemany
Silvia Alemany, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Helga Ask
Helga Ask, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Qi Chen
Qi Chen, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Robin P Corley
Robin P Corley, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Erik A Ehli
Erik A Ehli, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Luke M Evans
Luke M Evans, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Alexandra Havdahl
Alexandra Havdahl, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Fiona A Hagenbeek
Fiona A Hagenbeek, MSc
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Christian Hakulinen
Christian Hakulinen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Anjali K Henders
Anjali K Henders, BSc (HONS)
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Jouke Jan Hottenga
Jouke Jan Hottenga, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Tellervo Korhonen
Tellervo Korhonen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Abdullah Mamun
Abdullah Mamun, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Shelby Marrington
Shelby Marrington, BBehSc, BArts (HONS)
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Alexander Neumann
Alexander Neumann, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Kaili Rimfeld
Kaili Rimfeld, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Fernando Rivadeneira
Fernando Rivadeneira, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Judy L Silberg
Judy L Silberg, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Catharina E van Beijsterveldt
Catharina E van Beijsterveldt, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Eero Vuoksimaa
Eero Vuoksimaa, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Alyce M Whipp
Alyce M Whipp, MPH
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Xiaoran Tong
Xiaoran Tong, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ole A Andreassen
Ole A Andreassen, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Dorret I Boomsma
Dorret I Boomsma, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Sandra A Brown
Sandra A Brown, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
S Alexandra Burt
S Alexandra Burt, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
William Copeland
William Copeland, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Danielle M Dick
Danielle M Dick, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
K Paige Harden
K Paige Harden, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Kathleen Mullan Harris
Kathleen Mullan Harris, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Catharina A Hartman
Catharina A Hartman, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Joachim Heinrich
Joachim Heinrich, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
John K Hewitt
John K Hewitt, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Christian Hopfer
Christian Hopfer, MD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Elina Hypponen
Elina Hypponen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Marjo-Riitta Jarvelin
Marjo-Riitta Jarvelin, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Jaakko Kaprio
Jaakko Kaprio, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Liisa Keltikangas-Järvinen
Liisa Keltikangas-Järvinen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Kelly L Klump
Kelly L Klump, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Kenneth Krauter
Kenneth Krauter, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ralf Kuja-Halkola
Ralf Kuja-Halkola, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Henrik Larsson
Henrik Larsson, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Terho Lehtimäki
Terho Lehtimäki, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Paul Lichtenstein
Paul Lichtenstein, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Sebastian Lundström
Sebastian Lundström, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Hermine H Maes
Hermine H Maes, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Per Magnus
Per Magnus, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Marcus R Munafò
Marcus R Munafò, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Jake M Najman
Jake M Najman, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Pål R Njølstad
Pål R Njølstad, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Albertine J Oldehinkel
Albertine J Oldehinkel, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Craig E Pennell
Craig E Pennell, MBBS, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Robert Plomin
Robert Plomin, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Ted Reichborn-Kjennerud
Ted Reichborn-Kjennerud, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Chandra Reynolds
Chandra Reynolds, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Richard J Rose
Richard J Rose, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Andrew Smolen
Andrew Smolen, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Harold Snieder
Harold Snieder, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Michael Stallings
Michael Stallings, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Marie Standl
Marie Standl, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Jordi Sunyer
Jordi Sunyer, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Henning Tiemeier
Henning Tiemeier, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Sally J Wadsworth
Sally J Wadsworth, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Tamara L Wall
Tamara L Wall, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Andrew JO Whitehouse
Andrew JO Whitehouse, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Gail M Williams
Gail M Williams, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Eivind Ystrøm
Eivind Ystrøm, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Michel G Nivard
Michel G Nivard, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Meike Bartels
Meike Bartels, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1,
Christel M Middeldorp
Christel M Middeldorp, MD, PhD
1Mss. Jami and Hagenbeek, Mr. Ip, and Drs. Hammerschlag, Hottenga, Beijsterveldt, Boomsma, Nivard, Bartels, and Middeldorp are with Vrije Universiteit Amsterdam, Amsterdam, the Netherlands. Ms. Jami and Dr. Allegrini are with University College London, London, United Kingdom. Drs. Hammerschlag, Hagenbeek, and Bartels are also with Amsterdam Public Health Research Institute, Amsterdam, the Netherlands. Drs. Hammerschlag and Middeldorp are also with the Child Health Research Centre, University of Queensland, Brisbane, Australia. Dr. Middeldorp is also with the Child and Youth Mental Health Service, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia. Dr. Allegrini, Rimfeld, and Plomin are with the Social, Genetic and Developmental Psychiatry Centre, King’s College London, London, United Kingdom. Drs. Benyamin, Zhou, and Hypponen are with the University of South Australia, Adelaide, Australia. Drs. Benyamin and Hypponen are also with South Australian Health and Medical Research Institute, Adelaide, Australia. Drs. Border, Corley, Evans, Hewitt, Smolen, Stallings, and Wadsworth are with the Institute for Behavioral Genetics, University of Colorado Boulder. Drs. Diemer, Vilor-Tejedor, Neumann, Rivadeneira, and Tiemeier are with Erasmus University Medical Center, Rotterdam, the Netherlands. Dr. Neumann is also with the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Canada. Drs. Diemer, Vilor-Tejedor and Tiemeier are with Harvard T.H. Chan School of Public Health, Boston, Massachusetts. Mr. Jiang and Drs. Q. Lu, Tong, Burt, and Klump are with Michigan State University, East Lansing. Mr. Jiang and Dr. Tong are also with the University of Florida, Gainesville. Dr. Karhunen is with Imperial College London, United Kingdom. Drs. Y. Lu, Chen, Kuja-Halkola, Larsson, Lichtenstein, and Ms. Tate are with Karolinska Institutet, Stockholm, Sweden. Mr. Mallard and Dr. Harden are with the University of Texas, Austin. Drs. Pashupati and Lehtimäki are with Tampere University, Tampere, Finland, and Fimlab Laboratories, Tampere, Finland. Drs. Nolte, Hartman, Oldehinkel, and Snieder are with the University of Groningen, University Medical Center Groningen, the Netherlands. Mr. Palviainen, Drs. Korhonen, Vuoksimaa, Kaprio, and Ms. Whipp are with the Institute for Molecular Medicine Finland - FIMM, University of Helsinki, Finland. Drs. Peterson, Silber, and Maes are with Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond. Dr. Maes is also with Massey Cancer Center, Virginia Commonwealth University, Richmond. Drs. Sallis and Munafò are with the School of Psychological Science, University of Bristol, United Kingdom, and Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, United Kingdom. Dr. Sallis is also with the Centre for Academic Mental Health, Population Health Sciences, University of Bristol, United Kingdom. Dr. Munafo is also with NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, United Kingdom. Drs. Shabalin and Adkins are with the University of Utah, Salt Lake City. Drs. Thiering, Heinrich, and Standl are with the Institute of Epidemiology, Helmholtz Zentrum Munchen - German Research Center for Environmental Health, Neuherberg, Germany. Drs. Thiering and Heinrich are also with the Ludwig-Maximilians-Universität, Munich, Germany. Dr. Heinrich is also with the Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia. Dr. Vilor-Tejedor is with the Erasmus University Medical Center, Rotterdam, the Netherlands; Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Barcelona, Spain; BarcelonaBeta Brain Research Center, (BBRC) Pasqual Maragall Foundation, Barcelona, Spain; and Universitat Pompeu Fabra (UPF), Barcelona, Spain. Dr. Vilor-Tejedor, Alemany, and Sunyer are with the Universitat Pompeu Fabra (UPF), Barcelona, Spain. Drs. Alemany and Sunyer are also with SGlobal, Barcelona Institute of Global Health, Barcelona, Spain; and CIBER Epidemiologıa y Salud Publica (CIBERESP), Spain. Dr. Sunyer is also with IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain. Ms. Wang and Dr. Pennell is with the School of Medicine and Public Health, University of Newcastle, Australia. Drs. Ask, Havdahl, Reichborn-Kjennerud, and Ystrøm are with the Norwegian Institute of Public Health, Oslo, Norway. Dr. Ystrøm is also with PROMENTA Research Center, University of Oslo, Norway. Dr. Ehli is with Avera Institute for Human Genetics, Avera McKennan Hospital & University Health Center, Sioux Falls, South Dakota. Drs. Hakulinen and Keltikangas- Järvinen are with the University of Helsinki, Helsinki, Finland. Ms. Henders is with the Institute for Molecular Biosciences, University of Queensland, Brisbane, Australia. Dr. Mamun is with the Institute for Social Science Research, University of Queensland, Brisbane, Australia. Ms. Marrington and Drs. Najman and Williams are with the School of Public Health, University of Queensland, Brisbane, Australia. Dr. Andreassen is with NORMENT Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; and Oslo University Hospital, Norway. Drs. Brown and Wall are with the University of California San Diego, La Jolla. Dr. Copeland is with the University of Vermont, Burlington. Dr. Dick is with Virginia Commonwealth University, Richmond. Dr. Harris is with the Carolina Population Center, University of North Carolina at Chapel Hill. Dr. Hopfer is with the University of Colorado, Aurora. Dr. Jarvelin is with MRC-PHE Centre for Environment and Health, Imperial College London, United Kingdom; the Center for Life Course Health Research, University of Oulu, Oulu, Finland; and Oulu University Hospital, Oulu, Finland. Dr. Krauter is with the University of Colorado Boulder. Dr. Lundstrom is with the University of Gothenburg, Swe-€ den. Dr. Magnus is with the Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway. Dr. Njølstad is with the Center for Diabetes Research, University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway. Drs. Reynolds and Rose are with the University of California at Riverside, California, and Indiana University, Bloomington, Indiana. Dr. Whitehouse is with Telethon Kids Institute, University of Western Australia, Perth.
1