Abstract
Comparative study of the structural asymmetry of the human and chimpanzee brain may shed light on the evolution of language and other cognitive abilities in humans. Here we report the results of vertex-wise and ROI-based analyses that compared surface area (SA) and cortical thickness (CT) asymmetries in 3D MR images obtained for 91 humans and 77 chimpanzees. The human brain is substantially more asymmetric than the chimpanzee brain. In particular, the human brain has 1) larger total SA in the right compared with the left cerebral hemisphere, 2) a global torque-like asymmetry pattern of widespread thicker cortex in the left compared with the right frontal and the right compared with the left temporo-parieto-occipital lobe, and 3) local asymmetries, most notably in medial occipital cortex and superior temporal gyrus, where rightward asymmetry is observed for both SA and CT. There is also 4) a prominent asymmetry specific to the chimpanzee brain, namely, rightward CT asymmetry of precentral cortex. These findings provide evidence of there being substantial differences in asymmetry between the human and chimpanzee brain. The unique asymmetries of the human brain are potential neural substrates for cognitive specializations, and the presence of significant CT asymmetry of precentral gyrus in the chimpanzee brain should be further investigated.
Keywords: brain asymmetry, chimpanzee, cortical thickness (CT), human, region of interest (ROI)-based analysis, surface area (SA), vertex-wise analysis
Introduction
A key feature of the human brain is the population-level functional and structural asymmetry. Clinical and experimental data obtained using a variety of methods have documented the left hemispheric specializations for linguistic and praxis functions (Knecht et al. 2000; Ocklenburg and Gunturkun 2018). Several structural asymmetries have also been identified in the human brain and are potential neural substrates for functional lateralization (Barrick et al. 2007; Josse et al. 2009). For example, the Sylvian fissure typically rises more steeply in the right cerebral hemisphere and extends further posteriorly in the left cerebral hemisphere (Eberstaller 1884, 1890; Cunningham 1892; Rubens et al. 1976; Yeni-Komshian and Benson 1976; Ide et al. 1996; Hou et al. 2018), and the planum temporale (PT), which is the flat surface of the superior temporal gyrus posterior to Heschl’s gyrus, is larger on the left compared with the right in the majority of humans (Geschwind and Levitsky 1968; Witelson and Kigar 1988; Shapleske et al. 1999; Barrick et al. 2005; Vadlamudi et al. 2006). Also notable in the human brain is the so-called torque whereby there is a global anticlockwise twist in the transverse plane. The torque has an exaggerated posterior component in terms of protrusion and rightward bending of the left occipital lobe (LeMay 1982; Witelson and Kigar 1988; Xiang et al. 2018) which is potentially related to Sylvian Fissue asymmetry (Hou et al. 2018) and greater posterior extension of the lateral ventricle in the left compared with the right cerebral hemisphere (Narr et al. 2001). A summary of findings from some of the brain structural asymmetry studies that have been performed in humans is provided in Table 1. Historically, asymmetries in brain structure and cognitive and motor functions have been considered uniquely human and presumed to have evolved after the split from the common ancestor of humans and great apes (Corballis 1992; Bradshaw and Rogers 1993; Corballis 2002; Crow 2010). However, research over the past 20–25 years has challenged this long-held view with a growing body of evidence demonstrating asymmetries in nonhuman animals (Gannon et al. 1998; Cantalupo and Hopkins 2001; Hopkins et al. 2008; Corballis 2009; Rogers et al. 2013; Ocklenburg and Gunturkun 2018). Nevertheless, fundamental questions that persist are whether some asymmetries of the human brain evolved after separation from the common ancestor and whether they underpin human specific adaptations and cognitive abilities? The search for human-specific features is best performed by comparative study with our closest living relative—the chimpanzee (Rilling et al. 2011).
Table 1.
Studies of cerebral asymmetry in the human brain
| Methods and measurements | Main findings of the study | |
|---|---|---|
| Lyttelton et al. (2009), 112 right-handed subjects | SBM-based vertex-wise analysis of SA and positional asymmetry | SA asymmetry to the left in supramarginal gyrus, Heschl’s gyrus, PT, anterior superior temporal, lateral orbital frontal cortex, and to the right in anterior occipital lobe, dorsal anterior cingulate, and medial orbital frontal; positional asymmetry in the pattern of the cerebral torque |
| Zhou et al. (2013), 274 right-handed subjects | SBM-based vertex-wise analysis of CT asymmetry | CT asymmetry emerges extensively after adolescence and becomes more pronounced with age |
| Meyer et al. (2014), 104 healthy subjects | Destrieux atlas–based ROI analysis of GMV, SA, and CT asymmetry | Global rightward asymmetry in GMV and SA but not CT; leftward SA asymmetry in auditory-related cortex and rightward CT asymmetry in primary and secondary auditory cortex |
| Koelkebeck et al. (2014), 101 right-handed subjects | Desikan-Killiany atlas–based ROI analysis of GMV, SA, and CT asymmetry | Different patterns of asymmetry in different measures; more prominent SA asymmetry compared with CT asymmetry |
| Maingault et al. (2016), 250 subjects (130 right-handed) | SBM-based vertex-wise analysis of GMV, SA, CT, and sulcal depth asymmetry | Global GMV, SA, and CT asymmetry to the right; no significant correlation between global SA and CT asymmetry; handedness is not associated with cortical asymmetries |
| Chiarello et al. (2016), 200 healthy subjects | Destrieux atlas–based ROI analysis of SA, CT, and LGI asymmetry | Extensive asymmetries of all three measures; substantial differences between different measures in both pattern and extent; regions with larger between-subject variability also show greater asymmetry |
| Kong et al. (2018), 17 141 healthy subjects | Desikan-Killiany atlas–based ROI analysis of SA, CT asymmetry | Global rightward asymmetry in SA and leftward asymmetry in CT; substantial and differential regional asymmetry in SA and CT which interacts with sex, age, and ICV; no overall correlation between SA and CT asymmetry; handedness is not associated with cortical asymmetries |
| Le Guen, Leroy, et al. (2018), 800+ subjects from the Human Connectome Project (HCP) | Novel ROI–based analysis of sulcal pit distribution asymmetry | Sulcal pit asymmetry in STS reported to be genetically determined |
Note: Gray Matter Volume (GMV), Local Gyrification Index (LGI) and Intra Cranial Volume (ICV).
As with many comparative brain studies, identifying species-specific features of lateralization is challenging given substantial differences in brain size and difficulties in defining potentially corresponding anatomical regions of interest (ROI) in different species (Keller et al. 2007; Keller et al. 2012). In addition, different computational methods may have been adopted for quantification of brain measures in different species, making comparison difficult. By using brain mapping techniques, surface-based morphometry (SBM) analysis enables the projection of brains of different size to a common standard allowing direct comparison between subjects across the whole cortical surface on a vertex-by-vertex basis. Additionally, Greve et al. (2013) proposed an approach to studying the inter-hemispheric correspondence of brain features by projecting both cerebral hemispheres to a left–right symmetric registration atlas. A relevant pipeline has been integrated in the FreeSurfer Image Analysis software (http://surfer.nmr.mgh.harvard.edu) and has gained popularity in the study of human brain asymmetry. However, there have been very few applications to the chimpanzee brain reported in the literature. In previous brain asymmetry studies performed in this laboratory (Xiang et al. 2018, 2019a, 2019b), Greve’s approach was used to compare inter-hemispheric positional brain asymmetry between humans and chimpanzees in the same analysis framework, and the findings of absence of cerebral torque in the chimpanzee brain contradicted some previous literature (LeMay 1982; Hopkins and Marino 2000; Balzeau and Gilissen 2010; Balzeau et al. 2012). In the present study, the analyses have been extended to examine the asymmetry of two fundamental measurements which characterize the cerebral cortex, namely, surface area (SA) and cortical thickness (CT).
Based on prelabeled atlases, such as the Desikan-Killiany atlas (Desikan et al. 2006), available in FreeSurfer software, brain measures can be extracted for specific ROIs. In particular, parcellation atlases have been independently constructed for the left and right cerebral hemispheres based on manual labeling of a series of brain images. These atlases have been employed to separately obtain values of quantities of interest for ROIs in each cerebral hemisphere, and with asymmetry computed as the difference in the value between the two cerebral hemispheres. Given that the procedure relies on two standards, that is, one atlas for each cerebral hemisphere, this conventional approach is referred to as a two-atlas parcellation scheme (TAPS). However, there is concern in using this approach that areal asymmetry inherent in the atlas may be propagated to the regional parcellation for individual subjects and systematically affect the result of studies in which the atlas is used. In a TAPS-based meta-analysis of a large population of healthy human subjects, Kong et al. (2018) reported much lower variability of SA asymmetry in comparison to CT asymmetry across many databases. The authors associated the observation with the computation scheme (i.e., TAPS). A new approach for the ROI-based asymmetry analysis is necessary if ROI analyses are to be considered robust.
The main objective of the present study is to perform a comparative analysis of SA and CT asymmetry between the human and chimpanzee brain. Firstly, inter-hemispheric asymmetry in the global values of SA and CT were compared between species. Secondly, SA and CT asymmetries were accessed on a vertex-by-vertex basis. Thirdly, a novel approach was developed for the ROI-based asymmetry analysis which we have named the single-atlas parcellation scheme (SAPS). Compared with TAPS, SAPS additionally incorporates determination of the vertex-wise correspondence between the left and right cerebral hemispheres and therefore is able to project the anatomical convention from a single parcellation atlas (e.g., left or right side of the parcellation atlas) to both cerebral hemispheres of individual subjects.
Methods
Subjects and MRI Data Acquisition
MR imaging of humans was performed at the Queen’s Medical Research Institute (QMRI), University of Edinburgh, United Kingdom, and the Oxford Centre for Magnetic Resonance (OCMR), University of Oxford, United Kingdom. Altogether, there are 91 healthy subjects (39 females and 52 males, average age 33.5 ± 12.0 years) in the study, 42 recruited in Edinburgh and 49 recruited in Oxford. Handedness information was recorded for 31 subjects in the Edinburgh group, in which 4 are left-handed, 2 have ambiguous handedness, and the rest are right-handed, and for 47 subjects in the Oxford group, in which 2 are left-handed and the rest are right-handed. MR imaging of chimpanzees was performed at Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia, USA. There are 77 chimpanzees (50 females and 27 males, average age 26.2 ± 14.0 years). Approval for the study was obtained separately at each site from the local Research Ethics Committee and human subjects provided fully informed written consent prior to taking part.
In Edinburgh the MR images of human subjects were acquired using a 3 T Verio MRI system (Siemens Healthineers), acquisition parameters for the 3D T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence are TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°, and the images have isotropic voxel resolution of 1 mm. In Oxford the MR images of human subjects were acquired using a 1.5 T Sonata MRI system (Siemens Healthineers), acquisition parameters for the 3D T1-weighted fast low-angle shot (FLASH) sequence are TR = 5400 ms, TE = 76 ms, flip angle = 90°, and the images have isotropic voxel resolution of 1 mm. In Atlanta the MR images for the chimpanzees were acquired using a Siemens 3 T Trio MRI system (Siemens Healthineers), acquisition parameters for the 3D T1-weighted MPRAGE sequence are TR = 2300 ms, TE = 4.4 ms, TI = 1100 ms, flip angle = 8°, and the images have isotropic voxel resolution of 0.6 mm. The chimpanzees were immobilized by ketamine injection (10 mg/kg) and subsequently anesthetized with propocol (40–60 mg/kg/hr) before transportation to the MRI facility where they were scanned supine with a human head coil and remained anesthetized (total time ~2 hours) for the MR imaging before returning to the home compound.
Image Analysis
All MR images were preprocessed in FSL (version 5.0.9, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) including skull strip, bias field correction, and brain normalization using a transformation with 7 degrees of freedom (i.e. 3 translations, 3 rotations and 1 uniform scaling) (Fischl et al. 1999). In the normalization step all brain volume images, including the chimpanzee brains, were co-registered with the standard human MNI152 template. Thereby all pre-processed brain images could be subsequently put through the same standard FreeSurfer pipeline (version 6.0, https://surfer.nmr.mgh.harvard.edu/). In FreeSurfer, an analysis was first performed to label the white matter of the brain and split the brain into two cerebral hemispheres. Secondly, a triangular mesh was generated and deformed to tightly cover the white matter compartment in each cerebral hemisphere via analysis of the intensity gradients between the white matter and gray matter, and this mesh is the so-called white matter surface. Thirdly, the white matter surface was expanded along the direction of the intensity gradients between the gray matter and CSF until it coincided with the external gray matter surface which is called the pial surface (Dale et al. 1999). All subjects included in this study passed automatic FreeSurfer quality control and visual inspection by LX.
Vertex-Wise Analysis of Brain Asymmetry
For vertex-wise analysis, as described in Greve et al. (2013) (see Fig. 1a), the interhemispheric and between-subject correspondences were established through a nonlinear registration that adjusts the vertex coordinates of both the left and right cerebral hemispheres of individual subjects to match the folding pattern (i.e., curvature) of a pretrained left–right symmetric registration atlas (i.e., lh.fsaverage_sym in FreeSurfer which refers to a symmetric atlas constructed based on an initial left hemispheric atlas) in spherical space. In the case of the human brain, the symmetric registration atlas was already available in FreeSurfer (i.e., lh.fsaverage_sym), whereas the atlas of the chimpanzee brain was specifically constructed for this study based on the procedure described in (Greve et al. 2013). In brief, for 30 brains selected at random from the chimpanzee cohort: 1) both cerebral hemispheres of each subject were co-registered to an initial human left–right symmetric atlas (i.e., lh.fsaverage_sym) and 2) a new chimpanzee atlas was generated by respectively averaging the aligned folding patterns of the left and right cerebral hemispheres for all the subjects in the training pool. To obtain a better symmetric atlas this process was repeated three times to produce the final left–right symmetric registration atlas for the chimpanzee brain. Based on the established correspondences, surface-based measures, of SA and CT resampled to the reference atlas space were compared between cerebral hemispheres and across subjects, and surface-based spatial smoothing was performed to increase the signal-to-noise ratio. As shown in Supplementary Figure S1, the asymmetry pattern remains the same under different filter sizes and a Gaussian filter with full-width half-maximum (FWHM) of 15 mm was chosen for the main analysis as this corresponds well with the size of brain petalia and gyri which are the features that are the focus of interest for study.
Figure 1 .
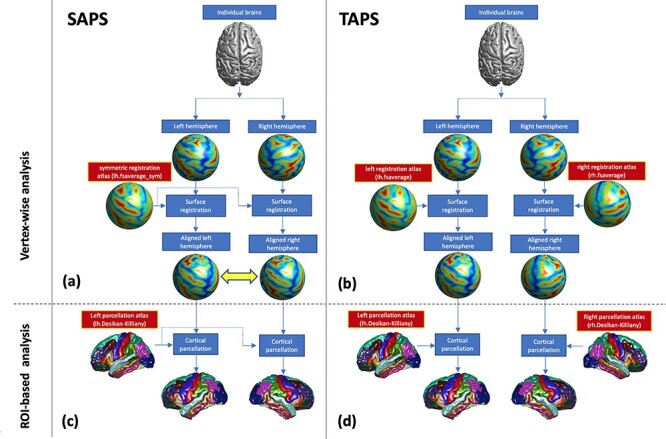
Flow diagrams of the steps in the application of the single-atlas parcellation scheme (SAPS), and two-atlas parcellation scheme (TAPS) are shown in the left and right columns, respectively, for the case of an individual in the chimpanzee cohort. For the vertex-wise analysis in SAPS (a) the interhemispheric and between-subject correspondences are established by coregistering both cerebral hemispheres of individual subjects to a symmetric registration atlas. For the corresponding vertex-wise analysis in TAPS (b) between-subject correspondences are established by separately coregistering left and right cerebral hemispheres of individual subjects to the relevant side of the atlas. For ROI-based analysis in SAPS (c) the parcellation of one cerebral hemisphere of the Desikan-Killiany atlas (e.g., left side) is first projected to both the ipsilateral and contralateral cerebral hemisphere of individual subjects. The pipeline is then repeated using the opposite side of the Desikan-Killiany atlas, and the two results are averaged. For ROI-based analysis in TAPS (d), the parcellation convention of each cerebral hemisphere in the Desikan-Killiany atlas is propagated separately to the corresponding hemisphere of individual subjects. The illustration is simplified by showing only one-half of the SAPS analysis pipeline for step-by-step comparison with TAPS.
ROI-Based Analysis of Brain Asymmetry
In Figure 1 the pipelines corresponding to the conventional TAPS (panels (b) and (d)) and new SAPS (panels (a) and (c)) ROI-based analysis of brain asymmetry are illustrated. As has been discussed the TAPS analysis can introduce bias. This is because there is significant areal difference between the left and right side of the Desikan-Killiany atlas that reflects the inherent asymmetry of the human brain, and different numbers of vertices are thereby assigned to corresponding ROIs in the left and right cerebral hemisphere of the atlas. The situation also exists for the Destrieux atlas (Destrieux et al. 2010) and for which there is a particular ROI referring to PT with 1454 vertices in the larger left PT in comparison to 1022 vertices for the smaller right PT. Thus, before performing any new analysis the influence of the atlas bias in the traditional TAPS-based analysis was investigated. In particular, an experiment was performed in which the Desikan-Killiany atlas (Supplementary Fig. S2) was applied to measure brain SA asymmeytry in a subset of 14 individuals randomly selected from the human cohort. Firstly, the TAPS analysis pipeline was applied to the original 3D MR images of the brain and then also to left-right flipped versions of the same 3D MR images. As demonstrated in Supplementary Figure S3, 23 of 34 (67.7%) ROIs have the same asymmetry direction in the analysis of both the un-flipped and flipped images, and these ROIs overlap with the regions showing the largest atlas bias in terms of number of vertices (see Supplementary Fig. S2), suggesting that the atlas bias significantly influences the results of analyses of brain asymmetry when TAPS is used. In addition, positive rather than negative correlation was found between the regional SA asymmetries computed for the original scans and their flipped versions (r = 0.79, P < 0.001), and both of which are highly positively correlated to the corresponding asymmetry of the Desikan-Killiany atlas (i.e., asymmetry of number of vertices in corresponding ROIs in the left and right cerebral hemisphere [r > 0.70, P < 0.001]), which may explain the high consistency of SA asymmetry across databases observed by Kong et al. (2018). The CT asymmetry is expected to be comparatively less severely affected because the atlas bias is inherently an areal difference between cerebral hemispheres and while the SA measures are computed as a sum of values at individual vertices within each ROI, the CT measures are computed by means of averaging the values at each vertex so that the bias is eliminated.
In order to address the above atlas bias in TAPS-based analysis, we developed a new SAPS-based analysis, in which only one reference parcellation is employed to subdivide both cerebral hemispheres of individual subjects. As demonstrated in Figure 1a and 1c, in SAPS the regional labels of the left side of the Desikan-Killiany atlas are assigned to both the ipsilateral and contralateral cerebral hemispheres of individual subjects based on their interhemispheric correspondence. This approach inherently sets a constraint whereby an identical number of vertices is assigned to the corresponding ROI in each cerebral hemisphere. However, in TAPS, as shown in Figure 1b and d, the parcellation convention of each cerebral hemisphere in the Desikan-Killiany atlas can only be projected to the corresponding hemisphere of individual subjects based on the between-subject correspondence (see Fig. 1d), since the correspondence between cerebral hemispheres is not known. Although vertex-wise analysis has been shown to be less influenced by the choice of left or right side of the registration atlas (i.e., lh.fsaverage_sym or rh.fsaverage_sym, Greve et al. 2013), the projection of a regional parcellation scheme to the contralateral cerebral hemisphere remains a concern. Therefore, to complete the SAPS-based analysis, the same analysis pipeline was repeated but now using the right side of the Desikan-Killiany atlas for brain parcellation and the right side of the FreeSurfer atlas (i.e., rh.fsaverage_sym which refers to a symmetric atlas constructed based on an initial right hemisphere atlas) for the inter-hemispheric co-registration. The ROI-based values of SA and CT were then calculated as the average of the values respectively computed based on the left and right cerebral hemisphere atlases. The parcellation results for all the human and chimpanzee subjects were checked by visual inspection. Examples for 10 randomly selected chimpanzees are shown in Supplementary Figure S4, which demonstrates that the Desikan-Killiany atlas provides good quality parcellation results for the chimpanzee brain.
Statistical Analysis
In all of the analyses (i.e., global, vertex-wise and ROI-based), the asymmetry index (AI) was defined as the normalized difference between values for the left and right cerebral hemisphere according to the formula AI = 2*(L−R)/(L + R). For the global and ROI-based analyses, two-tailed one-sample t-tests and multivariate analysis of variance (MANOVA) were respectively performed to test the significance of interhemispheric asymmetry of SA and CT for each species and to examine the effect of sex on asymmetries, using SPSS statistics for Mac, Version 22.0 (IBM Corp.). For the vertex-wise analysis, GLM in FreeSurfer was performed at each location on the cerebral surface to identify the clusters of significant SA and CT asymmetries for the human and chimpanzee brain, and the regions showing significant between-species difference, followed by a cluster-wise correction for multiple comparisons, with both the cluster-forming and cluster-wise significance levels set to P < 0.01. In addition, Spearman’s correlation analysis was performed to investigate the consistency of SA and CT directional asymmetry between the human and chimpanzee brain based on the ranked order of the asymmetry index across 34 ROIs of the Desikan-Killiany atlas.
Results
Global SA and CT Asymmetry
The mean values of SA and CT in the left and right cerebral hemispheres are presented in Table 2 for the human and chimpanzee brain. For the human brain, total SA is significantly larger in the right compared with the left cerebral hemisphere [t(90) = −4.10, P < 0.001] but there is no significant population-level asymmetry for CT. For the chimpanzee brain, no significant population-level asymmetry was found for either SA or CT. MANOVA showed no main effect of sex on SA or CT asymmetry in either the human [F(2,88) = 1.23, P = 0.30] or chimpanzee [F(2,74) = 0.67, P = 0.52] brain.
Table 2.
Statistics of the global SA and CT values for the human and chimpanzee brain showing 1) significant rightward SA, but not CT, asymmetry in the human brain and 2) no significant asymmetry for either SA or CT in the chimpanzee brain
| Human | Chimpanzee | ||||
|---|---|---|---|---|---|
| Hemisphere | SA (×1.0e+05 mm2) | CT (mm) | SA (×1.0e+04 mm2) | CT (mm) | |
| Left | 1.11 ± 0.11 | 2.29 ± 0.09 | 3.67 ± 0.29 | 1.39 ± 0.11 | |
| Right | 1.12 ± 0.11 | 2.30 ± 0.09 | 3.68 ± 0.29 | 1.40 ± 0.12 | |
| Asymmetry | t-stat | −3.82 | −1.10 | −0.39 | −1.31 |
| P-value (2-tailed) | < 0.001 | 0.27 | 0.70 | 0.19 | |
Vertex-Wise and ROI-Based SA Asymmetry
The results of the vertex-wise and SAPS-based ROI analysis of SA asymmetry are presented in Figure 2a, and Figure 3a, respectively. According to Figure 2a, the vertex-wise analysis for the human brain revealed significant rightward SA asymmetry in 1) STS extending to posterior insula, 2) inferior parietal, 3) medial frontal, and 4) medial occipital cortex. Significant leftward SA asymmetry was found in 5) supramarginal gyrus extending to PT and 6) anterior insula extending to Broca’s area and anterior and inferior temporal lobe. In comparison, the vertex-wise analysis for the chimpanzee brain showed significant leftward asymmetry in 1) Sylvian fissure extending from anterior superior temporal to supramarginal gyrus and 2) inferior parietal cortex and cuneus, however, there are no significant population-level rightward asymmetries. The comparative analysis revealed significant species difference in 1) STS, 2) posterior insula, 3) inferior parietal, 4) inferior temporal, 5) medial frontal, 6) medial occipital, and 7) supramarginal gyrus. The result of the ROI-based analysis of SA asymmetry is displayed in Figure 3a. There are a greater number of ROIs showing significant asymmetry in the human brain (i.e., in 10 of 34 ROIs) compared with the chimpanzee brain (i.e., in 2 of 34 ROIs). The statistics of regional SA asymmetries are summarized in Table 3 and the measurements of SA for the 34 ROIs per cerebral hemisphere of the Desikan-Killiany atlas are provided in Supplementary Table S1 for the human and chimpanzee brain.
Figure 2 .
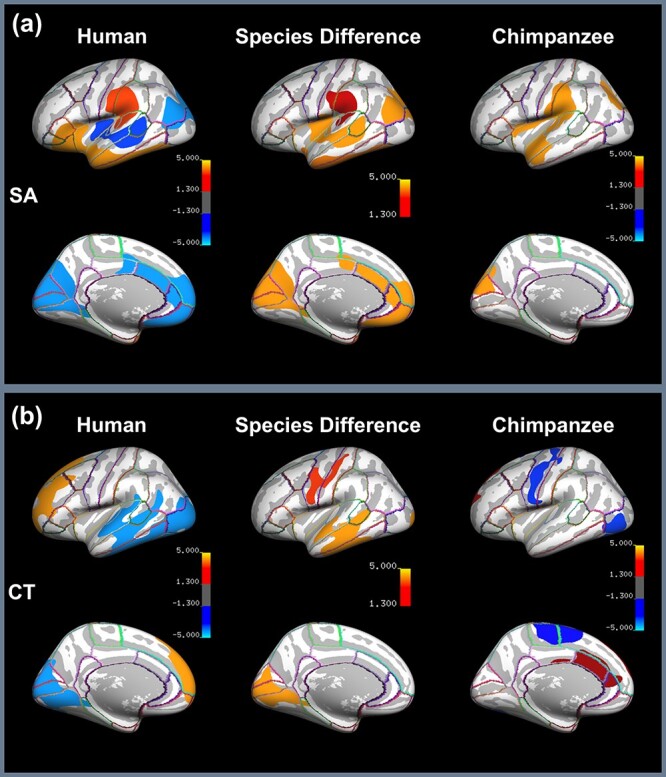
Vertex-wise results of (a) SA and (b) CT asymmetry illustrating 1) significant asymmetries for the human (left) and chimpanzee brain (right), in which hot colors refer to leftward asymmetry and cool colors to rightward asymmetry and 2) significant difference of asymmetry between the human and chimpanzee brain (middle), in which the intensity of the hot color indicates the significance level of the difference. The highlighted areas survive the cluster-wise correction for multiple comparisons and the significance level of the respective cluster-forming, and cluster-wise alphas are set as P < 0.01.
Figure 3 .
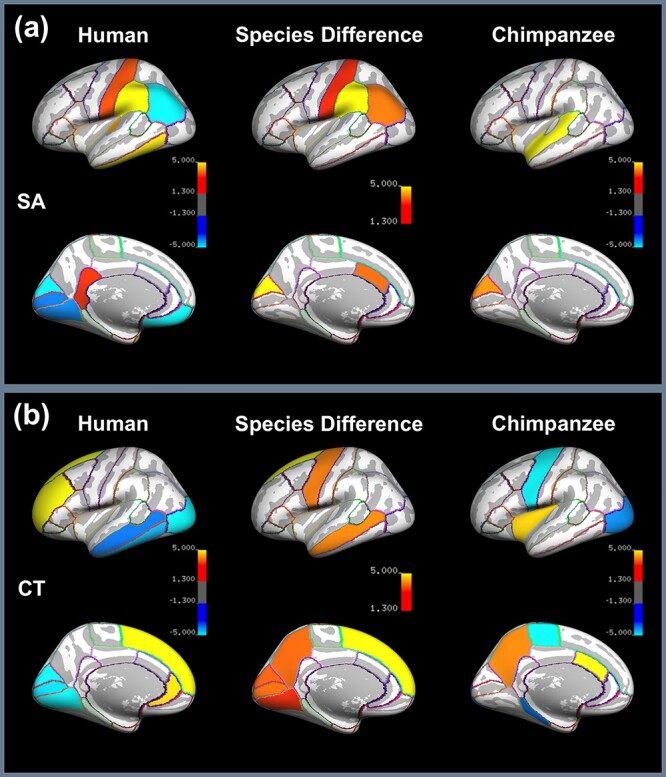
SAPS ROI-based results of (a) SA and (b) CT asymmetry illustrating 1) significant signed asymmetries across 34 ROIs for the human (left) and chimpanzee brain (right), in which hot colors refer to leftward asymmetry and cool colors to rightward asymmetry, and 2) differences between species (middle), in which the intensity of the hot color indicates the significance level of the difference. Bonferroni correction has been performed and the significance level is set as P < 0.05/34.
Table 3.
Statistics of ROI-based SA asymmetry index for the human and chimpanzee brain
| Surface area | Human | Chimpanzee | Species difference | |||
|---|---|---|---|---|---|---|
| ROIs | AI | P-value | AI | P-value | t-stats | P-value |
| Frontal | ||||||
| Superior frontal | −0.02 | −0.03* | 0.01 | 0.40 | −2.14 | 0.03* |
| Rostral middle frontal | −0.02 | −0.04* | −0.01 | −0.46 | −0.59 | 0.56 |
| Caudal middle frontal | 0.01 | 0.68 | 0.01 | 0.68 | 0.08 | 0.93 |
| Pars opercularis | 0.01 | 0.51 | 0.00 | 0.90 | 0.41 | 0.68 |
| Pars triangularis | 0.02 | 0.12 | −0.00 | −0.85 | 1.04 | 0.30 |
| Pars orbitalis | 0.01 | 0.32 | −0.02 | −0.45 | 1.17 | 0.24 |
| Lateral orbito frontal | 0.01 | 0.13 | 0.03 | 0.10 | −1.06 | 0.29 |
| Medial orbito frontal | −0.09 | −0.00** | 0.01 | 0.78 | −2.81 | 0.01* |
| Precentral | −0.01 | −0.06 | −0.01 | −0.15 | −0.58 | 0.56 |
| Paracentral | 0.01 | 0.36 | −0.02 | −0.02* | 1.72 | 0.09 |
| Frontal pole | −0.02 | −0.13 | 0.00 | 0.83 | −1.28 | 0.20 |
| Parietal | ||||||
| Superior parietal | −0.03 | −0.002* | 0.01 | 0.37 | −2.91 | 0.00* |
| Inferior parietal | −0.05 | −0.00** | −0.00 | −0.79 | −3.80 | 0.00** |
| Supramarginal | 0.10 | 0.00** | 0.01 | 0.10 | 5.47 | 0.00** |
| Postcentral | 0.03 | 0.00** | −0.00 | −0.68 | 3.25 | 0.00** |
| Precuneus | −0.01 | −0.16 | −0.01 | −0.51 | −0.30 | 0.76 |
| Temporal | ||||||
| Middle temporal | −0.00 | −0.58 | 0.01 | 0.39 | −1.03 | 0.30 |
| Inferior temporal | 0.04 | 0.00** | 0.01 | 0.31 | 1.86 | 0.06 |
| Superior temporal | 0.02 | 0.03* | 0.03 | 0.00** | −1.68 | 0.10 |
| Bankssts | −0.05 | −0.02* | −0.01 | −0.17 | −1.43 | 0.15 |
| Fusiform | −0.00 | −0.97 | 0.01 | 0.34 | −0.81 | 0.42 |
| Transverse temporal | 0.07 | 0.00** | 0.02 | 0.04* | 2.53 | 0.01* |
| Entorhinal | −0.02 | −0.23 | −0.06 | −0.13 | 0.83 | 0.41 |
| Temporal pole | 0.07 | 0.00** | 0.02 | 0.37 | 1.93 | 0.05 |
| Parahippocampal | −0.00 | −0.96 | −0.02 | −0.50 | 0.61 | 0.54 |
| Occipital | ||||||
| Lateral occipital | −0.01 | −0.24 | −0.02 | −0.08 | 0.53 | 0.60 |
| Lingual | −0.03 | −0.01* | −0.00 | −0.88 | −1.35 | 0.18 |
| Cuneus | −0.09 | −0.00** | 0.08 | 0.00** | −7.04 | 0.00** |
| Pericalcarine | −0.06 | −0.00** | 0.00 | 0.99 | −2.27 | 0.02* |
| Cingulate and insula | ||||||
| Caudal anterior cingulate | −0.09 | −0.003* | 0.05 | 0.02* | −3.69 | 0.00** |
| Isthmus cingulate | 0.07 | 0.00** | −0.00 | −0.99 | 2.16 | 0.03* |
| Posterior cingulate | −0.03 | −0.02* | 0.01 | 0.70 | −1.95 | 0.05 |
| Rostral anterior cingulate | 0.02 | 0.32 | 0.16 | 0.03* | −1.36 | 0.18 |
| Insula | −0.01 | 0.10 | 0.00 | 0.51 | −1.33 | 0.19 |
Note: *Denotes ROIs with significant asymmetry (i.e., P < 0.05 uncorrected for multiple comparisons), and **denotes ROIs with highly significant asymmetry that survived the Bonferroni correction (i.e., P < 0.05/34).
MANOVA revealed no significant main effect of sex on the overall SA asymmetry in either the human [F(34,56) = 1.51, P = 0.08] or chimpanzee [F(34,42) = 0.92, P = 0.60] brain. However, although subsequent univariate ANOVA showed a significant effect of sex on SA asymmetry in the superior temporal lobe [F(1,89) = 9.56, P = 0.003], cuneus [F(1,89) = 5.61, P = 0.02], pars opercularis [F(1,89) = 4.75, P = 0.03], and pars triangularis [F(1,89) = 8.19, P = 0.01] for the human brain and in bankssts [F(1,75) = 5.39, P = 0.02] and inferior parietal [F(1,75) = 4.11, P = 0.05] for the chimpanzee brain, none survived Bonferroni correction for multiple comparisons.
Vertex-Wise and ROI-Based CT Asymmetry
The results of the vertex-wise and SAPS-based ROI analysis of CT asymmetry are presented in Figure 2b, and Figure 3b, respectively. According to Figure 2b, the vertex-wise analysis for the human brain revealed significant rightward asymmetry in 1) temporal and 2) occipital lobe and significant leftward asymmetry in 3) superior and middle frontal gyrus. In comparison, the vertex-wise analysis for the chimpanzee brain revealed significant rightward asymmetry in 1) precentral gyrus, 2) paracentral gyrus, and significant leftward asymmetry in 3) dorsal anterior cingulate. The comparative analysis revealed significant species differences in 1) STS, 2) medial occipital, and 3) precentral cortex.
The results of the ROI-based analysis of CT asymmetry are displayed in Figure 3b. There are 9 out of 34 ROIs showing significant population-level asymmetry in the human brain compared with 7 out of 34 ROIs in the chimpanzee brain. The statistics of regional CT asymmetries are summarized in Table 4 and the measurements of CT for the 34 ROIs per cerebral hemisphere of the Desikan-Killiany atlas are summarized in Supplementary Table S2 for the human and chimpanzee brain. As was the case for the vertex-wise analysis, the ROI-based analysis also shows that the frontal lobe is thicker in the left compared with the right cerebral hemisphere and the temporo-parieto-occipital lobe is thicker in the right compared with the left cerebral hemisphere in the human brain, a torque-like pattern that is not present in the chimpanzee brain.
Table 4.
Statistics of ROI-based CT asymmetry index for the human and chimpanzee brain
| Cortical thickness | Human | Chimpanzee | Species difference | |||
|---|---|---|---|---|---|---|
| ROIs | AI | P-value | AI | P-value | t-stats | P-value |
| Frontal | ||||||
| Superior frontal | 0.02 | 0.00** | −0.00 | −0.94 | 4.75 | 0.00** |
| Rostral middle frontal | 0.03 | 0.00** | 0.02 | 0.01* | 0.95 | 0.34 |
| Caudal middle frontal | 0.00 | 0.34 | −0.01 | −0.45 | 1.20 | 0.23 |
| Pars opercularis | 0.00 | 0.38 | 0.00 | 0.87 | 0.38 | 0.70 |
| Pars triangularis | −0.00 | −0.61 | 0.01 | 0.31 | −1.16 | 0.25 |
| Pars orbitalis | −0.02 | −0.08 | −0.01 | −0.31 | −0.29 | 0.78 |
| Lateral orbito frontal | 0.01 | 0.16 | −0.01 | −0.11 | 2.15 | 0.03* |
| Medial orbito frontal | 0.00 | 0.93 | −0.01 | −0.41 | 0.72 | 0.47 |
| Precentral | 0.00 | 0.61 | −0.03 | −0.00** | 3.84 | 0.00** |
| Paracentral | −0.02 | −0.005* | −0.04 | −0.00** | 2.21 | 0.03* |
| Frontal pole | 0.02 | 0.07 | 0.00 | 0.99 | 1.10 | 0.27 |
| Parietal | ||||||
| Superior parietal | 0.01 | 0.14 | −0.00 | −0.42 | 1.58 | 0.12 |
| Inferior parietal | −0.01 | −0.02* | −0.00 | −0.65 | −0.94 | 0.35 |
| Supramarginal | 0.00 | 0.69 | 0.01 | 0.03* | −1.33 | 0.18 |
| Postcentral | 0.01 | 0.05 | −0.00 | −0.28 | 2.19 | 0.03* |
| Precuneus | −0.00 | −0.98 | 0.03 | 0.00** | −3.67 | 0.00** |
| Temporal | ||||||
| Middle temporal | −0.02 | −0.00** | 0.01 | 0.06 | −3.88 | 0.00** |
| Inferior temporal | −0.02 | −0.00** | −0.00 | −0.81 | −2.02 | 0.04* |
| Superior temporal | −0.02 | −0.002* | −0.00 | −0.64 | −1.67 | 0.10 |
| Bankssts | −0.03 | −0.00* | 0.00 | 0.83 | −2.09 | 0.04* |
| Fusiform | −0.01 | −0.03* | −0.01 | −0.26 | −0.26 | 0.79 |
| Transverse temporal | −0.01 | −0.55 | −0.03 | −0.01* | 1.83 | 0.07 |
| Entorhinal | −0.02 | −0.06 | −0.00 | −0.83 | −0.86 | 0.39 |
| Temporal pole | −0.00 | −0.75 | −0.01 | −0.71 | 0.16 | 0.88 |
| Parahippocampal | −0.02 | −0.01* | −0.04 | −0.00** | 1.28 | 0.20 |
| Occipital | ||||||
| Lateral occipital | −0.03 | −0.00** | −0.02 | −0.00** | −1.27 | 0.21 |
| Lingual | −0.04 | −0.00** | −0.01 | −0.16 | −3.26 | 0.00** |
| Cuneus | −0.03 | −0.00** | 0.00 | 0.97 | −3.63 | 0.00** |
| Pericalcarine | −0.04 | −0.00** | 0.01 | 0.38 | −3.70 | 0.00** |
| Cingulate and insula | ||||||
| Caudal anterior cingulate | 0.01 | 0.38 | 0.06 | 0.00** | −2.54 | 0.01* |
| Isthmus cingulate | −0.00 | −0.97 | −0.02 | −0.08 | 1.33 | 0.18 |
| Posterior cingulate | 0.01 | 0.11 | 0.00 | 0.62 | 0.68 | 0.50 |
| Rostral anterior cingulate | 0.06 | 0.00** | 0.05 | 0.01* | 0.53 | 0.60 |
| Insula | 0.00 | 0.49 | 0.02 | 0.00** | −2.52 | 0.01* |
Note: *Denotes ROIs with significant asymmetry (i.e., P < 0.05 uncorrected formultiple comparisons), and **denotes ROIs with highly significant asymmetry that survived the Bonferroni correction (i.e., P < 0.05/34).
MANOVA revealed no significant main effect of sex on CT asymmetry in either the human [F(34,56) = 0.79, P = 0.76] or chimpanzee [F(34,42) = 0.54, P = 0.97] brain, neither did subsequent ANOVA for any ROIs.
Detailed Analysis of the Significant Differences in Asymmetry Between the Human and Chimpanzee Brain
The significant differences in the vertex-wise asymmetry of the human and chimpanzee brain that are presented in the middle column of Figure 2a for SA, and the middle column of Figure 2b for CT, were analysed further to investigate whether the species difference had arisen from differences in the direction or magnitude of asymmetry. To facilitate this analysis, the average signed asymmetry maps for SA and CT are respectively displayed for individual species in Figure 4a and 4b, and yellow contours corresponding to the statistically significant clusters shown in the middle column of Figure 2a and 2b are drawn to indicate the boundary of the region of significant species difference in SA and CT asymmetry, respectively. Thus it is possible to assess whether significant species differences in SA and CT asymmetry correspond to places where the human and chimpanzee brain have an opposite direction of asymmetry or the same direction but different magnitude.
Figure 4 .
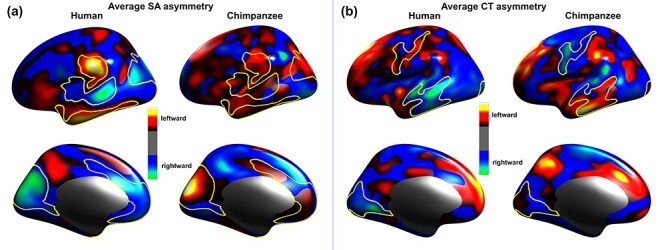
Vertex-wise average asymmetry maps of (a) SA and (b) CT. In each panel, the left column refers to the human brain and right column to the chimpanzee brain. The surface of the brain is color coded to represent the average signed asymmetry, with hot colors referring to significant leftward asymmetry and cool colors referring to significant rightward asymmetry. The yellow contours correspond to the boundaries of the red/yellow statistically significant clusters shown in the middle column of Figure 2a for SA, and middle column of Figure 2b for CT, asymmetry, respectively.
For the significant species differences in SA asymmetry (Fig. 4a), only the species difference in the supramarginal gyrus is due to a magnitude difference, with both species showing a consistent leftward asymmetry. In the remaining regions, SA asymmetries are in opposite directions in the human and chimpanzee brain. For the significant species differences in CT asymmetry (Fig. 4b), all asymmetries are in opposite directions in the human and chimpanzee brain.
Relationship between Asymmetries in the Human and Chimpanzee Brain
Correlation analyses were performed to investigate whether variation in the magnitude and direction of asymmetries in SA and CT are similar or different between the human and chimpanzee brain and whether the SA and CT asymmetries are related in each species. This revealed that for CT, but not SA, there is modest and marginally significant consistency in the magnitude and direction of asymmetries across 34 ROIs between humans and chimpanzees (CT: r = 0.34, P = 0.05; SA: r = 0.15, P = 0.40), whereas the correlation analysis between SA and CT asymmetry showed that on average there is no significant relationship between SA and CT asymmetry in either the human (r = 0.08, P = 0.64) or the chimpanzee (r = 0.31, P = 0.08) brain.
Discussion
In this study SA and CT asymmetries have been comprehensively investigated for the human and chimpanzee brain. Overall, the results revealed that asymmetries are more extensive in the human brain than the chimpanzee brain. There is significantly greater SA in the right compared with the left cerebral hemisphere in only the human brain globally. Population-level SA asymmetry was observed locally in 10 of 34 (29.4%) ROIs for SA and in 9 of 34 (26.5%) ROIs for CT in the human brain, compared with respective values of 2 of 34 (5.9%), and 7 of 34 (20.6%) ROIs, for the chimpanzee brain, reflecting different patterns of CT and SA asymmetry between the two species. In particular, human-specific population-level SA asymmetries were found in STS, insula, supramarginal gyrus, inferior parietal, medial occipital, medial orbital frontal, and anterior cingulate for SA and in middle temporal and medial occipital gyrus for CT, whereas chimpanzee-specific population-level asymmetry was observed only in the precentral gyrus for CT.
In the present study the potential influence of atlas bias in the conventional ROI-based analysis has been addressed by using a novel parcellation scheme called SAPS. The development of the SAPS approach, as compared with the TAPS approach, is broadly equivalent to the use of a symmetric compared with a standard atlas in voxel-based morphometry (VBM) studies. In particular, for TAPS, when an atlas is developed by using image registration techniques to combine the images of individuals in a cohort, if there is an average asymmetry in the population, this will appear in the atlas (see Supplementary Fig. S2). If the atlas is then used in a new study, this asymmetry can potentially add asymmetry to a population of individuals in which no asymmetry is present. Since our previous studies have shown that the human brain is significantly more asymmetric than the chimpanzee brain (e.g. Xiang et al., 2018), we had concerns that by using TAPS the inherent asymmetry of the human Desikan-Killiany atlas could be propagated to the chimpanzee brain. In SAPS, because an equivalent number of vertices is assigned to refer to each ROI in the left and right cerebral hemisphere, the propagation of asymmetry that may arise from there being different numbers of vertices associated with corresponding ROIs in the left and right cerebral hemisphere (see Supplementary Fig. S2b) is mitigated. Furthermore, for each ROI, measurements derived from the left and right sides of the parcellation atlas are averaged to avoid the possibility of bias which can occur if only one side is used. Demonstration that SAPS provides a less biased approach for the ROI-based analysis of cerebral asymmetry is provided in Supplementary Figure S3. In particular, as already described, the analysis pipeline of SAPS was applied to the original 3D MR images of 14 brains and also to left–right flipped versions of the same images. In the case when SAPS is used the results are almost completely reversed (see Supplementary Figure S3a) but not when TAPS is used (see Supplementary Figure S3b). This observation is supported quantitatively in that the SA asymmetry measurements remained in the same direction in 23 of 34 ROIs (i.e., 67.7%) when TAPS was used but only in 4 of 34 ROIs (11.8%) when SAPS was used. While the regional SA asymmetries computed using TAPS are influenced by the asymmetry in the Desikan-Killiany atlas (r > 0.7, P < 0.005), that is, asymmetry of number of vertices distributed in corresponding ROIs in the left and right cerebral hemisphere, this is not the case when using SAPS.
Regarding SA asymmetry in the human brain, in agreement with the ROI-based meta-analysis performed by Kong et al. (2018), total brain SA is significantly larger for the right compared with the left cerebral hemisphere, and there is also leftward asymmetry in the transverse temporal, superior temporal, inferior temporal and supramarginal gyrus, and rightward asymmetry in medial temporal, inferior parietal, cuneus and pericalcarine cortex. Contrary to the report of Kong et al. (2018) of rightward asymmetry in anterior and leftward asymmetry in posterior Broca's area, no significant asymmetry of Broca’s area was detected according to the SAPS ROI-based analysis of the present study, and it is notable that there is a large bias in the associated region of the Desikan-Killiany atlas (see Supplementary Fig. S2). However, the finding of significant leftward SA asymmetry of a region in the anterior Broca’s area extending to lateral orbital frontal and anterior temporal cortex is consistent with the observation in another vertex-wise analysis by Lyttelton et al. (2009), and significant leftward SA asymmetry of the parahippocampal gyrus and significant rightward SA asymmetry of medial orbital frontal cortex is consistent with the findings of Van Essen et al. (2012). In the case of CT asymmetry, a marked torque-like pattern was found in the human brain, corresponding to relatively thicker cortex in left compared with right frontal lobe and right compared with left temporo-parieto-occipital lobe. This finding is consistent with the findings of Plessen et al. (2014), Luders et al. (2006) and Le Guen, Leroy, et al. (2018), however, opposite to the findings of Zhou et al. (2013) and Maingault et al. (2016). Lateralization has also been reported for white matter underlying the gray matter. In particular, the arcuate fasciculus which links lateral temporal cortex and frontal lobe has been reported to be both structurally and functionally asymmetric (Trivedi et al. 2009; Takaya et al. 2015). Furthermore, Rilling et al. (2011) reported that there has been an augmentation of this dorsal language pathway in human evolution, which is more pronounced in the left hemisphere and is suggested to be related to the development of language. Study of the potential relationship between asymmetry of gray and white matter is an important topic for further investigation.
Two brain areas were found to be asymmetric in both the SA and CT analyses in the human brain. They are the medial occipital lobe and STS. Both showed reduction of SA and CT on the left compared with the right. In the case of the medial occipital lobe, the observation is compatible with the greater leftward posterior extension and rightward bending that were previously reported in the occipital lobe (Xiang et al. 2018), and rightward gyrification asymmetry in the region was also reported by Chiarello et al. (2016). In the case of STS, the finding supports the superior temporal asymmetrical pit (STAP) asymmetry identified by Leroy et al. (2015) as a “new human specific landmark,” which has been later found to be genetically constrained (Le Guen, Auzias, et al. 2018; Le Guen, Leroy, et al. 2018), advocating its potential role in the development of language during recent evolution. Of further interest with respect to the human brain is the finding of opposite directions of SA asymmetry in the anterior (i.e., leftward) and posterior insula (i.e., rightward). This finding may help to resolve the discrepancy between asymmetries previously reported for this brain region. In particular, Watkins et al. (2001) performed a VBM-based asymmetry study in 142 healthy subjects and reported significant rightward asymmetry, whereas Keller et al. (2012) performed a stereological analysis to measure insula volume in 25 subjects with confirmed hemisphere language dominance (HLD) and reported leftward insula asymmetry to be associated with left HLD. The respective blue- and red-colored regions overlying the anterior and posterior insula in the first panel of the top row of Figure 2 and which are compatible with the findings of Watkins et al. (2001) and Keller et al. (2012), respectively, are not present in the results of the ROI-based analysis of the corresponding panel in Figure 3, suggesting that the averaging inherent in the use of predefined ROIs may obscure findings of interest.
The chimpanzee brain shows fewer areas of significant population-level SA and CT asymmetry than the human brain and no significant population-level asymmetry in global SA and CT values. In contrast to previous postmortem studies by Hopkins and Avants (2013) and Cantalupo and Hopkins (2001), no extensive CT asymmetry in frontal, parietal, and temporal lobes, except for rightward asymmetry in the precentral gyrus and significant leftward SA asymmetry of Broca's area, was observed in the present study. The observation of significant rightward CT asymmetry in the precentral gyrus specific to the chimpanzee brain is a new finding. The primary motor region of the chimpanzee brain has the thinnest cortex across the whole cerebral surface (Hopkins and Avants 2013; Hopkins et al. 2016). After taking account of brain size, it is also disproportionately thinner than in humans (Hopkins et al. 2016). At the cellular level, a postmortem study of 18 chimpanzees revealed the density asymmetry of parvalbumin-immunoreactive interneurons in layers II and III of primary motor cortex to be significantly related to hand preference (Sherwood et al. 2007). These findings suggest that structural asymmetry of primary motor cortex may be related to the evolution of handedness (Hopkins 1995; Hopkins and Cantalupo 2004; Hopkins 2013; Hopkins et al., 2014). Nevertheless, caution is needed in interpreting the functional significance of this asymmetric feature since the present study showed humans, who have more lateralized handedness, do not possess any asymmetry in this region. Although there have been reports of a relationship between the structure of primary motor cortex and handedness in humans (Amunts et al., 1996) this has not always been observed (Good et al. 2001; Guadalupe et al. 2014; Kong et al. 2018; Wiberg et al. 2019).
The present study provides substantial evidence that humans and chimpanzees show different patterns of asymmetry. Especially, the direction of asymmetry, in the region showing significant species difference, is on average opposite between the human and chimpanzee brain (see Fig. 4). In addition, the species difference in most cases arises from significant asymmetry in the human brain which is absent on a population-level in the chimpanzee brain, with one exception in the precentral cortex. Divergence of asymmetry challenges the view that chimpanzees share the same pattern of asymmetry as humans but which only differs in a matter of magnitude (Gomez-Robles et al. 2013). The presence of asymmetries in the chimpanzee brain, though few in number, also provides further confirmation that population-level asymmetry is not unique to Homo sapiens. Population-level behavioral, functional, and anatomical asymmetries have been previously reported in a wide range of primates (Holloway and De La Costelareymondie 1982; Corballis 2009; Hopkins et al. 2015). Holloway and De La Costelareymondie (1982) were the first to study brain asymmetry in pongids (i.e., great apes) and hominids (i.e., humans and their fossil ancestors). They reported that while all taxa of hominoids (i.e., both groups) show asymmetries to various degrees, the patterns or combination of petalial asymmetries are very different. Only modern Homo and hominids (Australopithecus, Homo erectus, Neanderthals) showed a distinct left-occipital, right-frontal petalial asymmetry pattern. Of the pongids, gorilla showed leftward asymmetry of the occipital petalias. Subsequently, in a study of formalin-fixed brain specimens of five Old and New World Monkey species, Heilbroner and Holloway (1988) reported significantly greater Sylvian fissure length in the left compared with the right cerebral hemisphere, as is typical in humans (Hou et al. 2018), in four of the species. Corresponding population-level leftward asymmetries of PT have also been reported for chimpanzees (Zilles et al. 1996; Gannon et al. 1998; Hopkins and Nir 2010) and baboons (Marie et al. 2017) but not in macaque monkeys (Gannon et al. 2008; Lyn et al. 2011). Hopkins et al. (2015) have investigated whether hemispheric specialization evolved as a by-product of increasing brain size relative to the cross-sectional area of the corpus callosum in mid-sagittal section. They report that species with larger brains have a relatively smaller corpus callosum, suggesting that humans have increasingly “split” or “disconnected” hemispheres, followed by great apes, and then Old World monkeys. Nevertheless, as the present and previous studies have shown (Xiang et al. 2018, 2019a, 2019b), certain population-level asymmetries are unique to the human brain (Crow 2004, 2010). In particular, the asymmetries of the medial occipital lobe and STS in the human brain may be related to lateralization of cognitive abilities, such as left hemisphere dominance for language.
The failure to detect a significant correlation between SA and CT asymmetry in either humans or chimpanzees is consistent with previous studies of humans (Winkler et al. 2010) and chimpanzees (Hopkins and Avants 2013), suggesting that SA and CT have developmental phenotypes that are dependent upon different factors (Panizzon et al. 2009; Winkler et al. 2010). Interestingly, a marginally significant correlation was observed between asymmetries of the human and chimpanzee brain in CT (P = 0.05), but not SA. This finding suggests that the human brain can be better distinguished from the chimpanzee brain on the basis of SA rather than CT asymmetry, which is in line with the claim that there has been a more substantial change in SA than CT during the course of human evolution (Rakic 1995; Meyer et al. 2014; Lyall et al. 2015), and is also relevant to the observation that general cognitive ability is driven by SA rather than CT (Vuoksimaa et al. 2015). The search for a neural structural basis underlying superior human cognitive ability in comparative studies could be more fruitful if it is based on measurement of SA.
There is no significant sex effect on brain SA or CT asymmetries at the global level for either species. However, some evidence for an interesting sexual dimorphism of SA asymmetry is found in superior temporal lobe in the human brain. In particular, males were found to be significantly more leftwardly asymmetric in this brain region than females (P < 0.001), though the effects did not survive Bonferroni correction for multiple comparisons. Kong et al. (2018) also reported a sex difference in the same region.
A limitation of the present study is that the gyral boundaries used for parcellation of the chimpanzee brain are derived from the Desikan-Killiany neuro-anatomical atlas of the human brain (Desikan et al. 2006) which was constructed by averaging boundaries manually delineated for 34 ROIs on the basis of relevant gyri in each cerebral hemisphere of coregistered 3D MRI scans obtained for 40 individuals. In several previous studies, the brains of individual subjects in human and chimpanzee cohorts have been coregistered to a common reference space using the FreeSurfer pipeline (Xiang et al. 2018, 2019a, 2019b), and in one study the Desikan-Killiany atlas was applied to compare corresponding ROIs in the human and chimpanzee brain (Hopkins et al. 2016). In each case the coregistration using FreeSurfer was checked by using rigorous quality control procedures (Xiang et al. 2018), and the subsequent use of the Desikan-Killiany atlas is possible because the average pattern of the primary, and many of the secondary gyri, is closely similar in the human and chimpanzee brain. Thus although within the 34 ROIs in each cerebral hemisphere, there are undoubtedly variations in the gyral pattern, as is shown in Supplementary Figure S4 the bounding gyri show close correspondence between the human and chimpanzee brain. In addition, the result of the SAPS-based ROI analysis is highly consistent with the vertex-wise result that is less prone to the abovementioned atlas bias. In an alternative approach to atlas-based ROI analysis, Le Guen, Auzias, et al. (2018) and Le Guen, Leroy, et al. (2018) have proposed a novel strategy. In particular, the location of sulcal pits in the left and right cerebral hemispheres of individual subjects is determined, and then a watershed algorithm is applied to define mutual boundaries for new ROIs in each cerebral hemisphere. The method essentially generates a study-specific symmetric parcellation standard for subsequent regional analysis and can be readily employed in future studies of human cohorts. The approach is not, however, as suitable for use in the present comparative study as it is unlikely that humans and chimpanzees share the same pattern of distribution of sulcal pits. Another limitation is the lack of information on the effect of handedness on brain asymmetry, which could not be investigated because of the incompleteness of handedness information for the human cohort, although it is to be noted that no significant association between brain asymmetries and handedness was detected for any of the ROIs in the meta-analysis performed by Kong et al. (2018) (see also the vertex-wise SA and CT asymmetry analysis reported by Maingault et al. (2016)). Inconsistencies with results reported in previous studies could be related to 1) methodological differences such as use of SAPS versus TAPS, use of SBM rather than VBM, and use of automatic compared with manual methods (e.g., automatic parcellation versus manual outlining of ROIs), 2) spatial resolution [e.g., 163 842 vertices per cerebral hemisphere in the present study compared with 40 962 vertices per cerebral hemisphere in the study by Zhou et al. (2013)], 3) statistical methods, 4) in vivo versus in vitro studies, and 5) sample size considerations.
In summary, the present study represents the most comprehensive comparison so far available of SA and CT asymmetry between the human and chimpanzee brain. Overall, the human brain shows substantially greater asymmetry with distinct global and local features, whereas the chimpanzee brain is comparatively less asymmetric with seemingly only local asymmetries being present. In most regions where a significant difference between the human and chimpanzee brain was present, the sign of the average brain asymmetry is in opposite drections. Thus, it is probably not true that the two species share the same asymmetry but which is more prominent in humans (Gomez-Robles et al. 2013).
With regard to local asymmetries that are present in the human and chimpanzee brain, there is diminishing evidence for the one that was long predicted and expected to be found in Broca’s area and its homologue (Foundas et al. 1998; Keller et al. 2009a; Keller et al. 2009b), but on the other hand increasing evidence that both species share a common leftward asymmetry in PT and its homologue. Accordingly, against the backdrop of a global torque present in only the human brain is the interesting finding that the two species likely share a similar pattern of presence, and absence, of structural asymmetries in receptive, and expressive, “language” areas. Added to this are intriguing findings of structural asymmetries unique to each species, namely, the present study provides further support for the rightward STS asymmetry proposed by Leroy et al. (2015) to be a human-specific landmark, and observed for the first time a chimpanzee-specific asymmetry of the precentral gyrus and which could possibly provide information relevant to deciphering the brain changes that may have occurred related to the evolution of handedness.
In conclusion, after being highly sought after for well over a century but remaining somewhat enigmatic, a clearer picture is emerging regarding the nature of structural brain asymmetry and its evolution and many interesting lines of enquiry can now be more confidently pursued. The abovementioned asymmetries are all relatively subtle, but they are significant and they can be measured in unprecedented detail by using state-of-the-art MR imaging and image analysis techniques such as those used in the present study and which are becoming ever more refined and sophisticated. Coupled with advances in genetics, “Big Data,” and artificial intelligence, we anticipate the study of structural asymmetries of the human brain is poised to lead to new knowledge and new understanding regarding brain evolution and brain structural and functional organization, and we hope that the findings of the present study will provide further motivation to conduct these analyses.
Notes
We thank Prof. Stephen Lawrie for access to MRI scans of human subjects at the University of Edinburgh and staff at the University of Edinburgh, University of Oxford, and Yerkes National Primate Center for their support in acquiring the MRI data.
Funding
T.J. Crow Psychosis Research Trust (to L.X.); National Institutes of Health (grants NS-42867, HD-60563 to W.D.H.).
Supplementary Material
Contributor Information
Li Xiang, School of Clinical Sciences, University of Edinburgh, Edinburgh EH16 4TJ, UK.
Timothy J Crow, POWIC, Department of Psychiatry, Warneford Hospital, Oxford OX3 7JX, UK.
William D Hopkins, The University of Texas MD Anderson Cancer Center, Bastrop, TX 78602, USA.
Neil Roberts, School of Clinical Sciences, University of Edinburgh, Edinburgh EH16 4TJ, UK.
References
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. 1996. Asymmetry in the human motor cortex and handedness. Neuroimage. 4(3 Pt 1):216–222. [DOI] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E. 2010. Endocranial shape asymmetries in pan paniscus, pan troglodytes and Gorilla gorilla assessed via skull based landmark analysis. J Hum Evol. 59:54–69. [DOI] [PubMed] [Google Scholar]
- Balzeau A, Gilissen E, Grimaud-Herve D. 2012. Shared pattern of endocranial shape asymmetries among great apes, anatomically modern humans, and fossil hominins. PLoS One. 7(1):e29581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Mackay CE, Prima S, Maes F, Vandermeulen D, Crow TJ, Roberts N. 2005. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 24:678–691. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Lawes N, Mackay CE, Clark CA. 2007. White matter pathway asymmetry underlies functional lateralization. Cerebral Cortex. 17(3):591–598. [DOI] [PubMed] [Google Scholar]
- Bradshaw B, Rogers L. 1993. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego: Academic Press. [Google Scholar]
- Cantalupo C, Hopkins WD. 2001. Asymmetric Broca's area in great apes. Nature. 414(6863):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Vazquez D, Felton A, McDowell A. 2016. Structural asymmetry of the human cerebral cortex: regional and between-subject variability of surface area, cortical thickness, and local gyrification. Neuropsychologia. 93(Pt B):365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC. 1992. The lopsided brain: evolution of the generative mind. New York: Oxford University Press. [Google Scholar]
- Corballis MC. 2002. From hand to mouth: the origins of language. Princeton (NJ): Princeton University Press. [Google Scholar]
- Corballis MC. 2009. The evolution and genetics of cerebral asymmetry. Philos Trans R Soc Lond B Biol Sci. 364(1519):867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. 2004. Directional asymmetry is the key to the origin of modern homo sapiens (the Broca-Annett axiom): a reply to Rogers' review of the speciation of modern homo sapiens. Laterality. 9(2):233–242. [Google Scholar]
- Crow TJ. 2010. A theory of the origin of cerebral asymmetry: epigenetic variation superimposed on a fixed right-shift. Laterality. 15(3):289–303. [DOI] [PubMed] [Google Scholar]
- Cunningham DJ. 1892. Contribution to the surface anatomy of the cerebral hemispheres. Cunningham memoir, no. 7. Dublin: Academy House. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis I: segmentation and surface reconstruction. NeuroImage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BTet al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberstaller O. 1884. Zür oberflachen anatomie der grosshirn hemisphaeren. Wien Med. 7(479):642–644. [Google Scholar]
- Eberstaller O. 1890. Das stirnhirn. Ein beitrag zur anatomie der oberfläche des grosshirns. Urban and schwarzenberg: wien und leipzig. [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. 1999. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 8(4):272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. 1998. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis. Brain Lang. 64(3):282–296. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. 1998. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's brain language area homolog. Science. 279(5348):220–222. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Kheck N, Hof PR. 2008. Leftward interhemispheric asymmetry of macaque monkey temporal lobe language area homolog is evident at the cytoarchitectural, but not gross anatomic level. Brain Res. 1199:62–73. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. 1968. Human brain: left-right asymmetries in temporal speech region. Science. 161(3837):186–187. [DOI] [PubMed] [Google Scholar]
- Gomez-Robles A, Hopkins WD, Sherwood CC. 2013. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc Biol Sci. 280(1761):20130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. 2001. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 14(3):685–700. [DOI] [PubMed] [Google Scholar]
- Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, Brysbaert M. 2013. A surface-based analysis of language lateralization and cortical asymmetry. J Cogn Neurosci. 25(9):1477–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe T, Willems RM, Zwiers MP, Arias Vasquez A, Hoogman M, Hagoort P, Francks C. 2014. Differences in cerebral cortical anatomy of left- and right-handers. Front Psychol. 5:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbroner PL, Holloway RL. 1988. Anatomical brain asymmetries in New World and Old World monkeys: stages of temporal lobe development in primate evolution. Am J Phys Anthropol 76(1):39–48. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. 1995. Hand preferences for a coordinated bimanual task in 110 chimpanzees (pan troglodytes): cross-sectional analysis. J Comp Psychol. 109(3):291–297. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. 2013. Neuroanatomical asymmetries and handedness in chimpanzees (pan troglodytes): a case for continuity in the evolution of hemispheric specialization. Ann N Y Acad Sci. 1288:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Avants BB. 2013. Regional and hemispheric variation in cortical thickness in chimpanzees (pan troglodytes). J Neurosci. 33(12):5241–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. 2004. Handedness in chimpanzees (pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav Neurosci. 118(6):1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RL, De La Costelareymondie MC. 1982. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol. 58(1):101–110. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Li X, Crow T, Roberts N. 2016. Vertex- and atlas-based comparisons in measures of cortical thickness, gyrification and white matter volume between humans and chimpanzees. Brain Struct Funct. 222(1):229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L. 2000. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia. 38(4):493–499. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Meguerditchian A, Coulon O, Bogart S, Mangin JF, Sherwood CC, Grabowski MW, Bennett AJ, Pierre PJ, Fears Set al. 2014. Evolution of the central sulcus morphology in primates. Brain Behav Evol. 84(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Misiura M, Pope SM, Latash EM. 2015. Behavioral and brain asymmetries in primates: a preliminary evaluation of two evolutionary hypotheses. Ann N Y Acad Sci. 1359:65–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Nir TM. 2010. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): the effect of handedness and comparison within findings in humans. Behav Brain Res. 208(2):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Meguerditchian A, Nir T, Schenker NM, Sherwood CC. 2008. Grey matter asymmetries in chimpanzees as revealed by voxel-based morphometry. Neuroimage. 42(2):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Xiang L, Crow T, Leroy F, Riviere D, Mangin JF, Roberts N. 2018. Measurement of Sylvian fissure asymmetry and occipital bending in humans and pan troglodytes. Neuroimage. 184:855–870. [DOI] [PubMed] [Google Scholar]
- Ide A, Rodriguez E, Zaidel E, Aboitiz F. 1996. Bifurcation patterns in the human Sylvian fissure: hemispheric and sex differences. Cereb Cortex. 6(5):717–725. [DOI] [PubMed] [Google Scholar]
- Josse G, Kherif G, Flandin G, Seghier ML, Price CJ. 2009. Predicting language lateralization from gray matter. J Neurosci. 29(43):13516–13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. 2009a. Broca's area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 109(1):29–48. [DOI] [PubMed] [Google Scholar]
- Keller SS, Deppe M, Herbin M, Gilissen E. 2012. Variability and asymmetry of the sulcal contours defining Broca's area homologue in the chimpanzee brain. J Comp Neurol. 520(6):1165–1180. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N. 2007. Sulcal variability, stereological measurement and asymmetry of Broca's area on MR images. J Anat. 211(4):534–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins W. 2009b. A comparative magnetic resonance imaging study of the anatomy, variability, and asymmetry of Broca's area in the human and chimpanzee brain. J Neurosci. 29(46):14607–14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Henningsen H. 2000. Handedness and hemispheric language dominance in healthy humans. Brain. 123(12):2512–2518. [DOI] [PubMed] [Google Scholar]
- Koelkebeck K, Miyata J, Kubota M, Kohl W, Son S, Fukuyama H, Murai T. 2014. The contribution of cortical thickness and surface area to gray matter asymmetries in the healthy human brain. Hum Brain Mapp. 35(12):6011–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XZ, Mathias SR, Guadalupe T, Glahn DC, Franke B, Crivello F, Tzourio-Mazoyer N, Fisher SE, Thompson PM, Francks C. 2018. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the enigma consortium. Proc Natl Acad Sci U S A. 115(22):E5154–e5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen Y, Auzias G, Leroy F, Noulhiane M, Dehaene-Lambertz G, Duchesnay E, Frouin V. 2018. Genetic influence on the Sulcal pits: on the origin of the first cortical folds. Cereb Cortex. 28(6):1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen Y, Leroy F, Auzias G, Riviere D, Grigis A, Mangin JF, Frouin V. 2018. The chaotic morphology of the left superior temporal sulcus is genetically constrained. Neuroimage. 174:297–307. [DOI] [PubMed] [Google Scholar]
- LeMay M. 1982. Morphological aspects of human brain asymmetry: an evolutionary perspective. Trends in Neurosciences. 5:273–275. [Google Scholar]
- Leroy F, Cai Q, Bogart SL, Dubois J, Coulon O, Monzalvo K, Fischer C, Glasel H, Van der Haegen L, Benezit Aet al. 2015. New human-specific brain landmark: the depth asymmetry of superior temporal sulcus. Proc Natl Acad Sci U S A. 112(4):1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. 2006. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 16(8):1232–1238. [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D, Gilmore JH. 2015. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 25(8):2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyn H, Pierre P, Bennett AJ, Fears S, Woods R, Hopkins WD. 2011. Planum temporale grey matter asymmetries in chimpanzees (pan troglodytes), vervet (chlorocebus aethiops sabaeus), rhesus (macaca mulatta) and bonnet (macaca radiata) monkeys. Neuropsychologia. 49(7):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton OC, Karama S, Ad-Dab'bagh Y, Zatorre RJ, Carbonell F, Worsley K, Evans AC. 2009. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 46(4):895–903. [DOI] [PubMed] [Google Scholar]
- Maingault S, Tzourio-Mazoyer N, Mazoyer B, Crivello F. 2016. Regional correlations between cortical thickness and surface area asymmetries: a surface-based morphometry study of 250 adults. Neuropsychologia. 93(Pt B):350–364. [DOI] [PubMed] [Google Scholar]
- Marie D, Roth M, Lacoste R, Nazarian B, Bertello A, Anton JL, Meguerditchian A. 2017. Left brain asymmetry of the planum temporale in a nonhominid primate: redefining the origin of brain specialization for language. Cereb Cortex 28(5):1–8. [DOI] [PubMed] [Google Scholar]
- Meyer M, Liem F, Hirsiger S, Jancke L, Hanggi J. 2014. Cortical surface area and cortical thickness demonstrate differential structural asymmetry in auditory-related areas of the human cortex. Cereb Cortex. 24(10):2541–2552. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, Rayman J, Khaledy Met al. 2001. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatry. 50(2):84–97. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Gunturkun O. 2018. The lateralized brain: the neuroscience and evolution of hemispheric asymmetries. London: Academic Press. [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Kremen WS. 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19(11):2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Hugdahl K, Bansal R, Hao X, Peterson BS. 2014. Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. J Neurosci. 34(18):6294–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18(9):383–388. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Jbabdi S, Andersson J, Preuss TM. 2011. Continuity, divergence, and the evolution of brain language pathways. Front Evol Neurosci. 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LJ, Vallortigara G, Andrew RJ. 2013. Divided brains: the biology and behaviour of brain asymmetries. New York: Cambridge University Press. [Google Scholar]
- Rubens AB, Mahowald MW, Hutton JT. 1976. Asymmetry of the lateral (Sylvian) fissures in man. Neurology. 26(7):620–624. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. 1999. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 29:26–49. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Wahl E, Erwin JM, Hof PR, Hopkins WD. 2007. Histological asymmetries of primary motor cortex predict handedness in chimpanzees (Pan troglodytes). J Comp Neurol. 503(4):525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya S, Kuperberg GR, Liu H, Greve DN, Makris N, Stufflebeam SM. 2015. Asymmetric projections of the arcuate fasciculus to the temporal cortex underlie lateralized language function in the human brain. Front Neuroanat. 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Rathore RK, Saksena S, Tripathi RP, Malik GK, Gupta RK. 2009. Understanding development and lateralization of major cerebral fiber bundles in pediatric population through quantitative diffusion tensor tractography. Pediatr Res. 66(6):636–641. [DOI] [PubMed] [Google Scholar]
- Vadlamudi L, Hatton R, Byth K, Harasty J, Vogrin S, Cook MJ, Bleasel AF. 2006. Volumetric analysis of a specific language region- the planum temporale. J Clin Neurosci. 13:206–213. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. 2012. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 22(10):2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen CH, Fiecas M, Eyler LT, Fennema-Notestine C, Hagler DJ, Fischl B, Franz CE, Jak Aet al. 2015. The genetic association between neocortical volume and general cognitive ability is driven by global surface area rather than thickness. Cereb Cortex. 25(8):2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. 2001. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 11(9):868–877. [DOI] [PubMed] [Google Scholar]
- Wiberg A, Ng M, Al Omran Y, Alfaro-Almagro F, McCarthy P, Marchini J, Furniss D. 2019. Handedness, language areas and neuropsychiatric diseases: insights from brain imaging and genetics. Brain. 142(10):2938–2947.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53(3):1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson S, Kigar D. 1988. Asymmetry of brain function follows asymmetry in anatomical form: gross, microscopic, postmortem and imaging studies. In: Boller GJ, editor. Handbook of neuropsychology. Amsterdam: Elsevier, pp. 111–142. [Google Scholar]
- Xiang L, Crow T, Roberts N. 2019a. Automatic analysis of cross-sectional cerebral asymmetry on 3D in vivo MRI scans of human and chimpanzee. J Neurosci Res. 97(6):673–682. [DOI] [PubMed] [Google Scholar]
- Xiang L, Crow T, Roberts N. 2019b. Cerebral torque is human specific and unrelated to brain size. Brain Struct Funct. 224(3):1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Crow TJ, Hopkins WD, Gong Q, Roberts N. 2018. Human torque is not present in chimpanzee brain. Neuroimage. 165:285–293. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian G, Benson D. 1976. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 192:387–389. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Evans A, Beaulieu C. 2013. Cortical thickness asymmetry from childhood to older adulthood. Neuroimage. 83:66–74. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Steinmetz H. 1996. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 20:593–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


