Abstract
Background
Proton pump inhibitors (PPIs) are among the most commonly prescribed medications worldwide for acid-related disorders. While their short-term efficacy and safety are well-established, concerns regarding their long-term effects on bone health have emerged. This umbrella review aimed to synthesize the available findings on the associations between PPI use and bone metabolism outcomes.
Methods
An electronic search was conducted using PubMed, Web of Science, Embase, and the Cochrane Database up to September 16, 2023. Systematic reviews and meta-analyses of randomized controlled trials (RCTs) and observational studies that evaluated the relationship between PPIs and bone metabolism outcomes were included. Data extraction, quality appraisal, and synthesis were performed in line with the Joanna Briggs Institute and PRISMA guidelines. The strength of the evidence was graded using the GRADE criteria. Statistical analysis was performed in R version 4.3.
Results
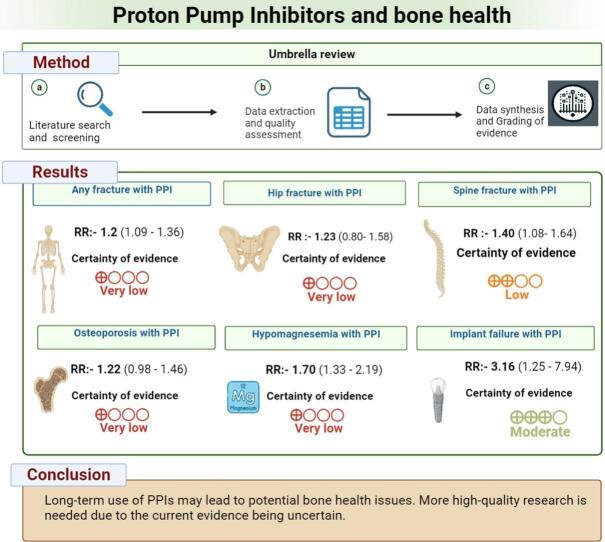
Out of 299 records, 27 studies met the inclusion criteria. The evidence indicated a statistically significant increased risk of fractures, notably hip, spine, and wrist fractures, in PPI users. PPI use was associated with changes in Bone Mineral Density (BMD) across various bones, though the clinical relevance of these changes remains uncertain. Furthermore, PPI-induced hypomagnesemia, which can influence bone health, was identified. A notable finding was the increased risk of dental implant failures in PPI users. However, the certainty of most of the evidence ranged from very low to low based on GRADE criteria.
Conclusion
The long-term use of PPIs may be associated with adverse bone health outcomes, including increased fracture risk, alterations in BMD, hypomagnesemia, and dental implant failure. While these findings highlight potential concerns for long-term PPI users, the current evidence's low certainty underscores the need for robust, high-quality research to clarify these associations.
Keywords: Proton pump inhibitors, Bone health, Fracture risk, Bone mineral density, Meta-analysis
Graphical abstract
1. Introduction
Proton pump inhibitors (PPIs) are pivotal in modern medical protocols for treating disorders related to gastric acid, having emerged as one of the most frequently utilized medications worldwide. The surge in the usage of compounds like esomeprazole is largely attributed to the escalating occurrences of conditions like gastroesophageal reflux disease and peptic ulcers (Aguilera-Castro et al., 2016). PPIs operate by causing an irreversible inhibition of the hydrogen/potassium adenosine triphosphatase enzyme system (the H+/K+ ATPase, or the gastric proton pump) located in the gastric parietal cells, effectively limiting the secretion of gastric acid (Malfertheiner et al., 2017). The global reliance on PPIs is evidenced by substantial prescription trends, with instances like the 16 million prescriptions recorded in France in 2015 (Lespessailles and Toumi, 2022), and a noted escalation in prescribing prevalence in Germany between 2005 and 2013 (Hoffmann et al., 2014). Their status further broadens the reach of PPIs as over-the-counter medications, rendering them accessible to a broader demographic (Curtiss, 2002; Forgacs and Loganayagam, 2008; Sattayalertyanyong et al., 2020).
Nonetheless, the extensive consumption of PPIs has invoked concerns and prompted extensive investigations into their safety. Although they are integral in managing and preventing a variety of acid-related conditions, emerging evidence points towards a possible link between extended PPI consumption and a range of adverse health implications such as clostridium difficile-associated diarrhea, occurrence of community-acquired pneumonia, and potentially, an elevated risk of certain cancers through intestinal dysbiosis (Kwok et al., 2012; Lambert et al., 2015; Vaezi and Choksi, 2017). Moreover, concerns have been raised about the long-term impacts of PPIs on bone health (Poly et al., 2019; Paik et al., 2018; Zhou et al., 2016), and some recent studies have uncovered associations between the initiation of certain PPIs and increased occurrences of knee replacement surgeries (Zeng et al., 2022). The evidence suggests potential adverse impacts of PPIs on bone health and metabolism. These impacts include an increased risk of fractures, the development of osteoporosis, and a decrease in bone mineral density (Lespessailles and Toumi, 2022). Furthermore, the effect of PPIs on dental implant failure is also a subject of ongoing debate (Rogoszinski et al., 2022). A myriad of systematic reviews has been conducted, exploring the correlations between PPI use and various aspects of bone metabolism, including but not limited to, risk of fractures, onset of osteoporosis, and alterations in bone mineral density. A noticeable increase in systematic reviews on this topic has been discerned in recent years, highlighting growing concern and focus in the medical community on these potential correlations (Poly et al., 2019; Aleraij et al., 2020; da Maia et al., 2022).
An umbrella review synthesizes evidence from multiple systematic reviews on a specific topic, offering a comprehensive overview of the existing research (Aromataris et al., 2015). This study aims to conduct an umbrella review of existing research on PPIs affect bone metabolism. It will compile and analyze data to understand the varied impacts of PPIs on bone health and determine the relationship between PPI use and changes in bone metabolism.
2. Methods
This umbrella review was conducted as per the methodology described by the Joanna Briggs Institute (JBI) (Aromataris et al., 2014) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1) (Page et al., 2021). The study is registered with PROSPERO under registration number: CRD42023465040.
2.1. Selection criteria
This umbrella review includes systematic reviews and meta-analyses of randomized controlled trials (RCTs) and observational studies that assessed the association between PPIs and bone metabolism and related outcomes such as fracture risk, bone mineral density changes, osteointegration of implants, hypomagnesemia, and osteoporosis. The following were excluded from this review: case reports, case series, animal studies, conference abstracts, and narrative reviews. Articles not available in English were also excluded. Refer to Table S2 for detailed inclusion criteria.
2.2. Literature search and screening
A literature search of the literature was undertaken in databases including Embase, PubMed, Web of Science, and the Cochrane Database to identify systematic reviews on the topic up to September 16, 2023. Keywords and MeSH terms related to “Proton pump inhibitors,” “systematic review,” and “meta-analysis” informed the search criteria. No restrictions were imposed on the publication year. The search strategy can be found in Table S3.
Two reviewers (PS, HA) independently assessed the search outcomes once duplicates had been removed via the Nested Knowledge software. The first level of screening focused on titles and abstracts, which was then followed by a comprehensive review of the full texts. Discrepancies in opinions about article inclusion were settled by seeking the input of a third reviewer (ASA).
2.3. Data extraction
Data extraction was performed by two reviewers (JKG, DM). They first extracted data from each eligible systematic review. Information such as author name, year of publication, databases and search year, objective of the study, type of participants, number and type of studies, risk of bias tools used and their results, outcomes of concern, effect size and confidence intervals (CI), p value, publication bias, and were obtained.
2.4. Quality appraisal
For assessing the quality of the included systematic reviews included in this study, JBI Checklist for Systematic Reviews and Research Syntheses was used (Aromataris et al., 2015). The JBI tool offers a comprehensive approach to appraise the quality of systematic reviews. It evaluates various aspects, including the clarity of the research question, the appropriateness of inclusion criteria, and the comprehensiveness of the search strategy, among others.
2.5. Data synthesis
The synthesis of evidence was presented in both narrative and tabular formats. We provided a table detailing the specifics of each systematic review included in our analysis. This encompassed information such as the number of primary studies and participants involved, outcomes assessed, and reported effect estimates, such as risk ratios (RR), odds ratios (OR), mean difference (MD), and Standardised mean difference (SMD). When available, their CIs, heterogeneity, publication bias, and final findings were also included. In addition, the table summarized the quality assessments and outlined the risk of bias identified in the primary studies. A narrative approach was employed to summarize the evidence for each outcome, complemented by tabular formats where applicable to ensure clarity. We prioritized the results of the systematic review rated highest by JBI tool.
A meta-analysis was conducted to determine pooled outcomes based on effect size such as RR, OR, and MD. We used a random effects model to pool results. The degree of heterogeneity among study findings was measured using I2 and tau-squared metrics (Langan et al., 2019). Both ranged from 0 % to 100 %, with higher values indicating greater inconsistency (Gandhi et al., 2023). A p-value below 0.05 was considered indicative of statistical significance. We calculated the tau-squared value using the maximum likelihood approach. The funnel plot was utilized to identify potential publication biases when >10 studies were available for each outcome. R software, version 4.3, was used for all statistical analyses (Shamim et al., 2023).
2.6. Certainty of evidence
The quality of evidence was determined using GRADE criteria (Grading of Recommendations, Assessment, Development, and Evaluations). Grading was performed by considering 5 domains, including risk of bias in the individual studies, inconsistency, indirectness, imprecision, and publication bias for each outcome (Langendam et al., 2013). We graded the strength of evidence as very low, low, moderate, or high (Table S4).
3. Results
3.1. Literature search
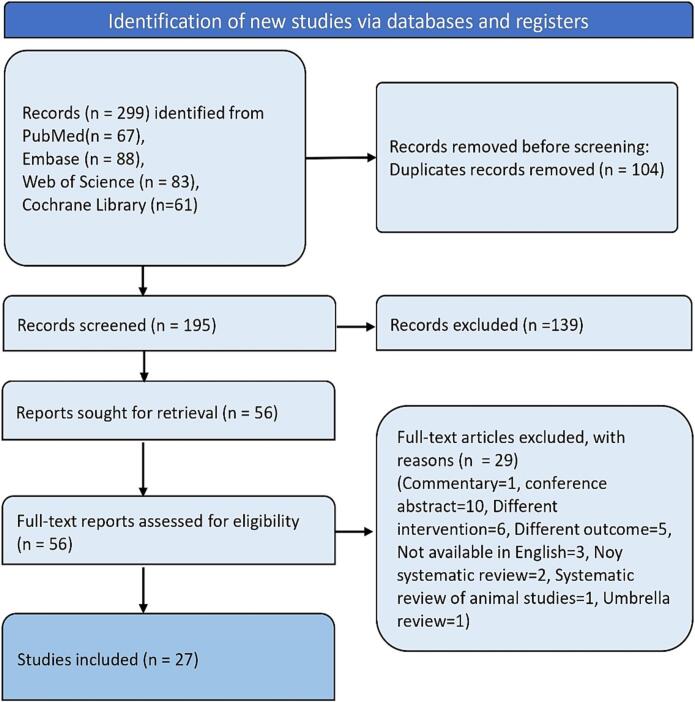
A total of 299 records appeared in the database search from all databases, of which 104 were duplicates. Out of these, 195 records were screened, and 56 articles underwent a full-text eligibility check. 29 studies were excluded for various reasons, such as being conference abstracts, having the wrong intervention, wrong outcome, being commentaries, not being systematic reviews, being systematic reviews of animal studies, or being umbrella reviews. Ultimately, 27 studies met the criteria and were included in this review. Fig. 1 depicts the flow diagram of the screening and selection process.
Fig. 1.
Flowchart showing screening and selection of articles.
3.2. Characteristics of included reviews
The important characteristics of included reviews are presented in Table 1. The systematic reviews focus on the association between PPIs and various bone-related outcomes, especially fracture risk, bone mineral density changes, and other drug-induced bone disorders. The research designs of the included studies ranged from prospective and retrospective cohort studies to case-control and nested case-control studies, with a few integrating cross-sectional and RCTs designs. These reviews covered diverse geographic locations, with a significant representation from the USA, UK, Canada, Denmark, and several European and Asian countries. The populations of interest varied from general patients to specific groups, such as menopausal women, children and young adults, hemodialysis patients, and patients undergoing dental implants. Predominantly, the outcomes of concern were related to fracture risk (hip, spine, wrist, and any fracture), bone mineral density (BMD) changes, dental implant failures, and other drug-related bone disorders. Risk of bias assessment tools, such as the Newcastle-Ottawa Scale (NOS), STROBE, Cochrane Adverse Effects Methods Group, NHLBI, and Cochrane RoB, were employed by different authors. The overall risk of bias in most studies ranged from low to moderate, although a few indicated high risk. Publication bias, when reported, was mainly assessed using techniques like Begg's test, Egger's test, and funnel plot asymmetry, with several studies showing no evidence of publication bias. The result of quality assessment is presented in Table S5.
Table 1.
Characteristics of included reviews.
| Study | Objective | Databases searched | Included Study designs | Number of studies | Country of included studies | Year of included studies | Population | Outcomes of concern | Risk of bias tool used | Overall risk of bias | Publication bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggarwal 2019 (Aggarwal et al., 2019) | To consolidate the available data on drug induced bone disorders | PubMed, Medline, Embase (July 2019) | Prospective and Nested case-control | NA | NA | NA | NA | Risk of fracture, hip fracture | NA | NA | NA |
| Aghaloo 2019 (Aghaloo et al., 2019) | To evaluate the effect of systemic disorders, other diseases, and drugs on implant osseointegration | PubMed upto July 2018 | Case control, prospective, retrospective studies | 2 (for PPI) | NA | 2001–2017 | General | Implant survival rate | NA | NA | NA |
| Aleraij 2020 (Aleraij et al., 2020) | To evaluate the association between the use of PPIs and changes in bone mineral density | PubMed/MEDLINE, EMBASE, Cochrane, CINAHL Up to March 2019 | Prospective and retrospective cohort studies | 10 | USA, the Republic of Kosovo, Canada, China, Turkey and South Korea | 2008–2018 | General | Mean annualized percent change in BMD, Mean difference in BMD | NOS | Not reported | NA |
| Cai 2015 (Cai et al., 2015) | To assess the relationship between use of antacid drugs and fracture risk | PubMed and Embase | Nested case control, case control, cohort | 18 | UK, USA, Spain, Canada, Netherlands, Europe, Sweden, Taiwan, Denmark | 1997–2014 | General | Hip fracture, any fracture, Spine fracture, wrist fracture | NA | NA | No publication bias |
| Chappuis 2018 (Chappuis et al., 2018) | To investigate the association between the intake of medications that may affect bone metabolism and implant outcomes | PubMed, MEDLINE (OVID), EMBASE (OVID), Cochrane Library, Web of Science, and SciVerse (Elsevier) up to May 2017 | Retrospective cohort studies | 2 | Canada, Sweden | 2017 | Adults wearing implant-supported prostheses | Implant Failure | STROBE and NOS | Low risk of bias, Studies scored 8 and 9 on NOS | NA |
| Da Maia 2022 (da Maia et al., 2022) | To assess whether there is a relationship between the use of PPIs and fractures in menopausal women | PubMed, Scopus, and Science Direct, 12 April 2021 | Observational prospective cohort studies | 5 | USA, Sweden, Germany, France, Australia | 2009–2014 | Menopausal women | Fractures | NOS | Overall NOS score 8–9, low risk of bias | NA |
| Eom 2011 (Eom et al., 2011) | To investigate the association between the use of PPIs or H2RAs and fracture risk | MEDLINE (PubMed), EMBASE, and the Cochrane Library from inception through December 2010 | Case-control, nested case-control, cohort | 10 (PPI) | USA, UK, Canada, Denmark, France, Taiwan, Netherlands | 2006–2011 (for PPI) | General | Any Fractures, Hip fracture, spine fracture, wrist fracture | NOS | moderate to high | No publication bias detected (Egger, p = 0.45) |
| Fan 2017 (Fan et al., 2017) | To evaluate the association between PPI use and risk of osteoporosis | PubMed, EMBASE and Web of Knowledge from inception up to March 2017. | Case-control, prospective studies | 27 | China, Germany, Taiwan, Australia, UK, USA, Korea, Denmark, Spain, Canada, Europe, Netherlands | 2006–2016 | General patient population | Osteoporosis, any Fracture, Hip fracture, Spine fracture | NOS | Moderate to high | No publication bias detected |
| Heidelbaugh2009 (Heidelbaugh et al., 2009) | To summarize adverse risks associated with long-term use of PPIs in the treatment of upper gastrointestinal disorders | MEDLINE (1966–2008) | Nested case-control, Case-control, Retrospective matched cohort | 3 | UK, Canada, Denmark | 2006 and 2008 | General Practice database from UK, Community based from Denmark and Canada | Bone fracture | NA | NA | NA |
| Hussain 2018 (Hussain et al., 2018) | Tor explore the association of PPI use and risk of hip fracture | MEDLINE via PubMed and Cochrane central | Cohort, Case control and nested case-control | 17 | USA, UK, Canada, Taiwan, Spain, Netherlands | 2006 to 2017 | Patients with PPI exposure | Hip fracture | NOS | Medium to high quality | NA |
| Islam 2018 (Islam et al., 2018) | To quantify the associations as presented in the literature and to also provide this information to healthcare professionals and patients about PPIs potentially adverse effects | Medline (PubMed), Embase, and the Cochrane Library (July 2016) | Case-control, cohort, and cross-sectional | 43 (12 studies on Hip fracture) | USA, UK, Spain, Taiwan, Korea, Netherlands | General | Hip fracture | Modified NOS | NA | NA | |
| Kwok 2011 (Kwok et al., 2011) | To perform a meta-analysis of fractures in patients taking PPIs and H2RAs | MEDLINE and Embase September 2010 | Case-control, prospective and retrospective cohort | 12 | USA, UK, Netherlands, Europe, Canada | 1997–2010 | General | Spine fracture, hip fracture, overall fracture | Cochrane Adverse Effects Methods Group | Unclear and low risk of biases | NA |
| Li 2021 (Li et al., 2021) | To evaluate the risk of fracture with PPIs and H2RAs use in children and young adults | PubMed, EMBASE database, Cochrane Library, and Web of Science (May 2021) | Retrospective cohort, cohort, case control | 6 | Sweden, Israel, UK, USA | 2015–2020 | Children and young adults | Risk of fracture | NOS | High | No publication bias detected |
| Liu 2019 (Liu et al., 2019) | To determine the link between PPI use and fractures, osteoporosis, and BMD loss | PubMed, EMBASE and the Cochrane Library from inception up to May 2018 | Case-control, Cohort and cross-sectional studies | 32 | USA, Canada, UK, France, Denmark, Netherland, Iran | 2006–2018 | General | Fracture/Osteoporosis/ Bone mineral loss | NOS | All studies scored moderate to high score for NOS | No publication bias detected for hip, any fracture, and osteoporosis (p = 0.54, 0.39, 0.07), Spine fracture showed publication bias (p = 0.03) |
| Mortensen 2020 (Mortensen et al., 2020) | To assess the impact of various classes of medications on the risk of fragility hip fracture | EMBASE, PubMed, Web of Science, and Cochrane Central and clinicaltrials.gov in September 2017. | NA | 38 | Denmark, USA, Norway, Netherlands, UK, Taiwan, Ireland, France, Greece, Canada, Austria, Germany | 1981 to 2017, | General | Hip fractures | NOS | High quality | No publication bias detected, Begg and Mazumdar test for rank correlation (p = 0.35) |
| Nassar 2018 (Nassar and Richter, 2018) | To evaluate the relation-ship between PPI use and fracture incidence | PubMed, Embase, and Google Scholar, Feb 2018 | Prospective or retrospective observational studies | 33 | NA | 2006–2017 | General and patient population | Fracture risk, Change in BMD | NA | NA | No publication bias detected. Begg's test (p = 0.15) |
| Ngamruengphong 2011 (Ngamruengphong et al., 2011) | To evaluate an association between the use of PPI and risks for fracture | MEDLINE, EMBASE, and Cochrane Up to Aug 2010 | Cohort and Case-control studies | 10 | UK, Denmark, USA, Netherlands, Canada, Europe | 2006–2010 | Men and women aged >50 years, Men and women aged >43 years, Men and women aged >18 years, Men and women aged 50–79 years, Post-menopausal women | Hip fracture, Spine, wrist, Any fracture | Validity criteria suggested by Loke et al. (9) and Levine (10) | Overall Low-moderate Risk of bias, only one study had high bias | NA |
| Poly 2019 (Poly et al., 2019) | To gauge precisely the nature and magnitude of the association between PPIs and hip fracture risk | PubMed, EMBASE, Scopus, Google Scholar, and Web of Science (January 1990 and March 2018) | Cohort and case-control studies | 24 | USA, UK, Taiwan, Denmark, Korea, Netherlands, Canada, Finaland | 2006 to 2018 | Adults (aged 18 years or greater) | Development of hip fracture | NOS | Moderate to high | Egger’s regression test of the funnel asymmetry showed no observed significantpublication bias (p value = 0.75). |
| Srinutta 2019 (Srinutta et al., 2019) | To find out an association between PPI dose or treatment duration and the development of hypomagnesemia | MEDLINE, Scopus, Cochrane (1978 to June 2018) | Cross-sectional, case-control, cohort studies | 16 | North America = 7, Europe = 6, Asia = 3 | 2012–2018 | Patients in ambulatory settings, dialysis facilities, hospital settings | Hypomagnesemia | NHLBI | Fair to good | Not present Egger p value = 0.69 |
| Verma 2022 (Verma, 2022) | To determine the influence of PPIs on biomechanical efficiency of dental implants | PubMed, Cochrane database, EBSCO host, Web of Science and Scopus from 2010 upto Dec 2021 | RCTs | 6 | NA | 2017–2019 (human) | Patients undergoing dental implant treatment modality | Dental implant failure | Cochrane RoB | Low bias | NA |
| Vestergaard2020 (Vestergaard, 2020) | To perform systematic review of drugs inducing bone loss or associated with fracture risk | Medline | NA | 5 | NA | 2006–2019 | General | Bone loss | NA | NA | NA |
| Vinnakota 2020 (Vinnakota and Kamatham, 2020) | To find out the usage of PPIs in individuals undergoing dental implantation influence the success of an implant compared to controls | MEDLINE, Ovid and Cochrane Up to July 2019 | Retrospective cohort studies | 3 | Canada, Turkey, Sweden | 2017–2019 | Patients who underwent dental implant | Dental implant failure | NOS | Low risk of bias, good quality overall | NA |
| Yang 2022 (Yang et al., 2022) | To evaluate the risk of fracture in children and young adults exposed to ASDs | Cochrane Library, PubMed, and EMBASE (inception to December 2020) | Cohort, Nested case-control study | 6 | USA, UK, Israel, Sweden | 2015 to 2020 | Children, Young adults | Fracture risk | NOS | Four studies out of 6 scored high quality | NA |
| Ye 2011 (Ye et al., 2011) | To determine whether the association between PPIs and hip fracture exists quantitatively | PubMed and EMBASE, Cochrane up to June 2010 | Prospective and retrospective cohort studies, case-control studies | 7 | Netherlands, USA, UK, CANADA, Denmark | 2008–2011 | 18 and older, 50–79 aged, post-menopausal women | Hip fracture | STROBE | Not reported | No publication bias detected |
| Yu 2011 (Elaine et al., 2011) | To estimate the overall effect of PPI use on fracture rates | PubMed/MEDLINE, EMBASE, Web of Science, and BIOSIS Previews upto October 10, 2010 | Case-control, retrospective longitudinal cohort | 11 | USA, UK, Denmark, Europe, Netherlands, Taiwan | 2006–2010 | Adults (Predominantly postmenopausal women and older men) | Hip fracture, Any fracture, Spine fracture | NA | NA | No evidence of publication bias (Begg's test p = 0.22) |
| Zhang 2022 (Zhang et al., 2022) | To find put the impact of PPIs on Hemodialysis patient outcomes | Pubmed, Embase, Cochrane Library, and Web of Science, April 2022 | Prospective, Retrospective and cross-sectional studies | 12 | Japan, USA, Croatia, Denmark, Multicentre, Spain | 2013–2019 | Hemodialysis Patients | Fracture, Hypomagnesemia, Vascular calcification | NOS | Moderate to high | No publication bias detected |
| Zhou 2016 (Zhou et al., 2016) | To further clarify the association between PPI use and fracture risk | PubMed upto Feb 2015 | Cohort and case-control studies | 17 | UK, Denmark, Canada, USA, Europe, Sweden, Spain, Netherlands, Taiwan, Australia | 1987–2012 | General | Hip fracture, Spine and all site fracture | NOS | Good quality | No publication bias detected for any site fracture (p = 0.297), Detected for spine fracture (p = 0.038) |
Abbreviations: - ASDs - Acid Suppressing Drugs, BMD - Bone Mineral Density, H2RAs - Histamine-2 Receptor Antagonists, MEDLINE - Medical Literature Analysis and Retrieval System Online, NOS - Newcastle-Ottawa Scale, NHLBI - National Heart, Lung, and Blood Institute, PPI - Proton Pump Inhibitor, RCTs - Randomized Controlled Trials, RoB - Risk of Bias, STROBE - Strengthening The Reporting of Observational studies in Epidemiology.
3.3. Summary of outcomes
Table 2 presents the overall summary of results based on each outcome.
Table 2.
Summary of outcomes.
| Outcome | Number of studies | Type of effect estimate | Effect size | 95 % CI | Heterogeneity (I2) | Publication bias | Grade |
|---|---|---|---|---|---|---|---|
| Any fracture | 14 | RR | 1.2 | 1.09–1.36 | 85 % | No | Very Low |
| Fracture risk (children) | 5 | RR | 1.12 | 1.07–1.17 | 18 % | NA | Low |
| Fracture risk (young adults) | 2 | RR | 0.98 | 0.31–1.65 | 84 % | NA | Very Low |
| Hip fracture | 26 | RR | 1.2 | 1.13–1.27 | 64 % | No | Very Low |
| Hip fracture (post-menopausal women) | 5 | RR | 1.23 | 0.8–1.58 | 30 % | NA | Very Low |
| Spine fracture | 6 | RR | 1.4 | 1.18–1.64 | 36 % | NA | Low |
| Wrist fracture | 3 | RR | 1.08 | 0.7–1.44 | 62 % | NA | Very Low |
| Osteoporosis | 6 | RR | 1.22 | 0.98–1.46 | 92 % | NA | Very Low |
| BMD (all) | 7 | MD | 0.025 | 0.001–0.050 | 55 % | NA | Very Low |
| BMD (femur) | 5 | MD | −0.094 | −0.409- 0.022 | 72 % | NA | Very Low |
| BMD (spine) | 7 | MD | 0.025 | −0.047-0.097 | 48 % | NA | Very Low |
| BMD (hip) | 4 | MD | 0.018 | −0.030- 0.066 | 54 % | NA | Very Low |
| Hypomagnesemia | 12 | OR | 1.7 | 1.33–2.19 | 88 % | No | Very Low |
| Implant failure | 4 | RR | 3.16 | 1.25–7.94 | 96 % | NA | Moderate |
| Bone fracture (hemodialysis patients) | 3 | OR | 1.29 | 1.21–1.37 | 0 % | No | Low |
| Hip fracture (hemodialysis patients) | 3 | OR | 1.37 | 1.12–1.67 | 82 % | No | Very low |
| Hypomagnesemia (hemodialysis patients) | 4 | OR | 2.79 | 1.95–4.00 | 0 % | No | Moderate |
| Aortic calcifications (hemodialysis patients) | 2 | OR | 2.03 | 1.28–3.24 | 0 % | NA | Low |
3.3.1. Fracture risk
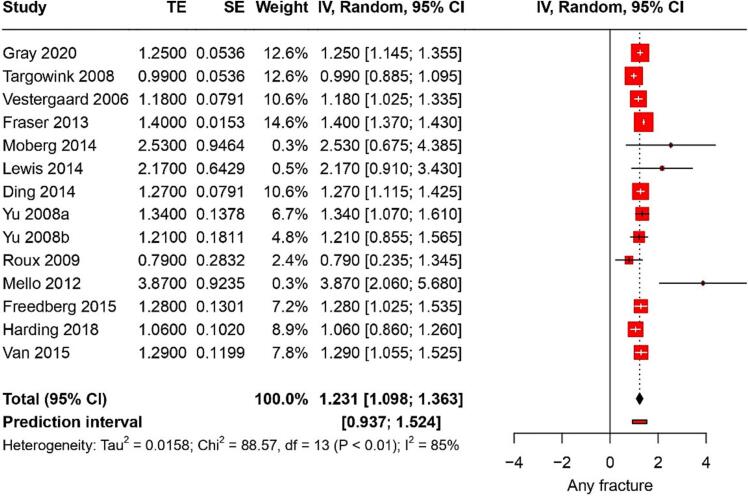
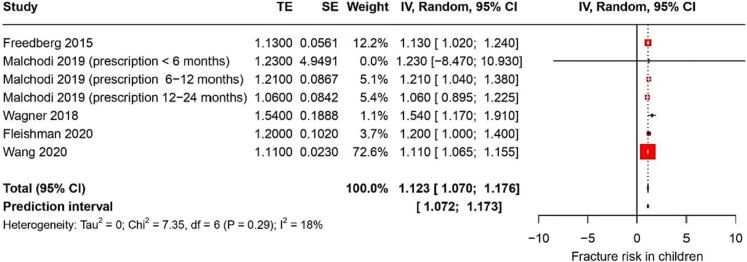
Fourteen studies reported on fracture risk. They identified a pooled RR of 1.2 (95 % CI: 1.09–1.36) with a heterogeneity of 85 % (Fig. 2). The certainty of this evidence was very low. In children, PPI use was associated with a RR of hip fractures of 1.12 (95 % CI: 1.07–1.17) (Fig. 3); the certainty of this evidence was low. Among young adults, PPI use was linked to a RR of hip fractures of 0.98 (95 % CI: 0.31–1.6); the certainty of this evidence was also very low.
Fig. 2.
Forest plot showing the pooled result of PPI use and risk of any fracture.
Fig. 3.
Forest plot showing the pooled result of PPI use and fracture risk in children.
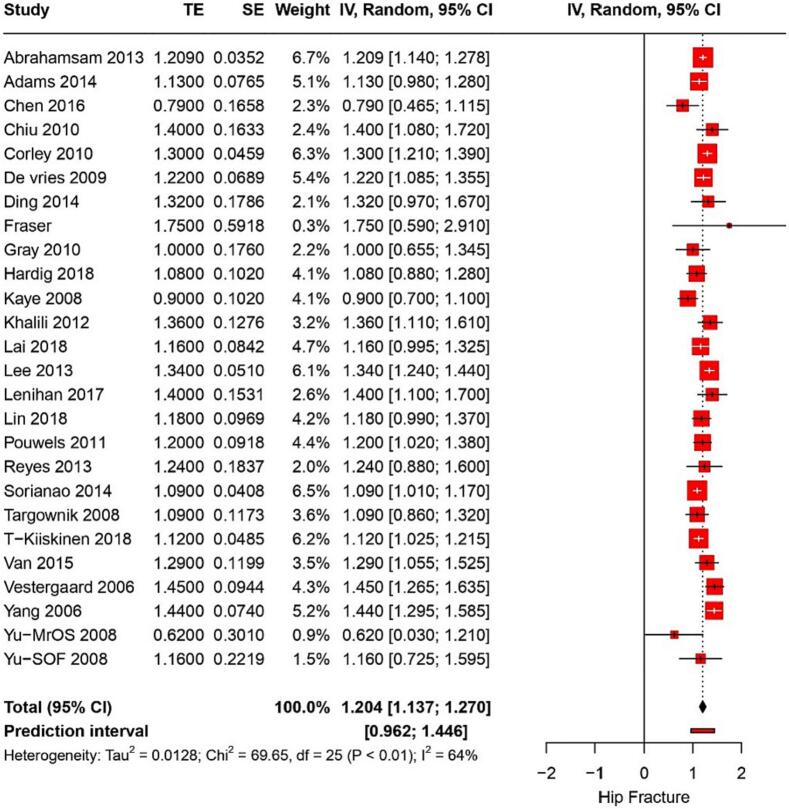
3.3.2. Hip fracture
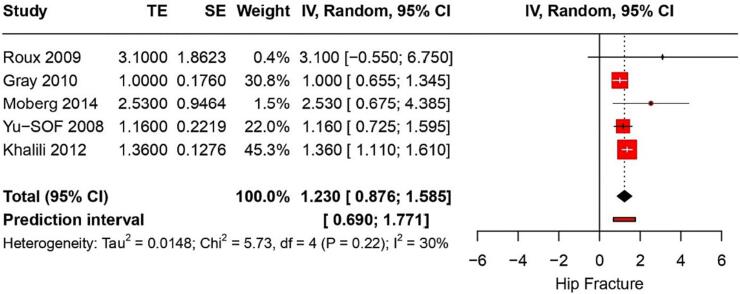
From 26 studies, the risk of hip fracture associated with PPI use was found to have a RR of 1.2 (95 % CI: 1.13–1.27) with a heterogeneity of I2 = 64 % (Fig. 4). The certainty of this evidence was very low. Five studies on postmenopausal women showed that PPI use was linked to a RR of hip fractures of 1.2 (95%CI: 0.87–1.5) (p < 0.006) (Fig. 5). The certainty of this evidence was very low. Furthermore, three studies reported hip fractures in hemodialysis patients with a pooled OR of 1.37 (95 % CI: 1.12–1.67) and I2 of 82 %; the evidence's certainty was very low.
Fig. 4.
Forest plot showing the pooled result of PPI use and hip fracture.
Fig. 5.
Forest plot showing the pooled result of PPI use and hip fracture risk in postmenopausal women.
3.3.3. Spine fracture
Six studies showed that the risk of spine fracture associated with PPI use had a RR of 1.4 (95 % CI: 1.18–1.64) with a heterogeneity of I2 = 36 %. The certainty of this evidence was very low.
3.3.4. Wrist fracture
From three studies, the risk of wrist fracture linked to PPI use was determined to have a RR of 1.08 (95 % CI: 0.71–1.44) with a heterogeneity of I2 = 62 %. The certainty of this evidence was very low.
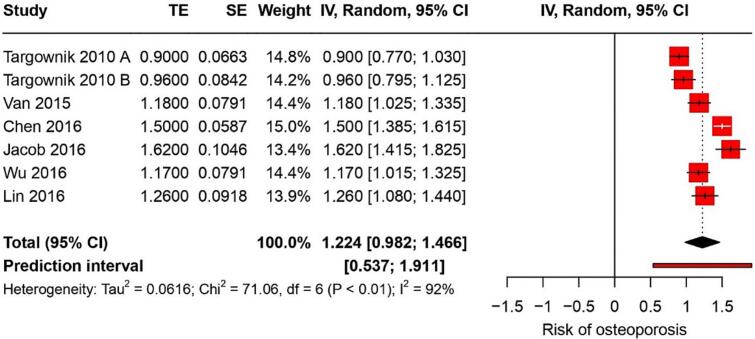
3.3.5. Osteoporosis
Six studies indicated that the risk of osteoporosis associated with PPI use had a RR of 1.22 (95 % CI: 0.98–1.46) with a high heterogeneity of I2 = 92 % (Fig. 6). The certainty of this evidence was very low.
Fig. 6.
Forest plot showing the pooled result of PPI use and risk of osteoporosis.
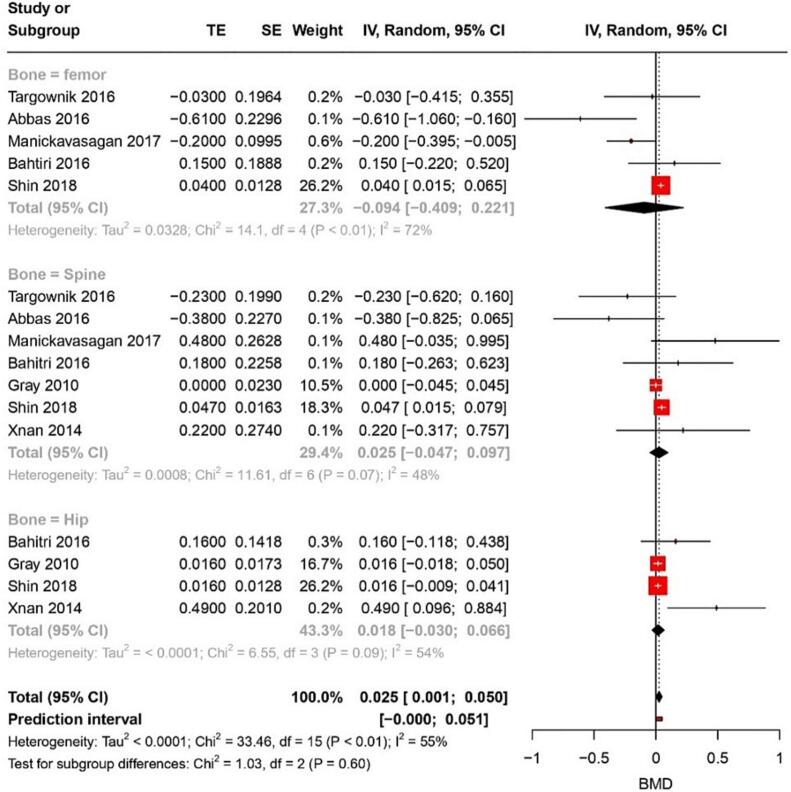
3.3.6. BMD
Seven studies reported a MD in BMD of 0.025 (95 % CI: 0.001–0.50) with an I2 of 55 % for all bone types combined (Fig. 7). The certainty of this evidence was very low. For femur BMD, five studies showed an MD of −0.094 (95 % CI: 0.409–0.22) with 48 % heterogeneity. Meanwhile, for the spine, five studies revealed an MD for BMD of 0.025 (95 % CI: 0.047–0.097). Lastly, for the hip bone, an MD of 0.018 (95 % CI: −0.030–0.66) was found from four studies with an I2 of 54 %.
Fig. 7.
Forest plot showing the pooled result of PPI use and change in BMD.
3.3.7. Hypomagnesemia
Twelve studies reported hypomagnesemia in the general population with PPI use, resulting in an OR of 1.7 (95 % CI: 1.33–2.19) and an I2 of 88 %. The certainty of this evidence was very low. In hemodynamic patients, PPI use led to an OR of 2.27 (95 % CI: 1.95–4.00) from four studies for hypomagnesemia, with the certainty of this evidence being moderate.
3.3.8. Implant failure
Four studies identified a RR of 3.15 (95 % CI: 1.25–7.94) for dental implant failure associated with PPI use. The heterogeneity was high with an I2 of 96 %. The certainty of this evidence was moderate.
4. Discussion
Among the most widely prescribed medications worldwide for the treatment of acid-related disorders are PPIs. While these medications are generally considered safe for short-term use, concerns regarding their long-term effects on bone health have been emerging. This umbrella review synthesized the available evidence from systematic reviews and meta-analyses to provide a comprehensive understanding of the associations between PPI use and alterations in bone metabolism. We could cover almost all outcomes related to bone metabolism.
Our findings highlight several associations of PPI use with bone-related outcomes. Predominantly, the evidence indicates a statistically significant, albeit modest, increased risk of fractures, including hip, spine, and wrist fractures, in individuals on PPIs. Notably, the fracture risk was found to be more pronounced in specific populations like children and post-menopausal women. These findings corroborate the concerns raised in earlier studies regarding the potential deleterious effects of acid suppression on bone health (Lespessailles and Toumi, 2022; Yu et al., 2008). The precise mechanisms underpinning this increased risk remain uncertain. However, it is postulated that long-term PPI use might interfere with calcium absorption due to the reduced stomach acid, thereby weakening bone strength (Ito and Jensen, 2010). Additionally, interference with osteoclast function, leading to altered bone remodelling, may play a role (Krüger et al., 2021). Besides fractures, our review identified a potential link between changes in BMD and PPI use. Although the mean differences in BMD across various bones were statistically significant in some studies, the clinical significance of these changes remains uncertain and necessitates further elucidation. It is worth noting that BMD is a crucial predictor of fracture risk, and even marginal reductions can culminate in clinically meaningful increases in fracture risk over time (Cefalu, 2004). Another most discussed is hypomagnesemia, a condition where blood magnesium levels are significantly reduced. Magnesium is not just an essential electrolyte for various physiological functions, but it also plays a pivotal role in bone health. Magnesium contributes to bone mineral density, serving as a cofactor in the enzymes that help deposit calcium into the bones. The etiology of PPI-induced hypomagnesemia is thought to be multifactorial, encompassing reduced intestinal absorption and increased renal magnesium wasting. A deficiency in magnesium can disrupt this balance, leading to weakened bones and an increased risk of fractures. Furthermore, magnesium deficiency has been linked to osteoporosis (Castiglioni et al., 2013). Given that PPI-induced hypomagnesemia might result in reduced magnesium availability for bone metabolism, the long-term use of these drugs could indirectly influence bone health. Interestingly, our review synthesized findings on a substantial risk of dental implant failure in PPI users. This underscores the broader implications of PPIs on skeletal health beyond the traditionally assessed outcomes. The underlying mechanisms for this observed association remain speculative but could be linked to altered bone metabolism and healing processes in PPI users (Rogoszinski et al., 2022). While there was a notable association between PPI use and outcomes related to bone health, the reliability of the existing evidence was deemed to be either very low or low for the majority of these outcomes as per the GRADE criteria. Such low certainty suggests that future research might change the estimates and our understanding. Several factors contribute to this uncertainty, including the high heterogeneity observed across the included reviews, and variations in study populations and designs. The extensive heterogeneity, in particular, makes interpretation challenging, as it hints at potential differences in study methodologies, populations, or both.
The adverse effects of PPIs are not limited to bone metabolism. For instance, A prior umbrella review highlighted a relation between the use of PPIs and various negative health effects, including those related to COVID-19, other infections, cardiovascular issues, bone-related complications, cancer, neurological, and renal problems (Veettil et al., 2022). In addition, another umbrella review explored the connection between PPI use and major adverse cardiovascular events (MACE), encompassing myocardial infarction, stroke and overall mortality (Teperikidis et al., 2023). The conclusions from these individual studies varied, with some indicating a direct relationship between MACE and PPI use, some finding no correlation, and some presenting inconclusive findings. Notably, a significant portion of the observational studies suggested a direct relation between PPI use and MACE.
Our findings together with previous reviews, highlight the importance of re-evaluating the risk-benefit profile of prescribing PPIs, particularly for extended durations (more than a year). The modest increase in fracture risk we identified, while statistically significant, carries different implications in clinical practice, depending on the risk factors of individual patients. These results should be interpreted within the context of each patient's overall fracture risk profileThe changes in BMD linked to PPI use, though statistically significant in some studies, may not lead to immediate clinical concerns. However, it's crucial to acknowledge that even minor reductions in BMD could, over time, result in a cumulative increase in fracture risks. Clinicians should be aware of the potential ramifications of PPIs on bone health, especially given the associations with increased fracture risks in vulnerable groups such as children and post-menopausal women, where even small decreases in bone density can have a significant impact on long-term bone health. It is crucial to weigh the therapeutic advantages against potential risks. PPIs, widely recognized for their efficacy in treating acid-related disorders, require careful consideration to ensure that the benefits of treatment adequately outweigh the risks. Tailored approaches and periodic re-assessment of PPI therapy are essential in managing the delicate balance between effective acid suppression and maintaining optimal bone health.
Periodic assessments of bone mineral density and serum magnesium levels in prolonged PPI users might be useful for early detection and intervention. The observed correlation between PPI use and dental implant failure adds another layer to clinical decision-making, necessitating additional counseling or alternative therapies for those undergoing dental procedures. Beyond bone health, the links between PPI use and other adverse health effects, including cardiovascular issues (Ariel and Cooke, 2019; Geng et al., 2023), underscore the need for a comprehensive approach in assessing the appropriateness of PPI therapy. A judicious and periodic re-evaluation of the necessity of ongoing PPI treatment, bearing in mind the potential cumulative risks, is advisable (Zhai et al., 2022).
Future research in this area should prioritize long-term, prospective studies to more clearly determine the causal relationship between PPI use and bone health outcomes. These studies need to encompass a range of diverse population groups and explore variations in PPI dosages and treatment durations. Additionally, there is a significant need for mechanistic studies to explore into how PPIs influence bone metabolism at the molecular and cellular levels, which could lead to new preventive strategies or alternative treatments. Complementing this, the use of real-world data in assessing the impact of PPIs across different clinical scenarios will offer a more comprehensive view of their effects on bone health. This approach will be particularly valuable in pinpointing patient groups more susceptible to adverse bone outcomes related to PPI use.
Our umbrella review has several strengths. Firstly, we undertook a comprehensive examination of all outcomes related to bone health associated with PPI use. Secondly, we employed the GRADE approach to assess the certainty of evidence, which ensures a systematic and rigorous evaluation of the available data. However, our review is not without limitations. As is inherent with all secondary research, our conclusions are bound by the quality and comprehensiveness of the primary systematic reviews we sourced. Moreover, the notable heterogeneity among the included studies points to potential variations in study design, participant populations, or both. These variations might raise concerns about the generalizability of our findings.
5. Conclusion
We could elucidate several potential adverse effects of PPIs on bone health, including increased risks of fractures, altered BMD, hypomagnesemia, and dental implant failure. These findings underscore the importance of the judicious use of PPIs, considering the potential risks against the benefits. Clinicians should be vigilant about the prolonged use of PPIs, especially in populations with already heightened fracture risks. Additionally, patients on long-term PPI therapy might benefit from regular bone health assessments. As the certainty of evidence for most outcomes remains low, further high-quality primary studies are essential to bolster our understanding of these associations and inform clinical practice more definitively.
Funding
This study received no funding.
CRediT authorship contribution statement
Abdullah S. Alanazi: Methodology, Investigation, Data curation, Conceptualization. Hadiah Almutairi: Project administration, Methodology, Investigation, Formal analysis. Jeetendra Kumar Gupta: Software, Resources, Project administration, Data curation, Conceptualization. Dibyalochan Mohanty: Validation, Supervision, Software, Investigation, Conceptualization. Deepankar Rath: Writing – review & editing, Investigation, Data curation. Ali A. AlOdan: Writing – review & editing, Supervision, Methodology, Data curation. Ahmed Mahal: Writing – review & editing, Supervision, Resources, Methodology. Mahalaqua Nazli Khatib: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Investigation, Formal analysis, Conceptualization. Shilpa Gaidhane: Writing – original draft, Visualization, Validation, Supervision, Data curation. Quazi Syed Zahiruddin: Software, Resources, Data curation, Conceptualization. Sarvesh Rustagi: Writing – review & editing, Supervision, Resources, Conceptualization. Prakasini Satapathy: Investigation, Formal analysis, Data curation, Conceptualization. Hashem Abu Serhan: Writing – review & editing, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The publication of this article was funded by Qatar National Library.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2024.101741.
Contributor Information
Abdullah S. Alanazi, Email: asdalananzi@ju.edu.sa.
Hadiah Almutairi, Email: hkalmutairi@uhb.edu.sa.
Jeetendra Kumar Gupta, Email: jk.gupta@gla.ac.in.
Dibyalochan Mohanty, Email: mohantypharmacy@anurag.edu.in.
Ahmed Mahal, Email: ahmed.mahal@cihanuniversity.edu.iq.
Sarvesh Rustagi, Email: sarveshrustagi@uumail.in.
Prakasini Satapathy, Email: prakasini.satapathy@gmail.com.
Hashem Abu Serhan, Email: habuserhan@hamad.qa.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Aggarwal A., Sharma M., Maisnam I., Ghosh S., Aggarwal S., Bhattacharya S., Dutta D. Drug-induced bone disorders: a systematic review. Indian J. Rheumatol. 2019;14(Suppl. 1):S44–S51. [Google Scholar]
- Aghaloo T., Pi-Anfruns J., Moshaverinia A., Sim D., Grogan T., Hadaya D. The effects of systemic diseases and medications on implant Osseointegration: a systematic review. International Journal of Oral & Maxillofacial Implants. 2019:34. doi: 10.11607/jomi.19suppl.g3. [DOI] [PubMed] [Google Scholar]
- Aguilera-Castro L., Martín-de-Argila-dePrados C., Albillos-Martínez A. Practical considerations in the management of proton-pump inhibitors. Rev. Esp. Enferm. Dig. 2016;108(3):145–153. doi: 10.17235/reed.2015.3812/2015. [DOI] [PubMed] [Google Scholar]
- Aleraij S., Alhowti S., Ferwana M., Abdulmajeed I., Mutawwam I.M. Effect of proton pump inhibitors on bone mineral density: a systematic review and meta-analysis of observational studies. Bone reports. 2020;13 doi: 10.1016/j.bonr.2020.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel H., Cooke J.P. Cardiovascular risk of proton pump inhibitors. Methodist Debakey Cardiovasc. J. 2019;15(3):214. doi: 10.14797/mdcj-15-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromataris E., Fernandez R.S., Godfrey C., Holly C., Khalil H., Tungpunkom P. 2014. Methodology for JBI Umbrella Reviews. [DOI] [PubMed] [Google Scholar]
- Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. JBI Evidence Implementation. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Cai D., Feng W., Jiang Q. Acid-suppressive medications and risk of fracture: an updated meta-analysis. Int. J. Clin. Exp. Med. 2015;8(6):8893. [PMC free article] [PubMed] [Google Scholar]
- Castiglioni S., Cazzaniga A., Albisetti W., Maier J.A. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5(8):3022–3033. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu C.A. Is bone mineral density predictive of fracture risk reduction? Curr. Med. Res. Opin. 2004;20(3):341–349. doi: 10.1185/030079903125003062. [DOI] [PubMed] [Google Scholar]
- Chappuis V., Avila-Ortiz G., Araújo M.G., Monje A. Medication-related dental implant failure: systematic review and meta-analysis. Clin. Oral Implants Res. 2018;29:55–68. doi: 10.1111/clr.13137. [DOI] [PubMed] [Google Scholar]
- Curtiss F.R. New generic and OTC drugs provide opportunities for drug benefit managers. Academy of Managed Care Pharmacy. 2002:520–521. doi: 10.18553/jmcp.2002.8.6.520a. [DOI] [PubMed] [Google Scholar]
- da Maia T.F., de Camargo B.G., Pereira M.E., de Oliveira C.S., Guiloski I.C. Increased risk of fractures and use of proton pump inhibitors in menopausal women: a systematic review and Meta-analysis. Int. J. Environ. Res. Public Health. 2022;19(20):13501. doi: 10.3390/ijerph192013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaine W.Y., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am. J. Med. 2011;124(6):519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom C.-S., Park S.M., Myung S.-K., Yun J.M., Ahn J.-S. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. The Annals of Family Medicine. 2011;9(3):257–267. doi: 10.1370/afm.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.-D., Ayom M.I.N., Sun W.-G., Yin P.-P., Wang X.-Y., Jia A., et al. An updated meta-analysis: the effect of proton pump inhibitor on risk of osteoporosis and fracture. Int. J. Clin. Exp. Med. 2017;10(11):15680–15695. [Google Scholar]
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. British Medical Journal Publishing Group; 2008. p. 2–3. [DOI] [PMC free article] [PubMed]
- Gandhi A.P., Shamim M.A., Padhi B.K. Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. The Evidence. 2023;1(1):44–59. [Google Scholar]
- Geng T., Chen J.-X., Zhou Y.-F., Lu Q., Wan Z., Liu L., et al. Proton pump inhibitor use and risks of cardiovascular disease and mortality in patients with type 2 diabetes. J. Clin. Endocrinol. Metabol. 2023;108(6):e216–e222. doi: 10.1210/clinem/dgac750. [DOI] [PubMed] [Google Scholar]
- Heidelbaugh J.J., Goldberg K.L., Inadomi J.M. Adverse risks associated with proton pump inhibitors: a systematic review. Gastroenterology & Hepatology. 2009;5(10):725. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F., Glaeske G., Schmiemann G. Increased prescribing of proton pump inhibitors in ambulatory care over the years 2005-2013. Zeitschrift fur Gastroenterologie. 2014;53(2):95–100. doi: 10.1055/s-0034-1384973. [DOI] [PubMed] [Google Scholar]
- Hussain S., Siddiqui A.N., Habib A., Hussain M.S., Najmi A.K. Proton pump inhibitors’ use and risk of hip fracture: a systematic review and meta-analysis. Rheumatol. Int. 2018;38(11):1999–2014. doi: 10.1007/s00296-018-4142-x. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Poly T.N., Walther B.A., Dubey N.K., Ningrum D.N.A., Shabbir S.-A., Li Y.-C.J. Adverse outcomes of long-term use of proton pump inhibitors: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018;30(12):1395–1405. doi: 10.1097/MEG.0000000000001198. [DOI] [PubMed] [Google Scholar]
- Ito T., Jensen R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B 12, iron, and magnesium. Curr. Gastroenterol. Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger T.B., Herlofson B.B., Lian A.M., Syversen U., Reseland J.E. Alendronate and omeprazole in combination reduce angiogenic and growth signals from osteoblasts. Bone Reports. 2021;14 doi: 10.1016/j.bonr.2021.100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C.S., Yeong J.K.-Y., Loke Y.K. Meta-analysis: risk of fractures with acid-suppressing medication. Bone. 2011;48(4):768–776. doi: 10.1016/j.bone.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Kwok C.S., Arthur A.K., Anibueze C.I., Singh S., Cavallazzi R., Loke Y.K. Risk ofClostridium difficileInfection with acid suppressing drugs and antibiotics: Meta-analysis. Official journal of the American College of Gastroenterology| ACG. 2012;107(7):1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- Lambert A.A., Lam J.O., Paik J.J., Ugarte-Gil C., Drummond M.B., Crowell T.A. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan D., Higgins J.P., Jackson D., Bowden J., Veroniki A.A., Kontopantelis E., et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res. Synth. Methods. 2019;10(1):83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- Langendam M.W., Akl E.A., Dahm P., Glasziou P., Guyatt G., Schünemann H.J. Assessing and presenting summaries of evidence in Cochrane reviews. Syst. Rev. 2013;2(1):1–9. doi: 10.1186/2046-4053-2-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespessailles E., Toumi H. Proton pump inhibitors and bone health: an update narrative review. Int. J. Mol. Sci. 2022;23(18):10733. doi: 10.3390/ijms231810733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xie X., Liu W., Gu F., Zhang K., Su Z., et al. Acid-suppressive drugs and risk of fracture in children and young adults: a meta-analysis of observational studies. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.712939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li X., Fan L., Yang J., Wang J., Sun J., Wang Z. Proton pump inhibitors therapy and risk of bone diseases: an update meta-analysis. Life Sci. 2019;218:213–223. doi: 10.1016/j.lfs.2018.12.058. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Kandulski A., Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 2017;14(12):697–710. doi: 10.1038/nrgastro.2017.117. [DOI] [PubMed] [Google Scholar]
- Mortensen S.J., Mohamadi A., Wright C.L., Chan J.J., Weaver M.J., von Keudell A., Nazarian A. Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif. Tissue Int. 2020;107:1–9. doi: 10.1007/s00223-020-00688-1. [DOI] [PubMed] [Google Scholar]
- Nassar Y., Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. Journal of bone metabolism. 2018;25(3):141–151. doi: 10.11005/jbm.2018.25.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamruengphong S., Leontiadis G.I., Radhi S., Dentino A., Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Official journal of the American College of Gastroenterology| ACG. 2011;106(7):1209–1218. doi: 10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- Paik J.M., Rosen H.N., Gordon C.M., Curhan G.C. Proton pump inhibitor use, H 2-receptor antagonist use, and risk of incident clinical vertebral fracture in women. Calcif. Tissue Int. 2018;103:380–387. doi: 10.1007/s00223-018-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poly T., Islam M., Yang H.-C., Wu C., Li Y.-C. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos. Int. 2019;30:103–114. doi: 10.1007/s00198-018-4788-y. [DOI] [PubMed] [Google Scholar]
- Rogoszinski T., Dazen C., Rekawek P., Coburn J.F., Carr B.R., Boggess W., et al. Are proton pump inhibitors associated with implant failure and peri-implantitis? Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133(1):15–20. doi: 10.1016/j.oooo.2021.05.002. [DOI] [PubMed] [Google Scholar]
- Sattayalertyanyong O., Thitilertdecha P., Auesomwang C. The inappropriate use of proton pump inhibitors during admission and after discharge: a prospective cross-sectional study. Int. J. Clin. Pharm. 2020;42:174–183. doi: 10.1007/s11096-019-00955-8. [DOI] [PubMed] [Google Scholar]
- Shamim M.A., Gandhi A.P., Dwivedi P., Padhi B.K. How to perform meta-analysis in R: a simple yet comprehensive guide. The Evidence. 2023;1(1):60–80. [Google Scholar]
- Srinutta T., Chewcharat A., Takkavatakarn K., Praditpornsilpa K., Eiam-Ong S., Jaber B.L., Susantitaphong P. Proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine. 2019;98(44) doi: 10.1097/MD.0000000000017788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperikidis E., Boulmpou A., Potoupni V., Kundu S., Singh B., Papadopoulos C. Does the long-term administration of proton pump inhibitors increase the risk of adverse cardiovascular outcomes? A ChatGPT powered umbrella review. Acta Cardiol. 2023;1-9 doi: 10.1080/00015385.2023.2231299. [DOI] [PubMed] [Google Scholar]
- Vaezi M.F., Choksi Y. Mucosal impedance: a new way to diagnose reflux disease and how it could change your practice. Am. J. Gastroenterol. 2017;112(1):4. doi: 10.1038/ajg.2016.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veettil S.K., Sadoyu S., Bald E.M., Chandran V.P., Khuu S.A.T., Pitak P., et al. Association of proton-pump inhibitor use with adverse health outcomes: a systematic umbrella review of meta-analyses of cohort studies and randomised controlled trials. Br. J. Clin. Pharmacol. 2022;88(4):1551–1566. doi: 10.1111/bcp.15103. [DOI] [PubMed] [Google Scholar]
- Verma V. Do proton pump inhibitors affect the biomechanical efficiency of implant?-a systematic review. J. Oral Biol. Craniofac. Res. 2022;12(5):656–661. doi: 10.1016/j.jobcr.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard P. Drugs causing bone loss. Bone regulators and osteoporosis. Therapy. 2020:475–497. doi: 10.1007/164_2019_340. [DOI] [PubMed] [Google Scholar]
- Vinnakota D.N., Kamatham R. Effect of proton pump inhibitors on dental implants: a systematic review and meta-analysis. The Journal of the Indian Prosthodontic Society. 2020;20(3):228. doi: 10.4103/jips.jips_283_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhou T.-j., Yang J., Bao D.-n. Use of acid-suppressive drugs and risk of fracture in children and young adults: a meta-analysis of observational studies. Eur. J. Clin. Pharmacol. 2022:1–9. doi: 10.1007/s00228-021-03245-3. [DOI] [PubMed] [Google Scholar]
- Ye X., Liu H., Wu C., Qin Y., Zang J., Gao Q., et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2011;23(9):794–800. doi: 10.1097/MEG.0b013e328348a56a. [DOI] [PubMed] [Google Scholar]
- Yu E.W., Blackwell T., Ensrud K.E., Hillier T.A., Lane N.E., Orwoll E., Bauer D.C. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif. Tissue Int. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C., Neogi T., Chan A.T., Wei J., Misra D., Lu N., et al. Proton pump inhibitor therapy and risk of knee replacement surgery: a general population-based cohort study. Osteoarthr. Cartil. 2022;30(4):559–569. doi: 10.1016/j.joca.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Ye X., Hu F., Xu J., Guo X., Lin Z., et al. Updated insights on cardiac and vascular risks of proton pump inhibitors: a real-world pharmacovigilance study. Frontiers in Cardiovascular Medicine. 2022;9 doi: 10.3389/fcvm.2022.767987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Deng D., Zhang R., Yi J., Dong J., Sha L. Relationship between proton pump inhibitors and adverse effects in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press. Res. 2022;47(9):545–555. doi: 10.1159/000526122. [DOI] [PubMed] [Google Scholar]
- Zhou B., Huang Y., Li H., Sun W., Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos. Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.