Abstract
Purpose
The incidence of heatstroke (HS) is not particularly high; however, once it occurs, the consequences are serious. It is reported that calcitonin gene-related peptide (CGRP) is protective against brain injury in HS rats, but detailed molecular mechanisms need to be further investigated. In this study, we further explored whether CGRP inhibited neuronal apoptosis in HS rats via protein kinase A (PKA)/p-cAMP response element-binding protein (p-CREB) pathway.
Methods
We established a HS rat model in a pre-warmed artificial climate chamber with a temperature of (35.5 ± 0.5) °C and a relative humidity of 60% ± 5%. Heatstress was stopped once core body temperature reaches above 41 °C. A total of 25 rats were randomly divided into 5 groups with 5 animals each: control group, HS group, HS+CGRP group, HS+CGRP antagonist (CGRP8-37) group, and HS+CGRP+PKA/p-CREB pathway blocker (H89) group. A bolus injection of CGRP was administered to each rat in HS+CGRP group, CGRP8-37 (antagonist of CGRP) in HS+CGRP8-37 group, and CGRP with H89 in HS+CGRP+H89 group. Electroencephalograms were recorded and the serum concentration of S100B, neuron-specific enolase (NSE), neuron apoptosis, activated caspase-3 and CGRP expression, as well as pathological morphology of brain tissue were detected at 2 h, 6 h, and 24 h after HS in vivo. The expression of PKA, p-CREB, and Bcl-2 in rat neurons were also detected at 2 h after HS in vitro. Exogenous CGRP, CGRP8-37, or H89 were used to determine whether CGRP plays a protective role in brain injury via PKA/p-CREB pathway. The unpaired t-test was used between the 2 samples, and the mean ± SD was used for multiple samples. Double-tailed p < 0.05 was considered statistically significant.
Results
Electroencephalogram showed significant alteration of θ (54.50 ± 11.51 vs. 31.30 ± 8.71, F = 6.790, p = 0.005) and α wave (16.60 ± 3.21 vs. 35.40 ± 11.28, F = 4.549, p = 0.020) in HS group compared to the control group 2 h after HS. The results of triphosphate gap terminal labeling (TUNEL) showed that the neuronal apoptosis of HS rats was increased in the cortex (9.67 ± 3.16 vs. 1.80 ± 1.10, F = 11.002, p = 0.001) and hippocampus (15.73 ± 8.92 vs. 2.00 ± 1.00, F = 4.089, p = 0.028), the expression of activated caspase-3 was increased in the cortex (61.76 ± 25.13 vs. 19.57 ± 17.88, F = 5.695, p = 0.009) and hippocampus (58.60 ± 23.30 vs. 17.80 ± 17.62, F = 4.628, p = 0.019); meanwhile the expression of serum NSE (5.77 ± 1.78 vs. 2.35 ± 0.56, F = 5.174, p = 0.013) and S100B (2.86 ± 0.69 vs. 1.35 ± 0.34, F = 10.982, p = 0.001) were increased significantly under HS. Exogenous CGRP decreased the concentrations of NSE and S100B, and activated the expression of caspase-3 (0.41 ± 0.09 vs. 0.23 ± 0.04, F = 32.387, p < 0.001) under HS; while CGRP8-37 increased NSE (3.99 ± 0.47 vs. 2.40 ± 0.50, F = 11.991, p = 0.000) and S100B (2.19 ± 0.43 vs. 1.42 ± 0.30, F = 4.078, p = 0.025), and activated the expression caspase-3 (0.79 ± 0.10 vs. 0.23 ± 0.04, F = 32.387, p < 0.001). For the cell experiment, CGRP increased Bcl-2 (2.01 ± 0.73 vs. 2.15 ± 0.74, F = 8.993, p < 0.001), PKA (0.88 ± 0.08 vs. 0.37 ± 0.14, F = 20.370, p < 0.001), and p-CREB (0.87 ± 0.13 vs. 0.29 ± 0.10, F = 16.759, p < 0.001) levels; while H89, a blocker of the PKA/p-CREB pathway reversed the expression.
Conclusions
CGRP can protect against HS-induced neuron apoptosis via PKA/p-CREB pathway and reduce activation of caspase-3 by regulating Bcl-2. Thus CGRP may be a new target for the treatment of brain injury in HS.
Keywords: Heatstroke, Calcitonin gene-related peptide, Brain injury, Apoptosis
1. Introduction
Heatstroke (HS) is a life-threatening heat-related disease. If multiple organ dysfunction syndrome develops, the mortality can reach 40% – 70%.1 As an important type of multiple organ dysfunction syndrome, about 80.3% – 100% of these patients are complicated with brain injury.2 However, there is a lack of prevention and treatment of brain injury under HS. Neuropeptides are signaling molecules in the central nervous system and involved in a variety of biological effects, including morphine peptide, enkephalin, corticotropin-releasing hormone, cholecystokinin, somatostatin, neuropeptide Y, and vasoactive intestinal peptide in the prefrontal cortex.3,4 Meanwhile, calcitonin gene-related peptide (CGRP) is a 37-amino acid novel endogenous neuroactive peptide, which is produced mainly by neurons and coexists with acetylcholine as a neuromodulator at the nerve-muscle junction and plays a neuroregulatory role related to brain injury.5 CGRP receptor is a G-protein-coupled receptor with 7 transmembrane functional domains. Recent studies have shown that CGRP can rapidly activate adenylate cyclase, enrich intracellular cAMP, and up-regulate intracellular protein kinase A (PKA). PKA phosphorylates the downstream cAMP response element-binding protein (CREB), of which PKA/p-CREB pathway plays an important role in chronic migraine and Alzheimer.6,7 Neurons release a variety of substances under HS, including neuropeptides and neurotransmitters, but whether they can play a protective role in brain injury is worth further investigation. Our previous studies have shown that CGRP is protective against brain injury in HS rats,8 while the insight into molecular mechanism is unclear. This study aims to further explore the potential mechanisms of CGRP's protective effect on brain injury of rats with HS.
2. Methods
2.1. Animals
Twenty-five male Wistar rats weighing 200 – 300 g, aged 10 – 12 weeks purchased from Southern Medical University, Guangzhou, China were used in this experiment. The rats were housed in controlled environmental conditions (12 h light/dark cycle, humidity 35% ± 5%, and temperature 25 °C) at the Experimental Animal Center of General Hospital of Southern Theatre Command of PLA and were given free access to standard laboratory chow and water. The study was approved by the animal ethics committee of the General Hospital of Southern Theatre Command of PLA.
2.2. Rats HS model and cooling treatment
The model of heat-stressed rats were established according to our previously reported method.7 A total of 25 rats were randomly divided into 5 groups with 5 animals each: control group, HS group, HS+CGRP group (2 μg/mL, Abcam, Cambridge, MA, USA), HS+CGRP8-37 group (30 nmol/kg, Sigma-Aldrich; Merck KGaA; Darmstadt, Germany), and HS+CGRP+H89 group (10 μmol/L, Abcam, Cambridge, MA, USA). CGRP8-37 is an antagonist of CGRP, and H89 is a PKA/p-CREB pathway blocker. Rats in the control group were maintained at (25.0 ± 0.5) °C and humidity of 35% ± 5%. In HS group, rats were placed in a pre-warmed incubator at (35.5 ± 0.5) °C and relative humidity of 60% ± 5% in the absence of food and water. The rectal core temperature (Tc) was continuously monitored. Rats were removed from the incubator and cooled at an ambient temperature of (25.0 ± 0.5) °C for 2 h, 6 h, and 24 h, after the Tc reached 41 °C.
2.3. Cell culture and treatment
Wistar rat cortical neuronal cells were purchased in Shanghai Saiqi Biotechnology Co., LTD and cultured in an incubator at 37 °C with 5% CO2. Experiments are performed after cell culture to the logarithmic growth phase. Culture dishes containing cells in a 37 °C cell incubator as a control group, and the experimental group is maintained at 43 °C for 2 h and then rewarmed at 37 °C for 24 h.
2.4. Drug treatment
All rats were injected intraperitoneally with sodium pentobarbital (50 mg/kg, Sigma-Aldrich, Merck KGaA) to eliminate the pain reflex. For the animal experiment, CGRP (2 μg/mL, 0.5 mL) and CGRP8-37 (30 nmol/kg, 0.5 mL) were administered via a carotid cannula (24-gauge) before the onset of HS. A comparable volume of normal saline was injected into the rats in the control group. Electroencephalogram (EEG) was examined on all rats 2 h after heating (duration: 30 mm/s, Gain: 0.5 cm = 50 μV). Further histopathological analysis and tests were performed. The cell experiments were divided into the control group, HS group, HS+CGRP group, HS+CGRP+CGRP8-37 group, and HS+CGRP+H89 group. In HS+CGRP+CGRP8-37 group and HS+CGRP+H89 group, CGRP8-37 (10−7 mol/L) and H89 (10 μmol/L, Abcam) were pretreated for 0.5 h, and then 10−6 mol/L CGRP was pretreated for 1 h.
2.5. Brain tissue sampling
The rats were sacrificed with a decapitation device, and the samples used for the histopathological test were fixed in 10% neutral formaldehyde fixation solution for 24 – 48 h. The paraffin-embedded tissue was cut into 3 μm thickness for subsequent hematoxylin-eosin (HE) staining, immunohistochemistry, and triphosphate gap terminal labeling (TUNEL). Liquid nitrogen was added to 50 mg of fresh brain tissue and ground. One milliliter of radio immunoprecipitation assay lysis buffer was then added to the ground powder to homogenize it, followed by chilling on ice for 20 min, centrifuging at 4 °C and 13,000 rpm for 20 min. The supernatant was then tested for protein quantification after centrifugation.
2.6. HE staining
The paraffin-embedded brain tissue block was coronally sectioned with a thickness of about 3 μm, and conventionally dewaxed. Then the following processes were: (1) hematoxylin staining for about 5 min, and rinsed with tap water; (2) differentiating with 1% hydrochloric acid and alcohol for 30 s, and rinsed with tap water; (3) differentiating with 1% ammonia back to blue for 2 s, and then rinse with tap water; and (4) 0.5% eosin staining for 30 s. Dehydration, transparency, sealing, microscope observation were performed.
2.7. Immunohistochemistry
The sections on the slides were heated at 60 °C for 2 h followed by incubations with xylene and rehydration through a graded ethanol series, then which were subjected to antigen retrieval (citrate buffer) and block (5% normal goat serum), and incubated overnight at 4 °C with the anti-activated caspase-3 (9446, Cell Signaling Technology, Massachusetts, MA) and anti-CGRP antibody (Ab47027, Abcam, Cambridge, UK;1:500 dilution). Sections were incubated with the secondary goat anti-rabbit antibody conjugated by horseradish peroxidase and using diaminobenzidine rendering. After washing the slides, hematoxylin was added as a counterstain. Slides were dehydrated with sequential ethanol washes. After sealing the slides, the sections were analyzed by optical microscopy. The mean optical density of each section was measured using Image-Pro Plus (Media Cybernetics) from 4 different fields of view. The medical image quantitative analysis system was applied to determine the area ratio (positive area/total area) and optical density of the positive expression products in the sliced cerebral cortex and hippocampus. Immunohistochemical positivity index = area ratio of positive areas × optical density (OD). The size of the immunohistochemical positivity index reflects the amount of reaction products. Three fields of view were examined per section and the average was taken.
2.8. TUNEL assay
TUNEL assay kit (Nanjing KeyGen Biotech, Nanjing, China) was used to detect neuron apoptosis. The samples were then counterstained with hematoxylin. Apoptosis was observed using a microscope (Olympus, Tokyo, Japan), and 3 visual fields of view were randomly selected to count the cells with positive staining.
2.9. Enzyme-linked immunosorbent assay (ELISA)
The levels of serum neuron-specific enolase (NSE) and S100B were assessed by the ELISA kit (Shanghai Gefan Biotechnology Co, LTD, China), and the method described by the manufacturer was followed. The OD value of each well was read at 450 nm by a Thermo spectrophotometer after the final color rendered. Taking the concentration of standard as abscissa and the OD value of standard as ordinate, the standard curve was drawn by a computer software package to calculate and determine the concentration of the sample.
2.10. Real-time quantitative polymerase chain reaction (PCR)
Total RNA (1 μg) was collected by RNeasy kit (QIAGEN) for reverse transcriptase reaction with SuperScript reverse transcriptase kit (LifeTechnologies). Thermal cycling reaction was carried out in an abi-7500 real-time quantitative fluorescent PCR machine (Applied Biosystems) using SYBR Green real-time quantitative fluorescent PCR kit (Life Technologies). The qPCR analysis was performed in 3 replicates as follows: CGRP: (forward: 5’ - CTTAGAAAGCAGCCCAGGC -3’; reverse: 5’ -CAAAGTTGTCCTTCACCACC -3’).
2.11. Western blot analysis
Protein samples were prepared from rat's brain tissues of each group using a lysis buffer (9803, Cell Signaling Technology, Massachusetts, MA). Protein samples (40 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. Protein expression was determined using specific antibodies to activated caspase-3 (9664), β-actin (4970), PKA (5842T), p-CREB (9198s) and Bcl-2 (3498T; all from Cell Signaling Technology, Massachusetts, MA and with a 1:1000 dilution), and analyzed using the grayscale.
2.12. Flow cytometric analysis
Cell apoptosis was measured by flow cytometry using propidium iodide/annexin V Apoptosis kit (Invitrogen, USA). Cells were re-suspended in the binding buffer at 1 × 106 cells/well. One hundred microliters of the solution were transferred to the culture tube, and 0.5 μl of propidium iodide and 5 μl annexin V were added. After the cells were incubated for 15 min at room temperature in the dark, cell apoptosis was analyzed by flow cytometry (BD FACSCalibur).
2.13. Statistical analysis
If the data fit the normal distribution, the unpaired t-test was used between the 2 samples, and the mean ± SD was used for multiple samples. The data were analyzed using SPSS19.0 statistical software. Double-tailed p < 0.05 was considered statistically significant.
3. Results
3.1. HS-induced brain injury in rats
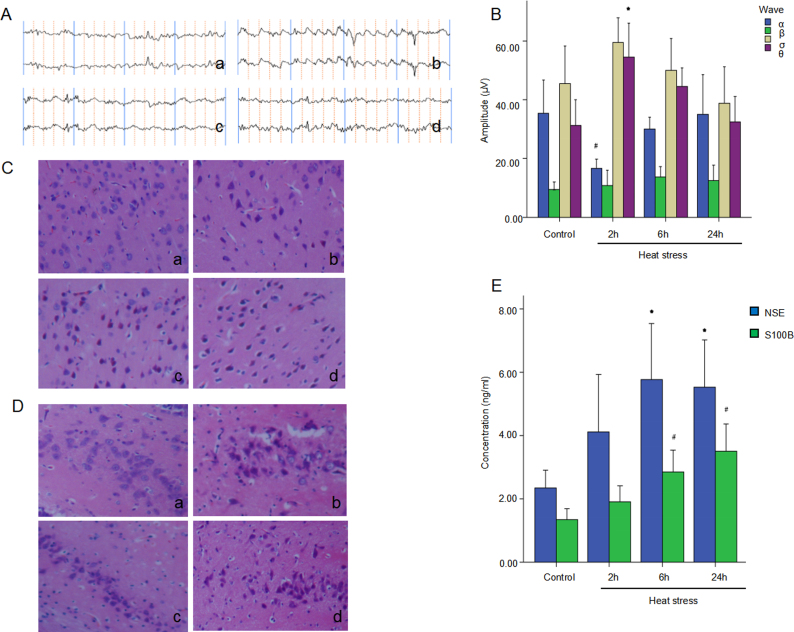
After HS and cooling treatments, EEG, HE staining, and ELISA were performed to evaluate the injury degree in the cerebral cortex and hippocampus. The corresponding neurological injuries were graded and the scores were assigned in a blinded manner by 2 certified veterinary pathologists. HS increased the number and amplitude of slow waves in the EEG of rats, compared to the control group. And at 2 h after heat, the EEG amplitude significantly changed, lasting at 5 Hz, 40 – 65 μV θ wave. The amplitude was significantly increased than that of the control group (p = 0.005). The amplitude of wave δ increased, while wave α significantly decreased (p = 0.020) (Fig. 1A and B). HS induced injuries in the cerebral cortex and hippocampus neurons are characterized by a decrease in the number of Nissl bodies with an uneven distribution. Gaps appeared around nerves and blood vessels at 2 h after HS with HE staining. At 6 h and 24 h, the lesions were further aggravated, the neurons shrank, part of the nuclear membrane disappeared, and cell necrosis appeared (Fig. 1C and D). NSE is a glycolytic enzyme that is specifically concentrated in neurons, while S100 is an acidic soluble protein that is mainly concentrated in astrocytes and oligodendrocytes. When brain injury occurs, NSE and S100 proteins are released. Therefore, serum NSE and S100B are considered markers of brain injury. HS increased the serum NSE and S100B at 6 h and 24 h significantly (p < 0.05) (Fig. 1E).
Fig. 1.
Heatstroke induces brain injury in rats. Male Wistar rats were subject to heat stress, and the rats were then cooled at an ambient temperature of (25 ± 0.5) °C for 2, 6, 24 h after the Tc reached 41 °C. Rats in the control group were maintained at (25 ± 0.5) °C and a humidity of 35% ± 5%. (A & B) EEG waves and corresponding quantifications of 4 waves were calculated and shown. ∗p < 0.05 vs. control group, #p < 0.05 vs. control group. (C & D) HE staining of cerebral cortex and hippocampus are shown, respectively. (E) The levels of Serum NSE and S100B in each group are shown. ∗p < 0.05 vs. control group, #p < 0.05 vs. control group.
Tc: core temperature; EEG: electroencephalogram; HE: hematoxylin-eosin; NSE: neuron-specific enolase.
3.2. HS-induced neuron apoptosis
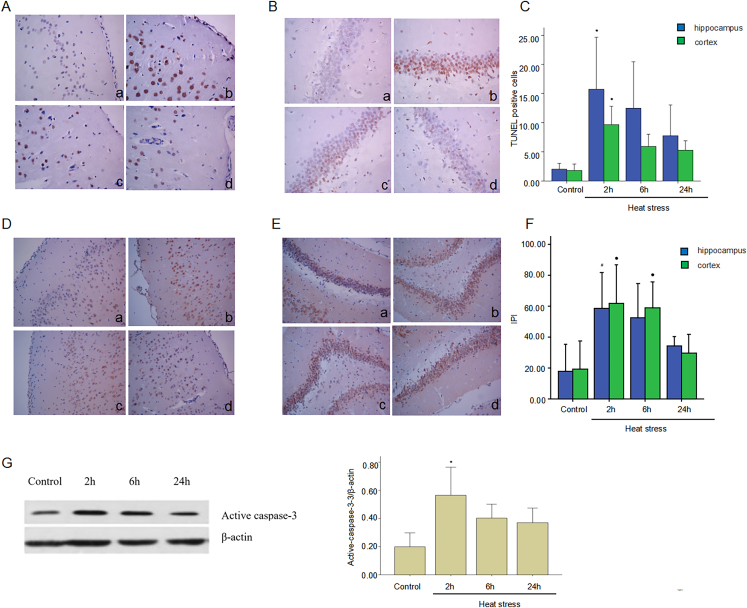
TUNEL and immunohistochemistry were used to assess neuron apoptosis. Compared with the control group, a large number of apoptotic cells were observed in both cortex and hippocampus at 2 h after HS (p < 0.05) (Fig. 2A–C). Caspase is an intracellular cysteine protease involved in the regulation of cell death and inflammation. Activated caspase-3 is a major mediator of both apoptosis and necrosis. Immunohistochemistry results showed activated caspase-3 expression increased in the cortex and hippocampus reaching a peak at 2 h that gradually decreased. The activated caspase-3 expression difference was statistically significant (p < 0.05) (Fig. 2D–F). Increased activated caspase-3 expression was also evident in HS rats' brain tissue by western blot analysis suggesting that HS significantly increased neuron apoptosis (Fig. 2G).
Fig. 2.
Heatstroke induces neuron apoptosis. Heatstroke rat model was prepared with a Tc that reached 41 °C with subsequent cooling at ambient temperature for 2 h, 6 h, and 24 h (A & B) TUNEL was used to detect the apoptosis of cerebral cortex and hippocampus. (C) Corresponding quantifications of TUNEL positive cells were calculated and are shown. ∗p < 0.05 vs. control group. (D & E) Active caspase-3 expression was detected by immunohistochemistry in cerebral cortex and hippocampus. Corresponding quantifications of positive cells were calculated and are shown in F. ∗p < 0.05 vs. control group. ∗p < 0.05 vs. control group, #p < 0.05 vs. control group. (G) Expression level of active caspase-3 was detected by western blot. Left: diagram of gel electrophoresis. Right: statistical graph. ∗p < 0.05 vs. control group.
Tc: core temperature; TUNEL: triphosphate gap terminal labeling; IPI: immunohistochemical positive index.
3.3. CGRP alleviated brain injury after HS
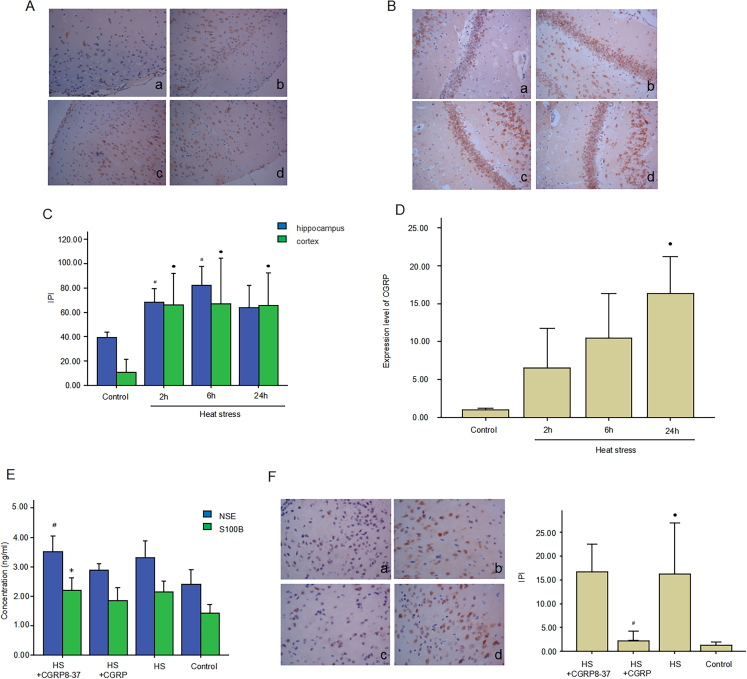
CGRP expression was detected by immunohistochemistry and real-time fluorescence quantitative PCR. The results showed that CGRP protein (Fig. 3A–C) and mRNA (Fig. 3D) levels increased in the HS group at 2 h in both cortex and hippocampus, with a peak appearing at 6 h in HS group compared to the control group. The difference was statistically significant (p < 0.05). To explore the protective role of CGRP in brain injury under HS, we administrated the CGRP antagonist and CGRP8-37 via carotid cannula. To further verify the protective effect of CGRP in brain injury under HS, CGRP8-37 as a kind of CGRP antagonist, was administrated on experiment rats via a carotid cannula. We used the ELISA to test the concentration of NSE and S100B in rat serum, and western blot to determine the expressions of activated caspase-3 in rat brain. As shown in Fig. 3E, serum NSE and S100B level significantly decreased in the HS+CGRP group, while significantly increased in HS+CGRP8-37 group (p < 0.05). As shown in Fig. 3F and G, activated caspase-3 expression had the same change tested by immunohistochemistry.
Fig. 3.
CGRP alleviates brain injury in heatstroke. (A & B) CGRP expression was detected by immunohistochemistry in cerebral cortex and hippocampus. (C) Corresponding quantifications of positive cells were calculated. ∗p < 0.05 vs. control group, #p < 0.05 vs. control group. (D) CGRP mRNA expression was detected by qPCR. ∗p < 0.05 vs. control group. (E) Serum NSE and S100B were detected by ELISA. ∗p < 0.05 vs. control group, #p < 0.05 vs. HS group. (F) Active caspase-3 expression was detected by immunohistochemistry in cerebral cortex of Control, HS, HS+CGRP, HS+CGRP8-37 group rats. (G) Corresponding quantifications of positive cells were calculated and shown. ∗p < 0.05 vs. control group, #p < 0.05 vs. HS group.
CGRP: calcitonin gene-related peptide; NSE: neuron-specific enolase; ELISA: Enzyme linked immunosorbent assay; HS: heatstroke.
3.4. CGRP inhibited neuron apoptosis via PKA/p-CREB
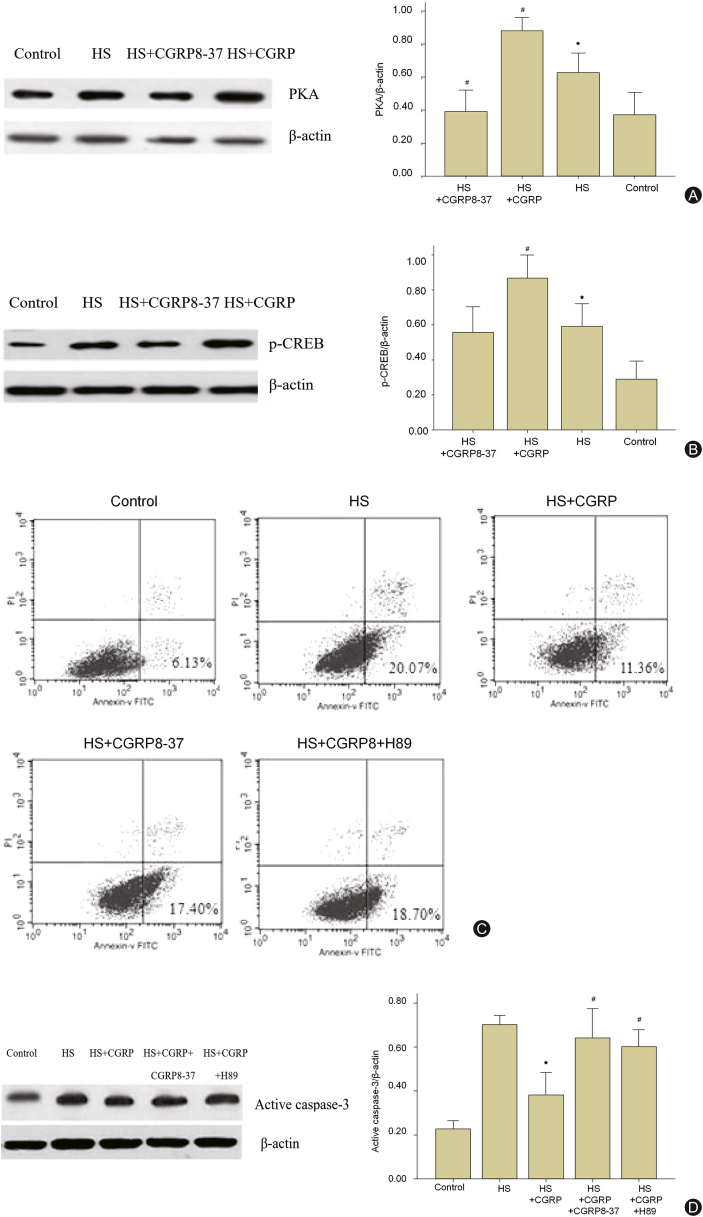
CGRP binds to the receptor and activates adenylate cyclase, resulting in intracellular cAMP enrichment and then signaling via PKA. As a member of the leucine-rich zipper-like structure superfamily, p-CREB can be activated by PKA. Hence, PKA/pCREB may involve in neuron apoptosis regulated by CGRP. To explore the intrinsic mechanism of CGRP inhibiting neuron apoptosis in HS, in the following experiment, we detected the expression of PKA and p-CREB in Wistar rat cortical neuronal cells in vitro by western blot. As shown in Fig. 4A and B, the expression of PKA and p-CREB increased after HS and more increased in HS+CGRP group, which could be reversed by CGRP antagonist and GRP8-37. Meanwhile, to further clarify the effect of PKA/CREB on cell fate in a high temperature and humidity simulated in HS environment, the Wistar rat cortical neurons were pretreated with 10 μmol/L H89 before HS simulation. Flow cytometry results showed apoptosis rate of the neuron was increased in the control group significantly, but decreased in HS+CGRP group after HS. Interestingly, the neuron apoptosis both in CGRP+CGRP8-37 group and CGRP+H89 group were at a high level compared with the CGRP group, which meant both CGRP8-37 and H89 could abolish the anti-apoptotic effect of CGRP (Fig. 4C). Activated caspase-3 expression had the same change, suggesting that CGRP plays an inhibitory effect on neuron apoptosis via PKA/p-CREB pathway (Fig. 4D).
Fig. 4.
CGRP inhibits neuron apoptosis via PKA/p-CREB. (A) Expression of PKA on cerebral cortex neurons in Wistar rats in each group detecting by western blot. Left: diagram of gel electrophoresis. Right: statistical graph. (B) Expression of p-CREB on Wistar rat cortical neuronal cells in each group detecting by western blot. Left: diagram of gel electrophoresis. Right: statistical graph. ∗p < 0.05 vs. control group, #p < 0.05 vs. HS group. (C) Apoptosis rate was detected by flow cytometry. A1-A5 corresponded to control group, HS group, HS+CGRP group, HS+CGRP8-37 group, and HS+CGRP+H89 group. (D) Expression of active caspase-3 on Wistar rat cortical neuronal cells in each group was detected by western blot. Left: diagram of gel electrophoresis. Right: statistical graph. ∗p < 0.05 vs. control group, #p < 0.05 vs. HS group.
CGRP: calcitonin gene-related peptide; CREB: cAMP response element-binding protein; PKA: protein kinase A; HS: heatstroke.
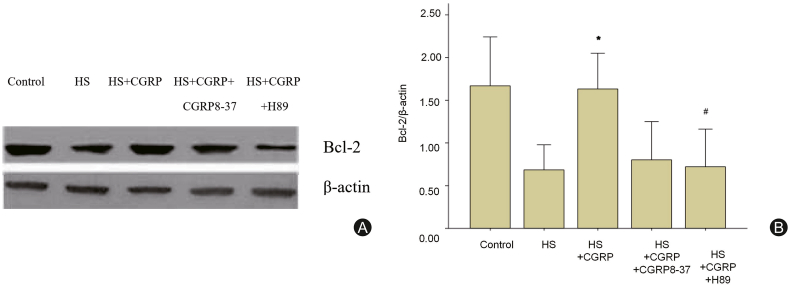
3.5. CGRP affected Bcl-2/caspase-3 mediated neuron apoptosis via PKA/p-CREB pathway
Caspase-3 is regulated by several genes including Bcl-2, which is an important anti-apoptotic protein and can be activated by CREB. To further explore the mechanism of CGRP in regulating neuron apoptosis, Bcl-2 expression was assessed by western blot. The expression of Bcl-2 in HS+CGRP group was significantly increased compared with that in HS group (p < 0.05), while the enhanced Bcl-2 was reversed in HS+CGRP+H89 group (p < 0.05). It indicated that blocking PKA/p-CREB pathway could affect the expression of Bcl-2, which suggested that CGRP might regulate the expression of Bcl-2 via PKA/p-CREB (Fig. 5).
Fig. 5.
CGRP affects the expression of Bcl-2, which is involved in inhibiting Wistar rat cortical neurons apoptosis. Expression of Bcl-2 on brain tissue in control group, HS group, HS+CGRP group, HS+CGRP8-37 group, HS+CGRP+H89 group was detected by western blot. ∗p < 0.05 vs. control group, #p < 0.05 vs. HS group.
CGRP: calcitonin gene-related peptide; HS: heatstroke.
4. Discussion
This study mainly explored the protective effect of CGRP and its molecular mechanism on brain injury in HS rats, and we found that CGRP may protect from neuron apoptosis via PKA/p-CREB signaling and then depressed caspase-3 activation. Thus CGRP may be a new target for the treatment of brain injury in HS.
HS is a systemic inflammatory response related to heat, which can be caused not only by exposure to a high temperature but also by vigorous exercise of muscles.9 It can cause multiple organ dysfunction and failure, characterized by central nervous system damage.10 Since the brain is sensitive to hyperthermia, a high-temperature environment can enhance the brain metabolic rate, reduce the blood flow in the brain, and increase the permeability of the blood-brain barrier during HS, so inflammatory factors and pathogens are prone to enter the brain tissue. Behavioral and mental changes even occur in the early stage and result in death from a cerebral hemorrhage in the later stage.12,11 Recent documents indicated that as the strongest vasodilator-activated peptide in vivo and having a wide range of effects, CGRP has a neuroprotective effect in various types of brain injury and ischemia injury of multiple organs.14,13 Most studies on CGRP focused on post-traumatic brain injury, and some studies showed that the level of serum CGRP was negatively correlated with the degree of post-traumatic brain injury.15 It could alleviate brain injury by inhibiting neuron apoptosis and autophagy.16 Meanwhile, the increase of CGRP was related to the reduction of cerebral vasospasm and good prognosis.17 Studies showed that CGRP played a self-protective role in sepsis and other brain injuries. Therefore this study confirmed that CGRP had protective effects in severe HS-induced brain injury, which was involved in inhibiting neuron apoptosis via PKA/p-CREB pathway.
Our previous study has shown that CGRP could reduce neuron apoptosis, thereby alleviating brain injury caused by HS, but its molecular mechanism was still unknown.8 In this study, we used EEG, histochemistry, and serum brain injury biomarkers to examine the damaging effect of HS on brain tissue. The EEG of rats showed a prolonged θ wave of 5 Hz, 40–65 μV, and the amplitude increased significantly. We found hippocampus and cerebral cortex neurons swelled, part of the nuclear membrane disappeared, and cells apoptosis. Accordingly, serum markers of brain injury NSE and S100B increased significantly in this study. Given CGRP inhibits apoptosis of intestinal epithelium and reduces the level of caspase-3 in intestinal ischemia-reperfusion injury in rats.18 We further explored whether CGRP affected caspase-3 and neuron apoptosis in HS. Activated caspase-3 increased in the cortex and hippocampus of rats under HS, while CGRP could reduce neuron apoptosis and the serum level of NSE and S100B, which were biomarkers of brain injury.19 According to other research, CGRP rapidly activates adenylate cyclase after binding to its receptor, resulting in intracellular cAMP enrichment, intracellular PKA up-regulation, and then phosphorylation of downstream CREB.20 Therefore, we further explored the relationship between the PKA/p-CREB pathway and neuron apoptosis. In our study, CGRP antagonist CGRP8-37 reversed the anti-apoptotic effect of CGRP. Moreover, the addition of PKA/p-CREB pathway blocker H89 increased neuron apoptosis and reduced the expression of anti-apoptotic protein Bcl-2, which then affected the expression of activated caspase-3.
In conclusion, this study confirmed the brain injury in HS and the protective effect of CGRP on brain injury in HS rats. This effect maybe caused by the influence of the PKA/p-CREB pathway on Bcl-2 and thus reducing the activation of caspase-3, and ultimately decreasing neuron apoptosis. However, as a neuroprotective protein, expression of CGRP in HS was not completely consistent with the changes in brain injury degree, indicating CGRP was not the only protective factor. Whether CGRP can be used as an early diagnostic marker of brain injury in HS is worth further investigation. And in vivo, whether CGRP can affect vascular endothelial cells, and then affect ischemia-reperfusion injury and coagulation dysfunction caused by HS is still unclear. Further studies are worth being done to determine the role and the regulating mechanisms of CGRP in brain injury under HS.
Funding
This work was supported by grants from the grants from Natural Science Foundation of Guangdong Province of China [NO. 2021A1515010170], the grants from the PLA Logistics Research Project of China [2022-JCJQ-ZD-097-12, 2022-JCJQ-ZQ-019].
Ethical statement
The study was approved by the Research Ethics Commission of General Hospital of Southern Theater Command of PLA and the requirement for informed consent was waived by the Ethics Commission.
Declaration of competing interest
The authors declare that they have no competing interests.
Author contributions
Study concept and design (Zhi-Feng Liu, Cheng-Xiang Lu), data collecting (Jie Zhu, Ya-Hong Chen, Jing-Jing Ji, Cheng-Xiang Lu), statistical analysis (Zhi-Feng Liu, Cheng-Xiang Lu, Jie Zhu), manuscript drafting (Jie Zhu, Zhi-Feng Liu).
Acknowledgments
This work was supported by grants from the grants from Natural Science Foundation of Guangdong Province of China [NO. 2021A1515010170], the grants from the PLA Medical Research Projects of China [2022-JCJQ-ZD-097-12, 2022-JCJQ-ZQ-019].
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Abriat A., Brosset C., Brégigeon M., et al. Report of 182 cases of exertional heatstroke in the French Armed Forces. Mil Med. 2014;179:309–314. doi: 10.7205/MILMED-D-13-00315. [DOI] [PubMed] [Google Scholar]
- 2.Ji J.J., Liu Z.F., Hong X.X., et al. Protective effects of rolipram on endotoxic cardiac dysfunction via inhibition of the inflammatory response in cardiac fibroblasts. BMC Cardiovasc Disord. 2020;20:242. doi: 10.1186/s12872-020-01529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockway D.F., Crowley N.A. Turning the 'tides on neuropsychiatric diseases: the role of peptides in the prefrontal cortex. Front Behav Neurosci. 2020;14 doi: 10.3389/fnbeh.2020.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casello S.M., Flores R.J., Yarur H.E., et al. Neuropeptide system regulation of prefrontal cortex circuitry: implications for neuropsychiatric disorders. Front Neural Circ. 2022;16 doi: 10.3389/fncir.2022.796443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos-Lasaosa S., Belvís R., Cuadrado M.L., et al. Calcitonin gene-related peptide in migraine: from pathophysiology to treatment. Neurologia. 2022;37:390–402. doi: 10.1016/j.nrleng.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Tan J., Yang L., Wang P.F., et al. Exogenous CGRP regulates apoptosis and autophagy to alleviate traumatic brain injury through Akt/mTOR signalling pathway. Neurochem Res. 2020;45:2926–2938. doi: 10.1007/s11064-020-03141-9. [DOI] [PubMed] [Google Scholar]
- 7.Singh Y., Gupta G., Shrivastava B., et al. Calcitonin gene-related peptide (CGRP): a novel target for Alzheimer's disease. CNS Neurosci Ther. 2017;23:457–461. doi: 10.1111/cns.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C.X., Qiu T., Liu Z.F., et al. Calcitonin gene-related peptide has protective effect on brain injury induced by heat stroke in rats. Exp Ther Med. 2017;14:4935–4941. doi: 10.3892/etm.2017.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leon L.R., Bouchama A. Heat stroke. Compr Physiol. 2015;5:611–647. doi: 10.1002/cphy.c140017. [DOI] [PubMed] [Google Scholar]
- 10.King M.A., Leon L.R., Morse D.A., et al. Unique cytokine and chemokine responses to exertional heat stroke in mice. J Appl Physiol. 1985;122:296–306. doi: 10.1152/japplphysiol.00667.2016. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Ji J.J., Hong X.X., Su L., et al. Proteomic identification of hippocalcin and its protective role in heatstroke-induced hypothalamic injury in mice. J Cell Physiol. 2019;234:3775–3789. doi: 10.1002/jcp.27143. Epub 2018 Sep. 7. [DOI] [PubMed] [Google Scholar]
- 12.Li L., Liu Z.F., Gu Z.T., et al. Research progress in the pathogenesis of central nervous system on severe heat stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:570–572. [PubMed] [Google Scholar]
- 13.Edvinsson L. The journey to establish CGRP as a migraine target: a retrospective view. Headache. 2015;55:1249–1255. doi: 10.1111/head.12656. [DOI] [PubMed] [Google Scholar]
- 14.Borkum J.M. CGRP and brain functioning: cautions for migraine treatment. Headache. 2019;59:1339–1357. doi: 10.1111/head.13591. [DOI] [PubMed] [Google Scholar]
- 15.Sorby-Adams A.J., Marcoionni A.M., Dempsey E.R., et al. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int J Mol Sci. 2017;18:1788. doi: 10.3390/ijms18081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian J., Yang L., Wang P.F., et al. Exogenous CGRP regulates apoptosis and autophagy to alleviate traumatic brain injury through Akt/mTOR signalling pathway. Neurochem Res. 2020;45:2926–2938. doi: 10.1007/s11064-020-03141-9. [DOI] [PubMed] [Google Scholar]
- 17.Schebesch K.M., Herbst A., Bele S., et al. Calcitonin-gene related peptide and cerebral vasospasm. J Clin Neurosci. 2013;20:584–586. doi: 10.1016/j.jocn.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Luo C.C., Huang C.S., Ming Y.C., et al. Calcitonin gene-related peptide downregulates expression of inducible nitride oxide synthase and caspase-3 after intestinal ischemia-reperfusion injury in rats. Pediatr Neonatol. 2016;57:474–479. doi: 10.1016/j.pedneo.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Conner A.C., Simms J., Barwell J., et al. Ligand binding and activation of the CGRP receptor. Biochem Soc Trans. 2007;35:729–732. doi: 10.1042/BST0350729. [DOI] [PubMed] [Google Scholar]
- 20.Tian R.M., Zhang Y., Pan Q., et al. Calcitonin gene-related peptide receptor antagonist BIBN4096BS regulates synaptic transmission in the vestibular nucleus and improves vestibular function via PKC/ERK/CREB pathway in an experimental chronic migraine rat model. J Headache Pain. 2022;23:35. doi: 10.1186/s10194-022-01403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]