Graphical abstract
Keywords: Salicylate derivatives, Paw edema test, LD50 value, Anti-inflammatory assessment, MD simulation
Abstract
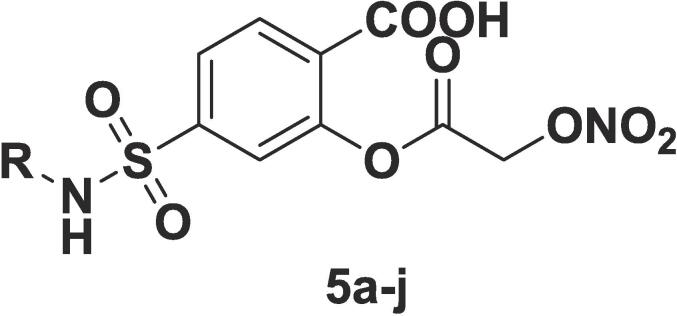
The series of newer salicylate derivatives incorporating nitroxy functionality were synthesized and evaluated for their potential effect in gastrointestinal (GI) related toxicity produced by aspirin. The synthesized compounds (5a-j) were subjected to %NO (nitric oxide) release study, in-vitro anti-inflammatory potential, % inhibition of carrageenan-induced paw edema and the obtained results were validated by in-silico studies including molecular docking, MD simulations and in-silico ADME (absorption, distribution, metabolism, and elimination) calculations. Compounds 5a (20.86 %) and 5g (18.20 %) displayed the highest percentage of NO release in all the tested compounds. Similarly, 5a and 5h were found to have (77.11 % and 79.53 %) &(78.56 % and 66.10 %) inhibition in carrageenan induced paw edema in animal mode which were relatively higher than ibuprofen (standard used). The obtained results were validated by molecular docking and MD simulations studies. The molecular docking study of 5a and 5h revealed that docking scores were also obtained in very close proximity of −8.35, −9.67 and −8.48 for ibuprofen, 5g and 5h respectively. In MD simulations studies, the calculated lower RMSD (root mean square deviation) values 2.8 Å and 5.6 Å for 5g and 5h, respectively indicated the stability of ligand-protein complexes. Similarly lower RSMF (root mean square fluctuation) values indicated the molecules remained in the active pocket throughout the entire MD simulations run. Further, in-silico ADME calculations were determined and all compounds obey the Lipinski’s rule of five and it was predicted that these molecules would be orally active without any serious toxic effect.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used to control in arthritis and infections-based pain (Crofford, 2013). Patients with rheumatoid arthritis are frequently prescribed for NSAIDs as their first-line of treatment for chronic disease treatment. However, their anti-inflammatory and analgesic effects are associated with the upper gastrointestinal side effects (GI) (Laine, 2003). According to the FDA, 1–2 % of patients taking NSAIDs for three months or less and 2–5 % of patients taking them for a year, have reported symptomatic ulcers, bleeding, and perforation (McDonald, 2019). The report of PAN-COX enzyme inhibition appears to be linked with the development of GI toxicity caused by these medications. In maximum cases, NSAIDs causes two severe GI toxicity conditions such as (a) inhibition of platelet cyclooxygenase, which leads to prolonged bleeding time and (b) inhibition of cyclooxygenase in the gastrointestinal mucosa, which causes decreased synthesis of cytoprotective gastric prostaglandins. The selectivity profiling of NSAIDs was responsible for its side effects such as selective COX-1 inhibitors (aspirin, indomethacin, piroxicame, and ketoprofene) to produce GI toxicity whereas selectivity towards COX-2 enzyme led to increased risk of cardiotoxicity (Wallace, 1992). To overcome these side effects, the second isoform of cyclooxygenase (COX-2) have led to the development of specialized COX-2 inhibitors, resulting in powerful anti-inflammatory drugs with very low GI toxicity (Hawkey, 1999, Hawkey and Langman, 2003, Hilário et al., 2006). However, many reports on GI toxicity caused by these selective COX-2 inhibitors have been published and researches for effective and safer NSAIDs are still continued. Recently, COX-I enzyme was reported as relatively important proinflammatory enzyme as compared to COX-2. Aspirin is a commonly used COX-I inhibitor and even its repurposing with other clinical condition increases its therapeutic benefits. However, the toxicity issues are still prime concern. Many researchers have attempted for different substitutions on aspirin and the optimization is still under investigation for better alternatives. In order to find better-tolerated nonsteroidal anti-inflammatory medicines, new compounds with a nitric oxide-releasing group and a hydrogen sulfide-releasing group have been synthesized. NO is an important gaseous messenger in addition to its role in gastro-protection. NCX-4016 is a prototype molecule reported earlier and synthesized by adding nitric oxide-releasing moiety to aspirin. However, aspirin and NO releasing group are connected vial ester linker group in the molecule. Furthermore, the metabolism of ester group may generate acidic group and lead to GI toxicity. The search of NO releasing group directly attached to aspirin moiety without any linker would be beneficial (Fiorucci et al., 2003, Brzozowski et al., 2005, Kodela et al., 2012). The directly attached NO releasing group can be used to create new, more effective, and safer anti-inflammatory agents. COX-1 is inhibited in the gastric mucosa as a result of anti-inflammatory drugs, which reduces blood flow, mucus secretion, neutrophil activation, and inflammation. NO shields it from these negative effects. It is also antiplatelet, antithrombotic, and vasodilating. Because of its ability to acetylate the Ser530 hydroxyl group in the primary COX binding site of COX-1 and COX-2, aspirin is a unique nonselective COX inhibitor. In this regard, aspirin is a 10- to 100-fold more potent COX-1 inhibitor than COX-2 (Catella-Lawson et al., 2001, Green, 2001, Vane and Botting, 2003). Despite being on the market for over a century, the adverse gastric and intestinal complications using aspirin (acetylsalicylic acid), still remain a significant limitation to prescribe frequently. A number of solutions, including the “gaseous solution,” have been proposed to address this issue (Hirsh et al., 1989, Kedir et al., 2021) This method entails conjugating a NO-releasing moiety with aspirin via an ester link. NO protects the gastric mucosa from the negative effects of NSAID-induced COX-1 inhibition, such as decreased mucosal blood flow, decreased mucus and bicarbonate secretion, increased neutrophil adherence and activation, and modulation of inflammatory mediators (Wallace et al., 2002, Fiorucci, 2003, Antoniades et al., 2007). The main objective of study is to design synthesis and evaluate newer salicylate derivative by incorporating nitroxy functional group to reduce the GI side effects (associated with aspirin) and also substitution at fifth position to improve anti-inflammatory activity as shown in Fig. 1. The GI protective effect was expected based upon the extent of NO release which prevents the GI mucosa from the common side effects of NSAIDs. The derivatization at carbon number 5 was supposed to check the alteration in pharmacological effect of aspirin.
Fig. 1.
Study design for NO releasing derivatives of Aspirin.
2. Materials and methods
2.1. Materials
Solvents and chemicals were procured from Sigma-Aldrich, and Spectro-Chem, S.D. Fine Chemicals, Mumbai, India. Precoated silica gel on aluminium sheets TLC (thin layer chromatography) plates (60 F254) were used to monitor the chemical reactions and visualized under UV chamber or with iodine fumes. The melting points were measured manually on Thiel's tube and were uncorrected. DRS (Diffuse Reflectance Spectroscopy) of Shimadzu 1000 FTIR (Fourier Transform Infrared) instrument was used to acquire infrared spectra in the 4000–400 cm−1 range. Proton resonance magnetic spectra (1H NMR) were recorded downfield using TMS (tetramethylsilane) as an internal standard. DMSO (dimethyl sulfoxide) as a solvent on a Bruker 400 MHz spectrophotometer with and chemical shift represented in ppm (parts per million). Mass spectra (MS) were obtained using an LCMS (liquid chromatography mass spectroscopy) system with a Q-TOF detector. An in-vitro NO release study was conducted using Griess reagent. In vitro anti-inflammatory studies with RBC (red blood cells) membrane stabilization were conducted. LD50 (lethal dose at 50% killing) of newly synthesized molecules were determined using acute toxicity study. Anti-inflammatory potency of synthesized compounds was determined by carrageenan induced paw oedema method. One-way ANOVA (analysis of variance) was used to test the statistics of obtained results followed by Dunnett's test. The cytotoxic potential of a few chemicals was assessed using a Brine shrimp lethality test. Then, to rationalize the obtained results molecular modelling studies were performed. Glide utility of Schrodinger LLC suit was used for molecular docking analysis. Similarly, Desmond (DE Shaw research group) was employed for molecular dynamic study. This software was running on HP Xeon workstation. Hardware support was provided by NVIDIA company limited under the NVIDIA Applied Research Accelerator Program.
2.2. Methods
2.2.1. Synthesis
Synthesis of 5-(chlorosulfonyl) 2-hydroxy benzoic acid (2): Chlorosulfonic acid (12.1 mL, 18.1 mmol) was transferred to an RBF. With the aid of a dropping funnel, a solution of salicylic acid (5 g, 3.62 mmol) in N,N-dimethyl formamide (20 mL) was slowly added to this and allowed for constant stirring at room temperature. TLC was used to monitor the completion of reaction. After the completion, the reaction mixture was poured on crushed ice and formed precipitate was filtered out and recrystallized it from chloroform (1.97 g, 39.4 percent), melting point: 164–165 °C.
Synthesis of various derivatives by Substitution of different anilines to 5-(chlorosulfonyl) 2- hydroxyl benzoic acid (3a-j): At 0 °C, 2 g of 2-hydroxy-5 (chloro sulfonyl) benzoic acid (2) (0.8456 mmol) in ethanol, was added to a pyridine solution of substituted arylamines. After the completion of reaction, the reaction mixture was transferred into a beaker containing ice-cold water (50 mL) followed by extraction using ethyl acetate (30 mL). The solvent was evaporated after ethyl acetate layer was dried over sodium sulphate. The residue was recrystallized using chloroform or dichloromethane solvent.
Synthesis of different 2- hydroxyl-5-(phenyl sulfamoyl) benzoic acids derivatives 4 (a-j): The 2-hydroxy-5-(phenyl sulfamoyl) benzoic acids 3(a-j) were dissolved in THF (tetrahydrofuran) contained in an RBF (round bottom flask) (5 mL). The reaction mixture was cooled to less than 5 °C and the calculated amount (2 equivalence) of triethylamine was added. When the temperature of the solution reached to 0 °C, chloroacetyl chloride was added to it dropwise and the reaction was monitor by TLC. After the completion of reaction, the mixture was added to crushed ice and acidified with HCl (hydrochloric acid). The formed precipitate was filtered, washed with water and recrystallized from chloroform.
Synthesis of final compounds 5 (a-j): Chloroalkyl derivatives were dissolved in acetonitrile (ACN) and a fixed portion of this (2 mL) was treated with silver nitrate (AgNO3) solution in dry ACN (5 mL). Then, the whole mixture was stirred at 25 °C for 12 to 24 h. Intermittently, TLC was utilized to confirm the completion of reaction. Then, the reaction mixture was added to the ice-cold water and obtained precipitate was filtered, washed with water, and dried.
2.2.2. Acute toxicity study
The LD50 (lethal dose to kill 50% of the tested animal) of the synthesised compounds was determined using the Up and Down Method in an acute toxicity study. The LD50 is the most commonly used parameter for determining acute toxicity. The acute oral toxicity test attempts to determine the lethal dose on the same strain and species. It is the median effective dose that causes death in 50 % of the animals of the same species and strain. As a result, the compounds toxicological effects were assessed using the Up and Down method (Jonsson et al., 2013, Jonsson et al., 2013).
2.2.2.1. Animal species
To investigate in vivo study, Swiss albino mice were used weighing about 20–25 g of either sex. They were housed in an air-conditioned room with free access to water and food. Animals were quarantined for 24 h before commencement of experiment and they were on fast condition for 18 h. Animals were approved by BKC M.E.T's Institute of Pharmacy, Nashik (CPCSEA Registration no. 1344/ac/10/CPCSEA), India.
2.2.2.2. Method of toxicity testing
AOT425 software was used to administer graded doses ranging from 1.75 mg/kg to 2000 mg/kg intraperitonially (Acute Oral Toxicity Guideline 425). Animals were observed for gross behavioral and toxic effects (such as slowed body movements, loss of body movements, body swellings, loss of fur, mortality, and so on) at once during the first 30 min, and then every 30 min for the next 24 h. After observing mortalities and behavioral profiles for the specified time, the maximal safe dose for the study was determined by plotting the dose versus percent death graph by obtaining the LD50 value. The ED50 value was calculated using the obtained LD50 value.
2.2.2.3. In-vitro studies
Nitric oxide (NO) release study: To estimate NO release (in vitro) in the presence of l-cysteine, animal serum (rat) or pig liver esterase is quantified by measuring produced NO as a result of the Griess reaction using the earlier reported method (Coneski and Schoenfisch, 2012).
Blank Solution: A blank control solution of 2.4 mL of PBS containing 5 % DMSO was kept at 37 °C for 1 h with constant stirring. The Griess reagent (0.8 mL) was then transferred to the solution and the resulting mixture was gently stirred for 30 min at 37 °C.
Standard solution: To prepare a working standard solution of NaNO2, an accurately weighed amount of NaNO2 (0.1 mmol) was dissolved in the same blank solution. A measured amount (0.8 mL) of the Griess reagent was transferred to the same mixture followed by stirring at 37 °C for 30 min and then incubated. The mixture was serially diluted for varied concentrations using Griess reagent solution as a diluent (32 mL of Griess reagent and 96 mL of blank solution). Thus, varied concentrations of NaNO2 were used to measure absorbance to establish a working standard calibration curve using UV Vis spectrophotometer at 540 nm. The study was replicated for mean and standard deviation (n = 3).
Test compound solutions: 0.2 mmol solutions in DMSO were prepared to determine the % of NO released from the test compounds. A portion of this solution was diluted with PBS (phosphate buffer solution) containing l-cysteine to achieve a final concentration of 0.1 mmol. This solution was gently stirred at 37 °C for 60 min. The reagent (0.8 mL) was transferred and the mixed solution was gently stirred at 37 °C for 30 min. Absorbance value of each solution was measured at 540 nm using UV Visible spectrophotometer wherein blank solution was used as reference (blank). Each test compound's absorbance value was corrected using blank control absorbance and subtracting from the test absorbance. To quantify the content of nitrite concentration, a standard nitrite concentration-absorbance curve was plotted. The NO% released from each test compound was calculated accordingly (Coneski and Schoenfisch, 2012).
2.2.2.4. In vitro RBC membrane stabilization method
A variety of disorders are caused due to lysosome-based enzymes secreted while inflammatory responses. These enzymatic extracellular activities are related to inflammation (acute or chronic). RBC membrane was used as an in vitro model for inflammation assessment. The membrane composition is similar to lysosomal membrane. The inhibition of hypotonicity-induced RBC membrane lysis is applied to assess the anti-inflammatory property of the drug. To study this, blood sample was collected in a blood collection tube from healthy rats. Then, the collected volume of the blood was mixed with a sterilized Alsever's solution (1:1). The packed cells were washed with isotonic NaCl (0.85 %, pH 7.2) after the blood was centrifuged at 1500 rpm, and a 10 % v/v suspension of packed cells was made with isotonic NaCl. The assay mixture included the drug (conc. i.e., 25 µg/mL, 50 µg/mL, 100 µg/mL, 120 µg/mL), 1 mL of phosphate buffer (0.15 M, pH = 7.4), 2 mL of hypotonic NaCl (0.36 percent), and 0.5 mL of RBC suspension. Aspirin (100 µg/mL) was used as the control. For the control, distilled water (2 mL) was utilized instead of hypotonic NaCl solution. The assay mixture was centrifuged after 30 min of incubation at 37 °C. A spectrophotometer set to 500 nm was used to estimate the haemoglobin content of the supernatant solution. The % hemolysis was estimated using the following formula (1) and distilled water-based hemolysis was considered as 100 % lysis (Padmaja et al., 2002, Padmaja et al., 2002).
| (1) |
where OD represents the optical density for the test and control samples.
2.2.2.5. Cytotoxic study
In preliminary assessment of the compound for anticancer potential, an in vitro cell line study was performed using a brine shrimp lethality bioassay. The model is simple, cost-effective, and least need of the test sample. This renders a first-line screening of the compound for cytotoxicity potential followed by specific, and expensive bioassays examination.
Brine solution: In order to get a brine solution, a measured quantity of iodized sodium chloride (38 g) was completely dissolved in distilled water (1 L) and filtered. The obtained solution was clear and stable.
Hatching of Artemia salina shrimps: Brine shrimp (Artemia salina) was allowed to hatch in the artificial sea water under constant aeration for 48 h. The active nauplii and larvae of shrimps were separated by collection for the experiment.
Sample solution: To prepare a stock solution of compound PB1, 10 g was completely dissolved in DMSO (10 mL) to get 1000 µg/mL. This was serially diluted to get various concentrations in the range of 1–100 µg/mL. To avoid possible toxicity caused by DMSO, stock solutions were diluted in suggested volume. The toxicity assay used pure DMSO as a positive control.
Application of test solution and larvae to the test tubes: Each test tube received about 5 mL of brine solution. The test substance was diluted to the appropriate concentration. The test tubes were filled with 0.05 mL of diluted test solution. Each test tube received 30 active shrimp larvae. The solution should be thoroughly mixed. After 24 h, the surviving (larvae) shrimps were counted, and the lethality concentration LC50 was determined (Padmaja et al., 2002, Padmaja et al., 2002, Ved et al., 2010).
2.2.2.6. In-vivo studies
The animals were procured from Haffkin's Institute in Parel, Mumbai and the details of animals and study design including grouping of animals have been summerized in Table 6. The study was conducted at BKC M.E.T's Institute of Pharmacy, Nashik with CPCSEA registration no. 1344/ac/10/CPCSEA using Swiss albino mice (20–25 g) of either sex of age of 6–9 months. They were housed at colony cages with proper environmental conditions at 25 °C. The experiments were conducted during the day (10:00–16:00 h).
Table 6.
Materials for anti-inflammatory activity.
| Sr. No. | Group | Treatment | Dose | Route | Number of mice |
|---|---|---|---|---|---|
| 1. | Normal | Tween 80 | 2 % | Intraplantar | 6 |
| 2. | Positive control | Carrageenan | 0.1 mL | Intraplantar | 6 |
| 3. | Carrageenan + standard | Ibuprofen | 5 mg/kg | Intraperitoneal | 6 |
| 4. | Carrageenan + Sample 1 | 5g | 1.75 mg/kg | Intraperitoneal | 6 |
| 5. | Carrageenan + Sample 1 | 5g | 3.5 mg/kg | Intraperitoneal | 6 |
| 6. | Carrageenan + Sample 2 | 5h | 10 mg/kg | Intraperitoneal | 6 |
| 7. | Carrageenan + Sample 2 | 5h | 15 mg/kg | Intraperitoneal | 6 |
2.2.2.7. Anti-inflammatory activity
See Table 6.
2.2.2.8. In vivo method
Induced paw edema model in mice was used to assess the anti-inflammatory property of synthesized compounds (5a-j). The test compound was used to heal induced paw edema of the right leg of mice following phlogistic agent injection. Typically, the volume of the injected paw is measured before and after the irritant is applied, and the paw volume of the treated animals is compared to that of the controls. This experiment was carried out in accordance with the method described before (Vadivu and Lakshmi, 2008). The mice were on fast for 16 hr . Using digital vernier callipers, the paw thickness (zero h) was measured in millimeters (Mitutoyo, Japan). The test substances and standard drug were given half an hour before the intraperitoneal injection of a phlogistic agent. A day before the study, the phlogistic agent carrageenan was prepared as a 1 percent suspension in sterile normal saline. Carrageenan (0.1 mL) was injected subcutaneously into each mouse's right hind paw. The thickness of the injected paw was measured at 0 h, 0.5 h, 1 h, 2 h, and 3 h after the carrageenan injection. By subtracting the zero-hour reading from the three-hour reading, the edema thickness (mm) was calculated. The percentage inhibition of edema between the treated and the control groups was calculated using the mean edema volume. The synthesized derivatives were administered orally via oral gavage in the form of a suspension containing tween 80.
Where, VC and Vt represent the average paw volume in the control and treated groups, respectively (Vadivu and Lakshmi, 2008).
Molecular docking: Molecular docking studies were performed using glide utility of Schrodinger module running on HP workstation. The co-crystallized protein structure of COX-2 enzyme complexed with ibuprofen (pdb id: 4HP9) and COX-I (3KK6) was downloaded from the scientific repository of protein data bank (Orlando et al., 2015, Rose et al., 2016). The downloaded raw protein was devoid of H atom with different missing residues. The downloaded protein was pentamer with five subunits. Therefore, this was subjected to protein preparation wizard where H atoms were added and the other four different units B-E were deleted. The unit A contains the co-crystallized ligand ibuprofen. Water molecules beyond 5 Å were deleted and amide groups were reoriented to acid group. The heterostates of internal ligand was generated and best suitable position was selected for further processing. The optimization was run at pH 7 using Epik and lastly energy minimization was performed using OPLS-2005. Receptor grid was generated using the receptor grid generation utility and by selecting the centroid of the co-crystallized ligand. The compound ibuprofen, 5g and 5h were sketched in Maestro and processed through ligprep utility to generate the different conformers and tautomers with least minimized energy conformations. These molecules were used for molecular docking study against the COX-2 protein (Bell et al., 2012).
MD Simulations: The ligand–protein complexes obtained in molecular docking study were used for MD simulations. Desmond V3 developed by DE Shaw group running HP workstation was used for simulation study. The obtained ligand–protein complexes were prepared before they were subjected to generate an orthorhombic simulation box using system builder utility of Desmond tool. Simple Point-Charge (SPC) explicit water model was used to generate simulation box by keeping surface distance of 10 Å between the solvent and protein and it was neutralized. Then, 0.15 M physiological salt content was kept and this equilibrated system was used for MD simulation study. At constant temperature (310.15 K) and pressure (1.0 bar) MD run was conducted for 100 ns. Simulation interaction analysis tool was used to analyze the file obtained after the successful MD run. MD trajectory was built using 1000 frames generated during the simulation process. However, to analyze the ligand–protein complex stability, only initial protein backbone frames were used. Then, RMSD (root mean square deviation), RMSF (root mean square fluctuation) values, and % ligand and amino acid interactions study were carried out to analyze the MD simulation results (Klepeis et al., 2009).
In-silico ADME calculation: The compounds 5g and 5h with ibuprofen were subjected to in-silico ADME calculation using qikprop module. The physicochemical properties like PKa, PSA, Mol, Lipinski’s rule of five (Ro5), Ro3, % oral absorption, and % BBB were considered for ADME calculation. The obtained values were analyzed and discussed in discussion section.
2.2.2.9. Experimental
2-(2-(nitrooxy)acetoxy)-5-(N-phenylsulfamoyl)ben zoic acid: Compound (5a) was obtained by the reaction of 2-(2-chloroacetoxy)-5-(N-phenyl sulfamoyl) benzoic acid (4a) (1.0 g, 0.270 mmol), silver nitrate (2.29 g, 1.353 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.47 g, 47 %), m.p. 90 °C. TLC: Rf −0.45 (n-hexane: ethyl acetate, 16:4); IR 3454 cm−1 (N–H), 3228 cm−1 (carboxylic OH), 3546 cm−1 (Ar-OH), 1681 cm−1 (C = O acid), 1753 cm−1 (C = O ester), 1377 & 1211.3 cm−1 (SO2), 1296 cm−1 (ONO2), 1614 & 1483 cm−1 (C = C aromatic), 3061 cm−1 (C–H aromatic), 1078 cm−1 (C-O), 1354 cm−1 (C-N), 698 & 758 cm−1 (monosubstitution); NMR: 11.29 δ (broad singlet, 1H, CO-OH, Ha), 4.79 δ (s, 1H, CH2, Hb), 6.89 δ (m, 2H, Ar–H, Hc & Hd), 8.61 δ (s, 1H, Ar–H, He), 3.89 δ (s, 1H, N–H, Hf), 8.06 δ (m, 2H, Ar–H, Hg & Hh), 7.76 δ (t, 1H, Ar–H, Hi); ESI + m/z calculated 397.03; found 397.9.
5-(N-(4-bromophenyl) sulfamoyl)-2-(2-(nitrooxy)acetoxy) benzoic acid: Compound (5b) was obtained by the reaction of 5-(N-(4-bromophenyl) sulfamoyl)-2-(2-chloroacetoxy) benzoic acid (4b) (1.0 g, 0.2285 mmol), silver nitrate (1.94 g, 1.142 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.51 g, 51 %), m.p. 98–102 °C. TLC: Rf −0.39 (n-hexane: ethyl acetate, 16:4); IR: 3734 cm−1 (N–H), 3566 cm−1 (carboxylic OH), 3647 cm−1 (Ar–OH), 1672 cm−1 (C = O acid), 1751 cm−1 (C = O ester), 1379 & 1157 cm−1 (SO2), 1282 cm−1(ONO2), 1608 & 1487 cm−1 (C = C aromatic), 3116 cm−1 (C–H aromatic), 1072 cm−1 (C–O), 1247 cm−1 (C–N), 802 cm−1 (para disubstitution), 677 cm−1(C–Br) MS: Calculated: 474.94, found 475.44 (476.00 Br isotopic peak).
5-(N-(4-chlorophenyl)sulfamoyl)-2-(2-(nitrooxy)acetoxy)benzoic acid: Compound (5c) was obtained by the reaction of 5-(N-(4-chlorophenyl) sulfamoyl)-2-(2-chloroacetoxy) benzoic acid (4c) (0.65 g,0.166 mmol), silver nitrate (1.42 g, 0.8333 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.321 g, 49.38 %), m.p. 104–107 °C. TLC: Rf −0.46 (Chloroform) IR: 3626 cm−1 (N–H), 3564 cm−1 (carboxylic OH), 3583 cm−1 (Ar-OH), 1714 cm−1 (C = O acid),1753 cm−1 (C = O ester),1379 & 1215 cm−1 (SO2), 1282 cm−1(ONO2), 1614 & 1485 cm−1 (C = C aromatic), 3062 cm−1 (C–H aromatic), 1078 cm−1 (C–O), 1338 cm−1 (C-N), 802 cm−1 (para disubstitution), 783 cm−1 (C–Cl) NMR: 12.68 δ (broad singlet,1H, CO-OH, Ha), 4.48 δ (s, 1H, CH2, Hb), 6.81 δ (m, 3H, Ar–H, Hc, Hd, He), 6.79 δ (s, 1H, N–H, Hf), 7.34 δ (d, 1H, Ar–H, Hg), 7.79 δ (t, 1H, Ar–H, Hh).
2-(2-(nitrooxy)acetoxy)-5-(N-(o-tolyl)sulfamoyl)benzoic acid: Compound (5d) was obtained by the reaction of 2-(2-chloroacetoxy)-5-(N-(o-tolyl) sulfamoyl) benzoic acid (4d) (0.5 g, 0.1345 mmol), silver nitrate (1.14 g, 0.6729 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.247 g, 49.4 %), m.p. 90 °C. TLC: Rf −0.374 (n-Hexane: ethyl acetate, 16:4) IR: 3434 cm−1 (N–H), 3215 cm−1 (carboxylic OH), 3261 cm−1 (Ar–OH), 1680 cm−1 (C = O acid), 1751 cm−1 (C = O ester), 1381 & 1215 cm−1 (SO2), 1284 cm−1 (ONO2), 1614 & 1487 cm−1 (C = C aromatic), 3074 cm−1 (C–H aromatic), 1112 cm−1 (C–O), 1303 cm−1 (C–N), 750 cm−1 (ortho disubstitution).
2-(2-(nitrooxy)acetoxy)-5-(N-(m-tolyl)sulfamoyl)benzoic acid: Compound (5e) was obtained by the reaction of 2-(2-chloroacetoxy)-5-(N-(m-tolyl)sulfamoyl)benzoic acid (4e) (1.0 g, 0.2691 mmol), silver nitrate (2.29 g, 1.345 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.386 g, 38.6 %), m.p. 120–125 °C. TLC: Rf −0.2592 (Chloroform) IR: 3745 cm−1 (N–H), 3566 cm−1 (carboxylic OH), 3657 cm−1 (Ar–OH), 1687 cm−1 (C = O acid), 1753 cm−1 (C = O ester), 1386 & 1157 cm−1 (SO2), 1282 cm−1 (ONO2), 1614 & 1506 cm−1 (C = C aromatic), 3064 cm−1 (C–H aromatic), 1076 cm−1 (C–O), 1157 cm−1 (C–N), 690 & 802 cm−1 (meta disubstitution).
2-(2-(nitrooxy)acetoxy)-5-(N-(p-tolyl)sulfamoyl)benzoic acid: Compound (5f) was obtained by the reaction of 2-(2-chloroacetoxy)-5-(N-(p-tolyl) sulfamoyl) benzoic acid (4f) (0.8 g, 0.2691 mmol), silver nitrate (2.29 g, 1.345 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.397 g, 49.625 %), m.p. 132 °C. TLC: Rf −0.3448 (n-hexane:ethyl acetate, 10:10) IR: 3566 cm−1 (N–H), 3273 cm−1 (carboxylic OH), 3523 cm−1 (Ar-OH),1683 cm−1 (C = O acid),1751 cm−1 (C = O ester),1375 & 1195 cm−1 (SO2), 1292 cm−1 (ONO2), 1616 & 1489 cm−1 (C = C aromatic), 3088 cm−1 (C–H aromatic), 1070 cm−1 (C–O), 1354 cm−1 (C–N), 802 cm−1 (para disubstitution).
5-(N-(naphthalen-2-yl) sulfamoyl)-2-(2-(nitrooxy)acetoxy) benzoic acid: Compound (5g) was obtained by the reaction of 2-(2-chloroacetoxy)-5-(N-(naphthalen-2-yl) sulfamoyl) benzoic acid (4g) (1.0 g, 0.2192 mmol), silver nitrate (1.86 g, 1.096 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.645 g, 64.5 %), m.p. 96–98 °C. TLC: Rf −0.352 (n-hexane: ethyl acetate, 16:4) IR: 3583 cm−1 (N–H), 3523 cm−1 (carboxylic OH), 3583 cm−1 (Ar–OH), 1714 cm−1 (C = O acid),1737 cm−1 (C = O ester),1377 & 1211 cm−1 (SO2), 1296 cm−1 (ONO2), 1614 & 1483 cm−1 (C = C aromatic), 3061 cm−1 (C–H aromatic), 1089 cm−1 (C–O), 1157 cm−1 (C–N), 698 & 759 cm−1(monosubstitution) MS: m/z 446.84 (Molecular ion peak), 279.99 (Base peak).
2-(2-(nitrooxy)acetoxy)-5-((2-phenylhydrazinyl)sulfonyl) benzoic acid: Compound (5 h) was obtained by the reaction of 2-(2-chloroacetoxy)-5-((2-phenylhydrazinyl) sulfonyl) benzoic acid (4 h) (0.8 g, 0.2080 mmol), silver nitrate (1.76 g, 1.040 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.483 g, 60.375 %), m.p. 76–80 °C.TLC: Rf −0.56 (n-hexane: ethyl acetate, 10:10) IR: 3533 cm−1 (N–H), 3230 cm−1 (carboxylic OH), 3516 cm−1 (Ar–OH), 1708 cm−1 (C = O acid), 1753 cm−1 (C = O ester), 1381 & 1209 cm−1 (SO2), 1294 cm−1 (ONO2), 1610 & 1483 cm−1 (C = C aromatic), 3049 cm−1 (C–H aromatic), 1089 cm−1 (C-O), 1357 cm−1 (C–N), 698 & 759 cm−1 (monosubstitution).
5-(N-(4-carboxyphenyl) sulfamoyl)-2-(2-(nitrooxy)acetoxy) benzoic acid: Compound (5i) was obtained by the reaction of 5-(N-(4-carboxyphenyl) sulfamoyl)-2-(2-chloroacetoxy) benzoic acid (4i) (0.7 g, 0.169 mmol), silver nitrate (1.59 g, 0.8 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.367 g, 52.42 %), m.p. 92–95 °C. TLC: Rf −0.341 (n-hexane: ethyl acetate, 10:10) IR: 3543 cm−1 (N–H), 33452 cm−1 (carboxylic OH), 3516 cm−1 (Ar-OH), 1703 cm−1 (C = O acid), 1737 cm−1 (C = O ester), 1371 & 1188 cm−1 (SO2), 1282 cm−1 (ONO2), 1608 & 1485 cm−1 (C = C aromatic), 3105 cm−1 (C–H aromatic), 1085 cm−1 (C–O), 1151 cm−1 (C–N), 800 cm−1 (para disubstitution).
5-(N-(4-acetamidophenyl) sulfamoyl)-2-(2-(nitrooxy)acetoxy) benzoic acid: Compound (5j) was obtained by the reaction of 5-(N-(4-acetamidophenyl) sulfamoyl)-2-(2-chloroacetoxy) benzoic acid (4j) (0.8 g, 0.1875 mmol), silver nitrate (1.59 g, 0.9375 mmol) and acetonitrile (5 mL) as per general procedure mentioned above, (0.517 g, 64.62 %), m.p. 98 °C. TLC: Rf −0.333 (n-hexane: ethyl acetate, 10:10) IR: 3572 cm−1 (N–H), 3506 cm−1 (carboxylic OH), 3549 cm−1 (Ar-OH), 1710 cm−1 (C = O acid), 1741 cm−1 (C = O ester),1381 & 1207 cm−1 (SO2), 1292 cm−1 (ONO2), 1608 & 1483 cm−1 (C = C aromatic), 3084 cm−1 (C–H aromatic), 1157 cm−1 (C-O), 1247.94 cm−1 (C–N), 698 & 748 cm−1 (monosubstitution).
3. Results
3.1. Chemistry
A series of NO releasing aspirin derivatives were synthesized using the procedure outlined in scheme 1 (Fig. 2). In short, salicylic acid was reacted with chlorosulphonic acid to yield 4-cholorosulphonyl salicylic acid (2). This intermediate was further subjected to coupling with aniline to give compound (3). Then, it was reacted with chloroacetyl chloride which yielded chloroacetate derivatives (4a-j). These intermediates on reaction with acetonitrile and subsequent oxidation gave final compounds (5a-j). All the final products were characterized by their physicochemical properties and spectral analysis. The melting point of all synthesized compounds were assessed and it was found within the range from 90 to 110 °C as compared to aspirin (135 °C).
Fig. 2.
Scheme 1: The synthetic route for the proposed NO releasing aspirin derivatives.
3.2. Pharmacological evaluation
3.2.1. Assessment of acute toxicity
Understanding the molecular basis of therapeutic functionality for the synthesized compounds is the most important domain in the new drug development processes. Moreover, the preclinical acute toxicity studies would be used for predicting organ specific toxicity, dose calculation and dose specific side effects of newer molecule at explored dose and dosing frequency. Hence, the acute toxicity test was performed using two potent synthesized compounds (5g and 5h) to determine the lethal dose for 50 % of population (Walum, 1998, Jonsson et al., 2013, Jonsson et al., 2013). The experimental dose was chosen between the lowest effective dose and the highest non-lethal dose.
3.2.2. In-vitro studies
3.2.2.1. Nitric oxide release study
The NO protects the gastric mucosa against the toxic effect produced by commonly used NSAIDs. Hence, the extent of NO release would provide the greater protection to gastric mucosa towards the side effects of NSAIDs. Considering the results (Table 1, Fig. 3), all of the compounds 5 (a-j) release significant amounts of nitric oxide. Compounds 5a, 5g and 5h tend to release a higher percentage of NO as 20.86 %, 18.20 % and 17.82 %, respectively as compared to other compounds.
Table 1.
Values showing the % Prevention of lysis and % NO release of synthesized compound 5a-j.
| Compound Code | R |
Activity (% prevention of lysis) |
% NO release | |||
|---|---|---|---|---|---|---|
| 25 µg/mL | 25 µg/mL | 25 µg/mL | 25 µg/mL | |||
| 5a | H | 56.64 | 57.08 | 61.20 | 62.26 | 20.86 |
| 5b | 4-Br-Ph- | 38.53 | 43.66 | 58.61 | 67.11 | 11.23 |
| 5c | 4-Cl-Ph- | 42.98 | 51.46 | 56.50 | 65.39 | 14.31 |
| 5d | 2-CH3-Ph- | 52.34 | 54.62 | 55.87 | 55.60 | 11.26 |
| 5e | 3-CH3-Ph- | 51.21 | 51.96 | 60.20 | 61.22 | 13.02 |
| 5f | 4-CH3-Ph- | 39.94 | 46.46 | 49.58 | 52.29 | 11.51 |
| 5g | 1-Naphthyl- | 50.01 | 51.07 | 62.36 | 53.86 | 18.20 |
| 5h | –NH-Ph | 53.88 | 54.98 | 61.34 | 58.50 | 17.82 |
| 5i | 4-COOH-Ph- | nd | nd | nd | nd | nd |
| 5j | 4-CH3CONH-Ph- | 55.07 | 56.00 | 60.05 | 66.89 | 15.72 |
| Aspirin | 36.02 | 54.44 | 62.06 | 76.06 | – | |
Fig. 3.
Basic structure for synthesized aspirin derivatives (5a-j).
3.2.2.2. In-vitro RBC membrane stabilization study
Considering the fact that RBC membrane stabilization could stabilize the lysosomes because of similarity in RBC and lysosomal membrane. If any compound can stabilize the lysosomes membrane it means it may show potent anti-inflammatory activity. Thus, it can be used to assess the anti-inflammatory potential of synthesized compounds. The synthesized compounds 5a-j were subjected to in-vitro RBC membrane stabilization study to check the anti-inflammatory activity. The anti-inflammatory activity of derived compounds was found to be superior at explored concentration (25 µg/mL) (Fig. 4). At a concentration of 25 µg/mL, the reference medicine aspirin provides 36.02 percent lysis protection (Fig. 4A), but other synthetic substances provided greater lysis prevention than 36.02 percent (Fig. 4A-C). At 50 µg/mL, the anti-inflammatory activity of 5a and 5j were 57.08 percent and 56.00 percent, respectively, whereas aspirin was 54.44 percent. The activity of 5d and 5h was found to be the same as that of aspirin, which is 54.44 percent lysis prevention (Fig. 4).
Fig. 4.
Graphical representation of Prevention of lysis by A) compound 5a, 5b, 5c; B) 5d, 5e, 5f; C) 5g, 5h & 5i; Aspirin was used as a reference compounds. Different colors indicate the different compounds from 5a to 5i in all three figures.
3.2.2.3. Cytotoxic study
A bioassay for brine shrimp lethality was carried out in the laboratory. The synthesized compound was poorly soluble in water. Therefore, it was dissolved in dimethyl sulfoxide (5 % DMSO) for further studies. The LD50 was estimated by calculating the generated data (Fig. 5 and Table 2). These findings were compared to aspirin. DMSO served as a positive control. Then, 30 active shrimps (larvae) were transferred to each test tube. These viable larval shrimps were counted after incubation (24 h) and 50 % lethal concentration (LD50) was estimated (Padmaja et al., 2002, Padmaja et al., 2002, Ved et al., 2010). In brine shrimp lethality bioassay compound 5j displayed the least mortality of shrimp indicating LC50 value of 1079.91 µg/mL compared to aspirin (2380.95 µg/mL). Similarly, compound, 5g, 5a and 5h were also lesser lethal than aspirin compounds with LC50 value of 1138.95, 1362.31, and 1457.72 µg/mL, respectively.
Fig. 5.
A) Graphical representation showing activity of compounds with respect to Aspirin. B) Graphical representation showing LC50 values of compounds with respect to Aspirin.
Table 2.
Brine shrimp lethality bioassay.
| Entry | Comp. | Conc. (µg/mL) |
Mortality of shrimps |
% Mean Mortality | LC50 µg/mL | ||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| 1. | Aspirin | 1000 | 7 | 5 | 6 | 20.00 | 2380.95 |
| 100 | 3 | 4 | 5 | 13.33 | |||
| 10 | 3 | 2 | 2 | 7.78 | |||
| 1 | 1 | 2 | 2 | 5.56 | |||
| 2. | 5a | 1000 | 10 | 12 | 9 | 34.44 | 1362.31 |
| 100 | 8 | 7 | 7 | 24.44 | |||
| 10 | 3 | 5 | 4 | 13.33 | |||
| 1 | 3 | 1 | 1 | 5.56 | |||
| 3. | 5g | 1000 | 14 | 12 | 11 | 41.11 | 1138.95 |
| 100 | 10 | 8 | 9 | 30.00 | |||
| 10 | 5 | 5 | 6 | 17.78 | |||
| 1 | 2 | 1 | 3 | 6.67 | |||
| 4. | 5h | 1000 | 10 | 9 | 10 | 32.22 | 1457.72 |
| 100 | 7 | 6 | 7 | 22.22 | |||
| 10 | 5 | 5 | 6 | 17.78 | |||
| 1 | 3 | 2 | 1 | 6.67 | |||
| 5. | 5j | 1000 | 13 | 14 | 12 | 43.33 | 1079.91 |
| 100 | 10 | 10 | 9 | 32.22 | |||
| 10 | 7 | 6 | 5 | 20.00 | |||
| 1 | 3 | 4 | 3 | 11.11 | |||
Positive control with DMSO has shown mortality of zero shrimps. No. of shrimp taken: 30.
3.2.3. In-vivo studies
3.2.3.1. Anti-inflammatory activity
The carrageenan-induced paw edema model was used to test the anti-inflammatory activity of two different compounds as an in vivo assessment. Table 3 summarized the values of edema inhibition of control, ibuprofen, 5g, and 5h. The paw volume was measured with a vernier calliper for 4 h, 30 min after the carrageenan injection and the percent protection was calculated. The obtained data was plotted on a graph, and it was discovered that the compounds had comparable anti-inflammatory activity to the standard.
Table 3.
Anti-inflammatory activity shown by the compounds in carrageenan-induced paw edema.
| Enrty | Compound No. |
Edema Inhibition (Mean ± SEM) Standard Error of the Mean |
% Inhibition | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | ½ h | 1 h | 2 h | 3 h | ||||
| 1 | Control | 2.42 ± 0.131 | 4.25 ± 0.264 | 4.56 ± 0.296 | 4.7 ± 00.297 | 4.84 ± 0.320 | 0 | |
| Carrageenan (positive control) | 2.41 ± 0.213 | 5.63 ± 0.142 | 5.75 ± 0.750 | 5.71 ± 00.652 | 5.61 ± 0.369 | 100 | ||
| 2 | Ibuprofen 5 mg/kg | 2.57 ± 0.118 | 4.24 ± 0.244 | 3.8 ± 0.207 | 3.32 ± 0.154 | 3.52 ± 0.277 | 66.90 | |
| 3 | 5g | Dose I (1.75 mg/kg) | 2.61 ± 0.110 | 3.62 ± 0.080 | 3.37 ± 0.073 | 3.13 ± 0.021 | 3.57 ± 0.140 | 77.11 |
| Dose II (3.5 mg/kg) | 2.58 ± 0.180 | 3.57 ± 0.081 | 3.23 ± 0.071 | 3.03 ± 0.033 | 3.25 ± 0.092 | 79.53 | ||
| 4 | 5h | Dose I (10 mg/kg) | 2.79 ± 0.043 | 4.00 ± 0.084 | 3.52 ± 0.086 | 3.28 ± 0.040 | 3.30 ± 0.125 | 78.65 |
| Dose II (15 mg/kg) | 2.68 ± 0.095 | 4.13 ± 0.065 | 3.66 ± 0.163 | 3.45 ± 0.085 | 3.52 ± 0.033 | 66.10 | ||
4. Molecular modelling studies
4.1. Molecular docking
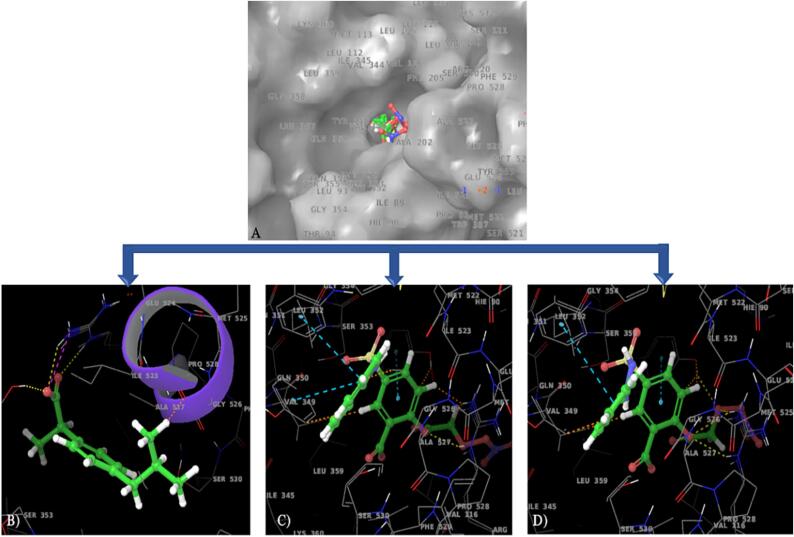
COX-2 enzyme is considered to be rate limiting step in the biosynthesis of inflammatory neurotransmitter prostaglandin and acts as a crucial target for anti-inflammatory and analgesic drugs. In the present study, we used abuprofen, the most commonly used anti-inflammatory drug as a reference molecule. Hence, for molecular modelling study, the co-crystallized structure of ibuprofen with COX-2 enzyme (pdb id: 4PH9) was used. The molecular docking studies were conducted to understand the ligand–protein interactions and binding affinity with minimum free energy which is needed to form a stable complex. The molecular docking study of ibuprofen and identified potent salicylate derivatives such as 5g and 5h were conducted and their binding pattern was analyzed. The results were obtained and different types of interactions with active site residues were summarized in Table 4. The Fig. 6 indicated the overlay of 2D and 3D interactive diagrams of ibuprofen (B), 5g (C), and 5h (D). Fig. 6A represents the molecular surface diagram of protein with superimposed structures of ibuprofen, 5g and 5h. The comparative analysis of 5g and 5h with ibuprofen revealed that synthesized compounds occupied the same binding cavity as that of ibuprofen. The docking scores for the tested compounds were found to be better than ibuprofen indicating the 5g-COX-2 and 5 h-COX2 complexes were more stable and had the good interactions with active site residues of COX-2 enzyme. The different crucial interactions observed are H bond, hydrophobic, π–π, and polar interactions. All three compounds formed the H bond with Arg121 & Tyr356 residues and these H bonds were stabilized by hydrophobic interactions with Phe358, Tyr356, Phe519, Phe382, Tyr349, Tyr386, Trp388, Phe206, Tyr116, Leu360, Leu353, Val350, Val345, Ile346, and Leu93 residues. Compared to ibuprofen, 5g and 5h had the larger number of hydrophobic interactions with active site residues indicating the more receptor affinity and potential binding at active site of COX-2 enzyme. While the molecules were displayed the polar interactions with Gln351, Ser354 and Ser531 residues except 5g and 5h had the additional Hie90 residues. Apart from these interactions, π–π interactions were observed in 5g and 5h with Tyr356. Thus, the greater number of interactions in 5g and 5h indicated the more affinity towards the COX-2 receptor with lesser binding free energy. Here, we aimed to minimize the GI related toxicities associated with aspirin which is associated with COX-I inhibitory properties. Considering the selective binding and inhibition by the synthesized compounds to the COX-2 enzyme, it is prudent to predict 5g and 5h with remarkably low in vivo toxicity as compared to aspirin. Hence, 5g and 5h molecules with reference aspirin were docked against COX-I enzyme using 3KK6 pdb id. The obtained results were displayed in Fig. 7 and different types of interactions were summarized in Table 4. The 5g and 5h molecule shown very poor docking score such as −5.151 and −3.545, respectively indicating that these two molecules selectively inhibited the COX-2 with better potency than aspirin. The lesser interactions of 5g and 5h with COX-I enzyme indicated the compounds are lesser toxic than aspirin and our hypothesis was also proven for GI protective effect with the synthesis of aspirin derivatives.
Table 4.
Summary of different types of crucial interactions displayed by Ibuprofen, 5g, 5h with the active site residues of COX-2 enzyme (PDB ID: 4PH9) and COX-1 (PDB ID 3KK6).
| Enrty | Molecules | Docking score | π–π stacking |
Interaction types |
||
|---|---|---|---|---|---|---|
| H bond | Hydrophobic | Polar | ||||
| COX-2 Enzyme | ||||||
| 1. | Reference (Ibuprofen) | −8.35 | Nil | Arg121 (2H Bonds), Tyr356 | Tyr356, Tyr349, Tyr386, Phe382, Phe519, Trp388, Val117, Leu526, Val524, Leu353 | Gln351, Ser354, Ser531 |
| 2. | 5g | −9.67 | Tyr356 | Arg121 (2H Bonds), Tyr356, | Phe358, Tyr356, Phe519, Phe382, Tyr349, Tyr386, Trp388, Phe206, Tyr116, Leu360, Leu353, Val350, Val345, Ile346, Leu93 | Ser120, Ser354, Ser531, Hie90 |
| 3. | 5h | −8.48 | Tyr356 | Arg121, Tyr356, | Phe358, Tyr356, Phe519, Phe382, Tyr349, Tyr386, Trp388, Phe206, Tyr116, Leu360, Leu353, Val350, Val345, Ile346, Leu93 | Ser120, Ser354, Ser531, Hie90 |
| COX-1 Enzyme | ||||||
| 1. | Reference (Ibuprofen) | −8.58 | Nil | Arg120, Tyr355 | Phe381, Tyr348, Tyr385, Trp387, Phe518, Pro528, Tyr355, Val349, Met525, Ala527, Leu357 | Ser353, Ser530 |
| 2. | 5g | −5.151 | Arg120 | Tyr355, Tyr385, Trp387 | Tyr348, Phe381, Phe518, Phe205, Tyr355, Trp387, Leu352, Leu507, Val119 | HIE90, Ser353, Ser530 |
| 3. | 5h | −3.545 | Trp387, Tyr355 | Arg120, Tyr355 | Phe381, Phe205, Tyr348, Phe205 Tyr355, Trp387, Phe518, Tyr355, Val349, | HIE90, Ser353, Ser530 |
Fig. 6.
Molecular docking analysis of Ibuprofen, 5g &5 h with the active site residues of COX-2 enzyme (PBD ID:4PH9); Fig. 6A: Molecular surface diagram of COX-2 enzyme with superimposed structure of all three compounds, 3D and 2D interactive diagrams of 6B) Ibuprofen, 6C) 5g, 6D) 5h, respectively.
Fig. 7.
Molecular docking analysis of Ibuprofen, 5g & 5h with the active site residues of COX-1 enzyme (PBD ID:3KK6); A) Molecular surface diagram of COX-1 enzyme with superimposed structure of all three compounds, 3D interactive diagrams of B) Ibuprofen, C) 5g, D) 5h respectively.
4.2. MD simulations
The docking study considered the rigid structure of protein. Hence, for better understanding of ligand–protein complexe,s molecular dynamics simulations studies (MD) was performed. It is done to analyze the movement of atoms in a complex over time which can be used to predict the stability of ligand–protein complex. For these, the docked complexes of all three molecules were simulated over 100 ns run of trajectory by using Desmond software. Different types of biophysical interactions between molecules and different atoms of protein were analyzed. RMSD and RMSF of molecules and protein backbone were used for the analysis of results. Fig. 8 represents the RMSD and RMSF values of ibuprofen (a), 5h (b) and 5g (c). The time bound analysis of different types of interaction between molecules and amino acid residues were also performed and results obtained were depicted in Fig. 9. The RMSD values were found to be as 1.6 Å, 2.8 Å and 5.6 Å for ibuprofen, 5h, and 5g, respectively. The results analysis also suggested that the ligand–protein complexes were quite stable over the entire run of 100 ns trajectory for all three molecules. None of the molecules deviated from protein active site over entire run. The lower RMSF values also indicated the molecules were not fluctuated from the active site and indicated the stability of docked complexes for ibuprofen, 5h, and 5g. The % interaction analysis of 5h revealed that H bond interactions with Arg121 (2H Bonds) observed in molecular docking study was conserved and remained for 97 % time over 100 ns run of MD trajectory. Similarly, with the 5g molecule, the H bond with Arg121 was retained for 98 % time. Overall results indicated that 5g-COX2 and 5h-COX2 complexes obtained in docking were quite stable over entire 100 ns run of trajectory. Thus, 5g and 5h are capable of doing conformational changes in COX-2 enzyme and formed the basis of potent and selective COX-2 inhibitory activity.
Fig. 8.
Structural changes observed (RMSD) relative to the first frame of protein backbone of C, C- and N of COX-2 enzyme a) Ibuprofen b) 5h c) 5g; RMSF (Root Mean Square Fluctuation) plot by residue of COX-2 protein for: a) Ibuprofen, b) 5h, c) 5g respectively.
Fig. 9.
Ligand-protein contacts histogram and % ligand–protein interactions with the COX-2 enzyme: (a) Ibuprofen b) 5h c) 5g respectively.
4.3. In-silico ADME calculations
Poor pharmacokinetic profiling and later stage toxicities of many drug molecules led to failure in later stage of drug discovery processes. Hence, early-stage prediction and determination of its pharmacokinetic and toxicity profiling are helpful for drug likeliness properties of lead molecules. The ibuprofen and the selected molecules (5g and 5h) were subjected to in-silico ADME calculation and results obtained were summarized in Table 5. These 5g and 5h molecules displayed 1 Ro5 and Ro3 violations as compared to ibuprofen. The other obtained values also indicated about the drug likeliness properties of 5g and 5h. The polar surface area of 5g and 5h was quite large. Hence, % human oral absorption of these molecules was low as compared to ibuprofen. Then, the toxicity profiling was predicted by online using freely available webserver Protox-II followed by their toxicities against different human organ. The obtained results were tabulated in Table 5. The results suggested 5g and 5h were quite safe to use and did not show any sever toxicity at explored dose.
Table 5.
In-silico ADME properties of reference molecule and 5g and 5h.
| Sr No. | Parameters | Reference | 5g | 5h |
|---|---|---|---|---|
| 1. | HB Donor | 1 | 2 | 3 |
| 2. | HB Acceptor | 2 | 13 | 14 |
| 3. | LogPo/w | 3.496 | 0.072 | −0.973 |
| 4. | logBBB | −0.487 | −3.772 | −3.716 |
| 5. | Metabolism | 2 | 1 | 2 |
| 6. | % Human oral Absorption | 92.674 | 15.211 | 8.391 |
| 7. | PSA | 46.701 | 202.748 | 212.002 |
| 8. | Ro5 Violation | 0 | 1 | 1 |
| 9. | Ro3 Violation | 0 | 1 | 1 |
| 10. | Molecular Weight | 206.284 | 462.387 | 427.342 |
5. Discussion
As per the proposed hypothesis, a total 10 (5a-j) NO releasing aspirin derivatives were synthesized. The hypothesis was designed by considering the fact that NO can protect the gastric mucosa from the side effects of NSAIDs. The synthesized compounds were characterized by their physicochemical properties such as melting points, mass spectral analysis, and NMR spectroscopic techniques. The (M + 1) peaks observed for synthesized compounds in positive mode ionization technique e.g. m/z ratio of compound 5a was found at 397.9 which indicates the formation of desired compound. In 1H NMR spectroscopy peak 11.29 δ ppm for carboxylic H was observed whereas aromatic protons were appeared at the region of 6.89 δ to 8.06 δ ppm. Similarly, IR spectrum of synthesized compounds exhibited all the peaks for different functional groups like 1753 cm − 1 (C = O ester), 3454 cm−1 (N–H), 3228 cm−1 (carboxylic OH) and 1296 cm−1 (–ONO2) indicating the formations of desired compounds. Then, the synthesized compounds "5a-5j" were subjected to biological evaluation. The acute toxicity of any compound indicates the adverse effect produced by any molecule either on single use or multiple use. It is prerequisite for preclinical study of any compound to check the acute toxicity of the newer compounds. In preclinical acute toxicity studies, two potent compounds such as 5g and 5h with the lowest effective dose and the highest non-lethal dose were tested to confirm safety concern. This study necessitated the use of 18 Swiss albino mice. Thus, the LD50 was 98.11 mg/kg and 17.5 mg/kg for compound 5g were calculated suggesting the compounds were safer to use for in-vivo studies (Walum, 1998, Jonsson et al., 2013, Jonsson et al., 2013) Rationally, NO acted as a protective agent to protect the gastric mucosa. The extent of NO release would provide the relative protection for gastric mucosa against the side effects of NSAIDs. %NO release for the compounds 5g and 5 h were found to be 18.20 and 17.82, respectively. Hence, these two compounds were subjected to further biological screening and in-silico studies. Taking RBC membrane as anti-inflammatory model, compound 5a and 5j were found to be superior in terms of protective nature at explored concentration (25 µg/mL) as compared to aspirin. At 100 µg/mL, the compound 5g has higher anti-inflammatory activity than aspirin. Compounds 5a and 5h have nearly equal activity to aspirin. Compound 5e and 5j showed comparable activity with the reference drug at 100 µg/mL. After establishing LD50 in viable larval shrimps, cytotoxic potential was assessed (Padmaja et al., 2002, Padmaja et al., 2002, Ved et al., 2010). The cytotoxic potential of selected compounds (5a, 5g, 5h, and 5j) was assessed and compared to aspirin. The results obtained were summerized in Table 2. Compared to aspirin, compound 5j demonstrated greater cytotoxic potential. Moreover, results obtained from carrageenan-induced paw oedema study, the compounds had comparable anti-inflammatory activity to the standard (ibuprofen). Compound 5g shows more potent activity than standard drug ibuprofen at the two selected dose levels (1.75 and 3.5 mg/kg). Compound 5 h shows more activity at dose I (10 mg/kg) and mostly equal activity at dose II (15 mg/kg). Hence, both the compounds were found to be more potent than ibuprofen. In molecular docking, COX-2 acts as a crucial target for anti-inflammatory drugs and identified potent salicylate derivatives 5g and 5h were docked for comparative assessment taking ibuprofen as reference. It was observed that the compound 5g and 5h occupied the same binding pocket and similar fashion like ibuprofen. Even the different types of interactions like H bond and hydrophobic interactions were also identical to ibuprofen. All three molecules displayed the H-Bond interactions with Arg121 (2H Bonds) and Tyr356 residues. These H-bond interactions were stabilized by hydrophobic interactions with Tyr356, Tyr349, Tyr386, Phe382, Phe519, Trp388, Val117, Leu526, Val524, and Leu353 active site residues. All these interactions were common in ibuprofen and our identified potent anti-inflammatory molecules 5g and 5h. Biological evaluation of these two molecules performed better than reference molecules and molecular docking study confirmed extra hydrophobic interactions with Phe206, Tyr116 residues. Moreover, stacking interactions were also observed in 5g and 5h which made the complex more stable than that of ibuprofen. These interactions were missing in ibuprofen complex. These extra interactions were considered to be responsible for more potent anti-inflammatory activity of newer salicylate derivatives. To reduce GI associated side effects as observed in aspirin, the derivatives (5g and 5h) selectively bind to COX-2 enzyme with possibly mitigated toxicity. Thus, both compounds executed poor docking score with COX-1 binding site. It indicated that docked complexes (5g and 5h molecules) were not stable and the molecules would not be potent inhibitors of COX-I enzyme whereas 5g and 5h have shown the good docking score −9.67 and −8.48, respectively against COX-2 enzyme. Hence, we can say that these docked complexes are stable and molecules can selectively inhibit the COX-2 enzyme. On the basis of poor docking profile against COX-I, we can say that the GI toxicity of synthesized molecules would be lesser than aspirin. Furthermore, to see the stability of docked complexes of ibuprofen, 5g and 5h MD simulations studies were performed. Furthermore, it was mandatory to validate the molecular docking and to assess the stability of docked complex after simulation. The results analysis also suggested the ligand–protein complexes were quite stable over the entire run for all three molecules without deviation from protein active sites. Similarly, 5g molecule has H bond with Arg121 and it was retained for 98 % time. By considering the RMSD, RMSF values and % interaction of 5g and 5h molecules with active site residues, we can suggest that these two complexes were quite stable and responsible for the potent anti-inflammatory activities of newer salicylate derivatives (5g and 5h). In new drug development and its subsequent success from laboratory to clinical bed include a long journey period and a huge burden of financial budget. Therefore, in silico prediction of a newly synthesized molecules gives an idea about various pharmacokinetic and pharmacodynamics properties for newly synthesized lead compound. In our case, it was necessary to predict the success of pharmacokinetic and pharmacodynamics outcomes based on simulation with reduced toxicity. Thus, ibuprofen and lead compounds such as 5g and 5h were predicted in in-silico program for ADME (absorption, distribution, metabolism, and excretion). Results were quite convincing for safe delivery at explored dose in the treatment of inflammation. These computational in-silico ADME data do not correlate the real and complex human biology hence it is further needed to validate by in-vitro and in-vivo pharmacokinetic studies.
6. Conclusion
GIT (gastro intestinal tract) toxicities of commonly used NSAIDs are serious problem nowadays. To mitigate the GI toxicity of aspirin, newer NO releasing derivatives of aspirin (5a-j) were synthesized and evaluated. Out of these 10 synthesized derivatives 5a, 5g, 5h, and 5j were found to be more toxic than aspirin. In NO releasing assay, compound 5a was associated with the highest nitric oxide release (20.86 %), followed by compound 5g (18.20 %). Compound 5g and 5h were tested for carrageenan induced paw oedema wherein 5g inhibited by 77.11 % and 79.53 % & 5h by 78.56 % and 66.10 %- of produced paw oedema. Compared with standard drug ibuprofen which inhibited by 66.90 % Thus, 5g and 5h were found to be more potent than ibuprofen. The obtained biological results were validated by in-silico docking (−8.35, −9.67, −8.48 for ibuprofen, 5a and 5h, respectively) and MD simulations studies. Compound 5a and 5h displayed the better docking score than ibuprofen and the interactions with receptors were also found to be better than ibuprofen. MD simulations studies indicated that 5a-COX-2 and 5 h-COX-2 complexes were quite stable. Thus, by considering all these data we can take these two molecules in further stages of drug discovery processes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education”, in Saudi Arabia, for funding this research (IFKSUOR3-068-3).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.101925.
Contributor Information
Afzal Hussain, Email: amohammed2@ksu.edu.sa.
Zahid R. Bhat, Email: zrbhat@mdanderson.org.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- C. Antoniades, D. Tousoulis, C. Stefanadis, 2007. Nitric oxide-releasing aspirin: Will it say NO to atherothrombosis?, Elsevier. 118: 170-172. [DOI] [PubMed]
- J.A. Bell, Y. Cao, J. R. Gunn, et al., 2012. PrimeX and the Schrödinger computational chemistry suite of programs.
- T. Brzozowski, P. Konturek, S. Konturek, et al., 2005. Role of prostaglandins in gastroprotection and gastric adaptation. 56, 33-55. [PubMed]
- Catella-Lawson F., Reilly M.P., Kapoor S.C., et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- Coneski P.N., Schoenfisch M.H. Nitric oxide release: part III. Measurement and reporting. Chem. Soc. Rev. 2012;41:3753–3758. doi: 10.1039/c2cs15271a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013;15:1–10. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S., Santucci L., Gresele P., et al. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- S. Fiorucci, P.J.D. Del Soldato, L. Disease, 2003. NO-aspirin: mechanism of action and gastrointestinal safety. 35, S9-S19. [DOI] [PubMed]
- Green G.A. Understanding NSAIDs: from aspirin to COX-2. Clin. Cornerstone. 2001;3:50–59. doi: 10.1016/s1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- Hawkey C.J. COX-2 inhibitors. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- Hawkey C.J., Langman M.J.S. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut. 2003;52:600–608. doi: 10.1136/gut.52.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilário M.O.E., Terreri M.T., Len C.A. Nonsteroidal anti-inflammatory drugs: cyclooxygenase 2 inhibitors. J. Pediatr. 2006;82:S206–S212. doi: 10.2223/JPED.1560. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Salzman E.W., Harker L., et al. Aspirin and other platelet active drugs: relationship among dose, effectiveness, and side effects. Chest. 1989;95:12S–18S. [PubMed] [Google Scholar]
- M. Jonsson, M. Jestoi, A. V. Nathanail, et al., 2013. Application of OECD Guideline 423 in assessing the acute oral toxicity of moniliformin. 53, 27-32. [DOI] [PubMed]
- Jonsson M., Jestoi M., Nathanail A.V., et al. Application of OECD Guideline 423 in assessing the acute oral toxicity of moniliformin. Food Chem. Toxicol. 2013;53:27–32. doi: 10.1016/j.fct.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Kedir H.M., Sisay E.A., Abiye A.A. Enteric-coated aspirin and the risk of gastrointestinal side effects: a systematic review. Int. J. Gen. Med. 2021:4757–4763. doi: 10.2147/IJGM.S326929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepeis J.L., Lindorff-Larsen K., Dror R.O., et al. Long-timescale molecular dynamics simulations of protein structure and function. Curr. Opin. Struct. Biol. 2009;19:120–127. doi: 10.1016/j.sbi.2009.03.004. [DOI] [PubMed] [Google Scholar]
- R. Kodela, M. Chattopadhyay, K.J.A.m.c.l. Kashfi, 2012. NOSH-Aspirin: A novel nitric oxide–hydrogen sulfide-releasing hybrid: A new class of anti-inflammatory pharmaceuticals. 3, 257-262. [DOI] [PMC free article] [PubMed]
- Laine L. Gastrointestinal effects of NSAIDs and coxibs. J. Pain Symptom Manage. 2003;25:32–40. doi: 10.1016/s0885-3924(02)00629-2. [DOI] [PubMed] [Google Scholar]
- McDonald D.D. Predictors of gastrointestinal bleeding in older persons taking nonsteroidal anti-inflammatory drugs: Results from the FDA adverse events reporting system. J. Am. Assoc. Nurse Pract. 2019;31:206–213. doi: 10.1097/JXX.0000000000000130. [DOI] [PubMed] [Google Scholar]
- Orlando B.J., Lucido M.J., Malkowski M.G. The structure of ibuprofen bound to cyclooxygenase-2. J. Struct. Biol. 2015;189:62–66. doi: 10.1016/j.jsb.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmaja R., Arun P.C., Prashanth D., et al. Brine shrimp lethality bioassay of selected Indian medicinal plants. Fitoterapia. 2002;73:508–510. doi: 10.1016/s0367-326x(02)00182-x. [DOI] [PubMed] [Google Scholar]
- R. Padmaja, P. Arun, D. Prashanth, et al., 2002. Brine shrimp lethality bioassay of selected Indian medicinal plants. 73, 508-510. [DOI] [PubMed]
- Rose P.W., Prlić A., Altunkaya A., et al. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Vadivu, K. S. Lakshmi, 2008. In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp Laurina. ||| Bangladesh Journal of Pharmacology. 3, 121-124.
- Vane J.R., Botting R.M. The mechanism of action of aspirin. Thromb. Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- C.H. Ved, N. S. More, S. S. Bharate, et al., 2010. Cytotoxicity screening of selected Indian medicinal plants using brine-shrimp lethality bioassay. 4, 389-396.
- Ved C.H., More N.S., Bharate S.S., et al. Cytotoxicity screening of selected Indian medicinal plants using brine-shrimp lethality bioassay. Adv. Nat. Appl. Sci. 2010;4:389–396. [Google Scholar]
- Wallace J.L. Prostaglandins, NSAIDs, and cytoprotection. Gastroenterol. Clin. North Am. 1992;21:631–641. [PubMed] [Google Scholar]
- Wallace J.L., Ignarro L.J., Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat. Rev. Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- Walum E. Acute oral toxicity. Environ. Health Perspect. 1998;106:497–503. doi: 10.1289/ehp.98106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E.J.E.h.p. Walum, 1998. Acute oral toxicity. 106, 497-503. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.