Abstract
Background
Physical frailty is a condition where a person has decreased physical reserve and resilience to stressors. Oral frailty, on the other hand, refers to a decline in oral function in conjunction with reductions in cognitive and physical functioning. Poor oral health, encompassing factors such as functional, physiological, psychosocial, and therapeutic aspects, can lead to physical frailty.
Objectives
Assess the prevalence of physical and oral frailty in geriatric patients attending health centres in Kerala, India.
Methodology
.
Design
Cross-sectional study.
Setting
Amrita Institute of Medical Sciences (Kochi), Amrita Kripa Charitable Hospital (Wayanad) and Amrita Urban Health Centre in (Kaloor).
Participants
250 geriatric participants above 60 years.
Measurements
The participants' physical frailty was evaluated using Fried's Frailty Phenotype, the Reported Edmonton Frail Scale, and sarcopenia screening. The assessment of oral frailty was based on several factors such as current dental status, chewing ability, tongue pressure, the Repetitive Saliva-Swallowing Test, Oral Diadokinetic rates, Xerostomia, and the Oral and Maxillofacial Index. The evaluation was using a questionnaire and clinical examination. Bivariate analysis was performed for additional variables, and multivariate analysis was utilized to examine the relationship between oral and physical frailty.
Results
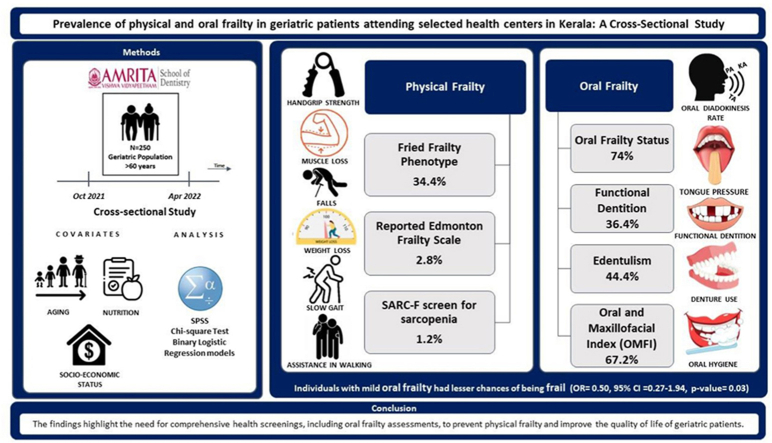
56 % of study participants were males, and the mean age was 68 ± 6.02 years. 34.4 % were physically frail, and the remaining were pre-frail using Fried Frailty Phenotype. 67 % showed oral frailty using the Oral and Maxillofacial Frailty Index (OMFI). Using six domains of the oral frailty status (Tanaka) showed that 74 % of individuals had an increased risk of new onset of physical frailty. In the adjusted model, individuals with mild oral frailty had lesser chances of being frail (OR = 0.509, 95 % CI = 0.274–1.946, p-value = 0.033).
Conclusion
The prevalence of physical frailty was 34.4 %, and oral frailty status was 74 %. The findings implied a need to include oral frailty assessments in the comprehensive general health screening for geriatric patients.
Keywords: Physical frailty, Oral frailty, Geriatric, Aging, Oral health, Longevity
Graphical abstract
1. Introduction
Aging is a global concern as per the Sustainable Development Goals.1 To address this concern, the “United Nations Decade of Healthy Ageing (2021–2030)1 initiates a global effort focusing on older people to uplift their lives along with their families, and the communities in which they live”. By 2030, one out of every six individuals globally will be 60 years or older.1 In 2021, the longest life expectancy was 85 years in Hong Kong and 70 years in India. The percentage of the elderly population in India is increasing by 2 % every decade.2 As of 2021, Kerala has the highest percentage (16.5 %) of elderly individuals in its population among all states in India.3 The increasing elderly population thereby increases this population's healthcare needs.
Physical frailty refers to a medical condition resulting from multiple factors, leading to heightened vulnerability in individuals, making them more susceptible to dependence and mortality. Frail individuals are at a higher risk of experiencing adverse health consequences, including falls, illnesses, physical and mental dependency, hospitalization, and ultimately, death.4 Frailty has been linked to various factors, including age, low body mass index, female gender, living alone, lack of physical activity, polypharmacy, smoking and drinking habits, low vitamin D levels, and malnutrition.5 In Japan, the overall prevalence of frailty is reported to be 7.4 %.6,7 The prevalence of physical frailty in India ranges from 16.3 % to 55.5 %.8 Efforts to reduce the risk and prevalence of frailty are crucial for extending healthy life expectancy among the elderly population.9
In 2013, Japan introduced a new concept called “oral frailty.”10 Oral frailty is defined (Japan Dental Association 2020) as “a series of events and processes contribute to changes in oral conditions (number of teeth, oral hygiene, oral functions, etc.) as a result of aging. This is accompanied by a decrease in interest in oral health, reduced physical and mental capacity, and an increase in oral frailty, leading to eating dysfunction. The outcome is a decline in both physical and mental function”.10 In frail patients, there is a rapid decline in the occlusal force, thickness of the masseter muscle, rate of oral diadochokinesis, and oral motor skills associated with speaking.11 The prevalence of oral frailty varies widely worldwide, with estimates ranging from 4.1 % to 63.7 %.12
Physical frailty and oral hygiene status are bidirectional. Physical frailty can have an impact on oral hygiene and the condition of the remaining teeth in elderly patients. Poor oral health is linked to frailty by four mechanisms, these include functional, physiological, psychosocial, and therapeutic factors.13 Various studies have found that the number of teeth is significantly related to frailty, with participants having 20 or more teeth showing a reduced risk of frailty compared to those who are edentulous.14 The number of remaining teeth is a risk factor for malnutrition, speech disability, loss of weight, physical strength, mobility, and poor mental health.15
Natural teeth are positively correlated with the quality of life in frail elderly persons. Additionally, the maintenance of teeth promotes a positive body image and self-worth.16 Men generally cared less about having natural teeth than women did in a gender-specific study, regardless of how frail they were.17,24 Frailty and oral health are thought to be related via the physiological/inflammatory pathway. Studies have indicated that periodontitis was associated with frailty incidence.17, 18, 19 Five significant longitudinal relationships between oral and physical frailty were found in a recent systematic study by Hakeem FF et al., which was published in 2019.20 This finding emphasizes the significance of oral health as a predictor of frailty.20,21
Currently, literature shows little or no evidence in India assessing the prevalence of physical frailty and oral frailty or oral frailty alone. Due to the increasing number of elderly populations in the state of Kerala, there is a need for early or timely screening and assessments to evaluate, interpret and diagnose physical and oral frailty, which improves their quality of life. Therefore, the current study aimed to give baseline information on physical and oral frailty in the geriatric population in Kerala and to incorporate oral examinations in geriatric departments of health institutes.
2. Methodology
2.1. Study design and participants
A hospital based cross-sectional study was designed. Ethical approval was received from the Institutional Ethics Committee of Amrita Institute of Medical Sciences in Kerala, India (ECASM-AIMS-2021-176, Date:23-02-2021). The selected three health centres, consisted of Amrita Institute of Medical Sciences in Kochi, Amrita Kripa Charitable Hospital in Kainatty, Kalpetta, Wayanad, and Amrita Urban Health Centre in Kaloor, Kochi. The study participants included 250 elderly patients from the chosen health centres. All participants provided written consent to participate and were informed of the details of the study. To be eligible for the study, patients had to be over 60 years of age, ambulant, and able to respond to the questionnaire, regardless of any underlying medical conditions. Participants who were unresponsive or unwilling to take part in the study were excluded.
A close-ended questionnaire, an oral examination, and a physical examination to assess frailty and associated factors were designed for the interview. The questionnaire was divided into six domains, including a detailed assessment of the demographic details, general examination, oral hygiene habits, dietary habits, physical frailty and oral frailty.
2.2. Determination of physical frailty
Physical frailty was measured using four scales: Fried's Frailty Phenotype,22 Reported Edmonton Frail Scale (REFS)23 and SARC-F sarcopenia.24
-
1.
Fried's Frailty Phenotype- Suppose three of the five domains are positive. In that case, it is defined as frail, with Prefrail-impairments in one or two of the five domains, Robust -without impairments in any of the five domains (Domains are: Weight loss; Weakness; Endurance/Exhaustion; Slowness; Low level of physical activity).
Unintentional weight loss was determined as a loss of at least 2 kg or at least 5 % of one's body weight over the past six months. Handgrip strength, an indicator of weakness, was measured using a hand-held dynamometer. Slowness was measured by assessing gait speed over a distance of 5 m. Exhaustion was assessed using two self-reported questions. Low activity was defined as engaging in less than 30 min of moderate physical activity, such as walking, or less than 20 min of vigorous exercise, for three days per week.
-
2.
Reported Edmonton Frail Scale (REFS)- Subjective measurements were taken in the following areas: cognition, overall health, functional independence, social support, medication use, nutrition, mood, and self-reported performance. The total score for these measures was 18.
-
3.
The SARC-F sarcopenia screening tool was assessed based on five components: strength, assistance in walking, rising from a chair, climbing stairs, and falls. The scores for each component range from 0 to 2 points, and the total score ranges from 0 to 10.
-
4.
Other physical frailty measures included the assessment of Body Mass Index (BMI), and Mini Nutritional Assessment (MNA).25
2.3. Determination of oral frailty
-
1.
Oral functions26: The ability to chew at least five peanuts was used as a marker of overall chewing performance, known as chewing ability. The frequency of Xerostomia (never, sometimes, often, always) were also evaluated. The Tongueometer™ instrument is used to assess tongue endurance and strength. The air-filled bulb of the gadget is positioned between the tongue and the hard palate. A disposable sleeve was used to cover the bulb before recording. To obtain maximum pressure values, subjects were instructed to press their tongues against the palate as hard as they could for 7 s. The readings were collected from the mobile phone application connected to the device using Bluetooth. The Repetitive Saliva Swallowing Test, or RSST, was first used in Japan by Oguchi et al.27 The patient in this screening test is asked to repeatedly swallow their saliva within a 30-s time frame, while the assessor counts the number of swallows by either feeling the larynx or observing it. The diadochokinetic rate, which is measured through this test, has been utilized in the assessment, diagnosis, and treatment of various oral conditions. Changes in the anatomy and physiology of the central nervous system and peripheral oral and speech production components can be observed through the diadochokinetic rate. The Count-by-Time test was used for data collection. The syllables “pa,” “ta,” and “ka” was repeated for 15 s and recorded. Three subjective measures were taken from the 25-item questionnaire of the Kihon Checklist to assess frailty. At baseline, an oral frailty score ranging from zero to six was associated with an increase in risk of physical frailty and sarcopenia.
-
2.
Dental status: Number of teeth present and absent, Mobility (score: 0, 1, 2, 3), Wasting diseases (Attrition, Abrasion, Erosion), Tender on percussion (Present/Absent), Fractured teeth, Denture. The Oral and Maxillofacial Index consisted of ten items that evaluated various aspects of oral health, including tooth or gum pain and bleeding, difficulties in chewing, the need for water when eating dry food, jaw pain or difficulty opening the mouth, intra-oral pain or ulceration, altered or impaired taste perception, limitations in jaw or tongue movements, difficulty speaking or pronouncing words, difficulties with swallowing, and limitations in facial expressions.
2.4. Additional variables
The socio-demographic characteristics included age, gender, marital status, living situation, and socioeconomic status (Kuppuswamy scale28-assesses income, education, and occupation). Other independent variables like oral hygiene habits, smoking habits and alcohol consumption were also collected. Oral hygiene habits included methods used for cleaning teeth, materials used for cleaning teeth, fluoridated toothpaste, frequency of cleaning teeth, the approximate duration of use, changes to the toothbrush, tongue cleaning aid, and frequency of dental visits. Dietary habits were collected using a Mini Nutritional Assessment (MNA) scale by the Nestle Nutrition Institute. Sleep apnoea was recorded using Obesity, Snoring Apneas in 50 years or the OSA 50 scale.
2.5. Sample size estimation
Based on Biritwum et al.29 on physical frailty, the prevalence of frailty was 55.5 %. Based on a study by Cakmur et al.,30 the prevalence of oral frailty was 57.1 %.
On applying the formula:
Sample size: (n)= (Z1−α/2)2 p (1-p)/d2.
n = Desired sample size.
Z 1−α/2 = Critical value and a standard value for the corresponding level of confidence. (At 95 % CI or 5 % level of significance (type-I error), it is 1.96).
P = Expected prevalence or based on previous research
q = 1-p
d = Margin of error or precision.
With 95 % confidence and 10 % allowable error, the minimum sample size comes to 92 and 94, respectively. So, the minimum sample size comes to 186. The final sample size was rounded to a total sample of 250.
2.6. Statistical analyses
Descriptive analyses was performed. In the study, categorical variables were analysed using frequency distribution while continuous variables were presented as the mean and standard deviation. To examine the relationship between oral and physical frailty markers, a chi-square test was employed. Hypotheses were tested using binary logistic regression models, and the results of the associations were reported as odds ratios (OR) with 95 % confidence intervals (95 % CI) and p-values. Statistical significance was determined if the p-value <0.05. Collected data was recorded in Microsoft Excel sheet and all statistical analyses were performed in IBM's SPSS version 23 (IBM SPSS for Windows, SPSS INC., Chicago, IL, USA).
3. Results
3.1. Descriptive statistics
A total of 250 geriatric participants were included in this study (Table 1), among which 56 % were males, and the mean age was 68 ± 6.02 years. Among the selected health centres, 57.2 % of the participants were from the tertiary health centre, and 42.8 % were from the satellite centres. Based on the Kuppuswamy28 socioeconomic status scale, 31.6 % belong to the upper middle class, 27.6 % to the upper lower class, 22.4 % to the lower middle class, 14.4 % to the lower class, and 4 % to the upper class. Around 50 % of the participants fall under the normal weight category, followed by 32 % being overweight, 8.8 % underweight, and 9 % obese.
Table 1.
Characteristics of study population.
| Variables | n | % | |
|---|---|---|---|
| Health Centre | Centre 1a | 143 | 57.2 |
| Centre 2b | 12 | 4.8 | |
| Centre 3c | 95 | 38 | |
| Age in years | 60–70 | 177 | 70.8 |
| Above 70 | 73 | 29.8 | |
| Gender | Male | 141 | 56.4 |
| Female | 109 | 43.6 | |
| Religion | Hindu | 175 | 70 |
| Muslim | 32 | 12.8 | |
| Christian | 43 | 17.2 | |
| Marital status | Married | 238 | 95.2 |
| Unmarried | 12 | 4.8 | |
| Living alone | Yes | 21 | 8.4 |
| No | 229 | 91.6 | |
| Socioeconomic status (Kuppuswamy Scale) | Upper | 10 | 4 |
| Upper middle | 79 | 31.6 | |
| Lower middle | 56 | 22.4 | |
| Upper lower | 69 | 27.6 | |
| Lower | 36 | 14.4 | |
| Medical history | Less than 1 | 95 | 38 |
| More than 1 | 83 | 33 | |
| NRHd | 72 | 28.8 | |
| Sleep Apnea – Obesity, Snoring, Apneas – 50 years (OSA-50) | No risk of Sleep Apnoea | 22 1 |
88.4 |
| Risk of Sleep Apnoea | 29 | 11.6 | |
| BMI (kg/m2) | Underweight | 22 | 8.8 |
| Normal weight | 125 | 50 | |
| Overweight | 80 | 32 | |
| Obese | 23 | 9 | |
| Waist circumference (cm) | Normal | 182 | 2.8 |
| Abnormal obesity | 68 | 27.2 | |
| Mini Nutritional Assessment (MNA) | Normal nutritional status | 163 | 65.2 |
| At risk of malnutrition | 87 | 34.8 | |
| Malnourished | 0 | 0 | |
Centre 1: Amrita Institute of Medical Sciences.
Centre 2: Kaloor.
Centre 3: Kalpetta.
NRH: No Relevant History.
3.2. Association between oral frailty with physical frailty
The results showed that people with physical frailty were 1.51 times more likely to experience oral frailty (OR = 1.51, 95 % CI = 0.84–2.71, p-value = 0.159) compared to those without physical frailty. However, it was not statistically significant. Association between the oral and maxillofacial index and physical frailty showed that according to the Fried frailty phenotype, individuals with physical frailty had a 50 % lesser chance of getting mild oral frailty (OR = 0.499, 95 % CI = 0.276–0.901, p-value = 0.020) than individuals without physical frailty. A statistically significant difference in physical frailty is shown in Table 2.
Table 2.
Association between oral frailty status and physical frailty measures.
| Variables |
Oral Frailty score |
Number of missing teeth |
OMFI |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Less than 2 |
More than 2 |
OR (95 % CI) |
Below 20 |
Above 20 |
OR (95 % CI) |
Normal |
Mild |
OR (95 % CI) |
|||||||
| n | % | N | % | n | % | N | % | n | % | N | % | ||||
| Fried's Frailty Phenotype (FFP) | |||||||||||||||
| Frail | 27 | 10.8 | 59 | 23.6 | 1.51 | 48 | 19.2 | 38 | 15.2 | 1.01 | 20 | 8 | 66 | 24.8 | 0.49 |
| Prefrail | 38 | 15.2 | 126 | 50.4 | (0.15–2.71) | 91 | 36.4 | 73 | 29.2 | (0.59–1.71) | 62 | 24.8 | 102 | 40.8 | (0.27–0.90) |
| Reported Edmonton Frail Scale (REFS) | |||||||||||||||
| Apparently Vulnerable | 1 | 0.4 | 6 | 6.8 | 2.14 | 3 | 1.2 | 4 | 1.6 | 1.69 | 2 | 0.8 | 5 | 2 | 1.22 |
| No frail | 64 | 25.6 | 179 | 71.6 | (0.47–18.16) | 136 | 54.4 | 107 | 42.8 | (0.37–7.73) | 80 | 32 | 163 | 65.2 | (0.23–6.46) |
| Sarcopenia | |||||||||||||||
| Risk of sarcopenia | 1 | 0.4 | 2 | 0.8 | 0.69 | 1 | 0.4 | 2 | 0.8 | 2.53 | 0 | 0 | 3 | 1.2 | 1.01 |
| No risk of sarcopenia | 64 | 25.6 | 183 | 73.2 | (0.77–7.84) | 138 | 55.2 | 109 | 43.6 | (0.22–28.29) | 82 | 32.8 | 165 | 66 | (0.99–1.03) |
Multivariate logistic regression analysis (Table 3) revealed that in the adjusted model, individuals with oral frailly had 1.6 times more likely to have physical frailty (OR = 1.662, 95 % CI = 0.903–3.057, p-value = 0.103) than individuals without physical frailty. However, it was insignificant. In the adjusted model, individuals with mild oral frailty had 50 % lesser chances of having physical frailty and were statistically significant (OR = 0.509, 95 % CI = 0.274–1.946, p-value = 0.033) than individuals without physical frailty.
Table 3.
Multivariate analysis.
| Model 1 Fried's Frailty Phenotype (FFP) and Oral Frailty Status |
Model 2 Fried's Frailty Phenotype (FFP) and Oral and Maxillofacial Index (OMFI) |
|||
|---|---|---|---|---|
| Variable | Reference category | Crude OR | Adj OR (95 % CI) | Adj OR (95 % CI) |
| Age (years) (Above 70) | 60–70 | 0.61 | 0.50 (0.27–0.93) | 0.55 (0.30–1.02) |
| Gender (Female) | Male | 0.77 | 0.62 (0.35–1.09) | 0.64 (0.36–1.13) |
| BMI (kg/m2) (Abnormal Weights) | Normal | 1 | 1.06 (0.61–1.83) | 0.99 (0.57–1.73) |
| Sleep Apnoea (More than 5) | Less than 5 | 1.43 | 1.70 (0.68–4.24) | 1.71 (0.68–4.26) |
| Oral Frailty Score (More than 2) | Less than 2 | 0.08 | 1.66 (0.90–3.05) | 0.50 (0.27–0.94) |
| Prosthesis | ||||
| <20 teeth with prosthesis | >20 teeth with prosthesis | 0.79 | 0.75 (0.39–1.42) | 0.77 (0.40–1.46) |
| <20 teeth without prosthesis | 1.5 | 1.38 (0.12–15.24) | 1.51 (0.14–15.94) | |
| >20 teeth without prosthesis | 1.33 | 1.50 (0.69–3.27) | 1.34 (0.61–2.95) | |
| No prosthesis | 0.5 | 0.46 (0.13–1.61) | 0.41 (0.11–1.44) | |
Dependant variable is Physical frailty (FFP) categorised into frail and prefrail.
4. Discussion
The current study assessed the prevalence of physical and oral frailty in geriatric patients attending selected health centres in Kerala. The study aimed to find the correlation between the physical and oral frailty.
We assessed Physical frailty using different scales, like the Fried frailty Phenotype, the Reported Edmonton frail scale (multidimensional presentations of frailty), and the SARC-F scale (sarcopenia). Using the fried frailty phenotype, prevalence of physical frailty in our study was 34.4 %. The data from Longitudinal Ageing Study in India (35 Indian states and union territories) showed that the prevalence of physical frailty was 35.5 % (Irshad et al., 2022) and 29.94 % (Thakkar et al., 2022). A study by Kamdem et al. (132) in Switzerland showed that the prevalence of frailty was 35.4 % which was similar to our finding. A study by Iwasaki et al. (2017) in Japan showed less prevalence (22 %) of physical frailty as they have healthier lifestyle and long-life expectancy.20 Studies by (Ramsay et al., 2019) in Australia and UK respectively showed a prevalence of 19 %.19 Using Reported Edmonton Frail Sale (REFS), prevalence of physical frailty was 2.8 %. A study by Rath et al., 2021 in Haryana, India showed a prevalence of 47.3 %. A study by Shwe et al., 2019 in Australia showed a prevalence of 66 %.12 The difference in prevalence is due to the geographical and culturally diverse nature of participants. Using SARC- F screen for sarcopenia, our findings showed less prevalence (1.2 %) compared to results from India (Shaikh.,2020) and Singapore (Lim.,2020) as it shows multi-dimensional state of vulnerability arising from a complex combination of biological, cognitive, and social factors.31
As of 2021, the global prevalence of oral frailty studies ranged from 4.1 % to 63.7 %.12 Oral frailty studies have used different types of tools or have assessed individual parameters. Most studies have used Oral Frailty Status (Tanaka et al.) which includes six measures namely: masticatory function, tongue pressure, oral diadochokinesis, difficulty swallowing, difficulty chewing, dry mouth.26 Some of the individual parameters assessed are, number of teeth present, missing teeth/edentulism, periodontal status, and dental prosthesis. Our study showed the prevalence of oral frailty as 74 % using the Oral Frailty Status. Similar findings to our study were observed in a study by Hironaka et al. (2020)32 in Japan, where the prevalence of Oral Frailty was 63.7 % (n = 632). However, Tanaka et al. (2018)26 in Japan showed a prevalence of oral frailty of 16 % (n = 1151). A study by Torres et al. (2020)33 in Brazil showed that of 689 participants, 47 % had edentulism (n = 315), and 16 % had 20 or more teeth. Our study showed 44.4 % edentulism and 36.4 % had 20 or more teeth. A study by Andrade et al. (2013)34 in Brazil showed that of 1374 participants, 45 % of the individuals were edentulous, and 79.5 % used a dental prosthesis. Our study showed that 26.4 % had used a dental prosthesis, and 75 % needed a dental prosthesis. Variations of prevalence in oral frailty are due to the differences in parameters attributed to each of the studies across the countries with differences in oral health approach and nutritional changes. When Oral and Maxillofacial Frailty Index (OMFI) was used to assess the frailty, our study showed a prevalence of 67.2 %. There are no other studies in India or globally to compare the prevalence using the OMFI tool. The variation in the prevalence of oral frailty may be due to the lack of standardized parameters or tools in assessment of oral frailty. This observation strengthens the needs of development and validation of the oral frailty assessment tools.
The relationship between oral frailty and physical frailty are associated with increased mortality, disability, and decreased quality of life. On using the Fried frailty phenotype, our study found that frail individuals had greater odds of having oral frailty. However, another study26 found that oral frailty was significantly associated with physical frailty. These results suggest that the relationship between oral frailty and physical frailty may be complex and multifactorial, with different measures of frailty showing different associations. On using the Reported Edmonton Frail Scale to explore the relationship between oral frailty and physical frailty, our study found that individuals vulnerable to frailty had greater odds of having oral frailty. Furthermore, self-reported oral health was found to have an independent negative association with frailty.11 This result emphasizes the importance of maintaining good oral health in older adults to prevent frailty. Therefore, our study suggests that oral frailty and physical frailty are closely related and may share common risk factors. Maintaining good oral health and using prosthesis may play an important role in preventing frailty in older adults. These findings highlight the need for a multidisciplinary approach to the management of frailty that includes dental care as an essential component.
Frailty is a condition that often accompanies aging and is characterized by a decreased ability to function physically and physiologically.35 Our study showed that, with a mean age of 68 years, 22 % of the population in the 60–70 age category were classified as frail according to Fried's Frailty Phenotype, and 50 % of the study population had oral frailty. This suggests that frailty is a significant issue in this age group. Additionally, Shimada et al. reported that the prevalence of frailty among Japanese adults aged 65 or older was estimated to be 11.3 % in 2013.35 The reason for the differences in the prevalence of frailty in different populations and age groups can be attributed to several factors. One major factor is genetic differences, which can affect the physiological changes that occur with aging and increase the risk of frailty. Environmental factors such as nutrition, exercise, and access to healthcare can also play a role in the development of frailty.
There is a correlation between frailty and socioeconomic status (SES), with frailty being more prevalent among people with lower SES. Our study mentioned the use of Kuppuswamy scale and found that 27 % of the study population were in the upper and lower classes. In contrast, a study by Felix et al.36 reported that 75 % of their study population belonged to the lower class. This indicates that the relationship between frailty and SES is significant and should be taken into consideration when developing interventions to prevent and manage frailty. Socioeconomic factors like income, education, and social support can also influence the prevalence of frailty. For instance, individuals with lower income and education levels may be more likely to experience frailty due to limited access to healthcare and resources that support healthy aging. The link between nutrition and frailty is well-established, with long-term malnutrition, insufficient protein, and energy intake being significant risk factors for developing frailty.11 In our study, we used the Mini Nutritional Assessment (MNA)25 to assess nutrition, and the results showed that 34.5 % of the population was at risk of malnutrition. This finding highlights the importance of proper nutrition in maintaining health and preventing frailty in older adults. Cultural differences may also contribute to the differences in the prevalence of frailty. For example, the prevalence of frailty in Japan may differ from that in other countries due to differences in lifestyle and cultural norms.
Strengths of the study was that the participants from two geographical locations (tribal and urban) were included in this study. The prevalence of physical frailty has been studied32, 33, 34, 35, 36 over the years in the Indian geriatric population. The study is the first to assess oral frailty in India. For assessing the oral frailty, we used all the tools available as there was no standardized tools available. Among them, this was the first study to assess Oral and Maxillofacial Frailty Index (OMFI). One of the parameters in assessing oral frailty was tongue pressure which was objectively measured using a Tongueometer™ device.
Some of the limitations of the study was that the population was selected from patients attending the health centres, the prevalence of physical and oral frailty may be higher than those in the community. A cohort design would be appropriate to assess the temporality between physical and oral frailty.
5. Conclusion
The study found that oral frailty was highly prevalent at 74 % compared to physical frailty at 34.4 %. This study emphasizes the importance of addressing oral health in the comprehensive general health screening for geriatric patients. Healthcare providers must recognize the significance of oral health in preventing and managing physical frailty. Early identification and targeted interventions can improve the overall health and well-being of older adults. The study highlights that unaddressed dental needs can lead to malnutrition, sarcopenia, and physical frailty, which is especially relevant in the Indian healthcare system where oral healthcare is not prioritized. Therefore, geriatric oral healthcare examinations must be included in routine medical-dental check-ups to identify oral frailty and prevent further health complications. Medical-dental collaboration in geriatric medicine can enhance health outcomes for older adults. It is crucial to establish a strategy for advocating for the inclusion of oral frailty assessments in geriatric health assessments and improve dental-medical collaboration. Ultimately, the integration of oral health assessments in routine health screenings for older adults can lead to better overall health outcomes.
Funding
This work was supported by the Kerala State Council for Science, Technology, and Environment [grant number: 00459/SPS 65/2021/KSCSTE].
Declaration of competing interest
The authors declare that they have no conflicts of interest.
References
- 1.World Population Ageing, 2019 Highlights. United Nations, Department of Economic and Social Affairs, Population Division; 2020. www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report Accessed on: February 1, 2023. Available on: [Google Scholar]
- 2.WHO’s work on the UN Decade of Healthy Ageing 2021-2030. https://www.who.int/initiatives/decade-of-healthy-ageing Accessed on: February 1, 2023. Available on:
- 3.Report of the Technical group of Population Projection Ministry of health and family welfare, GOI. July 2020. https://main.mohfw.gov.in/reports-0 (2011-2036)
- 4.Ko F.C., Walston J.D. What is frailty? Evid Based Pract Palliat Med. 2012:363–370. [Google Scholar]
- 5.Wang X., Hu J., Wu D. Risk factors for frailty in older adults. Medicine (Baltimore) 2022;101(34) doi: 10.1097/MD.0000000000030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima G., Liljas A.E.M., Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Pol. 2019;12:23–30. doi: 10.2147/RMHP.S168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada H., Makizako H., Doi T., et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc. 2013;14(7):518–524. doi: 10.1016/j.jamda.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Kashikar Y., Nagarkar A. Prevalence and determinants of frailty in older adults in India. Ind J Gerontol. 2016;30(3) [Google Scholar]
- 9.Makizako H., Nishita Y., Jeong S., et al. Trends in the prevalence of frailty in Japan: a meta-analysis from the ILSA-J. J Frailty Aging. 2021;10:211–218. doi: 10.14283/jfa.2020.68. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe Y., Okada K., Kondo M., Matsushita T., Nakazawa S., Yamazaki Y. Oral health for achieving longevity. Geriatr Gerontol Int. 2020;20(6):526–538. doi: 10.1111/ggi.13921. [DOI] [PubMed] [Google Scholar]
- 11.Shwe P.S., Ward S.A., Thein P.M., Junckerstorff R. Frailty, oral health and nutrition in geriatrics inpatients: a cross‐sectional study. Gerodontology. 2019;36(3):223–228. doi: 10.1111/ger.12397. [DOI] [PubMed] [Google Scholar]
- 12.Ayoob A.K., Neelamana S.K., Janakiram C. Impact of oral frailty on general frailty in geriatric population: a scoping review. J Indian Assoc Public Health Dent. 2022;20(1):9–15. [Google Scholar]
- 13.Castreján-Pérez R.C., Borges-Yẫez S.A., Gutiérrez-Robledo L.M., Ávila-Funes J.A. Oral health conditions and frailty in Mexican community-dwelling elderly: a cross sectional analysis. BMC Publ Health. 2012;12(1) doi: 10.1186/1471-2458-12-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer J.M., Kaiser M.J., Sieber C.C. Sarcopenia in nursing home residents. J Am Med Dir Assoc. 2008;9(8):545–551. doi: 10.1016/j.jamda.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Tôrres L.H., Tellez M., Hilgert J.B., Hugo F.N., de Sousa M.D., Ismail A.I. Frailty, frailty components, and oral health: a systematic review. J Am Geriatr Soc. 2015;63(12):2555–2562. doi: 10.1111/jgs.13826. 2015. [DOI] [PubMed] [Google Scholar]
- 16.Landi F., Liperoti R., Fusco D., et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Neyens J.C., van Haastregt J.C., Dijcks B.P., et al. Effectiveness and implementation aspects of interventions for preventing falls in elderly people in long-term care facilities: a systematic review of RCTs. J Am Med Dir Assoc. 2011;12(6):410–425. doi: 10.1016/j.jamda.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay S.E., Papachristou E., Watt R.G., et al. Influence of poor oral health on physical frailty: a population-based cohort study of older British men. J Am Geriatr Soc. 2018;66(3):473–479. doi: 10.1111/jgs.15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki M., Yoshihara A., Sato N., et al. A 5‐year longitudinal study of association of maximum bite force with development of frailty in community‐dwelling older adults. J Oral Rehabil. 2018;45(1):17–24. doi: 10.1111/joor.12578. [DOI] [PubMed] [Google Scholar]
- 20.Hakeem F.F., Bernabé E., Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36(3):205–215. doi: 10.1111/ger.12406. [DOI] [PubMed] [Google Scholar]
- 21.Kimble R., Papacosta A.O., Lennon L.T., et al. The relationship of oral health with progression of physical frailty among older adults: a longitudinal study composed of two cohorts of older adults from the United Kingdom and United States. J Am Med Dir Assoc. 2023;24(4):468–474. doi: 10.1016/j.jamda.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 23.Rose M., Yang A., Welz M., Masik A., Staples M. Novel modification of the reported Edmonton frail scale. Australas J Ageing. 2018;37(4):305–308. doi: 10.1111/ajag.12533. [DOI] [PubMed] [Google Scholar]
- 24.Malmstrom T.K., Morley J.E. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Vellas B., Guigoz Y., Garry P.J., et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T., Takahashi K., Hirano H., et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73(12):1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 27.Oguchi K., Saitoh E., Baba M., Kusudo S., Tanaka T., Onogi K. The repetitive saliva swallowing test (RSST) as a screening test of functional dysphagia (2) validity of RSST. Jpn J Rehabil Med. 2000;37(6):383–388. [Google Scholar]
- 28.Ain S.N., Khan Z.A., Gilani M.A. Revised kuppuswamy scale for 2021 based on new consumer price index and use of conversion factors. Indian J Publ Health. 2021;65(4):418. doi: 10.4103/ijph.ijph_1108_21. [DOI] [PubMed] [Google Scholar]
- 29.Biritwum R.B., Minicuci N., Yawson A.E., et al. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas. 2016;91:8–18. doi: 10.1016/j.maturitas.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Çakmur H. Frailty among elderly adults in a rural area of Turkey. Med Sci Mon Int Med J Exp Clin Res. 2015;21:1232–1242. doi: 10.12659/MSM.893400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaikh N., Harshitha R., Bhargava M. Prevalence of sarcopenia in an elderly population in rural South India: a cross-sectional study. F1000Res. 2020;9(175):175. [Google Scholar]
- 32.Hironaka S., Kugimiya Y., Watanabe Y., et al. Association between oral, social, and physical frailty in community-dwelling older adults. Arch Gerontol Geriatr. 2020;89 doi: 10.1016/j.archger.2020.104105. [DOI] [PubMed] [Google Scholar]
- 33.Tôrres L.H., da Silva D.D., Neri A.L., Hilgert J.B., Hugo F.N., Sousa M.L. Association between underweight and overweight/obesity with oral health among independently living Brazilian elderly. Nutrition. 2013;29(1):152–157. doi: 10.1016/j.nut.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 34.de Andrade F.B., Lebrão M.L., Santos J.L.F., de Oliveira Duarte Y.A. Relationship between oral health and frailty in community-dwelling elderly individuals in Brazil. J Am Geriatr Soc. 2013;61(5):809–814. doi: 10.1111/jgs.12221. [DOI] [PubMed] [Google Scholar]
- 35.Xue Q.L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felix R., Mishra R., Thomas J., Wilson B., Belavendra A., Gopal G. Is handgrip strength a useful tool to detect slow walking speed in older Indian adults: a cross-sectional study among geriatric outpatients in a tertiary care hospital in South India. J frailty, sarcopenia and falls. 2022;7:183–191. doi: 10.22540/JFSF-07-183. [DOI] [PMC free article] [PubMed] [Google Scholar]