Abstract
Osteoporosis is a metabolic bone disorder which increases fragility fracture risk. Elderly individuals, especially postmenopausal women, are particularly susceptible to osteoporosis. Although rare, osteoporosis in children and young adults is becoming increasingly evident, highlighting the need for timely diagnosis, management and follow-up. Early-onset osteoporosis is defined as the presence of a low BMD (Z-score of ≤ −2.0 in individuals aged < 20 years; T-score of ≤ −2.5 in those aged between 20 to 50 years) accompanied by a clinically significant fracture history, or the presence of low-energy vertebral compression fractures even in the absence of osteoporosis. Affected children and young adults should undergo a thorough diagnostic workup, including collection of clinical history, radiography, biochemical investigation and possibly bone biopsy. Once secondary factors and comorbidities are excluded, genetic testing should be considered to determine the possibility of an underlying monogenic cause. Defects in genes related to type I collagen biosynthesis are the commonest contributors of primary osteoporosis, followed by loss-of-function variants in genes encoding key regulatory proteins of canonical WNT signalling (specifically LRP5 and WNT1), the actin-binding plastin-3 protein (encoded by PLS3) resulting in X-linked osteoporosis, and the more recent sphingomyelin synthase 2 (encoded by SGMS2) which is critical for signal transduction affecting sphingomyelin metabolism. Despite these discoveries, genetic causes and underlying mechanisms in early-onset osteoporosis remain largely unknown, and if no causal gene is identified, early-onset osteoporosis is deemed idiopathic. This calls for further research to unravel the molecular mechanisms driving early-onset osteoporosis that consequently will aid in patient management and individualised targeted therapy.
Keywords: Early-onset osteoporosis, Bone mass, DXA, Osteogenesis imperfecta, Secondary osteoporosis, Fragility fractures, Genetic testing, Idiopathic osteoporosis
Introduction
Osteoporosis is a progressive, multifactorial systemic skeletal disease characterised by low bone mass, microarchitectural deterioration of bone tissue and reduced bone strength that culminates in increased fracture risk [1, 2]. Fractures of the hip and vertebrae are the most common, debilitating and costly and occasionally can lead to death in 20% of affected individuals within the first year of fracture [3–6]. Although osteoporosis is considered a disease of the elderly, affecting particularly postmenopausal women, increased clinical attention is being given to low bone mass disorders in children and young adults —whether primary or secondary in nature especially with the advent of improved or new diagnostic techniques [7, 8].
Bone is a physiologically dynamic organ exhibiting exceptional properties, ranging from mechanical to metabolic and endocrine functions. It is a complex living tissue encompassing a variety of different cells (osteoblasts, osteocytes, bone lining cells and osteoclasts) within a mineralised matrix, all of which contribute towards maintaining a healthy bone status [9]. Mechanically, the skeleton supports the body and protects the vital organs. Metabolically, this endocrine organ is primarily a major source of minerals, growth factors, hormones and fatty acids. Bone is composed of an inorganic portion (50–70%) consisting of hydroxyapatite (Ca10(PO4)6(OH)2), an organic matrix (20–40%) being chiefly made up of type I collagen, water (5–10%) and impurities [9, 10]. The degree of mineralisation influences mechanical resistance and rigidity of bones, enabling them to withstand compression forces and loading, whereas the collagenous matrix allows for elasticity and movement. At the microarchitectural level, bone consists of cortical (making up approximately 80% of bone) or trabecular bone differing in structural organisation, function and site distribution. Cortical bone is made up of densely packed collagen fibrils forming concentric bone lamellae parallel to and around central Haversian canals through which blood and lymphatic vessels, nerves and connective tissue flow. Trabecular bone is composed of irregularly organised rod and plate-like networks of trabeculae forming 3D lattices arranged along the lines of stress. Despite constituting 20% of the skeleton, trabecular bone harbours a higher surface area relative to cortical bone and undergoes more active remodelling making it more susceptible to pathogenesis [11, 12].
The precise and proper balance between bone formation and resorption is imperative in the shaping and development of bones, maintaining the integrity of the skeleton and in systemic mineral homeostasis. Bone modelling is prominent in childhood and helps to define bone structure, shaping, expansion and movement through space in response to the combined effect of mechanical loading, hormonal control and genetic factors affecting osteoblast and osteoclast function [13, 14]. Conversely, remodelling is a self-regeneration process involving the coordinated action or ‘coupling’, between osteoblastic bone formation and osteoclast-mediated bone resorption, which must be timely and quantitatively balanced by paracrine and endocrine factors and immune cells. Remodelling takes place in stages starting by osteoclast activation and resorption of existing damaged bone, reversal whereby osteoblasts are recruited to the bone surface and bone formation by osteoblasts that lay down osteoid which becomes mineralised forming mature bone [11, 13]. The coupling between resorption and formation is balanced and relatively stable during peak adult mass. However, it decreases over time with ageing increasing the risk of low bone mass and fracture susceptibility [11, 13, 15].
In this review, we describe the recent definition of early-onset osteoporosis and its aetiology, the clinical diagnostic evaluation including genetic testing methods to confirm the presence of an underlying monogenic cause and treatment options for affected individuals.
Definition of early-onset osteoporosis
Bone mineral density (BMD) measurement by dual-energy X-ray absorptiometry (DXA) can be used to diagnose osteoporosis in postmenopausal women and men aged > 50 years. The World Health Organization defines osteoporosis in these populations as a BMD at the spine, hip or forearm of 2.5 or more standard deviations below the young adult mean (T-score ≤ -2.5) [16–18]. Additionally, in all cases of unusual fracture, pathologies such as osteomalacia (e.g. due to severe vitamin D deficiency, hypophosphataemia), malignancy or fibrous dysplasia should be ruled out [7, 17–21]. Subsequently, any fracture of low-to-moderate energy trauma (aside from a fracture of the digits, skull or face) that occurs from a standing height or less can be considered a low-trauma or fragility fracture [17, 22]. Such individuals may have decreased bone strength and may be considered to have osteoporosis, irrespective of BMD.
However, the diagnostic guidelines of osteoporosis in children and young adults are different (Table 1). The International Society for Clinical Densitometry (ISCD) recommends the use of BMD Z-scores in these populations (compared with age-matched norms) [23, 24]. In premenopausal women and men aged < 50 years, a Z-score ≤ − 2.0 is interpreted as below the expected range for age and a Z-score > − 2.0 as within the expected range for age [25, 26]. In this age group, osteoporosis diagnosis should not be based only on low BMD, but also on a history of low-trauma fracture or a secondary cause of osteoporosis. In children, the values should be properly adjusted for short stature and/or delayed or advanced timing of puberty [23]. In the absence of vertebral compression fractures, the diagnosis of osteoporosis is indicated by the presence of both a clinically significant fracture history and BMD Z-score ≤ − 2.0. A clinically significant fracture history is one or more of the following: (1) two or more long bone fractures up to age of 10 years, (2) three or more long bone fractures up to age of 19 years [23, 24]. Additionally, in this age group, one or more vertebral compression fractures is indicative of osteoporosis, in the absence of local disease or high-energy trauma, even if the BMD Z-score is not subnormal. The International Osteoporosis Foundation (IOF) defines low bone mass as a Z-score of ≤ − 2.0 in subjects aged < 20 years and in those aged > 20 years with delayed puberty [27]. The IOF suggests the use of a T-score < − 2.5 to define osteoporosis in subjects aged 20–50 years in association with a low-trauma fracture history or a secondary cause of osteoporosis.
Table 1.
Definition of osteoporosis in children and young adults
| International society for clinical densitometry (ISCD) |
|
Children ≥ 1 vertebral compression fractures in the absence of local disease or high-energy trauma, or Clinically significant fracture history and BMD Z-score ≤ − 2.0 with ≥ 2 long bone fractures up to age 10 years and/or ≥ 3 long bone fractures up to age 19 years |
|
Premenopausal women and men aged < 50 years BMD Z-score ≤ − 2.0 and low-trauma fracture or secondary cause of osteoporosis |
| International Osteoporosis Foundation (IOF) |
|
Young adults aged 20–50 years T-score ≤ − 2.5 and low-trauma fracture or secondary cause of osteoporosis |
BMD bone mineral density
Aetiology of early-onset osteoporosis: identifying the underlying cause
Low bone mass may be related to either inadequate peak bone mass acquisition and/or ongoing bone loss. BMD depends primarily upon achievement of peak bone mass which is defined as the maximum BMD achieved by age 40 years [28, 29]. Importantly, 95–100% of peak bone mass is acquired by the late teen years [30–33] making this a crucial period for the proper formation of a robust musculoskeletal system.
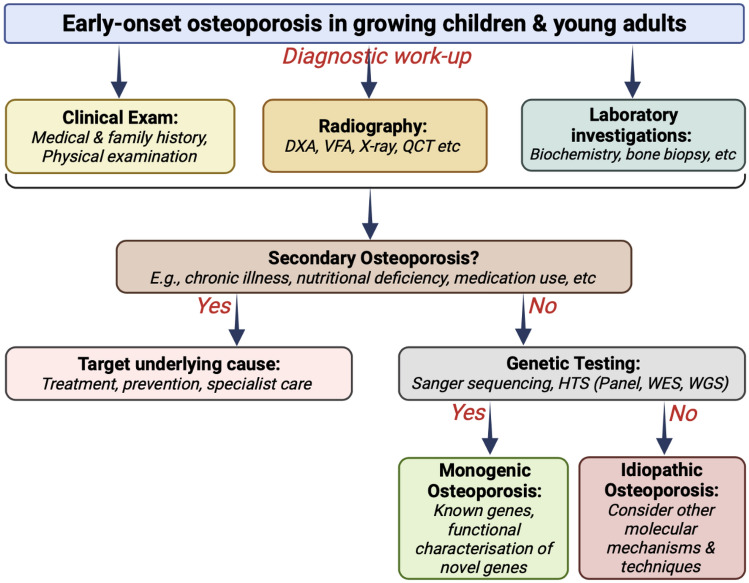
Bone loss and/or fragility fractures in children and young adults can be attributed to a secondary cause which needs to be carefully looked for. If no such cause is identified, bone fragility may then be regarded primary and potentially related to rare gene variants [34]. If there is still no apparent aetiology, bone loss and/or fractures are considered idiopathic (Fig. 1).
Fig. 1.
Flowchart showing the diagnostic workup of a growing child or young adult with suspected early-onset osteoporosis. DXA dual-energy X-ray absorptiometry, VFA vertebral fracture assessment, QCT quantitative computed tomography, HTS High-throughput sequencing, WES whole-exome sequencing, WGS whole-genome sequencing. Figure created using BioRender (https://biorender.com)
Secondary causes
Many secondary risk factors are similar to those for postmenopausal osteoporosis and osteoporosis in men. Table 2 includes secondary causes of osteoporosis in children and young adults and some of the more common conditions are described below.
Table 2.
Secondary causes of low bone mass/fractures in children and young adults
| Endocrine diseases | Medications |
| Cushing’s syndrome (ACTH, non-ACTH dependent) | Anticonvulsants |
| Diabetes mellitus | Aromatase inhibitors |
| GH deficiency | Chemotherapy |
| Hypercalciuria | Depot medroxyprogesterone acetate |
| Hyperparathyroidism | Excess levothyroxine |
| Hyperprolactinaemia | Glucocorticoids |
| Hyperthyroidism | GnRH agonists |
| Hypogonadism (hypogonadotropic, hypergonadotropic) | Heparin |
| Hypophosphatasia | Immunosuppressants |
| Hypophosphataemia | Proton pump inhibitors |
| Vitamin D and/or calcium deficiency | SSRI |
| Thiazolidinediones | |
| Haematologic diseases | Metabolic diseases |
| Bone marrow transplantation | Gaucher’s disease |
| Haemophilia | Glycogen storage disease |
| Hereditary haemochromatosis | Homocystinuria |
| Leukaemia | Mucopolysaccharidoses |
| Lymphoma | Malnutrition/malabsorption |
| Mastocytosis | Anorexia nervosa |
| Multiple myeloma | Celiac disease |
| Thalassemia major | Gastrointestinal surgery |
| Chronic inflammatory diseases | Other |
| Inflammatory bowel disease | Alcoholism |
| Liver diseases | Cystic fibrosis |
| Lung diseases | Duchene muscular dystrophy |
| Kidney diseases | Excessive exercise |
| Rheumatic diseases | HIV |
| Skin diseases | Pregnancy and lactation |
ACTH adrenocorticotropic hormone, GH growth hormone, GnRH gonadotropin-releasing hormone, HIV human immunodeficiency virus, SSRI selective serotonin reuptake inhibitor
Chronic inflammatory diseases
The aetiology of low BMD and fragility fractures in chronic inflammatory diseases, such as rheumatic diseases, lung diseases, inflammatory bowel disease, liver and kidney diseases, and skin diseases includes effects of the disease itself, systemic inflammation, glucocorticoids use, low body weight, malabsorption, low physical activity, delayed puberty and/or secondary amenorrhoea [17, 34, 35].
Glucocorticoid use
The negative effects of the glucocorticoids on bone include increased apoptosis of osteoblasts and osteocytes, decreased apoptosis of osteoclasts, negative effects on muscle function, decreased calcium absorption in the gut and decreased calcium re-absorption in the kidney [17]. The American College of Rheumatology advises to assess clinical fracture risk in all children and young adults within 6 months of starting glucocorticoids and to perform DXA in adults aged < 40 years when there is a history of osteoporotic fracture or other risk factors for fracture [36, 37]. In adults aged > 40 years, FRAX should be used with glucocorticoid dose correction and BMD should be tested within 6 months of starting glucocorticoids.
Oestrogen deficiency
Examples of premenopausal oestrogen deficiency include Hypogonadotropic hypogonadism due to low body weight, anorexia nervosa, excessive exercise, hyperprolactinaemia and hypopituitarism and Hypergonadotropic hypogonadism (premature ovarian insufficiency) due to chromosomal abnormalities (e.g. Turner syndrome, fragile X syndrome), chemotherapy, radiation and autoimmune diseases [38, 39].
Pregnancy and lactation
Normal pregnancy can be associated with bone loss of approximately 3–5% at the spine and hip [40–42], significant decline only at the trochanter [41], or stable BMD [43]. Lactation has more consistent effects and is associated with bone loss of 3–10% at the spine and hip seen over 3–6 months [44, 45]. Bone loss is related to duration of lactation and amenorrhoea and is not prevented by calcium supplementation [46]. Parathyroid hormone-related protein (PTHrP), which is secreted by the mammary gland and controls calcium mobilisation from bone [47, 48], as well as oestrogen deficiency, may be involved in bone loss. Although there is a loss of bone mass in pregnancy and lactation, physiologically there is a partial recovery. Recovery from lactation-associated bone loss may continue for 18 months or longer [49, 50]. It has been found that parity and lactation have no adverse associations with clinical fragility or radiographic vertebral fractures, or the rate of BMD decline over 10 years [51].
Pregnancy and lactation-associated osteoporosis (PLO) is a rare condition in which women present with fractures, often vertebral, in the third trimester of pregnancy or in the early postpartum period [52, 53]. In most women, no known cause of osteoporosis is found [54]. Evaluation for secondary causes of osteoporosis should be undertaken. Skeletal fragility in PLO may result from abnormal pregnancy-related bone changes. In some women, an underlying genetic predisposition may be identified, suggesting a pre-existing monogenetic form of osteoporosis with an exacerbation due to pregnancy [55]. Abnormal osteoblast function or other bone formation defects may contribute to the pathophysiology of PLO [56]. Some patients will improve spontaneously, while others will need treatment with antiresorptive or anabolic treatment [39]. There is an increased risk of fracture recurrence (overall and within the context of another pregnancy); 24% of patients with PLO followed for 6 years had subsequent fractures, most were vertebral fractures and number of fractures at diagnosis predicted subsequent risk [57].
Genetic causes of osteoporosis
Osteogenesis imperfecta and other monogenic bone fragility disorders
Genetic factors play an important role in osteoporosis and determine up to 80% of BMD [2, 58]. Several contributing genes have been identified in genome-wide association studies (GWAS) and the risk is thought to depend on several gene variants, each with modest effect sizes [59–62]. In monogenic forms, osteoporosis is caused by a single variant in a gene that has a major role in the skeleton [63]. The most recent nosology of genetic skeletal disorders lists altogether 55 genetic and clinical entities with skeletal fragility [64]. Osteogenesis imperfecta (OI) is the most common of these monogenic disorders with skeletal fragility; it is usually caused by mutations in the genes regulating extracellular matrix, especially type I collagen [65–70]. Apart from extracellular matrix defects, other mechanisms may also lead to skeletal fragility. These include impaired osteoblast and osteoclast function, defective matrix mineralisation and defects in calcium and phosphate homeostasis.
Only a small number of genetic entities presenting with early-onset osteoporosis without the classical features of OI or syndromic features have been recognised [64]. These genetic forms are summarised in Table 3. The WNT signalling pathway plays a major role in skeletal homeostasis [71]. Biallelic mutations in the WNT receptor, LRP5, lead to severe childhood-onset osteoporosis and blindness, while heterozygous loss-of-function variants lead to milder forms of osteoporosis, often presenting later in childhood or in adulthood [72]. It has become apparent that WNT1 is the key ligand for the canonical WNT signalling pathway in bone [73, 74]. Similar to LRP5, biallelic and monoallelic WNT1 variants lead to different degrees of skeletal fragility. Children with biallelic WNT1 variants present with severe skeletal fragility mimicking OI type III, while heterozygous WNT1 variants lead to an osteoporosis phenotype that manifests often only later in childhood or in adulthood [75–79].
Table 3.
Genes linked to early-onset osteoporosis
| Gene | OMIM | Inheritance | Mutation | Protein | Function |
|---|---|---|---|---|---|
| LRP5 |
259770 166710 |
AR, AD | LoF | Low-density lipoprotein-related receptor 5 | WNT signalling |
| WNT1 | 615220 | AR, AD | LoF | Wingless-type MMTV integration site family, member 1 | WNT signalling |
| PLS3 | 300910 | XL | LoF | Plastin 3 | Formation of F-actin bundles |
| SGMS2 | 126650 | AD | LoF | Sphingomyelin synthase 2 | Mineralisation |
| ARHGAP25 | 610587 | AD | LoF | Rho GTPase-activating protein 25 | Bone cell function and bone metabolism |
AR autosomal recessive, AD autosomal dominant, XL X-linked, LoF loss of function
In 2013, mutations in PLS3 were identified as a cause for osteoporosis [80]. Due to the gene’s X-chromosomal location, PLS3 mutations affect males more and earlier than females, but mutation-positive females may also develop symptomatic osteoporosis already in childhood or later in adulthood [81, 82]. Regarding the nature of reported variants, the studies have identified both missense and nonsense variants but also partial or total deletions of the gene, as well as a partial duplication of the gene in individuals with early-onset osteoporosis [83]. The gene codes for Plastin3, an actin-binding and actin-bundling protein involved in cytoskeleton remodelling [80]. The function of PLS3 in bone is still unknown. Plastin3 may be involved in the process of mechanosensing by osteocytes [84]. Recent findings indicate that PLS3 may also play a role in bone mineralisation [85].
Several other novel forms of monogenic osteoporosis have been recently described, for example those caused by variants in SGMS2 and ARHGAP25 [86, 87]. Individuals with a heterozygous mutation in SGMS2, encoding sphingomyelin synthase 2 (SMS2), had since childhood multiple fractures and often calvarial hyperostotic lesions [87]. Bone biopsies showed low bone volume, impaired matrix mineralisation and abnormal bone lamellarity with areas of 'woven bone' and a significantly disturbed osteocyte canalicular network [88]. Several subjects displayed in addition to osteoporosis, neurological symptoms, e.g. transient facial nerve palsy, suggesting that these extra-skeletal manifestations may be a distinctive feature of SGMS2-related osteoporosis [87]. The recurrent SGMS2 p.Arg50* stop-gain variant was present in four unrelated families and has since then been reported in several additional cases [89, 90]. In two families, a missense mutation in the same gene led to a much more severe disorder with skeletal dysplasia, significant calvarial hyperostosis, severe short stature and skeletal fragility since early infancy [87].
Despite these discoveries, genetic causes and underlying mechanisms in early-onset osteoporosis remain largely unknown. The spectrum of genetic and cellular pathology is complex [34] and hence patient management also requires individualised treatment strategies. To optimise management, the characteristic skeletal and extra-skeletal pathology and the disease course in each genetic form need to be elucidated.
Idiopathic osteoporosis
Children and young adults experiencing repetitive fragility fractures in the presence of a low BMD are primarily investigated for an underlying secondary cause or a monogenic defect in known or novel genes. Only when such causes are appropriately ruled out should idiopathic osteoporosis be considered. Indeed, some of the previously thought idiopathic cases turned out to be monogenic in nature when more extensive genetic testing became available, particularly during the high-throughput sequencing (HTS) era [91, 92].
Idiopathic osteoporosis is likely to be a heterogeneous disorder given the fact that bone remodelling and bone formation rate can be high, normal, or low [93]. In fact, several studies exploring the potential genetic causes for idiopathic osteoporosis have shown a variable monogenic aetiology [94, 95]. It is likely that parallel to increasing genetic knowledge, improved genetic tools and more active screening for a genetic aetiology, the proportion of truly “idiopathic” osteoporosis cases will decline.
The following clinical features of idiopathic osteoporosis have been described [96–98] whereby males and females are equally affected, a family history of osteoporosis is common, the age at diagnosis is approximately 35 years, fractures are usually multiple occurring over 5–10 years and involve sites rich in cancellous bone, such as the vertebrae, and the hip is affected in approximately 10% of affected individuals.
Evaluation of early-onset osteoporosis
Medical history, physical examination and biochemical testing
Evaluation of low bone mass in children and young adults (Fig. 1) begins with obtaining medical history (e.g. personal and family history, fracture history, medications, chronic diseases, lifestyle factors) and performing physical examination (e.g. anthropometry, joint mobility, scoliosis, limb deformities, functional tests) and laboratory testing with the goal of searching for potential secondary causes [99]. A secondary cause of osteoporosis can be found in a substantial proportion of subjects [97]. Those with a fragility fracture require evaluation for secondary causes even in the absence of low BMD. Subjects who have suspicious findings on history and physical examination, and/or abnormalities on the basic laboratory testing, require additional laboratory tests (Table 4).
Table 4.
Laboratory testing in serum or urine for searching of secondary causes in children and young adults
|
Basic laboratory testing Blood cell count Calcium, albumin, phosphate, ALP (total and bone specific) 25-OH Vitamin D, PTH Creatinine ESR TSH, fT4, fT3 24 h urine calcium and creatinine (in children spot urine) |
|
Additional laboratory testing Bone turnover markers (e.g. PINP, CTX) Fasting glucose, HbA1c IGF1 Iron, ferritin, AST, ALT, tTG-IgA antibodies, anti-DGP- IgG antibodies LH, FSH, E2 LH, FSH, testosterone, SHBG Morning cortisol, ACTH, midnight cortisol, UFC, DST Prolactin Protein immunoelectrophoresis in serum/urine Tryptase |
ACTH adrenocorticotropic hormone, ALP alkaline phosphatase, ALT alanine transaminase, AST aspartate transaminase, CTX C-terminal telopeptide, DGP deamidated gliadin peptide, DST dexamethasone suppression test, E2 oestradiol, ESR erythrocyte sedimentation rate, FSH follicle-stimulating hormone, fT3 free T3, fT4 free T4, HbA1c glycosylated haemoglobin, 25-OH Vitamin D 25-hydroxy vitamin D, IGF1 insulin-like growth factor 1, LH luteinising hormone, PINP procollagen type I N-terminal propeptide, PTH parathyroid hormone, SHBG sex hormone-binding globulin, TSH thyroid-stimulating hormone, tTG tissue transglutaminase, UFC 24 h urinary free cortisol
Serum or urinary bone turnover markers (BTM) may provide useful information. If markers of resorption are elevated above the premenopausal range, excessive bone resorption is likely. However, the range of normal is wide, making interpretation difficult [100]. Bone resorption markers must be interpreted according to the patient’s age. Young adults are characterised by active bone remodelling and physiologic increases in BTMs [101, 102]. Additionally, elevated BTMs are observed after a recent fracture. Importantly, BTMs are more helpful in adults in monitoring disease course and treatment response.
Genetic testing: the key to unresolved cases
Genetic studies have provided valuable information on bone biology, pathophysiological processes governing disease development and progression, and the genetic architecture of bone mass disorders. Monogenic disorders, such as early-onset osteoporosis, are more likely to arise from rare, highly penetrant genetic alterations inherited in an autosomal (dominant or recessive) or X-linked manner that ultimately result in aberrant protein function [63]. The classical approach to identify candidate gene variants in affected singletons or multiplex families with an apparent monogenic bone mass phenotype is by Sanger sequencing which is still considered the gold standard of clinical diagnostic testing. Single nucleotide substitutions (missense, nonsense and splicing) and small insertions or deletions (creating frameshift variations) in known genes (e.g. LRP5, PLS3, WNT1, SGMS2) are clearly identified in this yet time-consuming and costly hypothesis-driven method. HTS in the form of targeted gene panels, whole-exome sequencing (WES) and whole-genome sequencing (WGS) has been instrumental in gene and variant identification of monogenic osteoporosis, improving on throughput, turnaround time and costs [63]. Yet, it is important to keep in mind that the gene panels used in clinical practice are often limited and may not be up to date, considering the rapidly expanding spectrum of monogenic osteoporosis. The current Nosology of Genetic Skeletal Disorders includes tens of genes and conditions that may be relevant [64]. With increasing access to reasonably priced exome analyses and even WGS, there is probably going to be a shift from gene panels to other methods, particularly long-read sequencing which is better adapted at identifying structural variants [103]. Indeed, gene defects may involve deletions or duplications that can be easily missed when using diagnostic gene panels and short-read sequencing. Several cases of copy number variation (CNV)-related osteoporosis have been reportedly linked to, for example, type I collagen genes and PLS3 [85, 104–106].
Bone imaging
DXA is the preferred method for assessing bone mineral content (BMC) and areal BMD in children [23, 24]. The posterior–anterior spine and total body less head (TBLH) are the preferred sites for BMC and areal BMD measurements in most paediatric subjects. Other sites (e.g. proximal femur, lateral distal femur, distal radius) may be useful depending on each individualised case. A scan in children and young adults is usually indicated after two or more fragility fractures, after a fracture at an unusual site (such as the spine or hip), or in the presence of a chronic illness or medication predisposing to osteoporosis [27, 39]. If a follow-up DXA scan is indicated, the minimum interval between scans is 6–12 months. DXA uses very low radiation and is also fast and fully automated [99]. However, DXA is a 2D examination and it does not provide information on bone microarchitecture or differentiate between trabecular and cortical compartments. Additionally, DXA BMD can be falsely increased by collapsed vertebrae or mineral deposits at sites. Importantly, interpretation of DXA images in children requires adjustment not only for age and sex, but also for body or bone size, and skeletal maturity (bone age or pubertal status).
DXA vertebral fracture assessment (VFA) of the thoracic and lumbar spine may be used as a substitute for spine radiography in the identification of symptomatic and asymptomatic vertebral fractures in paediatric patients [23, 24]. Then, the Genant semi-quantitative method should be used. Important advantages of the VFA are the lower radiation exposure compared to plain radiographs, and the combination of BMD and VFA information through performing the same examination. Quantitative computed tomography (QCT), pQCT (peripheral QCT) and HR-pQCT (high resolution QCT) are research techniques used to characterise bone deficits in children. They can be used clinically in these populations where appropriate reference data and expertise are available.
The power of bone biopsies
Bone biopsies could hold the key to diagnosing unclear and potentially complicated cases of young individuals presenting with early-onset osteoporosis. Information on the rate of bone resorption and remodelling, degree of mineralisation (hypo- vs hypermineralisation) defects, bone structure and material properties, and chronic comorbidities (e.g. presence of multiple myeloma) can be unveiled that consequently will aid in differential diagnosis and patient management, especially treatment. Labelling of the bone with a double or quadruple tetracycline that binds to the mineralised bone surface is recommended to calculate the rate of bone formation and turnover [107], and in so doing characterise different low bone mass causes (e.g. low-turnover osteoporosis versus osteomalacia). Anterior iliac crest is the preferred sampling site thanks to its accessibility, which circumvents the need for surgery [108]. Yet, routine use of this invasive procedure remains low in the clinical setting [34].
Other tools can be used to further analyse the sampled bone tissue providing data on the mineralised bone volume and extracellular matrix, bone properties and mechanical strength and osteocytes lacunae. Such tools include quantitative backscatter electron imaging (qEBI), small-angle X-ray scattering, vibrational spectroscopy, nanoindentation and X-ray tomography, reviewed in detail elsewhere [34]. Histomorphometry using Masson–Goldner trichrome staining enables tissue and morphological identification and helps quantify osteoid and mineralised bone. The presence of defective collagen fibrils, altered cross-linking or thinner fibrils can also be observed in the same stained tissue sections and may help distinguish different pathologies, including OI types [109–111].
Treatment options for early-onset osteoporosis
The low prevalence of children and young adults with early-onset osteoporosis has made it difficult to undertake large-scale clinical trials, particularly to investigate the effect of pharmacological intervention on fracture prevention. For this reason, there are currently no evidence-based guidelines for the treatment of affected individuals with early-onset osteoporosis. Instead, preventive measures and individualised treatment approaches are generally recommended (Fig. 2), as discussed below.
Fig. 2.

Proposed prevention strategies and targeted treatment options for children and young adults with early-onset osteoporosis
Proper supplementation of calcium and vitamin D should be given, especially in case of deficiency or insufficiency following laboratory investigations [17, 112, 113]. Lifestyle changes are advised in the form of increased physical activity, reduced alcohol intake, no smoking and sufficient protein intake, which have indeed resulted in improved BMD status in young affected individuals [114, 115]. Treatment of the underlying comorbidity is a must which will also have beneficial effects on bone health, for example, gluten-free diet in coeliac disease, treatment of amenorrhoea with oestrogen replacement therapy, treatment of inflammatory bowel disease and rheumatoid arthritis with anti-TNF alpha antibodies, etc. [39]. When treatment of the chronic disease is not feasible or repetitive fractures are sustained, antiresorptive or osteoanabolic therapy is considered. Increase in BMD following bisphosphonate treatment has been reported in young individuals with secondary osteoporosis [27], including patients with anorexia nervosa treated with risedronate [116], women with ovarian failure after allogeneic stem cell transplant treated with risedronate and zoledronic acid [117], individuals with cystic fibrosis treated with alendronate [118] or zoledronate [119], as well as Crohn’s disease [120] and ß-thalassaemia major [121], amongst others. However, bone pharmaceuticals in other risk groups might not be favourable (e.g. pregnancy and women of childbearing age) due to potential adverse effects. In summary, although most studies have demonstrated an improvement in BMD, studies that focus on decreased fractures as the primary outcome are yet to be conducted.
Treatments of monogenic forms of early-onset osteoporosis have also been investigated, but not extensively. Teriparatide treatment showed increased bone turnover in individuals with missense variants in LRP5, LRP6 and WNT1 [122, 123], and splicing variants in PLS3 [123]. Improvement in BMD Z-scores with reshaping of compressed vertebrae was also observed in zoledronate-treated individuals harbouring a large fragment deletion variant in PLS3 [106]. Positive outcomes were seen in patients with deleterious SGMS2 variants following bisphosphonate therapy, including improvement in back pain and quality of life, and fracture prevention [87]. However, the same cannot be said for individuals with WNT1 and LRP5 variants who exhibited no effect after bisphosphonate treatment [77, 124]. In conclusion, more large-scale and long-term studies are required to properly evaluate the effects of different antiresorptive and osteoanabolic treatment, including anti-sclerostin therapy and possible combinatory treatment modalities not just on BMD, but even fracture risk.
Concluding remarks
Early-onset osteoporosis, although rare, remains a significant disorder with considerable morbidity that presents with diagnostic challenges. If no genetic causal variants are identified following high-throughput DNA sequencing, then transcriptomics, metabolomics and proteomics should be considered enabling a multi-omics approach that can be coupled with machine learning tools. Identification of the underlying cause can inform about inheritance patterns, treatment options and patient monitoring, all of which are also beneficial to other potentially susceptible relatives. The need for collaborations between clinical, basic and translational researchers through international scientific consortia (e.g. GEFOS: http://www.gefos.org and GENOMOS: http://www.genomos.eu), COST Actions (e.g. GEMSTONE COST Action, CA18139: https://cost-gemstone.eu), European Reference Networks (e.g. European Network for Rare Bone Conditions, ERN BOND: https://ernbond.eu), rare bone disorder registries (e.g. Osteogenesis Imperfecta: https://oif.org/oiregistry), as well as patient organisations has become more evident to overcome diagnostic obstacles and provide timely care to patients.
The canonical WNT signalling pathway is presently regarded as a key regulator of bone metabolism. Its role in bone was discovered by studying monogenic diseases with low and high bone mass. These genetic and molecular discoveries led to the development of a new anabolic osteoporosis medication, sclerostin antibody [125]. Similarly, genetic and molecular discoveries in other rare genetic bone mass disorders such as pycnodysostosis (cathepsin K antibody), juvenile Paget’s disease (RANKL antibody), hypophosphataemic rickets (burosumab) and hypophosphatasia (asfotase alfa) have been of key importance in drug development [126–129].
It is likely that significant scientific advancements can still be made by studying patients and families with early-onset osteoporosis, leading to renewal of our understanding of bone metabolism and pathogenesis of skeletal fragility. In the long-term, research discoveries are likely to enable the development of new modes of osteoporosis therapy and provide new tools for improved diagnostics and follow-up of affected individuals.
Acknowledgements
MMF is supported by the Malta Council for Science & Technology (ZeEBRA R&I-2019-018T and GRIT R&I-2022-007L), for and on behalf of the Foundation for Science and Technology, through the FUSION: R&I Technology Development Programme and Technology Development Programme LITE; OM is supported by the Academy of Finland, Sigrid Jusélius Foundation, Folkhälsan Research Foundation, Novo Nordisk Foundation, Swedish Research Council, Stockholm County Council (ALF) and Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse. MMF, MAC and OM are members of the GEMSTONE COST Action (https://cost-gemstone.eu). The GEMSTONE initiative is funded by the COST action grant CA18139.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Data availability
Not applicable.
Declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Compston JE, McClung MR, Leslie WD. Osteoporosis Lancet. 2019;393:364–376. doi: 10.1016/s0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 2.Ralston SH, Uitterlinden AG. Genetics of Osteoporosis. Endocr Rev. 2010;31:629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C and Ferrari S. IOF Compendium of Osteoporosis. 2nd edition ed2019. p. 1–90.
- 4.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 5.Lespessailles E, Cortet B, Legrand E, Guggenbuhl P, Roux C. Low-trauma fractures without osteoporosis. Osteoporos Int. 2017;28:1771–1778. doi: 10.1007/s00198-017-3921-7. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitriou N, Tsilidis KK, Orfanos P, Benetou V, Ntzani EE, Soerjomataram I, Künn-Nelen A, Pettersson-Kymmer U, Eriksson S, Brenner H, et al. Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium Lancet. Public Health. 2017;2:e239–e246. doi: 10.1016/s2468-2667(17)30046-4. [DOI] [PubMed] [Google Scholar]
- 7.Mäkitie O. Causes, mechanisms and management of paediatric osteoporosis. Nat Rev Rheumatol. 2013 doi: 10.1038/nrrheum.2013.45. [DOI] [PubMed] [Google Scholar]
- 8.Mäkitie RE, Costantini A, Kämpe A, Alm JJ, Mäkitie O. New insights into monogenic causes of osteoporosis. Front Endocrinol (Lausanne) 2019;10:70. doi: 10.3389/fendo.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure function and factors that influence bone cells. Biomed Res Int. 2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–139. doi: 10.2215/cjn.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 12.Ott SM. Cortical or trabecular bone: what’s the difference? Am J Nephrol. 2018;47:373–375. doi: 10.1159/000489672. [DOI] [PubMed] [Google Scholar]
- 13.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8:225–235. doi: 10.1177/1759720x16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ominsky MS, Niu QT, Li C, Li X, Ke HZ. Tissue-level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res. 2014;29:1424–1430. doi: 10.1002/jbmr.2152. [DOI] [PubMed] [Google Scholar]
- 15.Farr JN, Khosla S. Skeletal changes through the lifespan–from growth to senescence. Nat Rev Endocrinol. 2015 doi: 10.1038/nrendo.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 17.Mäkitie O, Zillikens MC. Early-onset osteoporosis. Calcif Tissue Int. 2022;110:546–561. doi: 10.1007/s00223-021-00885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward LM, Weber DR, Munns CF, Högler W, Zemel BS. A contemporary view of the definition and diagnosis of osteoporosis in children and adolescents. J Clin Endocrinol Metab. 2020;105:e2088–2097. doi: 10.1210/clinem/dgz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthold O, Frericks B, John T, Clemens V, Fegert JM, Moers AV. Abuse as a cause of childhood fractures. Dtsch Arztebl Int. 2018;115:769–775. doi: 10.3238/arztebl.2018.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galindo-Zavala R, Bou-Torrent R, Magallares-López B, Mir-Perelló C, Palmou-Fontana N, Sevilla-Pérez B, Medrano-San Ildefonso M, González-Fernández MI, Román-Pascual A, Alcañiz-Rodríguez P, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. 2020;18:20. doi: 10.1186/s12969-020-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen AV, Mundbjerg E. Lauritsen JM and Faergemann C (2020) Development of the annual incidence rate of fracture in children 1980–2018: a population-based study of 32375 fractures. Acta Orthop. 2020 doi: 10.1080/17453674.2020.1772555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Gazzar A, Högler W. Mechanisms of bone fragility: from osteogenesis imperfecta to secondary osteoporosis. Int J Mol Sci. 2021 doi: 10.3390/ijms22020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O. Munns CF and Shaw N (2014) Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD. Pediatric Official Positions J Clin Densitom. 2013;17:275–280. doi: 10.1016/j.jocd.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Gordon CM, Leonard MB, Zemel BS. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Cohen A. Premenopausal osteoporosis endocrinol metab. Clin North Am. 2017;46:117–133. doi: 10.1016/j.ecl.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, et al. (2008) International society for clinical densitometry. Adult and Pediatric Official Positions Bone. 2007;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, Stepan JJ, de Vernejoul MC, Kaufman JM. Osteoporosis in young adults: pathophysiology diagnosis, and management. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2030-x. [DOI] [PubMed] [Google Scholar]
- 28.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010 doi: 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32:39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 30.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 31.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011 doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 32.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, Bonjour JP. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992 doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 33.Chevalley T, Rizzoli R. Acquisition of peak bone mass. Best Pract Res Clin Endocrinol Metab. 2022 doi: 10.1016/j.beem.2022.101616. [DOI] [PubMed] [Google Scholar]
- 34.Costantini A, Mäkitie RE, Hartmann MA, Fratzl-Zelman N, Zillikens MC, Kornak U, Søe K, Mäkitie O. Early-onset osteoporosis: rare monogenic forms elucidate the complexity of disease pathogenesis beyond Type I collagen. J Bone Miner Res. 2022;37:1623–1641. doi: 10.1002/jbmr.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciancia S, van Rijn RR, Högler W, Appelman-Dijkstra NM, Boot AM, Sas TCJ, Renes JS. Osteoporosis in children and adolescents: when to suspect and how to diagnose it. Eur J Pediatr. 2022;181:2549–2561. doi: 10.1007/s00431-022-04455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, et al. 2017 American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69:1521–1537. doi: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 37.Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547–2556. doi: 10.1056/NEJMcp1800214. [DOI] [PubMed] [Google Scholar]
- 38.Abraham A, Cohen A, Shane E. Premenopausal bone health: osteoporosis in premenopausal women. Clin Obstet Gynecol. 2013;56:722–729. doi: 10.1097/GRF.0b013e3182a8ae55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepe J, Body JJ, Hadji P, McCloskey E, Meier C, Obermayer-Pietsch B, Palermo A, Tsourdi E, Zillikens MC, Langdahl B, et al. Osteoporosis in premenopausal women a clinical narrative review by the ECTS and the IOF. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa306. [DOI] [PubMed] [Google Scholar]
- 40.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover calcium homeostasis and bone density in normal pregnancy. J Bone Miner Res. 2000 doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 41.Kaur M, Pearson D, Godber I, Lawson N, Baker P, Hosking D. Longitudinal changes in bone mineral density during normal pregnancy. Bone. 2003;32:449–454. doi: 10.1016/s8756-3282(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 42.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15:129–137. doi: 10.1359/jbmr.2000.15.1.129. [DOI] [PubMed] [Google Scholar]
- 43.Sowers M, Crutchfield M, Jannausch M, Updike S, Corton G. A prospective evaluation of bone mineral change in pregnancy. Obstet Gynecol. 1991;77:841–845. [PubMed] [Google Scholar]
- 44.Holmberg-Marttila D, Leino A, Sievänen H. Bone turnover markers during lactation, postpartum amenorrhea and resumption of menses. Osteoporos Int. 2003 doi: 10.1007/s00198-002-1320-0. [DOI] [PubMed] [Google Scholar]
- 45.Karlsson MK, Ahlborg HG, Karlsson C. Maternity and bone mineral density. Acta Orthop. 2005;76:2–13. doi: 10.1080/00016470510030274. [DOI] [PubMed] [Google Scholar]
- 46.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337:523–528. doi: 10.1056/nejm199708213370803. [DOI] [PubMed] [Google Scholar]
- 47.Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA. 1996;276:549–554. doi: 10.1001/jama.1996.03540070045029. [DOI] [PubMed] [Google Scholar]
- 48.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112:1429–1436. doi: 10.1172/jci19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond) 1998;94:405–412. doi: 10.1042/cs0940405. [DOI] [PubMed] [Google Scholar]
- 50.Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab. 1995;80:2210–2216. doi: 10.1210/jcem.80.7.7608281. [DOI] [PubMed] [Google Scholar]
- 51.Cooke-Hubley S, Gao Z, Mugford G, Kaiser SM, Goltzman D, Leslie WD, Davison KS, Brown JP, Probyn L, Lentle B, et al. Parity and lactation are not associated with incident fragility fractures or radiographic vertebral fractures over 16 years of follow-up: Canadian multicentre osteoporosis study (CaMos) Arch Osteoporos. 2019 doi: 10.1007/s11657-019-0601-6. [DOI] [PubMed] [Google Scholar]
- 52.Hadji P, Boekhoff J, Hahn M, Hellmeyer L, Hars O, Kyvernitakis I. Pregnancy-associated osteoporosis: a case-control study. Osteoporos Int. 2017;28:1393–1399. doi: 10.1007/s00198-016-3897-8. [DOI] [PubMed] [Google Scholar]
- 53.Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26:2223–2241. doi: 10.1007/s00198-015-3149-3. [DOI] [PubMed] [Google Scholar]
- 54.Dunne F, Walters B, Marshall T, Heath DA. Pregnancy associated osteoporosis. Clin Endocrinol (Oxf) 1993;39:487–490. doi: 10.1111/j.1365-2265.1993.tb02398.x. [DOI] [PubMed] [Google Scholar]
- 55.Campos-Obando N, Oei L, Hoefsloot LH, Kiewiet RM, Klaver CC, Simon ME, Zillikens MC. Osteoporotic vertebral fractures during pregnancy: be aware of a potential underlying genetic cause. J Clin Endocrinol Metab. 2014;99:1107–1111. doi: 10.1210/jc.2013-3238. [DOI] [PubMed] [Google Scholar]
- 56.Cohen A, Kamanda-Kosseh M, Dempster DW, Zhou H, Müller R, Goff E, Colon I, Bucovsky M, Stubby J, Nickolas TL, et al. Women with pregnancy and lactation-associated osteoporosis (PLO) have low bone remodeling rates at the tissue level. J Bone Miner Res. 2019 doi: 10.1002/jbmr.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kyvernitakis I, Reuter TC, Hellmeyer L, Hars O, Hadji P. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporos Int. 2018 doi: 10.1007/s00198-017-4239-1. [DOI] [PubMed] [Google Scholar]
- 58.Rivadeneira F, Mäkitie O. Osteoporosis and bone mass disorders: from gene pathways to treatments. Trends Endocrinol Metab. 2016;27:262–281. doi: 10.1016/j.tem.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012 doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rauner M, Foessl I, Formosa MM, Kague E, Prijatelj V, Lopez NA, Banerjee B, Bergen D, Busse B, Calado Â, et al. Perspective of the GEMSTONE consortium on current and future approaches to functional validation for skeletal genetic disease using cellular. Molecular and Animal-Modeling Techniques Front Endocrinol (Lausanne) 2021 doi: 10.3389/fendo.2021.731217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB, Rivadeneira F. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. 2018 doi: 10.1136/bmj.k3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Formosa MM, Bergen DJM, Gregson CL, Maurizi A, Kämpe A, Garcia-Giralt N, Zhou W, Grinberg D, Ovejero Crespo D, Zillikens MC, et al. A Roadmap to gene discoveries and novel therapies in monogenic low and high bone mass disorders. Front Endocrinol (Lausanne) 2021 doi: 10.3389/fendo.2021.709711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, Cohn DH, Cormier-Daire V, Girisha KM, Hall C, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023 doi: 10.1002/ajmg.a.63132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gistelinck C, Weis M, Rai J, Schwarze U, Niyazov D, Song KM, Byers PH, Eyre DR. Abnormal bone collagen cross-linking in osteogenesis imperfecta/bruck syndrome caused by compound heterozygous PLOD2 mutations. JBMR Plus. 2021 doi: 10.1002/jbm4.10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jovanovic M, Guterman-Ram G, Marini JC. Osteogenesis Imperfecta: mechanisms and signaling pathways connecting classical and rare OI types. Endocr Rev. 2022;43:61–90. doi: 10.1210/endrev/bnab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2010 doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 69.Marom R, Rabenhorst BM, Morello R. Osteogenesis imperfecta: an update on clinical features and therapies. Eur J Endocrinol. 2020;183:R95–r106. doi: 10.1530/eje-20-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martínez-Glez V, Valencia M, Caparrós-Martín JA, Aglan M, Temtamy S, Tenorio J, Pulido V, Lindert U, Rohrbach M, Eyre D, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012 doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 72.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001 doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 73.Keupp K, Beleggia F, Kayserili H, Barnes AM, Steiner M, Semler O, Fischer B, Yigit G, Janda CY, Becker J, et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet. 2013;92:565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu J, Lin X, Gao P, Zhang Q, Zhou B, Wang O, Jiang Y, Xia W, Xing X, Li M. Genotypic and phenotypic spectrum and pathogenesis of WNT1 variants in a large cohort of patients with OI/osteoporosis. J Clin Endocrinol Metab. 2023 doi: 10.1210/clinem/dgac752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mäkitie RE, Haanpää M, Valta H, Pekkinen M, Laine CM, Lehesjoki AE, Schalin-Jäntti C, Mäkitie O. Skeletal characteristics of WNT1 osteoporosis in children and young adults. J Bone Miner Res. 2016;31:1734–1742. doi: 10.1002/jbmr.2841. [DOI] [PubMed] [Google Scholar]
- 77.Mäkitie RE, Niinimäki T, Nieminen MT, Schalin-Jäntti C, Niinimäki J, Mäkitie O. Impaired WNT signaling and the spine-heterozygous WNT1 mutation causes severe age-related spinal pathology. Bone. 2017;101:3–9. doi: 10.1016/j.bone.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Nampoothiri S, Guillemyn B, Elcioglu N, Jagadeesh S, Yesodharan D, Suresh B, Turan S, Symoens S, Malfait F. Ptosis as a unique hallmark for autosomal recessive WNT1-associated osteogenesis imperfecta. Am J Med Genet A. 2019;179:908–914. doi: 10.1002/ajmg.a.61119. [DOI] [PubMed] [Google Scholar]
- 79.Peris P, Monegal A, Mäkitie RE, Guañabens N, González-Roca E. Osteoporosis related to WNT1 variants: a not infrequent cause of osteoporosis. Osteoporos Int. 2023;34:405–411. doi: 10.1007/s00198-022-06609-2. [DOI] [PubMed] [Google Scholar]
- 80.van Dijk FS, Zillikens MC, Micha D, Riessland M, Marcelis CL, de Die-Smulders CE, Milbradt J, Franken AA, Harsevoort AJ, Lichtenbelt KD, et al. PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med. 2013;369:1529–1536. doi: 10.1056/NEJMoa1308223. [DOI] [PubMed] [Google Scholar]
- 81.Laine CM, Wessman M, Toiviainen-Salo S, Kaunisto MA, Mäyränpää MK, Laine T, Pekkinen M, Kröger H, Välimäki VV, Välimäki MJ, et al. A novel splice mutation in PLS3 causes X-linked early onset low-turnover osteoporosis. J Bone Miner Res. 2015;30:510–518. doi: 10.1002/jbmr.2355. [DOI] [PubMed] [Google Scholar]
- 82.Mäkitie RE, Niinimäki T, Suo-Palosaari M, Kämpe A, Costantini A, Toiviainen-Salo S, Niinimäki J, Mäkitie O. PLS3 Mutations cause severe age and sex-related spinal pathology. Front Endocrinol (Lausanne) 2020;11:393. doi: 10.3389/fendo.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff L, Strathmann EA, Müller I, Mählich D, Veltman C, Niehoff A, Wirth B. Plastin 3 in health and disease: a matter of balance. Cell Mol Life Sci. 2021;78:5275–5301. doi: 10.1007/s00018-021-03843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wesseling-Perry K, Mäkitie RE, Välimäki VV, Laine T, Laine CM, Välimäki MJ, Pereira RC, Mäkitie O. Osteocyte protein expression is altered in low-turnover osteoporosis caused by mutations in WNT1 and PLS3. J Clin Endocrinol Metab. 2017;102:2340–2348. doi: 10.1210/jc.2017-00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kämpe AJ, Costantini A, Levy-Shraga Y, Zeitlin L, Roschger P, Taylan F, Lindstrand A, Paschalis EP, Gamsjaeger S, Raas-Rothschild A, et al. PLS3 Deletions lead to severe spinal osteoporosis and disturbed bone matrix mineralization. J Bone Miner Res. 2017;32:2394–2404. doi: 10.1002/jbmr.3233. [DOI] [PubMed] [Google Scholar]
- 86.Mäkitie RE, Henning P, Jiu Y, Kämpe A, Kogan K, Costantini A, Välimäki VV, Medina-Gomez C, Pekkinen M, Salusky IB, et al. An ARHGAP25 variant links aberrant Rac1 function to early-onset skeletal fragility. JBMR Plus. 2021;5:e10509. doi: 10.1002/jbm4.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pekkinen M, Terhal PA, Botto LD, Henning P, Mäkitie RE, Roschger P, Jain A, Kol M, Kjellberg MA, Paschalis EP, et al. Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight. 2019 doi: 10.1172/jci.insight.126180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mäkitie RE, Blouin S, Välimäki VV, Pihlström S, Määttä K, Pekkinen M, Fratzl-Zelman N, Mäkitie O, Hartmann MA. Abnormal bone tissue organization and osteocyte Lacunocanalicular network in early-onset osteoporosis due to SGMS2 mutations. JBMR Plus. 2021;5:e10537. doi: 10.1002/jbm4.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basalom S, Fiscaletti M, Miranda V, Huber C, Couture G, Drouin R, Monceau É, Wavrant S, Dubé J, Mäkitie O, et al. Calvarial doughnut lesions with bone fragility in a French-Canadian family; case report and review of the literature. Bone Rep. 2021;15:101121. doi: 10.1016/j.bonr.2021.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson ME, Bardai G, Veilleux LN, Glorieux FH, Rauch F. Musculoskeletal phenotype in two unrelated individuals with a recurrent nonsense variant in SGMS2. Bone. 2020;134:115261. doi: 10.1016/j.bone.2020.115261. [DOI] [PubMed] [Google Scholar]
- 91.Collet C, Ostertag A, Ricquebourg M, Delecourt M, Tueur G, Isidor B, Guillot P, Schaefer E, Javier RM, Funck-Brentano T, et al. Primary osteoporosis in young adults: genetic basis and identification of novel variants in causal genes. JBMR Plus. 2018;2:12–21. doi: 10.1002/jbm4.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrari SL, Deutsch S, Baudoin C, Cohen-Solal M, Ostertag A, Antonarakis SE, Rizzoli R, de Vernejoul MC. LRP5 gene polymorphisms and idiopathic osteoporosis in men. Bone. 2005;37:770–775. doi: 10.1016/j.bone.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 93.Cohen A, Dempster DW, Recker RR, Stein EM, Lappe JM, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, et al. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011;96:3095–3105. doi: 10.1210/jc.2011-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hartikka H, Mäkitie O, Männikkö M, Doria AS, Daneman A, Cole WG, Ala-Kokko L, Sochett EB. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res. 2005;20:783–789. doi: 10.1359/jbmr.050101. [DOI] [PubMed] [Google Scholar]
- 95.Rouleau C, Malorie M, Collet C, Porquet-Bordes V, Gennero I, Eddiry S, Laroche M, Salles JP, Couture G, Edouard T. Diagnostic yield of bone fragility gene panel sequencing in children and young adults referred for idiopathic primary osteoporosis at a single regional reference centre. Bone Rep. 2022;16:101176. doi: 10.1016/j.bonr.2022.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peris P, Guañabens N, Martínez de Osaba MJ, Monegal A, Alvarez L, Pons F, Ros I, Cerdá D and Muñoz-Gómez J, (2002) Clinical characteristics and etiologic factors of premenopausal osteoporosis in a group of Spanish women Semin Arthritis Rheum 32:64-70. 10.1053/sarh.2002.33725 [DOI] [PubMed]
- 97.Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ., 3rd Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15:551–555. doi: 10.1016/8756-3282(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 98.Laine CM, Koltin D, Susic M, Varley TL, Daneman A, Moineddin R, Cole WG, Mäkitie O, Sochett E. Primary osteoporosis without features of OI in children and adolescents: clinical and genetic characteristics. Am J Med Gene A. 2012 doi: 10.1002/ajmg.a.35278. [DOI] [PubMed] [Google Scholar]
- 99.Foessl I, Bassett JHD, Bjørnerem Å, Busse B, Calado Â, Chavassieux P, Christou M, Douni E, Fiedler IAK, Fonseca JE, et al. Bone phenotyping approaches in human, Mice and Zebrafish - Expert Overview of the EU Cost Action GEMSTONE (“Genomics of musculoskeletal traits translational network”) Front Endocrinol (Lausanne) 2021 doi: 10.3389/fendo.2021.720728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubin MR, Schussheim DH, Kulak CA, Kurland ES, Rosen CJ, Bilezikian JP, Shane E. Idiopathic osteoporosis in premenopausal women. Osteoporos Int. 2005;16:526–533. doi: 10.1007/s00198-004-1716-0. [DOI] [PubMed] [Google Scholar]
- 101.Rand MS, Diemar SS, Møllehave LT, Heidemann M, Thuesen BH, Petersen JH, Johannesen J, Schou AJ, Wedderkopp N, Mølgaard C, et al. Z-scores of bone turnover markers calculated from new established sex- and age-specific reference curves are associated to future change in BMD in children and adolescents. Bone. 2023 doi: 10.1016/j.bone.2022.116641. [DOI] [PubMed] [Google Scholar]
- 102.Schini M, Vilaca T, Gossiel F, Salam S, Eastell R. Bone turnover markers: basic biology to clinical applications. Endocr Rev. 2023;44:417–473. doi: 10.1210/endrev/bnac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ho SS, Urban AE, Mills RE. Structural variation in the sequencing era. Nat Rev Genet. 2020;21:171–189. doi: 10.1038/s41576-019-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Apperley LJ, Albaba S, Dharmaraj P, Balasubramanian M. PLS3 whole gene deletion as a cause of X-linked osteoporosis: clinical report with review of published PLS3 literature. Clin Dysmorphol. 2023;32:43–47. doi: 10.1097/mcd.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 105.Costantini A, Skarp S, Kämpe A, Mäkitie RE, Pettersson M, Männikkö M, Jiao H, Taylan F, Lindstrand A, Mäkitie O. Rare copy number variants in array-based comparative genomic hybridization in early-onset skeletal fragility front. Endocrinol (Lausanne) 2018;9:380. doi: 10.3389/fendo.2018.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lv F, Ma M, Liu W, Xu X, Song Y, Li L, Jiang Y, Wang O, Xia W, Xing X, et al. A novel large fragment deletion in PLS3 causes rare X-linked early-onset osteoporosis and response to zoledronic acid. Osteoporos Int. 2017;28:2691–2700. doi: 10.1007/s00198-017-4094-0. [DOI] [PubMed] [Google Scholar]
- 107.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster D. Double and quadruple tetracycline labeling of bone: impact of the label itself. J Bone Miner Res. 2013;28:222–223. doi: 10.1002/jbmr.1818. [DOI] [PubMed] [Google Scholar]
- 108.Kann PH, Pfützner A, Delling G, Schulz G, Meyer S. Transiliac bone biopsy in osteoporosis: frequency, indications, consequences and complications. An evaluation of 99 consecutive cases over a period of 14 years. Clin Rheumatol. 2006 doi: 10.1007/s10067-005-1132-7. [DOI] [PubMed] [Google Scholar]
- 109.Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 110.Razi H, Predan J, Fischer FD, Kolednik O, Fratzl P. Damage tolerance of lamellar bone. Bone. 2020 doi: 10.1016/j.bone.2019.115102. [DOI] [PubMed] [Google Scholar]
- 111.Shapiro F, Maguire K, Swami S, Zhu H, Flynn E, Wang J, Wu JY. Histopathology of Osteogenesis imperfecta bone. Supramolecular assessment of cells and matrices in the context of woven and lamellar bone formation using light, polarization and ultrastructural microscopy. Bone Rep. 2021 doi: 10.1016/j.bonr.2020.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Islam MZ, Shamim AA, Viljakainen HT, Akhtaruzzaman M, Jehan AH, Khan HU, Al-Arif FA, Lamberg-Allardt C. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: a double-blinded, randomised, placebo-controlled 1-year intervention. Br J Nutr. 2010;104:241–247. doi: 10.1017/s0007114510000437. [DOI] [PubMed] [Google Scholar]
- 113.Peris P, Monegal A, Martínez MA, Moll C, Pons F, Guañabens N. Bone mineral density evolution in young premenopausal women with idiopathic osteoporosis. Clin Rheumatol. 2007;26:958–961. doi: 10.1007/s10067-006-0405-0. [DOI] [PubMed] [Google Scholar]
- 114.Lorentzon M, Mellström D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD study. J Bone Miner Res. 2005;20:1936–1943. doi: 10.1359/jbmr.050709. [DOI] [PubMed] [Google Scholar]
- 115.Zhu K, Prince RL. Lifestyle and osteoporosis. Curr Osteoporos Rep. 2015;13:52–59. doi: 10.1007/s11914-014-0248-6. [DOI] [PubMed] [Google Scholar]
- 116.Miller KK, Grieco KA, Mulder J, Grinspoon S, Mickley D, Yehezkel R, Herzog DB, Klibanski A. Effects of risedronate on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2004;89:3903–3906. doi: 10.1210/jc.2003-031885. [DOI] [PubMed] [Google Scholar]
- 117.Tauchmanovà L, De Simone G, Musella T, Orio F, Ricci P, Nappi C, Lombardi G, Colao A, Rotoli B, Selleri C. Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:81–88. doi: 10.1038/sj.bmt.1705196. [DOI] [PubMed] [Google Scholar]
- 118.Papaioannou A, Kennedy CC, Freitag A, Ioannidis G, O'Neill J, Webber C, Pui M, Berthiaume Y, Rabin HR, Paterson N, et al. Alendronate once weekly for the prevention and treatment of bone loss in Canadian adult cystic fibrosis patients (CFOS trial) Chest. 2008;134:794–800. doi: 10.1378/chest.08-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chapman I, Greville H, Ebeling PR, King SJ, Kotsimbos T, Nugent P, Player R, Topliss DJ, Warner J, Wilson JW. Intravenous zoledronate improves bone density in adults with cystic fibrosis (CF) Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2008.03434.x. [DOI] [PubMed] [Google Scholar]
- 120.Pazianas M, Rhim AD, Weinberg AM, Su C, Lichtenstein GR. The effect of anti-TNF-alpha therapy on spinal bone mineral density in patients with Crohn’s disease. Ann N Y Acad Sci. 2006;1068:543–556. doi: 10.1196/annals.1346.055. [DOI] [PubMed] [Google Scholar]
- 121.Haidar R, Musallam KM, Taher AT. Bone disease and skeletal complications in patients with β thalassemia major. Bone. 2011;48:425–432. doi: 10.1016/j.bone.2010.10.173. [DOI] [PubMed] [Google Scholar]
- 122.Stürznickel J, Rolvien T, Delsmann A, Butscheidt S, Barvencik F, Mundlos S, Schinke T, Kornak U, Amling M, Oheim R. Clinical phenotype and relevance of LRP5 and LRP6 variants in patients with early-onset osteoporosis (EOOP) J Bone Miner Res. 2021;36:271–282. doi: 10.1002/jbmr.4197. [DOI] [PubMed] [Google Scholar]
- 123.Välimäki VV, Mäkitie O, Pereira R, Laine C, Wesseling-Perry K, Määttä J, Kirjavainen M, Viljakainen H, Välimäki MJ. Teriparatide treatment in patients With WNT1 or PLS3 mutation-related early-onset osteoporosis: a pilot study. J Clin Endocrinol Metab. 2017;102:535–544. doi: 10.1210/jc.2016-2423. [DOI] [PubMed] [Google Scholar]
- 124.Kruk M, Ralston SH and Albagha OM (2009) LRP5 Polymorphisms and response to risedronate treatment in osteoporotic men Calcif Tissue Int 84:171–179. [DOI] [PubMed]
- 125.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 126.Binkley N, Orwoll E, Chapurlat R, Langdahl BL, Scott BB, Giezek H, Santora AC. Randomized, controlled trial to assess the safety and efficacy of odanacatib in the treatment of men with osteoporosis. Osteoporos Int. 2021 doi: 10.1007/s00198-020-05701-9. [DOI] [PubMed] [Google Scholar]
- 127.Carpenter TO, Whyte MP, Imel EA, Boot AM, Högler W, Linglart A, Padidela R, Van’t Hoff W, Mao M, Chen CY, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 128.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 129.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, Van Sickle BJ, Simmons JH, Edgar TS, Bauer ML, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.