Abstract
Malignant tumours of the digestive system cover a wide range of diseases that affect the health of people to a large extent. Angiogenesis is indispensable in the development, and metastasis of tumours, mainly in two ways: occupation or formation. Vessels can provide nutrients, oxygen, and growth factors for tumours to encourage growth and metastasis, so cancer progression depends on simultaneous angiogenesis. Recently, exosomes have been proven to participate in the angiogenesis of tumours. They influence angiogenesis by binding to tyrosine kinase receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 with different affinities, regulating Yap-VEGF pathway, Akt pathway or other signaling pathway. Additionally, exosomes are potential therapeutic vectors that can deliver many types of cargoes to different cells. In this review, we summarize the roles of exosomes in the angiogenesis of digestive system tumours and highlight the clinical application prospects, directly used as targers or delivery vehicles, in antiangiogenic therapy.
Keywords: Exosome, Angiogenesis, Antiangiogenic therapy, Digestive system tumour
Background
The digestive system is one of the most important systems in our body, and related tumours frequently occur [1]. Digestive system malignant tumours can be classified into oesophageal, gastric, small intestinal, colon, rectal, appendiceal, anal, hepatic and pancreatic cancer based on the organ from which the abnormal cell proliferation is derived [2]. These tumours cause adverse effects on the physical and mental health of high-risk individuals [3, 4]. According to the report released by the International Agency for Research on Cancer, for both sexes combined, four digestive system cancer types are in the top 10 cancer types for worldwide incidence (23.4% of total cases) and five are in the top 10 for worldwide mortality (35.6% of total cases) [5]. Due to the lack of typical early symptoms, early detection is often difficult and patients often miss the opportunity for optimal treatment [6, 7]. Although endoscopy, cholangiopancreatography and needle aspiration techniques have improved, the invasiveness, uncertainty of operation and high cost restrict large-scale screening [8, 9]. At present, serum carcinoembryonic antigen (CEA), Carbohydrate antigen 19–9 (CA19-9), and Carbohydrate antigen 125 (CA125) are normally used in clinical diagnosis, but both their sensitivity and specificity are low [10–12]. Therefore, noninvasive and highly accurate tumour biomarkers are urgently needed to strongly support the screening of digestive system tumours, even in the early stages.
Exosomes refer to a specific subtype of secreted derived vesicles with a lipid bilayer membrane structure, a size of approximately 30–150 nm in diameter [13]. Exosomes are secreted by almost all human cells and have been found in numerous biological fluids, such as sperm, blood (serum and/or plasma), breast milk, urine, amniotic fluid, saliva, cerebrospinal fluid, nasal secretions, bronchoalveolar lavage, bile, synovial fluid, malignant effusions, pleural effusions and ascites [14]. In both physiological and pathological processes, exosomes play a crucial role in cell–cell communication [15]. Due to their special structural characteristics and capacity to carry many cargos, [16] exosomes have become hot research topics in recent years.
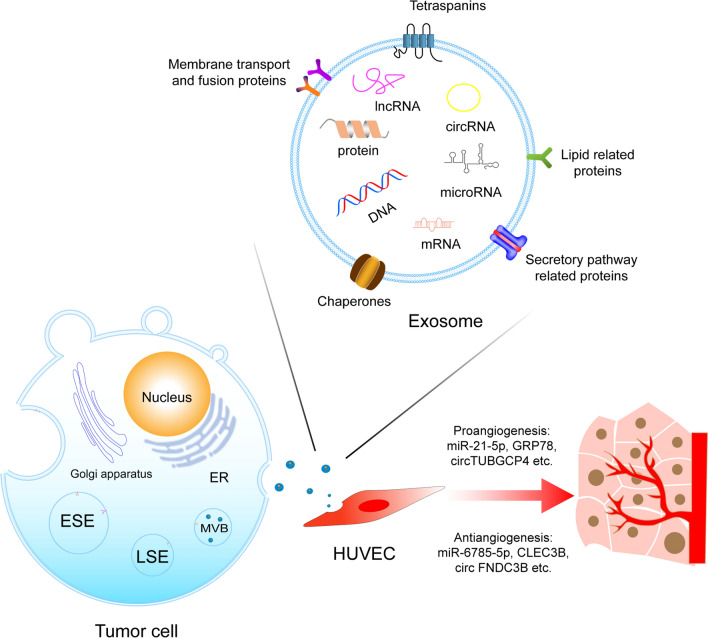
Vessels can provide nutrients, oxygen, and growth factors for tumours to encourage growth and metastasis, so cancer progression depends on simultaneous angiogenesis, which means tumours and angiogenesis are supplementary to each other [17]. The process by which new capillaries arise from preexisting blood vessels is named angiogenesis [18]. The balance between pro- and antiangiogenic factors controls the process of angiogenesis [19]. Tumour angiogenesis and pathological activation of the endothelium, tumour vessel leakiness and hypoxia-induced apoptosis/necrosis in the tumour core have a vital role in recruiting and activating many more inflammatory cells, such as lymphocytes, neutrophils, macrophages and mast cells [20]. These infiltrating immune cells secrete growth and angiogenic factors into the microenvironment to enhance cancer growth and subsequent resistance to therapy [20]. The tumour microenvironment (TME) is a dynamic environment in which multiple components interact and influence each other to regulate the process of the disease [21]. Exosomes participate in the angiogenesis of cancer by influencing endothelial cells through growth factors or direct interactions [22] (Fig. 1). This review focuses on exosomes in the context of tumour angiogenesis in digestive system tumours and describes their therapeutic properties as targets for future antiangiogenic drugs.
Fig. 1.
Tumour-derived exosomes are generated through ESE, LSE, and ILVs in MVBs. After being secreted, exosomes can participate in the pro- and anti-angiogenesis of cancer by the cargos. ESE early-sorting endosome, LSE late-sorting endosome, ILVs intraluminal vesicles, MVBs multivesicular bodies, HUVEC human umbilical vein endothelial cell
Exosome biogenesis, composition and regulation
Exosomes are a subtype of small extracellular vesicles (sEVs) [23]. Exosomes are generated through the early-sorting endosome (ESE), late-sorting endosome (LSE), and intraluminal vesicle (ILVs) phases in multivesicular bodies (MVBs) before they exit the cell [24]. ESEs arise from the inward budding of the cell’s plasma membrane with the help of the trans-Golgi network and endoplasmic reticulum [25]. Then, they mature into LSEs and eventually invaginate from the endosomal limiting membrane to form MVBs, which contain several ILVs (future exosomes) [26]. MVBs are finally either sent to lysosomes to be degraded or fused with the cell plasma membrane to release the contained ILVs as exosomes (Fig. 1) [25]. The mechanisms of exosome biogenesis and formation and vesicle scission are diverse. The endosomal sorting complex needed for transport (ESCRT)-dependent and ESCRT-independent machinery are widely studied [27, 28]. Other factors, such as Vps60 [29], IST1 [30], and synaptotagmin 7 (SYT7) [31], have also been reported.
The contents of exosomes are complex and include microRNAs, circular RNAs, long noncoding RNAs (lncRNAs), DNA fragments, proteins, lipids and metabolites (Fig. 1) [32, 33]. These cargoes determine the function of exosomes to a great degree [34, 35]. Exosomes modulate the tissue microenvironment by mediating autocrine, juxtacrine and paracrine interactions, and they also participate in the suppression of antitumour immune responses, cancer angiogenesis, and tumour progression [36]. Exosome uptake by target cells is processed by lipid raft-mediated endocytosis, receptor-mediated endocytosis, phagocytosis, micropinocytosis and membrane fusion [37]. However, the detailed mechanisms remain unclear [32].
Exosomes need to be isolated from biological fluids for various studies [38–40]. Until now, differential ultracentrifugation has been the most commonly used method for exosome separation [41–43]. Density gradient centrifugation, filtration, size-exclusion chromatography, and liquid chromatography techniques have also been applied [44]. The identification of exosomes is similarly important. Since exosomes are a type of sEV, they are suitable for routine detection and analysis [45]. Specifically, they are positive for at least one transmembrane/lipid-bound protein (usually CD9, CD63, CD83 and integrin), and at least one cytosolic protein can be recovered from EVs (usually ALIX, TSG101, syntenin and HSP70) [46, 47]. At the same time, the levels of at least one negative protein, such as albumin, lipoproteins, and ribosomal proteins, should also be determined [46, 47]. In addition, analysis of functional proteins, such as histones, cytochrome C, calnexin or Grp94, is needed for specific sEVs [46, 47]. Therefore, western blotting, fluorescence microscopy, flow cytometry and mass spectrometry are helpful to identify these proteins [48]. Every technique has both advantages and disadvantages with regard to sensitivity, specificity, time and cost [49]. Combinations of methodologies may be increasingly accepted for the absolute isolation of exosomes.
Mechanism of angiogenesis
The formation of cancer blood vessels involves two aspects. One is the incorporation of host blood vessels into tumours, and the other is the ability of cancer cells to express the endothelial cell (EC) phenotype and form similar vessels [50]. At the initial stage of tumour progression, vessels may not be essential, and tumour cells acquire oxygen, nutrients, and growth factors from nearby vascular tubes through diffusion [51–53]. Alter the phrase. Although it is generally correlated with increased necrosis, the requirement for angiogenesis is more correlated with the oxygen gradient within the tumor, with tumor inner cells exposed to lower levels of oxygen resulting in necrosis [54]. However, newly generated vasculature in tumours is variable in size, shape, architecture, and arrangement compared to normal vessels [55]. These irregular, immature, tortuous, and distended vessels are produced because extracellular signals, such as hypoxia, low pH, a deregulated and disorganized extracellular matrix (ECM), mechanical stresses, and soluble mediators released by surrounding tumour and stromal cells, destroy the balance of pro- and antiangiogenic factors [56]. At least four mechanisms underlie angiogenesis, namely, sprouting angiogenesis (SA), splitting angiogenesis, also known as intussusceptive angiogenesis (IA), vessel co-option (VC) and vascular mimicry (VM) [57]. SA depends on angiogenic factors, such as growth factors, chemokines, angiopoietins, endostatin, interferons, and NO [58]. Angiogenic factors and the absence of blood flow mediate blood vessel destabilization to create conditions for a new sprout. Then, tip and stalk cells are differentiated and combine to sprout. Next, the apical membrane of stalk cells forms and extends new lumenized vascular tubes through tight and adhering connections with neighbouring sprouts or blood vessels [59]. SA completes when blood vessels stabilize, mature and prune [60]. During IA, intussusceptive pillars are generated from contacted endothelial cells that extend processes into the vascular lumen and fuse with the assistance of pericytes, fibroblasts, and other supporting cells to split vessels [61]. For VC, tumours hijack preexisting blood vessels of the nonmalignant tissue that they colonize by migrating along the abluminal surface of preexisting vessels and/or infiltrating the tissue space between pre-existing vessels [62]. It is described that cancer cells can utilize their capacity for epithelial-to-mesenchymal transition and acquire cancer stem cell-like behaviour to assume an endothelial-like phenotype, ultimately forming matrix-embedded vascular structures, including plasma and blood cells. This process is called VM [63, 64].
Although these four mechanisms of angiogenesis are known, various regulators and signalling pathways involved in the mechanisms are still being researched [65–67]. The classical factor is vascular endothelial growth factor A (VEGF-A). It is in a family that includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, virally encoded VEGF-E, and placental growth factor (PLGF). They influence angiogenesis by binding to tyrosine kinase receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 with different affinities [68]. The matrix metalloproteinase (MMP) family can modulate the dynamic remodelling of the ECM, the VEGF pathway and vascular permeability through different signalling axes [69–71]. For example, the MMP-1/protease-activated receptor-1 (PAR1) signalling axis induces vascular permeability to participate in tumour angiogenesis [72]. Under similar conditions, Forkhead Box M1 D (FOXM1D) upregulated VEGFA expression by binding to pyruvate kinase M2 (PKM2) and the NF-κB subunits p65 and p50 to promote their nuclear translocation [73]. Furthermore, FOXM1D increased the release of VEGFA and exosomes by interacting with VPS11 [73]. The platelet-derived growth factor (PDGF) family consists of four heparin-binding polypeptide growth factors (A, B, C, and D). It promotes vessel maturation, the recruitment of pericytes and VEGF upregulation [74]. The PDGF-B/PDGFRβ axis has been extensively explored [75]. The fibroblast growth factor (FGF) family has garnered interest. It consists of 22 members, most of which interact with the tyrosine kinase receptors FGFR-1, FGFR-2, FGFR-3, FGFR-4. The FGF-dependent control of c-MYC expression regulates levels of the glycolytic enzyme hexokinase 2 (HK2) to participate in vascular growth and development [76]. Angiopoietin is another noteworthy angiogenic factor. Angiopoietins 1–4 act through the tyrosine kinase receptors Tie-1 and Tie-2. Ang1 inhibits Forkhead Box O (Foxo), a negative regulator of angiogenesis, through Akt activation to produce vessels [77]. Ang2 can induce endothelial cell migration and sprout formation by binding to integrins in Tie2-dependent or Tie2-independent signalling [78]. In addition, many antiangiogenetic factors, such as thrombospondin-1 (TSP-1), vasostatin, and interferon, are being studied. The imbalance of pro- and antiangiogenic factors is also influenced by environmental elements, such as hypoxia, cellular nutrient deficiency, hypoglycaemia, and metabolic acidosis, which often act at the gene level [53].
Recently, tumour-derived exosomes (TEX) have been shown to markedly affect angiogenesis [79–81]. Data show that miR-23a from hypoxic tumour cell colonies increased the expression levels of angiogenic marker genes, such as VEGF, VEGFR2, and MMP9, by modulating the levels of SIRT1 [82]. Both in vitro and in vivo studies revealed that TEX carrying enzymatically active CD39/CD73 and adenosine (ADO) enhanced the secretion of angiogenic factors by directly interacting with ADO receptors or promoting A2BR-mediated polarization of macrophages towards an M2-like phenotype [83]. In addition, experiments using hypoxic papillary thyroid cancer cells showed that exosomal miR-181a promoted angiogenesis by downregulating histone-lysine N-methyltransferase-3 (MLL3) and disheveled binding antagonist of beta-catenin 2 (DACT2), as well as activating the YAP-VEGF pathway [84].
Exosomes in angiogenesis of digestive system tumours
Numerous studies have convincingly proven that TEX induces molecular and genetic programs in EC changes and thus promotes the process of angiogenesis [85]. Most studies verified the relationship between exosomes and angiogenesis by coculturing exosomes with human umbilical vein endothelial cells (HUVESs) in vitro and creating an in vivo nude mouse xenograft model [86]. A list of the tumour-derived exosome cargoes involved in the stimulation of angiogenesis according to the several mentioned studies is shown in Table 1.
Table 1.
Exosomes in angiogenesis of digestive system tumours
| Cancer | Function | Type | Contents | Reference |
|---|---|---|---|---|
| Oesophageal cancer | Proangiogenesis | lncRNA | FAM225A | [88] |
| microRNA | miR-21-5p | [90] | ||
| miR-301a-3p | [91] | |||
| Antiangiogenesis | microRNA | miR-154-5p | [93] | |
| Gastric cancer | Proangiogenesis | protein | YB-1 | [96] |
| protein | GRP78 | [101] | ||
| circular RNA | circSHKBP1 | [95] | ||
| circular RNA | circ29 | [98] | ||
| microRNA | miR-23a | [94] | ||
| microRNA | miR-10a-5p | [99] | ||
| microRNA | miR-519a-3p | [187] | ||
| ncRNA | X26nt | [97] | ||
| Antiangiogenesis | microRNA | miR-6785-5p | [188] | |
| Colorectal cancer | Proangiogenesis | protein | Angiopoietin-like 7(ANGPTL7) | [104] |
| protein | Wnt4 | [106] | ||
| protein | Glucose-regulated protein 78 (GRP78) | [107] | ||
| protein | dipeptidyl peptidase IV (DPP4) | [115] | ||
| protein | growth/differentiation factor 15 (GDF15) | [116] | ||
| circular RNA | circTUBGCP4 | [117] | ||
| circular RNA | circ_0007334 | [114] | ||
| microRNA | miR‑1246 | [105] | ||
| microRNA | miR-25-3p | [108] | ||
| microRNA | miR-1229 | [109] | ||
| microRNA | miR-92a-3p | [110, 111] | ||
| microRNA | miR-183-5p | [112] | ||
| microRNA | miR-21-5p | [113] | ||
| microRNA | miR-135b-5p | [118] | ||
| Antiangiogenesis | circular RNA | circ FNDC3B | [120] | |
| microRNA | miR-126 | [121] | ||
| microRNA | miR-125a-3p | [121] | ||
| microRNA | miR-125a-5p | [121] | ||
| microRNA | miR-34a | [122] | ||
| Hepatic cancer | Proangiogenesi | protein | lysyl oxidase-like 1–4 (LOXL1–4) | [130] |
| protein | Angiopoietin-2 (ANGPT2) | [132] | ||
| protein | RAB13 | [140] | ||
| lncRNA | lncRNA H19 | [123] | ||
| lncRNA | LINC00161 | [133] | ||
| lncRNA | small nucleolar RNA host gene 16 (SNHG16) | [139] | ||
| circular RNA | circRNA-100,338 | [131] | ||
| circular RNA | circCMTM3 | [138] | ||
| microRNA | miR-32-5p | [124] | ||
| microRNA | miR-210 | [125] | ||
| microRNA | miR-155 | [126] | ||
| microRNA | miR-21 | [127] | ||
| microRNA | miR-378b | [136] | ||
| microRNA | miR-1290 | [137] | ||
| Antiangiogenesis | protein | C-Type Lectin Domain Family 3 Member B (CLEC3B) | [141] | |
| microRNA | miR-200b-3p | [142] | ||
| microRNA | miR-3682-3p | [143] | ||
| Pancreatic cance | Proangiogenesis | protein | annexin A1 (ANXA1) | [144] |
| lncRNA | lncRNA SNHG11 | [146] | ||
| lncRNA | lncRNA UCA1 | [148] | ||
| microRNA | miR-27a | [145] | ||
| microRNA | miR-30b-5p | [149] | ||
| microRNA | miR-210 | [150] | ||
| microRNA | miR-501-3p | [152] | ||
| microRNA | miRNAs 155-5p and 221-5p | [153] | ||
| Antiangiogenesis | microRNA | miR-29b | [151] |
Oesophageal cancer
Several studies have described the role of exosomes in the angiogenesis of oesophageal cancer. Yu Mao et al. performed a Matrigel tube formation assay, examined the recruitment of vasculature into subcutaneously implanted Matrigel plugs containing exosomes and concluded that exosomes promote endothelial cell recruitment and vascular organization both in vitro and in vivo, especially hypoxic exosomes [87]. Recently, the exosome-mediated transfer of lncRNA FAM225A was revealed to upregulate NETO2 and FOXP1 expression by sponging miR-206 and accelerating oesophageal cancer progression and angiogenesis [88]. Furthermore, poly (A) binding protein cytoplasmic 1 (PABPC1) activates the IFN/IFI27 signalling pathway to enhance oesophageal squamous cell carcinoma (ESCC) proliferation; at the same time, PABPC1/IFI27 can increase exosomal miRNA-21-5p to promote angiogenesis by inhibiting CXCL10 [89]. Moreover, a review has shown that miRNA-21-5p accelerates angiogenesis by activating programmed cell death 4 and downregulating the signalling pathway or the PTEN (phosphatase and tensin homologue)/Akt signalling pathway [90]. ESCC-derived exosomal miR-301a-3p induced macrophage polarization into the M2 type via the inhibition of PTEN and activation of the PI3K/AKT signalling pathway, subsequently promoting angiogenesis via the secretion of VEGFA and MMP9 [91]. In addition to proangiogenesis, the antiangiogenesis of exosomes in ESCC cells has also been described [92]. Specifically, miR-154-5p overexpression in exosomes inhibited angiogenesis in vitro, as indicated by the inhibition of tube formation in HUVECs. This inhibition may have been mediated by a reduction in kinesin family member 14 (KIF14) expression in ESCC cells via the direct targeting of the KIF14 3ʹUTR. [93] Additionally, many exosomes are secreted by oesophageal cancer cells, but whether they play key roles in the formation of vessels still needs rigorous experiments.
Gastric cancer
Angiogenesis has been researched in gastric cancer (GC). Evidence has indicated that exosomal miR-23a is a proangiogenic factor because it negatively regulates PTEN, which is a tumour angiogenesis suppressor [94]. A series of analysis assays showed that exosomal circSHKBP1 enhanced VEGF mRNA stability and induced VEGF translation by decreasing miR-582-3p to increase HUR expression [95]. YB-1 is considered to be conducive to the neovasculature of GC via exosomes, which may directly or indirectly influence proangiogenic factors [96]. Xiaocui Chen et al. revealed that exosomal X26nt, which is a 26-nt-long ncRNA spliced from inositol- requiring enzyme 1 alpha (IRE1α)- induced unspliced XBP1, directly combined with the 3′UTR of VE- cadherin mRNA in HUVECs to enhance vascular permeability and accelerate angiogenesis [97]. A recently published study showed that exosomal circ29 in GC regulated angiogenesis via the VEGF pathway as a sponge of miR-29a [98]. Subsequently, miR-10a-5p downregulated zinc finger MYND-type containing 11 (ZMYND11) to enhance the viability and migration of HUVECs, which are packaged into GC cell-derived exosomes [99]. A novel study revealed that circFCHO2 was overexpressed in the serum exosomes of GC patients, which might enhance the progression of GC by activating the JAK1/STAT3 signalling pathway by sponging miR-194-5p [100]. In another study, exosomal glucose-regulated protein 78 (GRP78) was found to promote the TME and induce angiogenesis. [101] GC-derived exo-miR-519a-3p are mainly accumulates in the liver and is internalized by intrahepatic macrophages, activating the MAPK/ERK pathway by targeting DUSP2, thereby causing M2-like polarization of macrophages [102]. M2-like polarized macrophages induce angiogenesis and promote intrahepatic premetastatic niche formation to accelerate liver metastasis in gastric cancer patients [103]. These exosomal cargoes, which promote or suppress GC angiogenesis, provide new treatment options, but their mechanism should be further investigated.
Colorectal cancer
Cancer of the colon, retum and anus are classified as colorectal cancer. Experimental evidence supports that angiopoietin-like 7 (ANGPTL7) plays a role in proangiogenesis and vascularization in colorectal cancer, and this process is partially associated with exosomes [104]. Colorectal cancer (CRC) cells can transfer angiogenesis‑promoting miR‑1246 to endothelial cells via exosome transfer [105]. By targeting promyelocytic leukaemia (PML) protein, Smad 1/5/8 signalling is activated, enhancing angiogenesis [105]. Wnt4 loaded in cancer-derived exosomes is conducive to the angiogenesis of cancer via the β-catenin signalling pathway [106]. GRP78 is a known proangiogenic factor in CRC that can be secreted into the microenvironment by tumour cells through the exosome pathway [107]. Moreover, miR-25-3p, which is carried by exosomes derived from CRC, reportedly increases the expression of VEGFR2 and decreases the levels of ZO-1, occludin and claudin-5 in endothelial cells by targeting Krüppel-like factor 2 (KLF2) and Krüppel-like factor 4 (KLF4), consequently promoting vascular permeability and angiogenesis [108]. Exosomal miR-1229 could facilitate angiogenesis by repressing the protein expression of HIPK2 to activate the VEGF pathway [109]. The pro-angiogenic function of miR-92a-3p is attributed to the decreasing expression of at least two target genes, Dickkopf-3 (Dkk-3) [110] and claudin-11(CLDN11), which may induce partial endothelial-to mesenchymal transition [111]. A cell coculture model was used to show that exosomes loaded with miR-183-5p promoted the tube formation abilities of CRC by inhibiting FOXO1 [112]. Compelling evidence has demonstrated that exosomal miR-21-5p suppressed Krev interaction trapped protein 1 (KRIT1) in recipient HUVECs and subsequently activated the β-catenin signalling pathway and increased their downstream targets VEGFa and Ccnd1, enhancing angiogenesis and vascular permeability in CRC [113]. Exosomal circ_0007334 in CRC cells was found to directly bind to miR-577 and target Krüppel-like Factor 12 (KLF12) to promote angiogenesis and tumour growth [114]. Exosomes also paly roles in drug-resistant colon cancer cells. Research has shown that dipeptidyl peptidase IV (DPP4)-enriched exosomes, which are secreted by 5-fluorouracil-resistant colon cancer cells, mediate angiogenesis by increasing the expression and secretion of periostin (POSTN) (a proangiogenic extracellular matrix protein) via Twist1 nuclear translocation or activating the Smad signalling pathway [115]. However, exosomal growth/differentiation factor 15 (GDF15) plays an essential role in the effects of angiogenesis, which increases POSTN levels by inhibiting the Smad signalling pathway [116]. Exosomal circTUBGCP4 upregulated PDK2 to activate the Akt signalling pathway by sponging miR-146b-3p, which causes vascular endothelial cell tipping to promote angiogenesis and tumour metastasis [117]. In addition to TEX, cancer-associated fibroblast (CAF)-derived exosomes (CAF-exos) can also transmit microRNAs (miRNAs) to CRC. Recently, miR-135b-5p, which is transported by CAF-exos, was reported to downregulate thioredoxin-interacting protein (TXNIP) to promote angiogenesis [118]. Experiments have also confirmed that CAF-Exos deliver VEGFA to promote the viability, apoptosis, DDP resistance, and angiogenesis of CRC [119]. In contrast, an antiangiogenic role has been demonstrated for circular FNDC3B, which has been found to be involved in microvesicles secreted by CRC cells and suppresses angiogenesis by directly regulating miR-937-5p to induce the expression level of the tumour-suppressor TIMP3, which inhibits the angiogenesis of CRC [120]. Other anti-angiogenic exosomal miRNAs have been described, such as miR-126, miR-125a-3p and miR-125a-5p, which target different regulators in CRC [121]. Both pro- and antiangiogenic exosomes provide predictive biomarkers for antiangiogenic treatment.
Recently, a murine model of colorectal cancer was used to explore whether TEX-miR-34a can serve as a favourable therapeutic option in CRC combinational therapies, one function of which is inhibiting angiogenesis by targeting VEGF [122]. The results of this study were promising and suggested that exosomes can be used as a treatment for cancer.
Hepatic cancer
Exosomes participate in many mechanisms that promote hepatic tumour angiogenesis. Existing literature has indicated that the lncRNA H19, released via exosomes from CD90 + liver cancer cells, increases the expression of VEGF and the production of VEGF-R1, hence enhancing angiogenesis [123]. A large body of evidence has documented that exosomal microRNA-32-5p increases angiogenesis by activating the PTEN/PI3K/Akt pathway [124]. Moreover, miR-210 transferred by exosomes enhanced angiogenesis by directly repressing the expression of SNAD4 and STAT6 [125]. A series of experiments have been performed to show that miR-155 mediates angiogenic activity via exosomes under hypoxia and that it may be associated with proangiogenic factors [126]. Hepatocellular carcinoma (HCC) cell-derived exosomal miRNA-21 directly targeted PTEN (gene of phosphate and tension homology deleted on chromosome ten), leading to the activation of PDK1/AKT signalling in Hepatic stellate cells (HSCs), which are then transformed into CAFs [127]. Activated CAFs further promote cancer angiogenesis by secreting angiogenic cytokines, including VEGF, MMP2, MMP9, bFGF and TGF-β [128]. Furthmore, miR-21 can also shape a vascular microenvironment for HCC via the STAT3/VEGF signalling pathway, and miR-221 can activate the SAND/NF-κB pathway to upregulate the expression of CXCL16, which is an angiogenic factor [129]. Lysyl oxidase-like 1–4 (LOXL1–4), which was secreted by HCC-derived exosomes in a paracrine mechanism, was also reported to promote angiogenesis [130]. Exosomal circRNA-100,338 was found to be upregulated and could increase angiogenesis of HCC cells [131]. Moreover, circRNA-100,338 might decrease the expression of VE-cadherin and ZO-1 in HUEVCs to promote vascular endothelial cell permeability [131]. A pro‑angiogenic role has also been demonstrated for angiopoietin-2 (ANGPT2), which has been found to be contained in exosomes secreted by HCC cells and activates the Tie2-independent, AKT/eNOS and AKT/β-catenin pathways. [132] Research has shown that HCC cell-derived exosomes carrying LINC00161 activate the ROCK2 signalling pathway by inhibiting miR-590-3p and strengthen the tube-forming ability of HUVECs [133]. Hiroshi Yukawa et al. verified that HepG2-exosomes activated lumen formation by HUVECs [134]. Exosomes contain both upregulated and downregulated miRNAs, but their detailed influence is unknown [134]. Shihua Wang et al. found that HCC cell HepG2-derived exosomes could activate various kinases, such as AKT, STAT5α, GSK3 alpha/beta, p38 alpha, and ERK1/2, as well as the NF-κB signalling pathway in adipocytes to promote tube formation [135]. Recent experimental evidence has highlighted the role of exosomal microRNA-378b in HCC. HepG2 cell-derived exosomal miR-378b enhanced HCC cell angiogenesis by increasing MMP9, FGF2 and VEGFA expression, which may be linked with TGFBR3 [136]. Exosomal miR-1290 enhanced tube formation by directly targeting SMEK1 to alleviate the suppression of VEGFR2 phosphorylation [137]. In addition, HCC-cell-derived exosomes can be used as carriers to deliver CircCMTM3 to HUVECs. CircCMTM3 regulates SOX9 expression in HUVECs by sponging miR‐3619‐5p, which promotes angiogenesis and HCC cell tumorigenesis [138]. Moreover, exosome-delivered small nucleolar RNA host gene 16 (SNHG16) regulates GALNT1 expression by sponging miR-4500 via the PI3K/Akt/mTOR pathway to activate the formation of new HCC blood vessels, thus promoting the progression of HCC [139]. Exosomal RAB13, a potential regulator of HCC metastasis, was also associated with VEGF levels, microvessel density, and tube formation by vascular endothelial cells in both in vitro and in vivo models, suggesting that it promotes angiogenesis [140]. Moreover, HCC cell-derived exosomes containing C-Type Lectin Domain Family 3 Member B (CLEC3B) were able to inhibit the angiogenic ability of HMVECs via the repression of VEGF by activating AMPK signalling [141]. By targeting the transcription factor ERG (erythroblast transformation‑specific (ETS)‑related gene), exosomal miR-200b-3p plays a negative role in angiogenesis [142]. Similarly, exosomal miR-3682-3p targeted angiopoietin-1 (ANGPT1) via RAS-MEK1/2-ERK1/2 signalling, and attenuated angiogenesis [143]. All of these findings may indicate potential therapeutic targets for antiangiogenic therapy.
Pancreatic cancer
Several studies have reported that exosomes released from pancreatic cancer (PC) cells contribute to enhancing cancer angiogenesis. Emanuela Pessolano et al. found that exosome-related annexin A1 (ANXA1) could induce angiogenesis in PC [144], but the mechanism remains unknown. PC cell-derived exosomal miR-27a, along with regulated B‐cell translocation gene 2 (BTG2), enhanced tumour angiogenesis [145]. LncRNA SNHG11 (SNHG11) has also been found to be highly expressed in the serum of PC patients, and is carried by exosomes. Mechanistically, SNHG11 acts as a ceRNA for miR‐324‐3p, therefore upregulating VEGFA, which has previously been implicated in tumour angiogenesis [146]. Environmental factors, such as hypoxia, play a prominent role in tumour angiogenesis [147]. The lncRNA UCA1, acting as a sponge of miR-96-5p, alleviated the repressive effects of miR-96-5p on the expression of its target gene AMOTL2 to enhance angiogenesis [148]. MiR-30b-5p was significantly enriched in hypoxic pancreatic ductal adenocarcinoma (PDAC) cell-derived exosomes, which could be transferred to HUVECs, resulting in the upregulation of tube formation and endothelial cell migration via downregulation of the gap junction protein GJA1 [149]. Emerging studies have proposed that hypoxia-induced miR-210 facilitates tumour angiogenesis and cellular permeability in PDAC, negatively regulating EFNA3 expression and participating in the PI3K/AKT/VEGFA or Wnt/β-catenin/RHOA pathways [150]. In contrast, miR-29b-containing exosomes in PC cells reduced angiogenesis migration and tube formation of HUVECs by targeting ROBO1 and SRGAP2 [151].
In addition to tumour cells, tumour-associated macrophages (TAMs) have recently been reported to enhance the angiogenic ability of endothelial cells. For example, Yin et al. experimented with TAM-derived exosomes, which are highly enriched in miRNA-501-3p, showing that incubating human microvascular endothelial cells with these exosomes promotes angiogenesis [152]. This effect may be related to the upregulation of VEGFA, VEGFR2, ANG2, and PLGF at the protein level and the enhancement of the angiogenic ability of human microvascular endothelial cells in vitro [152]. TAMs also overexpress miRNAs 155-5p and 221-5p [153]. These miRNAs can be secreted by exosomes and then delivered to endothelial cells to enhance tube formation ability, which is mediated by downregulation of the transcription factor E2F2 [153].
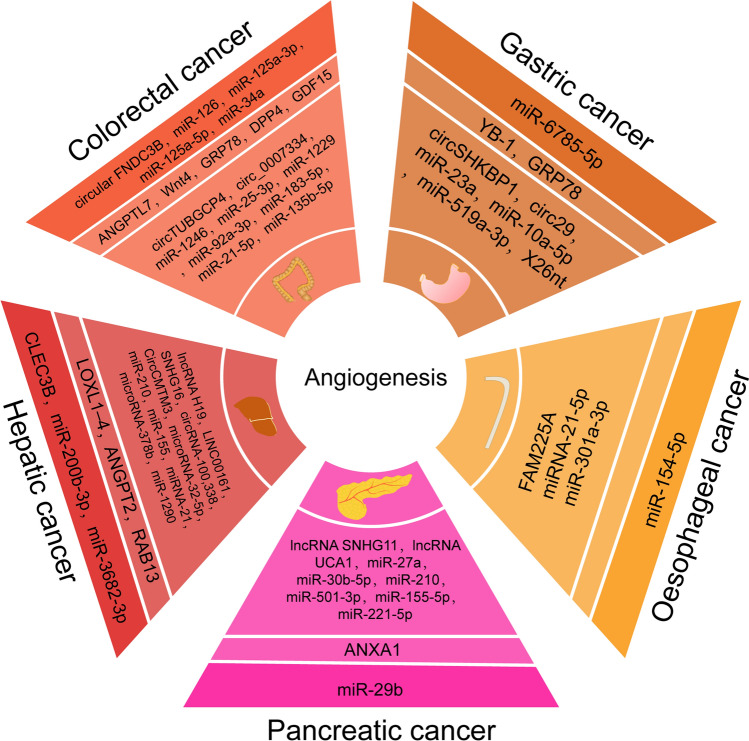
Although many exosomes have been identified, as described above (Fig. 2), many are yet to be discovered and may have therapeutic potential.
Fig. 2.
Exosomes in angiogenesis of digestive system tumours. The two light colored circles are factors of proangiogenesis. The first inner circle is protein; the outer circle is RNA. The factors in outermost dark circle are antiangiogenesis
Anti-angiogenic therapy
As mentioned above, angiogenesis is one of the most important processes for tumour development. Therefore, antiangiogenic therapy (AAT) has attracted attention, and many drugs are being studied [154–156]. Four main strategies are considered to develop antiangiogenic agents: inhibiting endogenous factors promoting angiogenesis, identifying and applying natural angiogenesis inhibitors, inhibiting the molecules that promote the invasion of the surrounding tissue through tumour blood vessels, and incapacitating actively proliferating endothelial cells [53]. Bevacizumab, ramucirumab and aflibercept, which are antiangiogenic agents, are indicated for most colorectal cancer patients [157]. Clinical trials have certified that sorafenib and lenvatinib are multikinase inhibitors, regorafenib and cabozantinib are tyrosine kinase inhibitors, and ramucirumab is a monoclonal antibody, and all of them target the factors of the VEGF axis to block tube formation in hepatocellular cancer [158]. Several preclinical and clinical studies have demonstrated the efficacy of anti-VEGF-A therapies in pancreatic cancer, and some agents have emerged, such as bevacizumab and sunitinib [159]. Any drug has side effects [160] and prolonged treatment will ultimately lead to resistance. The main mechanisms of antiangiogenic drug-related resistance involve revascularization, tumour vasculature protection, accentuated invasiveness of tumour cells, and increased metastasis through different modes of vascularization [161]. Therefore, novel antiangiogenic strategies, the combination of antiangiogenesis agents and chemotherapy or immunotherapy, have been developed [160].
In recent years, nanotechnology-based theranostic strategies have gained great interest for their unique properties or delivery of antiangiogenic agents to tumour sites by active (ligand-mediated) or passive (enhanced EPR effect of antiangiogenic agents to the tumour site) targeting [162]. Although many nanoparticles employed for the transmission of antiangiogenic agents have been studied, such as cerium oxide, gold, silver, copper, silicate, carbon, and peptide-conjugated nanoparticles, only 0.7% of the administered nanoparticles are considered to reach the targeted site [163]. Although nanoparticles have shown great potential in biology and medicine for their wide range of applications, their potential toxicity, non-specific uptake and clearance, as well as the conditions during their production, currently limit their use in these fields. Many experiments in animal models and clinical studies are ongoing to prove that the nanomedicine approach is feasible as a new antiangiogenic treatment [164].
Exosomes in anti-angiogenic therapy
With the advancement of 3D culture models, it is now possible to artificially manipulate the cell architecture by introducing various components, including collagen, fibronectin, and cytokines [165]. These alterations have the potential to induce variations in the content and cargo of exosomes [166, 167]. Consequently, the manipulation of the cellular environment using 3D culture models presents novel prospects for comprehending and harnessing exosomes as biomarkers in the realms of diagnosis and treatment [168]. Exosomes carry unique substances in distinct cancers and their levels reflect the clinical staging and prognosis to some degree [169–172]. At the same time, the transfection or knockdown of exosomes can have opposing effects on cancer cells [173]. For example, exosome-encapsulated hepatocyte growth factor (HGF) siRNA could exert inhibitory effects on the proliferation and migration of vascular cells via the repression of HGF and VEGF [174]. A recent experiment with liver cancer HepaRG cells treated with camel milk exosomes resulted in a significant downregulation of VEGF [175], and another antiangiogenic method, apatinib monotherapy, showed a reduction in exosome secretion in colorectal cancer cells [176]. Thus, exosomes have enormous therapeutic potential.
With in-depth research, exosomes have been confirmed to affect antiangiogenic drug-related resistance. Ying Gao et al. used both in vivo and in vitro assays to conclude that exosomal miR-494-3p derived from Golgi phosphoprotein 3 (GOLPH3)-overexpressing HCC cells promoted the angiogenesis ability of HUVECs and induced sorafenib resistance in HCC cells, which has important potential clinical value in improving therapeutic efficiency in HCC patients [177].
In addition to being targets, exosomes can be carriers. Compared with synthetic nanoparticles, exosomes are specific, safe, of cell-origin, and natural carriers that have a long half-life and nonimmunogenic properties for drug delivery systems [178–180]. Specifically, exosomes have a lipid bilayer and a hydrophilic core; thus, they can transmit both lipid- and water-soluble drugs [181]. Exosome-based nanocarriers can be designed in direct or indirect engineering processes [182]. Notably, some researchers have attempted to engineer exosomes in the antiangiogenic treatment of ovarian cancer, alone or combined with apatinib [183]. In a similar case, some studies have shown that anticancer drug DOX bound to exosomes plays roles in lung cancer or colorectal cancer cells, and studies have shown that exosomes can affect the central nervous system (CNS) as carriers of proteins and RNAs [184].
Conclusion and future perspectives
Malignant tumours of the digestive system occur in daily life. Angiogenesis is an important process in tumour development that is controlled by the balance of pro- and anti-angiogenic factors. Exosomes have a marked effect. To date, a multitude of exosomes carrying mRNA/lncRNA/protein have been discovered. Correspondingly, directly targeting them by enhancing their secretion or decreasing their expression is an anti-angiogenic therapeutic strategy. Alternatively, exosomes can be used as delivery vehicles for drugs that can promote or inhibit angiogenesis. Simultaneously, exosome functions have recently attracted researchers’ attention, especially regarding angiogenesis. However, the mechanism by which exosomes selectively package their cargo remains unclear. This limitation has led to the exploration of improving treatments, such as chemotherapy, immunotherapy, or combination therapy [185]. Advancements in technology will advance the use of exosomes in the digestive system.
However, the clinical application of exosomes remains subject to limitations. The methods used to isolate, purify, and identify exosomes in the laboratory cannot be used in clinical research because of inconvenience. Firstly, the extraction process of exosomes is relatively complicated, requiring multiple methods such as ultracentrifugation, precipitation, ultrafiltration, and so on. The operation process is also quite cumbersome. Secondly, the number of exosomes extracted is usually small, and we need large volumes of body fluids to obtain sufficient exosomes to meet clinical requirements. Additionally, the identification of exosomes is also relatively complex, necessitating methods such as transmission electron microscopy, particle size analysis, protein markers, etc., to ensure their purity and biological activity [186]. Therefore, improving the efficiency of exosome isolation methods and developing strategies to obtain a larger yield of exosomes from limited fluid sources is necessary to facilitate their clinical translation. As a result, the application of exosomes in the diagnosis and therapy of cancer remains difficult. Exosomes will be widely used shortly.
Abbreviations
- AAT
Antiangiogenic therapy
- ADO
Adenosine
- ANGPT1
Angiopoietin-1
- ANGPT2
Angiopoietin-2
- ANGPTL7
Angiopoietin-like 7
- ANXA1
Annexin A1
- ARF6
ADP ribosylation factor 6
- BTG2
B‐cell translocation gene 2
- CA125
Carbohydrate antigen 125
- CA19-9
Carbohydrate antigen 19–9
- CAF
Cancer-associated fibroblast
- CEA
Carcinoembryonic antigen
- CLDN11
Claudin-11
- CLEC3B
C-Type Lectin Domain Family 3 Member B
- CNS
Central nervous system
- CRC
Colorectal cancer
- DACT2
Disheveled binding antagonist of beta-catenin 2
- Dkk-3
Dickkopf-3
- DPP4
Dipeptidyl peptidase IV
- EC
Endothelial cell
- ECM
Extracellular matrix
- ERG
Erythroblast transformation specific (ETS) related gene
- ESCC
Oesophageal squamous cell carcinoma
- ESCRT
Endosomal sorting complex needed for transport
- ESE
Early-sorting endosome
- FGF
Fibroblast growth factor
- FOXM1D
Forkhead Box M1 D
- Foxo
Forkhead Box O
- GC
Gastric cancer
- GDF15
Growth/differentiation factor 15
- GOLPH3
Golgi phosphoprotein 3
- GRP78
Glucose-regulated protein 78
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HK2
Hexokinase 2
- HSCs
Hepatic stellate cells
- HUVECs
Human umbilical vein endothelial cells
- IA
Intussusceptive angiogenesis
- ILVs
Intraluminal vesicle
- IRE1
Inositol- requiring enzyme 1 alpha
- KIF14
Kinesin family member 14
- KLF12
Krüppel-like factor 12
- KLF2
Krüppel-like factor 2
- KLF4
Krüppel-like factor 4
- KRIT1
Krev interaction trapped protein 1
- LncRNAs
Long noncoding RNAs
- LOXL1–4
Lysyl oxidase-like 1–4
- LSE
Late-sorting endosome
- MLL3
Histone-lysine N-methyltransferase-3
- MMP
Matrix metalloproteinase
- MVBs
Multivesicular bodies
- NSF
N-ethyl- maleimide-sensitive factor
- PAR1
Protease-activated receptor-1
- PABPC1
Poly (A) binding protein cytoplasmic 1
- PC
Pancreatic cancer
- PDAC
Pancreatic ductal adenocarcinoma
- PDGF
Platelet-derived growth factor
- PKM2
Pyruvate kinase M2
- PLD2
Phospholipase D2
- PLGF
Placental growth factor
- PML
Promyelocytic leukaemia
- POSTN
Periostin
- PTEN
Phosphatase and tensin homologue
- SA
Sprouting angiogenesis
- sEVs
Small extracellular vesicles
- SNARE
Soluble N-ethyl- maleimide-sensitive factor-attachment protein receptor complex
- SNHG16
Small nucleolar RNA host gene 16
- SYT7
Synaptotagmin 7
- TAMs
Tumour-associated macrophages
- TEX
Tumour-derived exosomes
- TME
Tumour microenvironment
- TSP-1
Thrombospondin-1
- TXNIP
Thioredoxin-interacting protein
- VC
Vessel co-option
- VEGF-A
Vascular endothelial growth factor A
- VM
Vascular mimicry
- ZMYND11
Zinc finger MYND-type containing 11
Author contributions
YL and HW had the idea for the article, YL and HW performed the literature search and finished the manuscript; YL, WC and YDS finished the figures and tables; WC, LS and LPL made critical revisions and proofread the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82203854, 82102702, 82103322), Key Research and Development Program of Shandong Province (No.2021CXGC011104; No.2019JZZY010104; No.2019GSF108146), Academic promotion programme of Shandong First Medical University (2019QL021), and Special Foundation for Taishan Scholars Program of Shandong Province (No.ts20190978), Natural Science Foundation of Shandong Province of China (ZR2020QH180, ZR2021QH141), Youth Innovation Science and Technology Program of Shandong Provincial Universities (2022KJ187), China postdoctoral science foundation (2022M711970), Clinical Medical Science and Technology Innovation Project of Jinan (202225046).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Chong, Email: chongwei@sdfmu.edu.cn, Email: chongwei.good@163.com.
Liang Shang, Email: docshang@163.com.
Leping Li, Email: lileping@medmail.com.cn, Email: lileping@mail.sdu.edu.cn.
References
- 1.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- 2.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. Board WHOCoTE: the 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomsen MK, Jørgensen MD, Pedersen L, Erichsen R, Sørensen HT, Mikkelsen EM. Mental disorders, participation, and trajectories in the Danish colorectal cancer programme: a population-based cohort study. Lancet Psychiatry. 2023;10:518–527. doi: 10.1016/S2215-0366(23)00179-7. [DOI] [PubMed] [Google Scholar]
- 4.Housini M, Dariya B, Ahmed N, Stevens A, Fiadjoe H, Nagaraju GP, Basha R. Colorectal cancer: genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene. 2024 doi: 10.1016/j.gene.2023.147857. [DOI] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158:418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Correa P. Gastric cancer: overview. Gastroenterol Clin N Am. 2013;42:211. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang YB, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2021;15:2580–2599. doi: 10.1002/1878-0261.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baiu I, Visser B. Endoscopic retrograde cholangiopancreatography. JAMA. 2018;320:2050. doi: 10.1001/jama.2018.14481. [DOI] [PubMed] [Google Scholar]
- 10.Gu YL, Lan C, Pei H, Yang SN, Liu YF, Xiao LL. Applicative value of serum CA19-9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16:6569–6573. doi: 10.7314/APJCP.2015.16.15.6569. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 2018;8:2732. doi: 10.1038/s41598-018-21048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essola JM, Zhang MJ, Yang HY, Li FZ, Xia BZ, Mavoungou JF, Hussain A, Huang YY. Exosome regulation of immune response mechanism: pros and cons in immunotherapy. Bioactive Mater. 2024;32:124–146. doi: 10.1016/j.bioactmat.2023.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes—structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015;81:2–10. doi: 10.1111/sji.12247. [DOI] [PubMed] [Google Scholar]
- 15.McCoy-Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int J Biochem Cell Biol. 2016;71:44–54. doi: 10.1016/j.biocel.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Tang B, Ma W, Lin Y. Emerging applications of anti-angiogenic nanomaterials in oncotherapy. J Control Release. 2023;364:61–78. doi: 10.1016/j.jconrel.2023.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Maiborodin I, Mansurova A, Chernyavskiy A, Romanov A, Voitcitctkii V, Kedrova A, Tarkhov A, Chernyshova A, Krasil'nikov S. Cancer angiogenesis and opportunity of influence on tumor by changing vascularization. J Pers Med. 2022 doi: 10.3390/jpm12030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes. 2013;2:54–59. doi: 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J, Chen Y, Lin Z. Exosomes: mediators in microenvironment of colorectal cancer. Int J Cancer. 2023 doi: 10.1002/ijc.34471. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi S, Yoshioka Y, Prieto-Vila M, Ochiya T. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int J Mol Sci. 2019 doi: 10.3390/ijms20102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Fu M, Liu J, Chong W, Fang Z, Du F, Liu Y, Shang L, Li L. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20:71. doi: 10.1186/s12943-021-01365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlton JG, Baum B. Roles of ESCRT-III polymers in cell division across the tree of life. Curr Opin Cell Biol. 2023;85:102274. doi: 10.1016/j.ceb.2023.102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfitzner A-K, Zivkovic H, Bernat-Silvestre C, West M, Peltier T, Humbert F, Odorizzi G, Roux A. Vps60 initiates alternative ESCRT-III filaments. J Cell Biol. 2023 doi: 10.1083/jcb.202206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clippinger AK, Naismith TV, Yoo W, Jansen S, Kast DJ, Hanson PI. IST1 regulates select recycling pathways. Traffic. 2023 doi: 10.1111/tra.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Li R, Chen X, Yao J, Wang Q, Zhang J, Jiang Y, Qu Y. SYT7 is a key player in increasing exosome secretion and promoting angiogenesis in non-small-cell lung cancer. Cancer Lett. 2023;577:216400. doi: 10.1016/j.canlet.2023.216400. [DOI] [PubMed] [Google Scholar]
- 32.Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P. Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. 2019;234:16885–16903. doi: 10.1002/jcp.28374. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, Guo T, Sheng H, Chen J, Zheng Q, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. 2019;65:798–808. doi: 10.1373/clinchem.2018.301291. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, TanTai J. Exosomes secreted by metastatic cancer cells promotes epithelial mesenchymal transition in small cell lung carcinoma: the key role of Src/TGF-β1 axis. Gene. 2024;892:147873. doi: 10.1016/j.gene.2023.147873. [DOI] [PubMed] [Google Scholar]
- 35.Park AY, Lee JO, Jang Y, Kim Y-J, Lee JM, Kim S-Y, Kim BJ, Yoo KH. Exosomes derived from human dermal fibroblasts protect against UVB-induced skin photoaging. Int J Mol Med. 2023 doi: 10.3892/ijmm.2023.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22:409–417. doi: 10.1080/14728222.2018.1464141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabatke B, Rossi IV, Sana A, Bonato LB, Ramirez MI. Extracellular vesicles biogenesis and uptake concepts: a comprehensive guide to studying host-pathogen communication. Mol Microbiol. 2023 doi: 10.1111/mmi.15168. [DOI] [PubMed] [Google Scholar]
- 38.Özdemir S, Çomaklı S, Küçükler S, Aksungur N, Altundaş N, Kara S, Korkut E, Aydın Ş, Bağcı B, Çulha MH, Öztürk G. Integrative analysis of serum-derived exosomal lncRNA profiles of alveolar echinococcosis patients. Gene. 2024;892:147884. doi: 10.1016/j.gene.2023.147884. [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Liu Z, Xiao H, Yang X, Li T, Huang W, Zhou H. Effect of tumor exosome-derived Lnc RNA HOTAIR on the growth and metastasis of gastric cancer. Clin Trans Oncol. 2023;25:3447–3459. doi: 10.1007/s12094-023-03208-3. [DOI] [PubMed] [Google Scholar]
- 40.Liao M, Qin M, Liu L, Huang H, Chen N, Du H, Huang D, Wang P, Zhou H, Tong G. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024;122:155091. doi: 10.1016/j.phymed.2023.155091. [DOI] [PubMed] [Google Scholar]
- 41.Yang P, Huang Y, Zhu Y, Wang Q, Guo Y, Li L. Plasma exosomes proteome profiling discovers protein markers associated with the therapeutic effect of Chaihu-Longgu-Muli decoction on temporal lobe epilepsy. J Ethnopharmacol. 2024;318:116928. doi: 10.1016/j.jep.2023.116928. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y-X, Deng Z-H, Xue G, Qiang D, Juan Y, Chen G-H, Li J-G, Zhao Y-M, Zhang H-T, Zhang G-X, Qian J-X. Exosomes derived from mesenchymal stromal cells exert a therapeutic effect on hypoxia-induced pulmonary hypertension by modulating the YAP1/SPP1 signaling pathway. Biomed Pharmacother. 2023;168:115816. doi: 10.1016/j.biopha.2023.115816. [DOI] [PubMed] [Google Scholar]
- 43.Tavasolian F, Lively S, Pastrello C, Tang M, Lim M, Pacheco A, Qaiyum Z, Yau E, Baskurt Z, Jurisica I, et al. Proteomic and genomic profiling of plasma exosomes from patients with ankylosing spondylitis. Ann Rheum Dis. 2023;82:1429–1443. doi: 10.1136/ard-2022-223791. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altıntaş Ö, Saylan Y. Exploring the versatility of exosomes: a review on isolation, characterization, detection methods, and diverse applications. Anal Chem. 2023;95:16029–16048. doi: 10.1021/acs.analchem.3c02224. [DOI] [PubMed] [Google Scholar]
- 46.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witwer KW, Goberdhan DC, O'Driscoll L, Théry C, Welsh JA, Blenkiron C, Buzás EI, Di Vizio D, Erdbrügger U, Falcón-Pérez JM, et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182. doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv Q, Wang Y, Tian W, Liu Y, Gu M, Jiang X, Cai Y, Huo R, Li Y, Li L, Wang X. Exosomal miR-146a-5p derived from human umbilical cord mesenchymal stem cells can alleviate antiphospholipid antibody-induced trophoblast injury and placental dysfunction by regulating the TRAF6/NF-κB axis. J Nanobiotechnol. 2023;21:419. doi: 10.1186/s12951-023-02179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong L, Zieren RC, Horie K, Kim CJ, Mallick E, Jing YZ, Feng MX, Kuczler MD, Green J, Amend SR, et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J Extracell Vesicles. 2020 doi: 10.1002/jev2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B, Yang H, Song Y-S, Sorenson CM, Sheibani N. Thrombospondin-1 in vascular development, vascular function, and vascular disease. Semin Cell Dev Biol. 2024;155:32–44. doi: 10.1016/j.semcdb.2023.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moshe DL, Baghaie L, Leroy F, Skapinker E, Szewczuk MR. Metamorphic effect of angiogenic switch in tumor development: conundrum of tumor angiogenesis toward progression and metastatic potential. Biomedicines. 2023 doi: 10.3390/biomedicines11082142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watabe T, Takahashi K, Pietras K, Yoshimatsu Y. Roles of TGF-β signals in tumor microenvironment via regulation of the formation and plasticity of vascular system. Semin Cancer Biol. 2023;92:130–138. doi: 10.1016/j.semcancer.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci. 2020 doi: 10.3390/ijms21165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S, Sharife H, Kreisel T, Bar-Lev L, Grunewald M, Keshet E. Isolation of tumor cells based on their distance from blood vessels. Bio-Protoc. 2020 doi: 10.2176/BioProtoc.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Z-W, Yu Z-L, Chen G, Jia J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023;114:2739–2749. doi: 10.1111/cas.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. doi: 10.1007/978-3-319-95294-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudley AC, Griffioen AW. The modes of angiogenesis: an updated perspective. Angiogenesis. 2023;26:477–480. doi: 10.1007/s10456-023-09895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaBelle SA, Poulson AM, Maas SA, Rauff A, Ateshian GA, Weiss JA. Spatial configurations of 3D extracellular matrix collagen density and anisotropy simultaneously guide angiogenesis. PLoS Comput Biol. 2023;19:e1011553. doi: 10.1371/journal.pcbi.1011553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Zeng X, Chen P, Du W, Pei Y, Wang G, Liu B-F. On-chip-angiogenesis based on a high-throughput biomimetic three-dimensional cell spheroid culture system. Analyst. 2023;148:3870–3875. doi: 10.1039/D3AN00817G. [DOI] [PubMed] [Google Scholar]
- 60.Betz C, Lenard A, Belting HG, Affolter M. Cell behaviors and dynamics during angiogenesis. Development. 2016;143:2249–2260. doi: 10.1242/dev.135616. [DOI] [PubMed] [Google Scholar]
- 61.Weinstein N, Mendoza L, Gitler I, Klapp J. A network model to explore the effect of the micro-environment on endothelial cell behavior during angiogenesis. Front Physiol. 2017;8:960. doi: 10.3389/fphys.2017.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 63.Eelen G, Treps L, Li X, Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020;127:310–329. doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- 64.Angara K, Borin TF, Arbab AS. Vascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl Oncol. 2017;10:650–660. doi: 10.1016/j.tranon.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao XY, Xue YB, Ma Q, Bai YJ, Jia P, Zhang YM, Lai BC, He ST, Ma Q, Zhang JB, et al. 221S–1a inhibits endothelial proliferation in pathological angiogenesis through ERK/c-Myc signaling. Eur J Pharmacol. 2023 doi: 10.1016/j.ejphar.2023.175805. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Hong Y, Mao S, Pan J, Cui Y, Lu J, Wen T, Wang X, Luo Y. Downregulation of miR-124-3p suppresses the development of the deep retinal blood vessels by enhancing the Stat1/Ripk1 pathway in mouse retinal microglia. Exp Eye Res. 2023;233:109551. doi: 10.1016/j.exer.2023.109551. [DOI] [PubMed] [Google Scholar]
- 67.Huang J, Wang C, Hou Y, Tian Y, Li Y, Zhang H, Zhang L, Li W. Molecular mechanisms of thrombospondin-2 modulates tumor vasculogenic mimicry by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 2023;167:115455. doi: 10.1016/j.biopha.2023.115455. [DOI] [PubMed] [Google Scholar]
- 68.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha K, Parwez S, Mv S, Yadav A, Siddiqi MI, Banerjee D. Machine learning and biological evaluation-based identification of a potential MMP-9 inhibitor, effective against ovarian cancer cells SKOV3. J Biomol Struct Dyn. 2023 doi: 10.1080/07391102.2023.2240416. [DOI] [PubMed] [Google Scholar]
- 70.Chillà A, Anceschi C, Frediani E, Scavone F, Del Rosso T, Pelagio G, Tufaro A, De Palma G, Del Rosso M, Fibbi G, et al. Inhibition of MMPs supports amoeboid angiogenesis hampering VEGF-targeted therapies via MLC and ERK 1/2 signaling. J Transl Med. 2023;21:102. doi: 10.1186/s12967-023-03954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi H, Dong Z, Gan Q, Tang X, Xing J, Sheng X, Zhan W. Matrix metalloproteinase 9 modulates immune response along with the formation of extracellular traps in flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2023;133:108570. doi: 10.1016/j.fsi.2023.108570. [DOI] [PubMed] [Google Scholar]
- 72.Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V, Lara-Riegos J, Ramirez-Camacho MA, Alvarez-Sanchez ME. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Zhang X, Huang S, Chen J, Ding P, Wang Q, Li L, Lv X, Li L, Zhang P, et al. FOXM1D potentiates PKM2-mediated tumor glycolysis and angiogenesis. Mol Oncol. 2021;15:1466–1485. doi: 10.1002/1878-0261.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ge Z, Zhang Q, Lin W, Jiang X, Zhang Y. The role of angiogenic growth factors in the immune microenvironment of glioma. Front Oncol. 2023;13:1254694. doi: 10.3389/fonc.2023.1254694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thijssen VL, Paulis YW, Nowak-Sliwinska P, Deumelandt KL, Hosaka K, Soetekouw PM, Cimpean AM, Raica M, Pauwels P, van den Oord JJ, et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J Pathol. 2018;246:447–458. doi: 10.1002/path.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, et al. FGF-dependent metabolic control of vascular development. Nature. 2017;545:224–228. doi: 10.1038/nature22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Sheikh MM, Aziz MM, Abdelrahman SSM, Mohmad MAEH. The protective effect of crocin against testicular toxicity induced by ionizing radiation via AKT/FOXO pathway. Environ Toxicol. 2023 doi: 10.1002/tox.23932. [DOI] [PubMed] [Google Scholar]
- 78.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019 doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang T, Zhu Z, Zhang J, Chen M, Chen S. Role of tumor-derived exosomes in metastasis, drug resistance and diagnosis of clear cell renal cell carcinoma. Front Oncol. 2022;12:1066288. doi: 10.3389/fonc.2022.1066288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gholipour E, Kahroba H, Soltani N, Samadi P, Sarvarian P, Vakili-Samiani S, Hosein Pour Feizi AA, Soltani-Zangbar MS, Baghersalimi A, Darbandi B, et al. Paediatric pre-B acute lymphoblastic leukaemia-derived exosomes regulate immune function in human T cells. J Cell Mol Med. 2022;26:4566–4576. doi: 10.1111/jcmm.17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bai S, Wei Y, Liu R, Xu R, Xiang L, Du J. Role of tumour-derived exosomes in metastasis. Biomed Pharmacother. 2022;147:112657. doi: 10.1016/j.biopha.2022.112657. [DOI] [PubMed] [Google Scholar]
- 82.Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V, Ramachandran V, Poyyakkara A, Kumar SVB. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J Cell Physiol. 2018;233:3498–3514. doi: 10.1002/jcp.26202. [DOI] [PubMed] [Google Scholar]
- 83.Ludwig N, Yerneni SS, Azambuja JH, Gillespie DG, Menshikova EV, Jackson EK, Whiteside TL. Tumor-derived exosomes promote angiogenesis via adenosine A2B receptor signaling. Angiogenesis. 2020;23:599–610. doi: 10.1007/s10456-020-09728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Cen A, Yang Y, Ye H, Li J, Liu S, Zhao L. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Mol Ther Nucleic Acids. 2021;24:610–621. doi: 10.1016/j.omtn.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang R, Lian T, Liu J, Du F, Chen Z, Zhang R, Wang Q. Dendritic cell-derived exosomes stimulated by treponema pallidum induce endothelial cell inflammatory response through the TLR4/MyD88/NF-κB signaling pathway. ACS Infect Dis. 2023;9:2299–2305. doi: 10.1021/acsinfecdis.3c00348. [DOI] [PubMed] [Google Scholar]
- 87.Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. doi: 10.1186/s13046-019-1384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Luo Y, Cao J, Wang X, Miao Z, Shao G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020;9:8600–8611. doi: 10.1002/cam4.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Chen C, Liu Z, Guo H, Lu W, Hu W, Lin Z. PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 2022;41:111. doi: 10.1186/s13046-022-02339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z, Yang S, Zhou A, Li X, Fang R, Zhang S, Zhao G, Li P. Small extracellular vesicles in the development, diagnosis, and possible therapeutic application of esophageal squamous cell carcinoma. Front Oncol. 2021;11:732702. doi: 10.3389/fonc.2021.732702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shou Y, Wang X, Chen C, Liang Y, Yang C, Xiao Q, Li H, Wang S, Shu J, Tian X, Chen K. Exosomal miR-301a-3p from esophageal squamous cell carcinoma cells promotes angiogenesis by inducing M2 polarization of macrophages via the PTEN/PI3K/AKT signaling pathway. Cancer Cell Int. 2022;22:153. doi: 10.1186/s12935-022-02570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao L, Yu L, Wang X, He J, Zhu X, Zhang R, Yang A. Mechanisms of function and clinical potential of exosomes in esophageal squamous cell carcinoma. Cancer Lett. 2023;553:215993. doi: 10.1016/j.canlet.2022.215993. [DOI] [PubMed] [Google Scholar]
- 93.Shou Y, Wang X, Liang Y, Liu X, Chen K. Exosomes-derived miR-154-5p attenuates esophageal squamous cell carcinoma progression and angiogenesis by targeting kinesin family member 14. Bioengineered. 2022;13:4610–4620. doi: 10.1080/21655979.2022.2037322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du J, Liang Y, Li J, Zhao JM, Wang ZN, Lin XY. Gastric cancer cell-derived exosomal microRNA-23a promotes angiogenesis by targeting PTEN. Front Oncol. 2020;10:326. doi: 10.3389/fonc.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y, Xu T. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19:112. doi: 10.1186/s12943-020-01208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xue X, Huang J, Yu K, Chen X, He Y, Qi D, Wu Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer. 2020;20:996. doi: 10.1186/s12885-020-07509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Zhang S, Du K, Zheng N, Liu Y, Chen H, Xie G, Ma Y, Zhou Y, Zheng Y, et al. Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2021;112:1839–1852. doi: 10.1111/cas.14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, Li J, Zhang H, Zhang Y, Wang X, Yang H, Zhou Z, Hao X, Ying G, Ba Y. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem Biophys Res Commun. 2021;560:37–44. doi: 10.1016/j.bbrc.2021.04.099. [DOI] [PubMed] [Google Scholar]
- 99.Zhu J, Du S, Zhang J, Huang G, Dong L, Ren E, Liu D. microRNA-10a-5p from gastric cancer cell-derived exosomes enhances viability and migration of human umbilical vein endothelial cells by targeting zinc finger MYND-type containing 11. Bioengineered. 2022;13:496–507. doi: 10.1080/21655979.2021.2009962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Zhang Z, Sun C, Zheng Y, Gong Y. circFCHO2 promotes gastric cancer progression by activating the JAK1/STAT3 pathway via sponging miR-194-5p. Cell Cycle. 2022;21:2145–2164. doi: 10.1080/15384101.2022.2087280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iha K, Sato A, Tsai HY, Sonoda H, Watabe S, Yoshimura T, Lin MW, Ito E. Gastric cancer cell-derived exosomal GRP78 enhances angiogenesis upon stimulation of vascular endothelial cells. Curr Issues Mol Biol. 2022;44:6145–6157. doi: 10.3390/cimb44120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res CR. 2022;41:296. doi: 10.1186/s13046-022-02499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L, Zheng H, Yu X, Liu L, Li H, Zhu H, Zhang Z, Lei P, Shen G. Tumor-secreted GRP78 promotes the establishment of a pre-metastatic niche in the liver microenvironment. Front Immunol. 2020;11:584458. doi: 10.3389/fimmu.2020.584458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parri M, Pietrovito L, Grandi A, Campagnoli S, De Camilli E, Bianchini F, Marchio S, Bussolino F, Jin B, Sarmientos P, et al. Angiopoietin-like 7, a novel pro-angiogenetic factor over-expressed in cancer. Angiogenesis. 2014;17:881–896. doi: 10.1007/s10456-014-9435-4. [DOI] [PubMed] [Google Scholar]
- 105.Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, Naoe T, Akao Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839:1256–1272. doi: 10.1016/j.bbagrm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Huang Z, Feng Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced beta-catenin signaling in endothelial cells. Oncol Res. 2017;25:651–661. doi: 10.3727/096504016X14752792816791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y, Zhang L, La X, Li Z, Li H, Guo S. Salvianolic acid A inhibits tumor-associated angiogenesis by blocking GRP78 secretion. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:467–480. doi: 10.1007/s00210-018-1585-2. [DOI] [PubMed] [Google Scholar]
- 108.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY, Yang Y, Chen Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int J Biol Macromol. 2019;132:470–477. doi: 10.1016/j.ijbiomac.2019.03.221. [DOI] [PubMed] [Google Scholar]
- 110.Yamada N, Nakagawa Y, Tsujimura N, Kumazaki M, Noguchi S, Mori T, Hirata I, Maruo K, Akao Y. Role of intracellular and extracellular microRNA-92a in colorectal cancer. Transl Oncol. 2013;6:482–492. doi: 10.1593/tlo.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamada NO, Heishima K, Akao Y, Senda T. Extracellular vesicles containing MicroRNA-92a-3p facilitate partial endothelial-mesenchymal transition and angiogenesis in endothelial cells. Int J Mol Sci. 2019 doi: 10.3390/ijms20184406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shang A, Wang X, Gu C, Liu W, Sun J, Zeng B, Chen C, Ji P, Wu J, Quan W, et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging. 2020;12:8352–8371. doi: 10.18632/aging.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He Q, Ye A, Ye W, Liao X, Qin G, Xu Y, Yin Y, Luo H, Yi M, Xian L, et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576. doi: 10.1038/s41419-021-03803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bai L, Gao Z, Jiang A, Ren S, Wang B. Circular noncoding RNA circ_0007334 sequestrates miR-577 to derepress KLF12 and accelerate colorectal cancer progression. Anticancer Drugs. 2022;33:e409–e422. doi: 10.1097/CAD.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 115.Zheng X, Liu J, Li X, Tian R, Shang K, Dong X, Cao B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021;497:190–201. doi: 10.1016/j.canlet.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Zheng X, Ma N, Wang X, Hu J, Ma X, Wang J, Cao B. Exosomes derived from 5-fluorouracil-resistant colon cancer cells are enriched in GDF15 and can promote angiogenesis. J Cancer. 2020;11:7116–7126. doi: 10.7150/jca.49224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen C, Liu Y, Liu L, Si C, Xu Y, Wu X, Wang C, Sun Z, Kang Q. Exosomal circTUBGCP4 promotes vascular endothelial cell tipping and colorectal cancer metastasis by activating Akt signaling pathway. J Exp Clin Cancer Res. 2023;42:46. doi: 10.1186/s13046-023-02619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yin H, Yu S, Xie Y, Dai X, Dong M, Sheng C, Hu J. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell Signal. 2021;84:110029. doi: 10.1016/j.cellsig.2021.110029. [DOI] [PubMed] [Google Scholar]
- 119.Shi Y, Zhu H, Jiang H, Yue H, Yuan F, Wang F. Cancer-associated fibroblasts-derived exosomes from chemoresistant patients regulate cisplatin resistance and angiogenesis by delivering VEGFA in colorectal cancer. Anticancer Drugs. 2023;34:422–430. doi: 10.1097/CAD.0000000000001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng W, Liu Y, Li WT, Li Y, Zhu JF. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol Oncol. 2020;14:2960–2984. doi: 10.1002/1878-0261.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Danac JMC, Uy AGG, Garcia RL. Exosomal microRNAs in colorectal cancer: overcoming barriers of the metastatic cascade (Review) Int J Mol Med. 2021 doi: 10.3892/ijmm.2021.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hosseini M, Baghaei K, Hajivalili M, Zali MR, Ebtekar M, Amani D. The anti-tumor effects of CT-26 derived exosomes enriched by MicroRNA-34a on murine model of colorectal cancer. Life Sci. 2022;290:120234. doi: 10.1016/j.lfs.2021.120234. [DOI] [PubMed] [Google Scholar]
- 123.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:52. doi: 10.1186/s13046-018-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, Zhuang SM. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matsuura Y, Wada H, Eguchi H, Gotoh K, Kobayashi S, Kinoshita M, Kubo M, Hayashi K, Iwagami Y, Yamada D, et al. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig Dis Sci. 2019;64:792–802. doi: 10.1007/s10620-018-5380-1. [DOI] [PubMed] [Google Scholar]
- 127.Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Q, Wang X, Zheng Y, Ye T, Liu J, Wang J-Q, Zhang W-F, Wu F-L, Bo H, Shao H, et al. Cancer-associated fibroblasts contribute to the immunosuppressive landscape and influence the efficacy of the combination therapy of PD-1 inhibitors and antiangiogenic agents in hepatocellular carcinoma. Cancer. 2023;129:3405–3416. doi: 10.1002/cncr.34935. [DOI] [PubMed] [Google Scholar]
- 129.Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. doi: 10.1186/s13045-019-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, Xia Q, Zhang Z, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18. doi: 10.1186/s12943-019-0948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie JY, Wei JX, Lv LH, Han QF, Yang WB, Li GL, Wang PX, Wu SB, Duan JX, Zhuo WF, et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 2020;18:46. doi: 10.1186/s12964-020-00535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.You LN, Tai QW, Xu L, Hao Y, Guo WJ, Zhang Q, Tong Q, Zhang H, Huang WK. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021;28:719–736. doi: 10.1038/s41417-020-00269-2. [DOI] [PubMed] [Google Scholar]
- 134.Yukawa H, Suzuki K, Aoki K, Arimoto T, Yasui T, Kaji N, Ishikawa T, Ochiya T, Baba Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci Rep. 2018;8:6765. doi: 10.1038/s41598-018-24563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S, Xu M, Li X, Su X, Xiao X, Keating A, Zhao RC. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 2018;11:82. doi: 10.1186/s13045-018-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen W, Huang L, Liang J, Ye Y, He S, Niu J. Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci. 2021;273:119184. doi: 10.1016/j.lfs.2021.119184. [DOI] [PubMed] [Google Scholar]
- 137.Wang Q, Wang G, Niu L, Zhao S, Li J, Zhang Z, Jiang H, Zhang Q, Wang H, Sun P, et al. Exosomal MiR-1290 promotes angiogenesis of hepatocellular carcinoma via targeting SMEK1. J Oncol. 2021;2021:6617700. doi: 10.1155/2021/6617700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hu K, Li NF, Li JR, Chen ZG, Wang JH, Sheng LQ. Exosome circCMTM3 promotes angiogenesis and tumorigenesis of hepatocellular carcinoma through miR-3619-5p/SOX9. Hepatol Res. 2021;51:1139–1152. doi: 10.1111/hepr.13692. [DOI] [PubMed] [Google Scholar]
- 139.Li S, Qi Y, Huang Y, Guo Y, Huang T, Jia L. Exosome-derived SNHG16 sponging miR-4500 activates HUVEC angiogenesis by targeting GALNT1 via PI3K/Akt/mTOR pathway in hepatocellular carcinoma. J Physiol Biochem. 2021;77:667–682. doi: 10.1007/s13105-021-00833-w. [DOI] [PubMed] [Google Scholar]
- 140.Huang XY, Zhang JT, Li F, Li TT, Shi XJ, Huang J, Huang XY, Zhou J, Tang ZY, Huang ZL. Exosomal proteomics identifies RAB13 as a potential regulator of metastasis for HCC. Hepatol Commun. 2023;7:e0006. doi: 10.1097/HC9.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dai W, Wang Y, Yang T, Wang J, Wu W, Gu J. Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun Signal. 2019;17:113. doi: 10.1186/s12964-019-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moh-Moh-Aung A, Fujisawa M, Ito S, Katayama H, Ohara T, Ota Y, Yoshimura T, Matsukawa A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci Rep. 2020;10:10418. doi: 10.1038/s41598-020-67425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dong SS, Dong DD, Yang ZF, Zhu GQ, Gao DM, Chen J, Zhao Y, Liu BB. Exosomal miR-3682-3p suppresses angiogenesis by targeting ANGPT1 via the RAS-MEK1/2-ERK1/2 pathway in hepatocellular carcinoma. Front Cell Dev Biol. 2021;9:633358. doi: 10.3389/fcell.2021.633358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pessolano E, Belvedere R, Bizzarro V, Franco P, Marco I, Porta A, Tosco A, Parente L, Perretti M, Petrella A. Annexin A1 may induce pancreatic cancer progression as a key player of extracellular vesicles effects as evidenced in the in vitro MIA PaCa-2 model system. Int J Mol Sci. 2018 doi: 10.3390/ijms19123878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shang D, Xie C, Hu J, Tan J, Yuan Y, Liu Z, Yang Z. Pancreatic cancer cell-derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J Cell Mol Med. 2020;24:588–604. doi: 10.1111/jcmm.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fang X, Cai Y, Xu Y, Zhang H. Exosome-mediated lncRNA SNHG11 regulates angiogenesis in pancreatic carcinoma through miR-324-3p/VEGFA axis. Cell Biol Int. 2022;46:106–117. doi: 10.1002/cbin.11703. [DOI] [PubMed] [Google Scholar]
- 147.Manuelli V, Pecorari C, Filomeni G, Zito E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022;289:5413–5425. doi: 10.1111/febs.16110. [DOI] [PubMed] [Google Scholar]
- 148.Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, Huang C, Zhao Q, Qiu Z. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol Ther Nucleic Acids. 2020;22:179–195. doi: 10.1016/j.omtn.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen K, Wang Q, Liu X, Wang F, Yang Y, Tian X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int J Biol Sci. 2022;18:1220–1237. doi: 10.7150/ijbs.67675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu G, Ding X, Quan G, Xiong J, Li Q, Li Z, Wang Y. Hypoxia-induced miR-210 promotes endothelial cell permeability and angiogenesis via exosomes in pancreatic ductal adenocarcinoma. Biochem Res Int. 2022;2022:7752277. doi: 10.1155/2022/7752277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang L, Yang L, Zhuang T, Shi X. Tumor-derived exosomal miR-29b reduces angiogenesis in pancreatic cancer by silencing ROBO1 and SRGAP2. J Immunol Res. 2022;2022:4769385. doi: 10.1155/2022/4769385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, Zhang K, Teng B, Cao J, Wu W, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. 2021;29:1226–1238. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Borriello R, Cerrito L, Gasbarrini A, Ponziani FR. Pharmacokinetic considerations for angiogenesis inhibitors used to treat hepatocellular carcinoma: an overview. Expert Opin Drug Metab Toxicol. 2023 doi: 10.1080/17425255.2023.2272598. [DOI] [PubMed] [Google Scholar]
- 155.Gadre S, M M, Chakraborty G, Rayrikar A, Paul S, Patra C, Patra M. Development of a highly in vivo efficacious dual antitumor and antiangiogenic organoiridium complex as a potential anti-lung cancer agent. J Med Chem. 2023;66:13481–13500. doi: 10.1021/acs.jmedchem.3c00704. [DOI] [PubMed] [Google Scholar]
- 156.Wang C, Huang M, Lin Y, Zhang Y, Pan J, Jiang C, Cheng M, Li S, He W, Li Z, et al. ENO2-derived phosphoenolpyruvate functions as an endogenous inhibitor of HDAC1 and confers resistance to antiangiogenic therapy. Nat Metab. 2023;5:1765–1786. doi: 10.1038/s42255-023-00883-y. [DOI] [PubMed] [Google Scholar]
- 157.Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70–83. doi: 10.1016/j.ejca.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 158.Moawad AW, Szklaruk J, Lall C, Blair KJ, Kaseb AO, Kamath A, Rohren SA, Elsayes KM. Angiogenesis in hepatocellular carcinoma; pathophysiology, targeted therapy, and role of imaging. J Hepatocell Carcinoma. 2020;7:77–89. doi: 10.2147/JHC.S224471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Annese T, Tamma R, Ruggieri S, Ribatti D. Angiogenesis in pancreatic cancer: pre-clinical and clinical studies. Cancers. 2019 doi: 10.3390/cancers11030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017 doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance. J Res Med Sci. 2017;22:117. doi: 10.4103/jrms.JRMS_182_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hameed S, Bhattarai P, Dai Z. Nanotherapeutic approaches targeting angiogenesis and immune dysfunction in tumor microenvironment. Sci China Life Sci. 2018;61:380–391. doi: 10.1007/s11427-017-9256-1. [DOI] [PubMed] [Google Scholar]
- 163.Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2019 doi: 10.3390/jcm9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale. 2016;8:12444–12470. doi: 10.1039/C5NR07887C. [DOI] [PubMed] [Google Scholar]
- 165.Franchi M, Piperigkou Z, Karamanos KA, Franchi L, Masola V. Extracellular matrix-mediated breast cancer cells morphological alterations, invasiveness, and microvesicles/exosomes release. Cells. 2020 doi: 10.3390/cells9092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eguchi T, Sogawa C, Okusha Y, Uchibe K, Iinuma R, Ono K, Nakano K, Murakami J, Itoh M, Arai K, et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE. 2018;13:e0191109. doi: 10.1371/journal.pone.0191109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:13012. doi: 10.1038/s41598-019-49671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bordanaba-Florit G, Madarieta I, Olalde B, Falcon-Perez JM, Royo F. 3D cell cultures as prospective models to study extracellular vesicles in cancer. Cancers. 2021 doi: 10.3390/cancers13020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ariston Gabriel AN, Wang F, Jiao Q, Yvette U, Yang X, Al-Ameri SA, Du L, Wang YS, Wang C. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol Cancer. 2020;19:132. doi: 10.1186/s12943-020-01245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li X, Li C, Zhang L, Wu M, Cao K, Jiang F, Chen D, Li N, Li W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol Cancer. 2020;19:1. doi: 10.1186/s12943-019-1085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, Ge S, Li J, Ning T, Deng T, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–641. doi: 10.1111/cas.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.El-Kattawy AM, Algezawy O, Alfaifi MY, Noseer EA, Hawsawi YM, Alzahrani OR, Algarni A, Kahilo KA, El-Magd MA. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed Pharmacother. 2021;143:112220. doi: 10.1016/j.biopha.2021.112220. [DOI] [PubMed] [Google Scholar]
- 176.Zhao L, Yu Q, Gao C, Xiang J, Zheng B, Feng Y, Li R, Zhang W, Hong X, Zhan YY, et al. Studies of the efficacy of low-dose apatinib monotherapy as third-line treatment in patients with metastatic colorectal cancer and apatinib's novel anticancer effect by inhibiting tumor-derived exosome secretion. Cancers. 2022 doi: 10.3390/cancers14102492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao Y, Yin Z, Qi Y, Peng H, Ma W, Wang R, Li W. Golgi phosphoprotein 3 promotes angiogenesis and sorafenib resistance in hepatocellular carcinoma via upregulating exosomal miR-494-3p. Cancer Cell Int. 2022;22:35. doi: 10.1186/s12935-022-02462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang Y-H, Wen C-S, Kuo Y-L, Fu S-L, Lin T-Y, Chen C-M, Wu P-K, Chen W-M, Wang J-Y. GuiLu-ErXian glue extract promotes mesenchymal stem cells (MSC)-induced chondrogenesis via exosomes release and delays aging in the MSC senescence process. J Ethnopharmacol. 2023;317:116784. doi: 10.1016/j.jep.2023.116784. [DOI] [PubMed] [Google Scholar]
- 179.Alam MR, Rahman MM, Li Z. The link between intracellular calcium signaling and exosomal PD-L1 in cancer progression and immunotherapy. Genes Dis. 2024;11:321–334. doi: 10.1016/j.gendis.2023.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kang C, He H, Liu P, Liu Y, Li X, Zhang J, Ran H, Zeng X, Zhao H, Liu J, Qiu S. Role of dendritic cell-derived exosomes in allergic rhinitis (Review) Int J Mol Med. 2023 doi: 10.3892/ijmm.2023.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahmadi M, Rezaie J. Tumor cells derived-exosomes as angiogenenic agents: possible therapeutic implications. J Transl Med. 2020;18:249. doi: 10.1186/s12967-020-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang J, Wang C, Li Y, Li M, Zhu T, Shen Z, Wang H, Lv W, Wang X, Cheng X, Xie X. Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin Transl Med. 2021;11:e425. doi: 10.1002/ctm2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rizk NI, Abulsoud AI, Kamal MM, Kassem DH, Hamdy NM. Exosomal-long non-coding RNAs journey in colorectal cancer: Evil and goodness faces of key players. Life Sci. 2022;292:120325. doi: 10.1016/j.lfs.2022.120325. [DOI] [PubMed] [Google Scholar]
- 185.Katayama Y, Uchino J, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Tumor neovascularization and developments in therapeutics. Cancers. 2019 doi: 10.3390/cancers11030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu R, Greening DW, Zhu H-J, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Investig. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41:296. doi: 10.1186/s13046-022-02499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Z, Xie Y, Chen W, Li T, Chen X, Liu B. microRNA-6785-5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021;284:119222. doi: 10.1016/j.lfs.2021.119222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.